Synthesis, characterization, and antibacterial activity of a new poly azo compound containing N-arylsuccinimid and dibenzobarrelene moieties
-
Njoya Abdou Salamou
, Tamokou Jean-de-Dieu
, Sopbué Fondjo Emmanuel
, Matsuete Takongmo Germaine
, Apollinaire Tsopmo
Abstract
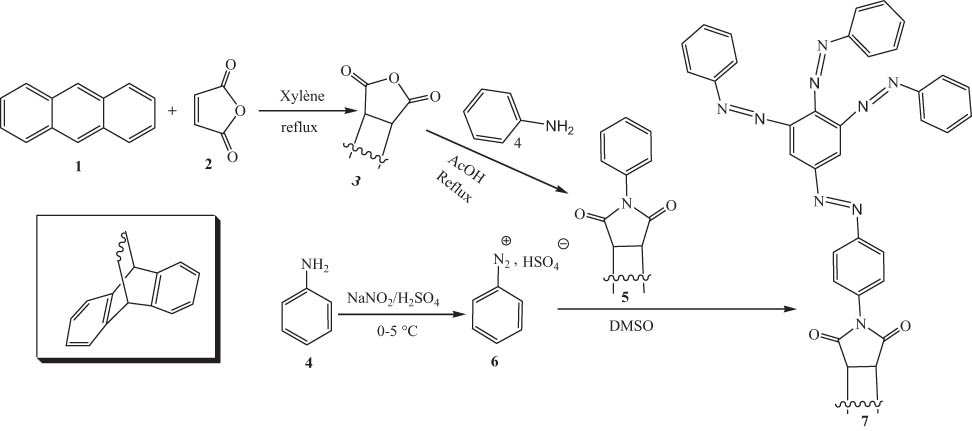
The present work describes the synthesis, characterization, and evaluation of the antibacterial activity of a new poly azo compound resulting from the coupling of a previously reported N-arylsuccinimid precursor 5 with the diazonium ion of aniline. This azo compound was characterized using its physical, elemental, and 1D and 2D spectroscopic data. The novel azo compound 7 (minimum inhibitory concentration [MIC] = 16–32 μg/mL) showed higher antibacterial activity than its precursor 5 (MIC = 32–64 μg/mL), although it was low compared to the reference drug ciprofloxacin (MIC = 0.5–4 μg/mL).
Graphical abstract
1 Introduction
Substituted succinimids such as N-arylsuccinimid (Figure 1) are important compounds in the manufacture of many drugs [1]. This importance is due to the fact that these compounds possess several pharmacological properties, such as antibacterial [2], analgesic [3], antitumor [4], anticancer [5], antispasmodic [6], bacteriostatic [7], antifungal [8], anticonvulsant [9], and antitubercular [10].
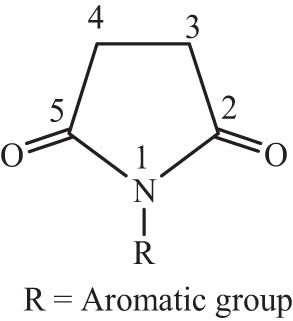
General structure of N-arylsuccinimid.
Azo compounds are characterized by an azo group (–N═N–), a chromophore whose nitrogen atoms are each bonded to sp2 hybridized carbon atoms. Most of the aromatic azo compounds are colored due to the presence of the azo group’s extended π electron system and are often used as dyes [11]. They are one of the classes of compounds in chemistry useful in many areas of life. Numerous biological activities make azo compounds very attractive agents medically. They are compounds with several activities, such as antimicrobial [12,13,14,15], antidiabetic [16], antioxidant [17], analgesic [18], anti-inflammatory [19], antiviral [20], antitubercular [21], and antitumor [22].
Here, we report the results of the coupling of the N-arylsuccinimid, 13-phenyl-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dicarboximide (5) containing the dibenzobarrelene ring (Figure 2) with the diazonium salt of aniline. The resulting product 7 was fully characterized by its elemental and spectroscopic data. In addition, the antibacterial activities of the novel azo compound were evaluated on four strains of bacteria, namely Staphylococcus aureus ATCC 25923, Klebsiella pneumoniae 22, Salmonella 68R, and Salmonella Typhi ATCC 6539.
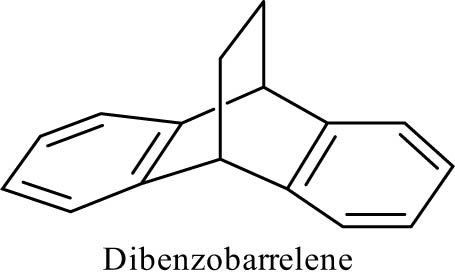
Basic structure of dibenzobarrelene.
The comparison of the activity of compound 7 with that of the precursor 5 could allow us to establish the effect of the azo fragments (–N═N–) on the antimicrobial potential of the newly prepared azo compound.
2 Results and discussion
2.1 Chemistry
The preparation of compound 7 was done according to a three-step procedure (Scheme 1): it starts with the synthesis of compound 3 by reaction between anthracene 1 and maleic anhydride 2, and then compound 3 is condensed with aniline to give the desired N-arylsuccinimid precursor 5. The final involves coupling of N-arylsuccinimid 5 with four equivalents of the diazonium ion of aniline.
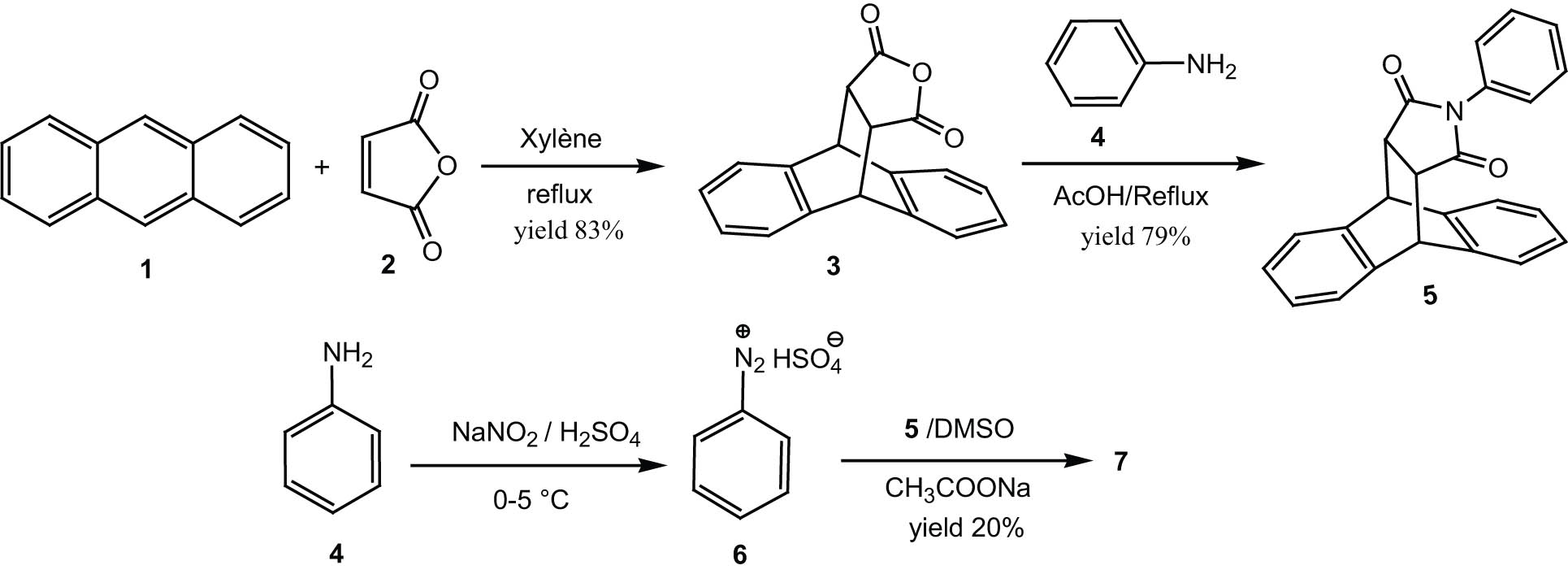
Reactions sequences to azo compound 7.
Compound 7 is the product of a substitution of four diazonium ion units on N-arylsuccinimid 5. The optimized 3D view of compound 7 is presented in Figure 3.
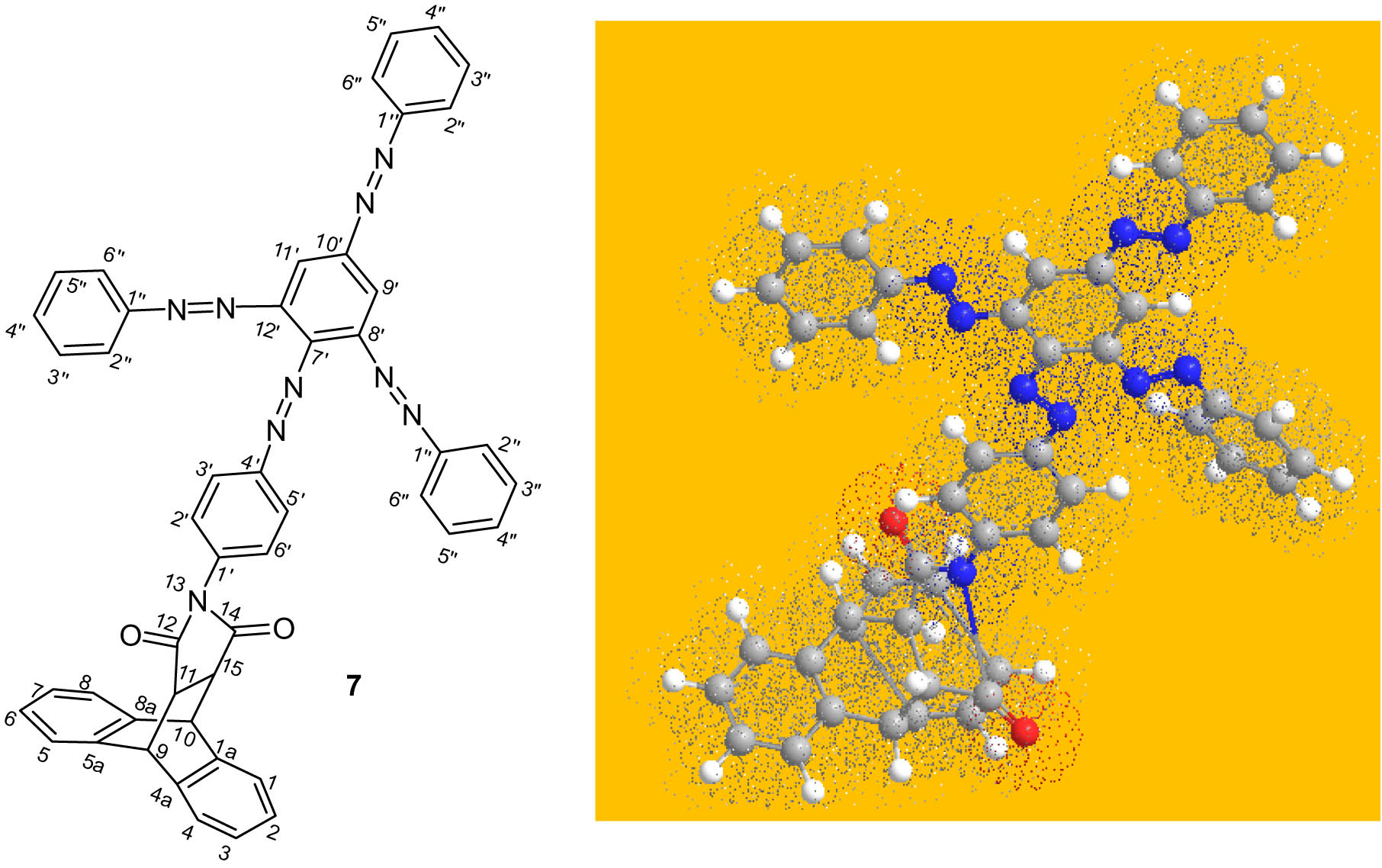
Optimized 3D view of compound 7.
The proton nuclear magnetic resonance (1H-NMR) spectrum of compound 7, contained core proton signals of the dibenzobarrelene moiety [23,24,25,26] at δ H 7.51 (2H, dd, J = 5.3 and 3.2 Hz), 7.29 (2H, dd, J = 5.3 and 3.3 Hz), 7.21 and 7.19 (4H, m), 4.84 (2H, s), and 3.36 (2H, s). In addition, the weak field region displayed an AA′BB′ proton system of two types of ortho-coupled benzene proton signals at 7.34 (2H, d, J = 8.1 Hz) and 6.43 (2H, d, J = 8.0 Hz) attributable to the H-2′, H-6′ and H-3′, H-5′ protons, respectively. Other strongly deshielded signals at 8.58, 8.09, and 7.51 are those of the H-2″, H-3″, H-4″, H-5″, and H-6″ protons of the diazonium ion of aniline with an integrated value equivalent to nearly 15 protons. Its Correlation Spectroscopy (COSY) 1H–1H spectrum showed through the correlation squares the couplings between the H-2′, H-6′ and H-3′, H-5′ protons; the H-4″ and H-3″, H-5″ protons; and the H-3″, H-5″ and H-2″, H-6″ protons.
On its carbon-13 nuclear magnetic resonance (13C-NMR) spectrum, after the identification of the signals of the carbons of dibenzobarrelene, two intense signals observed at δ C 128.5 and 126.0 were attributed to carbons C-3″, C-5″ and C-2″, C-6″, respectively. The C-4″ carbons were identified by the presence of a signal at 126.5 ppm. The NMR, distortionless enhancement by polarization transfer (DEPT), heteronuclear single quantum correlation (HSQC), and heteronuclear multiple bond correlation (HMBC) spectra allowed us to assign chemical shift values to the different carbons and protons of the molecule, and the data are grouped in Table 1. The important HMBC correlations are exhibited in Table 1 and Figure 4.
1H-NMR (DMSO-d 6, 600 MHz) and 13C-NMR (DMSO-d 6, 150 MHz) chemical shifts of azo compound 7 (δ in ppm, multiplet, J in Hz)
| N° C | δ C | δ H/HSQC (H → C) | HMBC (H → C) |
|---|---|---|---|
| 12, 14 | 176.3 | ||
| 4a, 8a | 142.1 | ||
| 1a, 5a | 139.8 | ||
| 6′ | 132.3 | ||
| 4′ | 132.1 | ||
| 1′′, 8′, 12′ | 131.7 | ||
| 7′ | 131.0 | ||
| 3′, 5′ | 129.3 | 7.33 (2H, d, J = 8.1 Hz) | C-2′, C-4′ |
| 1′ | 129.3 | ||
| 9′, 11′ | 128.9 | 7.33 (2H, s) | |
| 3′′, 5′′ | 128.4 | 8.09 (6H, dd, J = 5.3 and 3.2 Hz) | C-2′′/C-6′′, C-4′′, C-1′′ |
| 1, 5 | 127.1 | 7.20 (2H, dd, J = 5.3 and 3.2 Hz) | C-2/C-6, C-3/C-7 |
| 2′, 6′ | 127.0 | 6.43 (2H, d, J = 8.2 Hz) | C-3′, C-1′ |
| 4, 8 | 126.8 | 7.22 (2H, dd, J = 5.3 and 3.2 Hz) | C-2/C-6, C-3/C-7 |
| 4′′ | 126.5 | 8.58 (3H, m) | C-2′′, C-3′′, C-1′′ |
| 2′′, 6′′ | 126.0 | 7.51 (6H, dd, J = 5.3 and 3.2 Hz) | C-3′′/C-5′′, C-4′′, C-1′′ |
| 3, 7 | 125.2 | 7.51 (2H, dd, J = 5.3 and 3.2 Hz) | C-1/C-5, C-3/C-7 |
| 2, 6 | 124.8 | 7.31 (2H, dd, J = 5.3 and 3.3 Hz) | C-1/C-5 |
| 11, 15 | 47.0 | 3.43 (2H, s) | C-9/C-10,C-1a/C-5a, C-4a/C-8a, C-12/C-14 |
| 9, 10 | 45.2 | 4.87 (2H, s) | C-11/C-15, C-1a/C-5a, C-12/C-14 |
N.B.: The carbon atoms numbering in structure 7 does not correspond to the international union of pure and applied chemistry Nomenclature.
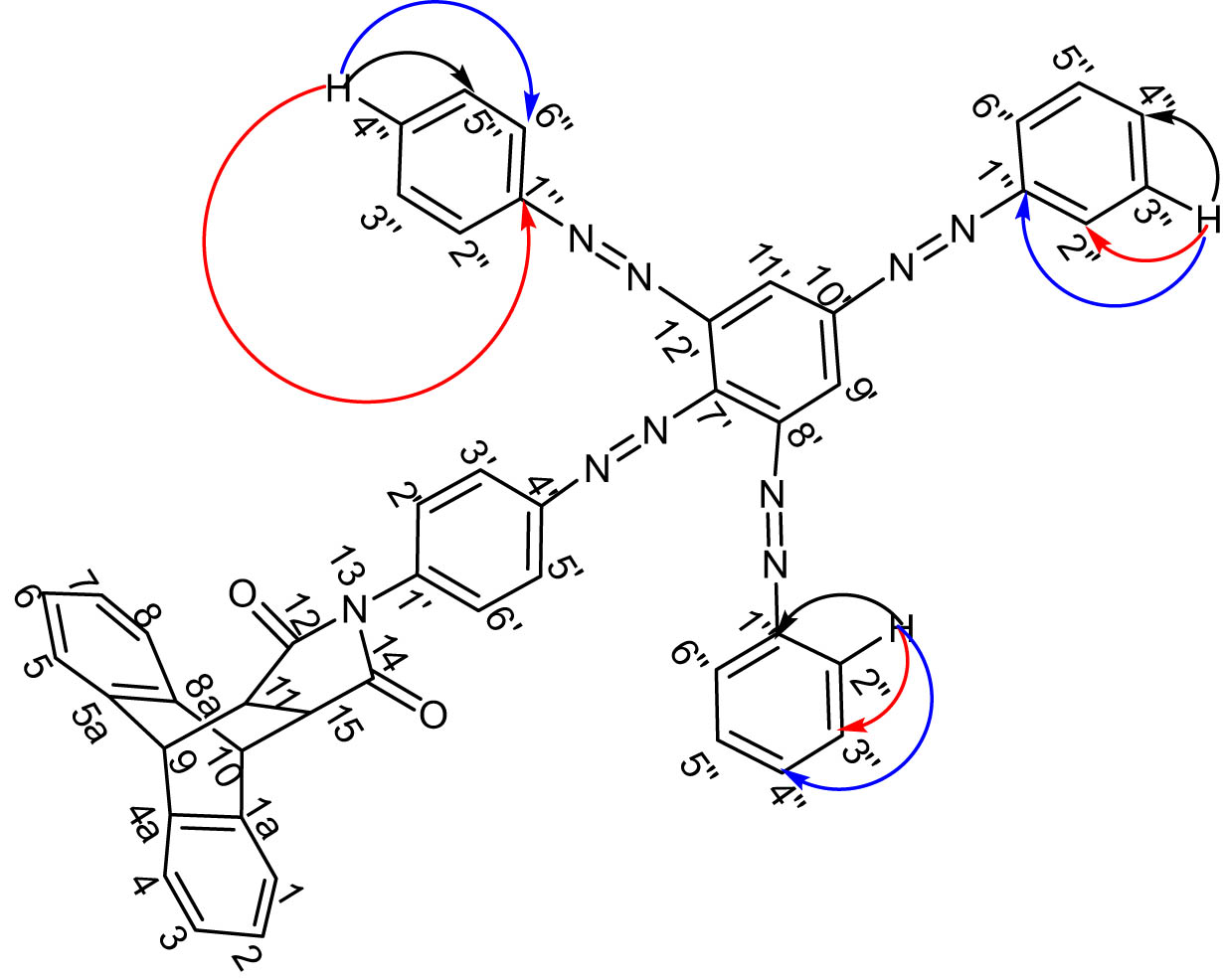
Some important HMBC correlations in compound 7.
2.2 Biology
2.2.1 Antimicrobial activity
The antibacterial activities of azo compound 7 as well as its precursor 5 were evaluated on one Gram-positive bacterium (S. aureus ATCC 25923) and three Gram-negative bacteria (K. pneumoniae 22, Salmonella 68R, and Salmonella Typhi ATCC 6539) and the data are depicted in Table 2. The newly synthesized azo compound 7 (minimum inhibitory concentration [MIC] = 16–32 μg/mL) and its precursor 5 (MIC = 32–64 μg/mL) showed antibacterial activity on all tested microorganisms. However, these activities are moderate compared to the reference molecule ciprofloxacin (MIC = 0.5–4 μg/mL). Nevertheless, the azo compound 7 displayed better activity than N-arylsuccinimid 5. It is reasonable to assume that the substitution reaction of the diazonium ion on N-arylsuccinimid has resulted in the improved activity of the azo compound 7 on the tested bacteria. This activity was most pronounced on S. aureus ATCC 25923 and Salmonella Typhi ATCC 6539, whose MIC decreased from 64 μg/mL for N-arylsuccinimid to 16 μg/mL for the azo compound. As previously demonstrated by Mkpenie et al. [27], the increase in antibacterial activity in the azo compound would be due to the azo (–N═N–) chromophore present in the molecule. Moreover, the association of four azo groups should have a cumulative effect on the antibacterial activity in the azo compound. We also noticed the bactericidal effect (minimum bactericidal concentration [MBC]/MIC ≤4) of the azo compound on all microorganisms just like ciprofloxacin, while N-arylsuccinimid showed a bactericidal effect only on three bacteria and a bacteriostatic effect (MBC/MIC >4) against Salmonella 68R.
Antibacterial activity (MIC and MBC in µg/mL) of synthesized compounds 5 and 7 as well as reference antibiotic drug
| Compounds | Inhibition parameters | S. aureus ATCC 25923 | K. pneumoniae 22 | Salmonella 68R | Salmonella Typhi ATCC 6539 |
|---|---|---|---|---|---|
| 5 | MIC | 64 | 32 | 64 | 64 |
| MBC | 64 | 64 | >256 | 128 | |
| MBC/MIC | 1 | 2 | / | 2 | |
| 7 | MIC | 16 | 16 | 32 | 16 |
| MBC | 32 | 32 | 64 | 32 | |
| MBC/MIC | 2 | 2 | 2 | 2 | |
| Ciprofloxacin | MIC | 0.50 | 1 | 4 | 2 |
| MBC | 0.50 | 1 | 4 | 2 | |
| MBC/MIC | 1 | 1 | 1 | 1 |
/: not determined.
2.2.2 Cytotoxic activity
To investigate the potential use of compounds 5 and 7, the cytotoxicity also has to be evaluated. In this study, none of the tested samples showed hemolytic activities against red blood cells at concentrations up to 128 µg/mL. However, at the highest concentration tested in this study (256 μg/mL), the azo compound 7 (3.88 ± 0.004%) has a hemolytic activity three times greater than that of its precursor 5 (1.26 ± 0.002%). This finding highlights that the test compounds are slightly hemolytic at 256 μg/mL. The highest cytotoxic activity of the azo compound would in fact be attributed to the azo groups present in the latter, thus confirming its function as a pharmacophore responsible for the observed activities [27].
3 Conclusion
In summary, a new poly azo dye containing four diazo bridges and incorporating a dibenzobarrelene backbone and four phenyl substituents has been synthesized by coupling the N-aryl succinimid fused to dibenzobarrelene with phenyl diazonium hydrogenosulfate. The structure of the new azo compound 7 was fully assigned on the basis of the available spectroscopic data. The improvements noted in the antimicrobial activities of the new compound compared to its precursor are without any doubt attributable to the cumulative pharmacophore effects of the diazo bridges introduced in the hybrid.
4 Materials and methods
4.1 Instrumental method
All melting points are corrected and were determined with a STUART SCIENTIFIC Melting Point Apparatus Model SMP3. The thin layer chromatography were carried out on Eastman Chromatogram Silica Gel Sheets (13,181; 6,060) with fluorescent indicators. A mixture of hexane and ethyl acetate (1:2) was used as eluent and sulfuric acid (10%) was used as a revelator for the chromatograms. The infrared (IR) spectra were measured with a Fourier-transform infrared spectrometer Bruker Alpha. The ultraviolet (UV) spectra were recorded with a JENWAY 6715 UV-Vis Spectrophotometer. Combustion analyses were carried out with a C, H, N, and S Euro EA from Hekatech Company, their results were found to be in good agreement (±0.3%) with the calculated values. High-resolution electrospray ionization mass spectrometry spectra were performed with a spectrometer (QTOF Bruker, Germany) equipped with an high-resolution electrospray ionization source. The spectrometer operates in positive ion mode (mass range: 100–1,500, with a scan rate of 1.00) with automatic gain control to provide high-accuracy mass measurements within 0.40 ppm deviation using Na formate as calibrant. The following parameters were used for experiments: spray voltage of 4.5 kV, capillary temperature of 200°C. Nitrogen was used as sheath gas (10 L/min). 1H-NMR spectra and carbon nuclear magnetic resonance (13C-NMR) spectra were recorded in deuterated dimethylsulfoxide (DMSO) on a Bruker SF spectrometer operating at 600 and 150 MHz, respectively; TMS was used as an internal reference.
4.1.1 Synthesis of 9,10-dihydro-9,10-ethanoanthracene-11,12-dicarboxylic anhydride (3)
The synthesis procedure of compound 3 is similar to the one previously described [26]. Electrospray ionisation mass spectrometry (ESI-MS): 299 (M + Na, 100%). IR (KBr) υmax (cm−1) 3,049 (═CAr–H); 1,770 (C═O); 1,460, 1,477 (CAr═CAr); 1,245 (C–O). Anal. Calcd. for C18H12O3 (276.08): C, 78.12; H, 4.53; found: C, 78.25; H, 4.38.
4.1.2 Synthesis of 13-phenyl-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dicarboximide (5)
About 4.5 g (0.0163 mol) of compound 3 is dissolved in 50 mL of acetic acid at elevated temperature, and 4.5 g (0.0483 mol) of aniline is added dropwise and the mixture is heated to reflux for 3 h and subsequently cooled to room temperature. The resulted precipitate is filtered and washed with aqueous ethanol (50%) to give 4.5 g of the whitish product; mp: 220°C (Lit [28]: 242°C from DMF), yield 79% (Lit [28]: 70%); ESI-MS: 374 (M + Na, 100%). IR (KBr) υmax (cm−1) 2,965 (═CAr–H); 1,709 (C═O); 1,621, 1,532 (CAr═CAr). Anal. Calcd. for C18H12O3 (351.13): C, 82.03; H, 4.88; N, 3.99; found: C, 81.91; H, 4.93; N, 3.87.
4.1.3 Synthesis of 13-(4-(2,4,6-tris(phenyldiazenyl)phenyl)diazenylphenyl)-9,10-dihydro-9,10-ethanoanthracene-12,14-dicarboximide
4.1.3.1 Preparation of diazonium salt solution
This preparation is similar to that previously described [26]. In an Erlenmeyer flask, 1 g (10.75 mmol) of aniline is dissolved in 5 mL of DMSO. An excess of 1.5 g (21 mmol) of sodium nitrite is added, and the mixture is homogenized and introduced into a salted ice bath to achieve a temperature between 0 and 5°C. A volume of 5 mL of concentrated sulfuric acid (98%) is added dropwise under stirring for 30 min while maintaining the temperature between 0 and 5°C.
4.1.3.2 Coupling of diazonium ion with N-arylsuccinimid
To the diazonium solution prepared at 4.1.3.1 is added dropwise over 30 min 0.5 g (1.42 mmol) of N-arylsuccinimid 5 dissolved in 5 mL of DMSO. The mixture is then stirred for another 30 min to complete the reaction. At the end of the reaction, 10 mL of aqueous sodium acetate (10%) is also introduced by a small portion in order to neutralize the excess sulfuric acid. The precipitate obtained after 30 min is then filtered and washed first with cold water and finally with hot water. After crystallization from ethanol (98%), 213 mg of compound 7 was obtained as a brown powder; yield 20%; mp: 148.1–149°C. UV-Vis: λ max (ethanol) 268, 340, 355, 375 nm. ESI-MS: 790 (M + Na, 100%). IR (KBr) υmax (cm−1) 3,034 (═CAr–H), 1,711 (C═O), 1,593 (CAr═CAr), and 1,485 (N═N). 1H-NMR (DMSO-d 6) δ (ppm): 8.58 (3H, m, H-4″), 8.09 (6H, dd, J = 5.3 and 3.2 Hz, H-3″, H-5″), 7.51 (6H, dd, J = 5.3 and 3.2 Hz, H-3, H-7, H-2″, H-6″), 7.33 (2H, d, J = 8.1 Hz, H-3′, H-5′), 7.33 (2H, s, H-9′, H-11′), 7.22 (2H, dd, J = 5.3 and 3.2 Hz, H-4, H-8) 7.20 (2H, dd, J = 5.3 and 3.2 Hz, H-1, H-5), 7.31 (2H, dd, J = 5.3 and 3.3 Hz, H-2, H-6), 6.43 (2H, d, J = 8.2 Hz, H-2′, H-6′), 4.87 (2H, s, H-9, H-10), and 3.43 (2H, s, H-11, H-15). 13C-NMR (DMSO) δ (ppm): 176.3 (C-12, C-14), 142.1 (C-4a, C-8a), 139.8 (C-1a, C-5a), 132.3 (C-6′), 132.1 (C-4′), 131.7 (C-1″, C-8′, C-12′), 131.0 (C-7′), 129.3 (C-3′, C-5′, C-1′), 128.9 (C-9′, C-11′), 128.4 (C-3″, C-5″), 127.1 (C-1, C-5), 127.0 (C-2′, C-6′), 126.8 (C-4, C-8), 126.5 (C-4″), 126.0 (C-2″, C-6″), 125.2 (C-3, C-7), 124.8 (C-2, C-6), 47.0 (C-11, C-15), and 45.2 (C-9, C-10). Anal. Calcd. for C48H33N9O2 (767.28): C, 74.83; H, 4.57; N, 16.61; found: C, 75.08; H, 4.33; N, 16.42.
4.2 Biology assay
4.2.1 Tested bacteria
Antibacterial activity was performed against one Gram-positive bacterium (S. aureus ATCC 25923) and three Gram-negative bacteria (K. pneumoniae 22, Salmonella 68R, and Salmonella Typhi ATCC 6539). These microorganisms were collected from the Research Unit of Microbiology and Antimicrobial Substances. These bacterial species were grown at 37°C and maintained on nutrient agar plates (NA, Conda Madrid, Spain).
4.2.2 Antibacterial assay
Antibacterial activity was performed by determining MIC and MBC as described previously [29]. The MICs of synthesized compounds 5 and 7 were determined using the broth microdilution method. Each test sample was dissolved in DMSO to obtain a stock solution. This was serially diluted twice in Mueller–Hinton broth (MHB) to obtain a concentration range from 512 to 0.25 μg/mL. Then, 100 µL of each sample concentration was added to the respective wells (96-well microplate) containing 90 µL of MHB and 10 µL of inoculum at 106 CFU/mL to obtain a final concentration range of 256–0.125 μg/mL. Dilutions of ciprofloxacin (Sigma-Aldrich, Steinheim, Germany) were used as positive controls. Broth with 10 µL of DMSO was used as a negative control. MICs were assessed visually and were considered as the lowest concentration of the sample at which there was no growth or virtually no growth. The lowest concentration that resulted in no growth after subculture was considered the MIC. All tests were repeated three times [29].
4.2.3 Hemolytic assay
Whole blood (10 mL) from albino rats was collected by cardiac puncture into a conical tube containing ethylenediaminetetraacetic acid as an anticoagulant. The study was conducted according to the ethical guidelines of the Committee for Control and Supervision of Experiments on Animals (Registration no. 173/CPCSEA, dated 28 January 2000), Government of India, on the use of animals for scientific research. Erythrocytes were harvested by centrifugation at room temperature for 10 min at 1,000 × g and were washed three times in phosphate-buffered saline buffer [30]. The cytotoxicity was evaluated as previously reported [30].
Acknowledgments
Emmanuel Sopbué Fondjo gratefully acknowledges the financial support from DAAD (grant no. 91691265). Additional financial support for the work was obtained from the University of Dschang’s research grant committee and the Cameroonian Ministry of Higher Education special research allocation.
-
Ethical approval: The research related to animal use has been complied with all the relevant national regulations and institutional policies for the care and use of animals, and have been approved by the Cameroon National Ethical Committee (Reg. No. FWA-IRB00001954) and in compliance with the ARRIVE guidelines.
-
Funding information: Financial support was provided by DAAD (grant no. 91691265), the University of Dschang, and the Cameroonian Ministry of Higher Education.
-
Author contributions: E.S.F. designed the research topic, provided the reagents and supervised the chemistry experimental work as well as the compilation of the chemistry parts of the paper; A.S.N. carried out the chemistry part of the experimental work in the lab, contributed to the exploitation of the data and the paper drafting; J.D.D.T and G.M.T. performed the antibacterial and antifungal assays and contributed the compilation of the biological parts of the paper; G.D. performed the powder diffraction XRD measurements, helped in the interpretation of the XRD data and contributed in reviewing the whole manuscript’s draft; P.F.W.S. performed the GC-MS measurements, helped in the interpretation of the spectra and contributed in reviewing the whole manuscript’s draft; B.N.L. performed the LC-MS measurements, helped in the interpretation of the spectra and contributed in reviewing the whole manuscript’s draft; A.T. and G.T.B.M. performed the NMR measurements, helped in the interpretation of the spectra and contributed in reviewing the whole manuscript’s draft; J.R.K. supervised the biological screening experiments and contributed in reviewing the whole manuscript’s draft. All authors have given approval to the final version of the paper. All authors read and approved the final manuscript.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Rajput SS, Patil MM. Succinimides: Synthesis, reaction and biological activity. Int J Pharm Sci. 2014;6(11):8–14.Search in Google Scholar
[2] Zentz F, Valla A, Guillou RL, Labia R, Mathot AG, Sirot D. Synthesis and antimicrobial activities of N-substituted imides. IL Farmaco. 2002;57:421–6.10.1016/S0014-827X(02)01217-XSearch in Google Scholar
[3] Corrêa R, Filho VC, Rosa PW, Pereira CI, Schlemper V, Nunes RJ. Synthesis of new succinimides and sulphonated derivatives with analgesic action in mice. Pharm Sci. 1997;3:67–71.Search in Google Scholar
[4] Hall IH, Wong OT, Scovill JP. The cytotoxicity of N-pyridinyl and N-quinolinyl substituted derivatives of phthalimide and succinimide. Biomed Pharmacother. 1995;49:251–8.10.1016/0753-3322(96)82631-XSearch in Google Scholar PubMed
[5] Crider AM, Kolczynski TM, Yates KM. Synthesis and anticancer activity of nitroso urea derivatives of phensuximide. J Med Chem. 1980;23:324–6.10.1021/jm00177a024Search in Google Scholar PubMed
[6] Filho VC, Nunes RJ, Calixto JB, Yunes RA. Inhibition of Guineapig ileum contraction by phyllanthimide analogues: Structure-activity relationships. Pharma Sci. 1995;1:399–401.Search in Google Scholar
[7] Johnston TP, Piper JR, Stringfellow CR. 1. Terminal dicarboximido analogs of S-2-omega-aminoalkylamino) ethyl dihydrogen phosphorothioates and related compounds as potential antiradiation agents. 2. Succinimides, glutarimides, and cis-1,2-cyclo hexane dicarboximides. J Med Chem. 1971;14:345–50.10.1021/jm00286a019Search in Google Scholar PubMed
[8] Hazra BG, Vandana SP, Sanjeev KD, Suchitra D, Mahendra P, Darokar DS, et al. Bile acid amides derived from chiral amino alcohols: novel antimicrobials and antifungals. Bioorg Med Chem Lett. 2004;14:773–7.10.1016/j.bmcl.2003.11.018Search in Google Scholar PubMed
[9] Kornet MJ, Crider AM, Magarian EO. Potential long-acting anticonvulsants; synthesis and activity of succinimides containing an Alkylating group at the 2 position. J Med Chem. 1977;20:405–9.10.1021/jm00213a018Search in Google Scholar PubMed
[10] Isaka M, Prathumpai W, Wongsa P, Tanticharoen M, Hirsutellone FS, Hirsutellone F. A dimer of antitubercular alkaloids from the Seed Fungus Trichoderma Species BCC 7579. Org Lett. 2006;8:2815–7.10.1021/ol060926xSearch in Google Scholar PubMed
[11] Lokhande S, Thakare N. Synthesis and characterization of azo compounds containing disubstituted phenolic moities. Int J Adv Sci Res Manag. 2019;4:127–9.10.36282/IJASRM/4.3.2019.1297Search in Google Scholar
[12] Tsemeugne J, Sopbué FE, Tamokou JDD, Ngongang DA, Kuiate JR, Sondengam BL. Synthesis and antimicrobial activities of some novel thiophene containing azo compound. Heterocycl Commun. 2013;19:253–9.10.1515/hc-2013-0096Search in Google Scholar
[13] Tsemeugne J, Sopbué FE, Tamokou JDD, Rohand T, Djintchui AN, Kuiate J-R, et al. Synthesis, characterization, and antimicrobial activity of a novel trisazo dye from 3-amino-4H-thieno[3,4-c][1]benzopyran-4-one. Int J Med Chem. 2018;2018(9197821):8.10.1155/2018/9197821Search in Google Scholar PubMed PubMed Central
[14] Tsemeugne J, Nangmo KP, Mkounga P, Tamokou JDD, Kengne CI, Edwards G, et al. Synthesis, characteristic fragmentation patterns, and antibacterial activity of new azo compounds from the coupling reaction of diazobenzothiazo ions and acetaminophen. Heterocycl Commun. 2021;27:1–11. 10.1515/hc-2020-0127.Search in Google Scholar
[15] Rajiv AS, Dipti KD. Synthesis and antimicrobial evaluation of novel azo compounds. World J Pharm Pharm Sci. 2016;5(9):1149–54.Search in Google Scholar
[16] Garg HG, Prakash C. Potential antidiabetics, 11. Preparation of 4-arylazo-3,5- disubstituted-(2H)-1, 2, 6-thiadiazine 1,1-Dioxides. J Med Chem. 1972;15:435–6.10.1021/jm00274a035Search in Google Scholar PubMed
[17] Kribaa L, Atia S, Boudehane A, Maher RMB, Gherraf N, Lounas A. Synthesis, characterization and antioxidant activity studies of new azo-compounds. J Biochem Technol. 2019;10:85–90.Search in Google Scholar
[18] Malar SJS, Reji TFAF. Biological studies of synthetised azo compounds of indole: A comparative study. IJIAS. 2016;18:602–6.Search in Google Scholar
[19] Rani P, Srivastava VK, Kumar A. Synthesis and anti-inflammatory activity of heterocyclic indole derivatives. Eur J Med Chem. 2004;39:449–52.10.1016/j.ejmech.2003.11.002Search in Google Scholar PubMed
[20] Tonelli M, Vazzana I, Tasso B, Boido V, Sparatore F, Fermeglia M, et al. Antiviral and cytotoxic activities of amino-arylazo compounds and aryltriazene derivatives. Bioorg Med Chem. 2009;17:4425–40.10.1016/j.bmc.2009.05.020Search in Google Scholar PubMed PubMed Central
[21] Rohini RM, Kalpana D, Simi D. Synthesis of novel phenyl azo chalcone derivatives for antitubercular, antiinflammatory and antioxidant activity. Der Pharma Chem. 2015;7:77–83.Search in Google Scholar
[22] Fadda AA, Abdel-Latif E, El-Mekawy RE. Synthesis of some new arylazothiophene and arylazopyrazole derivatives as antitumor agents. Pharmacol Pharm. 2012;3:148–57.10.4236/pp.2012.32022Search in Google Scholar
[23] Madhurambal G, Ramasamy P, Srinivasan PA, Mojumdar SC. Synthesis and characterization of a novel non-linear optical (NLO) material; endo-anthracene maleic Anhydride-Diels-Alder adduct of anthracene. J Therm Anal Calorim. 2006;86(3):601–4. 10.1007/s10973-006-7714-z.Search in Google Scholar
[24] Arya S, Kumar S, Rani R, Kumar N, Roy P, Sondhi SM. Synthesis, anti-inflammatory, and cytotoxicity evaluation of 9,10-dihydroanthracene-9,10-α,β-succinimide and bis-succinimide derivatives. Med Chem Res. 2013;22:4278–85. 10.1007/s00044-012-0439-6.Search in Google Scholar
[25] İrfan Ç, Süleyman S. Synthesis of novel aza-heterocyclic derivatives from diester and diacid chlorides having the dibenzobarrelene skeleton. Synth Commun. 2018;48(10):1164–71. 10.1080/00397911.2018.1437449.Search in Google Scholar
[26] Sopbué FE, Njoya AS, Tamokou JDD, Doungmo G, Lenta NB, Tsopmo A, et al. Synthesis, characterization and antimicrobial properties of two derivatives of pyrrolidine-2,5-dione fused at positions-3,4 to a dibenzobarrelene backbone. BMC Chem. 2022;16(8):10. 10.1186/s13065-022-00801-5.Search in Google Scholar PubMed PubMed Central
[27] Mkpenie VG, Ebong IBO, Obot B, Abasiekong B. Evaluation of the effect of azo group on the biological activity of 1-(4-methylphenylazo)-2-naphthol. E J Chem. 2008;5(3):431–4.10.1155/2008/438946Search in Google Scholar
[28] Weber E, Finge S, Csoregh I. Modular design of hosts involving a rigid succinimide framework and N-bonded lateral groups. Crystalline inclusion properties and crystal structures of inclusion compounds with dioxane, MeOH, and DMF. J Org Chem. 1990;56:7281–8.10.1021/jo00026a018Search in Google Scholar
[29] Tamokou JD, Tala FM, Wabo KH, Kuiate J-R, Tane P. Antimicrobial activities of methanol extract and compounds from stem bark of Vismia rubescens. J Ethnopharmacol. 2009;124:571–5.10.1016/j.jep.2009.04.062Search in Google Scholar PubMed
[30] Situ H, Bobek LA. In vitro assessment of antifungal therapeutic potential of salivary histatin-5, two variants of histatin-5, and salivary mucin (MUC7) domain 1. Antimicrob Agents Chemother. 2000;44:1485–93.10.1128/AAC.44.6.1485-1493.2000Search in Google Scholar PubMed PubMed Central
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Synthesis, characterization, and antibacterial activity of a new poly azo compound containing N-arylsuccinimid and dibenzobarrelene moieties
- Design, synthesis, and antiviral activities evaluation of novel quinazoline derivatives containing sulfonamide moiety
- Design, synthesis, and anticancer activity of novel 4,6-dimorpholinyl-1,3,5-triazine compounds
- Design, synthesis, biological evaluation, and bio-computational modeling of imidazo, thieno, pyrimidopyrimidine, pyrimidodiazepene, and motifs as antimicrobial agents
- Synthesis of a novel phosphate-containing ligand rhodium catalyst and exploration of its optimal reaction conditions and mechanism for the polymerization of phenylacetylene
- Design, synthesis, and antiproliferative activity of novel 1,2,4-triazole-chalcone compounds
- Synthesis of metal–organic nanofiber/rGO nanocomposite as the sensing element for electrochemical determination of hypoxanthine
- Design and synthesis of various 1,3,4-oxadiazoles as AChE and LOX enzyme inhibitors
- Bis(2-cyanoacetohydrazide) as precursors for synthesis of novel azoles/azines and their biological evaluation
- Synthesis, characterization, and biological target prediction of novel 1,3-dithiolo[4,5-b]quinoxaline and thiazolo[4,5-b]quinoxaline derivatives
- Sustainable conversion of carbon dioxide into novel 5-aryldiazenyl-1,2,4-triazol-3-ones using Fe3O4@SP-vanillin-TGA nanocomposite
- Erratum
- Erratum to “Design, synthesis and study of antibacterial and antitubercular activity of quinoline hydrazone hybrids”
- SI: Undergraduate Research in the Synthesis of Biologically Active Small Molecules and Their Applications
- Preparation of novel acyl pyrazoles and triazoles by means of oxidative functionalization reactions
- Synthesis and conformational analysis of N-BOC-protected-3,5-bis(arylidene)-4-piperidone EF-24 analogs as anti-cancer agents
- SI: Development of Heterocycles for Biomedical and Bioanalytical Applications
- Influence of octreotide on apoptosis and metabolome expression in lipopolysaccharide-induced A549 cells
- Crude extract of J1 fermentation promotes apoptosis of cervical cancer cells
Articles in the same Issue
- Research Articles
- Synthesis, characterization, and antibacterial activity of a new poly azo compound containing N-arylsuccinimid and dibenzobarrelene moieties
- Design, synthesis, and antiviral activities evaluation of novel quinazoline derivatives containing sulfonamide moiety
- Design, synthesis, and anticancer activity of novel 4,6-dimorpholinyl-1,3,5-triazine compounds
- Design, synthesis, biological evaluation, and bio-computational modeling of imidazo, thieno, pyrimidopyrimidine, pyrimidodiazepene, and motifs as antimicrobial agents
- Synthesis of a novel phosphate-containing ligand rhodium catalyst and exploration of its optimal reaction conditions and mechanism for the polymerization of phenylacetylene
- Design, synthesis, and antiproliferative activity of novel 1,2,4-triazole-chalcone compounds
- Synthesis of metal–organic nanofiber/rGO nanocomposite as the sensing element for electrochemical determination of hypoxanthine
- Design and synthesis of various 1,3,4-oxadiazoles as AChE and LOX enzyme inhibitors
- Bis(2-cyanoacetohydrazide) as precursors for synthesis of novel azoles/azines and their biological evaluation
- Synthesis, characterization, and biological target prediction of novel 1,3-dithiolo[4,5-b]quinoxaline and thiazolo[4,5-b]quinoxaline derivatives
- Sustainable conversion of carbon dioxide into novel 5-aryldiazenyl-1,2,4-triazol-3-ones using Fe3O4@SP-vanillin-TGA nanocomposite
- Erratum
- Erratum to “Design, synthesis and study of antibacterial and antitubercular activity of quinoline hydrazone hybrids”
- SI: Undergraduate Research in the Synthesis of Biologically Active Small Molecules and Their Applications
- Preparation of novel acyl pyrazoles and triazoles by means of oxidative functionalization reactions
- Synthesis and conformational analysis of N-BOC-protected-3,5-bis(arylidene)-4-piperidone EF-24 analogs as anti-cancer agents
- SI: Development of Heterocycles for Biomedical and Bioanalytical Applications
- Influence of octreotide on apoptosis and metabolome expression in lipopolysaccharide-induced A549 cells
- Crude extract of J1 fermentation promotes apoptosis of cervical cancer cells


