Abstract
Stress granules are cytosolic, membraneless RNA-protein complexes that form in the cytosol in response to various stressors. Stress granules form through a process termed liquid-liquid phase separation, which increases the local concentration of RNA and protein within the granules, creates dynamic sorting stations for mRNAs and associated proteins, and modulates the availability of mRNA for protein translation. We introduce the concept that neuronal stress granules act as dynamic cytosolic microcompartments in which their components differentially cycle in and out, monitoring the cellular environment. We discuss that neuronal stress granules have distinctive features and contain substructures in which individual components interact transiently. We describe that neuronal stress granules modulate protein expression at multiple levels and affect the proteoform profile of the cytoskeletal protein tau. We argue that a better knowledge of the regulation of stress granule dynamics in neurons and the modulation of their material state is necessary to understand their function during physiological and pathological stress responses. Finally, we delineate approaches to determine the behavior and regulation of critical stress granule organizers and the physical state of stress granules in living neurons.
Stress granules are membraneless microcompartments that contain RNAs and proteins
Stress granules are membraneless structures visible by light microscopy that form under the influence of various stressors such as oxidative stress, heat shock or viral infection (Figure 1A) (Riggs et al. 2020). They were first described in plant cells (Nover et al. 1983) and later also in mammalian cells (Kedersha et al. 1999). Stress granules are ribonucleoprotein (RNP) granules composed of RNA and proteins and they assemble through liquid-liquid phase separation (LLPS), a process in which macromolecules condense into a dense phase that coexists with a dilute phase (Alberti et al. 2019).
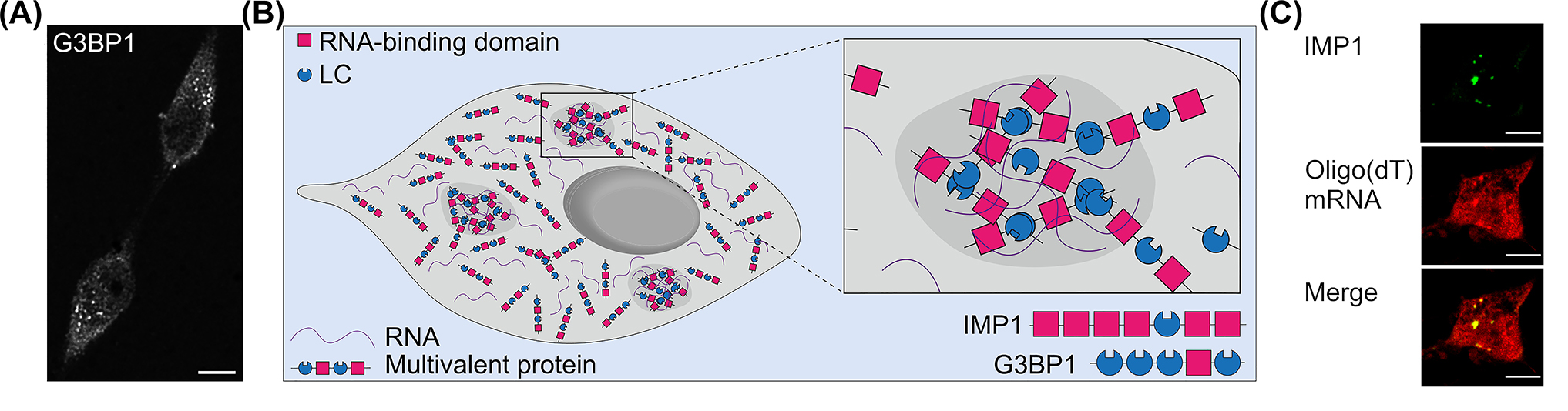
Stress granules in nerve cells. (A) Fluorescence micrograph showing stress granules in arsenite-treated model neurons (neuronally differentiated PC12 cells). The granules were stained against the stress granule marker G3BP1. Note the distribution of the granules with heterogeneous shape throughout the cytoplasm of the cells. Scale bar, 10 µm. (B) Schematic representation of stress granule formation by liquid-liquid phase separation of RNA and multivalent proteins. The schematic structure of the stress granule proteins G3BP1 and IMP1 is shown below right. (C) Fluorescence micrographs showing the localization of a fraction of mRNAs (stained by in situ hybridization with an oligo (dT) probe) to granules induced by exogenous expression of the stress granule protein IMP1. Scale bar, 10 µm.
The main function of stress granules appears to reshape the translatome of a cell at stress conditions. Since protein synthesis is energetically expensive, translation of the cell is reprogrammed by inhibiting general translation, in particular the expression of housekeeping genes, and promoting the translation of stress-responsive pro-survival proteins (Advani and Ivanov 2019). Such transient reprogramming is thought to improve cell survival and promote successful adaptation to stressful conditions (Hofmann et al. 2012; Maharjan et al. 2017). As such, stress granules are dynamic in that they form rapidly under stress, for example after exposure to a potent stressor like sodium arsenite, which is commonly used in laboratory settings (Figure 1A) (Moschner et al. 2014). On the other hand, stress granules also dissipate quickly once the stress has subsided.
Many stress granule-related proteins have been identified over the past 20 years, and recent proximity labeling techniques have identified a large stress granule interactome of approximately 100 proteins (Youn et al. 2018). Many stress granule nucleating proteins are multivalent proteins that promote phase separation and stress granule formation by mediating various weak protein-protein and protein-RNA interactions (Figure 1B). Often, stress granule proteins are also enriched in low complexity regions (LCRs), which are characterized by high structural plasticity and are known to mediate interactions with many binding partners (Kastano et al. 2021). Thus, LCRs may provide a mechanism for increased binding promiscuity and enhanced ability of stress granules to respond rapidly to changes in the environment. A paradigmatic multivalent RNA-binding protein (RBP) is the Ras GTPase-activating protein-binding protein 1 (G3BP1). It contains an RNA recognition motif (RRM) and four LCRs according to SMART analysis (Simple Modular Architecture Research Tool; http://smart.embl.de/) and is present in arsenite-induced stress granules (Figure 1B). Overexpression of G3BP1 and other multivalent mRNA-binding proteins, such as insulin-like growth factor II mRNA-binding protein 1 (IMP1) leads to the formation of RBP granules in cells, even in the absence of stress, thereby concentrating mRNAs in these granules (Figure 1C).
Thus, both stress granules and overexpression-induced RBP granules appear to be functional units that locally concentrate specific RNAs and proteins. As such, they qualify as cytosolic microcompartments in terms of creating dynamic sorting stations for mRNAs and associated proteins (Brandt et al. 2013; Brandt and Paululat 2013).
Stress granules are dynamic and their components shuttle in and out differentially
Stress granules not only rapidly assemble and disassemble in response to the induction of stress conditions or their removal, but they can also exchange their molecular components due to the lack of an enclosing membrane. Shuttling has been clearly demonstrated for stress granule proteins, but it is less clear for the RNA part. Mollet et al. reported a short residence time of mRNAs in stress granules, on the order of a minute, although stress granules can persist for extended periods (Mollet et al. 2008). However, it is unclear whether this short residence time is due to the particular construct, the β-Gal reporter mRNA. It could also be due to the binding of the fluorescence reporter, the MS2-GFP used in the experiment. The results are more plentiful for specific stress granule proteins, where exchange rates ranging from seconds to minutes have been reported (Bley et al. 2015; Kedersha et al. 2000). Using fluorescence decay after photoactivation (FDAP) experiments, in which the fluorescence of PAGFP tagged to a stress granule protein is activated in a single stress granule and the dissipation from the activation site is recorded, the residence time of different stress granule proteins can be determined (Sohnel et al. 2022) (Figure 2). With such an approach, we determined a t 1/2 of ∼20s for G3BP1, which was much shorter than the respective t 1/2 of IMP1 (Niewidok et al. 2018). Thus, the data indicate that the exchange dynamics of stress granule proteins can be highly variable and dependent on the number and strength of the individual RNA- and protein-interaction domains. According to SMART analysis, G3BP1 contains four putative protein-protein interaction domains (LCRs) and a single RNA-binding region, while IMP1 contains six RNA-binding domains and only a single LCR. One might speculate that the RNA-binding regions may anchor the RBPs more to the stress granules due to their stronger binding to the mRNAs.
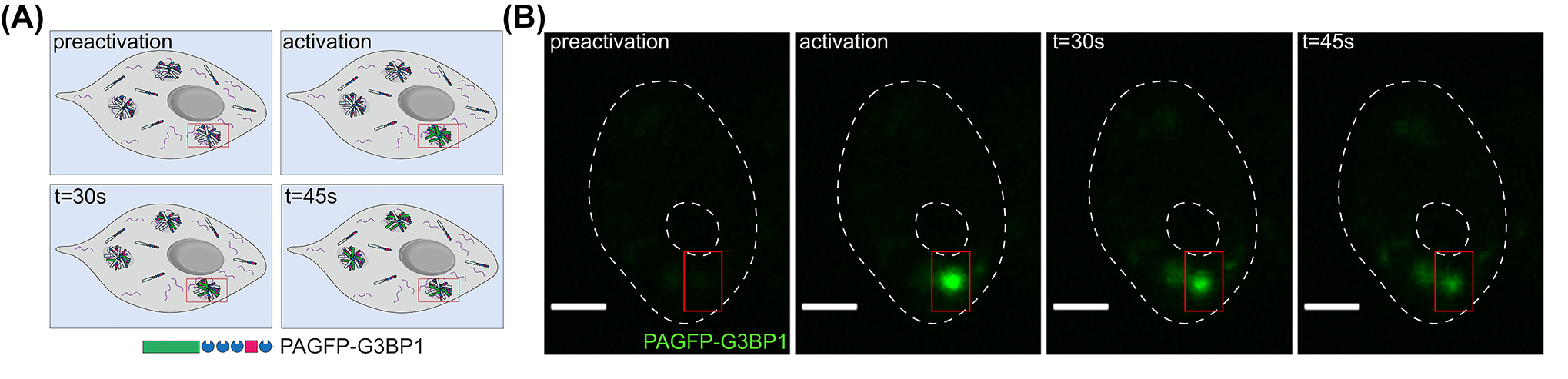
Shuttling of RNA-binding proteins between stress granules. (A) Schematic representation of a typical fluorescence photoactivation experiment. A tagged stress granule protein is photoactivated in an individual stress granule and distribution to other granules is monitored over time. (B) Time-lapse images after photoactivation of the stress granule marker G3BP1 tagged with photoactivatable GFP (PAGFP). Note the fluorescence decay of the protein over time due to the shuttling of the protein to other granules. Scale bar, 5 µm.
Post-translational modifications of stress granule proteins can also affect their function and shuttle dynamics. For example, it has been reported that phosphorylation at serine 149 impairs the ability of G3BP1 to induce stress granule formation (Tourriere et al. 2003). However, this observation was later questioned (Panas et al. 2019). Several post-translational modifications have also been shown to affect LLPS, at least in vitro. These include arginine methylation, which appears to repress LLPS (Hofweber et al. 2018; Ryan et al. 2018). Also, phosphorylation can affect phase separation and reduces LLPS of the FUS (Fused in Sarcoma) RNA binding protein and TDP-43 (TAR DNA–binding protein of 43 kDa) (Monahan et al. 2017; Wang et al. 2018).
Thus, stress granules are highly dynamic and their components shuttle in and out differentially. This dynamic exchange allows them to constantly monitor the cellular environment and respond quickly to changes in the activity of signaling molecules. Therefore, stress granules qualify as physiologically relevant microcompartments whose function can be locally directed by the cellular environment.
Neuronal stress granules have distinctive features
Stress granules appear to be heterogeneous microcompartments and their properties can vary in different cell types. This may be due to different protein and RNA composition depending on the particular cell type and condition of the cell. In Drosophila neurons, it has been predicted that 398 different RNAs are present in stress granules (van Leeuwen et al. 2022) that can recruit multivalent RBPs to direct granule assembly and disassembly (Campos-Melo et al. 2021). There is also a molecular overlap between components of stress granules with other phase-separated microcompartments such as processing bodies that can influence their activity (Youn et al. 2018).
Neurons have special properties that distinguish them from other cell types. They are postmitotic, develop distinct polarity with extensive processes, and exhibit high energy expenditure. Neurons also undergo degeneration in various neurodegenerative diseases, many of which are characterized by the formation of protein aggregates from molecules that are also known to phase separate. These include α-synuclein, which aggregates in Lewy bodies in patients with Parkinson’s disease, the microtubule-associated protein tau, which forms neurofibrillary tangles in AD and other tauopathies, and TDP-43, which is implicated in several diseases including amyotrophic lateral sclerosis (ALS) (Zbinden et al. 2020). Disease-associated proteins such as survival of motor neuron (SMN), TDP-43, RNA-binding protein FUS, TIA1, and tau have also been identified in stress granules (Advani and Ivanov 2020; Jeon and Lee 2021), and in some cases it has been shown that disease-associated mutations show an increased tendency to phase separation (Mackenzie et al. 2017). All of these proteins contain LCRs that provide structural plasticity, conformational adaptability, and binding promiscuity (Uversky 2015), which are also typical features of stress granule-associated proteins. Therefore, aggregation of some stress granule-associated proteins may be related to dysregulated stress granules, and this seems to be a characteristic feature of neurons. In support of such a hypothesis, modulation of TDP-43 recruitment to stress granules prevents TDP-43 accumulation in ALS (Fang et al. 2019), which also emphasizes that a better understanding of stress granule regulation in neurons and the modulation of neuronal stress granule interactions could provide approaches to prevent neurodegeneration.
Functional analysis of stress granule dynamics in cultured primary neurons and astrocytes showed that cortical neurons are more resistant to arsenite-induced stress granule formation than astrocytes. On the other hand, the stress granules persisted longer once formed (Khalfallah et al. 2018). This suggests that neuronal stress granules are resilient to a certain level of stress and confirms that neuronal stress granules have distinctive features that distinguish them from stress granules in other cell types. Khalfallah et al. also reported that SG dynamics are modulated by the amount of TDP-43, suggesting that differences in the protein composition of neuronal stress granules may be responsible for their distinctive features.
Neuronal stress granules contain substructures
In contrast to condensates produced in vitro, stress granules are not perfectly round but have a more irregular shape (Figure 1A). This indicates that stress granules contain substructures. Indeed, it has been proposed that stress granules have a biphasic structure consisting of a stable core surrounded by a less concentrated shell (Wheeler et al. 2016). The shell structure can behave more like a condensate formed by weak protein-protein and protein-RNA interactions, while the inner core is less dynamic and consists of more stable assemblies (Jain et al. 2016). This may also indicate a sequence of events where the first structures are formed by LLPS and then more stable core structures are formed over time. Such a mechanism, where more stable interactions are formed through an aging process, would also be relevant to a potential formation of disease-associated stable amyloid structures, which have been hypothesized to be the result of abnormally persistent stress granules (Jeon and Lee 2021). While such a hypothesis appears attractive, experimental support is scarce. In fact, stress granule dynamics and core size were found to remain unchanged during prolonged stress (Wheeler et al. 2016), suggesting that additional factors might be involved in triggering the structural changes that lead to more stable stress granules.
It is also important to note that stress granules are present in all eukaryotic kingdoms, but may differ in their structure between organisms. For example, it has been suggested that the core of yeast stress granules is more rigid than the core of mammalian stress granules (Jain et al. 2016). In addition, the composition and structure of stress granules can differ depending on the particular stressor. Indeed, mammalian cells have been shown to assemble different types of stress granules in a stress-specific manner, and differences between stress granules induced by heat shock, proteasome inhibition, and UV irradiation have been reported (Aulas et al. 2017).
Single-molecule tracking of selected stress granule proteins can be an excellent method to determine their behavior in stress granules of living cells. Tracking the proteins should then allow stress granule regions with higher liquidity (shell region) to be distinguished from more stable core regions. We used this approach and expressed paradigmatic multivalent stress granule proteins such as G3BP1 and IMP1 in model neurons. Proteins were tagged to allow substoichiometric labelling with a fluorescent marker and stress was induced with sodium arsenite. Indeed, imaging revealed a biphasic distribution of both proteins within stress granules (Figure 3). However, we did not observe a distinct core domain but distributed hotspots of immobilized G3BP1 and IMP1, reflecting the presence of relatively immobile nanometer-sized nanocores (Niewidok et al. 2018). Furthermore, between the liquid-like diffusion, the molecules transiently interacted with the nanocores (with a mean lifetime of 200–300 ms). Thus, at least in the cell type analyzed and with arsenite as a stressor, our results argue for the presence of transient interactions of RBPs in distributed nanocores without a stable core structure. Such an organization may facilitate the functioning of stress granules as dynamic sorting stations under acute stress conditions.
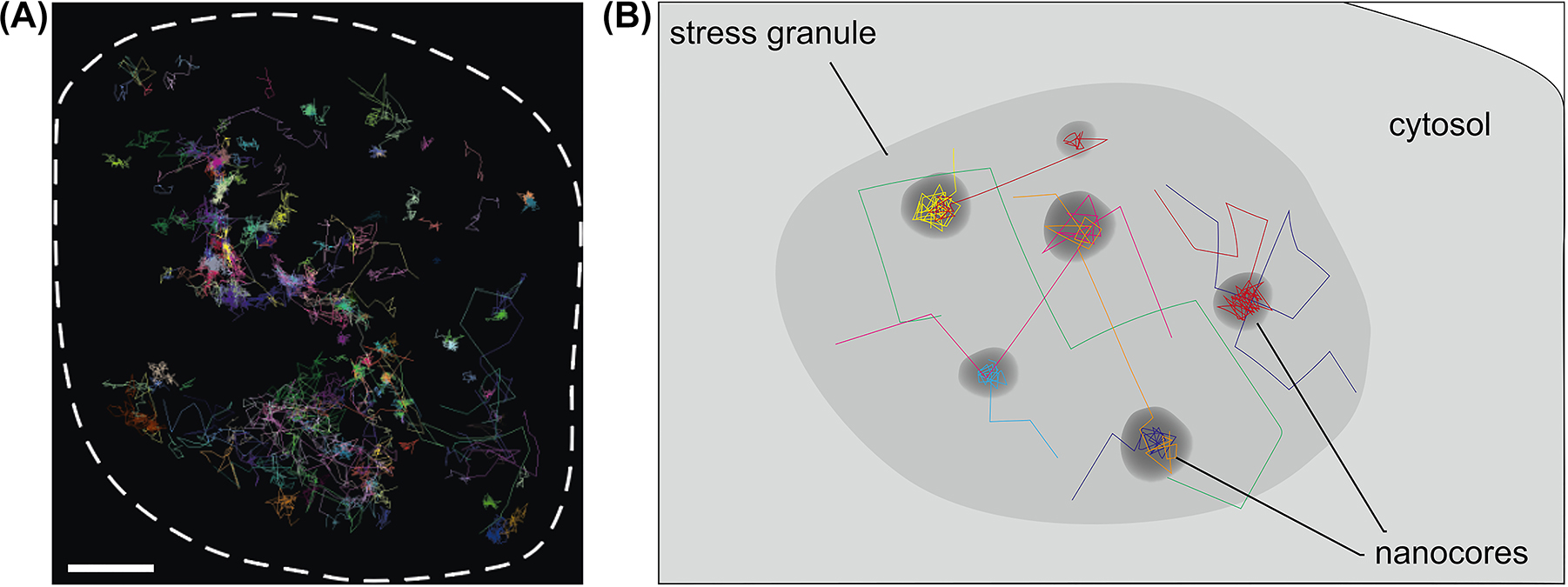
Nanoscale organization of neuronal stress granules. (A) Trajectories of individual molecules of the stress granule protein G3BP1 in a single stress granule. For the tracking experiment, HaloTag-G3BP1 was expressed in the cells and substoichiometric staining was performed with TMR. Single-molecule localization microscopy was performed using Total Internal Reflection Fluorescence (TIRF) microscopy in the Highly Inclined and Laminated Optical sheet (HILO) mode. For experimental details see Niewidok et al. (2018). The boundary of the stress granule is indicated by a dashed line. Note the biphasic distribution in a freely diffusing phase and a bound phase, which reflects the presence of distributed nanocores within the stress granules. Scale bar, 0.5 μm. (B) Schematic representation of the trajectories of single molecules showing the transient interaction with distributed nanocores in a stress granule.
Single-molecule tracking also allowed the determination of the diffusion behavior of the investigated protein during the unbound time. The corresponding analysis revealed that the proteins exhibit anomalous diffusion in the mobile phase of stress granules with an anomaly exponent α of 0.5–0.7 (Niewidok et al. 2018). Since the anomaly exponent decreases with increasing molecular crowding (α would be 1.0 in the absence of molecular crowding), the data indicate that stress granules have a much higher protein concentration than the surrounding cytoplasm. These findings support that stress granules result from a condensation event leading to the concentration of the molecular components in the microcompartments.
Thus, the data indicate that, in contrast to liquid-liquid-phase condensates formed in vitro, neuronal stress granules are less homogenous and contain substructures with different material states. This may indicate that their reaction to cellular changes is complex, and that components in the more liquid phase are likely to shuttle more dynamically than those present in the more solid core regions.
Neuronal stress granules modulate protein expression at multiple levels
A major function of stress granules is believed to be to adapt the translation to the adverse conditions of acute stress. However, the mechanism of how stress granules act on the RNAs they contain is less clear. A translational switch of certain mRNAs can occur through various mechanisms, such as protecting and storing mRNAs, which prevents translation. This would conform to the classical concept of stress granules as mRNA-protein assemblies formed from non-translating mRNAs that are assembled from mRNAs stalled in translation initiation (Protter and Parker 2016). However, recent evidence suggests that at least some mRNAs within stress granules are not silenced and single-molecule imaging indicates the presence of active translation of mRNA within stress granules (Mateju et al. 2020). Notably, this study found that translating and non-translating mRNAs switch between the cytosol and stress granules without associated changes in translation status. Therefore, it would also be conceptually possible that localization to stress granules promotes the translation of specific mRNAs by bringing them close to regulatory factors.
Notably, most mRNAs are also not concentrated in stress granules and analysis of the stress granule transcriptome revealed that only 10% of all mRNA molecules accumulate in stress granules (Khong et al. 2017). In fact, only a comparatively small number of genes have been found to have more than 50% of their mRNA molecules contained in stress granules. This obviously questions the direct involvement of stress granules in the control of translation of the mRNAs.
The regulation can not only take place at the level of repression or promotion of translation, but can also occur in an isoform-specific manner, which would lead to a change in the proteoform profile of the respective protein. In such a scenario, even a limited number of mRNAs of a particular species could evoke the presence of a particular protein isoform with a particular function. In support of such a possibility, we observed that the presence of stress granules caused a shift in expression of a neuronal microtubule-modulating protein, the microtubule-associated protein tau, towards a longer isoform, big tau (Moschner et al. 2014). Again, much of the tau mRNA was not localized in RNP granules, suggesting that the localization of tau mRNA is not quantitatively important for the massive change in isoform expression. It has been hypothesized that “big tau” has an increased ability to stabilize the microtubule network in peripheral neurons (Fischer and Baas 2020), consistent with our observation that the shift in isoform expression leads to the formation of longer processes (Figure 4).
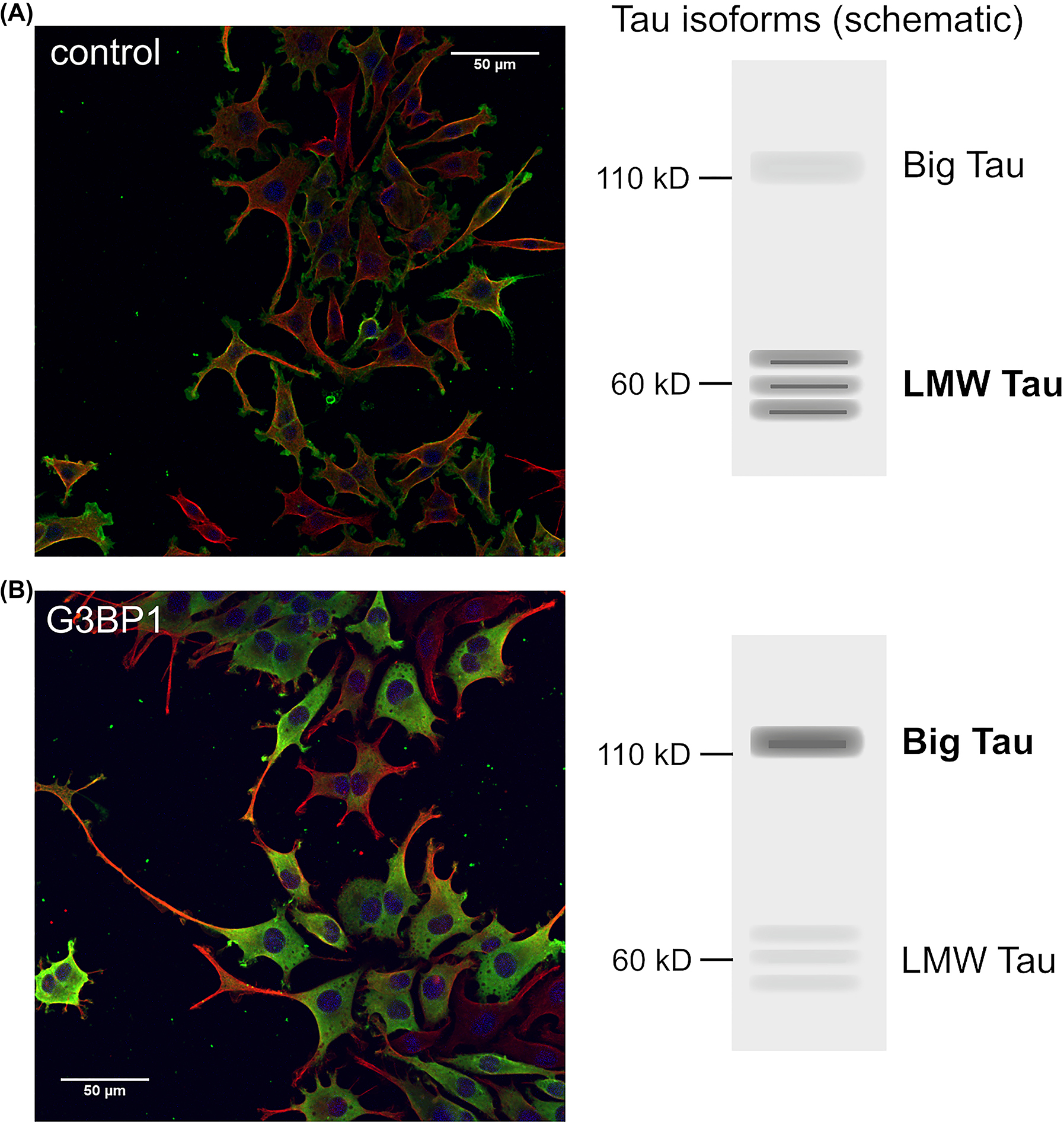
Stress granule formation alters isoform-expression of the microtubule-associated protein tau. (A, B) Expression of a stress granule-associated protein changes the expression of the isoforms of microtubule-associated protein tau from low molecular weight (LMW) tau to longer isoforms (big tau) and increases process outgrowth. Model neurons (neuronally differentiated PC12 cells) stably transfected to express farnesylated PAGFP (A) or the stress granule protein G3BP1 tagged with PAGFP (B) are shown. A schematic representation of the expressed tau isoforms is shown on the right. The cells were treated for 24 h with NGF, fixed and stained against GFP (green) and tubulin (red). Nuclei were stained with DAPI (blue). For experimental details see Moschner et al. (2014).
Overall, current data may suggest that the impact of stress granules on mRNA expression is more complex than initially thought. It can affect the proteoform (Smith and Kelleher 2013), i.e. the different molecular forms in which the protein product of a single gene can be found, including changes due to alternatively spliced RNA transcripts. In neurons, influencing the proteoform profile of cytoskeletal proteins can contribute to morphogenetic effects involved in the regulation of neuronal regeneration and plasticity.
Towards a better understanding of the structure and function of neuronal stress granules
As presented above, stress granules are dynamic and physiologically relevant subcellular specializations that meet the criteria for typical microcompartments. They are functional units that locally concentrate certain RNAs and proteins in the cytosol. They are dynamic and their components shuttle in and out differently, constantly scanning and responding to the cellular environment. However, neuronal stress granules in particular are more complex than simple phase-separated condensates, they have distinctive features that distinguish them from stress granules in other cell types, and they contain substructures that are likely to affect their function. Neuronal stress granules also appear to influence the expression of mRNAs in more complex ways than originally thought.
Thus, important aspects of the neuronal stress granules remain in the dark. A major unsolved question is how changes in cellular signal transduction mechanisms are communicated to the stress granules, which molecules of the neuronal stress granule are involved, and what changes result. A possible mechanism is that changes in the phosphorylation or other post-translational modifications of central stress granule organizer proteins affect the dynamics of stress granules and their material state. However, it is still unclear whether and how post-translational modifications of stress granule proteins affect the dynamic shuttling of the proteins in an authentic cytosolic environment and how this is related to stress granule function. A live-cell imaging assay to monitor and quantify the dynamics of stress granule components in live neuronal cells by FDAP, as we recently developed (Sohnel et al. 2022) may be helpful to identify and functionally characterize critical posttranslational modifications.
A second important question is how the material state of neuronal stress granules affects their functionality and how changes in the material state are regulated. Because stress granules are highly dynamic, the analysis of isolated stress granules can be problematic as it can bias the interpretation towards the more stable components that survive the isolation procedure. Therefore, ideally, the material state of stress granules would need to be analyzed in the context of living cells. Due to the small size of stress granules, ranging in diameter from a few hundred nanometers up to the low µm scale, standard light microscopy approaches are not sufficient. On the other hand, the electron microscopic analysis cannot capture the dynamics of the system, which is a characteristic feature of this microcompartment. A potential solution could be super-resolution microscopy approaches like single-molecule tracking of individual stress granule components, as we recently developed for stress granules in model neurons (Niewidok et al. 2020). Such an approach allows quantitative determination of the proportion of freely diffusing and nanocore-bound proteins within individual stress granules, calculation of the lifetime of binding in nanocores, and determination of diffusive behaviour in the liquid phase. The parameters for diffusion allow the determination of viscosity and the extent of molecular crowding within stress granules, thereby monitoring potential changes towards higher insolubility. This could also be of pathological relevance, since the inappropriate formation of neuronal stress granules or changes from a more fluid-like, dynamic phase to higher insolubility of their components have been associated with aging and pathological processes when more stable protein aggregates formed (Alberti and Hyman 2016; Wolozin and Ivanov 2019).
Of course, the most critical questions relate to the function of the neuronal stress granules. How do stress granules alter the proteome of a neuron and the proteoforms of key neuronal proteins? How is this activity regulated at the molecular level and what role does the subgranular organization of neuronal stress granules play?
Funding source: Deutsche Forschungsgemeinschaft
Award Identifier / Grant number: SFB 944, Project P1
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work has been supported by the Deutsche Forschungsgemeinschaft (DFG Grant SFB 944, Project P1 (to R.B.)).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
Advani, V.M. and Ivanov, P. (2019). Translational control under stress: reshaping the translatome. Bioessays 41: e1900009, https://doi.org/10.1002/bies.201900009.Search in Google Scholar PubMed PubMed Central
Advani, V.M. and Ivanov, P. (2020). Stress granule subtypes: an emerging link to neurodegeneration. Cell. Mol. Life Sci. 77: 4827–4845, https://doi.org/10.1007/s00018-020-03565-0.Search in Google Scholar PubMed PubMed Central
Alberti, S., Gladfelter, A., and Mittag, T. (2019). Considerations and challenges in studying liquid-liquid phase separation and biomolecular condensates. Cell 176: 419–434, https://doi.org/10.1016/j.cell.2018.12.035.Search in Google Scholar PubMed PubMed Central
Alberti, S. and Hyman, A.A. (2016). Are aberrant phase transitions a driver of cellular aging? Bioessays 38: 959–968, https://doi.org/10.1002/bies.201600042.Search in Google Scholar PubMed PubMed Central
Aulas, A., Fay, M.M., Lyons, S.M., Achorn, C.A., Kedersha, N., Anderson, P., and Ivanov, P. (2017). Stress-specific differences in assembly and composition of stress granules and related foci. J. Cell Sci. 130: 927–937, https://doi.org/10.1242/jcs.199240.Search in Google Scholar PubMed PubMed Central
Bley, N., Lederer, M., Pfalz, B., Reinke, C., Fuchs, T., Glass, M., Moller, B., and Huttelmaier, S. (2015). Stress granules are dispensable for mRNA stabilization during cellular stress. Nucleic Acids Res. 43: e26, https://doi.org/10.1093/nar/gku1275.Search in Google Scholar PubMed PubMed Central
Brandt, R., Klare, J., Steinhoff, H.J., and Ungermann, C. (2013). Highlight: the physiology and dynamics of cellular microcompartments. Biol. Chem. 394: 149–150, https://doi.org/10.1515/hsz-2012-0349.Search in Google Scholar PubMed
Brandt, R. and Paululat, A. (2013). Microcompartments in the Drosophila heart and the mammalian brain: general features and common principles. Biol. Chem. 394: 217–230, https://doi.org/10.1515/hsz-2012-0261.Search in Google Scholar PubMed
Campos-Melo, D., Hawley, Z.C.E., Droppelmann, C.A., and Strong, M.J. (2021). The integral role of RNA in stress granule formation and function. Front. Cell Dev. Biol. 9: 621779, https://doi.org/10.3389/fcell.2021.621779.Search in Google Scholar PubMed PubMed Central
Fang, M.Y., Markmiller, S., Vu, A.Q., Javaherian, A., Dowdle, W.E., Jolivet, P., Bushway, P.J., Castello, N.A., Baral, A., Chan, M.Y., et al.. (2019). Small-molecule modulation of TDP-43 recruitment to stress granules prevents persistent TDP-43 accumulation in ALS/FTD. Neuron 103: 802–819.e811, https://doi.org/10.1016/j.neuron.2019.05.048.Search in Google Scholar PubMed PubMed Central
Fischer, I. and Baas, P.W. (2020). Resurrecting the mysteries of big tau. Trends Neurosci. 43: 493–504, https://doi.org/10.1016/j.tins.2020.04.007.Search in Google Scholar PubMed PubMed Central
Hofmann, S., Cherkasova, V., Bankhead, P., Bukau, B., and Stoecklin, G. (2012). Translation suppression promotes stress granule formation and cell survival in response to cold shock. Mol. Biol. Cell 23: 3786–3800, https://doi.org/10.1091/mbc.e12-04-0296.Search in Google Scholar
Hofweber, M., Hutten, S., Bourgeois, B., Spreitzer, E., Niedner-Boblenz, A., Schifferer, M., Ruepp, M.D., Simons, M., Niessing, D., Madl, T., et al.. (2018). Phase separation of FUS is suppressed by its nuclear import receptor and arginine methylation. Cell 173: 706–719.e713, https://doi.org/10.1016/j.cell.2018.03.004.Search in Google Scholar PubMed
Jain, S., Wheeler, J.R., Walters, R.W., Agrawal, A., Barsic, A., and Parker, R. (2016). ATPase-modulated stress granules contain a diverse proteome and substructure. Cell 164: 487–498, https://doi.org/10.1016/j.cell.2015.12.038.Search in Google Scholar PubMed PubMed Central
Jeon, P. and Lee, J.A. (2021). Dr. Jekyll and Mr. Hyde? Physiology and pathology of neuronal stress granules. Front. Cell Dev. Biol. 9: 609698, https://doi.org/10.3389/fcell.2021.609698.Search in Google Scholar PubMed PubMed Central
Kastano, K., Mier, P., and Andrade-Navarro, M.A. (2021). The role of low complexity regions in protein interaction modes: an illustration in huntingtin. Int. J. Mol. Sci. 22: 1727, https://doi.org/10.3390/ijms22041727.Search in Google Scholar PubMed PubMed Central
Kedersha, N., Cho, M.R., Li, W., Yacono, P.W., Chen, S., Gilks, N., Golan, D.E., and Anderson, P. (2000). Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J. Cell Biol. 151: 1257–1268, https://doi.org/10.1083/jcb.151.6.1257.Search in Google Scholar PubMed PubMed Central
Kedersha, N.L., Gupta, M., Li, W., Miller, I., and Anderson, P. (1999). RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J. Cell Biol. 147: 1431–1442, https://doi.org/10.1083/jcb.147.7.1431.Search in Google Scholar PubMed PubMed Central
Khalfallah, Y., Kuta, R., Grasmuck, C., Prat, A., Durham, H.D., and Vande Velde, C. (2018). TDP-43 regulation of stress granule dynamics in neurodegenerative disease-relevant cell types. Sci. Rep. 8: 7551, https://doi.org/10.1038/s41598-018-25767-0.Search in Google Scholar PubMed PubMed Central
Khong, A., Matheny, T., Jain, S., Mitchell, S.F., Wheeler, J.R., and Parker, R. (2017). The stress granule transcriptome reveals principles of mRNA accumulation in stress granules. Mol. Cell. 68: 808–820.e805, https://doi.org/10.1016/j.molcel.2017.10.015.Search in Google Scholar PubMed PubMed Central
Mackenzie, I.R., Nicholson, A.M., Sarkar, M., Messing, J., Purice, M.D., Pottier, C., Annu, K., Baker, M., Perkerson, R.B., Kurti, A., et al.. (2017). TIA1 mutations in amyotrophic lateral sclerosis and frontotemporal dementia promote phase separation and alter stress granule dynamics. Neuron 95: 808–816.e809, https://doi.org/10.1016/j.neuron.2017.07.025.Search in Google Scholar PubMed PubMed Central
Maharjan, N., Kunzli, C., Buthey, K., and Saxena, S. (2017). C9ORF72 regulates stress granule formation and its deficiency impairs stress granule assembly, hypersensitizing cells to stress. Mol. Neurobiol. 54: 3062–3077, https://doi.org/10.1007/s12035-016-9850-1.Search in Google Scholar PubMed
Mateju, D., Eichenberger, B., Voigt, F., Eglinger, J., Roth, G., and Chao, J.A. (2020). Single-molecule imaging reveals translation of mRNAs localized to stress granules. Cell 183: 1801–1812.e1813, https://doi.org/10.1016/j.cell.2020.11.010.Search in Google Scholar PubMed
Mollet, S., Cougot, N., Wilczynska, A., Dautry, F., Kress, M., Bertrand, E., and Weil, D. (2008). Translationally repressed mRNA transiently cycles through stress granules during stress. Mol. Biol. Cell 19: 4469–4479, https://doi.org/10.1091/mbc.e08-05-0499.Search in Google Scholar PubMed PubMed Central
Monahan, Z., Ryan, V.H., Janke, A.M., Burke, K.A., Rhoads, S.N., Zerze, G.H., O’Meally, R., Dignon, G.L., Conicella, A.E., Zheng, W., et al.. (2017). Phosphorylation of the FUS low-complexity domain disrupts phase separation, aggregation, and toxicity. EMBO J. 36: 2951–2967, https://doi.org/10.15252/embj.201696394.Search in Google Scholar PubMed PubMed Central
Moschner, K., Sundermann, F., Meyer, H., da Graca, A.P., Appel, N., Paululat, A., Bakota, L., and Brandt, R. (2014). RNA protein granules modulate tau isoform expression and induce neuronal sprouting. J. Biol. Chem. 289: 16814–16825, https://doi.org/10.1074/jbc.m113.541425.Search in Google Scholar PubMed PubMed Central
Niewidok, B., Igaev, M., Pereira da Graca, A., Strassner, A., Lenzen, C., Richter, C.P., Piehler, J., Kurre, R., and Brandt, R. (2018). Single-molecule imaging reveals dynamic biphasic partition of RNA-binding proteins in stress granules. J. Cell Biol. 217: 1303–1318, https://doi.org/10.1083/jcb.201709007.Search in Google Scholar PubMed PubMed Central
Niewidok, B., Kurre, R., and Brandt, R. (2020). Nanocores and Liquid Droplets: single-molecule microscopy of neuronal stress granule components. Neuromethods 154: 39–57.10.1007/978-1-0716-0532-5_3Search in Google Scholar
Nover, L., Scharf, K.D., and Neumann, D. (1983). Formation of cytoplasmic heat shock granules in tomato cell cultures and leaves. Mol. Cell Biol. 3: 1648–1655, https://doi.org/10.1128/mcb.3.9.1648.Search in Google Scholar
Panas, M.D., Kedersha, N., Schulte, T., Branca, R.M., Ivanov, P., and Anderson, P. (2019). Phosphorylation of G3BP1-S149 does not influence stress granule assembly. J. Cell Biol. 218: 2425–2432, https://doi.org/10.1083/jcb.201801214.Search in Google Scholar PubMed PubMed Central
Protter, D.S. and Parker, R. (2016). Principles and properties of stress granules. Trends Cell Biol. 26: 668–679, https://doi.org/10.1016/j.tcb.2016.05.004.Search in Google Scholar PubMed PubMed Central
Riggs, C.L., Kedersha, N., Ivanov, P., and Anderson, P. (2020). Mammalian stress granules and P bodies at a glance. J. Cell Sci. 133: jcs242487, https://doi.org/10.1242/jcs.242487.Search in Google Scholar PubMed
Ryan, V.H., Dignon, G.L., Zerze, G.H., Chabata, C.V., Silva, R., Conicella, A.E., Amaya, J., Burke, K.A., Mittal, J., and Fawzi, N.L. (2018). Mechanistic view of hnRNPA2 low-complexity domain structure, interactions, and phase separation altered by mutation and arginine methylation. Mol. Cell. 69: 465–479.e467, https://doi.org/10.1016/j.molcel.2017.12.022.Search in Google Scholar PubMed PubMed Central
Smith, L.M. and Kelleher, N.L. (2013). Consortium for Top Down, PProteoform: a single term describing protein complexity. Nat. Methods 10: 186–187, https://doi.org/10.1038/nmeth.2369.Search in Google Scholar PubMed PubMed Central
Sohnel, A.C., Trushina, N.I., and Brandt, R. (2022). Monitoring and quantification of the dynamics of stress granule components in living cells by fluorescence decay after photoactivation. Methods Mol. Biol. 2428: 243–259, https://doi.org/10.1007/978-1-0716-1975-9_15.Search in Google Scholar PubMed
Tourriere, H., Chebli, K., Zekri, L., Courselaud, B., Blanchard, J.M., Bertrand, E., and Tazi, J. (2003). The RasGAP-associated endoribonuclease G3BP assembles stress granules. J. Cell Biol. 160: 823–831, https://doi.org/10.1083/jcb.200212128.Search in Google Scholar PubMed PubMed Central
Uversky, V.N. (2015). Intrinsically disordered proteins and their (disordered) proteomes in neurodegenerative disorders. Front. Aging Neurosci. 7: 18, https://doi.org/10.3389/fnagi.2015.00018.Search in Google Scholar PubMed PubMed Central
van Leeuwen, W., VanInsberghe, M., Battich, N., Salmen, F., van Oudenaarden, A., and Rabouille, C. (2022). Identification of the stress granule transcriptome via RNA-editing in single cells and in vivo. Cell Rep. Methods 2: 100235, https://doi.org/10.1016/j.crmeth.2022.100235.Search in Google Scholar PubMed PubMed Central
Wang, A., Conicella, A.E., Schmidt, H.B., Martin, E.W., Rhoads, S.N., Reeb, A.N., Nourse, A., Ramirez Montero, D., Ryan, V.H., Rohatgi, R., et al.. (2018). A single N-terminal phosphomimic disrupts TDP-43 polymerization, phase separation, and RNA splicing. EMBO J. 37: e97452, https://doi.org/10.15252/embj.201797452.Search in Google Scholar PubMed PubMed Central
Wheeler, J.R., Matheny, T., Jain, S., Abrisch, R., and Parker, R. (2016). Distinct stages in stress granule assembly and disassembly. Elife 5, https://doi.org/10.7554/elife.18413.Search in Google Scholar
Wolozin, B. and Ivanov, P. (2019). Stress granules and neurodegeneration. Nat. Rev. Neurosci. 20: 649–666, https://doi.org/10.1038/s41583-019-0222-5.Search in Google Scholar PubMed PubMed Central
Youn, J.Y., Dunham, W.H., Hong, S.J., Knight, J.D.R., Bashkurov, M., Chen, G.I., Bagci, H., Rathod, B., MacLeod, G., Eng, S.W.M., et al.. (2018). High-density proximity mapping reveals the subcellular organization of mRNA-associated granules and bodies. Mol. Cell. 69: 517–532.e511, https://doi.org/10.1016/j.molcel.2017.12.020.Search in Google Scholar PubMed
Zbinden, A., Perez-Berlanga, M., De Rossi, P., and Polymenidou, M. (2020). Phase separation and neurodegenerative diseases: a disturbance in the force. Dev. Cell 55: 45–68, https://doi.org/10.1016/j.devcel.2020.09.014.Search in Google Scholar PubMed
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Highlight: Physiology and Dynamics of Cellular Microcompartments
- Highlight: on the past and the future of cellular microcompartments
- Nuclear redox processes in land plant development and stress adaptation
- The readily retrievable pool of synaptic vesicles
- Loss of respiratory complex I subunit NDUFB10 affects complex I assembly and supercomplex formation
- Modulation of self-organizing circuits at deforming membranes by intracellular and extracellular factors
- Computational resolution in single molecule localization – impact of noise level and emitter density
- Setting up a data management infrastructure for bioimaging
- Molecular insights into endolysosomal microcompartment formation and maintenance
- The role of lysosomes in lipid homeostasis
- Membrane damage and repair: a thin line between life and death
- Neuronal stress granules as dynamic microcompartments: current concepts and open questions
- Molecular determinants of protein half-life in chloroplasts with focus on the Clp protease system
- Neprilysin 4: an essential peptidase with multifaceted physiological relevance
- Determinants of synergistic cell-cell interactions in bacteria
- Drosophila collagens in specialised extracellular matrices
Articles in the same Issue
- Frontmatter
- Highlight: Physiology and Dynamics of Cellular Microcompartments
- Highlight: on the past and the future of cellular microcompartments
- Nuclear redox processes in land plant development and stress adaptation
- The readily retrievable pool of synaptic vesicles
- Loss of respiratory complex I subunit NDUFB10 affects complex I assembly and supercomplex formation
- Modulation of self-organizing circuits at deforming membranes by intracellular and extracellular factors
- Computational resolution in single molecule localization – impact of noise level and emitter density
- Setting up a data management infrastructure for bioimaging
- Molecular insights into endolysosomal microcompartment formation and maintenance
- The role of lysosomes in lipid homeostasis
- Membrane damage and repair: a thin line between life and death
- Neuronal stress granules as dynamic microcompartments: current concepts and open questions
- Molecular determinants of protein half-life in chloroplasts with focus on the Clp protease system
- Neprilysin 4: an essential peptidase with multifaceted physiological relevance
- Determinants of synergistic cell-cell interactions in bacteria
- Drosophila collagens in specialised extracellular matrices

