Abstract
Objectives
Pulmonary hypertension (PH) is a complication of bronchopulmonary dysplasia (BPD) and associated with increased mortality and morbidity. Our aim was to identify, in infants with BPD, the effect of PH on health-care utilisation and health related cost of care.
Methods
An electronic data recording system was used to identify infants ≤32 weeks of gestation who developed BPD. PH was classified as early (≤28 days after birth) or late (>28 days after birth).
Results
In the study period, 182 infants developed BPD; 22 (12.1%) developed late PH. Development of late PH was associated with a lower gestational age [24.6 (23.9–26.9) weeks, p=0.001] and a greater need for positive pressure ventilation on day 28 after birth (100%) compared to infants without late PH (51.9%) (odds ratio (OR) 19.5, 95% CI: 2.6–148), p<0.001. Late PH was associated with increased mortality (36.4%) compared those who did not develop late PH (1.9%) after adjusting for gestational age and ventilation duration (OR: 26.9, 95% CI: 3.8–189.4), p<0.001. In infants who survived to discharge, late PH development was associated with a prolonged duration of stay [147 (118–189) days] compared to the infants that did not develop late PH [109 (85–149) days] (p=0.03 after adjusting for gestational age). Infants who had late PH had a higher cost of stay compared to infants with BPD who did not develop late PH (median £113,494 vs. £78,677, p=0.016 after adjusting for gestational age).
Conclusions
Development of late PH was associated with increased mortality, a prolonged duration of stay and higher healthcare cost.
Introduction
The survival rates of extremely premature infants have improved significantly in the last few decades, but are associated with long term morbidities [1], [2], [3]. Bronchopulmonary dysplasia (BPD) is the most common adverse outcome and can result in complications which may extend into adulthood [4]. One of the significant contributory factors for morbidity and mortality in infants with BPD is the development of pulmonary hypertension (PH) [5].
The incidence of pulmonary hypertension in infants with BPD ranges from 17 to 37% [6], [7], [8]. The pathogenesis of pulmonary vascular disease associated with BPD is multifactorial and can be attributed to the interaction of maternal, genetic and postnatal factors. Inhibition of angiogenesis has been associated with decreased alveolarization and pulmonary vascular growth in an animal model [9]. The arrest of vascular growth and the reduction in the alveolar-capillary surface result in impaired gas exchange. Thus, the lungs are exposed to prolonged ventilation and high oxygen levels along with hemodynamic and inflammatory stressors. This causes pulmonary arterial remodelling leading to fibrosis of the vessel walls [10]. The risk factors associated with the development of pulmonary hypertension include intrauterine growth restriction, preeclampsia, maternal substance abuse, lower gestational age and birthweight, patent ductus arteriosus (PDA) requiring treatment and sepsis [8, 11], [12], [13], [14].
The gold standard to diagnose pulmonary hypertension is cardiac catheterization, but this is often difficult to perform in BPD infants who may have significant vascular and haemodynamic complications [15]. Two-dimensional echocardiography, however, is a valuable tool for screening and diagnosis of PH in such patients [10]. Various studies have investigated the risk factors, outcomes [5], [6], [7], [8, 11, 14, 16], [17], [18], [19], [20], [21] and evolution of pulmonary hypertension in BPD infants [7, 8, 14, 19], [20], [21]. The identified risk factors include early pulmonary hypertension, postnatal steroid administration, tracheostomy and tracheitis [5, 16, 17]. Outcomes associated with PH in BPD infants include increased mortality and morbidity including a prolonged duration of invasive ventilation, increased occurrence of severe IVH and prolonged hospitalization [5, 7, 8, 14, 19], [20], [21]. Our aim was to identify, in infants with BPD, the effect of PH on health-care utilisation and the health related cost of care.
Materials and methods
Study subjects
An observational study was conducted of infants admitted to the neonatal unit at a London hospital, United Kingdom (UK) between January 2017 and December 2020. Badgernet, an electronic patient record database, was used to identify study subjects. The criteria for inclusion were (i) infants born at or prior to 32 weeks’ gestation (ii) birthweight less than 1,500 g and (iii) development of BPD (oxygen requirement for at least 28 days from birth) [22]. Infants who died before 36 weeks of gestation, but required oxygen for at least 28 days were included in the study [23].
Infants had an echocardiogram when there was a clinical suspicion of pulmonary hypertension as evidenced by different pre-ductal and post-ductal saturations, a persistently high oxygen requirement above 60% and/or requiring increased mean airway pressures to maintain oxygenation. Some infants had an echocardiogram for assessment of whether they had a patent ductus arteriosus (PDA). Infants whose echocardiogram reports were not available, who were admitted for short surgical procedures such as inguinal hernia and laser photocoagulation and those with major respiratory and cardiac anomalies were excluded from the study.
Ethical approval
The study was registered with the local Clinical Governance and Audit Department and did not require informed parental consent.
Assessment of pulmonary hypertension
The echocardiograms of the infants were reviewed by one author, a neonatologist trained in echocardiography and further reviewed by a paediatric cardiologist. The presence of PH was defined based on quantitative or qualitative assessments. Quantitative assessment included the presence of tricuspid regurgitant jet velocity (TRJV)>3 m/s (right ventricular systolic pressure (RVSP)>36 mm Hg). In the absence of tricuspid regurgitation, qualitative assessment was used to detect PH using the following parameters: (i) flattened or left deviated intraventricular septum in ventricular systole (ii) right to left shunting across a patent foramen ovale, atrial septal defect, ventricular septal defect or patent ductus arteriosus, if present (iii) right ventricular hypertrophy or dysfunction [15].
Definitions and risk factors
BPD was defined as per the Jobe and Bancalari criteria [22]. Early PH was defined as PH diagnosed within 28 days after birth, while late PH diagnosed after 28 days [16]. Severe intraventricular haemorrhage was defined as Grade 3 or 4 as described by Papile [24].
The last echocardiogram during the admission was used to identify the presence of PH at discharge or death. Risk factors for late PH assessed included maternal preeclampsia, substance abuse, small for gestational age (SGA) (defined as birthweight less than 10th percentile or less than −1.28 standard deviation for gestational age [25]), positive pressure ventilation (PPV) at 28 days after birth, medically or surgically treated patent ductus arteriosus (PDA), sepsis (positive blood culture requiring intravenous antibiotics) and presence of early PH.
Outcomes
We assessed the mortality in the cohort and the total duration of stay in the survivors. Additionally, the total cost of stay was calculated based on national average costs of neonatal unit stay. We also compared the length and cost of stay in infants whom PH resolved prior to discharge against the ones in whom PH did not resolve prior to death or discharge. The average daily cost ranges from £437 to £1,218 was graded based on the intensity of care coded as intensive care, high dependency and special care (Table 1) [26]. The duration of care at each level of care was extracted from Badgernet and used to estimate the total cost of stay in the neonatal unit.
National schedule of reference costs in neonatal units in UK.
| Level of intensity | National average unit cost per day (interquartile range) |
|---|---|
| Intensive care | £1,218 (£959–£1,399) |
| High dependency | £872 (£746–£984) |
| Special care | £560 (£455–£645) |
Statistical analysis
On testing for normality using Shapiro-Wilk test, the data was found to be non- normally distributed. Therefore, the Mann-Whitney U test was used for comparing the continuous variables and Chi-square test for dichotomous variables. Multiple logistic or linear regression was used to adjust for confounding variables. All the risk factors were adjusted for gestational age, birthweight z score and 10 min Apgar score. The effect on mortality and duration of hospitalisation were further adjusted for gestational age and the duration of invasive ventilation. The effect of total cost of stay was further adjusted for gestational age. IBM SPSS Statistics for Windows, Version 25.0 (SPSS Inc. Chicago, IL) was used to analyse the data.
Results
A total of 428 premature infants were admitted to the neonatal unit during the study period, 211 (49.3%) subsequently developed BPD; 189 infants were included in the analysis and 29 infants were excluded (Figure 1).
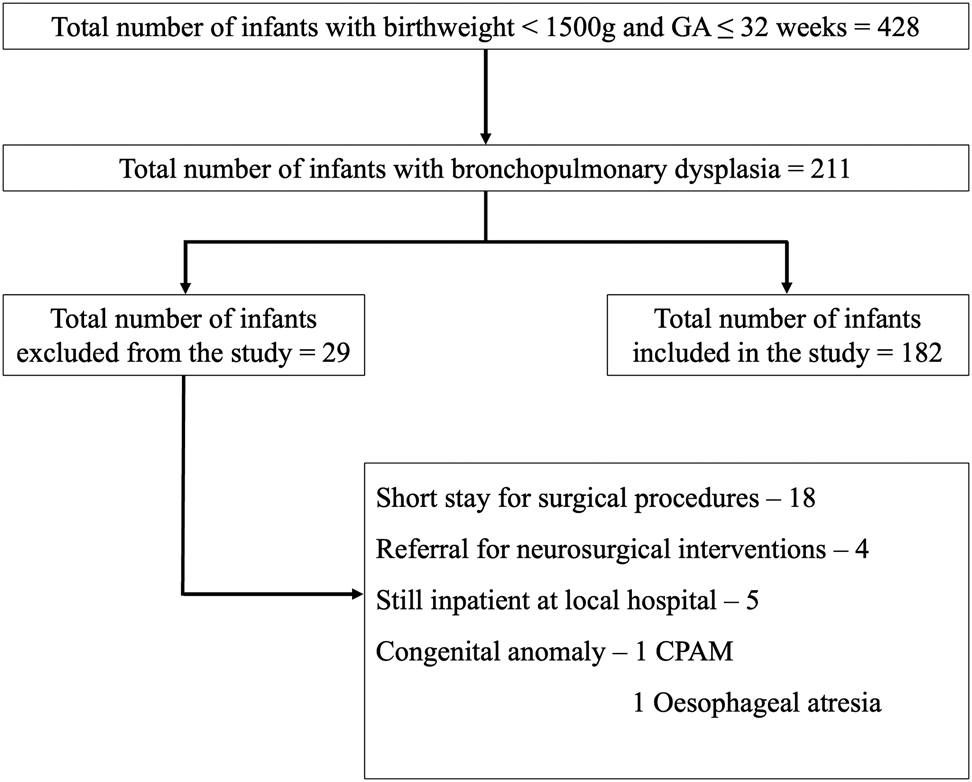
Flowchart of selected cases.
The included infants had a median (interquartile range) gestational age of 25.9 (24.9–27.7) weeks and birthweight of 769 (667–930) grams (Table 2). Twenty-one (11.5%) infants had early PH of which six (28.5%) subsequently developed late PH. Twenty-two (12%) infants had late PH. Amongst the 22 infants with late PH, pulmonary hypertension resolved in 10 infants, four had pulmonary hypertension at discharge from neonatal care and eight died with pulmonary hypertension (Figure 2).
Demographics of the study population.
| Median (interquartile range) | n (%) | |
|---|---|---|
| Gestational age | 25.9 (24.9–27.7) | |
| Birthweight | 769 (667–930) | |
| Small for gestational age | 36 (19.8) | |
| Sex | Male: 94 (51.6) | |
| Ethnicity (maternal) | White: 71 (39) | |
| Black: 64 (35.2) | ||
| Asian: 14 (7.7) | ||
| Mixed: 3 (1.6) | ||
| Other: 7 (3.8) | ||
| Mode of delivery | Emergency caesarean: 75 (41.2) | |
| Elective caesarean: 6 (3.3) | ||
| Spontaneous vaginal: 97 (53.3) | ||
| Instrumental: 4 (2.2) | ||
| Antenatal steroids (at least one dose) | 169 (92.9) | |
| Pre-eclampsia | 30 (16.5) | |
| Maternal substance abuse | 4 (2.2) | |
| Clinical chorioamnionitis | 9 (4.9) | |
| Early PH | 21 (11.5) | |
| Late PH | 22 (12.1) | |
| PH at death or discharge | 12 (6.6) | |
| Positive pressure ventilation on day 28 | 105 (57.7) | |
| Inhaled nitric oxide | 54 (29.7) | |
| Inotrope administration | 100 (54.9) | |
| PDA requiring treatment | 73 (40.1) | |
| Culture proven sepsis | 78 (42.9) | |
| Surgical NEC | 18 (9.9) | |
| Severe IVH | 44 (24.2) | |
| Severe ROP | 34 (18.7) | |
| Mortality | 11 (6.0) |
-
PH, pulmonary hypertension; PDA, patent ductus arteriosus.
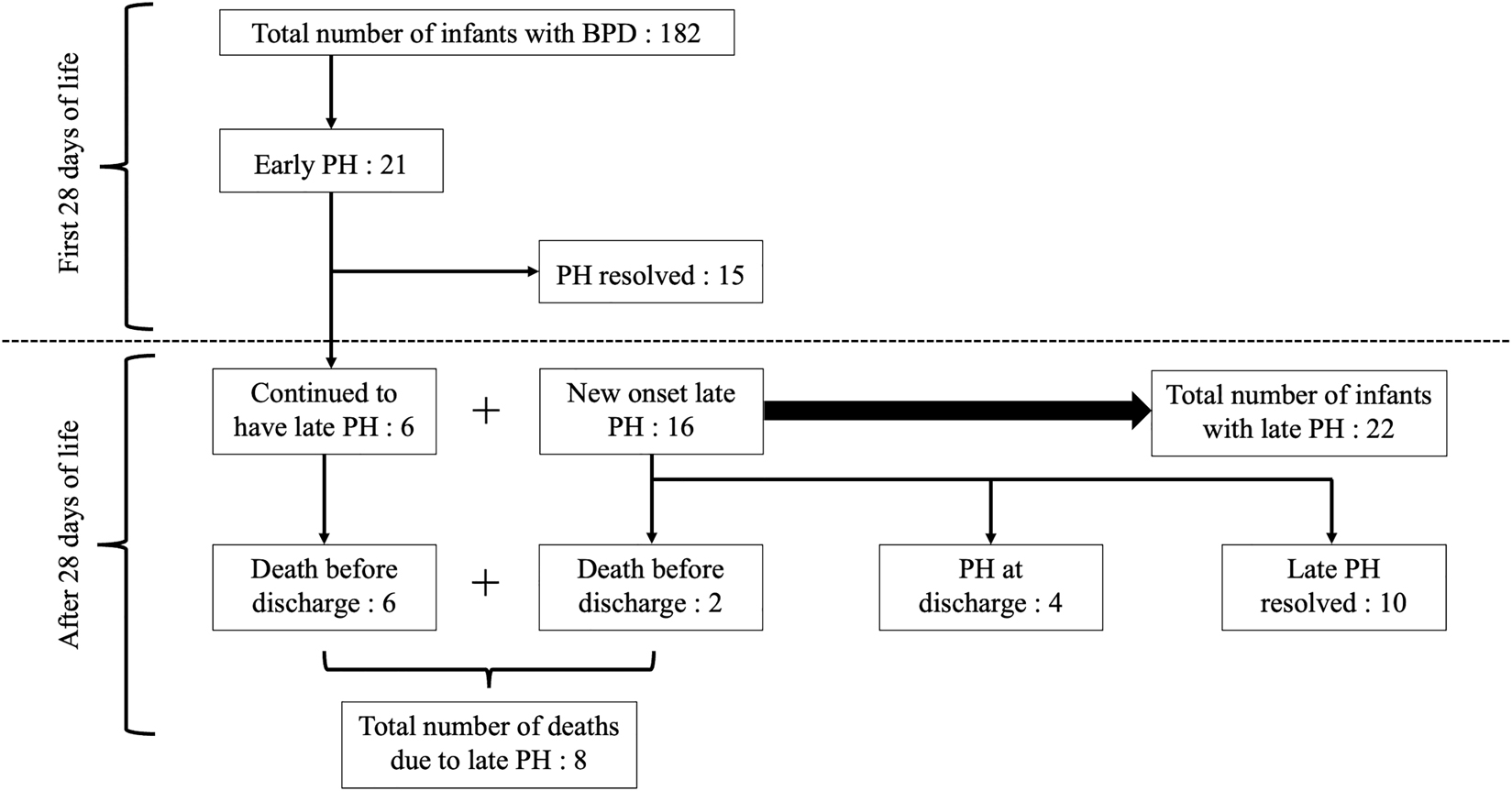
Development and evolution of pulmonary hypertension during neonatal stay.
The characteristics of infants who developed early PH, late PH, both early and late and no PH have been detailed in Table 3. The infants who developed both early and late PH had a lower (median) gestational age (24.4 weeks) compared to those who had only early PH (25.7 weeks), late PH (24.6 weeks) or no PH (26.1 weeks, p=0.02). Furthermore, infants who developed both early and late PH had a lower (median) birthweight (603 g) compared to those who had early PH (780 g), late PH (673 g) or no PH (818 g, p<0.001).
Demographics of infants by early PH, late PH, both early + late and no PH.
| Characteristics | Early PH (n=15), n (%) or median (IQR) | Late PH (n=16), n (%) or median (IQR) | Early + late PH (n=6), n (%) or median (IQR) | No PH (n=145), n (%) or median (IQR) | p-Value |
|---|---|---|---|---|---|
| Gestational age, weeks | 25.7 (25.0–28.1) | 24.6 (24.2–26.5) | 24.4 (23.5–27.4) | 26.1 (25–27.9) | 0.02 |
| Birthweight, grams | 780 (704–890) | 673 (627–769) | 603 (535–650) | 818 (692.5–962) | <0.001 |
| Birthweight z scores | −0.6 (−1.2 to 0.23) | −0.6 (−1.4 to 0.31) | −0.7 (−2.5 to 0.04) | −0.3 (−1.1 to 0.19) | 0.66 |
| Apgar at 10 min | 8 (7–9) | 8 (7–9) | 8 (8–9) | 9 (8–10) | 0.15 |
| Small for gestational age | 2 (19) | 4 (25.0) | 2 (33.3) | 28 (19.3) | 0.71 |
| Sex, male | 8 (53.3) | 8 (50.0) | 4 (66.7) | 74 (51.0) | 0.90 |
| Antenatal steroids | 13 (86.7) | 16 (100) | 5 (83.3) | 135 (93.1) | 0.40 |
| Pre-eclampsia | 3 (20.0) | 3 (18.8) | 0 | 24 (16.6) | 0.71 |
| Maternal substance abuse | 1 (6.7) | 0 | 0 | 3 (2.1) | 0.59 |
-
PH, pulmonary hypertension; IQR, interquartile range.
The infants who developed late PH had a lower gestational age (24.6 vs. 26.1 weeks, p=0.002) and lower birthweight (642.5 vs. 816.5 g, p<0.001) compared to those who did not have late PH. The infants who developed late PH were more often mechanically ventilated on day 28 after birth compared to the ones who did not develop late PH [100 vs. 51.9%, p<0.001; OR: 19.5 (CI: 2.6–148)]. The infants who developed late PH had a longer median duration of mechanical ventilation compared to the infants without late PH (53.5 vs. 27.5 days, p<0.001) [Table 4].
Demographics of infants by late PH status.
| Characteristics | Late PH (n=22), n (%) or median (IQR) | No late PH (n=160), n (%) or median (IQR) | p-Value |
|---|---|---|---|
| Gestational age, weeks | 24.6 (23.9–26.9) | 26.1 (25–27.9) | 0.002 |
| Birthweight, grams | 642.5 (610.3–718.9) | 816.5 (696.3–960) | <0.001 |
| Birthweight z scores | −0.6 (−1.4 to 0.13) | −0.34 (−1.1 to 0.2) | 0.38 |
| Apgar at 10 min | 8 (7–9) | 9 (8–10) | 0.084 |
| Small for gestational age | 6 (27.2) | 30 (18.8) | 0.35 |
| Sex, male | 12 (54.5) | 82 (51.2) | 0.77 |
| Antenatal steroids | 21 (95.5) | 148 (92.5) | 0.61 |
| Pre-eclampsia | 3 (13.6) | 27 (16.9) | 0.70 |
| Maternal substance abuse | 0 | 4 (2.5) | 0.45 |
| Positive pressure ventilation on day 28 | 22 (100) | 83 (51.9) | <0.001 |
| PDA requiring treatment (medical/surgical) | 12 (54.5) | 61 (38.1) | 0.141 |
| Duration of invasive ventilation, days | 53.5 (42–65.8) | 27.5 (8–48) | <0.001 |
| Culture proven sepsis | 13 (59.1) | 65 (40.6) | 0.08 |
| Presence of early PH | 6 (27.3) | 15 (9.4) | 0.19 |
| Surgical NEC | 2 (9.1) | 16 (10) | 0.89 |
| Severe IVH | 6 (27.3) | 38 (23.8) | 0.72 |
-
PH, pulmonary hypertension; IQR, interquartile range; PDA, patent ductus arteriosus.
Eleven infants (6%) died in the study population. Eight had pulmonary hypertension at death, of those eight, six had developed PH within 28 days after birth (Figure 2). The presence of late PH was independently associated with increased mortality [OR: 21.4 (4.1–111.3), adjusted p=0.001]. Similarly, the presence of late PH in surviving infants was independently associated with a longer duration of hospital stay [147 vs. 109 days, adjusted p: 0.03] (Table 5).
Late PH and mortality and total cost of stay.
| Late PH (n=22), n (%) or median (IQR) | No late PH (n=160), n (%) or median (IQR) | p-Value | Odds ratio (95% CI) | Adjusted p-value | Adjusted OR (95% CI) | |
|---|---|---|---|---|---|---|
| Mortality | 8 (36.4) | 3 (1.9) | <0.001 | 29.9 (7.1–125.6) | 0.001 | 26.9 (3.8–189.4) |
| Length of hospitalisation, days | 147 (118–189) | 109 (85–149) | 0.002 | 0.034 | ||
| Total cost of hospital stay | £113,494 (72,650–169,798) | £78,677 (48,476–115,483) | 0.018 | 0.016 |
-
PH, pulmonary hypertension; IQR, interquartile range.
The total cost of stay for the 182 infants was £17,164,290 ($23,957,659). The median (IQR) total cost of stay in infants with late PH was £113,494 (72,650–169,798) compared to £78,677 (48,476–115,483) in infants with BPD, but no late PH after adjusting for gestational age (p=0.016) [Table 5]. The median cost of stay in infants with BPD and PH was approximately £35,000 higher (44% higher) compared to that of infants with BPD without PH.
The median length of hospitalisation was similar in infants whom PH resolved before discharge [147 (118–189) days] compared to the infants in whom PH did not resolve before discharge or died with PH [147 (72–259) days, p=0.81]. The total cost of stay was not significantly different in infants in whom PH resolved before discharge [£85,992 (67,524–121,939)] compared to the infants in whom PH did not resolve before discharge [£148,458 (80,185–252,455), p=0.07].
Discussion
We have demonstrated late PH was associated with a higher mortality and a longer duration of stay compared to infants with BPD without PH. Furthermore, we found that the total cost of stay for infants with late PH and BPD was higher compared to those who did not develop late PH.
Our study showed that while the incidence of overall mortality was 6% in the study population, the presence of late PH was associated with a 36% increase in mortality. Studies have described the association of PH and mortality with an incidence ranging from 10 to 38% [7, 8, 14, 17, 18, 27]. Notably, among the eight infants who died with pulmonary hypertension, six of them had developed PH within 28 days (Figure 2). Two studies have shown that early PH increases the risk for severe BPD and development of late PH in infants with BPD, but did not describe the impact of this on mortality [16, 17]. This highlights the need for early as well as regular screening for pulmonary hypertension via echocardiographic assessment in infants with BPD.
One study reported the healthcare utilization of infants with PH associated with BPD presenting after two months of age into a BPD clinic and found that the infants were hospitalised for a longer duration and had more respiratory morbidity than infants with BPD but no PH [28]. Our study reported on the total cost of stay based on national average in infants with BPD and PH and to our knowledge this is the first study which specifically looked into the effect of pulmonary hypertension in infants with bronchopulmonary dysplasia on health care costs.
Our study has strengths and some limitations. In our study population we used echocardiography to identify pulmonary hypertension; echocardiography is considered to be an effective and reliable screening tool for detection of pulmonary hypertension in infants with BPD [29]. A limitation, however, was that the infants had echocardiograms at various time intervals according to clinical need rather than at specific intervals, but this makes our results generalisable to clinical practice.
In conclusion, infants with late PH had a longer duration of hospitalization and increased mortality compared to the infants without late PH. The presence of late PH in infants with BPD resulted in increased healthcare costs.
Funding source: NIHR Biomedical Research Centre based at Guy’s and St Thomas NHS Foundation Trusts and King’s College London
Award Identifier / Grant number: N/A
-
Research funding: The research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
-
Author contributions: All authors were involved in the conception of the study. FA-T, MN and AB were involved in the data collection. FA-T, TD and AG were involved in the analysis of the data. All authors were involved in the preparation of the manuscript and approved the final version.
-
Competing interests: Authors state no conflict of interest.
-
Informed consent: Not applicable.
-
Ethical approval: The study was registered with the King’s College Hospital NHS Foundation Trust Clinical Governance and Audit Department and did not require informed parental consent.
References
1. Santhakumaran, S, Statnikov, Y, Gray, D, Battersby, C, Ashby, D, Modi, N. Survival of very preterm infants admitted to neonatal care in England 2008–2014: time trends and regional variation. Arch Dis Child Fetal Neonatal Ed 2018;103:F208–15. https://doi.org/10.1136/archdischild-2017-312748.Search in Google Scholar PubMed PubMed Central
2. Stoll, BJ, Hansen, NI, Bell, EF, Walsh, MC, Carlo, WA, Shankaran, S, et al.. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. J Am Med Assoc 2015;314:1039. https://doi.org/10.1001/jama.2015.10244.Search in Google Scholar PubMed PubMed Central
3. Siffel, C, Kistler, KD, Lewis, JFM, Sarda, SP. Global incidence of bronchopulmonary dysplasia among extremely preterm infants: a systematic literature review. J Matern Fetal Neonatal Med 2021;34:1721–31. https://doi.org/10.1080/14767058.2019.1646240.Search in Google Scholar PubMed
4. Zivanovic, S, Peacock, J, Alcazar-Paris, M, Lo, JW, Lunt, A, Marlow, N, et al.. Late outcomes of a randomized trial of high-frequency oscillation in neonates. N Engl J Med 2014;370:1121–30. https://doi.org/10.1056/nejmoa1309220.Search in Google Scholar
5. Altit, G, Bhombal, S, Hopper, RK, Tacy, TA, Feinstein, J. Death or resolution: the “natural history” of pulmonary hypertension in bronchopulmonary dysplasia. J Perinatol 2019;39:415–25. https://doi.org/10.1038/s41372-018-0303-8.Search in Google Scholar PubMed
6. Al-Ghanem, G, Shah, P, Thomas, S, Banfield, L, El Helou, S, Fusch, C, et al.. Bronchopulmonary dysplasia and pulmonary hypertension: a meta-analysis. J Perinatol 2017;37:414–9. https://doi.org/10.1038/jp.2016.250.Search in Google Scholar PubMed
7. Bhat, R, Salas, AA, Foster, C, Carlo, WA, Ambalavanan, N. Prospective analysis of pulmonary hypertension in extremely low birth weight infants. Pediatrics 2012;129:e682–9. https://doi.org/10.1542/peds.2011-1827.Search in Google Scholar PubMed PubMed Central
8. Slaughter, JL, Pakrashi, T, Jones, DE, South, AP, Shah, TA. Echocardiographic detection of pulmonary hypertension in extremely low birth weight infants with bronchopulmonary dysplasia requiring prolonged positive pressure ventilation. J Perinatol 2011;31:635–40. https://doi.org/10.1038/jp.2010.213.Search in Google Scholar PubMed
9. Jakkula, M, Le Cras, TD, Gebb, S, Hirth, KP, Tuder, RM, Voelkel, NF, et al.. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol 2000;279:L600–7. https://doi.org/10.1152/ajplung.2000.279.3.l600.Search in Google Scholar PubMed
10. Mourani, PM, Abman, SH. Pulmonary hypertension and vascular abnormalities in bronchopulmonary dysplasia. Clin Perinatol 2015;42:839–55. https://doi.org/10.1016/j.clp.2015.08.010.Search in Google Scholar PubMed PubMed Central
11. Check, J, Gotteiner, N, Liu, X, Su, E, Porta, N, Steinhorn, R, et al.. Fetal growth restriction and pulmonary hypertension in premature infants with bronchopulmonary dysplasia. J Perinatol 2013;33:553–7. https://doi.org/10.1038/jp.2012.164.Search in Google Scholar PubMed PubMed Central
12. Mestan, KK, Check, J, Minturn, L, Yallapragada, S, Farrow, KN, Liu, X, et al.. Placental pathologic changes of maternal vascular underperfusion in bronchopulmonary dysplasia and pulmonary hypertension. Placenta 2014;35:570–4. https://doi.org/10.1016/j.placenta.2014.05.003.Search in Google Scholar PubMed PubMed Central
13. Hansen, AR, Barnés, CM, Folkman, J, McElrath, TF. Maternal preeclampsia predicts the development of bronchopulmonary dysplasia. J Pediatr 2010;156:532–6. https://doi.org/10.1016/j.jpeds.2009.10.018.Search in Google Scholar PubMed
14. An, HS, Bae, EJ, Kim, GB, Kwon, BS, Beak, JS, Kim, EK, et al.. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Korean Circ J 2010;40:131–6. https://doi.org/10.4070/kcj.2010.40.3.131.Search in Google Scholar PubMed PubMed Central
15. Krishnan, U, Feinstein, JA, Adatia, I, Austin, ED, Mullen, MP, Hopper, RK, et al.. Evaluation and management of pulmonary hypertension in children with bronchopulmonary dysplasia. J Pediatr 2017;188:24–34. https://doi.org/10.1016/j.jpeds.2017.05.029.Search in Google Scholar PubMed
16. Sheth, S, Goto, L, Bhandari, V, Abraham, B, Mowes, A. Factors associated with development of early and late pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. J Perinatol 2020;40:138–48. https://doi.org/10.1038/s41372-019-0549-9.Search in Google Scholar PubMed PubMed Central
17. Mourani, PM, Sontag, MK, Younoszai, A, Miller, JI, Kinsella, JP, Baker, CD, et al.. Early pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. Am J Respir Crit Care Med 2015;191:87–95. https://doi.org/10.1164/rccm.201409-1594oc.Search in Google Scholar PubMed PubMed Central
18. Khemani, E, McElhinney, DB, Rhein, L, Andrade, O, Lacro, RV, Thomas, KC, et al.. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics 2007;120:1260–9. https://doi.org/10.1542/peds.2007-0971.Search in Google Scholar PubMed
19. Mirza, H, Ziegler, J, Ford, S, Padbury, J, Tucker, R, Laptook, A. Pulmonary hypertension in preterm infants: prevalence and association with bronchopulmonary dysplasia. J Pediatr 2014;165:909–14. https://doi.org/10.1016/j.jpeds.2014.07.040.Search in Google Scholar PubMed
20. Del Cerro, MJ, Sabaté Rotés, A, Cartón, A, Deiros, L, Bret, M, Cordeiro, M, et al.. Pulmonary hypertension in bronchopulmonary dysplasia: clinical findings, cardiovascular anomalies and outcomes. Pediatr Pulmonol 2014;49:49–59. https://doi.org/10.1002/ppul.22797.Search in Google Scholar PubMed
21. Kwon, HW, Kim, H-S, An, HS, Kwon, BS, Kim, GB, Shin, SH, et al.. Long-term outcomes of pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Neonatology 2016;110:181–9. https://doi.org/10.1159/000445476.Search in Google Scholar
22. Jobe, AH, Bancalari, E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163:1723–9. https://doi.org/10.1164/ajrccm.163.7.2011060.Search in Google Scholar
23. Poindexter, BB, Feng, R, Schmidt, B, Aschner, JL, Ballard, RA, Hamvas, A, et al.. Comparisons and limitations of current definitions of bronchopulmonary dysplasia for the prematurity and respiratory outcomes program. Ann Am Thorac Soc 2015;12:1822–30. https://doi.org/10.1513/annalsats.201504-218oc.Search in Google Scholar
24. Papile, L-A, Burstein, J, Burstein, R, Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 1978;92:529–34. https://doi.org/10.1016/s0022-3476(78)80282-0.Search in Google Scholar
25. Royal College of Obstetricians and Gynaecologists (RCOG). Small-for-gestational-age fetus, investigation and management (green-top guideline no. 31). London: RCOG; 2002 (update 2013). Available from: www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg31/.Search in Google Scholar
26. Department of Health: reference costs guidance 2015–2016; national schedule of reference costs – main schedule. London, UK: Department of Health and Social Care; 2016:1–59 pp.Search in Google Scholar
27. Aswani, R, Hayman, L, Nichols, G, Luciano, AA, Amankwah, EK, Leshko, JL, et al.. Oxygen requirement as a screening tool for the detection of late pulmonary hypertension in extremely low birth weight infants. Cardiol Young 2016;26:521–7. https://doi.org/10.1017/s1047951115000608.Search in Google Scholar
28. Stuart, BD, Sekar, P, Coulson, JD, Choi, SE, McGrath-Morrow, SA, Collaco, JM. Health-care utilization and respiratory morbidities in preterm infants with pulmonary hypertension. J Perinatol 2013;33:543–7. https://doi.org/10.1038/jp.2012.170.Search in Google Scholar PubMed
29. Nagiub, M, Lee, S, Guglani, L. Echocardiographic assessment of pulmonary hypertension in infants with bronchopulmonary dysplasia: systematic review of literature and a proposed algorithm for assessment. Echocardiography 2015;32:819–33. https://doi.org/10.1111/echo.12738.Search in Google Scholar PubMed
© 2021 Fahad M.S. Arattu Thodika et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Corner of Academy
- Cost of providing cell-free DNA screening for Down syndrome in Finland using different strategies
- Adverse perinatal outcomes following the prenatal diagnosis of isolated single umbilical artery in singleton pregnancies: a systematic review and meta-analysis
- Original Articles – Obstetrics
- Perinatal outcomes in women with severe acute respiratory syndrome coronavirus 2 infection: comparison with contemporary and matched pre-COVID-19 controls
- The postpartum period during the COVID-19 pandemic: investigating Turkish women’s postpartum support and postpartum-specific anxiety
- First-line noninvasive management of cytomegalovirus primary infection in pregnancy
- Ultrasound and magnetic resonance imaging in the diagnosis of clinically significant placenta accreta spectrum disorders
- Improved management of placenta accreta spectrum disorders: experience from a single institution
- A randomized controlled trial of two-doses of vaginal progesterone 400 vs. 200 mg for prevention of preterm labor in twin gestations
- The impact of preimplantation genetic testing for aneuploidy on prenatal screening
- Original Articles – Fetus
- Myocardial deformation analysis in late-onset small-for-gestational-age and growth-restricted fetuses using two-dimensional speckle tracking echocardiography: a prospective cohort study
- HDlive Flow Silhouette with spatiotemporal image correlation for assessment of fetal cardiac structures at 12 to 14 + 6 weeks of gestation
- Umbilical artery pulsatility index and half-peak systolic velocity in second- and third-trimester fetuses with trisomy 18 and 13
- Original Articles – Neonates
- Pulmonary hypertension in infants with bronchopulmonary dysplasia: risk factors, mortality and duration of hospitalisation
- Outcomes from birth to 6 months of publicly insured infants born to mothers with severe acute respiratory syndrome coronavirus 2 infection in the United States
- Placental findings are not associated with neurodevelopmental outcome in neonates with hypoxic-ischemic encephalopathy – an 11-year single-center experience
- High frequency band limits in spectral analysis of heart rate variability in preterm infants
- Bacterial DNA detection in very preterm infants assessed for risk of early onset sepsis
- Short Communication
- Healthcare workers’ attitudes about vaccination of pregnant women and those wishing to become pregnant
- Letter to the Editors
- Comment on Abdel Wahab et al.: A randomized controlled trial of two-doses of vaginal progesterone 400 vs. 200 mg for prevention of preterm labor in twin gestations
- Re: Comment on Abdel Wahab et al.: A randomized controlled trial of two-doses of vaginal progesterone 400 vs. 200 mg for prevention of preterm labor in twin gestations
- SARS-CoV-2 behavior, through the eyes of a perinatologist?
- Re: SARS-CoV-2 behavior, through the eyes of a perinatologist?
Articles in the same Issue
- Frontmatter
- Corner of Academy
- Cost of providing cell-free DNA screening for Down syndrome in Finland using different strategies
- Adverse perinatal outcomes following the prenatal diagnosis of isolated single umbilical artery in singleton pregnancies: a systematic review and meta-analysis
- Original Articles – Obstetrics
- Perinatal outcomes in women with severe acute respiratory syndrome coronavirus 2 infection: comparison with contemporary and matched pre-COVID-19 controls
- The postpartum period during the COVID-19 pandemic: investigating Turkish women’s postpartum support and postpartum-specific anxiety
- First-line noninvasive management of cytomegalovirus primary infection in pregnancy
- Ultrasound and magnetic resonance imaging in the diagnosis of clinically significant placenta accreta spectrum disorders
- Improved management of placenta accreta spectrum disorders: experience from a single institution
- A randomized controlled trial of two-doses of vaginal progesterone 400 vs. 200 mg for prevention of preterm labor in twin gestations
- The impact of preimplantation genetic testing for aneuploidy on prenatal screening
- Original Articles – Fetus
- Myocardial deformation analysis in late-onset small-for-gestational-age and growth-restricted fetuses using two-dimensional speckle tracking echocardiography: a prospective cohort study
- HDlive Flow Silhouette with spatiotemporal image correlation for assessment of fetal cardiac structures at 12 to 14 + 6 weeks of gestation
- Umbilical artery pulsatility index and half-peak systolic velocity in second- and third-trimester fetuses with trisomy 18 and 13
- Original Articles – Neonates
- Pulmonary hypertension in infants with bronchopulmonary dysplasia: risk factors, mortality and duration of hospitalisation
- Outcomes from birth to 6 months of publicly insured infants born to mothers with severe acute respiratory syndrome coronavirus 2 infection in the United States
- Placental findings are not associated with neurodevelopmental outcome in neonates with hypoxic-ischemic encephalopathy – an 11-year single-center experience
- High frequency band limits in spectral analysis of heart rate variability in preterm infants
- Bacterial DNA detection in very preterm infants assessed for risk of early onset sepsis
- Short Communication
- Healthcare workers’ attitudes about vaccination of pregnant women and those wishing to become pregnant
- Letter to the Editors
- Comment on Abdel Wahab et al.: A randomized controlled trial of two-doses of vaginal progesterone 400 vs. 200 mg for prevention of preterm labor in twin gestations
- Re: Comment on Abdel Wahab et al.: A randomized controlled trial of two-doses of vaginal progesterone 400 vs. 200 mg for prevention of preterm labor in twin gestations
- SARS-CoV-2 behavior, through the eyes of a perinatologist?
- Re: SARS-CoV-2 behavior, through the eyes of a perinatologist?

