Synthesis of a novel phosphate-containing ligand rhodium catalyst and exploration of its optimal reaction conditions and mechanism for the polymerization of phenylacetylene
-
Mingyu Zhang
and Hongge Jia
Abstract
Due to the chemical properties of Group VIII transition metals and their importance in catalytic processes, the reactions of rhodium and its neighboring organic complexes have received much attention as model systems. In the present experiments, we successfully prepared two new rhodium complexes named [Rh(cod)(TTP)Cl] and [Rh(cod)(CDP)Cl] using tri(o-tolyl)phosphine and clohexyldiphenylphosphine as ligands, respectively, and the proofs of their structures were completed using 1H NMR and 13C NMR. Subsequently, it was applied to the polymerization reaction of phenylacetylene (PA), and the effects of different solvents, time, and temperature on the yield and molecular weight of the polymerization reaction products were discussed. The experimental results show that both complexes can play a catalytic role in the polymerization of PA. From the point of view of polymer molecular weight, the best reaction conditions for both catalysts were 3 h at 20°C in THF solvent (molecular weight up to 2.40 × 105). From the point of view of yield, the best reaction conditions for both catalysts were 4 h at 35°C in THF solvent (yield up to 89.2%).
1 Introduction
In recent years, polyphenylacetylene (PPA) and its derivatives have been widely used as alkyne polymeric materials in many applications, such as electronic products, automotive parts, and toys. The application of these polymers has undoubtedly driven the research process of catalysts. For this reason, various transition metal catalysts have been developed, and the design and development of new catalysts for alkyne polymerization are a relatively active research area [1,2,3,4]. Rhodium complexes are generally known as versatile and efficient catalysts for the (co-)polymerization of functional alkyne monomers [5,6,7]. As a catalyst for olefin polymerization, rhodium catalysts are stable in air and can bind to a wide range of available monomers. Therefore, it has been attracted by an increasing number of researchers [8,9,10].
In the family of catalysts, rhodium catalysts containing phosphine ligands were one of them, and these had been widely used in alkyne polymerization. In 2018, Tan et al. [11] had synthesized three new rhodium–vinyl complexes, Rh(nbd)(CPh═CFlu)P(4-FC6H4)3, Rh(nbd)(CPh═CFlu)P(4-CF3C6H4)3, and Rh(nbd)(CPh═CFlu)P[3,5-(CF3)2C6H3]3 (nbd = 2,5-norbornadiene; fluorenyl (Flu)), containing Flu with fluorine-functionalized phosphine ligands. The synthesis and purity of these complexes were proved by NMR (1H, 31P, 19F, and 103Rh). These complexes had high activity as initiators in phenylacetylene (PA) polymerization experiments, with initiation efficiencies about 56%. The polymers had high molecular weight (8.35 × 104) and low dispersion (1.18) under optimal experimental conditions.
In 2019, Angoy [12] had synthesized the amino functional phosphine ligands and then combined with rhodium metal to prepare several catalysts such as [Rh2(diene){μ-NH(CH2)3PPh2}2], and cationic phosphine-amions, [Rh(diene){Ph2P(CH2)3NHR}]+ (diene = cod, nbd or tfb) and [Rh{Ph2P(CH2)3NHR}2]+. The binuclear complexes bearing π-acceptor diene ligands (nbd or tfb) showed a significant catalytic activity in PA polymerization, with high molecular weight M w = 3.25 × 106 and medium dispersion index. The binuclear complexes had higher catalytic activity than the mononuclear catalysts, and they had different polymerization mechanisms. The molecular weight of the polymer could be raised by binuclear complexes, which would be reached 1.19 × 106 in a very short time (15 min). However, the catalysts without the diene ligands showed no activity in the polymerization of PA.
In 2021, Tan et al. [13] had prepared and synthesized catalysts [Rh(tfb)(biph)(PAr3)] (biph = biphenyl compounds). The structures and stability of the catalyst were proved by various testing methods (103Rh, 2D 31P-103Rh{1H} HMQC NMR and single-crystal X-ray). The molecular weight distribution and dispersibility of PPA under different polymerization conditions were observed to prove the catalytic activity and efficiency. The molecular weight of the polymer was up to 2.4 × 104, and the dispersion coefficient was mostly normal (Đ ≈ 1.12). Compared with different diene ligands (nbd or tfb), the binding ability and catalytic effect of them were significantly enhanced. In addition, the catalytic polymerization of different arylacetylene monomers and the mechanism of catalyst-mediated insertion polymerization of PA were also studied.
In addition to the aforementioned reports, there had been many studies of phosphine ligands and rhodium catalysts in the past. Jiménez et al. [14] prepared and synthesized a series of cationic complexes [Rh(diene){Ph2P(CH2)nZ}]+ [BF4]− containing functional phosphine ligands of the type Ph2P(CH2) n Z (n = 2 or 3; Z = OMe, NMe2 or SMe), and ligands were screened and optimized to obtain better catalytic activity for the polymerization of PA. Shiotsuki et al. [15] had synthesized a series of complexes [(tfb)Rh(L)]BPh4] (L = PPh3 or dppt). Multiple experiments screened out catalysts with higher activity by changing different reaction conditions and carried out the polymerization reaction of PA. Tan et al. [16] prepared (2-(naphthalen-2-yl)phenyl) rhodium(I) complex, which proposed the migration of metal atoms inside the molecule. The prepared rhodium catalyst could obtain PPA with a high degree of stereoregularity during polymerization. Pell and Ozerov [17] had prepared and synthesized a series of aryl/bis(phosphine/phosphinite) PCP or POCOP pincer–ligand complexes for study as potential catalysts for alkyne dimerization.
In this article, the novel catalysts, [Rh(cod)(TTP)Cl] (cod = 1,5-cyclooctadiene, tri(o-tolyl)phosphine (TTP)), [Rh(cod)(CDP)Cl] clohexyldiphenylphosphine (CDP), were synthesized and purified by recrystallization. The changes in molecular weight and yield of polymers when the two new catalysts are used for alkyne polymerization under different conditions were also discussed separately.
2 Materials and equipment
All solvents were dried using standard methods. RhCl3·3H2O, 1,5-cyclooctadiene (cod), TTP, CDP, and PA were purchased from Aladdin Reagent (Shanghai) Co, Ltd. The molecular structures of catalysts were determined by 1H NMR (600 MHz, Bruker, Germany). The molecular weight of polymers was measured by gel permeation chromatography (Polymer Laboratory, UK); the structure of the polymer was characterized by Fourier transform infrared spectrometer (FT-IR) (Spectrum Two, PE company, Waltham, Massachusetts, USA) and Thermal Gravimetric (TG) Analyzer (Diamond, PE company, USA).
2.1 Synthesis of [Rh(cod)(TTP)Cl]:
The solution of NaOH (0.06 mmol, 2.40 mg) in H2O (0.30 mL) was added to the solution of [Rh(cod)Cl]2 (0.03 mmol, 15.00 mg) in THF (1.00 mL) under N2 atmosphere. After being stirred at 20℃ for 10 min, the solution of TTP (0.12 mmol, 36.51 mg) in THF (1.00 mL) was added. The mixture was stirred for 4 h at 20°C and then concentrated to 2.00 mL and recrystallized under 4℃. After the mixture was filtered and dried, the product was obtained as a yellow powder (yield = 50.72%). 1H NMR (600 MHz, DMSO-d 6) δ (ppm) 7.31 (q, 12H, J = 6 Hz 4H PPh-4,5-H), 7.14 (t, 6H,PPh-3-H), 6.61 (d, 6H,PPPh-6-H), 4.46 (s, J = 12 Hz, 4H, ═CH 2), 2.43 (t, 4H, cod-CH 2 (in)), 2.31 (s, 18H,CH 3), 2.00 (d, 4H cod-CH 2 (out). 13C NMR 600 MHz, δ (ppm) 20.73 (–CH3), 30.18 (cod-CH2), 126.35 (Ph-5-C), 129 (Ph-3,4-C), 130.26 (Ph-1-C),132.3 (Ph-2-C),133.73 (═CH4), 141.81 (Ph-5-C)).
2.2 Synthesis of [Rh(cod)(CDP)Cl]
It was prepared by the similar method as that of [Rh(cod)(TTP)Cl]. The only difference was that the solvent THF was changed to acetone (yield = 43.63%). 1H NMR 600 MHz, δ (ppm) 7.55 (d, 4H, PPh-2-H), 7.43 (q, 6H, PPh-3,4-H), 5.20 (m, J = 18 Hz, 4H, ═CH 2), 3.03 (t, J = 6 Hz, 4H, cod-CH 2 (in)), 2.63 (t, J = 12 Hz 4H,cod-CH 2 (out)), 2.30 (m 4H,PPh-10-H), 2.11 (t 4H, PPh-8-H), 1.32 (m, 2H, PPh-9-H), 1.15 (m, 1H, PPh-10-H), 13C NMR 600 MHz, δ (ppm) 24.08 (cyclohexane-1-C), 26.22 (cyclohexane-3-C), 27.24 (cyclohexane-2-C), 27.98 (cyclohexane-4-C) 30.16 (cod-CH2), 128.65 (Ph-3-C) 128.88 (Ph-4-C), 130.36 (Ph-1-C), 131.04 (═CH4), 134.07 (Ph-2-C)).
2.3 Polymerization of PA
The PA solution (1.05 mL, 9.56 mmol) was added to the THF solution of the [Rh(cod)(CDP)Cl] (0.24 mg, 0.48 µmol) or [Rh(cod)(TTP)Cl] (0.39 mg, 0.48 µmol) reacted for 3 h under the inert atmosphere at 20°C. The polymer produced precipitates in a large amount in the methanol solution; the sediment was separated and finally dried to constant weight in vacuum.
3 Results and discussion
Rhodium catalysts, [Rh(cod)(TTP)Cl] and [Rh(cod)(CDP)Cl], were successfully synthesized in this study. 1H NMR spectrum of [Rh(cod)(TTP)Cl] is shown in Figure 1. Due to the influence of –CH2– conformation, the resonance frequency of the atomic nucleus was different, and the density of the electron cloud around the hydrogen nucleus was reduced. And the effect of Rh coordination bond on the attraction and repulsion of two hydrogen atoms led to two splitting peaks (b1 and b2) in different directions (Schemes 1 and 2).
![Figure 1
1H NMR spectrum of [Rh(cod)(TTP)Cl] in DMSO-d
6.](/document/doi/10.1515/hc-2022-0163/asset/graphic/j_hc-2022-0163_fig_001.jpg)
1H NMR spectrum of [Rh(cod)(TTP)Cl] in DMSO-d 6.

Synthesis of rhodium catalysts.

Polymerization of PA.
1H NMR spectrum of [Rh(cod)(CDP)Cl] is shown in Figure 2. Cyclohexane had two different conformations: the boat conformation and the chair conformation. Among them, the chair conformation was relatively stable and cross-type, which contains a small torsional tension. Moreover, the C–H bond of the upright type was not completely parallel, but it was outward, and it had an angle with the sp3 hybrid orbital. The substituents had a great influence on the balance between the cyclohexane chairs. The vertical and horizontal bonds in the chair conformation directly transition to each other. Therefore, at different rotational speeds, the position and shape of the spectral peak will be affected, and the i hydrogen spectrum would appear.
![Figure 2
1H NMR spectrum of [Rh(cod)(CDP)Cl] in DMSO-d
6.](/document/doi/10.1515/hc-2022-0163/asset/graphic/j_hc-2022-0163_fig_002.jpg)
1H NMR spectrum of [Rh(cod)(CDP)Cl] in DMSO-d 6.
13C NMR spectrum of [Rh(cod)(TTP)Cl] is shown in Figure 3. At 30.18 ppm for cod methylene carbon absorption peak, 133.73 ppm place for cod on the double bond CH═CH on carbon absorption peak, 20.73 ppm methyl absorption peak on benzene ring, and 126.35, 129.00, 130.26, 133.73, and 141.81 ppm are the absorption peaks of the benzene ring.
![Figure 3
13C NMR spectrum of [Rh(cod)(TTP)Cl] in DMSO-d
6.](/document/doi/10.1515/hc-2022-0163/asset/graphic/j_hc-2022-0163_fig_003.jpg)
13C NMR spectrum of [Rh(cod)(TTP)Cl] in DMSO-d 6.
Figure 4 shows the mass spectrum of the [Rh(cod)(TTP)Cl], C29H31PRhCl ([M + Na]+). The theoretical value is 573.6356, and the actual molecular weight is 573.6312, which proves the successful synthesis product.
![Figure 4
Mass spectrum of [Rh(cod)(TTP)Cl].](/document/doi/10.1515/hc-2022-0163/asset/graphic/j_hc-2022-0163_fig_004.jpg)
Mass spectrum of [Rh(cod)(TTP)Cl].
13C NMR spectrum of [Rh(cod)(CDP)Cl] is shown in Figure 5. At 30.16 ppm for cod methylene carbon absorption peak, 131.10 ppm place for cod on the double bond CH═CH on carbon absorption peak, and 24.08, 26.22, 2.24, and 27.98 ppm are the absorption peaks of cyclohexyl; 128.65, 128.88, 130.36, and 134.07 ppm are the absorption peaks of the benzene ring.
![Figure 5
13C NMR spectrum of [Rh(cod)(CDP)Cl] in DMSO-d
6.](/document/doi/10.1515/hc-2022-0163/asset/graphic/j_hc-2022-0163_fig_005.jpg)
13C NMR spectrum of [Rh(cod)(CDP)Cl] in DMSO-d 6.
Figure 6 shows the mass spectrum of the [Rh(cod)(CDP)Cl], C26H32PRhCl ([M + Na]+). The theoretical value is 573.0961, and the actual molecular weight is 37.3655, which proves the successful synthesis product.
![Figure 6
Mass spectrum of [Rh(cod)(CDP)Cl].](/document/doi/10.1515/hc-2022-0163/asset/graphic/j_hc-2022-0163_fig_006.jpg)
Mass spectrum of [Rh(cod)(CDP)Cl].
3.1 Polymerization of PA
After determining the chemical structure of the catalyst, the influence of reaction conditions on the yield and molecular weight was further studied. The reaction time and solvent also had some influence on the reaction process (Table 1). The optimal reaction conditions were selected by adjusting the time and solvent.
Polymerization of PA by using different catalyzing conditionsa
| No. | Catalyst | Solvent | Time (h) | M w (×105)b | PD | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | [Rh(cod)(TTP)Cl] | THF | 2 | 1.71 | 1.94 | 77.5 |
| 2 | 3 | 2.40 | 1.31 | 84.9 | ||
| 3 | 4 | 1.10 | 2.46 | 89.2 | ||
| 4 | DMAC | 2 | 1.11 | 1.56 | 77.1 | |
| 5 | 3 | 1.30 | 1.30 | 77.7 | ||
| 6 | 4 | 1.13 | 1.56 | 87.5 | ||
| 7 | Acetone | 2 | 1.28 | 1.74 | 75.7 | |
| 8 | 3 | 1.34 | 1.37 | 78.0 | ||
| 9 | 4 | 1.13 | 1.51 | 79.1 | ||
| 10 | Toluene | 2 | 1.14 | 1.56 | 77.1 | |
| 11 | 3 | 1.37 | 1.54 | 87.1 | ||
| 12 | 4 | 1.26 | 1.74 | 87.7 | ||
| 13 | [Rh(cod)(CDP)Cl] | THF | 2 | 1.62 | 1.65 | 73.3 |
| 14 | 3 | 1.73 | 1.45 | 74.6 | ||
| 15 | 4 | 1.64 | 2.10 | 74.8 | ||
| 16 | DMAC | 2 | 1.07 | 1.64 | 77.8 | |
| 17 | 3 | 1.46 | 1.43 | 79.2 | ||
| 18 | 4 | 1.31 | 1.53 | 80.1 | ||
| 19 | Acetone | 2 | 1.30 | 1.64 | 74.9 | |
| 20 | 3 | 1.37 | 1.59 | 84.6 | ||
| 21 | 4 | 1.08 | 1.77 | 85.1 | ||
| 22 | Toluene | 2 | 1.06 | 1.78 | 70.8 | |
| 23 | 3 | 1.25 | 1.72 | 80.6 | ||
| 24 | 4 | 1.02 | 1.76 | 84.1 | ||
| 25 | [Rh(Dbcot)PPh3]Clc | Toluene | 1 | 0.12 | 2.79 | 9.00 |
| 26 | THF | 0.07 | 1.83 | 49.0 |
a[PA] = 0.008 mmol/L, [Rh]/[PA] = 1:20,000, at 20°C.
bDetermined by gel permeation chromatography (GPC) in THF.
cDATA from the study of Zhang et al. [8].
As can be seen from Table 1, the yield gradually increased with the increase in reaction time in any reaction solvent. When the reaction time was about 2 h, the yield did not reach a high level. It means that the reaction was carried out incomplete. In contrast, when the reaction time was 3 h, the molecular weight of the polymer reached its highest and the dispersion was normal. At 4 h of polymerization, the molecular weight of the polymer decreases, because the extension of reaction time in polymerization will lead to the termination and fracture of the chain reaction, resulting in the reduction of the number of monomers in the system, slowing down the polymerization rate and increasing the decomposition rate, thus affecting the molecular weight of the polymer.
In a previous study, Zhang et al. [8] prepared a rhodium catalyst [Rh(Dbcot)PPh3]Cl containing a phosphine ligand. With the reaction conditions of a reaction time of 1 h, a temperature of 25°C, and the solvent as THF and toluene, the highest yield obtained was 49.0%. The molecular weight of the polymer was M w = 0.12 × 105 in the solvent of toluene. Compared to the previous study [8], the new catalysts [Rh(cod)(TTP)Cl] and [Rh(CDP)(cod)Cl], which were prepared by us, had exhibited a significant reaction activity and achieved higher yields (89.2%) and molecular weights (M w = 2.40 × 105).
In order to optimize the polymerization conditions, the catalyst catalyzed the polymerization of PA at different temperatures (Table 2). A comparison of the molecular weight of the products obtained at three different reaction temperatures in Table 2 shows that the polymers have the highest molecular weight at 20°C (No. 2 and No. 5 in Table 2). Their molecular weights were about 2.35 × 105 and 1.73 × 105, respectively, corresponding to dispersion coefficients of about 1.31 and 1.45. In terms of molecular weight, at 0°C, the energy of the system was low, and the chain reaction was incomplete; the resulting polymer had a lower molecular weight. When the temperature was 20°C, the activity of the catalyst reached the best, the stability of the free radicals dissociated by the molecules in the system was high, and the molecular weight of the polymer reached the highest. As the temperature continued to rise, the obtained polymer had an increased carbon content and a decreased hydrogen content, and the molecular structure of the polymer undergoes significant changes, with the original chain structure gradually transforming into a ring structure. At the same time, the polymer molecules may also undergo partial cleavage, forming small molecules. Eventually, this leads to a decrease in the molecular weight of the polymer. In addition, the yield increased with the increase in temperature (No. 3 and 6 in Table 2). The maximum yields of PPA were both about 91.4 and 84.6%.
Polymerization of PA by different rhodium catalysts in different temperaturesa
| No. | Catalyst | Temperature (°C) | M w (×105)b | PD | Yield (%) |
|---|---|---|---|---|---|
| 1 | [Rh(cod)(TTP)Cl] | 0 | 1.31 | 1.41 | 84.4 |
| 2 | 20 | 2.35 | 1.31 | 84.9 | |
| 3 | 35 | 1.21 | 1.42 | 91.4 | |
| 4 | [Rh(cod)(CDP)Cl] | 0 | 0.86 | 1.54 | 71.0 |
| 5 | 20 | 1.73 | 1.45 | 74.6 | |
| 6 | 35 | 0.66 | 2.18 | 84.6 |
a[PA] = 0.008 mmol/L, [Rh]/[PA] = 1:20,000, in THF for 3 h.
bDetermined by GPC in THF.
3.2 Characterization of PPA
In order to facilitate the characterization of the obtained prepared PPAs, we used the molecular weight size as a screening criterion and selected the PPA with the largest molecular weight for the relevant tests.
According to the infrared spectrum of Figure 7, it can be seen that the C≡C of benzene acetylene (PA) had a very obvious absorption peak at about 2,100 cm−1, the infrared spectrum of the polymer PPA showed that the C≡C absorption peak disappeared obviously, and the absorption peak appeared at the position of 2,500–3,000 and 500–1,500 cm−1, indicating that the obtained polymer was cis–trans structure.

FT-IR spectrum of PA and PPA.
In order to explore the thermal stability of the polymer, TG analysis in N2 gas environment was also used to analyze and determine the stability of the polymer, as shown in Figure 8. We can see that the decomposition occurred at 100°C, with a loss of about 3%, representing the evaporation of water from the PPA. At 100–300°C, PPA almost did not decompose and the mass loss was very small. When the temperature was higher than 300°C, PPA was decomposed obviously. As the temperature increased, the weight loss of the polymer accelerated, because PPA contained a large number of C═C, C═C was easy to crack at high temperature, molecular chain would move, polymer internal structure would be changed, so there was a continuous weight loss phenomenon. When the temperature rose to 450°C, the weight loss of the polymer tends to flatten out, and PPA was basically completely decomposed. The aforementioned results show that PPA polymer had good thermal stability.

TG curve of the PPA.
4 Exploration of the reaction mechanism
This article also explores the possible mechanisms for the application of rhodium catalysts in the polymerization reaction of PA, as shown in Figure 5. In the process of polymerization, 2 was formed by the rhodium atom in 1 coordinate with one PA molecule. Then, the PA in 2 was restructured and produced a carbon-free radical as 3. Due to a PA replacing the phosphine ligand in 3 to obtain 4, the carbon-free radical in 4 attacked the coordination bonds on concurrently as 5. The two PA molecules were inserted and polymerized into the interior. In addition, α-C· was reserved as 6. Similarly, as the PA was joined in the reaction system on until the activity of carbon radical was not to support subsequent system reaction, until the PPA molecules emerge from chemical reactions (Figure 9).
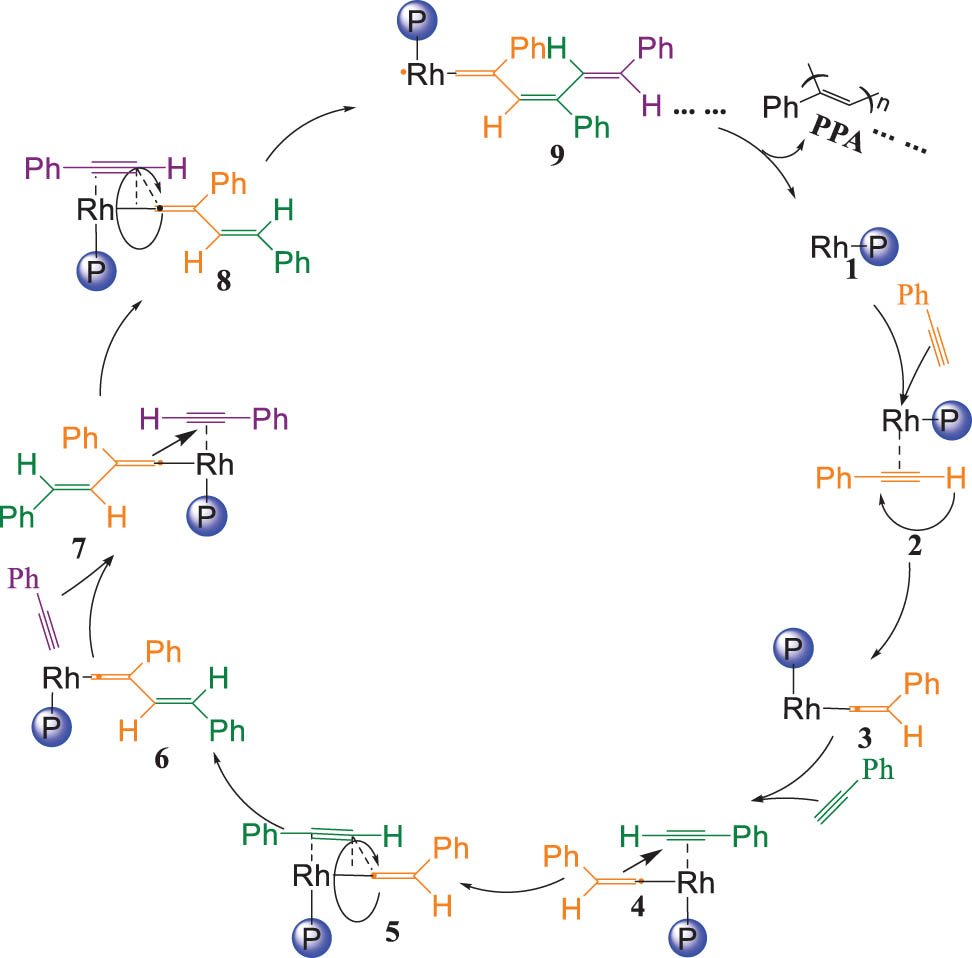
Plausible mechanism for the polymerization of PA by catalysts.
5 Conclusion
In conclusion, two novel catalysts had been successfully prepared in this study, which could be used in the polymerization of PA. And the effects of different solvents, time, and temperature on the molecular weight and yield of the polymerization reaction products were discussed in the subsequent catalytic reactions. The results showed that from the perspective of molecular weight of polymer, the optimal reaction condition of the two catalysts was reaction at 20°C for 3 h in THF solvent (molecular weight up to 2.40 × 105). From the point of view of yield, the best reaction conditions for both catalysts were 4 h at 35°C in THF solvent (yield up to 89.2%).
-
Funding information: This work was supported by National Natural Science Foundation of China (52203093), Scientific Research Project of Provincial University, Education Department of Heilongjiang Province, China (CLKFKT2021B12) and (135309350).
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on a reasonable request.
References
[1] Sanda M, Shiotsuki M, Masuda T. Controlled polymerization of phenylacetylenes using well-defined rhodium catalysts. Ion Polymerization Part II. 2015;350:67–75.10.1002/masy.201400026Search in Google Scholar
[2] Chen J, Cai S, Wang R, Wang S, Zhang J, Wan X. Polymerization-induced self-assembly of conjugated block copoly(phenylacetylene)s. Macromolecules. 2020;53:1638–44.10.1021/acs.macromol.9b02504Search in Google Scholar
[3] Onishi N, Shiotsuki M, Masuda T, Sano N, Sanda F. Polymerization of phenylacetylenes using rhodium catalysts coordinated by norbornadiene linked to a phosphino or amino group. Organometallics. 2013;32:846–53.10.1021/om301147nSearch in Google Scholar
[4] Angoy M, Victoria Jiménez M, Javier Modrego F, Luis AO, Vincenzo P, Jesús J, et al. Mechanistic investigation on the polymerization of phenylacetylene by 2-diphenylphosphinopyridine rhodium(i) catalysts: Understanding the role of the cocatalyst and alkynyl intermediates. Organometallics. 2018;37:2778–94.10.1021/acs.organomet.8b00430Search in Google Scholar
[5] Tan N, Nealon G, Turner G, Moggach S, Ogden M, Massi M, et al. Rh(I)(2,5-norbornadiene)(biphenyl)(tris(4-fluorophenyl)phosphine): Synthesis, characterization, and application as an initiator in the stereoregular (Co)polymerization of phenylacetylenes. ACS Macro Lett. 2020;9:56–60.10.1021/acsmacrolett.9b00975Search in Google Scholar PubMed
[6] Misumi Y, Kanki K, Miyake M, Masuda T. Living polymerization of phenylacetylene by rhodium-based ternary catalysts, (diene) Rh (I) complex/vinyllithium/phosphorus ligand. Effects of catalyst components. Macromol Chem & Phys. 2000;201:2239–44.10.1002/1521-3935(20001101)201:17<2239::AID-MACP2239>3.0.CO;2-PSearch in Google Scholar
[7] Kai H, Tabata M, Mawatari Y, Miyasaka A, Ishii K. Origin of the color of piconjugated columnar polymers: Poly(p-n-octylthiophenyl)acetylene prepared using a [Rh(norbornadiene)Cl]2 catalyst. J Polym Sci Part A Polym Chem. 2010;43:2836–50.10.1002/pola.20751Search in Google Scholar
[8] Zhang P, Wang H, Shi X, Yan X, Wu X, Zhang S, et al. On-water polymerization of phenylacetylene catalyzed by Rh complexes bearing strong π-acidic dibenzo [a,e] cyclooctatetraene ligand. J Polym Sci A Polym Chem. 2017;55:716–25.10.1002/pola.28417Search in Google Scholar
[9] Onitsuka K, Yamamoto M, Mori T, Takei F, Takahashi S. Living polymerization of Bulky aryl isocyanide with arylrhodium compexes. Organometallics. 2006;25:1270–8.10.1021/om0509692Search in Google Scholar
[10] Gil W, Trzeciak AM, Ziółkowski JJ. Catalytic polymerization of phenylacetylene with dimeric [Rh(OMe)(cod)]2 complex in ionic liquids. Appl Organomet Chem. 2006;20:776–0.10.1002/aoc.1138Search in Google Scholar
[11] Tan N, Simpson P, Nealon G, Sobolev A, Raiteri P, Massi M, et al. Rhodium(I)-α-phenylvinylfluorenyl complexes: Synthesis, characterization, and evaluation as initiators in the stereospecific polymerization of phenylacetylene. Eur J Inorg Chem. 2019;592–601.10.1002/ejic.201801411Search in Google Scholar
[12] Angoy M, Jimenez MV, Orduna PG, Oro LA, Vispe E, Perez-Torrente JJ. Dinuclear phosphine-amido [Rh2(diene){μ-NH(CH2)3PPh2}2] complexes as efficient catalyst precursors for phenylacetylene polymerization. Organometallics. 2019;38:1991–2006.10.1021/acs.organomet.9b00078Search in Google Scholar
[13] Tan N, Nealon G, Moggach S, Lynam J, Ogden M, Massi M, et al. 4-Tetrafluorobenzobarrelene)-η1-((tri-4-fluorophenyl)phosphine)-η1-(2-phenylphenyl) rhodium(i): A catalyst for the living polymerization of phenylacetylenes. Macromolecules. 2021;54:6191–6203.10.1021/acs.macromol.1c00906Search in Google Scholar
[14] Jiménez M, Pérez-Torrente J, Bartolomé M, Vispe E, Lahoz F, Oro L. Cationic rhodium complexes with hemilabile phosphine ligands as polymerization catalyst for high molecular weight stereoregular poly(phenylacetylene). Macromolecules. 2009;42:8146–56.10.1021/ma901549gSearch in Google Scholar
[15] Shiotsuki M, Onishi N, Sanda F, Masuda T. Living polymerization of phenylacetylenes catalyzed by cationic rhodium complexes bearing tetrafluorobenzobarrelene. Polym J. 2011;43:51–7.10.1038/pj.2010.98Search in Google Scholar
[16] Tan N, Nealon G, Lynam J, Sobolev A, Rowles M, Ogden M, et al. A (2-(naphthalen-2-yl)phenyl)rhodium(i) complex formed by a proposed intramolecular 1,4-ortho-to-ortho Rh metal-atom migration and its efficacy as an initiator in the controlled stereospecific polymerisation of phenylacetylene. Dalton Trans. 2019;48:16437–47.10.1039/C9DT02953BSearch in Google Scholar
[17] Pell C, Ozerov O. A series of pincer-ligated rhodium complexes as catalysts for the dimerization of terminal alkynes. ACS Catal. 2014;4:3470–80.10.1021/cs5009317Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Synthesis, characterization, and antibacterial activity of a new poly azo compound containing N-arylsuccinimid and dibenzobarrelene moieties
- Design, synthesis, and antiviral activities evaluation of novel quinazoline derivatives containing sulfonamide moiety
- Design, synthesis, and anticancer activity of novel 4,6-dimorpholinyl-1,3,5-triazine compounds
- Design, synthesis, biological evaluation, and bio-computational modeling of imidazo, thieno, pyrimidopyrimidine, pyrimidodiazepene, and motifs as antimicrobial agents
- Synthesis of a novel phosphate-containing ligand rhodium catalyst and exploration of its optimal reaction conditions and mechanism for the polymerization of phenylacetylene
- Design, synthesis, and antiproliferative activity of novel 1,2,4-triazole-chalcone compounds
- Synthesis of metal–organic nanofiber/rGO nanocomposite as the sensing element for electrochemical determination of hypoxanthine
- Design and synthesis of various 1,3,4-oxadiazoles as AChE and LOX enzyme inhibitors
- Bis(2-cyanoacetohydrazide) as precursors for synthesis of novel azoles/azines and their biological evaluation
- Synthesis, characterization, and biological target prediction of novel 1,3-dithiolo[4,5-b]quinoxaline and thiazolo[4,5-b]quinoxaline derivatives
- Sustainable conversion of carbon dioxide into novel 5-aryldiazenyl-1,2,4-triazol-3-ones using Fe3O4@SP-vanillin-TGA nanocomposite
- Erratum
- Erratum to “Design, synthesis and study of antibacterial and antitubercular activity of quinoline hydrazone hybrids”
- SI: Undergraduate Research in the Synthesis of Biologically Active Small Molecules and Their Applications
- Preparation of novel acyl pyrazoles and triazoles by means of oxidative functionalization reactions
- Synthesis and conformational analysis of N-BOC-protected-3,5-bis(arylidene)-4-piperidone EF-24 analogs as anti-cancer agents
- SI: Development of Heterocycles for Biomedical and Bioanalytical Applications
- Influence of octreotide on apoptosis and metabolome expression in lipopolysaccharide-induced A549 cells
- Crude extract of J1 fermentation promotes apoptosis of cervical cancer cells
Articles in the same Issue
- Research Articles
- Synthesis, characterization, and antibacterial activity of a new poly azo compound containing N-arylsuccinimid and dibenzobarrelene moieties
- Design, synthesis, and antiviral activities evaluation of novel quinazoline derivatives containing sulfonamide moiety
- Design, synthesis, and anticancer activity of novel 4,6-dimorpholinyl-1,3,5-triazine compounds
- Design, synthesis, biological evaluation, and bio-computational modeling of imidazo, thieno, pyrimidopyrimidine, pyrimidodiazepene, and motifs as antimicrobial agents
- Synthesis of a novel phosphate-containing ligand rhodium catalyst and exploration of its optimal reaction conditions and mechanism for the polymerization of phenylacetylene
- Design, synthesis, and antiproliferative activity of novel 1,2,4-triazole-chalcone compounds
- Synthesis of metal–organic nanofiber/rGO nanocomposite as the sensing element for electrochemical determination of hypoxanthine
- Design and synthesis of various 1,3,4-oxadiazoles as AChE and LOX enzyme inhibitors
- Bis(2-cyanoacetohydrazide) as precursors for synthesis of novel azoles/azines and their biological evaluation
- Synthesis, characterization, and biological target prediction of novel 1,3-dithiolo[4,5-b]quinoxaline and thiazolo[4,5-b]quinoxaline derivatives
- Sustainable conversion of carbon dioxide into novel 5-aryldiazenyl-1,2,4-triazol-3-ones using Fe3O4@SP-vanillin-TGA nanocomposite
- Erratum
- Erratum to “Design, synthesis and study of antibacterial and antitubercular activity of quinoline hydrazone hybrids”
- SI: Undergraduate Research in the Synthesis of Biologically Active Small Molecules and Their Applications
- Preparation of novel acyl pyrazoles and triazoles by means of oxidative functionalization reactions
- Synthesis and conformational analysis of N-BOC-protected-3,5-bis(arylidene)-4-piperidone EF-24 analogs as anti-cancer agents
- SI: Development of Heterocycles for Biomedical and Bioanalytical Applications
- Influence of octreotide on apoptosis and metabolome expression in lipopolysaccharide-induced A549 cells
- Crude extract of J1 fermentation promotes apoptosis of cervical cancer cells

