Abstract
The reaction of (CO)5MnBr with phenoxatellurine (PT) provided the octahedral complex fac-(CO)3(PT)2MnBr in which the two PT ligands are situated in cis-position.
Hieber’s pioneering work on metal carbonyls laid the foundation for the field of organometallic chemistry (Hieber, 1970). Whilst researching the substitution of CO by alternative ligands, his group also studied the reaction of (CO)5MnCl with phenoxatellurine (PT), which provided (CO)3(PT)2MnCl (1) as octahedral complex with an unknown constitution (Scheme 1) (Hieber and Kruck, 1962).
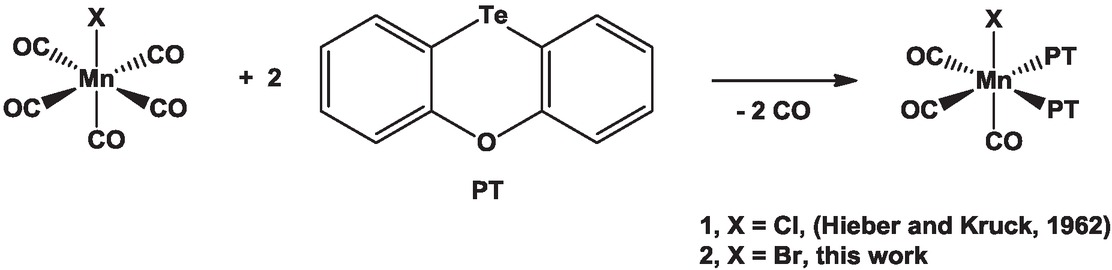
Reaction of (CO)5MnX (X = Cl, Br) with phenoxatellurine (PT).
Recently, we (re-)investigated the single-electron oxidation of PT (Mostaghimi et al., 2019a, 2019b) and the preparation of charge-transfer complexes with PT (Chulanova et al., 2017), which prompted us study the closely related reaction of (CO)5MnBr with PT that afforded an analogous complex fac-(CO)3(PT)2MnBr (2) as colourless, low-melting solid (Scheme 1). Although 2 was reasonably soluble in many solvents including chloroform and dichloromethane, no reasonable NMR spectra were acquired, which was tentatively attributed to the paramagnetic nature of the sample. The molecular structure of 2 is shown in Figure 1 and selected bond parameters are collected in the caption of the figure. Crystal and refinement data are listed in Table 1.
![Figure 1 Molecular structure of fac-(CO)3(PT)2MnBr (2) showing 50% probability ellipsoids and the crystallographic numbering scheme. Selected bond parameters [Å]: Mn1-Te1 2.650(6), Mn1-Te2 2.612(8), Mn1-C1 1.814(4), Mn1-C2 1.811(1), Mn1-C3 1.774(4), Mn1-Br1 2.524(2), Te1-Mn1-Te2 89.01(6), Te1-Mn1-Br1 86.19(0), Te1-Mn1-C1 87.61(4), Te1-Mn1-C2 175.32(5), Te1-Mn1-C3 95.18(0), Te2-Mn1-Br1 83.52(7), Te2-Mn1-C1 175.64(3), Te2-Mn1-C2 91.26(5), Te2-Mn1-C3 92.91(9).](/document/doi/10.1515/mgmc-2020-0022/asset/graphic/j_mgmc-2020-0022_fig_002.jpg)
Molecular structure of fac-(CO)3(PT)2MnBr (2) showing 50% probability ellipsoids and the crystallographic numbering scheme. Selected bond parameters [Å]: Mn1-Te1 2.650(6), Mn1-Te2 2.612(8), Mn1-C1 1.814(4), Mn1-C2 1.811(1), Mn1-C3 1.774(4), Mn1-Br1 2.524(2), Te1-Mn1-Te2 89.01(6), Te1-Mn1-Br1 86.19(0), Te1-Mn1-C1 87.61(4), Te1-Mn1-C2 175.32(5), Te1-Mn1-C3 95.18(0), Te2-Mn1-Br1 83.52(7), Te2-Mn1-C1 175.64(3), Te2-Mn1-C2 91.26(5), Te2-Mn1-C3 92.91(9).
The spatial arrangement of the manganese atom is octahedral and defined by a C3Te2Br donor set. The three CO ligands are arranged in a facial manner, which is consistent with the observation of three intense CO stretching vibrations at ῦ = 2007, 1943 and 1905 cm–1. For the starting material (CO)5MnBr, the IR spectrum shows two intense CO stretching vibrations at t ῦ = 2055 and 1999 cm–1 and in addition six minor intense CO stretching vibrations (Kaesz, et al., 1967). The two PT ligands adopt butterfly conformations and are situated in cis-position to each other. The degree of folding within the butterfly conformation may be quantified by the fold angle α between the planes defined by the two phenyl rings and the Te and O atoms. The fold angles α of 2 (Te1: 34.4°, Te2: 37.8°) are very similar to than in the free PT (37.5°) (Mostaghimi et al, 2019a). The Te-Mn bond lengths of 2 (2.612(8) and 2.650(6) Å) are substantially longer than those of peri-substituted acenaphthyl (Ace)-based complexes fac-(6-Ph2P-Ace-5-)2 TeMn(CO)3Br (2.599(1) Å) and (6-Ph2P-Ace-5-)2Mn(CO)2Br (2.546(1) Å) (Do et al., 2018).
Experimental
Synthesis of fac-(CO)3(PT)2MnBr (2)
In a 25 mL J. Young tube, a solution of PT (470 mg, 1.6 mmol) in absolute ethanol (20 mL) was added to (CO)5MnBr (200 mg, 0.7 mmol). The mixture was heated to 50°C. After the (CO)5MnBr had entirely dissolved, the solvent was evaporated to dryness. The solid residue was dissolved in the minimum amount of dry dichloromethane. This solution was carefully layered by the same volume of hexane. Slow diffusion of the solvents induced crystallization of the product, which was obtained as orange needles (450 mg, 79% yield; Mp.: 75°C dec.).
MS (ESI, positive, CH2Cl2/CH3CN 1:10, 3μL/min): m/z (rel. Int) = 537 (100%) [M-PT+Na], 521 (56%) [M-PT+Li], 437 (55%) [Mn(CO)3PT].
X-ray crystallography
Intensity data of 2 was collected on a Bruker Venture D8 diffractometer with graphite-monochromated Mo-Kα (0.7107 Å) radiation. The structure was solved by direct methods and difference Fourier synthesis with subsequent Full-matrix least-squares refinements on F2, using all data (Dolomanov, 2009). All non-hydrogen atoms were refined using anisotropic displacement parameters. Hydrogen atoms were included in geometrically calculated positions using a riding model. Crystal and refinement data are collected in Table 1. Figure 1 was created using DIAMOND (Brandenburg and Putz, 2006). Crystallographic data for the structural analysis has been deposited with the Cambridge Crystallographic Data Centre, CCDC numbers 2017546.
Crystal data and structure refinement of (C12H8OTe)2(CO)3MnBr.
| Formula | C27H16BrMnO5Te2 |
| Formula weight, g mol–1 | 810.45 |
| Crystal system | orthorhombic |
| Crystal size, mm | 0.60 × 0.60 × 0.50 |
| Space group | Pna21 |
| a, Å | 19.208(5) |
| b, Å | 20.513(5) |
| c, Å | 6.428(5) |
| V, Å3 | 2533(2) |
| Z | 4 |
| ρcalcd, Mg m–3 | 2.125 |
| T, K | 100 |
| μ (Mo Kα), mm–1 | 4.394 |
| F(000) | 1528 |
| θ range, deg | 2.25 to 30.08 |
| Index ranges | −27 ≤ h ≤ 27 |
| −28 ≤ k ≤ 28 | |
| −9 ≤ 1 ≤ 9 | |
| No. of reflns collected | 229503 |
| Completeness to θmax | 99.9% |
| No. indep. Reflns | 7419 |
| No. obsd reflns with (I>2σ(I)) | 7227 |
| No. refined params | 130 |
| GooF (F2) | 1.047 |
| R1 (F) (I > 2σ(I)) | 0.0119 |
| wR2 (F2) (all data) | 0.0252 |
| Flack parameter | 0.008(2) |
| (Δ/σ)max | < 0.001 |
| Largest diff peak/hole, e Å–3 | 0.261 / –0.492 |
Copies of this information may be obtained free of charge from The Director, CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (Fax: +44-1223-336033; e-mail: deposit@ccdc. cam.ac.uk or http://www.ccdc.cam.ac.uk)
Conflict of interest: One of the authors (Jens Beckmann) is a member of the Editorial Board of Main Group Metal Chemistry.
References
Brandenburg K., Putz H., DIAMOND V3.1d, Crystal Impact GbR, 2006.Search in Google Scholar
Chulanova E.A., Pritchina E.A., Malaspina L.A., Grabowsky S., Mostaghimi F., Beckmann J., et al., Novel Charge-Transfer Complexes with 1,2,5-Thiadiazoles as Both Electron Acceptors and Donors Featuring an Unprecedented Addition Reaction. Chem. Eur. J., 2017, 23, 852-864.10.1002/chem.201604121Search in Google Scholar
Do T.G., Hupf E., Lork E., Beckmann J., Bis(6-diphenylphosphino-acenaphth-5-yl)telluride as Ligand Towards Manganese and Rhenium Carbonyls. Molecules, 2018, 23, 2805.10.3390/molecules23112805Search in Google Scholar
Dolomanov O.V., Bourhis L.J., Gildea R.J., Howard A.K., Puschmann H., OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Cryst., 2009, 42, 339-341.10.1107/S0021889808042726Search in Google Scholar
Hieber W., Kruck T., Tellurorganyl-haltige Metallcarbonyle. Chem. Ber., 1962, 95, 2027-2041.10.1002/cber.19620950826Search in Google Scholar
Hieber W., Metal Carbonyls, forty years of research. Adv. Organomet. Chem., 1970, 8, 1-28.10.1016/S0065-3055(08)60632-2Search in Google Scholar
Kaesz H.D., Bau R., Hendrickson D., Smith J.M., Spectroscopic Studies of Isotopically Substituted Metal Carbonyls. I. Vibrational Analysis of Metal Pentacarbonyl Halides. J. Am. Chem. Soc., 1967, 89, 2844-2851.10.1021/ja00988a009Search in Google Scholar
Mostaghimi F., Bolsinger J., Lork E., Beckmann J., New Insights into the Oxidation of Phenoxatellurine with Sulfuric Acid. Main Group Met. Chem., 2019a, 42, 150-152.10.1515/mgmc-2019-0017Search in Google Scholar
Mostaghimi F., Lork E., Hong I., Roemmele T.L., Boeré R.T., Mebs S., et al., The Reaction of Phenoxatellurine with Single-Electron Oxidizers Revisited. New J. Chem., 2019b, 43, 12754-12766.10.1039/C9NJ02401HSearch in Google Scholar
© 2020 Mostaghimi et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- An accelerated and effective synthesis of zinc borate from zinc sulfate using sonochemistry
- A new approach on lithium-induced neurotoxicity using rat neuronal cortical culture: Involvement of oxidative stress and lysosomal/mitochondrial toxic Cross-Talk
- Green synthesis and characterization of hexaferrite strontium-perovskite strontium photocatalyst nanocomposites
- Assessment of content and chemical forms of arsenic, copper, lead, and chromium in sewage sludge compost as affected by various bulking agents
- Preparation of skeletally diverse quinazoline-2,4(1H,3H)-diones using Na2SiO3/SnFe2O4 catalytic system through a four-component reaction
- Efficient photocatalytic degradation of organic dye from aqueous solutions over zinc oxide incorporated nanocellulose under visible light irradiation
- Synthesis of pyrimidines by Fe3O4@SiO2-L-proline nanoparticles
- Abnormally aggregation-induced emissions observed from hydrogen- and silyl-substituted siloles
- Organodiphosphines in PtP2X2 (X = As, Ge or Te) derivatives – Structural aspects
- Synthesis and structural characterization of dialkyltin complexes of N-salicylidene-L-valine
- Ultrasound-promoted solvent-free synthesis of some new α-aminophosphonates as potential antioxidants
- Occupational exposure in lead and zinc mines induces oxidative stress in miners lymphocytes: Role of mitochondrial/lysosomal damage
- Eccentric topological properties of a graph associated to a finite dimensional vector space
- Magnetically recoverable nanostructured Pd complex of dendrimeric type ligand on the MCM-41: Preparation, characterization and catalytic activity in the Heck reaction
- Short Communications
- The crystal structure of the first ether solvate of hexaphenyldistannane [(Ph3Sn)2 • 2 THF]
- New crystal structures of alkali metal tetrakis(pentafluorophenyl)borates
- s-Block metal scorpionates – A new sodium hydrido-tris(3,5-dimethyl-1-pyrazolyl)borate salt showing an unusual core stabilized by bridging and terminal O-bonded DMSO ligands
- Reduction of a 1,4-diazabutadiene and 2,2’-bipyridine using magnesium(I) compounds
- fac-Bis(phenoxatellurine) tricarbonyl manganese(I) bromide
- A new 2D dibutyltin coordination polymer with 3,5-dinitrosalicylate and 4,4’-bipyridine ligands
- Review
- Structures of Pt(0)P3, Pt(0)P4 and Pt(II)P4 – Distortion isomers
- Special Issue: Topological descriptors of chemical networks: Theoretical studies (Guest Editors: Muhammad Imran and Muhammad Javaid)
- Modified Zagreb connection indices of the T-sum graphs
- Topological properties of metal-organic frameworks
- Eccentricity based topological indices of siloxane and POPAM dendrimers
- On topological aspects of degree based entropy for two carbon nanosheets
- On multiplicative degree based topological indices for planar octahedron networks
- Computing entire Zagreb indices of some dendrimer structures
Articles in the same Issue
- Research Articles
- An accelerated and effective synthesis of zinc borate from zinc sulfate using sonochemistry
- A new approach on lithium-induced neurotoxicity using rat neuronal cortical culture: Involvement of oxidative stress and lysosomal/mitochondrial toxic Cross-Talk
- Green synthesis and characterization of hexaferrite strontium-perovskite strontium photocatalyst nanocomposites
- Assessment of content and chemical forms of arsenic, copper, lead, and chromium in sewage sludge compost as affected by various bulking agents
- Preparation of skeletally diverse quinazoline-2,4(1H,3H)-diones using Na2SiO3/SnFe2O4 catalytic system through a four-component reaction
- Efficient photocatalytic degradation of organic dye from aqueous solutions over zinc oxide incorporated nanocellulose under visible light irradiation
- Synthesis of pyrimidines by Fe3O4@SiO2-L-proline nanoparticles
- Abnormally aggregation-induced emissions observed from hydrogen- and silyl-substituted siloles
- Organodiphosphines in PtP2X2 (X = As, Ge or Te) derivatives – Structural aspects
- Synthesis and structural characterization of dialkyltin complexes of N-salicylidene-L-valine
- Ultrasound-promoted solvent-free synthesis of some new α-aminophosphonates as potential antioxidants
- Occupational exposure in lead and zinc mines induces oxidative stress in miners lymphocytes: Role of mitochondrial/lysosomal damage
- Eccentric topological properties of a graph associated to a finite dimensional vector space
- Magnetically recoverable nanostructured Pd complex of dendrimeric type ligand on the MCM-41: Preparation, characterization and catalytic activity in the Heck reaction
- Short Communications
- The crystal structure of the first ether solvate of hexaphenyldistannane [(Ph3Sn)2 • 2 THF]
- New crystal structures of alkali metal tetrakis(pentafluorophenyl)borates
- s-Block metal scorpionates – A new sodium hydrido-tris(3,5-dimethyl-1-pyrazolyl)borate salt showing an unusual core stabilized by bridging and terminal O-bonded DMSO ligands
- Reduction of a 1,4-diazabutadiene and 2,2’-bipyridine using magnesium(I) compounds
- fac-Bis(phenoxatellurine) tricarbonyl manganese(I) bromide
- A new 2D dibutyltin coordination polymer with 3,5-dinitrosalicylate and 4,4’-bipyridine ligands
- Review
- Structures of Pt(0)P3, Pt(0)P4 and Pt(II)P4 – Distortion isomers
- Special Issue: Topological descriptors of chemical networks: Theoretical studies (Guest Editors: Muhammad Imran and Muhammad Javaid)
- Modified Zagreb connection indices of the T-sum graphs
- Topological properties of metal-organic frameworks
- Eccentricity based topological indices of siloxane and POPAM dendrimers
- On topological aspects of degree based entropy for two carbon nanosheets
- On multiplicative degree based topological indices for planar octahedron networks
- Computing entire Zagreb indices of some dendrimer structures

