Abstract
A series of novel 4,6-dimorpholinyl-1,3,5-triazine derivatives 6a–6r were obtained through N-substitution and Claisen-Schmidt condensation. 1H NMR, 13C NMR, and mass spectrometry were used to characterize the molecular structures of the derivatives. The in vitro antiproliferation activity of derivatives was evaluated using the MTT assay against SW620 (human colon cancer cells), A549 (human nonsmall cell lung cancer cells), HeLa (human cervical cancer cells), and MCF-7 (human breast cancer cells). Compound 6o bearing a pyridyl group exhibited good cytotoxicity against four cancer cells, with IC50 values of 8.71, 9.55, 15.67, and 21.77 μM, sequentially. In addition, compound 6a showed some selectivity against SW620.
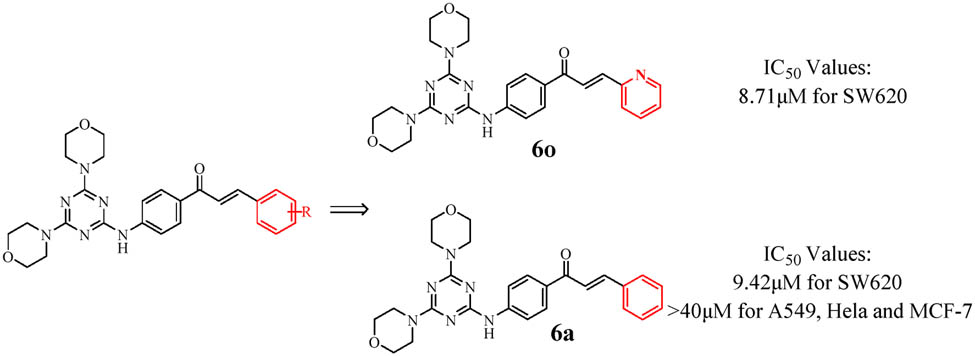
Graphical abstract
The chalcone structure was introduced into the 4,6-dimorpholinyl-1,3,5-triazine molecule through the C-N bond, and a series of new compounds were obtained. Among them, the pyridyl-containing 6o exhibits anti-proliferation activity similar to that of cisplatin on SW620. Interestingly, the phenyl-containing 6a exhibits a certain selectivity for the anti-proliferation activity of SW620.
1 Introduction
The phosphoinositide 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) signaling pathway is one of the most significant intracellular signaling pathways for mammals and regulates angiogenesis, cell proliferation, motillity, and metabolism [1,2,3]. Dysregulation of the PI3K/mTOR signaling pathway can promote the development and the growth of cancers such as breast cancer and hematologic malignancies [4,5]. Therefore, the PI3K/mTOR signaling pathway has emerged as a key target in the contemporary anticancer therapy research. Over 100 chemical patents on novel mTOR inhibitors have been published since 2012 [6]. Unfortunately, when mTOR is selectively inhibited, negative feedback regulation due to SK61 generally leads to abnormal activation of the PI3K/mTOR signaling pathway [7,8]. This suggests that the development of dual PI3K/mTOR inhibitors is significant and urgently needed. Currently, a number of compounds with dual PI3K/mTOR inhibitory activity have been reported (Figure 1) [9,10,11,12].
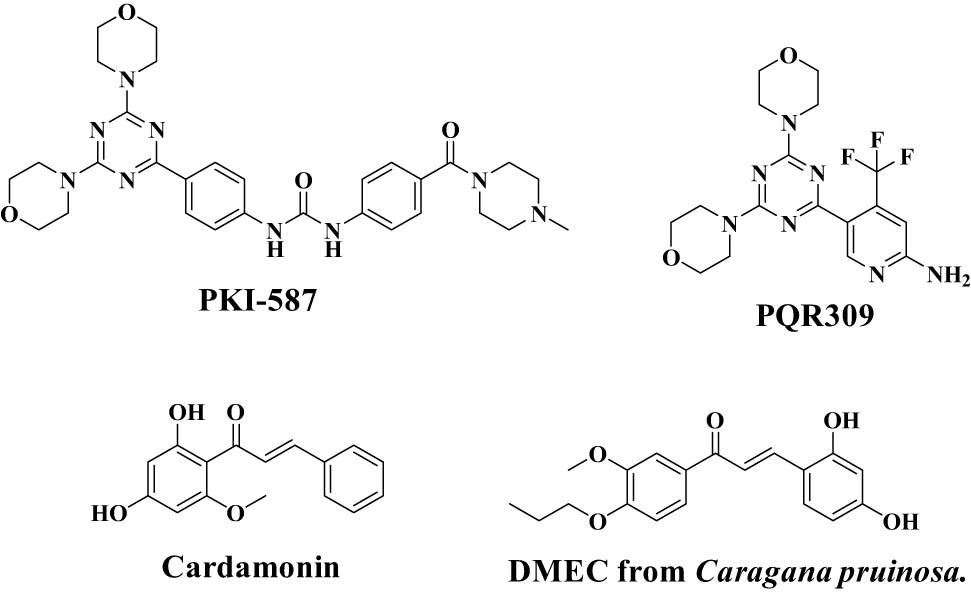
Structure of compounds with dual PI3K/mTOR kinase inhibitory activity.
Nitrogen-containing heterocycles are significant in cancer-fighting medicines [13,14]. Compounds with a 1,3,5-triazine skeleton rich in N, in particular, exhibit a wide range of biological activities, including antibacterial [15], antimalarial [16], anti-inflammatory [17], anticancer [18], and tubulin polymerization inhibition [19]. Furthermore, 1,3,5-triazine scaffolds with multiple substitution sites can bring in multiple active groups, facilitating drug design.
It is critical that compounds with a skeleton structure of 4,6-dimorpholino-1,3,5-triazine (Figure 2) can produce antitumor effects by effectively controlling the PI3K signaling pathway [20]. The 4,6-dimorpholino-1,3,5-triazine skeleton structure has distinct advantages according to our findings. On the one hand, the unique bismorpholine group can effectively prevent the monomorphline group from failing due to oxidation during the drug metabolism process, while the remaining morpholine group can still hydrogen bond with the PI3K catalytic domain [21,22,23]. The presence of three nitrogen atoms in the 1,3,5-triazine core, on the other hand, can effectively reduce the overall molecular polarity.
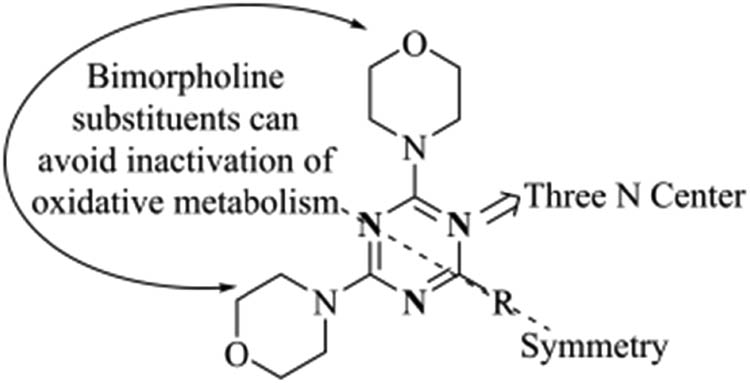
4,6-Dimorpholino-1,3,5-triazine skeleton structure.
Therefore, the 4,6-dimorpholino-1,3,5-triazine nucleus has emerged as a key research target for dual PI3K/mTOR kinase inhibitors. There have been numerous reports on dual PI3K/mTOR kinase inhibitors with a 4,6-dimorpholino-1,3,5-triazine nucleus [24]. However, these compounds still have flaws such as low bioavailability, mTOR inhibitory activity, and permeability [25,26]. Therefore, structural modifications to 4,6-dimorpholino-1,3,5-triazine compounds are required.
One of the main approaches for new drug development is to design and rationally synthesize natural product libraries based on the molecular structure of active natural products, from which lead compounds with high efficiency, high selectivity, and lower toxicity and side effects are screened for preclinical research on antitumor drugs [27].
Chalcones are special small molecular compounds with α,β-unsaturated carbonyl structures that have medium flexibility and can bind to multiple biological receptors at the same time and are found in many natural products [28,29]. For this reason, these chalcone derivatives have a wide range of biological activities, including antibacterial [30], insecticide [31], antihypertensive [32], antispasmodic [33], antiplatelet [34], antidiabetic [35], antituberculosis [36], anti-angiogenesis [37], anti-oxidation [38], lowering blood lipids [39], anti-asthma [40], antiretroviral [41], antimalarial [42], antiulcer [43], anti-arrhythmia [44], anti-invasion [45], antihistamine [46], anti-AChE [47], anticancer [48,49,50,51,52], and so on. Especially in terms of anticancer, previous studies have found that chalcone derivatives can act on a variety of anticancer targets to exert anticancer effects such as breast cancer resistance protein, P-glycoprotein, epidermal growth factor receptor, vascular endothelial growth factor receptor 2, cyclin-dependent kinases, cytochrome P450 family 2 subfamily J member 2, protein kinase C, histone deacetylase, Notch signaling pathway, PI3K/AKT signaling pathway, WNT/β-cantenin signaling pathway, and mitochondrial-mediated apoptosis signaling pathway [32,53].
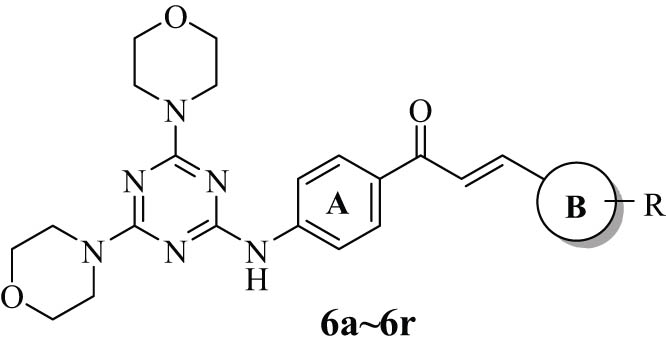
On the basis of the active substructure splicing principle, we have designed the structure of the target compounds as shown in Figure 3 [54]. The basic core was formed by connecting 4,6-dimorpholinyl-1,3,5-triazinyl and the chalcone structure through the C–N bond, and the 4,6-dimorpholino-1,3,5-triazine derivatives containing the chalcone structure were modified by introducing different groups into the B ring. Subsequently, we tested the in vitro anticancer activity of a series of synthesized 4,6-dimorpholinyl-1,3,5-triazine derivatives against four kinds of cancer cells by MTT assay.
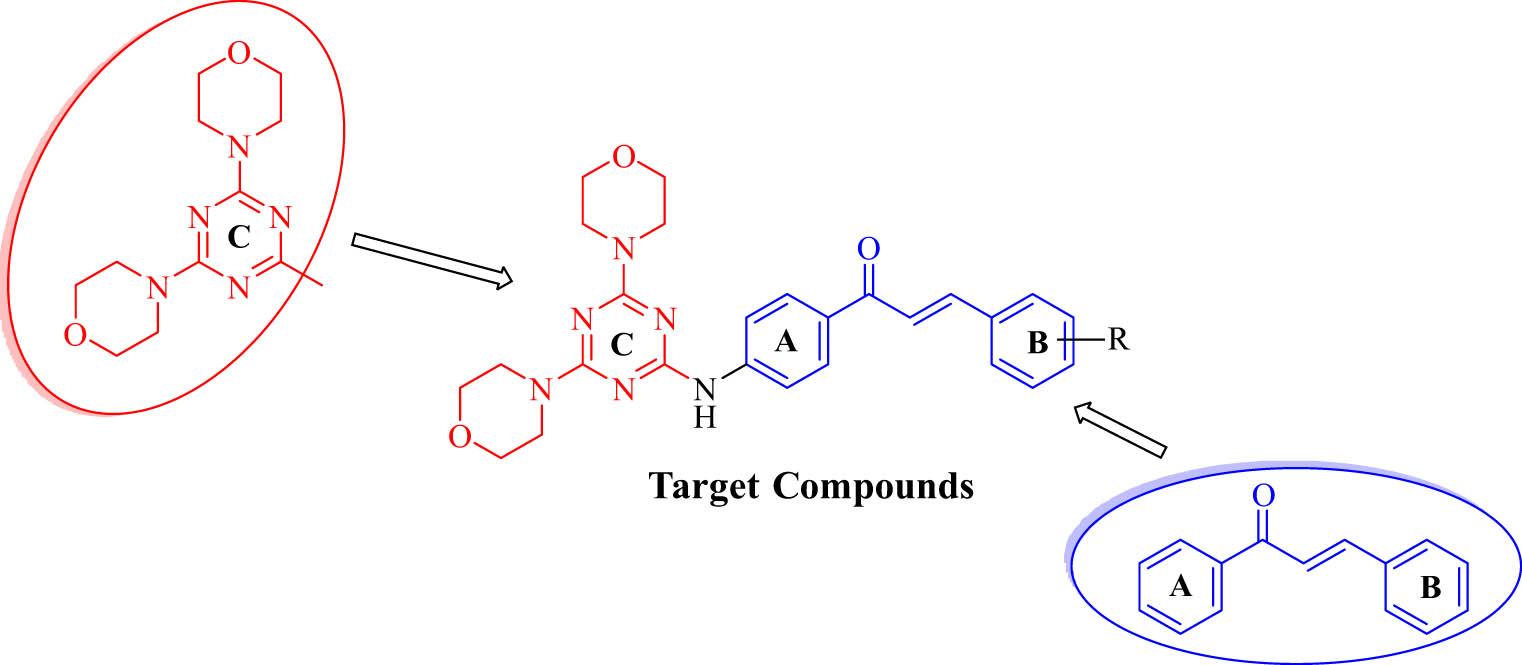
Design principle of the target compound.
2 Result and discussion
2.1 Synthesis
The target compounds were synthesized using the previously described method with a few changes [16]. Scheme 1 illustrates the production process for the 4,6-dimorpholinyl-1,3,5-triazine derivatives 6a–6r. In this method, initially, compound 3 was prepared by the equimolar amount of cyanuric chloride (1) and 4′-aminoacetophenone (2) in ethyl acetate at 0°C. Subsequently, compound 3 was treated with morpholine in 1,4-dioxane, which refluxed for 5–8 h to obtain compound 4. Compound 4 was used to make derivatives 6a–6r by reacting it with substituted benzaldehyde, substituted thiophene formaldehyde, furfural, pyrrole-2-formaldehyde, and pyridine-2-carboxaldehyde in methanol or dioxane. All the derivatives were obtained from the corresponding reactants in 75–98% yields. The structure, melting point, and yield data of the new derivatives 6a–6r are presented in Table 1.
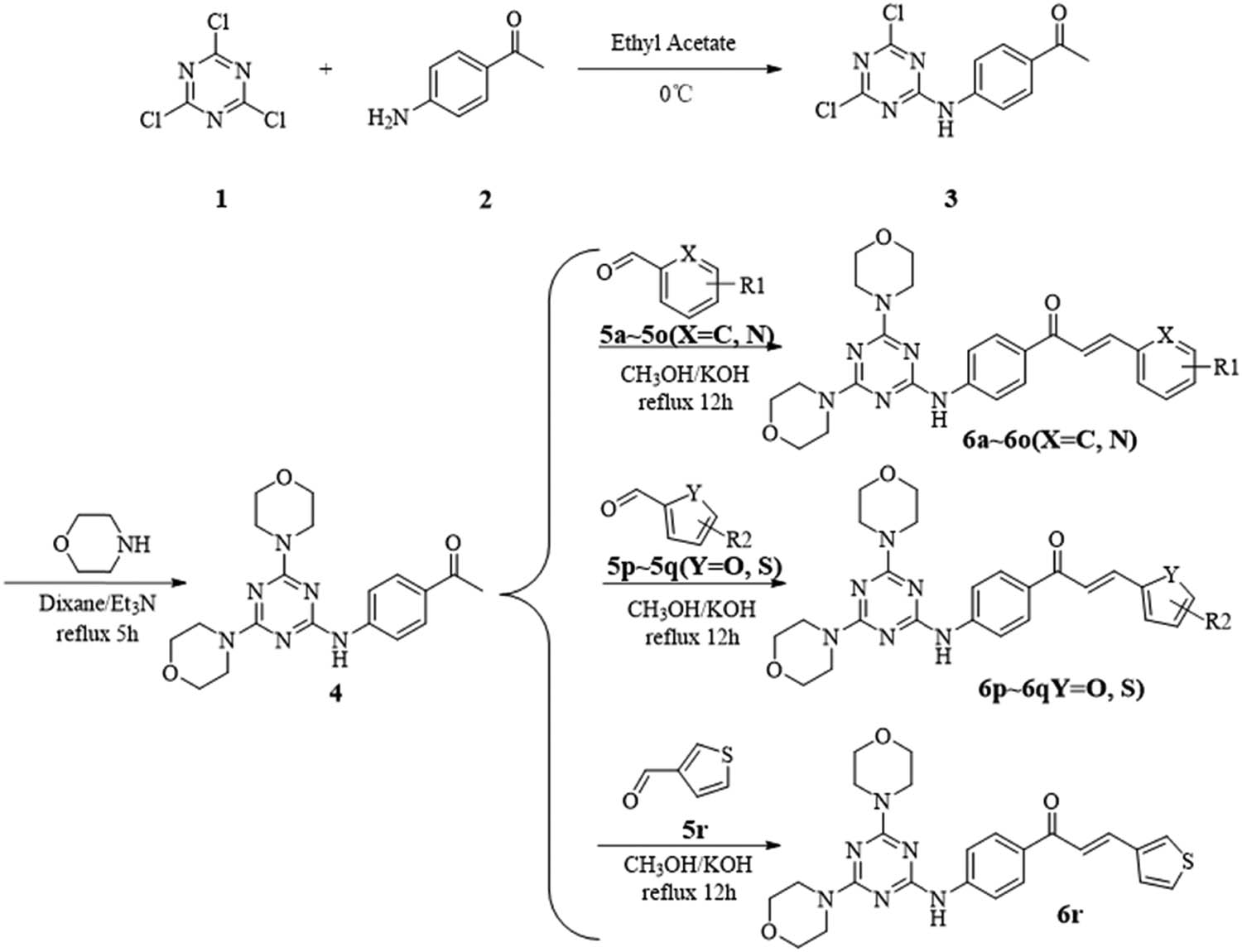
Preparation of 4,6-dimorpholinyl-1,3,5-triazine derivatives 6a–6r.
Physical data of 4,6-dimorpholinyl-1,3,5-triazine derivatives 6a–6r a
|
|||||||
|---|---|---|---|---|---|---|---|
| Compd. | B | Yield (%) | m.p./°C | Compd. | B | Yield (%) | m.p./°C |
| 6a | C6H5 | 79 | 194–196 | 6j | 2-FC6H4 | 98 | 226–227 |
| 6b | 4-CH3C6H4 | 92 | 246–247 | 6k | 4-Cl-2-FC6H3 | 93 | 220–221 |
| 6c | 2-CH3C6H4 | 80 | 232–234 | 6l | 4-CF3C6H4 | 91 | 251–253 |
| 6d | 4-(CH3)2CHC6H4 | 80 | 256–258 | 6m | 5-Br-2-ClC6H3 | 85 | 190–192 |
| 6e | 3-CH3OC6H4 | 91 | 119–122 | 6n | 2-Naphthyl | 93 | 238–239 |
| 6f | 2-CH3OC6H4 | 92 | 214–215 | 6o | 2-Pyridyl | 80 | 233–235 |
| 6g | 2,3,4-(OCH3)3C6H2 | 85 | 166–168 | 6p | 2-Furyl | 86 | 203–204 |
| 6h | 2-CH3CH2OC6H4 | 80 | 243–244 | 6q | 3-Methyl-2-thienyl | 90 | 267–269 |
| 6i | 4-(CH3)2NC6H4 | 75 | 265–268 | 6r | 3-Thienyl | 94 | 255–256 |
aisolated yields.
2.2 Spectra
The structures of the synthesized 4,6-dimorpholinyl-1,3,5-triazine derivatives 6a–6r were confirmed by 1H NMR, 13C NMR, and mass spectrometry (MS) spectral data. In the 1H NMR spectra, δ of H on the characteristic olefinic bond of the chalcone structure is located at 7.37–8.16 ppm, J > 15 Hz, indicating that the chalcone structure part of 6a–6r is in trans configuration.
2.3 Biological studies
All of the synthesized 4,6-dimorpholino-1,3,5-triazine derivatives were evaluated for anticancer activity in vitro against four cancer cell lines, SW620 (human colon cancer cells), A549 (human non-small cell lung cancer cells), HeLa (human cervical cancer cell), and MCF-7 (human breast cancer cell), using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay method. Table 2 displays the results of the experiments.
In vitro antiproliferation activity of the title compounds 6a–6r against SW620, A549, HeLa, and MCF-7 cancer cell lines
| IC50 a/μM | IC50 a/μM | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compd. | SW620 | A 549 | HeLa | MCF-7 | Compd. | SW620 | A 549 | HeLa | MCF-7 |
| 6a | 9.42 | 40.18 | >50 | >50 | 6j | 41.64 | 12.19 | 26.49 | >50 |
| 6b | >50 | >50 | >50 | >50 | 6k | 34.02 | 37.44 | >50 | >50 |
| 6c | 34.09 | >50 | 45.71 | 35.66 | 6l | 35.61 | 33.83 | 48.61 | 47.73 |
| 6d | >50 | >50 | >50 | >50 | 6m | >50 | 17.54 | >50 | >50 |
| 6e | 33.53 | >50 | >50 | >50 | 6n | >50 | >50 | >50 | >50 |
| 6f | 40.73 | >50 | >50 | >50 | 6o | 8.71 | 9.55 | 15.67 | 21.77 |
| 6g | 42.55 | >50 | >50 | >50 | 6p | >50 | >50 | 41.57 | 45.92 |
| 6h | 35.98 | >50 | 41.86 | >50 | 6q | 42.03 | 36.82 | 42.59 | 24.85 |
| 6i | >50 | >50 | >50 | >50 | 6r | >50 | 24.69 | >50 | 20.66 |
| Cisplatin | 6.82 | 4.40 | 3.76 | 10.44 | |||||
aAntiproliferation activity was assayed by exposure for 24 h to the tested substances and expressed as the concentration required to inhibit tumor cell proliferation by 50% (IC50).
The results demonstrated that the steric structure of the B ring of the chalcone structure and the type of its substituents have significant effects on its antiproliferation activity in vitro. It is worth noting that when the B ring of the chalcone structure is composed of a pyridine (6o) heterocycle in a state of electron deficiency, it exhibits strong antiproliferation activity against four cancer cells (SW620, A549, HeLa, and MCF-7 with IC50 values of 8.71, 9.55, 15.67, and 21.77 μM, respectively). When the B ring is composed of electron-rich heterocycles, such as the furan ring (6p) and thiophene ring (6q), the antiproliferation activity against the four types of cancer cells is inferior to that of 6o. This shows that when the B ring constituting the chalcone structure is in the electron-deficient state, it has a significant impact on its antiproliferation activity, which also was confirmed in vitro antiproliferation experiments on A549.
For A549, as shown in Table 2, when the benzene ring is used as the B ring to construct the chalcone structure, compounds with electron-withdrawing groups outperform compounds with electron-donating groups in terms of antiproliferation activity. Furthermore, the type of the substituents at the 2-position has a significant impact on antiproliferation activity against A549; for example, the antiproliferation activity of the 2-fluoro group (6j) (IC50 = 12.17 μM) is superior to that of 6c, 6f, or 6h. Interestingly, when other sites other than the 2-position are substituted, the target compounds’ antiproliferation activity against A549 is significantly reduced, which may be due to an increase in the steric volume of the B ring caused by the multiple substituents.
Surprisingly, the antiproliferation activity of unsubstituted 6a (IC50 = 9.42 μM) for SW620 is far superior to that of derivatives with electron-withdrawing or electron-donating substituents (IC50 > 33.53 μM). This could be due to the smaller steric volume on the B ring being more conducive to binding to the receptor. This finding significantly demonstrates that the steric structure of the target compounds’ B ring has a significant impact on their antiproliferation activity. Furthermore, 2-methyl (6c) has better antiproliferation activity against SW620 than 2-methoxy (6f), 2-ethoxy (6h), or 2-fluorine (6j). The importance of the 2-position substituent type in this series of compounds’ antiproliferation activity is further demonstrated.
It is worth noting that 6o has antiproliferation activity against SW620 that is comparable to the classic anticancer drug cisplatin (6o IC50 = 8.71 μM, cisplatin IC50 = 6.82 μM) and has the potential to be an anticancer drug. In HeLa, 6o has the strongest antiproliferation activity (IC50 = 15.67 μM), while 6j has good antiproliferation activity (IC50 = 26.49 μM), while the other compounds are not sensitive to HeLa (IC50 > 40.00 μM). In MCF-7, 6o and 6q showed antiproliferation activity in addition to the thiophene ring (6r), which has the strongest antiproliferation activity (IC50 = 20.66 μM). Interestingly, when the heteroatom in the 6r structure is changed from S to O (6p), the antiproliferation activity is significantly reduced, implying that the S atom may play an unexpectedly important role in inhibiting the HeLa growth.
3 Conclusions
We obtained a series of novel chalcone-containing 4,6-dimorpholinyl-1,3,5-triazine derivatives (6a–6r), of which 6b–6d, 6h, 6j–6o, and 6q–6r are previously unknown compounds. The 6o obtained from the chalcone structure constructed by the pyridyl group, a classical electron-deficient state group, had good antiproliferation activities on SW620, A549, HeLa, and MCF-7, according to the results of an in vitro antiproliferation activity test. This group of substances also exhibits some selectivity for various cancer cell lines. For instance, unsubstituted 6a on ring B in SW620 had substantial antiproliferation activity, in contrast to A549, where it exhibited much weaker antiproliferation activity than 6j. HeLa and MCM-7 are not susceptible to this class of compounds; only compounds that contain the heterocycles 6o, 6q, and 6r have any antiproliferation activity. Generally speaking, the stronger the antiproliferation activity, the smaller the steric volume of the portion of the chalcone structure that forms the B ring and the stronger the electron-withdrawing action of the substituents on it. We will continue to undertake in-depth study on the relationship between its structure–activity and mechanism of action in light of 6o’s strong antiproliferation activity and the uniqueness of its structure.
4 Experimental
4.1 Experimental details
Unless otherwise stated, materials were obtained from Shanghai Xian Ding Biotechnology Company and used without further purification. Thin-layer chromatography (TLC) was performed using silica gel 60 F254 and visualized using ultraviolet light. NMR spectra were obtained in a Bruker Avance spectrometer in CDCl3 using TMS as the internal standard. Fourier transform infrared spectroscopy (FT-IR) (Nicolet, Nexus-470, USA) was used to analyze functional groups of 6a–6r. ESI+ mode is used to obtain mass spectral information on compounds.
4.2 Synthesis of 1-(4-((4,6-dimorpholino-1,3,5-triazin-2-yl) amino) phenyl) ethan-1-one (4)
To a mixed solution of cyanuric chloride (1 mmol) in 10 mL of ethyl acetate being cooled to 0°C was added the equimolar amount of 4′-aminoacetophenone (1 mmol). Then, the reaction was kept at 0°C for 3 h, while TLC monitored the reaction process. After the reaction, compound 3 was obtained by filtration without further purification. The Et3N (2.5 mmol) was added to a mixture suspension of compound 3 (1 mmol) and morpholine (2 mmol) in 10 mL of 1,4-dixane. The mixture was heated to reflux for 5 h. TLC monitored the reaction process. After the reaction, 1,4-dioxane was removed by distillation under reduced pressure. The residue was added to 10 mL of water, stirred at room temperature for 0.5 h, and filtered to obtain compound 4 (yield: 70%). No further purification is required before the next reaction can be initiated.
White solid, 1H NMR (600 MHz, CDCl3) δ 7.93 (d, J = 6.9 Hz, 2H), 7.64 (d, J = 6.9 Hz, 2H), 6.99 (s, 1H), 3.80 (s, 8H), 3.74 (s, 8H), 2.57 (s, 3H).
4.3 General procedure for synthesis of (E)-chalcones (6)
KOH (2.5 mmol) was added to a mixture solution (or suspension) of compound 4 (1 mmol) and aldehyde (1 mmol) in methanol (10 mL). The mixture was heated to reflux for 5–8 h and monitored by TLC. Methanol was removed by distillation under reduced pressure after the reaction. The residue was added to 10 mL of water, stirred at room temperature for 0.5 h, and filtered to obtain the crude product, and then the pure product was obtained by recrystallization from ethyl acetate and 95% ethanol.
4.3.1 (E)-1-(4-((4,6-Dimorpholino-1,3,5-triazin-2-yl)amino)phenyl)-3-phenylprop-2-en-1-one (6a)
1-(4-((4,6-Dimorpholino-1,3,5-triazin-2-yl)amino)phenyl)ethan-1-one (1 mmol) and benzaldehyde (1 mmol) in 10 mL methanol were reacted according to the general procedure of compounds 6 to obtain product 6a. 1H NMR (400 MHz, CDCl3) δ 8.06 (d, J = 8.7 Hz, 2H), 7.84 (d, J = 15.7 Hz, 1H), 7.72 (d, J = 8.7 Hz, 2H), 7.67 (m, 2H), 7.59 (d, J = 15.7 Hz, 1H), 7.44 (m, 2H), 6.99 (s, 1H), 3.83–3.82 (m, 8H), 3.78–3.77 (m, 8H). 13C NMR (101 MHz, CDCl3) δ 188.7, 165.2, 164.2, 144.1, 144.0, 135.1, 131.9, 130.4, 123.0, 128.9, 128.4, 121.9, 118.6, 66.8, 43.8. FT-IR (KBr) ν: 3,409, 2,979, 2,898, 2,857, 1,654, 1,612, 1,508, 1,448, 1,392, 1,255, 1,220, 1,178, 1,112, 802, 765, 543 cm−1. MS (ESI+) calculated for C26H28N6O3 [M + H]+: 473.22; found 473.29.
4.3.2 (E)-1-(4-((4,6-Dimorpholino-1,3,5-triazin-2-yl)amino)phenyl)-3-(p-tolyl)prop-2-en-1-one (6b)
1H NMR (400 MHz, CDCl3) δ 8.06 (d, J = 8.7 Hz, 2H), 7.82 (d, J = 15.6 Hz, 1H), 7.72 (d, J = 8.7 Hz, 2H), 7.71–7.28 (m, 3H), 7.24 (d, J = 7.9 Hz, 2H), 7.00 (s, 1H), 3.84–3.83 (m, 8H), 3.78–3.77 (m, 8H), 2.42 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 188.8, 164.8, 163.7, 144.1, 143.8, 140.9, 132.4, 132.2, 129.9, 129.7, 128.4, 120.9, 118.7, 66.8, 43.9, 21.5. FT-IR (KBr) ν: 3,411, 2,979, 2,900, 2,858, 1,654, 1,612, 1,508, 1,411, 1,392, 1,359, 1,255, 1,176, 1,108, 802, 543 cm−1. MS (ESI+) calculated for C27H30N6O3 [M + H]+: 487.24; found 487.29.
4.3.3 (E)-1-(4-((4,6-Dimorpholino-1,3,5-triazin-2-yl)amino)phenyl)-3-(o-tolyl)prop-2-en-1-one (6c)
1H NMR (600 MHz, CDCl3) δ 8.11 (d, J = 15.5 Hz, 1H), 8.04 (d, J = 8.4 Hz, 2H), 7.69 (d, J = 8.4 Hz, 2H), 7.49 (d, J = 15.5 Hz, 1H), 7.32–7.19 (m, 5H), 3.82–3.79 (m, 8H), 3.76–3.72 (m, 8H), 2.47 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 188.6, 165.2, 164.2, 144.1, 141.7, 138.3, 134.2, 131.9, 130.9, 130.1, 123.0, 126.4, 126.3, 123.0, 118.6, 66.8, 43.8, 19.9. FT-IR (KBr) ν: 3,345, 2,964, 2,852, 1,735, 1,658, 1,610, 1,569, 1,211, 1,106, 865, 804, 578 cm−1. MS (ESI+) calculated for C27H30N6O3 [M + H]+: 487.24; found 487.29.
4.3.4 (E)-1-(4-((4,6-Dimorpholino-1,3,5-triazin-2-yl)amino)phenyl)-3-(4-isopropylphenyl)prop-2-en-1-one (6d)
1H NMR (400 MHz, CDCl3) δ 8.03 (d, J = 8.7 Hz, 2H), 7.81 (d, J = 15.6 Hz, 1H), 7.69 (d, J = 8.7 Hz, 2H), 7.58 (d, J = 8.1 Hz, 2H), 7.53 (d, J = 15.6 Hz, 1H), 7.27 (d, J = 9.7 Hz, 2H), 7.03 (s, 1H), 3.81–3.80 (m, 8H), 3.76–3.74 (m, 8H), 2.95 (seq, 1H), 1.27 (d, J = 6.9 Hz, 6H). 13C NMR (151 MHz, CDCl3) δ 188.8, 165.2, 164.2, 151.8, 144.1, 143.9, 132.8, 132.0, 129.9, 128.5, 127.1, 121.0, 118.5, 66.8, 43.8, 34.1, 23.8. FT-IR (KBr) ν: 3,440, 2,962, 2,856, 1,650, 1,610, 1,508, 1,394, 1,257, 1,172, 1,114, 1,025, 821, 804, 543 cm−1. MS (ESI+) calculated for C29H34N6O3 [M + H]+: 515.27; found 515.33.
4.3.5 (E)-1-(4-((4,6-Dimorpholino-1,3,5-triazin-2-yl)amino)phenyl)-3-(3-methoxyphenyl)prop-2-en-1-one (6e)
1H NMR (600 MHz, CDCl3) δ 8.03 (d, J = 8.5 Hz, 2H), 7.77 (d, J = 15.6 Hz, 1H), 7.69 (d, J = 8.5 Hz, 2H), 7.54 (d, J = 15.6 Hz, 1H), 7.36–7.21 (m, 3H), 7.15 (s, 1H), 6.96 (s, 1H), 3.85 (s, 3H), 3.82–3.79 (m, 8H), 3.75–3.72 (m, 8H). 13C NMR (151 MHz, CDCl3) δ 188.7, 165.1, 164.1, 159.9, 143.9, 136.5, 130.0, 129.9, 129.7, 122.2, 121.0, 118.6, 116.0, 113.5, 66.8, 55.4, 43.8. FT-IR (KBr) ν: 3,411, 2,977, 2,900, 2,857, 1,654, 1,612, 1,508, 1,392, 1,359, 1,255, 1,180, 1,112, 802, 541 cm−1. MS (ESI+) calculated for C27H30N6O4 [M + H]+: 503.23; found 503.29.
4.3.6 (E)-1-(4-((4,6-Dimorpholino-1,3,5-triazin-2-yl)amino)phenyl)-3-(2-methoxyphenyl)prop-2-en-1-one (6f)
1H NMR (600 MHz, CDCl3) δ 8.13 (d, J = 15.8 Hz, 1H), 8.03 (d, J = 8.3 Hz, 2H), 7.68 (d, J = 8.3 Hz, 2H), 7.66–7.60 (m, 2H), 7.37 (t, 1H), 7.02–6.92 (m, 3H), 3.92 (s, 3H), 3.83–3.79 (m, 8H), 3.76–3.73 (m, 8H). 13C NMR (101 MHz, CDCl3) δ 189.3, 165.2, 164.2, 158.8, 143.8, 139.5, 132.2, 131.6, 130.0, 129.0, 124.2, 122.7, 120.7, 118.6, 111.3, 66.8, 55.6, 43.8. FT-IR (KBr) ν: 3,316, 2,948, 2,850, 1,735, 1,649, 1,608, 1,486, 1,392, 1,218, 1,174, 1,110, 804, 742, 536 cm−1. MS (ESI+) calculated for C27H30N6O4 [M + H]+: 503.23; found 503.29.
4.3.7 (E)-1-(4-((4,6-Dimorpholino-1,3,5-triazin-2-yl)amino)phenyl)-3-(2,3,4-trimethoxyphenyl)prop-2-en-1-one (6g)
1H NMR (400 MHz, CDCl3) δ 8.03 (d, J = 8.7 Hz, 2H), 7.99 (d, J = 15.9 Hz, 1H), 7.68 (d, J = 8.7 Hz, 2H), 7.59 (d, J = 15.9 Hz, 1H), 7.39 (d, J = 8.8 Hz, 1H), 7.05 (s, 1H), 6.72 (d, J = 8.8 Hz, 1H), 3.95 (s, 3H), 3.91 (s, 3H), 3.90 (s, 3H), 3.84–3.78 (m, 8H), 3.77–3.72 (m, 8H). 13C NMR (101 MHz, CDCl3) δ 189.1, 165.2, 164.2, 155.7, 153.8, 143.8, 142.6, 139.3, 132.3, 129.9, 123.9, 122.3, 121.3, 118.6, 107.7, 66.8, 61.5, 61.0, 56.1, 43.8. FT-IR (KBr) ν: 3,480, 3,355, 2,964, 2,857, 1,652, 1,610, 1,581, 1,494, 1,257, 1,093, 862, 838, 800, 688, 543 cm−1. MS (ESI+) calculated for C29H34N6O6 [M + H]+: 563.25; found 563.34.
4.3.8 (E)-1-(4-((4,6-Dimorpholino-1,3,5-triazin-2-yl)amino)phenyl)-3-(2-ethoxyphenyl)prop-2-en-1-one (6h)
1H NMR (400 MHz, CDCl3) δ 8.14 (d, J = 15.9 Hz, 1H), 8.06 (d, J = 8.0 Hz, 2H), 7.75–7.65 (m, 4H), 7.39–7.35 (t, 1H), 7.02–6.95 (m, 3H), 4.16 (q, 2H), 3.83 (s, 8H), 3.77 (s, 8H), 1.55 (t, 3H). 13C NMR (101 MHz, CDCl3) δ 189.4, 165.3, 164.3, 158.3, 143.9, 139.9, 132.4, 131.6, 130.0, 129.5, 124.3, 122.8, 120.7, 118.6, 112.2, 66.9, 64.1, 43.9, 15.0. FT-IR (KBr) ν: 3,330, 2,958, 2,848, 1,654, 1,612, 1,573, 1,508, 1,392, 1,257, 1,172, 1,106, 862, 804, 750, 538 cm−1. MS (ESI+) calculated for C28H32N6O4 [M + H]+: 517.25; found 517.33.
4.3.9 (E)-3-(4-(Dimethylamino)phenyl)-1-(4-((4,6-dimorpholino-1,3,5-triazin-2-yl)amino)phenyl)prop-2-en-1-one (6i)
1H NMR (400 MHz, CDCl3) δ 8.05 (d, J = 8.7 Hz, 2H), 7.82 (d, J = 15.4 Hz, 1H), 7.69 (d, J = 8.7 Hz, 2H), 7.57 (d, J = 8.9 Hz, 2H), 7.40 (d, J = 15.4 Hz, 1H), 6.97 (s, 1H), 6.72 (d, J = 8.9 Hz, 2H), 3.84–3.82 (m, 8H), 3.78–3.76 (m, 8H), 3.07 (s, 6H). 13C NMR (151 MHz, CDCl3) δ 188.8, 165.2, 164.2, 151.9, 145.0, 143.4, 132.8, 130.3, 129.7, 122.9, 118.5, 116.7, 111.8, 66.8, 43.8, 40.2. FT-IR (KBr) ν: 3,423, 2,979, 2,898, 2,856, 1,643, 1,608, 1,577, 1,508, 1,396, 1,255, 1,166, 919, 804, 624, 542 cm−1. MS (ESI+) calculated for C28H33N7O3 [M + H]+: 516.26; found 516.33.
4.3.10 (E)-1-(4-((4,6-Dimorpholino-1,3,5-triazin-2-yl)amino)phenyl)-3-(2-fluorophenyl)prop-2-en-1-one (6j)
1H NMR (600 MHz, CDCl3) δ 8.04 (d, J = 8.4 Hz, 2H), 7.89 (d, J = 15.8 Hz, 1H), δ 7.70 (d, J = 8.4 Hz, 2H), δ 7.68 (d, J = 15.8 Hz, 1H), 7.64 (t, 1H), δ 7.38–3.76 (m, 1H), 7.19 (t, 1H), 7.16–7.10 (m, 2H), 3.81–3.80 (m, 8H), 3.75–3.74 (m, 8H). 13C NMR (151 MHz, CDCl3) δ 188.6, 165.2, 164.2, 162.6, 160.9, 144.2, 136.8, 131.6, 131.6, 130.1, 129.9, 124.5, 123.3, 123.2, 120.7, 118.6, 116.4, 116.2, 66.8, 43.8. FT-IR (KBr) ν: 3,340, 2,952, 2,854, 1,785, 1,735, 1,654, 1,616, 1,504, 1,392, 1,168, 923, 806, 761, 538 cm−1. MS (ESI+) calculated for C26H27FN6O3 [M + H]+: 491.22; found 491.29.
4.3.11 (E)-3-(4-Chloro-2-fluorophenyl)-1-(4-((4,6-dimorpholino-1,3,5-triazin-2-yl)amino)phenyl)prop-2-en-1-one (6k)
1H NMR (600 MHz, CDCl3) δ δ 8.03 (d, J = 8.7 Hz, 2H), 7.82 (d, J = 15.8 Hz, 1H), 7.70 (d, J = 8.7 Hz, 2H), 7.66 (d, J = 15.8 Hz, 1H), 7.58 (t, 1H), 7.21–7.15 (m, 2H), 6.94 (s, 1H), 3.82–3.79 (m, 8H), 3.76–3.73 (m, 8H). 13C NMR (101 MHz, CDCl3) δ 188.3, 165.2, 164.2, 160.1, 144.3, 136.7, 135.5, 131.5, 130.5, 130.5, 130.1, 125.1, 125.1, 124.8, 121.9, 118.5, 117.3, 117.0, 66.8, 43.8. FT-IR (KBr) ν: 3,421, 2,970, 2,854, 1,654, 1,606, 1,506, 1,409, 1,255, 1,115, 1,024, 804, 765, 538 cm−1. MS (ESI+) calculated for C26H26ClFN6O3 [M + H]+: 525.17; found 525.22.
4.3.12 (E)-1-(4-((4,6-Dimorpholino-1,3,5-triazin-2-yl)amino)phenyl)-3-(4-(trifluoromethyl)phenyl)prop-2-en-1-one (6l)
1H NMR (600 MHz, CDCl3) δ 8.05 (d, J = 8.7 Hz, 2H), 7.81 (d, J = 15.7 Hz, 1H), 7.74 (d, J = 8.2 Hz, 2H), 7.71 (d, J = 8.7 Hz, 2H), 7.67 (d, J = 8.2 Hz, 2H), 7.63 (d, J = 15.7 Hz, 1H), 6.98 (s, 1H), 3.82–3.79 (m, 8H), 3.77–3.72 (m, 8H). 13C NMR (151 MHz, CDCl3) δ 188.1, 165.1, 164.1, 144.4, 141.9, 138.5, 131.8, 131.6, 131.4, 130.1, 129.7, 128.4, 125.9, 125.9, 124.8, 124.1, 123.0, 118.6, 66.8, 43.8. FT-IR (KBr) ν: 3,351, 2,991, 2,964, 2,860, 1,662, 1,604, 1,509, 1,330, 1,255, 1,112, 863, 823, 804, 543 cm−1. MS (ESI+) calculated for C27H27F3N6O3 [M + H]+: 541.21; found 541.30.
4.3.13 (E)-3-(5-Bromo-2-chlorophenyl)-1-(4-((4,6-dimorpholino-1,3,5-triazin-2-yl)amino)phenyl)prop-2-en-1-one (6m)
1H NMR (400 MHz, CDCl3) δ 8.10 (d, J = 15.7 Hz, 1H), 8.06 (d, J = 8.8 Hz, 2H), 7.89 (d, J = 2.3 Hz, 1H), 7.73 (d, J = 8.7 Hz, 2H), 7.53 (d, J = 15.6 Hz, 1H), 7.46 (dd, J = 8.5, 2.3 Hz, 1H), 7.33 (d, J = 8.5 Hz, 1H), 7.04 (s, 1H), 3.83–3.82 (m, 8H), 3.78–3.77 (m, 8H). 13C NMR (101 MHz, CDCl3) δ 187.9, 165.2, 164.2, 144.4, 138.2, 135.4, 134.3, 133.6, 131.6, 131.3, 130.4, 130.2, 125.6, 120.0, 118.6, 66.8, 43.8. FT-IR (KBr) ν: 3,405, 2,944, 2,854, 1,664, 1,616, 1,496, 1,251, 1,114, 831, 802, 659, 603, 543 cm−1. MS (ESI+) calculated for C26H26BrClN6O3 [M + 2H]+: 587.10; found 587.15.
4.3.14 (E)-1-(4-((4,6-Dimorpholino-1,3,5-triazin-2-yl)amino)phenyl)-3-(naphthalen-2-yl)prop-2-en-1-one (6n)
1H NMR (400 MHz, CDCl3) δ 8.10 (d, J = 8.7 Hz, 1H), 8.05 (s, 1H), 8.00 (d, J = 15.6 Hz, 1H), 7.92–7.82 (m, 4H), 7.74 (d, J = 8.7 Hz, 2H), 7.71 (d, J = 15.6 Hz, 1H), 7.56–7.52 (m, 2H), 7.08 (s, 1H), 3.84–3.83 (m, 8H), 3.78–3.77 (m, 8H). 13C NMR (101 MHz, CDCl3) δ 188.6, 165.2, 164.2, 144.1, 134.3, 133.4, 132.6, 132.0, 130.4, 130.0, 128.7, 128.6, 127.8, 127.3, 126.8, 123.8, 122.0, 118.6, 66.8, 43.8. FT-IR (KBr) ν: 3,326, 2,958, 2,850, 1,648, 1,610, 1,506, 1,392, 1,255, 1,112, 1,024, 1,008, 804, 736, 626, 541, 474 cm−1. MS (ESI+) calculated for C30H30N6O3 [M + H]+: 523.24; found 523.30.
4.3.15 (E)-1-(4-((4,6-Dimorpholino-1,3,5-triazin-2-yl)amino)phenyl)-3-(pyridin-2-yl)prop-2-en-1-one (6o)
1H NMR (400 MHz, CDCl3) δ 8.71 (d, J = 3.7 Hz, 1H), 8.18 (d, J = 15.2 Hz, 1H), 8.13 (d, J = 8.8 Hz, 2H), 7.79 (d, J = 15.2 Hz, 1H), 7.75 (dd, J = 7.7, 1.8 Hz, 1H), 7.72 (d, J = 8.8 Hz, 2H), 7.49 (d, J = 7.7 Hz, 1H), 7.35–7.28 (m, 1H), 7.05 (s, 1H), 3.83–3.82 (m, 8H), 3.78–3.77 (m, 8H). 13C NMR (101 MHz, CDCl3) δ 188.6, 165.2, 164.2, 153.4, 150.1, 144.3, 141.9, 136.9, 131.6, 130.3, 125.6, 125.5, 124.3, 118.6, 66.8, 43.8. FT-IR (KBr) ν: 3,282, 3,176, 2,965, 2,850, 1,656, 1,604, 1,508, 1,411, 1,255, 1,182, 1,118, 838, 804, 779, 570 cm−1. MS (ESI+) calculated for C25H27N7O3 [M + H]+: 474.22; found 474.29.
4.3.16 (E)-1-(4-((4,6-Dimorpholino-1,3,5-triazin-2-yl)amino)phenyl)-3-(furan-2-yl)prop-2-en-1-one (6p)
1H NMR (600 MHz, CDCl3) δ 8.04 (d, J = 8.6 Hz, 2H), 7.69 (d, J = 8.6 Hz, 2H), 7.59 (d, J = 15.3 Hz, 1H), 7.55–7.42 (m, 2H), 6.92 (s, 1H), 6.70 (d, J = 3.1 Hz, 1H), 6.52–6.51 (m, 1H), 3.81–3.79 (m, 8H), 3.76–3.73 (m, 8H). 13C NMR (101 MHz, CDCl3) δ 188.1, 165.2, 164.2, 151.9, 144.7, 144.0, 131.9, 130.0, 129.9, 119.2, 118.5, 115.8, 112.6, 66.8, 43.8. FT-IR (KBr) ν: 3,405, 2,977, 2,861, 1,654, 1,614, 1,591, 1,508, 1,390, 1,255, 1,113, 1,010, 921, 802, 752, 536 cm−1. MS (ESI+) calculated for C24H26N6O4 [M + H]+: 463.20; found 463.25.
4.3.17 (E)-1-(4-((4,6-Dimorpholino-1,3,5-triazin-2-yl)amino)phenyl)-3-(3-methylthiophen-2-yl)prop-2-en-1-one (6q)
1H NMR (600 MHz, CDCl3) δ 8.04 (d, J = 15.1 Hz, 1H), 8.03 (d, J = 8.7 Hz, 2H), 7.69 (d, J = 8.7 Hz, 2H), 7.32 (d, J = 15.1 Hz, 1H), 7.29 (d, J = 5.0 Hz, 1H), 6.93 (s, 1H), 6.91 (d, J = 5.0 Hz, 1H), 3.83–3.78 (m, 8H), 3.76–3.73 (m, 8H), 2.40 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 188.0, 165.2, 164.2, 143.9, 142.5, 134.8, 134.7, 132.0, 131.4, 129.8, 127.0, 119.7, 118.5, 100.0, 66.8, 43.8, 14.3. FT-IR (KBr) ν: 3,326, 2,960, 2,848, 1,641, 1,610, 1,577, 1,506, 1,394, 1,255, 1,112, 1,025, 1,006, 825, 804, 621 cm−1. MS (ESI+) calculated for C25H28N6O3S [M + H]+: 493.19; found 493.25.
4.3.18 (E)-1-(4-((4,6-Dimorpholino-1,3,5-triazin-2-yl)amino)phenyl)-3-(thiophen-3-yl)prop-2-en-1-one (6r)
1H NMR (600 MHz, CDCl3) δ 8.02 (d, J = 8.7 Hz, 2H), 7.80 (d, J = 15.5 Hz, 1H), 7.69 (d, J = 8.7 Hz, 2H), 7.59 (d, J = 2.4 Hz, 1H), 7.44–7.36 (m, 3H), 6.95 (s, 1H), 3.82–3.79 (m, 8H), 3.76–3.72 (m, 8H). 13C NMR (151 MHz, CDCl3) δ 188.0, 165.2, 164.2, 142.5, 134.8, 134.7, 131.5, 129.8, 127.0, 119.6, 118.5, 66.8, 43.8. FT-IR (KBr) ν: 3,322, 3,083, 2,958, 2,854, 1,650, 1,612, 1,589, 1,508, 1,361, 1,255, 1,112, 1,024, 804, 607, 542 cm−1. MS (ESI+) calculated for C24H26N6O3S [M + H]+: 479.18; found 479.21.
4.4 Cytotoxic activity
The vitro antiproliferation activities of target compounds 6a–6r were detected by the MTT assay. SW620, A549, HeLa, and MCF-7 cells were harvested in the logarithmic growth phase and seeded in 96-well plates, and cultured at 37°C in a humidified atmosphere containing 5% CO2 in Dulbecco’s modified Eagle medium (DMEM) with 10% FBS for 24 h before any treatments. Tested compounds were dissolved in dimethyl sulfoxide (DMSO) and diluted in the culture fluid to get various concentrations. The cells were treated with target compounds subsequently and incubated for 24 h. Then 20 μL of MTT (5 mg/mL) was added in a 37°C, 5% CO2 incubator for 4 h. The medium was removed immediately, and MTT formazan was solubilized in 150 μL of DMSO. Its absorbance value (OD) was measured at a 490 nm wavelength using an enzyme-labeled instrument. The half maximal inhibitory concentration (IC50) value was calculated by the OD value.
-
Funding information: This work was supported by the basic research project of Heilongjiang basic scientific research operating expenses (No. 2018-KYYWF-0946) and Natural Science Foundation of Heilongjiang Province of China (LH2022H094).
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
1 Toker A, Cantley LC. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387(6634):673–6.10.1038/42648Search in Google Scholar PubMed
2 Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular function of phosphoinositide 3-kinases: Implications for development, immunity, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17(1):615–75.10.1146/annurev.cellbio.17.1.615Search in Google Scholar PubMed
3 Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7(8):606–19.10.1038/nrg1879Search in Google Scholar PubMed
4 Dong P, Konno Y, Watari H, Hosaka M, Noguchi M, Sakuragi N. The impact of microRNA-mediated PI3K/AKT signaling on epithelial-mesenchymal transition and cancer stemness in endometrial cancer. J Transl Med. 2014;12(1):231.10.1186/s12967-014-0231-0Search in Google Scholar PubMed PubMed Central
5 Benetatos L, Voulgaris E, Vartholomatos G. The crosstalk between long non-coding RNAs and PI3K in cancer. Med Oncol. 2017;34(3):39.10.1007/s12032-017-0897-2Search in Google Scholar PubMed
6 Workman P, Clarke PA, Guillard S, Raynaud FI. Drugging the PI3 kinome. Nat Biotechnol. 2006;24(7):794–6.10.1038/nbt0706-794Search in Google Scholar PubMed
7 Fan Q-W, Knight ZA, Goldenberg DD, Yu W, Mostov KE, Stokoe D, et al. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006;9(5):341–9.10.1016/j.ccr.2006.03.029Search in Google Scholar PubMed PubMed Central
8 Proschak E, Stark H, Merk D. Polypharmacology by design: a medicinal chemist’s perspective on multitargeting compounds. J Med Chem. 2019;62(2):420–44.10.1021/acs.jmedchem.8b00760Search in Google Scholar PubMed
9 Venkatesan AM, Dehnhardt CM, Delos Santos E, Chen Z, Dos Santos O, Ayral-Kaloustian S, et al. Bis(morpholino-1,3,5-triazine) derivatives: Potent adenosine 5′-triphosphate competitive phosphatidylinositol-3-kinase/mammalian target of rapamycin inhibitors: Discovery of compound 26 (pki-587), a highly efficacious dual inhibitor. J Med Chem. 2010;53(6):2636–45.10.1021/jm901830pSearch in Google Scholar PubMed
10 Beaufils F, Cmiljanovic N, Cmiljanovic V, Bohnacker T, Melone A, Marone R, et al. 5-(4,6-dimorpholino-1,3,5-triazin-2-yl)-4-(trifluoromethyl)pyridin-2-amine (pqr309), a potent, brain-penetrant, orally bioavailable, pan-class i pi3k/mtor inhibitor as clinical candidate in oncology. J Med Chem. 2017;60(17):7524–38.10.1021/acs.jmedchem.7b00930Search in Google Scholar PubMed PubMed Central
11 Zhou X, Zhou R, Li Q, Jie X, Hong J, Zong Y, et al. Cardamonin inhibits the proliferation and metastasis of non-small-cell lung cancer cells by suppressing the PI3K/Akt/mTOR pathway. Anti-Cancer Drugs. 2019;30(3):241–50.10.1097/CAD.0000000000000709Search in Google Scholar PubMed
12 Zhu F, Jiang D, Zhang M, Zhao B. 2,4-Dihydroxy-3′-methoxy-4′-ethoxychalcone suppresses cell proliferation and induces apoptosis of multiple myeloma via the PI3K/akt/mTOR signaling pathway. Pharm Biol. 2019;57(1):641–8.10.1080/13880209.2019.1662814Search in Google Scholar PubMed PubMed Central
13 Baillache DJ, Unciti-Broceta A. Recent developments in anticancer kinase inhibitors based on the pyrazolo[3,4-d]pyrimidine scaffold. RSC Med Chem. 2020;11(10):1112–35.10.1039/D0MD00227ESearch in Google Scholar PubMed PubMed Central
14 Mermer A, Keles T, Sirin Y. Recent studies of nitrogen containing heterocyclic compounds as novel antiviral agents: A review. Bioor Chem. 2021;114:105076.10.1016/j.bioorg.2021.105076Search in Google Scholar PubMed
15 Al-Zaydi KM, Khalil HH, El-Faham A, Khattab SN. Synthesis, characterization and evaluation of 1,3,5-triazine aminobenzoic acid derivatives for their antimicrobial activity. Chem Cent J. 2017;11(1):39.10.1186/s13065-017-0267-3Search in Google Scholar PubMed PubMed Central
16 Sahu S, Ghosh SK, Gahtori P, Pratap Singh U, Bhattacharyya DR, Bhat HR. In silico ADMET study, docking, synthesis and antimalarial evaluation of thiazole-1,3,5-triazine derivatives as Pf-DHFR inhibitor. Pharmacol Rep. 2019;71(5):762–7.10.1016/j.pharep.2019.04.006Search in Google Scholar PubMed
17 Elshemy HAH, Abdelall EKA, Azouz AA, Moawad A, Ali WAM, Safwat NM. Synthesis, anti-inflammatory, cyclooxygenases inhibitions assays and histopathological study of poly-substituted 1,3,5-triazines: Confirmation of regiospecific pyrazole cyclization by HMBC. Eur J Med Chem. 2017;127:10–21.10.1016/j.ejmech.2016.12.030Search in Google Scholar PubMed
18 Cascioferro S, Parrino B, Spanò V, Carbone A, Montalbano A, Barraja P, et al. 1,3,5-Triazines: A promising scaffold for anticancer drugs development. Eur J Med Chem. 2017;142:523–49.10.1016/j.ejmech.2017.09.035Search in Google Scholar PubMed
19 Narva S, Chitti S, Amaroju S, Bhattacharjee D, Rao BB, Jain N, et al. Design and synthesis of 4-morpholino-6-(1,2,3,6-tetrahydropyridin-4-yl)-N-(3,4,5-trimethoxyphenyl)-1,3,5-triazin-2-amine analogues as tubulin polymerization inhibitors. Bioorg Med Chem Lett. 2017;27(16):3794–801.10.1016/j.bmcl.2017.06.060Search in Google Scholar PubMed
20 Rageot D, Bohnacker T, Melone A, Langlois J-B, Borsari C, Hillmann P, et al. Discovery and preclinical characterization of 5-[4,6-bis({3-oxa-8-azabicyclo[3.2.1]octan-8-yl})-1,3,5-triazin-2-yl]-4-(difluoromethyl)pyridin-2-amine (PQR620), a highly potent and selective mtorc1/2 inhibitor for cancer and neurological disorders. J Med Chem. 2018;61(22):10084–105.10.1021/acs.jmedchem.8b01262Search in Google Scholar PubMed
21 Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, Wymann MP, et al. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol Cell. 2000;6(4):909–19.10.1016/S1097-2765(05)00089-4Search in Google Scholar PubMed
22 Burger MT, Pecchi S, Wagman A, Ni ZJ, Knapp M, Hendrickson T, et al. Identification of NVP-BKM120 as a potent, selective, orally bioavailable class I PI3 kinase inhibitor for treating cancer. ACS Med Chem Lett. 2011;2(10):774–9.10.1021/ml200156tSearch in Google Scholar PubMed PubMed Central
23 Bohnacker T, Prota AE, Beaufils F, Burke JE, Melone A, Inglis AJ, et al. Deconvolution of Buparlisib’s mechanism of action defines specific PI3K and tubulin inhibitors for therapeutic intervention. Nat Commun. 2017;8(1):14683.10.1038/ncomms14683Search in Google Scholar PubMed PubMed Central
24 Zhang JQ, Luo YJ, Xiong YS, Yu Y, Tu ZC, Long ZJ, et al. Design, synthesis, and biological evaluation of substituted pyrimidines as potential phosphatidylinositol 3-kinase (PI3K) inhibitors. J Med Chem. 2016;59(15):7268–74.10.1021/acs.jmedchem.6b00235Search in Google Scholar PubMed
25 Borsari C, Rageot D, Beaufils F, Bohnacker T, Keles E, Buslov I, et al. Preclinical development of PQR514, a highly potent PI3K inhibitor bearing a Difluoromethyl–Pyrimidine moiety. ACS Med Chem Lett. 2019;10(10):1473–9.10.1021/acsmedchemlett.9b00333Search in Google Scholar PubMed PubMed Central
26 Rageot D, Bohnacker T, Keles E, McPhail JA, Hoffmann RM, Melone A, et al. (s)-4-(Difluoromethyl)-5-(4-(3-methylmorpholino)-6-morpholino-1,3,5-triazin-2-yl)pyridin-2-amine (PQR530), a potent, orally bioavailable, and brain-penetrable dual inhibitor of class I PI3K and mTOR kinase. J Med Chem. 2019;62(13):6241–61.10.1021/acs.jmedchem.9b00525Search in Google Scholar PubMed
27 Rayan A, Raiyn J, Falah M. Nature is the best source of anticancer drugs: Indexing natural products for their anticancer bioactivity. PLoS One. 2017;12(11):e0187925.10.1371/journal.pone.0187925Search in Google Scholar PubMed PubMed Central
28 Gaonkar SL, Vignesh UN. Synthesis and pharmacological properties of chalcones: a review. Res Chem Intermed. 2017;43(11):6043–77.10.1007/s11164-017-2977-5Search in Google Scholar
29 Zhuang C, Zhang W, Sheng C, Zhang W, Xing C, Miao Z. Chalcone: A privileged structure in medicinal chemistry. Chem Rev. 2017;117(12):7762–810.10.1021/acs.chemrev.7b00020Search in Google Scholar PubMed PubMed Central
30 Ramírez–Prada J, Robledo SM, Vélez ID, Crespo MdP, Quiroga J, Abonia R, et al. Synthesis of novel quinoline–based 4,5–dihydro–1H–pyrazoles as potential anticancer, antifungal, antibacterial and antiprotozoal agents. Eur J Med Chem. 2017;131:237–54.10.1016/j.ejmech.2017.03.016Search in Google Scholar PubMed
31 Charris JE, Monasterios MC, Acosta ME, Rodríguez MA, Gamboa ND, Martínez GP, et al. Antimalarial, antiproliferative, and apoptotic activity of quinoline-chalcone and quinoline-pyrazoline hybrids. A dual action. Med Chem Res. 2019;28(11):2050–66.10.1007/s00044-019-02435-0Search in Google Scholar
32 Mahapatra DK, Asati V, Bharti SK. Chalcones and their therapeutic targets for the management of diabetes: Structural and pharmacological perspectives. Eur J Med Chem. 2015;92:839–65.10.1016/j.ejmech.2015.01.051Search in Google Scholar PubMed
33 Tukur A, Habila JD, Ayo RG-O, Iyun ORA. Design, synthesis, docking studies and antibiotic evaluation (in vitro) of some novel (E)-4-(3-(diphenylamino)phenyl)-1-(4-methoxyphenyl)-2-methylbut-3-en-1-one and their analogues. Bull Natl Res Cent. 2022;46(1):60.10.1186/s42269-022-00745-9Search in Google Scholar
34 Ohkura N, Ohnishi K, Taniguchi M, Nakayama A, Usuba Y, Fujita M, et al. Anti-platelet effects of chalcones from Angelica keiskei Koidzumi (Ashitaba) in vivo. Pharmazie. 2016;71(11):651–4.Search in Google Scholar
35 Rocha S, Ribeiro D, Fernandes E, Freitas M. A systematic review on anti-diabetic properties of chalcones. Curr Med Chem. 2020;27(14):2257–321.10.2174/0929867325666181001112226Search in Google Scholar PubMed
36 Gomes MN, Braga RC, Grzelak EM, Neves BJ, Muratov E, Ma R, et al. QSAR-driven design, synthesis and discovery of potent chalcone derivatives with antitubercular activity. Eur J Med Chem. 2017;137:126–38.10.1016/j.ejmech.2017.05.026Search in Google Scholar PubMed PubMed Central
37 Chen J, Liu C-F, Rao G-W. Progress in the synthesis, angiogenesis activity and mechanism of chalcone derivatives. Mini-Rev Org Chem. 2020;17(7):814–27.10.2174/1570193X17666191223161941Search in Google Scholar
38 Wang L, Chen H-C, Yang X, Tao J-J, Liang G, Wu J-Z, et al. The novel chalcone analog L2H17 protects retinal ganglion cells from oxidative stress-induced apoptosis. Neural Regener Res. 2018;13(9):1665–72.10.4103/1673-5374.237140Search in Google Scholar PubMed PubMed Central
39 Birari RB, Gupta S, Mohan CG, Bhutani KK. Antiobesity and lipid lowering effects of Glycyrrhiza chalcones: Experimental and computational studies. Phytomedicine. 2011;18(8):795–801.10.1016/j.phymed.2011.01.002Search in Google Scholar PubMed
40 Guzmán-Gutiérrez SL, Nieto-Camacho A, Castillo-Arellano JI, Huerta-Salazar E, Hernández-Pasteur G, Silva-Miranda M, et al. Mexican propolis: A source of antioxidants and anti-inflammatory compounds, and isolation of a novel chalcone and ε-caprolactone derivative. Molecules. 2018;23(2):334.10.3390/molecules23020334Search in Google Scholar PubMed PubMed Central
41 Wu J, Ao MT, Shao R, Wang HR, Yu D, Fang MJ, et al. A chalcone derivative reactivates latent HIV-1 transcription through activating P-TEFb and promoting Tat-SEC interaction on viral promoter. Sci Rep. 2017;7(1):10657.10.1038/s41598-017-10728-wSearch in Google Scholar PubMed PubMed Central
42 Cheng P, Yang L, Huang X, Wang X, Gong M. Chalcone hybrids and their antimalarial activity. Arch Pharm. 2020;353(4):1900350.10.1002/ardp.201900350Search in Google Scholar PubMed
43 Pallavi HM, Al-Ostoot FH, Vivek KH, Khanum SA. Synthesis, characterization, DFT, docking studies and molecular dynamics of some 3-phenyl-5-furan isoxazole derivatives as anti-inflammatory and anti-ulcer agents. J Mol Struct. 2022;1250(2):131812.10.1016/j.molstruc.2021.131812Search in Google Scholar
44 Xue X, Deng Y, Wang J, Zhou M, Liao L, Wang C, et al. Hydroxysafflor yellow A, a natural compound from carthamus tinctorius L with good effect of alleviating atherosclerosis. Phytomedicine. 2021;91:153694.10.1016/j.phymed.2021.153694Search in Google Scholar PubMed
45 Mendanha D, Vieira de Castro J, Moreira J, Costa BM, Cidade H, Pinto M, et al. A new chalcone derivative with promising antiproliferative and anti-invasion activities in glioblastoma cells. Molecules. 2021;26(11):3383.10.3390/molecules26113383Search in Google Scholar PubMed PubMed Central
46 Wang J, Wang N, Yao X, Kitanaka S. Structures and anti-histamine activity of chalcones & aurones compounds from bidens parviflora willd. Asian J Tradit Med. 2007;2(1):23–9.Search in Google Scholar
47 Stellenboom N. Comparison of the inhibitory potential towards carbonic anhydrase, acetylcholinesterase and butyrylcholinesterase of chalcone and chalcone epoxide. J Biochem Mol Toxicol. 2019;33(2):e22240.10.1002/jbt.22240Search in Google Scholar PubMed
48 Zhu C, Zuo Y, Wang R, Liang B, Yue X, Wen G, et al. Discovery of potent cytotoxic ortho-aryl chalcones as new scaffold targeting tubulin and mitosis with affinity-based fluorescence. J Med Chem. 2014;57(15):6364–82.10.1021/jm500024vSearch in Google Scholar PubMed
49 Pawlak A, Henklewska M, Hernández Suárez B, Łużny M, Kozłowska E, Obmińska-Mrukowicz B, et al. Chalcone methoxy derivatives exhibit antiproliferative and proapoptotic activity on canine lymphoma and leukemia cells. Molecules. 2020;25(19):4362.10.3390/molecules25194362Search in Google Scholar PubMed PubMed Central
50 Raghavender M, Kumar AK, Sunitha V, Vishnu T, Jalapathi P. Synthesis and cytotoxicity of chalcone based 1,2,3-triazole derivatives. Russ J Gen Chem. 2020;90(4):697–702.10.1134/S1070363220040210Search in Google Scholar
51 Wang G, Liu W, Gong Z, Huang Y, Li Y, Peng Z. Design, synthesis, biological evaluation and molecular docking studies of new chalcone derivatives containing diaryl ether moiety as potential anticancer agents and tubulin polymerization inhibitors. Bioorg Chem. 2020;95:103565.10.1016/j.bioorg.2019.103565Search in Google Scholar PubMed
52 Wang G, Liu W, Gong Z, Huang Y, Li Y, Peng Z. Synthesis, biological evaluation, and molecular modelling of new naphthalene-chalcone derivatives as potential anticancer agents on MCF-7 breast cancer cells by targeting tubulin colchicine binding site. J Enzyme Inhib Med Chem. 2020;35(1):139–44.10.1080/14756366.2019.1690479Search in Google Scholar PubMed PubMed Central
53 Ouyang Y, Li J, Chen X, Fu X, Sun S, Wu Q. Chalcone derivatives: Role in anticancer therapy. Biomolecules. 2021;11(6):894.10.3390/biom11060894Search in Google Scholar PubMed PubMed Central
54 Dai Hong MW, Liu J, Wu S, Qin X, Fang J. Synthesis and biological activity of novel chalcone derivatives containing 2-substituted-1,3-thiazolidine ring. Chin J Org Chem. 2012;32(9):1690–4.10.6023/cjoc1202211Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Synthesis, characterization, and antibacterial activity of a new poly azo compound containing N-arylsuccinimid and dibenzobarrelene moieties
- Design, synthesis, and antiviral activities evaluation of novel quinazoline derivatives containing sulfonamide moiety
- Design, synthesis, and anticancer activity of novel 4,6-dimorpholinyl-1,3,5-triazine compounds
- Design, synthesis, biological evaluation, and bio-computational modeling of imidazo, thieno, pyrimidopyrimidine, pyrimidodiazepene, and motifs as antimicrobial agents
- Synthesis of a novel phosphate-containing ligand rhodium catalyst and exploration of its optimal reaction conditions and mechanism for the polymerization of phenylacetylene
- Design, synthesis, and antiproliferative activity of novel 1,2,4-triazole-chalcone compounds
- Synthesis of metal–organic nanofiber/rGO nanocomposite as the sensing element for electrochemical determination of hypoxanthine
- Design and synthesis of various 1,3,4-oxadiazoles as AChE and LOX enzyme inhibitors
- Bis(2-cyanoacetohydrazide) as precursors for synthesis of novel azoles/azines and their biological evaluation
- Synthesis, characterization, and biological target prediction of novel 1,3-dithiolo[4,5-b]quinoxaline and thiazolo[4,5-b]quinoxaline derivatives
- Sustainable conversion of carbon dioxide into novel 5-aryldiazenyl-1,2,4-triazol-3-ones using Fe3O4@SP-vanillin-TGA nanocomposite
- Erratum
- Erratum to “Design, synthesis and study of antibacterial and antitubercular activity of quinoline hydrazone hybrids”
- SI: Undergraduate Research in the Synthesis of Biologically Active Small Molecules and Their Applications
- Preparation of novel acyl pyrazoles and triazoles by means of oxidative functionalization reactions
- Synthesis and conformational analysis of N-BOC-protected-3,5-bis(arylidene)-4-piperidone EF-24 analogs as anti-cancer agents
- SI: Development of Heterocycles for Biomedical and Bioanalytical Applications
- Influence of octreotide on apoptosis and metabolome expression in lipopolysaccharide-induced A549 cells
- Crude extract of J1 fermentation promotes apoptosis of cervical cancer cells
Articles in the same Issue
- Research Articles
- Synthesis, characterization, and antibacterial activity of a new poly azo compound containing N-arylsuccinimid and dibenzobarrelene moieties
- Design, synthesis, and antiviral activities evaluation of novel quinazoline derivatives containing sulfonamide moiety
- Design, synthesis, and anticancer activity of novel 4,6-dimorpholinyl-1,3,5-triazine compounds
- Design, synthesis, biological evaluation, and bio-computational modeling of imidazo, thieno, pyrimidopyrimidine, pyrimidodiazepene, and motifs as antimicrobial agents
- Synthesis of a novel phosphate-containing ligand rhodium catalyst and exploration of its optimal reaction conditions and mechanism for the polymerization of phenylacetylene
- Design, synthesis, and antiproliferative activity of novel 1,2,4-triazole-chalcone compounds
- Synthesis of metal–organic nanofiber/rGO nanocomposite as the sensing element for electrochemical determination of hypoxanthine
- Design and synthesis of various 1,3,4-oxadiazoles as AChE and LOX enzyme inhibitors
- Bis(2-cyanoacetohydrazide) as precursors for synthesis of novel azoles/azines and their biological evaluation
- Synthesis, characterization, and biological target prediction of novel 1,3-dithiolo[4,5-b]quinoxaline and thiazolo[4,5-b]quinoxaline derivatives
- Sustainable conversion of carbon dioxide into novel 5-aryldiazenyl-1,2,4-triazol-3-ones using Fe3O4@SP-vanillin-TGA nanocomposite
- Erratum
- Erratum to “Design, synthesis and study of antibacterial and antitubercular activity of quinoline hydrazone hybrids”
- SI: Undergraduate Research in the Synthesis of Biologically Active Small Molecules and Their Applications
- Preparation of novel acyl pyrazoles and triazoles by means of oxidative functionalization reactions
- Synthesis and conformational analysis of N-BOC-protected-3,5-bis(arylidene)-4-piperidone EF-24 analogs as anti-cancer agents
- SI: Development of Heterocycles for Biomedical and Bioanalytical Applications
- Influence of octreotide on apoptosis and metabolome expression in lipopolysaccharide-induced A549 cells
- Crude extract of J1 fermentation promotes apoptosis of cervical cancer cells

