Abstract
Nickel Chromite nanoparticles were successfully synthesized via a modified sol-gel method using nickel acetate and ammonium dichromate in melted stearic acid as a complexing agent. The diffractograms of the nanoparticles confirmed a pure formation of NiCr2O4 spinel without any minor phase. The coordination structure of as prepared nanoparticles shows a series of absorption bands below 1,000 cm−1 were evidenced the M-O (Cr-O, Ni-O) bond in the sample. Optical band gap, magnetic properties and color parameters (L*.a*.b*) indicates that the final nanoparticles are optically and magnetically active. The particle size of NiCr2O4 was calculated using Scherrer equation at about 24 nm. Optical band gap obtained at 1.7 eV indicating that NiCr2O4 nanoparticles are semiconductor material and can be used in electrical devices.
Introduction
Spinel with general formula AB2X4 (X=S, O, …) has a cubic structure with the crystal group Fd3m [1, 2]. Two major variants of the spinel crystal structure denoted as: normal and inverse [3, 4]. NiCr2O4 is a normal spinel (AB2O4) ferromagnetic compound. A- and B-sites are occupied by divalent Ni2+ ions and trivalent Cr3+ ions, respectively. Many applications of this structure have been reported in light, heat-sensitive micromechanical devices, catalytic materials and gas sensors [5–7]. In particular, nickel chromite is identified as a promising catalytic-size dependent-material for various industrial processes [8–11]. Among various methods for preparation spinel type materials, thermal treatment achieves higher attraction because of its ability to control the movement of the metal ions and oxygen atoms to occupy either A- or B- sites [12–14]. Some of the most well developed methods for preparation of metal oxides are sol-gel [15, 16], micro-emulsion [17], co-precipitation [18], supersonic radiation [19], Pechini’s method [20], hydrothermal synthesis [21], freeze-drying [22], and combustion synthesis [23–26].
In current study, a modified sol-gel method in combination with thermal treatment has been developed to synthesize nickel chromite. Dried spinel NiCr2O4 nanopowder was mixed thoroughly to ensure reproducibility of samples used for various characterizations.
Materials and method
Nickel acetate powder (99.99 %, Mw=248.84 g.mol−1) and Ammonium dichromate (97 %, Mw=252.06 g.mol−1) were supplied from Sigma-Aldrich (USA).
NiCr2O4 nanoparticles were prepared via a wet-chemistry synthesis method. An appropriate amount of stearic acid was first melted in a beaker at ~346 K. A fixed amount of nickel acetate and ammonium dichromate dissolved in deionized water and added to the melted stearic acid. The heating process of this dual phase materials was continued for complete diffusing metallic-cations from aqueous to organic phase [27], naturally cooling down to room temperature followed by drying in oven to obtain dried gel denoted as precursor. The precursor was finally calcined at 1,073 K for 4 h in air. The calcined nanoparticles were mixed up thoroughly for further investigations. Scheme 1 represents the synthesize, thermal treatment and diffusion (aqueous to organic phase) process.

Schematic representation of synthesize, thermal treatment and diffusion process of metallic cations from aqueous to organic phase.
Results and discussion
Structural analysis
The vibration spectrum of NiCr2O4 nanoparticles (Figure 1) was obtained by exposing the sample to infrared radiation (FT–IR, Perkin Elmer spectrum RX1, ϑ=4,000 to 400 cm−1) and recording the variation of the absorption with frequencies of metal-oxygen (M-O) and metal-metal (M-M) bonds. Sequentially absorptions at 616 and 495 cm−1 confirm the formation of M-O (Cr-O and Ni-O) bonds. Moreover, a single and sharp absorption at around 883 cm−1 indicates the metal-metal (Ni-Cr) vibrational frequencies.

FTIR spectrum of NiCr2O4 nanoparticles.
The formation of NiCr2O4 nanoparticles with an appropriate structure was analyzed using Rigaku X-ray diffractometer (XRD, PTS 3003, Cu-Kα 30 kV & 20 mA, angle =10–80°). Figure 2 shows the XRD pattern of NiCr2O4 nanoparticles. The XRD pattern of NiCr2O4 nanoparticles is clearly supported with literature (JCPDS 75–1728) without any minor phase. The mean nanoparticles diameter was calculated using Scherrer equation (1) from XRD pattern according to the line width of the (311) plane reflection peak,
where θ and
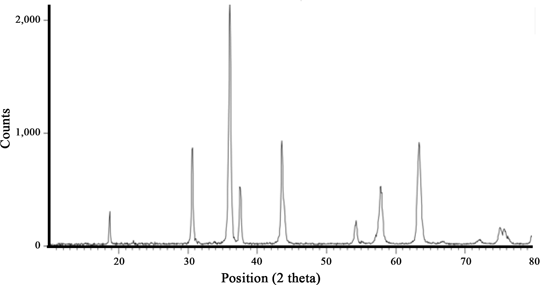
XRD pattern of NiCr2O4 calcined at 1,073 K for 4 h.
A general complementary technique for structural analysis of NiCr2O4 nanoparticles observed by 3D scanning of the sample using a scanning electron microscope (SEM, KYKY-EM 3200). The surface scanning of the nanoparticles (Figure 3) indicates that a narrow distribution of the particles with an average grain size about 27 nm.

SEM of the NiCr2O4 calcined at 1,073 K for 4 h.
Magnetic property
Vibrating sample magnetometer (VSM) system was used to measure the magnetic properties of NiCr2O4 nanoparticles (Figure 4) as a function of magnetic field. The magnetization parameter obtained ~0.2 emu/g in 8kOe applied field at 25 °C. It seems that the NiCr2O4 nanoparticles are paramagnetic; however, the nickel chromite in bulk is known as ferromagnetic materials [28].

VSM curve of the NiCr2O4 nanoparticles.
Diffuse reflectance spectroscopy
The optical band gap of NiCr2O4 nanoparticles carried out by diffuse reflectance spectroscopy (DRS, SCINCO S4100). DR spectra of synthesized NiCr2O4 nanoparticles were obtained between 200 and 1,000 nm with a DRS spectrophotometer shown in Figure 5. This curve shows a sharp absorbance peak around 310 nm.

DRS of NiCr2O4 nanoparticles.
The band gap, Eg, and absorption coefficient of a semiconductor are related to Tauc model [29]. In general, for direct band gap determination, plot of (αhν)2 versus hν is linear at higher values of (hν)2, but tends to deviate from linearity as hν approaches a lower value. Band gap value was obtained by extrapolating the straight portion of the graph, as indicated by solid line. The optical absorption curve of NiCr2O4 nanoparticles obtained from raw data’s of DRS results is shown in Figure 6. The optical absorption curve has been measured and the absorption edge shows that the band gap of NiCr2O4 is about 1.7 eV; hence, NiCr2O4 nanoparticles are semiconductor material applicable for photoelectric devices.

The optical band gap of NiCr2O4 nanoparticles.
Colorimetric coordinates (L*.a*.b*)
The most frequently used modern system is the CIE (L* a* b*) space which provides an effective means for visualizing color: the L* – axis represents lightness-darkness (ranging from 0 to 100), the a* – axis redness-greenness, and the b* axis yellowness-blueness. The color parameters (L*.a*.b*) of NiCr2O4 nanoparticles identified by Reflectance Spectrophotometer (RS, Ihara-spcam spectrophotometer) adopted by the Commission Internationaled’Eclairage (CIE) where L* is the luminance or lightness component, ranging from 0 to 100, a* (from green to red) and b* (from blue to yellow) are two chromatic components (–120 to+120) [30]. The L*.a*.b* color parameters of NiCr2O4 nanoparticles obtained in this study from reflectance spectroscopy are shown in Table 1. The relatively high values of L* (31.58) along with low a* and b* confirm full light absorption (Figure 7).
Color (L*.a*.b*) parameters of NiCr2O4 nanoparticles.
| Name | Illumination | L* | a* | b* |
|---|---|---|---|---|
| NiCr2O4 | D65 | 31.579 | −4.607 | 3.968 |

Arrangement of color attributes in the CIE 1976 (L*.a*.b*) color space.
Conclusion
Spinel NiCr2O4 was synthesized successfully via sol-gel technique. The spinel NiCr2O4 was identified as the main crystalline phase without any minor phase. The nanosize formation of NiCr2O4 confirmed by XRD and SEM in the average range about 24–27 nm. The asymmetric stretching vibration of Ni-O and Cr-O (ϑ=495 and 616 cm−1) affirmed the facets of metal oxide. The band gap of NiCr2O4 nanoparticles obtained at 1.7 eV; hence, NiCr2O4 nanoparticles can be used as semiconductor in photoelectric devices.
References
[1] H.S.C. O’Neill and A. Navrostky, Am. Miner., 68 (1983) 181.Suche in Google Scholar
[2] H.S.C. O’Neill and A. Navrotsky, Am. Miner., 69 (1984) 733.Suche in Google Scholar
[3] D. Levy, A. Pavese and M. Hanfland, Phys. Chem. Miner., 27 (2000) 638.10.1007/s002690000117Suche in Google Scholar
[4] R.M. Hazen and H. Yang, Am. Miner., 84 (1999) 1956.10.2138/am-1999-11-1224Suche in Google Scholar
[5] S. Klemme and J.C. Miltenburg, Phys. Chem. Miner., 29 (2002) 663.10.1007/s00269-002-0280-4Suche in Google Scholar
[6] O. Crottaz, F. Kubel and H. Schmid, J. Mater. Chem., 7 (1997) 143.10.1039/a604758kSuche in Google Scholar
[7] Z. Wang, S.K. Saxena, P. Lazor and H.S.C. O’Neill, J. Phys. Chem. Solids, 64 (2003) 425–431.10.1016/S0022-3697(02)00328-1Suche in Google Scholar
[8] M. Ptak, M. Maczka, A. Gągor, A. Pikul, L. Macalik and J. Hanuza, J. Solid State Chem., 201 (2013) 270–279.10.1016/j.jssc.2013.03.023Suche in Google Scholar
[9] N.H. Li, Y.H. Chen, C.Y. Hu, C.H. Hsieh and S.L. Lo, J. Hazard. Mater., 198 (2011) 356–361.10.1016/j.jhazmat.2011.10.077Suche in Google Scholar PubMed
[10] S. Klemme and J.V. Miltenburg, Phys. Chem. Miner., 29 (2002) 663–667.10.1007/s00269-002-0280-4Suche in Google Scholar
[11] S.M. El-Sheikh and M. Rabbah, Thermochim. Acta, 568 (2013) 13–19.10.1016/j.tca.2013.06.024Suche in Google Scholar
[12] E. Manova, T. Tsoncheva, C. Estournès, D. Paneva, K. Tenchev, I. Mitov and L. Petrov, Appl. Catal. A, 300 (2006) 170–180.10.1016/j.apcata.2005.11.005Suche in Google Scholar
[13] Y. He and K. Shih, Ceram. Int., 38 (2012) 3121–3128.10.1016/j.ceramint.2011.12.013Suche in Google Scholar
[14] M.G. Naseri, E.B. Saion, H.A. Ahangar, A.H. Shaari and M. Hashim, J. Nanomater. 75 (2010) 1–8.10.1155/2010/907686Suche in Google Scholar
[15] M.G. Naseri, E.B. Saion, H.A. Ahangar, M. Hashim and A.H. Shaari, Powder Technol., 212 (2011) 80–88.10.1016/j.powtec.2011.04.033Suche in Google Scholar
[16] C.O. Areán, M.P. Mentruit, A.J. López and J.B. Parra, Physicochem. Eng. Aspect., 180 (2001) 253–258.10.1016/S0927-7757(00)00590-2Suche in Google Scholar
[17] F. Meyer, R. Hemplemann, S. Mathur and M. Veith, J. Mater. Chem., 9 (1999) 1955–1966.Suche in Google Scholar
[18] Y. Cesteros, P. Salagre, F. Medina and J.E. Sueiras, Chem. Mater., 12 (2000) 331–339.10.1021/cm990154hSuche in Google Scholar
[19] P. Jeevanandam, Y. Kolty and A. Gedanken, Nano. Lett., 1 (2001) 263–270.10.1021/nl010003pSuche in Google Scholar
[20] F.M. Pontes, E. Longo, J.H. Rangel, M.I.B. Bernardi, E.R. Leite and J. Varela, J. Mater. Lett., 43 (2000) 249–253.10.1016/S0167-577X(99)00268-2Suche in Google Scholar
[21] S. Ono and S. Hironi, J. Am. Ceram. Soc., 80 (1997) 2533–2540.10.1111/j.1151-2916.1997.tb03155.xSuche in Google Scholar
[22] P.K. Gallagher and S.S.J. Warne, Termochem. Acta, 1 (1970) 465–473.10.1016/0040-6031(70)85017-1Suche in Google Scholar
[23] R.H.G.A. Kiminami, J. KONA, 19 (2001) 156–165.10.14356/kona.2001019Suche in Google Scholar
[24] J.J. Kingsley, K. Suresh and K.C. Patil, J. Mater. Sci., 25 (1990) 1305–1312.10.1007/BF00585441Suche in Google Scholar
[25] A.C.F.M. Costa, (in Portuguese) Thesis, Materials Engineering Dept., Federal University of São Carlos, Brazil, 2002.Suche in Google Scholar
[26] N. Soltani, A.B. Syuhada, W.M.M. Yunus, E. Saion and A. Bahrami, Solid State Comm., 192 (2014) 15–19.10.1016/j.ssc.2014.05.002Suche in Google Scholar
[27] M. Enhessari, Pig. Res. Tech., 42 (2013) 347–352.10.1108/PRT-09-2011-0079Suche in Google Scholar
[28] A.N. Goryaga, L.G. Antoshina, A.I. Kokorev and D.A. Chursin, Phys. Solid. State, 44 (2002) 759–762.10.1134/1.1470572Suche in Google Scholar
[29] M. Enhessari, M. Kargar Razi, L. Etemad, A. Parviz and M. Sakhaei, J. Exp. Nano Sci., 9 (2014) 167–176.10.1080/17458080.2011.649432Suche in Google Scholar
[30] S.E. Papadakis, S. Abdul-Malek, R.E. Kamdem and K.L. Yam, Food Tech., 54 (2000) 48–51.Suche in Google Scholar
©2017 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- Research Articles
- Preparation of Co3O4 Nanostructures via a Hydrothermal- Assisted Thermal Treatment Method by Using of New Precursors
- Transformation Temperatures, Shape Memory and Magnetic Properties of Hafnium Modified Ti-Ta Based High Temperature Shape Memory Alloys
- Modified Sol-Gel Processing of NiCr2O4 Nanoparticles; Structural Analysis and Optical Band Gap
- Effect of Proeutectoid Ferrite Morphology on the Microstructure and Mechanical Properties of Hot Rolled 60Si2MnA Spring Steel
- Uniaxial Properties versus Temperature, Creep and Impact Energy of an Austenitic Steel
- Effect of Rare Earth Cerium Addition on Microstructures and Mechanical Properties of Low Carbon High Manganese Steels
- Geometry and Material Constraint Effects on Creep Crack Growth Behavior in Welded Joints
- Preparation and Properties of ZrO2/Mo Alloys
- The Mechanical Properties of the Mo-0.5Ti and Mo-0.1Zr Alloys at Room Temperature and High Temperature Annealing
- Diffusion Kinetics of Chromium in a Novel Super304H Stainless Steel
- Mechanism of Selective Desulphurization in Iron Ore Sintering Process by Adding Urea
- The Characteristics and Generating Mechanism of Large Precipitates in Ti-Containing H13 Tool Steel
Artikel in diesem Heft
- Frontmatter
- Research Articles
- Preparation of Co3O4 Nanostructures via a Hydrothermal- Assisted Thermal Treatment Method by Using of New Precursors
- Transformation Temperatures, Shape Memory and Magnetic Properties of Hafnium Modified Ti-Ta Based High Temperature Shape Memory Alloys
- Modified Sol-Gel Processing of NiCr2O4 Nanoparticles; Structural Analysis and Optical Band Gap
- Effect of Proeutectoid Ferrite Morphology on the Microstructure and Mechanical Properties of Hot Rolled 60Si2MnA Spring Steel
- Uniaxial Properties versus Temperature, Creep and Impact Energy of an Austenitic Steel
- Effect of Rare Earth Cerium Addition on Microstructures and Mechanical Properties of Low Carbon High Manganese Steels
- Geometry and Material Constraint Effects on Creep Crack Growth Behavior in Welded Joints
- Preparation and Properties of ZrO2/Mo Alloys
- The Mechanical Properties of the Mo-0.5Ti and Mo-0.1Zr Alloys at Room Temperature and High Temperature Annealing
- Diffusion Kinetics of Chromium in a Novel Super304H Stainless Steel
- Mechanism of Selective Desulphurization in Iron Ore Sintering Process by Adding Urea
- The Characteristics and Generating Mechanism of Large Precipitates in Ti-Containing H13 Tool Steel

