Abstract
Fe3O4@SiO2-L-proline nanoparticles have been used as an effective catalyst for the preparation of pyrimidines by three-component reactions of 1,3-dimethylbarbituric acid, aromatic aldehydes and 4-methyl aniline or 4-methoxy aniline under reflux condition in ethanol. Fe3O4@SiO2-L-proline nanoparticles have been characterized by scanning electronic microscopy (SEM), powder X-ray diffraction (XRD), vibrating sample magnetometer (VSM), thermal gravimetric analysis (TGA), energy dispersive X-ray (EDS), dynamic light scattering (DLS) and FT-IR spectroscopy. This method provides several advantages including, the reusability of the catalyst, low catalyst loading, atom economy, short reaction times and high yields of products.
1 Introduction
Pyrimidines have many biological properties including anticancer (Singh et al., 2009), anti-diabetic (Barakat et al., 2015), antioxidan (Barakat et al., 2016), antibacterial, anti-fungal (Dhorajiya et al., 2014), and anticonvulsant (Andrews et al., 1979). The discovery impressive ways for the preparation of pyrimidines is a serious challenge (Maleki and Paydar, 2016; Shaabani et al., 2010; Maleki and Aghaei, 2017). The synthesis of pyrimidine derivatives have been developed by InCl3 (Sharma et al., 2015), H3PW12O40 (Panahi et al., 2013), K2CO3 (Azzam and Pasha, 2012), p-toluene sulfonic acid (Rahmati and Khalesi, 2012), [Bmim]PF6 (Shirvan et al., 2012). Each of these procedures may have its own advantages but also suffer from such apparent drawbacks as prolonged reaction times, complicated work-up, low yield, or hazardous reaction conditions. Despite the availability of these ways, there remains enough choice for a capable and reusable catalyst with high catalytic activity for the preparation of pyrimidines. Multicomponent reactions (MCRs) enhance the efficiency by combining several operational steps without isolation of intermediates or changing the reaction conditions (Maleki, 2013; Maleki et al., 2018a; Shaabani et al., 2007, 2009). Ideally, introducing neat processes and utilizing eco-friendly and green catalysts which can be simply recycled at the end of reactions has obtained great attention in recent years (Maleki, 2018). Magnetic nanoparticles (MNPs) have been utilized as a robust catalyst with a very significant feature of straightforward separation by external magnet bar (Chen et al., 2015; Elsayed et al., 2018; Fakheri-Vayeghan et al., 2018; Maleki et al., 2018b; Safaei-Ghomi et al., 2018). Magnetic materials have appeared as a useful group of heterogeneous catalysts owing to their various usages in synthesis and catalysis (Huang et al., 2016; Maleki, 2012, 2014; Shin et al., 2015; Tietze et al., 2015). The surface of magnetic nanoparticles can be functionalized by surface modifications to provide the attachment of a variety of eligible functionalities (Deatsch and Evans, 2014; Maleki and Firouzi-Haji, 2018; Zhang et al., 2012; Zheng et al., 2009). Herein, we report the use of nano-Fe3O4@SiO2-L-proline as an effective catalyst for the synthesis of pyrimidines by three-component reactions of 1,3-dimethylbarbituric acid, aromatic aldehydes and 4-methyl aniline or 4-methoxy aniline under reflux condition in ethanol (Scheme 1).

Synthesis of pyrimidines using Fe3O4@SiO2-L-proline nanoparticles.
2 Results and discussion
The process of the preparation Fe3O4@SiO2-L-proline nanoparticles is shown in Scheme 2.
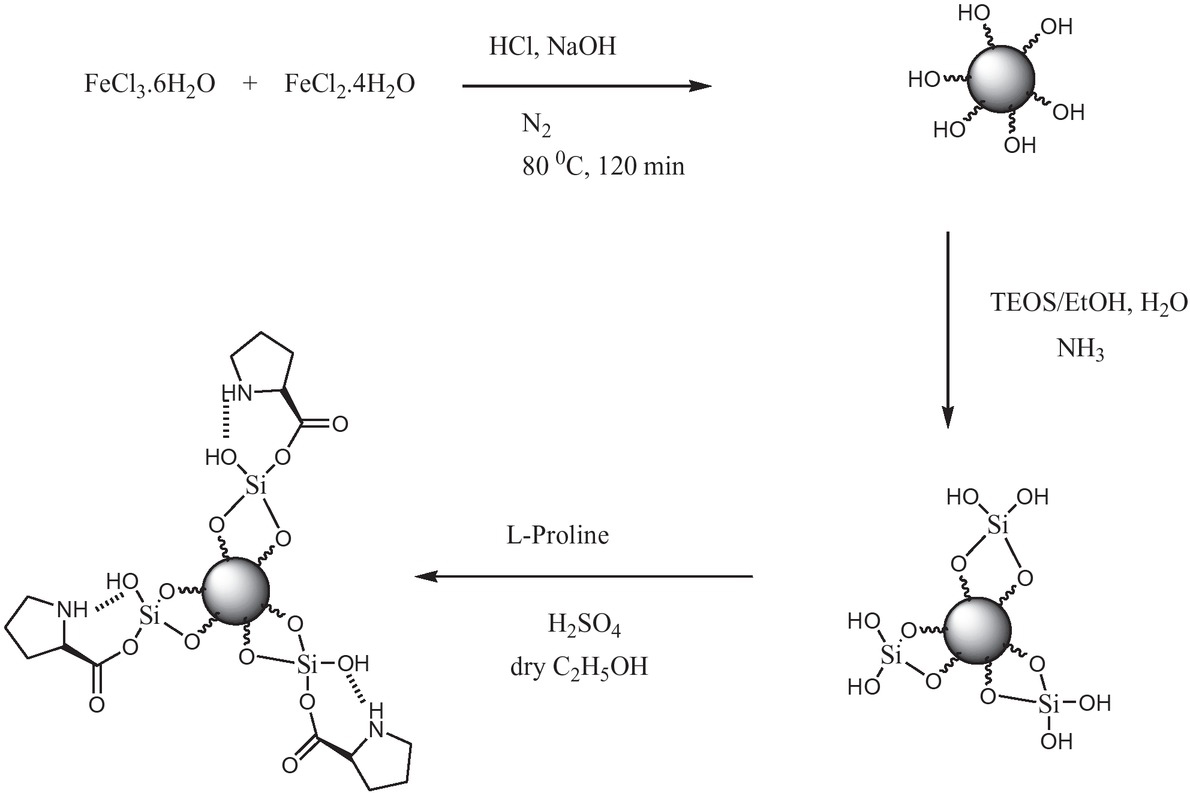
Schematic illustration of the preparation Fe3O4@SiO2-L-proline nanoparticles.
XRD pattern of Fe3O4 and Fe3O4@SiO2-L-proline nanoparticles is indicated in Figure 1. The pattern agrees with the reported pattern for Fe3O4 (JCPDS No. 75-1609). The crystallite size of Fe3O4@SiO2-L-proline was calculated by the Debye–Scherer equation is about 46-50 nm, in good agreement with the result gained by SEM.

The XRD pattern of (a) Fe3O4 and (b) Fe3O4@SiO2-L-proline nanoparticles.
The particle size and morphology of Fe3O4@SiO2-L-proline was indicated by SEM. The statistic of results from SEM images exhibit that the average size of Fe3O4@SiO2-L-proline is about 45-50 nm (Figure 2).

SEM image of Fe3O4@SiO2-L-proline nanoparticles.
Figure 3 displays FT-IR spectra of Fe3O4, Fe3O4@SiO2 and Fe3O4@SiO2-L-proline nanoparticles. The FT-IR spectra of Fe3O4@SiO2 and Fe3O4@SiO2-L-proline nanoparticles show the vibrations of Fe-O at 570-588 cm−1. The band at about 1050 cm−1 belongs to Si–O stretching vibrations. The peaks that appear at 1042, 1131 and 3320 cm−1 are related to the stretching of C–O, C–N and N-H bonds. A peak appears at about 1720 cm−1 due to the stretching of the C=O group in L-proline.

FT-IR spectra of (a) Fe3O4 (b) Fe3O4@SiO2 and (c) Fe3O4@ SiO2-L-proline nanoparticles.
The magnetic properties of Fe3O4, Fe3O4@SiO2 and Fe3O4 @SiO2-L-proline were determined using VSM (Figure 4). The amount of saturation magnetization for Fe3O4, Fe3O4@SiO2 and Fe3O4@SiO2-L-proline is 59.2, 41.3 and 14.5 emu/g. These results demonstrate that the magnetization property decreases by coating and functionalization.

The VSM curve of: (a) Fe3O4 (b) Fe3O4@SiO2 and (c) Fe3O4@ SiO2-L-proline nanoparticles.
TGA indicates the thermal stability of the Fe3O4@ SiO2-L-proline. These nanoparticles display appropriate thermal stability without a significant decrease in weight (Figure 5). The weight loss at temperatures below 200°C is owing to the removal of physically adsorbed solvent. The curve indicates a weight loss about 12.7% from 250°C to 600°C, resulting from the decomposition of the organic spacer grafting to the nanoparticles surface.

TGA curve of Fe3O4@SiO2-L-proline nanoparticles.
An EDS spectrum of Fe3O4@SiO2-L-proline (Figure 6) displays that the elemental compositions are carbon, silicon, oxygen, iron and nitrogen.

EDS spectrum of Fe3O4@SiO2-L-proline nanoparticles.
In order to investigate the size distribution of nanocatalysts, DLS (dynamic light scattering) measurements of the nanoparticles were showed in Fig ure 7. The size distribution is centered at a value of 49.2 nm. The dispersion for DLS analysis (1.25 g nanocatalyst at 25 mL ethanol) was prepared using an ultrasonic bath (50 W) for 20 min.

DLS of Fe3O4@SiO2-L-proline nanoparticles.
Initially, we had optimized different reaction parameters for the preparation of pyrimidines by three-component reactions of 1,3-dimethylbarbituric acid, 4-chlorobenzaldehyde and 4-methoxy aniline as a model reaction. The model reactions were performed using CAN, L-proline, Et3N, Fe3O4, Fe3O4@SiO2 and Fe3O4@SiO2-L-proline nanoparticles. Several reactions were investigated using diverse solvents such as ethanol, water, acetonitrile and dimethylformamide. The best results were achieved in EtOH and we found that the reaction gave satisfying results using Fe3O4@SiO2-L-proline which gave good yields of products (Table 1).
Optimization of reaction conditions using different catalysts.[a]
| Entry | Solvent (reflux) | Catalyst | Time (min) | Yield (%)[b] |
|---|---|---|---|---|
| 1 | EtOH | ------- | 500 | trace |
| 2 | EtOH | CAN (4 mol%) | 400 | 22 |
| 3 | EtOH | Et3N (10 mol%) | 300 | 35 |
| 4 | EtOH | L-proline (10 mol%) | 150 | 55 |
| 5 | EtOH | Fe3O4 NP (50 mg) | 200 | 25 |
| 6 | EtOH | Nano-Fe3O4@SiO2 (20 mol%) | 200 | 33 |
| 7 | H2O | Nano-Fe3O4@SiO2-L-proline (8 mg) | 120 | 62 |
| 8 | DMF | Nano-Fe3O4@SiO2-L-proline (8 mg) | 120 | 70 |
| 9 | CH3CN | Nano-Fe3O4@SiO2-L-proline (8 mg) | 120 | 85 |
| 10 | EtOH | Nano-Fe3O4@SiO2-L-proline (6 mg) | 120 | 90 |
| 11 | EtOH | Nano-Fe3O4@SiO2-L-proline (8 mg) | 120 | 92 |
| 12 | EtOH | Nano-Fe3O4@SiO2-L-proline (10 mg) | 120 | 92 |
The above results indicate the present catalytic way is extendable to a wide diversity of substrates to create pyrimidines. Investigations of the reaction scope showed that diverse aromatic aldehydes can be utilized in this method (Table 2). Turnover frequency (TOF) for this catalytic system is listed in Table 2.
Synthesis of pyrimidines by nano-Fe3O4@SiO2-L-proline (8 mg) under reflux condition.
| Entry | R (Aldehyde) | Rʹ (Aniline) | Product | Time (min) | Yield[a] (%) | M.p. (°C) | TOF (min–1) |
|---|---|---|---|---|---|---|---|
| 1 | 2-NO2 | 4-CH3 | 4a | 150 | 88 | 183-185 | 0.56 |
| 2 | 4-SCH3 | 4-OCH3 | 4b | 150 | 84 | 190-192 | 0.52 |
| 3 | 4-Cl | 4-OCH3 | 4c | 120 | 92 | 240-242 | 0.77 |
| 4 | 3-NO2 | 4-CH3 | 4d | 130 | 88 | 188-190 | 0.68 |
| 5 | 4-NO2 | 4-OCH3 | 4e | 120 | 93 | 237-239 | 0.78 |
| 6 | 2,4-di-Cl | 4-CH3 | 4f | 120 | 90 | 198-200 | 0.75 |
| 7 | 4-F | 4-OCH3 | 4g | 120 | 89 | 222-224 | 0.73 |
| 8 | 4-CH3 | 4-CH3 | 4h | 120 | 82 | 205-207 | 0.66 |
The reusability of Fe3O4@SiO2-L-proline nanoparticles was investigated for the reaction of 1,3-dimethylbarbituric acid, 4-chlorobenzaldehyde and 4-methoxy aniline. After completion of the reaction, the nanocatalyst was separated using an external magnet. The recovered magnetite nanoparticles were washed several times with ethanol and then dried at room temperature. Figure 8 shows that the nanocatalyst could be reused for six times with a minimal loss of activity.

Reusability of Fe3O4@SiO2-L-proline nanoparticles as catalyst for the synthesis of 4c.
FT-IR analysis before and after reuse of the Fe3O4@ SiO2-L-proline nanoparticles is shown in Figure 9. The nanoparticles remained unchanged before and after reaction. We suppose that, this is also the possible reason for the extreme stability of the Fe3O4@SiO2-L-proline nanoparticles presented herein. The extreme stability of the Fe3O4@SiO2-L-proline nanoparticles is mainspring of the continuous and high catalytic activity.

FT-IR analysis before and after reuse of the Fe3O4@SiO2-L-proline nanoparticles.
The proposed mechanism for the reaction is shown in Scheme 3. Initially, 1,3-dimethylbarbituric acid is reacted with aldehyde to form intermediate (I) via condensation reaction. Intermediate (I), in the presence of Fe3O4@SiO2- L-proline, is condensed with aniline derivatives to form intermediate (II). The migration of the hydrogen atom will provide the final product. It is assumed that catalytically active site of catalyst is contains SiO2 that acts as a Lewis acid and L-proline (−NH−) that acts as a Lewis basic. This proposed mechanism has also been supported by the literature (Fekri et al., 2019; Keshavarz et al., 2018; Safaei-Ghomi et al., 2017).

Proposed reaction pathway for the synthesis of pyrimidines by nano-Fe3O4@SiO2-L-proline.
3 Conclusions
In conclusion, we have developed an effective method for the synthesis of pyrimidines using Fe3O4@SiO2-L-proline. The method offers several advantages containing high yields, cleaner reaction profiles, shorter reaction times, easy availability, reusability of the catalyst and low catalyst loading.
Experimental
Preparation of Fe3O4@SiO2-L-proline nanocatalyst
Firstly, nano-Fe3O4@SiO2 (1 g) were dispersed in dry ethanol (10mL) using an ultrasonic bath for 30 min.
Subsequently, L-proline (0.6 g) and H2SO4 (1 mL) were added to the solution of Fe3O4@SiO2 (1 g) and heated under reflux conditions. Then, the mixture was stirred for a further 12 h to allow for the completion of the reaction. The resulting magnetic nanoparticles were separated by external magnet and washed with ethanol and water before being dried in an oven at 60°C to give Fe3O4@SiO2- L-proline as a light brown powder.
General procedure for the preparation of pyrimidines
A mixture of 1,3-dimethylbarbituric acid (1 mmol), aromatic aldehydes (1 mmol) and 4-methyl aniline or 4-methoxy aniline (1 mmol) and Fe3O4@SiO2-L-proline (8 mg) were heated in EtOH (10 mL) under reflux conditions. The completion of the mixture was monitored by TLC and the catalyst was separated from reaction before work up by an external magnet field. The precipitate was washed with EtOH to afford the pure product.
Spectral data of products
5-((2-amino-5-methylphenyl)(2-nitrophenyl)methyl)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione (4a): Yellow solid; m. p. 183°C-185°C, – IR (KBr): ν = 3323, 3318, 2922, 1689, 1550, 1350 cm–1. – 1H NMR (400 MHz, [D6]DMSO): δ (ppm) = 2.99 (s, 3H, CH3), 3.33 (s, 6H, 2CH3), 5.33 (d, J = 8.8 Hz, 1H, CH), 5.47 (d, J = 8.8 Hz, 1H, CH), 6.99-7.47 (m, 7H, ArH), 9.18 (s, 2H, NH2). – 13C NMR (100 MHz, [D6]DMSO): δ (ppm) = 25.15, 31.76, 32.19, 43.73, 54.14, 121.20, 121.21, 125.15, 129.07, 129.11, 136.80, 136.82, 139.08, 142.20, 148.19, 154.19, 170.01.– Analysis for C20H20N4O5: calcd. C, 60.60; H, 5.09; N, 14.13, Found C, 60.52; H, 5.03; N, 14.05%.
5-((2-amino-5-methoxyphenyl)(4-(methylthio)phenyl) methyl)-1,3-dimethylpyrimidine-2,4,6 (1H,3H,5H)-trione (4b): Yellow solid; m. p. 190°C-192°C, – IR (KBr): ν = 3414, 2917, 1680, 1490, 1442, 816 cm–1. – 1H NMR (400 MHz, [D6] DMSO): δ (ppm) = 2.04 (s, 3H, CH3), 2.49 (s, 3H, CH3), 2.81 (s, 6H, 2CH3), 4.93 (d, J = 9.2 Hz, 1H, CH), 5.10 (d, J = 9.2 Hz, 1H, CH), 6.31-7.23 (m, 9H, ArH and NH2). – 13C NMR (100 MHz, [D6]DMSO): δ (ppm) = 22.12, 27.90, 28.54, 50.51, 59.12, 64.58, 121.98, 124.92, 125.85, 126.05, 127.54, 127.92, 128.08, 135.05, 138.12, 139.83, 142.53, 148.77, 164.63, 169.61. – Analysis for C21H23N3O4S: calcd. C, 61.00; H, 5.61; N, 10.16; S, 7.75, Found C, 61.09; H, 5.75; N, 10.19; S, 7.82%.
5-((2-amino-5-methoxyphenyl)(4-chlorophenyl) methyl)-1,3-dimethylpyrimidine-2,4,6 (1H,3H, 5H)-trione (4c): Yellow solid; m. p. 240°C-242°C, – IR (KBr): ν = 3343, 2925, 1667, 1493, 528 cm–1. – 1H NMR (400 MHz, [D6]DMSO): δ (ppm) = 2.80 (s, 6H, 2CH3), 2.96 (s, 3H, CH3), 4.80 (d, J = 10.2 Hz, 1H, CH), 5.12 (d, J = 10.2 Hz, 1H, CH), 6.49-7.43 (m, 9H, ArH and NH2). – 13C NMR (100 MHz, [D6]DMSO): δ (ppm) = 27.15, 28.14, 44.00, 51.50, 59.15, 111.49, 117.32, 120.31, 129.84, 130.01, 135.51, 138.42, 139.50, 145.01, 153.30, 157.49, 161.96.– Analysis for C20H20ClN3O4: calcd. C, 59.78; H, 5.02; N, 10.46, Found C, 59.82; H, 5.15; N, 10.58%.
5-((2-amino-5-methylphenyl)(3-nitrophenyl)methyl)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione (4d): Yellow solid; m. p. 188°C-190°C, – IR (KBr): ν = 3405, 2916, 1681, 1532, 1350, 1112 cm–1. – 1H NMR (400 MHz, [D6]DMSO): δ (ppm) = 2.06 (s, 3H, CH3), 2.75 (s, 3H, CH3), 2.77 (s, 3H, CH3), 5.06 (d, J = 8.8 Hz, 1H, CH), 5.33 (d, J = 8.8 Hz, 1H, CH), 6.31-8.26 (m, 9H, ArH and NH2). – 13C NMR (100 MHz, [D6]DMSO): δ (ppm) = 20.78, 27.98, 28.66, 49.32, 64.11, 122.02, 123.51, 124.77, 125.15, 128.07, 130.44, 130.77, 134.56, 138.86, 139.55, 142.78, 142.20, 153.01, 168.81.– Analysis for C20H20N4O5: calcd. C, 60.60; H, 5.09; N, 14.13, Found C, 60.75; H, 5.19; N, 14.25%.
5-((2-amino-5-methoxyphenyl)(4-nitrophenyl) methyl)-1,3-dimethylpyrimidine-2,4,6(1H,3H, 5H)-trione (4e): Yellow solid; m. p. 237°C-239°C, – IR (KBr): ν = 3473, 3073, 2955, 1667, 1524, 1349 cm–1. – 1H NMR (400 MHz, [D6] DMSO): δ (ppm) = 2.79 (s, 6H, 2CH3), 3.51 (s, 3H, CH3), 4.99 (d, J = 8.2 Hz, 1H, CH), 5.36 (d, J = 8.2 Hz, 1H, CH), 6.70-8.25 (m, 9H, ArH and NH2). – 13C NMR (100 MHz, [D6]DMSO): δ (ppm) = 28.01, 28.69, 50.06, 55.69, 59.15, 113.50, 113.63, 116.23, 122.30, 123.99, 129.14, 132.01, 132.09, 145.53, 147.51, 148.22, 148.41, 151.50, 168.74.– Analysis for C20H20N4O6: calcd. C, 58.25; H, 4.89; N, 13.59, Found C, 58.35; H, 4.95; N, 13.68%.
5-((2-amino-5-methylphenyl)(2,4-dichlorophenyl) methyl)-1,3-dimethylpyrimidine-2,4,6(1H,3H, 5H)-trione (4f): Yellow solid; m. p. 198°C-200°C, – IR (KBr): ν = 3294, 3203, 3067, 2921, 1683, 1474 cm–1. – 1H NMR (400 MHz, [D6] DMSO): δ (ppm) = 2.11 (s, 3H, CH3), 3.72 (s, 3H, CH3), 2.88 (s, 3H, 2CH3), 4.90 (d, J = 10.8 Hz, 1H, CH), 4.94 (d, J = 10.8 Hz, 1H, CH), 6.34-7.94 (m, 7H, ArH), 9.02 (s, 2H, NH2). – 13C NMR (100 MHz, [D6]DMSO): δ (ppm) = 20.23, 30.34, 40.53, 40.55, 52.95, 115.63, 124.86, 124.91, 125.22, 128.48, 129.77, 134.42, 135.05, 138.51, 138.51, 139.45, 145.06, 149.96, 158.46, 171.98.– Analysis for C20H19Cl2N3O3: calcd. C, 57.15; H, 4.56; N, 10.00, Found C, 57.05; H, 4.42; N, 10.15%.
5-((2-amino-5-methoxyphenyl)(4-fluorophenyl) methyl)-1,3-dimethylpyrimidine-2,4,6(1H,3H, 5H)-trione (4g): Yellow solid; m. p. 222°C-224°C, – IR (KBr): ν = 3376, 1686, 1524, 1464, 473 cm–1. – 1H NMR (400 MHz, [D6]DMSO): δ (ppm) = 2.80 (s, 3H, CH3), 2.82 (s, 3H, CH3), 3.51 (s, 3H, CH3), 5.04 (d, J = 10.8 Hz, 1H, CH), 5.36 (d, J = 10.8 Hz, 1H, CH), 6.70-8.25 (m, 9H, ArH and NH2). – 13C NMR (100 MHz, [D6]DMSO): δ (ppm) = 28.05, 28.69, 50.66, 55.69, 59.15, 113.15, 113.63, 116.23, 122.30, 123.96, 129.14, 132.01, 132.09, 145.53, 147.51, 148.32, 149.41, 151.50, 168.74.– Analysis for C20H20FN3O4: calcd. C, 62.33; H, 5.23; N, 10.90, Found C, 62.45; H, 5.35; N, 10.98%.
5-((2-amino-5-methylphenyl)(p-tolyl)methyl)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione (4h): Yellow solid; m. p. 205°C-207°C, – IR (KBr): ν = 3376, 1686, 1524, 1464, 473 cm–1. – 1H NMR (400 MHz, [D6]DMSO): δ (ppm) = 2.82 (s, 3H, CH3), 2.84 (s, 3H, CH3), 2.92 (s, 6H, CH3), 4.95 (d, J = 10.2 Hz, 1H, CH), 5.04 (d, J = 10.2 Hz, 1H, CH), 6.72-7.50 (m, 9H, ArH and NH2). – 13C NMR (100 MHz, [D6] DMSO): δ (ppm) = 22.20, 25.03, 28.67, 51.66, 58.60, 60.15, 115.18, 116.73, 117.20, 122.32, 123.95, 129.14, 132.01, 132.09, 145.53, 147.51, 148.32, 149.41, 151.50, 168.74.– Analysis for C21H23N3O3: calcd. C, 69.02; H, 6.34; N, 11.50;, Found C, 69.15; H, 6.42; N, 11.65%.
Acknowledgement
The authors are grateful to University of Kashan for supporting this work by Grant NO: 159196/XXI.
References
Andrews P.R., Jones G.P., Lodge D., Convulsant, anticonvulsant and anaesthetic barbiturates. 5-Ethyl-5-(3′-methyl-but-2′-enyl)-barbituric acid and related compounds. Eur. J. Pharmacol., 1979, 55, 115-120.10.1016/0014-2999(79)90382-0Search in Google Scholar PubMed
Azzam S.H.S., Pasha M.A., Microwave-assisted, mild, facile, and rapid one-pot three-component synthesis of some novel pyrano [2, 3-d] pyrimidine-2, 4, 7-triones. Tetrahedron Lett., 2012, 53, 7056–7059.10.1016/j.tetlet.2012.10.056Search in Google Scholar
Barakat A., Islama M.S., Al-Majid A.M., Ghabbour H.A., Yousuf S., Ashraf M., et al., Synthesis of pyrimidine-2, 4, 6-trione derivatives: Anti-oxidant, anti-cancer, α-glucosidase, β-glucuronidase inhibition and their molecular docking studies. Bioorg. Chem., 2016, 68, 72-79.10.1016/j.bioorg.2016.07.009Search in Google Scholar PubMed
Barakat A., Soliman S.M., Al-Majid A.M., Lotfy G., Ghabbour H.A., Fun H.K., et al., Synthesis and structure investigation of novel pyrimidine-2, 4, 6-trione derivatives of highly potential biological activity as anti-diabetic agent. J. Mol. Struct., 2015, 1098, 365-376.10.1016/j.molstruc.2015.06.037Search in Google Scholar
Chen Y., Wang Y., Zhang H.B., Li X., Gui C.X., Yu Z.Z., Enhanced electromagnetic interference shielding efficiency of polystyrene/ graphene composites with magnetic Fe3O4 nanoparticles. Carbon, 2015, 82, 67-76.10.1016/j.carbon.2014.10.031Search in Google Scholar
Deatsch A.E., Evans B.A., Heating efficiency in magnetic nanoparticle hyperthermia. J. Magn. Magn. Mater., 2014, 354, 163-172.10.1016/j.jmmm.2013.11.006Search in Google Scholar
Dhorajiya B.D., Dholakiya B.Z., Mohareb R.M., Hybrid probes of aromatic amine and barbituric acid: highly promising leads for anti-bacterial, anti-fungal and anti-cancer activities. Med. Chem. Res., 2014, 23, 3941–3952.10.1007/s00044-014-0973-5Search in Google Scholar
Elsayed I., Mashaly M., Eltaweel F., Jackson M.A., Hassana E.B., Dehydration of glucose to 5-hydroxymethylfurfural by a core-shell Fe3O4@ SiO2-SO3H magnetic nanoparticle catalyst. Fuel, 2018, 221, 407-416.10.1016/j.fuel.2018.02.135Search in Google Scholar
Fakheri-Vayeghan S., Abdolmohammadi S., Kia-Kojoori R., An expedient synthesis of 6-amino-5-[(4-hydroxy-2-oxo-2H-chromen-3-yl)(aryl)methyl]-1,3-dimethyl 2,4,6(1H,3H)-pyrimidin edione derivatives using Fe3O4@TiO2 nanocomposite as an efficient, magnetically separable, and reusable catalyst. Z. Naturforsch. B., 2018, 73, 545-551.10.1515/znb-2018-0030Search in Google Scholar
Fekri L.Z., Nikpassand M., Khakshoor S.N., Green, effective and chromatography free synthesis of benzoimidazo[1,2-a]pyrimidine and tetrahydrobenzo [4,5]imidazo [1,2-d] quinazolin-1(2H)-one and their pyrazolyl moiety using Fe3O4@SiO2@L-proline reusable catalyst in aqueous media. J. Organomet. Chem., 2019, 894, 18-27.10.1016/j.jorganchem.2019.05.004Search in Google Scholar
Huang J., Li Y., Orza A., Lu Q., Guo P., Wang L., et al., Magnetic nanoparticle facilitated drug delivery for cancer therapy with targeted and image‐guided approaches. Adv. Funct. Mater., 2016, 26, 3818-3836.10.1002/adfm.201504185Search in Google Scholar PubMed PubMed Central
Keshavarz M., Ahmady A.Z., Vaccaro L., Kardani M., Non-covalent supported of L-proline on graphene oxide/Fe3O4 nanocomposite: a novel, highly efficient and superparamagnetically separable catalyst for the synthesis of bis-pyrazole derivatives. Molecules, 2018, 23, 330-346.10.3390/molecules23020330Search in Google Scholar PubMed PubMed Central
Maleki A., Fe3O4SiO2 nanoparticles: an efficient and magnetically recoverable nanocatalyst for the one-pot multicomponent synthesis of diazepines. Tetrahedron, 2012, 68, 7827-7833.10.1016/j.tet.2012.07.034Search in Google Scholar
Maleki A., One-pot multicomponent synthesis of diazepine derivatives using terminal alkynes in the presence of silica-supported superparamagnetic iron oxide nanoparticles. Tetrahedron Lett., 2013, 54, 2055–2059.10.1016/j.tetlet.2013.01.123Search in Google Scholar
Maleki A., One-pot three-component synthesis of pyrido[2´,1´:2,3] imidazo[4,5-c]isoquinolines using Fe3O4@SiO2–OSO3H as an efficient heterogeneous nanocatalyst. RSC Adv., 2014, 4, 64169-64173.10.1039/C4RA10856FSearch in Google Scholar
Maleki A., Green oxidation protocol: selective conversions of alcohols and alkenes to aldehydes, ketones and epoxides by using a new multiwall carbon nanotube-based hybrid nanocatalyst via ultrasound irradiation. Ultrason. Sonochem., 2018, 40, 460-464.10.1016/j.ultsonch.2017.07.020Search in Google Scholar PubMed
Maleki A., Paydar R., Bionanostructure-catalyzed one-pot three-component synthesis of 3,4-dihydropyrimidin-2(1H)-one derivatives under solvent-free conditions. React. Funct. Polym., 2016, 109, 120-124.10.1016/j.reactfunctpolym.2016.10.013Search in Google Scholar
Maleki A., Aghaei M., Ultrasonic assisted synergetic green synthesis of polycyclic imidazo (thiazolo)pyrimidines by using Fe3O4@clay core-shell. Ultrason. Sonochem., 2017, 38, 585-589.10.1016/j.ultsonch.2016.08.024Search in Google Scholar PubMed
Maleki A., Firouzi-Haji R., Hajizadeh Z., Magnetic guanidinylated chitosan nanobiocomposite: A green catalyst for the synthesis of 1,4-dihydropyridines. Int. J. Biol. Macromol., 2018a, 116, 320-326.10.1016/j.ijbiomac.2018.05.035Search in Google Scholar PubMed
Maleki A., Firouzi-Haji R., L-Proline functionalized magnetic nanoparticles: A novel magnetically reusable nanocatalyst for one-pot synthesis of 2,4,6-triarylpyridines. Sci. Rep.-UK, 2018, 8, 17303-17310.10.1038/s41598-018-35676-xSearch in Google Scholar PubMed PubMed Central
Maleki A., Hajizadeh Z., Firouzi-Haji R., Eco-friendly functionalization of magnetic halloysite nanotube with SO3H for synthesis of dihydropyrimidinones. Micropor. Mesopor. Mat., 2018b, 259, 46-53.10.1016/j.micromeso.2017.09.034Search in Google Scholar
Panahi F., Yousefi R., Mehraban M.H., Khalafi-Nezhad A., Synthesis of new pyrimidine-fused derivatives as potent and selective antidiabetic α-glucosidase inhibitors. Carbohydr. Res., 2013, 380, 81-91.10.1016/j.carres.2013.07.008Search in Google Scholar PubMed
Rahmati A., Khalesi Z., A one-pot, three-component synthesis of spiro [indoline-isoxazolo [4′, 3′: 5, 6] pyrido [2, 3-d] pyrimidine] triones in water. Tetrahedron, 2012, 68, 8472-8479.10.1016/j.tet.2012.07.073Search in Google Scholar
Safaei-Ghomi J., Aghagoli R., Shahbazi-Alavi H., Synthesis of hexahydro-4-phenylquinoline-3-carbonitriles using Fe3O4@ SiO2-SO3H nanoparticles as a superior and retrievable heterogeneous catalyst under ultrasonic irradiations. Z. Naturforsch. B., 2018, 73, 269-274.10.1515/znb-2017-0200Search in Google Scholar
Safaei-Ghomi J., Nazemzadeh S.H., Shahbazi-Alavi H., Synthesis of propargylamines catalyzed by nano-colloidal silica-tethered polyhedral oligomeric silsesquioxanes with eight branches of 3-aminopropyltriethoxysilane as an efficient catalyst. Main Group Met. Chem., 2017, 40, 129-135.10.1515/mgmc-2017-0039Search in Google Scholar
Shaabani A., Soleimani E., Maleki A., One-pot three-component synthesis of 3-aminoimidazo [1,2-a]pyridines and -pyrazines in the presence of silica sulfuric acid. Monatsh. Chem., 2007, 138, 73-76.10.1007/s00706-006-0561-6Search in Google Scholar
Shaabani A., Seyyedhamzeh M., Maleki A., Hajishaabanha F., Diketene as an alternative substrate for a new Biginelli-like multicomponent reaction: one-pot synthesis of 5-carboxamide substituted 3,4-dihydropyrimidine-2(1H)ones. Tetrahedron, 2010, 66, 4040-4042.10.1016/j.tet.2010.04.028Search in Google Scholar
Shaabani A., Seyyedhamzeh M., Maleki A., Behnam M., Rezazadeh F., Synthesis of fully substituted pyrazolo[3,4-b]pyridine-5-carboxamide derivatives via a one-pot four-component reaction. Tetrahedron Lett., 2009, 50, 2911-2913.10.1016/j.tetlet.2009.03.200Search in Google Scholar
Sharma M., Borah P., Bhuyan P.J., InCl3-Catalyzed, Three-component reactions for the synthesis of some novel functionalized/ annulated barbituric acids. Synth. Commun., 2015, 45, 1792-1798.10.1080/00397911.2015.1045081Search in Google Scholar
Shirvan S.A., Ghahremanzadeh R., Moghaddam M.M., Bazgir A., Zarnani A.H., Akhondi M.M., A novel method for the synthesis of spiro [indoline‐ Pyrazolo [4′,3′:5,6]pyrido[2,3‐d]pyrimidine] triones by alum as a reusable catalyst. J. Heterocyclic Chem., 2012, 49, 951-954.10.1002/jhet.898Search in Google Scholar
Singh P., Kaur M., Verma P., Design, synthesis and anticancer activities of hybrids of indole and barbituric acids – Identification of highly promising leads. Bioorg. Med. Chem. Lett., 2009, 19, 3054-3058.10.1016/j.bmcl.2009.04.014Search in Google Scholar PubMed
Shin T.H., Choi Y., Kim S. Cheon J., Recent advances in magnetic nanoparticle-based multi-modal imaging. J. Chem. Soc. Rev., 2015, 44, 4501-4516.10.1039/C4CS00345DSearch in Google Scholar PubMed
Tietze R., Zaloga J., Unterweger H., Lyer S., Friedrich R.P., Janko C., et al., Magnetic nanoparticle-based drug delivery for cancer therapy. Biochem. Biophys. Res. Commun., 2015, 468, 463-470.10.1016/j.bbrc.2015.08.022Search in Google Scholar PubMed
Zhang Q., Su H., Luo J., Wei Y., A magnetic nanoparticle supported dual acidic ionic liquid: a “quasi-homogeneous” catalyst for the one-pot synthesis of benzoxanthenes. Green Chem., 2012, 14, 201-208.10.1039/C1GC16031ASearch in Google Scholar
Zheng X., Luo S., Zhang L., Cheng J.P., Magnetic nanoparticle supported ionic liquid catalysts for CO2 cycloaddition reactions. Green Chem., 2009, 11, 455-458.10.1039/b823123kSearch in Google Scholar
© 2020 Safaei-Ghomi and Samadi, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- An accelerated and effective synthesis of zinc borate from zinc sulfate using sonochemistry
- A new approach on lithium-induced neurotoxicity using rat neuronal cortical culture: Involvement of oxidative stress and lysosomal/mitochondrial toxic Cross-Talk
- Green synthesis and characterization of hexaferrite strontium-perovskite strontium photocatalyst nanocomposites
- Assessment of content and chemical forms of arsenic, copper, lead, and chromium in sewage sludge compost as affected by various bulking agents
- Preparation of skeletally diverse quinazoline-2,4(1H,3H)-diones using Na2SiO3/SnFe2O4 catalytic system through a four-component reaction
- Efficient photocatalytic degradation of organic dye from aqueous solutions over zinc oxide incorporated nanocellulose under visible light irradiation
- Synthesis of pyrimidines by Fe3O4@SiO2-L-proline nanoparticles
- Abnormally aggregation-induced emissions observed from hydrogen- and silyl-substituted siloles
- Organodiphosphines in PtP2X2 (X = As, Ge or Te) derivatives – Structural aspects
- Synthesis and structural characterization of dialkyltin complexes of N-salicylidene-L-valine
- Ultrasound-promoted solvent-free synthesis of some new α-aminophosphonates as potential antioxidants
- Occupational exposure in lead and zinc mines induces oxidative stress in miners lymphocytes: Role of mitochondrial/lysosomal damage
- Eccentric topological properties of a graph associated to a finite dimensional vector space
- Magnetically recoverable nanostructured Pd complex of dendrimeric type ligand on the MCM-41: Preparation, characterization and catalytic activity in the Heck reaction
- Short Communications
- The crystal structure of the first ether solvate of hexaphenyldistannane [(Ph3Sn)2 • 2 THF]
- New crystal structures of alkali metal tetrakis(pentafluorophenyl)borates
- s-Block metal scorpionates – A new sodium hydrido-tris(3,5-dimethyl-1-pyrazolyl)borate salt showing an unusual core stabilized by bridging and terminal O-bonded DMSO ligands
- Reduction of a 1,4-diazabutadiene and 2,2’-bipyridine using magnesium(I) compounds
- fac-Bis(phenoxatellurine) tricarbonyl manganese(I) bromide
- A new 2D dibutyltin coordination polymer with 3,5-dinitrosalicylate and 4,4’-bipyridine ligands
- Review
- Structures of Pt(0)P3, Pt(0)P4 and Pt(II)P4 – Distortion isomers
- Special Issue: Topological descriptors of chemical networks: Theoretical studies (Guest Editors: Muhammad Imran and Muhammad Javaid)
- Modified Zagreb connection indices of the T-sum graphs
- Topological properties of metal-organic frameworks
- Eccentricity based topological indices of siloxane and POPAM dendrimers
- On topological aspects of degree based entropy for two carbon nanosheets
- On multiplicative degree based topological indices for planar octahedron networks
- Computing entire Zagreb indices of some dendrimer structures
Articles in the same Issue
- Research Articles
- An accelerated and effective synthesis of zinc borate from zinc sulfate using sonochemistry
- A new approach on lithium-induced neurotoxicity using rat neuronal cortical culture: Involvement of oxidative stress and lysosomal/mitochondrial toxic Cross-Talk
- Green synthesis and characterization of hexaferrite strontium-perovskite strontium photocatalyst nanocomposites
- Assessment of content and chemical forms of arsenic, copper, lead, and chromium in sewage sludge compost as affected by various bulking agents
- Preparation of skeletally diverse quinazoline-2,4(1H,3H)-diones using Na2SiO3/SnFe2O4 catalytic system through a four-component reaction
- Efficient photocatalytic degradation of organic dye from aqueous solutions over zinc oxide incorporated nanocellulose under visible light irradiation
- Synthesis of pyrimidines by Fe3O4@SiO2-L-proline nanoparticles
- Abnormally aggregation-induced emissions observed from hydrogen- and silyl-substituted siloles
- Organodiphosphines in PtP2X2 (X = As, Ge or Te) derivatives – Structural aspects
- Synthesis and structural characterization of dialkyltin complexes of N-salicylidene-L-valine
- Ultrasound-promoted solvent-free synthesis of some new α-aminophosphonates as potential antioxidants
- Occupational exposure in lead and zinc mines induces oxidative stress in miners lymphocytes: Role of mitochondrial/lysosomal damage
- Eccentric topological properties of a graph associated to a finite dimensional vector space
- Magnetically recoverable nanostructured Pd complex of dendrimeric type ligand on the MCM-41: Preparation, characterization and catalytic activity in the Heck reaction
- Short Communications
- The crystal structure of the first ether solvate of hexaphenyldistannane [(Ph3Sn)2 • 2 THF]
- New crystal structures of alkali metal tetrakis(pentafluorophenyl)borates
- s-Block metal scorpionates – A new sodium hydrido-tris(3,5-dimethyl-1-pyrazolyl)borate salt showing an unusual core stabilized by bridging and terminal O-bonded DMSO ligands
- Reduction of a 1,4-diazabutadiene and 2,2’-bipyridine using magnesium(I) compounds
- fac-Bis(phenoxatellurine) tricarbonyl manganese(I) bromide
- A new 2D dibutyltin coordination polymer with 3,5-dinitrosalicylate and 4,4’-bipyridine ligands
- Review
- Structures of Pt(0)P3, Pt(0)P4 and Pt(II)P4 – Distortion isomers
- Special Issue: Topological descriptors of chemical networks: Theoretical studies (Guest Editors: Muhammad Imran and Muhammad Javaid)
- Modified Zagreb connection indices of the T-sum graphs
- Topological properties of metal-organic frameworks
- Eccentricity based topological indices of siloxane and POPAM dendrimers
- On topological aspects of degree based entropy for two carbon nanosheets
- On multiplicative degree based topological indices for planar octahedron networks
- Computing entire Zagreb indices of some dendrimer structures

