Synthesis and conformational analysis of N-BOC-protected-3,5-bis(arylidene)-4-piperidone EF-24 analogs as anti-cancer agents
-
Robert B. Smith
Abstract
Natural products and their analogs have been explored to stop the progression of cancer cells without unwanted side effects. Chalcones, compounds synthesized naturally in plants, have demonstrated potential as anti-cancer treatments. A library of bis-chalcones that are similar in structure to EF-24, a bis-chalcone molecule known to have anti-cancer properties, has been synthesized in order to examine their medicinal properties. This report highlights the synthesis of ten novel analogs, their anti-cancer activities, and conformational analysis via cryo-NMR and corroboration by density functional theory calculations of the lead compound 1b, tert-butyl 3,5-bis((E)-4-methylbenzylidene)-4-oxopiperidine-1-carboxylate.
1 Introduction
In recent years, the use of natural products and their derivatives as methods to treat diseases has grown in popularity [1]. Of the 185 small molecule anti-cancer drugs approved by the United States Food and Drug Administration (FDA) from 1981 to 2019, greater than 84% were either natural products or derived from natural products, with only two fully synthetic drugs approved in the last 3 years [2]. For example, Taxol, an anti-cancer drug used to treat ovarian, breast, prostate, bladder, and non-small cell lung cancers (NSCLCs), is one of the most widely used anti-cancer drugs and is derived from the Pacific Yew (Taxus brevifolia) [3]. Curcumin (Figure 1), from the spice turmeric (Curcuma longa), has been studied extensively to determine its ability to inhibit cancer cell growth. The origin of curcumin use has its roots in ancient Indian and Chinese medicine and it is still in use today by many cultures in Eastern Asia to treat conditions such as acne, gastric ulcers, and the hallucinatory effects of psychotropic drugs [4]. According to Hatcher et al., curcumin is not used as frequently in Western medicine for these purposes, but it has been investigated as a chemotherapy alternative due to its ability to inhibit the progression and growth of cancer cells. Many animal studies have shown anti-cancer activity of curcumin, specifically in treating colon, stomach, esophageal, and oral cancer [5]. The FDA has labeled curcumin “Generally Recognized As Safe” and clinical trials have shown that the human body can tolerate it in doses ranging from 4,000 up to 8,000 mg/day. Several clinical trials for curcumin as an anti-cancer therapy have demonstrated increased patient quality of life, tumor shrinkage, decrease of oncogenic markers, and increased pro-apoptotic markers in tumors [6].
One of the main drawbacks to curcumin as a chemotherapeutic agent is poor bioavailability [7]. EF-24 (Figure 1), an analog of curcumin, was developed in an attempt to improve its bioavailability [8]. While EF-24 is considered a curcumin analog, the two compounds have distinct mechanisms of action. Curcumin has no demonstrated effect on microtubule stabilization, whereas EF-24 does [9]. A recent review by He et al. summarized the literature on EF-24 and concluded that: (1) EF-24 could effectively inhibit the growth of breast cancer tumors with little to no toxicity, which immediately showed its promise as a better alternative to curcumin in mice; (2) EF-24 may have a strong effect on multiple types of cancer including, breast, ovarian, colon, and pancreatic cancer; and (3) these effects can also be enhanced when used in tandem with other drugs and therapeutic agents [10]. EF-24 analogs have also displayed a broad range of other bioactivities, including anticholinesterase, anti-inflammatory, antifungal, antimalarial, and anti-obesity [11].
Several studies have demonstrated that the addition of substituents on the nitrogen can increase the potency of EF-24 analogs (Figure 1). For example, Dimmock et al. synthesized N-acryloyl analogs [12] and Ocasio-Malavé et al. recently reported N-BOC-3,5-bis(arylidene)-4-piperidones with methoxy and trifluoromethyl substituents on the ring [13]. Dimmock et al. also reported interesting intramolecular hydrogen bonding-like interactions with the methylene groups on the piperidone ring with the carbonyl of the acryloyl group. The aim of the current study was to see whether this effect would also be seen in N-BOC derivatives of EF-24, and if these complexes would be biologically active.
2 Results and discussion
2.1 Synthesis
Compounds 1a–j were synthesized through the Aldol Condensation of tert-butyl 4-oxopiperidine-1-carboxylate with two equivalents of commercially available aldehyde Scheme 1). The reaction was considered complete (typically within an hour) when a yellow precipitate formed, which was then filtered and characterized by 1H NMR. The identity of the product was confirmed by 1H NMR primarily through the appearance of a deshielded singlet at δ ∼ 7.5–8.0 ppm, which is the β-hydrogen in the α-β unsaturated system. This chemical shift is indicative of the trans enone, compared to the lower chemical shift of the cis enone [14]. If this peak was present, along with the absence of the aldehyde starting material peak δ ∼10 ppm, the reaction was considered successful. If impurities were detected by 1H NMR, the compound was recrystallized from methanol and H2O and analyzed by NMR again. This process was repeated until the compound was deemed pure. Upon confirmation of the formation of the product and its purity, 13C NMR, high-resolution mass spectrometry (HRMS), and melting point were performed (Table 1). Attempts at growing single crystals of these compounds for X-ray crystallography have proven unsuccessful.

Aldol Condensation to produce symmetrical N-BOC-protected-3,5-bis(arylidene)-4-piperidones.
Experimental and calculated properties of compounds and controls
| Number | Formula | Melting point (℃) | Yield (%) | Docking score (kcal/mol) |
|---|---|---|---|---|
| 1a | C28H34NO3 | 125–131 | 61 | −8.4 |
| 1b | C26H30NO3 | 184–189 | 68 | −9.6 |
| 1c | C26H30NO3 | 126–130 | 50 | −8.8 |
| 1d | C26H30NO3 | 122–126 | 12 | −8.9 |
| 1e | C20H22NO3S2 | 185–188 | 83 | −7.9 |
| 1f | C28H34NO3 | 133–143 | 54 | −9.1 |
| 1g | C28H34NO3 | 200–210 | 75 | −8.7 |
| 1h | C20H22NO5 | 171–176 | 69 | −8.2 |
| 1i | C20H22NO5 | 139–142 | 34 | −8.1 |
| 1j | C24H30NO3F2 | 141–145 | 17 | −10.3 |
| Nocodazole | −7.8 | |||
| EF24 | −9.0 |
2.2 Conformational analysis via dynamic NMR
The room temperature NMR characterization of the analogs displayed peak broadening for the methylene hydrogens of the piperidone ring in the 1H spectra (δ = 4.62 ppm, 4H). Heating the samples to 50 oC caused the peak to narrow, indicating that there were multiple conformations of the compounds in solution. A similar effect was seen in the 13C NMR, with the peaks on the alpha carbons being quite broad at room temperature. Thus, cryo-NMR was performed on lead compound 1b (Figure 2). The methylene hydrogens peak split into two well-resolved singlets at δ = 4.806 and 4.719 ppm (∆ν = 34.8 Hz, with a 1:1 ratio at coalescence temperature 298 K). In addition, the methyl hydrogens also began to separate at lower temperatures, which corresponds with X-ray data of EF24 that the two rings are not perfectly symmetrical, but are at a twisted angle and the piperidone ring adopts a sofa conformation [15]. The approximate rate constant, k, of the interconversion of the two conformations of 1b was calculated from the Gutowsky-Holm equation (equation (1)) which is valid for two states with equal populations, which is seen in the case of the two methylenes in 1b [16]. The free energy of activation for the interconversion was estimated from Eyring equation (2) to be 14.87 kcal/mol [17].
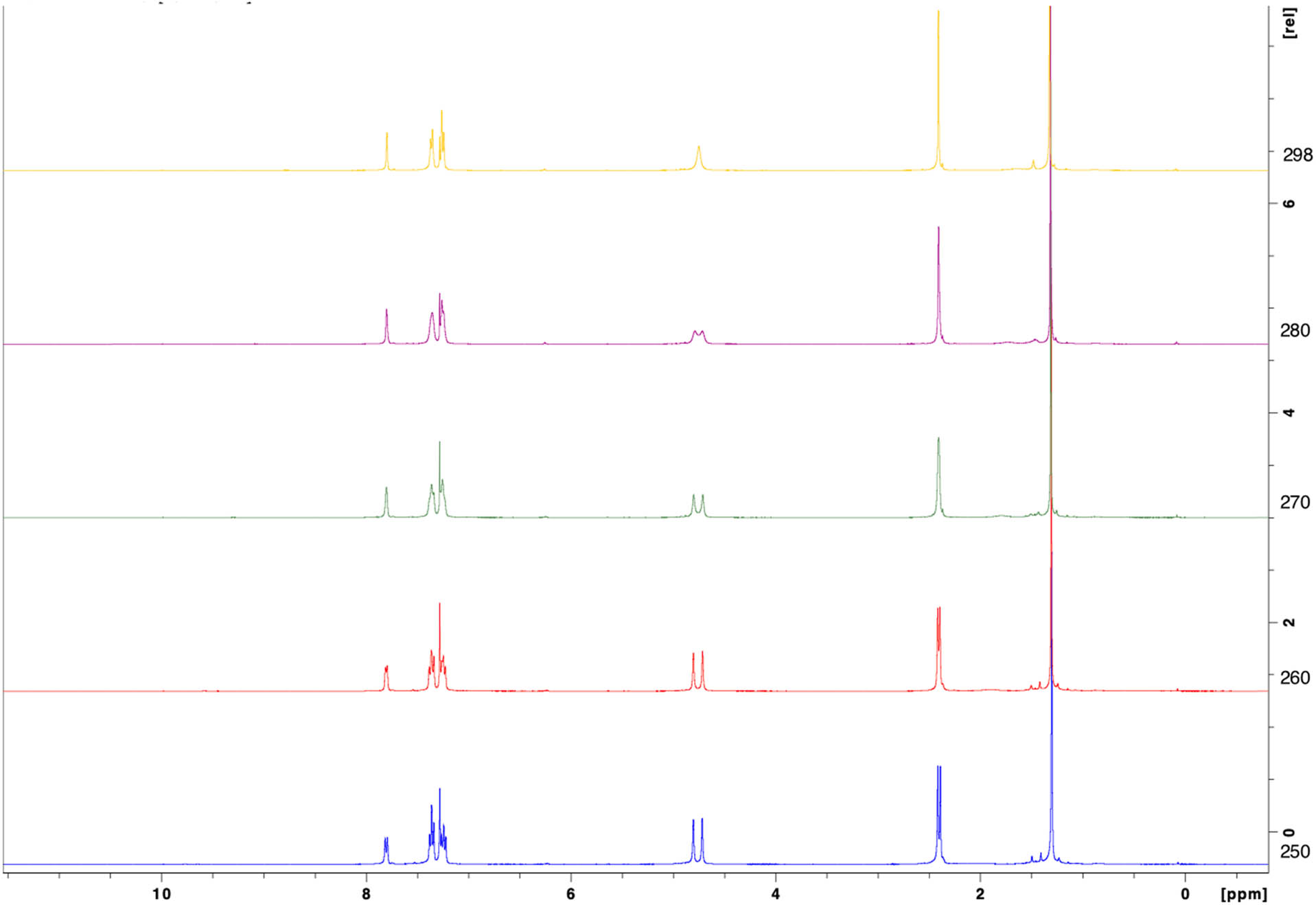
Temperature-dependent 1H NMR array of 1b indicating two conformations at lower temperature. Temperature is decreasing down the y axis.
2.3 Density functional theory (DFT) calculations of conformations
In order to better understand the structure of 1b and the process by which the methylene signals undergo exchange in solution, DFT calculations were carried out. The geometry of 1b was calculated at the MN15-L/def2-TZVP level of theory [18] with chloroform solvent modeled with a polarized solvent continuum model. The optimized geometry of 1b and the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) are shown in Figure 3. The HOMO is delocalized over the benzene rings, the alkene C═C π bonds, and the nitrogen atom. The LUMO is also delocalized onto the benzene rings, but is primarily centered on the carbonyl and the monosubstituted alkene carbons.

DFT-optimized geometry of 1b shown from two different angles, and the HOMO and LUMO shown at an isovalue of 0.04.
Given that the rotation of the BOC group was suspected to be involved in the changes observed in variable temperature NMR, a scan at the MN15-L/def2-SV(P) level of theory was performed. The highest energy state of the scan was used as an input for a MN15-L/def2-TZVP calculation of the transition state, which was optimized to the geometry shown in Figure 4. A single negative frequency was found (−40.57 cm−1), corresponding to the rotation of the BOC group, confirming that the geometry was a transition state. The transition state was calculated to be 15.6 kcal/mol higher in energy than 1b, in good agreement with the value calculated from the NMR experiments.

Transition state geometry found the rotation of the BOC group.
2.4 Biology
2.4.1 Single-dose assay
Ten compounds (1a–j) were screened by the National Cancer Institute (NCI) against 60 cell lines. The inhibition growth percent results obtained from testing the ten compounds against the different types of cancer are shown in Tables 2–10. A positive number greater than 100 indicates that there was some amount of growth promotion (undesirable), a positive number less than 100 indicates that there was some amount of growth inhibition (desirable), and a negative number indicates that the compound is lethal (undesirable). Inhibition is preferred over lethality because lethality may mean that normal cells could also be harmed in the process of killing the cancer cells.
NCI results from each compound tested against leukemia cell lines
 |
Growth promotion is highlighted in yellow, cytotoxicity is highlighted in red, growth inhibition of 50% or more is highlighted in cyan. ND indicates the compounds were not tested against those cell lines. The most potent compound for each cell line is bolded and italicized.
NCI results from each compound tested against NSCLC cell lines
 |
Growth promotion is highlighted in yellow, cytotoxicity is highlighted in red, growth inhibition of 50% or more is highlighted in cyan. The most potent compound for each cell line is bolded and italicized.
NCI results from each compound tested against colorectal cancer cell lines
 |
Growth promotion is highlighted in yellow, cytotoxicity is highlighted in red, growth inhibition of 50% or more is highlighted in cyan. The most potent compound for each cell line is bolded and italicized.
NCI results from each compound tested against central nervous system cancer cell lines
 |
Growth promotion is highlighted in yellow and growth inhibition of 50% or more is highlighted in cyan. ND indicates the compounds were not tested against those cell lines. The most potent compound for each cell line is bolded and italicized.
NCI results from each compound tested against melanoma cell lines
 |
Growth promotion is highlighted in yellow, cytotoxicity is highlighted in red, and growth inhibition of 50% or more is highlighted in cyan. ND indicates the compounds were not tested against those cell lines. The most potent compound for each cell line is bolded and italicized.
NCI results from each compound tested against ovarian cancer cell lines
 |
Growth promotion is highlighted in yellow, cytotoxicity is highlighted in red, growth inhibition of 50% or more is highlighted in cyan. The most potent compound for each cell line is bolded and italicized.
NCI results from each compound tested against renal cancer cell lines
 |
Growth promotion is highlighted in yellow, cytotoxicity is highlighted in red, growth inhibition of 50% or more is highlighted in cyan. ND indicates the compounds were not tested against those cell lines. The most potent compound for each cell line is bolded and italicized.
NCI results from each compound tested against prostate cancer cell lines
 |
Growth promotion is highlighted in yellow and growth inhibition of 50% or more is highlighted in cyan. The most potent compound for each cell line is bolded and italicized.
NCI results from each compound tested against breast cancer cell lines
 |
Growth promotion is highlighted in yellow and growth inhibition of 50% or more is highlighted in cyan. The most potent compound for each cell line is bolded and italicized.
2.4.1.1 Leukemia
The ten compounds were tested against six types of leukemia (Table 2). Compound 1e exhibits the lowest inhibition ability across all leukemia cell lines. Compounds 1j and 1h do not affect the growth of the cancer significantly. Compound 1g slightly promoted growth of all leukemia cell lines. Compounds 1b, 1c, and 1f significantly inhibited cell growth in all cell lines and were only mildly cytotoxic. Compound 1a was the most potent against RPMI-8226 (24.86% growth), compound 1b was the most potent against K-562 (3.47% growth), and compound 1c was the most potent against CCRF-CCM (2.95% growth). Compound 1f was the most potent compound against HL-60, MOLT-4, and SR (10.75, 7.40, and 0.12% growth, respectively), with its activity against SR being the most active of all compounds tested against all leukemia lines. Compounds 1i and 1d were active against most cell lines and compound 1a was slightly active against all cell lines tested. Generally, these compounds were cytotoxic to RPMI, and many were highly active against CCRF, K-562, and SR.
2.4.1.2 NSCLC
The ten compounds were tested against nine cell lines of NSCLC (Table 3). Compound 1c had the most inhibitory activity of all of the other compounds for every cell line except for HOP-92 and was only cytotoxic for cell line NCI-H460. Compound 1b showed very little inhibitory activity against any of the tested cell lines. Compounds 1a, 1d, and 1e showed very little inhibitory ability and slightly promoted growth in some cell lines. Compounds 1g, 1h, 1i, and 1j significantly promoted cell growth in almost all cell lines tested. Compound 1c showed the most inhibitory activity against cell lines A549/ATCC, EKVX, HOP-62, NCI-H226, NCI-H23, NCI-H322M, and NCI-H522 (49.11, 55.65, 48.22, 42.24, 8.22, 80.93, and 20.79% growth, respectively). Compound 1e showed the most growth inhibition for cell line HOP-92 (82.24% growth). Compound 1f showed the most growth inhibition for cell line NCI-H460 (45.31% growth). Cell growth in cell lines EKVX, HOP-92, and NCI-H322M was inhibited very little by every compound tested against them.
2.4.1.3 Colon cancer
The ten compounds were tested against seven different cell lines of colorectal cancer (Table 4). Compound 1c was the most active against all cell lines, but it was cytotoxic to all except for HCC-2998, HCT-15, and SW-620 (3.27, 1.66, and 5.37% growth, respectively). Compounds 1g and 1j significantly promoted cell growth in over half of the cell lines tested and had no notable level of inhibition. Compounds 1e and 1h showed little inhibitory activity. Compounds 1a and 1i showed very little inhibitory activity toward every cell line except for cell line HCT-116. Compound 1b showed the most inhibitory activity against cell line SW-620 (5.21% growth), compound 1c showed the most inhibitory activity against cell lines HCC-2988 and HCT-15 (3.27 and 1.66% growth, respectively), and compound 1d showed the most inhibitory activity against cell line HCT-116 (11.21% growth). Compound 1f showed the most inhibitory activity against cell lines COLO 205, HT29, and KM12 (30.89, 9.93, and 16.74% growth, respectively).
2.4.1.4 Central nervous system (CNS) cancer
The ten compounds were tested against six types of CNS cancer (Table 5). Compound 1c had the most inhibitory activity of all of the other tested compounds against cell lines SF-268, SF-295, SF-539, SNB-19, SNB-75, U251 (2.68, 24.57, 31.18, 39.06, 53.43, 0.09% growth, respectively). Compounds 1b and 1f showed significant inhibition ability against cell line U251 (12.37 and 15.88% growth, respectively). Compound 1c was the only compound to have a significant inhibitory effect on cell line SF-539; the other tested compounds either had little inhibitory effect or promoted cell growth. Cell line SF-539 was the cell line that experienced the most growth promotion. Cell line SNB-75 was affected very little by any of the compounds tested.
2.4.1.5 Melanoma
The ten compounds were tested against nine cell lines of melanoma (Table 6). Compound 1f was highly potent against all tested cell lines and was only cytotoxic to cell line MDA-MB-435. Compound 1c was the most potent against cell lines MDA-MB-435 and UACC-62 (12.04 and 28.67% growth, respectively), and compound 1f was the most potent against all of the others. Compound 1d was the only compound to show any inhibitory activity toward cell line LOX IMVI. Cell lines MALME-3M, SK-MEL-2, and UACC-257 were the least susceptible to growth inhibition. Cell line SK-MEL-28 was the most susceptible to growth promotion. Compound 1j had the weakest inhibition ability and promoted cell growth more than any other tested compound. Compounds 1a, 1e, 1g, 1h, and 1i were also weak inhibitors of every cell line. Compounds 1b, 1c, and 1f were the only compounds to exhibit any level of cytotoxicity.
2.4.1.6 Ovarian cancer
The compounds were tested against seven types of ovarian cancer (Table 7). Compound 1c had the strongest inhibitory ability out of all of the compounds tested, but was cytotoxic to cell lines OVCAR-3 and OVCAR-4. Compound 1f had a slightly stronger inhibitory ability than compound 1c but was not cytotoxic. Compound 1j had the least inhibitory activity of any other compound and promoted cell growth in every cell line except for cell line IGROV1. Compound 1b showed the most inhibitory activity toward cell line OVCAR-3 (7.18% growth). Compound 1c showed strong inhibitory activity against cell lines IGROV1, OVCAR-5, NCI/ADR-RES, and SK-OV-3 (25.99, 51.10, 15.66, and 74.92% growth, respectively). Compound 1f showed the most inhibitory activity against cell lines OVCAR-4 and OVCAR-8 (22.04 and 12.67% growth, respectively). Compounds 1a, 1d, 1e, 1g, 1h, and 1i showed no significant inhibitory activity toward any of the tested cell lines and slightly promoted cell growth. Cell line OVCAR-5 was the least susceptible to growth inhibition and was the most susceptible to growth promotion. Compound 1c was the only compound to be cytotoxic against any of the tested cell lines.
2.4.1.7 Renal cancer
The ten compounds were tested against eight types of renal cancer (Table 8). Compound 1c showed the most inhibitory activity against all cell lines and was cytotoxic against ACHN and SN12C. Compound 1h showed the weakest inhibitory activity toward all of the tested cell lines, and promoted cell growth in RXF 393, SN12C, and TK-10. Compound 1c showed the most inhibitory activity against cell lines 786-0, CAKI-1, RXF 393, TK-10, UO-31 (25.81, 28.75, 61.07, 30.31, and 31.91% growth, respectively). Compound 1f showed the most inhibitory activity against cell lines ACHN and SN12C (15.52 and 31.37% growth, respectively). Compound 1g showed the most inhibitory activity against cell line A498 (62.82% growth). Cell line RXF 393 was the least susceptible to growth inhibition and the most susceptible to growth promotion, but it was only tested against seven of the ten compounds. Cell line TK-10 only responded to treatment with compound 1c. Compounds 1b, 1c, and 1f were the only compounds to show any significant growth inhibition against any of the cell lines. Compound 1c was the only compound to show any degree of cytotoxicity. Compounds 1a, 1d, 1e, 1h, and 1j showed little growth inhibition and some growth promotion. Compound 1i was the only compound to show no significant levels of growth promotion or growth inhibition.
2.4.1.8 Prostate cancer
The ten compounds were tested against two types of prostate cancer (Table 9). Compound 1c was the most potent compound against both of the tested cell lines, PC-3 and DU-145 (21.91 and 9.09% growth, respectively). Compounds 1b and 1f were the only other compounds to exhibit any significant level of growth inhibition; all other compounds showed no significant amount of inhibition or promoted cell growth. Cell line DU-145 was more susceptible to growth promotion than inhibition.
2.4.1.9 Breast cancer
The ten compounds were tested against six types of breast cancer (Table 10). Compound 1c showed the most inhibitory activity out of all of the compounds tested, and compound 1j showed the least. Compound 1j also showed the most growth promotion out of any of the other tested compounds. Compound 1b was the most active against cell line MCF7 (15.72% growth). Compound 1c was the most potent against cell lines MDA-MB-231/ATCC, HS 578T, BT-549, T-47D, and MDA-MB-468 (42.69, 31.19, 70.26, 51.76, and 36.65% growth, respectively). Cell line MCF7 was the most susceptible to growth inhibition by all of the tested compounds except for compounds 1g, 1h, and 1j. Cell line BT-549 was the least susceptible to growth inhibition, and cell line MDA-MB-231/ATCC was the most susceptible to growth promotion. Compounds 1a, 1d, and 1e showed intermediate growth inhibition, and compounds 1d and 1e showed a small amount of growth promotion.
2.4.2 Multi-dose assay
Compounds 1b, 1c, and 1f were chosen for further dose-escalation studies by the NCI. The results are shown in Table 11. For these results, a low GI50 and IC50 are preferred. A low GI50 means that it takes a smaller concentration to inhibit 50% growth of a population, and a low IC50 means that it takes a lower dosage to kill 50% of a population of cells. Compounds 1b, 1c, and 1f were chosen because they were the most active of the compounds tested in the single dose assays. In the multi-dose assays, compound 1b had, by far, the strongest inhibitory effects as evidenced by its low GI50 and IC50 values. Compounds 1c and 1f showed similar degrees of inhibition, but neither were as potent as compound 1b. Compound 1f was not tested against all of the cell types as indicated by – in Table 11.
Heat map showing the log10 GI50 values for each EF-24 analog in the NCI-60 multi-dose screen, where high intensity (red) cells indicate high activity and low intensity (blue) cells indicate low activity
  |
2.5 Computational docking
To further explore the anti-cancer potential of these analogs, compounds and controls were subjected to flexible docking using AutoDock Vina [19]. Tubulin (PDB entry code: 4O2B) was prepared using AutoDock by removing the additional small molecules and tubulin chains. The grid box was centered on the colchicine binding site, as this site is the known binding site for compounds with similar structures and which exhibit tubulin disruption [20]. The results are compiled in Table 1. The docking indicated that compounds 1b, 1f, and 1j would have the best binding affinities to tubulin, which was higher than that of EF-24 and control compound nocodazole. These results correlate with the biological data from NCI for 1b and 1f; however, 1j which is the BOC version of EF-24 was not active in the anti-cancer assay.
3 Conclusions
Lead compound 1b was identified from this new series of N-BOC-protected EF-24 analogs. The addition of the BOC group caused two different conformations to be seen, with rotation of the BOC responsible for this effect. Interestingly, when the BOC group was introduced, the 2-fluoro direct analog of EF24 lost anti-cancer potency, although it was predicted to have better binding affinity to tubulin through our computational modeling. This indicates a different mechanism of action and/or binding site for these N-BOC-protected EF-24 analogs. Further investigations into this effect are underway.
4 Experimental
4.1 General methods
All commercial chemicals were reagent grade, obtained from Fisher Scientific, and were used without further purification. 1H and 13C spectra were obtained on a JEOL 300 NMR spectrometer at 300 and 75 MHz, respectively, in deuterated solvent with TMS (δ = 0.00 ppm) or residual proteo solvent signal in CDCl3 (δ = 7.27 ppm) as internal reference. Dynamic NMR was obtained on a Bruker Avance III HD 400 (AM-400) at the University of Minnesota NMR Laboratory. For all reactions, analytical grade solvent was used. TLC plates and silica gel were obtained from Sorbent Technologies. High-resolution mass spectra were obtained by the Proteomics & Mass Spectrometry Core Facility at the University of Georgia, Department of Chemistry on a Thermo-Fisher Orbitrap Elite (ESI) or at Georgia Southern University on a Shimadzu LCMS-9030 HR-HPLC-Q-TOF-MS (1j). Melting point was performed on MEL-TEMP II. DFT calculations were performed using Gaussian 16 (Revision C.01) [21] and results were visualized with GaussView 6.0.16 [22]. Yu, He, and Truhlar’s MN15-L functional [18] was used with Weigend and Alrichs’ def2-TZVP basis set [23] for 1b and the transition state. The chloroform solvent was modeled with the polarized continuum model (PCM) using the integral equation formalism variant (IEFPCM).
4.2 General procedure for Aldol Condensation
Reactions were performed on a millimole scale. Tert-butyl 4-oxopiperidine-1-carboxylate (1 mmol) was dissolved in absolute ethanol (5 mL). After the ketone dissolved the chosen aldehyde was added (2 mmol). NaOH (3 mL of 5 M solution) was added and the reaction was stirred until a yellow precipitate formed. The products were characterized using 1H NMR, 13C NMR, and HRMS. If any impurities were detected, the product was recrystallized from MeOH/H2O.
4.3 Synthesized compounds
Tert-butyl 3,5-bis((E)-2,4-dimethylbenzylidene)-4-oxopiperidine-1-carboxylate (1a) The bis-chalcone was obtained in 61% yield as a yellow solid; 1H NMR (300 MHz, CDCl3): δ 7.93 (s, 2H, vinyl-H), 7.09–7.01 (m, 6H, Ar-H), 4.56 (s, 4H, CH2), 2.34 (s, 12H, CH3), 1.26 (s, 9H, BOC) ppm; 13C NMR (75 MHz, CDCl3): δ 187.9, 154.4, 139.4, 138.3, 136.4, 132.3, 131.4, 131.1, 129.1, 126.5, 80.3, 44.9, 28.2, 21.3, 20.1 ppm; HRMS (ESI) m/z calculated for C28H34NO3 [(M + H)+] 432.2533, found 432.2536; melting point 125–131°C.
Tert-butyl 3,5-bis((E)-4-methylbenzylidene)-4-oxopiperidine-1-carboxylate (1b) The bis-chalcone was obtained in 68% yield as a yellow solid; 1H NMR (300 MHz, CDCl3, 50°C): δ 7.67 (s, 2H, vinyl-H), 7.23 (d, J = 6.9 Hz, 4H, Ar-H), 7.12 (d, J = 6.6 Hz, 4H, Ar-H), 4.62 (s, 4H, CH2), 2.27 (s, 6H, CH3), 1.21 (s, 9H, BOC) ppm; 13C NMR (75 MHz, CDCl3, 50°C): δ 187.3, 154.4, 139.6, 137.1, 132.2, 131.7, 130.5, 129.4, 80.4, 45.1, 28.1, 21.3 ppm; HRMS (ESI) m/z calculated for C26H30NO3 [(M + H)+] 404.2220, found 404.2223; melting point 184–189°C.
Tert-butyl 3,5-bis((E)-2-methylbenzylidene)-4-oxopiperidine-1-carboxylate (1c) The bis-chalcone was obtained in 50% yield as a yellow solid; 1H NMR (300 MHz, CDCl3, 50°C): δ 7.95 (s, 2H, vinyl-H), 7.25–7.21 (m, 8H, Ar-H), 4.54 (s, 4H, CH2), 2.37 (s, 6H, CH3), 1.27 (s, 9H, BOC) ppm; 13C NMR (75 MHz, CDCl3, 50°C): δ 187.6, 154.3, 138.0, 136.6, 134.1, 133.0, 130.5, 129.1, 128.9, 125.8, 80.3, 44.9, 28.1, 20.0 ppm; HRMS (ESI) m/z calculated for C26H30NO3 [(M + H)+] 404.2220, found 404.2224; melting point 126–130°C.
Tert-butyl 3,5-bis((E)-3-methylbenzylidene)-4-oxopiperidine-1-carboxylate (1d) The bis-chalcone was obtained in 12% yield as a yellow solid; 1H NMR (300 MHz, CDCl3): δ 7.77 (s, 2H, vinyl-H), 7.33–7.17 (m, 8H, Ar-H), 4.71 (s, 4H, CH2), 2.38 (s, 6H, CH3), 1.27 (s, 9H, BOC) ppm; 13C NMR (75 MHz, CDCl3, 50°C): δ 187.6, 154.5, 138.3, 137.5, 135.0, 132.4, 131.1, 130.1, 128.6, 127.5, 80.5, 45.1, 28.1, 21.4 ppm; HRMS (ESI) m/z calculated for C26H30NO3 [(M + H)+] 404.2220, found 404.2224; melting point 122–126°C.
Tert-butyl (3E,5E)-4-oxo-3,5-bis(thiophen-2-ylmethylene)piperidine-1-carboxylate (1e) The bis-chalcone was obtained in 83% yield as a yellow solid; 1H NMR (300 MHz, CDCl3): δ 7.96 (s, 2H, vinyl-H), 7.58 (d, J = 4.8 Hz, 2H, 5-thiophenyl-H), 7.37 (d, J = 3.9 Hz, 2H, 3-thiophenyl-H), 7.14 (dd, J = 3.9, 4.8 Hz, 2H, 4-thiophenyl-H), 4.78 (s, 4H, CH2), 1.42 (s, 9H, BOC) ppm; 13C NMR (75 MHz, CDCl3, 50°C): δ 185.8, 154.5, 138.6, 133.5, 130.8, 129.3, 128.1, 80.8, 45.1, 28.3 ppm; HRMS (ESI) m/z calculated for C20H22NO3S2 [(M + H)+] 388.1036 found 388.1039; melting point 185–188°C.
Tert-butyl 3,5-bis((E)-2,5-dimethylbenzylidene)-4-oxopiperidine-1-carboxylate (1f) The bis-chalcone was obtained in 54% yield as a yellow solid; 1H NMR (300 MHz, CDCl3, 50°C): δ 7.93 (s, 2H, vinyl), 7.12 (d, J = 7.8 Hz, 2H, aryl), 7.07 (d, J = 7.8 Hz, 2H, aryl), 6.99 (s, 2H, aryl), 4.54 (s, 4H, CH2), 2.34 (s, 6H, CH3), 2.32 (s, 6H, CH3), 1.26 (s, 9H, BOC) ppm; 13C NMR (75 MHz, CDCl3, 50°C): δ 187.6, 154.4, 136.8, 135.1, 134.9, 134.0, 132.8, 130.4, 129.9, 129.4, 80.2, 45.0, 28.1, 21.0, 19.5 ppm; HRMS (ESI) m/z calculated for C28H34NO3 [(M + H)+] 432.2533, found 432.2537; melting point 133–143°C.
Tert-butyl 3,5-bis((E)-2,6-dimethylbenzylidene)-4-oxopiperidine-1-carboxylate (1g) The bis-chalcone was obtained in 75% yield as a yellow solid. 1H NMR (300 MHz, CDCl3, 50°C): δ 7.83 (s, 2H, vinyl), 7.13–7.04 (m, 6H), 4.08 (s, 4H, CH2), 2.22 (s, 6H, CH3), 2.21 (s, 6H, CH3), 1.26 (s, 9H, BOC) ppm; 13C NMR (75 MHz, CDCl3, 50°C): δ 186.1, 153.9, 138.1, 135.4, 134.6, 134.2, 128.0, 127.6, 80.3, 44.5, 28.1, 20.2 ppm; HRMS (ESI) m/z calculated for C28H34NO3 [(M + H)+] 432.2533, found 432.2536; melting point 200–210°C.
Tert-butyl (3E,5E)-4-oxo-3,5-bis(thiophen-2-ylmethylene)piperidine-1-carboxylate (1h) The bis-chalcone was obtained in 69% yield as a yellow solid. 1H NMR (300 MHz, CDCl3): δ 7.56 (d, J = 1.5 Hz, 2H, 5-furanyl-H), 7.46 (s, 2H, vinyl-H), 6.68 (d, J = 3.3 Hz, 2H, 3-furanyl-H), 6.49 (t, J = 1.5 Hz, 2H, 4-furanyl-H), 4.85 (s, 4H, CH2), 1.38 (s, 9H, BOC) ppm; 13C NMR (75 MHz, CDCl3, 50°C): δ 186.1, 154.7, 152.0, 145.4, 129.1, 122.5, 117.5, 112.6, 80.4, 45.1, 28.3 ppm; HRMS (ESI) m/z calculated for C20H22NO5 [(M + H)+] 356.1492, found 356.1497; melting point 171–176°C.
Tert-butyl (3E,5E)-4-oxo-3,5-bis(thiophen-2-ylmethylene)piperidine-1-carboxylate (1i) The bis-chalcone was obtained in 34% yield as a brown solid. 1H NMR (300 MHz, CDCl3): δ 7.71 (s, 2H, 2-furanyl-H), 7.60 (s, 2H, vinyl-H), 7.49 (s, 2H, 5-furanyl-H), 6.61 (s, 2H, 4-furanyl-H), 4.64 (s, 4H, CH2), 1.41 (s, 9H, BOC) ppm; 13C NMR (75 MHz, CDCl3): δ 186.1, 154.5, 145.6, 144.2, 130.6, 127.6, 121.6, 110.8, 80.9, 44.9, 28.3 ppm; HRMS (ESI) m/z calculated for C20H22NO5 [(M + H)+] 356.1492, found 356.1496; melting point 139–142°C.
Tert-butyl 3,5-bis((E)-2,6-dimethylbenzylidene)-4-oxopiperidine-1-carboxylate (1j) The bis-chalcone was obtained in 17% yield as a yellow solid. 1H NMR (300 MHz, CDCl3, 50°C): δ 7.86 (s, 2H, vinyl), 7.38–7.33 (4H, m, aryl), 7.21–7.10 (m, 4H, aryl), 4.59 (s, 4H, CH2), 1.27 (s, 9H, BOC) ppm; 13C NMR (75 MHz, CDCl3, 50°C): δ 186.7, 161.0 (d, J C–F: 246 Hz), 154.3, 134.3 (broad), 131.1 (d, J C–F: 8 Hz), 130.8 (d, J C–F: 3 Hz), 130.2 (broad), 124.1 (d, J C–F: 4 Hz), 123.0 (d, J C–F: 13 Hz), 116.0 (d, J C–F: 22 Hz), 80.6, 45.2, 45.2, 28.1 ppm; HRMS (ESI) m/z calculated for C24H23F2NO3Na [(M + Na)+] 434.15437, found 434.15520; melting point 141–145°C.
4.4 Single dose anti-cancer assay
Anti-cancer screening was performed by the NCI (National Cancer Institute, Bethesda, MD). Cytotoxic and anti-proliferative activities were determined in a preliminary single high dose concentration (10−5 M) screening against the NCI-60 panel (60 cell lines derived from nine different human cancer types: leukemia, NSCLC, colon, CNS, melanoma, ovarian, renal, prostate, and breast cancer). The single dose results are expressed as the percent growth inhibition of treated cells at the test concentration of 10−5 M following 48 h of incubation [24].
4.5 Multi-dose anti-cancer assay
The cells were pipetted into a 96 well microtiter plate and left to sit for 24 h before the addition of the drugs. The drugs are dissolved in a solution that is 400 times larger than the desired maximum concentration. When ready for use, an aliquot is taken from the solution and diluted to twice the desired final maximum concentration. Four further 10-fold serial dilutions are prepared for use. Once the aliquots have been added, the plates are allowed to sit for 48 h before being analyzed. The results are expressed as percentage of inhibition at each concentration [24].
4.6 Docking
Two-dimensional structures of selected ligands were drawn in Chemdraw and converted from .cdx files to .pdb files using Chem3d. Ligand .pdb structures were then converted to .pdb using Python Molecular Viewer by importing as a ligand. The macromolecule was prepared by using PDB code 4O2B. Subunits C–F of tubulin were deleted, as well as the water and colchicine. Remaining subunits A + B were saved as a.pdbqt file. The grid was defined as x = 15, y = 65, z = 45 and size x = 60, y = 50, z = 40 centered on the colchicine binding site. Each compound was submitted to AutoDock Vina for docking and the lowest energy docking conformation and binding affinities were identified.
Acknowledgements
The anti-cancer screening was performed by the National Cancer Institute Discovery & Development Services. HRMS reported in this publication was supported by NIH grant S10RR028859 which was granted to the Proteomics and Mass Spectrometry Facility at the University of Georgia and NSF grant 2018774 awarded to the Chemistry Department at Georgia Southern University, PI Nathaniel Shank and key personnel Sarah Zingales. Dynamic NMR reported in this publication was supported by the Office of the Vice President of Research, College of Science and Engineering, and the Department of Chemistry at the University of Minnesota. Augsburg University Chemistry Donor Funds for financial support. The PIs would like to thank #ChemTwitter for connecting them.
-
Funding information: Authors state no external funding involved. Internal GSU funds to S.K.Z.; Internal USJ funds to M.U. and W.R.; Internal AU funds to M.T.W.
-
Author contributions: Robert B. Smith as undergraduate student at GSU: original product preparation and characterization, and writing – original draft; as graduate student at FSU: writing – reviewing and editing. William Roberts: graduate student, computational modeling, writing – docking section of original draft. Mary Upenieks: undergraduate student, scaling up product preparation, writing – reviewing. Maya Z. Gibson, as undergraduate student at GSU, original product preparation and characterization; as MD resident at Children’s Mercy Kansas City, writing – editing and reviewing. Michael T. Wentzel: dynamic NMR, writing – reviewing. Kyle A. Grice: running and interpreting DFT calculations, writing – DFT sections of the manuscript. Sarah K. Zingales: conceptualization, project administration, student supervision, review of lab work, and writing – original draft, review, and editing.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Salmerón-Manzano E, Garrido-Cardenas JA, Manzano-Agugliaro F. Worldwide research trends on medicinal plants. Int J Environ Res Public Health. 2020;17:3376. 10.3390/ijerph17103376.Search in Google Scholar PubMed PubMed Central
[2] Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. 2020;83:770–803. 10.1021/acs.jnatprod.9b01285.Search in Google Scholar PubMed
[3] Wani MC, Horwitz SB. Nature as a remarkable chemist: a personal story of the discovery and development of Taxol®. Anticancer Drugs. 2014;25:482.10.1097/CAD.0000000000000063Search in Google Scholar PubMed PubMed Central
[4] Kah G, Chandran R, Abrahamse H. Curcumin a natural phenol and its therapeutic role in cancer and photodynamic therapy: a review. Pharmaceutics. 2023;15:639. 10.3390/pharmaceutics15020639.Search in Google Scholar PubMed PubMed Central
[5] Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–52.10.1007/s00018-008-7452-4Search in Google Scholar PubMed PubMed Central
[6] Salehi B, Stojanović-Radić Z, Matejić J, Sharifi-Rad M, Kumar NVA, Martins N, et al. The therapeutic potential of curcumin: a review of clinical trials. Eur J Med Chem. 2019;163:527–45.10.1016/j.ejmech.2018.12.016Search in Google Scholar PubMed
[7] Liu W, Zhai Y, Heng X, Che FY, Chen W, Sun D, et al. Oral bioavailability of curcumin: problems and advancements. J Drug Target. 2016;24:694–702. 10.3109/1061186X.2016.1157883.Search in Google Scholar PubMed
[8] Adams BK, Ferstl EM, Davis MC, Herold M, Kurtkaya S, Camalier RF, et al. Synthesis and biological evaluation of novel curcumin analogs as anti-cancer and anti-angiogenesis agents. Bioorg Med Chem. 2004;12:3871–83.10.1016/j.bmc.2004.05.006Search in Google Scholar PubMed
[9] Thomas SL, Zhong D, Zhou W, Malik S, Liotta D, Snyder JP, et al. EF24, a novel curcumin analog, disrupts the microtubule cytoskeleton and inhibits HIF-1. Cell Cycle Georget Tex. 2008;7:2409–17. 10.4161/cc.6410.Search in Google Scholar PubMed PubMed Central
[10] He Y, Li W, Hu G, Sun H, Kong Q. Bioactivities of EF24, a novel curcumin analog: a review. Front Oncol. 2018;8:614.10.3389/fonc.2018.00614Search in Google Scholar PubMed PubMed Central
[11] Girgis AS, D’Arcy P, Aboshouk DR, Bekheit MS. Synthesis and bio-properties of 4-piperidone containing compounds as curcumin mimics. RSC Adv. 2022;12:31102–23. 10.1039/D2RA05518J.Search in Google Scholar
[12] Dimmock JR, Padmanilayam MP, Puthucode RN, Nazarali AJ, Motaganahalli NL, Zello GA, et al. A conformational and structure–activity relationship study of cytotoxic 3, 5-bis (arylidene)-4-piperidones and related N-acryloyl analogues. J Med Chem. 2001;44:586–93.10.1021/jm0002580Search in Google Scholar PubMed
[13] Ocasio-Malavé C, Donate MJ, Sánchez MM, Sosa-Rivera JM, Mooney JW, Pereles-De León TA, et al. Synthesis of novel 4-Boc-piperidone chalcones and evaluation of their cytotoxic activity against highly-metastatic cancer cells. Bioorg Med Chem Lett. 2020;30:126760. 10.1016/j.bmcl.2019.126760.Search in Google Scholar PubMed PubMed Central
[14] Smith PJ, Dimmock JR, Turner WA. Mass spectrometry of some substituted 2-benzylidenecyclohexanones and 2,6-bis-benzylidenecyclohexanones. Can J Chem. 1973;51:1458–70. 10.1139/v73-220.Search in Google Scholar
[15] Lagisetty P, Powell DR, Awasthi V. Synthesis and structural determination of 3,5-bis(2-fluorobenzylidene)-4-piperidone analogs of curcumin. J Mol Struct. 2009;936:23–8. 10.1016/j.molstruc.2009.07.016.Search in Google Scholar
[16] Raban M, Jones Jr FB. Stereochemistry at trivalent nitrogen. XI. Effect of polar substituents on the barrier to rotation about the sulfenyl sulfur–nitrogen bond in N-alkyl-N-arenesulfonylarenesulfenamides. J Am Chem Soc. 1971;93:2692–9. 10.1021/ja00740a017.Search in Google Scholar
[17] Modarresi-Alam AR, Keykha H, Khamooshi F, Dabbagh HA. Dynamic 1H NMR study of 4-methylphenoxyimidoyl azides: conformational or configurational isomerisation? Tetrahedron. 2004;60:1525–30. 10.1016/j.tet.2003.12.007.Search in Google Scholar
[18] Yu HS, He X, Truhlar DG. MN15-L: a new local exchange-correlation functional for Kohn–Sham density functional theory with broad accuracy for atoms, molecules, and solids. J Chem Theory Comput. 2016;12:1280–93. 10.1021/acs.jctc.5b01082.Search in Google Scholar PubMed
[19] Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455–61. 10.1002/jcc.21334.Search in Google Scholar PubMed PubMed Central
[20] Kim DY, Kim K-H, Kim ND, Lee KY, Han CK, Yoon JH, et al. Design and biological evaluation of novel tubulin inhibitors as antimitotic agents using a pharmacophore binding model with tubulin. J Med Chem. 2006;49:5664–70. 10.1021/jm050761i.Search in Google Scholar PubMed
[21] Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman HR, et al. Gaussian 16, Revision C.01. Wallingford, CT: Gaussian, Inc.; 2016.Search in Google Scholar
[22] Dennington R, Keith TA, Millam JM. GaussView 6.0. 16. Semichem Inc Shawnee Mission KS USA; 2016.Search in Google Scholar
[23] Weigend F, Ahlrichs R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys Chem Chem Phys. 2005;7:3297–305. 10.1039/B508541A.Search in Google Scholar
[24] National Cancer Institute: Division of Cancer Treatment and Diagnosis. NCI-60 Screening Methodology. https://dtp.cancer.gov/discovery_development/nci-60/methodology.htm. (accessed 3/30/23).Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Synthesis, characterization, and antibacterial activity of a new poly azo compound containing N-arylsuccinimid and dibenzobarrelene moieties
- Design, synthesis, and antiviral activities evaluation of novel quinazoline derivatives containing sulfonamide moiety
- Design, synthesis, and anticancer activity of novel 4,6-dimorpholinyl-1,3,5-triazine compounds
- Design, synthesis, biological evaluation, and bio-computational modeling of imidazo, thieno, pyrimidopyrimidine, pyrimidodiazepene, and motifs as antimicrobial agents
- Synthesis of a novel phosphate-containing ligand rhodium catalyst and exploration of its optimal reaction conditions and mechanism for the polymerization of phenylacetylene
- Design, synthesis, and antiproliferative activity of novel 1,2,4-triazole-chalcone compounds
- Synthesis of metal–organic nanofiber/rGO nanocomposite as the sensing element for electrochemical determination of hypoxanthine
- Design and synthesis of various 1,3,4-oxadiazoles as AChE and LOX enzyme inhibitors
- Bis(2-cyanoacetohydrazide) as precursors for synthesis of novel azoles/azines and their biological evaluation
- Synthesis, characterization, and biological target prediction of novel 1,3-dithiolo[4,5-b]quinoxaline and thiazolo[4,5-b]quinoxaline derivatives
- Sustainable conversion of carbon dioxide into novel 5-aryldiazenyl-1,2,4-triazol-3-ones using Fe3O4@SP-vanillin-TGA nanocomposite
- Erratum
- Erratum to “Design, synthesis and study of antibacterial and antitubercular activity of quinoline hydrazone hybrids”
- SI: Undergraduate Research in the Synthesis of Biologically Active Small Molecules and Their Applications
- Preparation of novel acyl pyrazoles and triazoles by means of oxidative functionalization reactions
- Synthesis and conformational analysis of N-BOC-protected-3,5-bis(arylidene)-4-piperidone EF-24 analogs as anti-cancer agents
- SI: Development of Heterocycles for Biomedical and Bioanalytical Applications
- Influence of octreotide on apoptosis and metabolome expression in lipopolysaccharide-induced A549 cells
- Crude extract of J1 fermentation promotes apoptosis of cervical cancer cells
Articles in the same Issue
- Research Articles
- Synthesis, characterization, and antibacterial activity of a new poly azo compound containing N-arylsuccinimid and dibenzobarrelene moieties
- Design, synthesis, and antiviral activities evaluation of novel quinazoline derivatives containing sulfonamide moiety
- Design, synthesis, and anticancer activity of novel 4,6-dimorpholinyl-1,3,5-triazine compounds
- Design, synthesis, biological evaluation, and bio-computational modeling of imidazo, thieno, pyrimidopyrimidine, pyrimidodiazepene, and motifs as antimicrobial agents
- Synthesis of a novel phosphate-containing ligand rhodium catalyst and exploration of its optimal reaction conditions and mechanism for the polymerization of phenylacetylene
- Design, synthesis, and antiproliferative activity of novel 1,2,4-triazole-chalcone compounds
- Synthesis of metal–organic nanofiber/rGO nanocomposite as the sensing element for electrochemical determination of hypoxanthine
- Design and synthesis of various 1,3,4-oxadiazoles as AChE and LOX enzyme inhibitors
- Bis(2-cyanoacetohydrazide) as precursors for synthesis of novel azoles/azines and their biological evaluation
- Synthesis, characterization, and biological target prediction of novel 1,3-dithiolo[4,5-b]quinoxaline and thiazolo[4,5-b]quinoxaline derivatives
- Sustainable conversion of carbon dioxide into novel 5-aryldiazenyl-1,2,4-triazol-3-ones using Fe3O4@SP-vanillin-TGA nanocomposite
- Erratum
- Erratum to “Design, synthesis and study of antibacterial and antitubercular activity of quinoline hydrazone hybrids”
- SI: Undergraduate Research in the Synthesis of Biologically Active Small Molecules and Their Applications
- Preparation of novel acyl pyrazoles and triazoles by means of oxidative functionalization reactions
- Synthesis and conformational analysis of N-BOC-protected-3,5-bis(arylidene)-4-piperidone EF-24 analogs as anti-cancer agents
- SI: Development of Heterocycles for Biomedical and Bioanalytical Applications
- Influence of octreotide on apoptosis and metabolome expression in lipopolysaccharide-induced A549 cells
- Crude extract of J1 fermentation promotes apoptosis of cervical cancer cells

![Figure 1
Curcumin, EF-24, N-acryloyl-3,5-bis(arylidene)-4-piperidones [12], and N-BOC-3,5-bis(arylidene)-4-piperidones [13].](/document/doi/10.1515/hc-2022-0162/asset/graphic/j_hc-2022-0162_fig_001.jpg)
