Abstract
A series of novel 5-aryldiazenyl-1,2,4-triazol-3-ones are synthesized at room temperature in short reaction time, and excellent yields via four-component reaction between 4-aryldiazenyl-salicylaldehydes, ammonium acetate, arylhydrazine, and carbon dioxide using bis(thioglycolic acid)-vanillin (2,2′-(((4-hydroxy-3-methoxyphenyl)methylene)bis(sulfanediyl))diacetic acid)‐functionalized silica-coated Fe3O4 magnetic nanocomposite (Fe3O4@SP-vanillin-TGA MNCs). The present protocol offers several advantages such as gentle reaction conditions, excellent performance, simple fabrication, separation methods, and reduction of detrimental environmental consequences. The catalyst could be easily recovered and reused for six runs with nearly steady activity. The structures of the synthesized 5-aryldiazenyl-1,2,4-triazol-3-ones have been confirmed with the aid of using 1H, 13C NMR, and fourier transform infrared spectral data and elemental analyses.
Graphical abstract

1 Introduction
Triazoles are important biochemical compounds with significant biological activities. The union of these structures is especially crucial since they have distinctive natural components; there are several complex drug compounds with triazole as building blocks. These structures are regularly found in common compounds representing pharmaceuticals and related vital substances. Essentially, these compounds have diverse applications such as erosion inhibitors, colors and dyes, sensors, and light stabilizers. Furthermore, triazoles serve as ligands, protein components, and bound fluorescent oligomeric nucleotides [1,2].
Various methods have been proposed for the synthesis of these important heterocyclic and pharmaceutical compounds. The use of 1-(phenylacetyl)thiosemicarbazide [3], the reaction of thiosemicarbazide, including a hydrazide acid and 2-halo benzyl isothiocyanate [4], reaction of methylpyridine-1-carboxylate with thiosemicarbazide [5], oxidation of cyclic aldehyde, and thiosemicarbazide in iron chloride-assisted preparation of 1,2,4-triazoline and 1,3,4-thiadiazine [6] are examples of the latest scientific articles that have been reported on the synthesis of triazoles.
The focus on environmental aspects is expanding rapidly in new chemistry explorations. This sustainable strategy strives to deploy greener techniques such as earth-abundant and inexpensive catalysts, solvent-free or non-harmful solvents in synthesis, multi-component and one-pot reactions, among others [7,8]. Emerging nanocatalysts [9,10] simultaneously have the advantages of both the heterogeneous and homogeneous catalysts and provide excellent conditions for deploying nanoparticles as catalysts. Since nanocatalysts have smaller particle sizes, they are exposed to more reagents and maximum efficiency can be achieved with small amounts of catalyst, especially utilizing single atom catalysts [11–13]. In addition, good selectivity in many nanocatalysts leads to the removal of by-products. Another advantage of nanocatalysts is their heterogeneity, which makes them easier to separate and reuse [14,15].
Carbon dioxide is one of the greenhouse gases that causes global warming and acidification of seawater and oceans [16]. The amount of this gas in the Earth’s atmosphere is about 300 billion tons, which is predicted to cause more severe changes in climate and increase the possibility of irreparable danger to life on Earth, according to research, and should be reduced. One important solution is to use the science and technology of carbon dioxide capture, storage, and utilization to reduce the devastating effects on the climate. Recently, many scientists worldwide have turned their attention to these techniques, and the development and optimization of ensued methods. On the other hands, carbon dioxide is a good source of carbon because of its availability, non-toxicity, and inexpensive nature. Therefore, the synthesis of different functional compounds using abundant carbon dioxide is a convenient and practical approach to consume this raw material [17].
In the continuation of our practical studies on development of newer methods for the preparation of novel heterocyclic compounds under greener and more environmentally eco-friendly protocols [18–25], a sustainable approach is presented for the synthesis of 5-aryldiazenyl-1,2,4-triazol-3-ones. The reaction is catalyzed by Fe3O4@SP-vanillin-TGA nanocomposite in a one-pot reaction of 4-aryldiazenyl-salicyladehydes, phenyl hydrazine, ammonium acetate, and CO2 at room temperature wherein Fe3O4@SP-vanillin-TGA serves as a recyclable magnetic nanocatalyst (Scheme 1).

One-pot synthesis of 5-aryldiazenyl-1,2,4-triazol-3-ones.
2 Experimental
2.1 Materials and methods
The required raw materials were acquired and utilized from Merck and Fluka companies. Electro-thermal 9100 devices were utilized to measure the melting points of the products. The Carlo-Erba EA1110CNNO-S analyzer was used to record the elemental analyses of materials. The FT-IR spectrum was recorded on a Shimadzu FT-IR-8400S spectrometer. Bruker DRX 400Avance spectrometer was used for 1H NMR and 13C NMR spectra in CDCl3 as solvent and with tetramethylsilane as internal standard. Nanostructures were detected and characterized by deploying a Philips Xpert X-ray powder diffraction (XRD) diffractometer (CuKα, radiation, λ = 0.154056), at a scanning speed of 2°/min from 10° to 80° (2θ). High resolution mass spectrum (HRMS) were performed with a Bruker MicroTOF-Q II spectrometer. Transmission electron microscopy (TEM) measurements were carried out on a Zeiss-EM10C-100 KV instrument.
2.2 General procedure for the synthesis of Fe3O4@SP-vanillin-TGA nanocomposite
The earlier synthesized Fe3O4 and Fe3O4@SP‐Cl MNCs were obtained by optimized processes documented [15].
Then, vanillin (0.2 g), NaOH 10% (5 mL), Fe3O4@SP‐Cl (0.2 g), and distilled H2O (15 mL) were added and the contents were stirred for 24 h. Next, thioglycolic acid (TGA) (1.5 mL) was added. After 5 h, Et3N (5 mL) was added, and after stirring with the magnet, the mixture was incubated in an oven at 50°C for 24 h (Figure 1). The structure of the MNCs was confirmed by TEM, XRD, vibrating sample magnetometry (VSM), and fourier transform infrared (FT-IR) spectroscopy (Figures 2–6).

Structure of Fe3O4@SP-vanillin-TGA MNCs.

FT-IR spectra of (a) Fe3O4 MNCs, (b) Fe3O4@SP-Cl, (c) Fe3O4@SP-vanillin MNCs, and (d) Fe3O4@SP-vanillin-TGA MNCs.
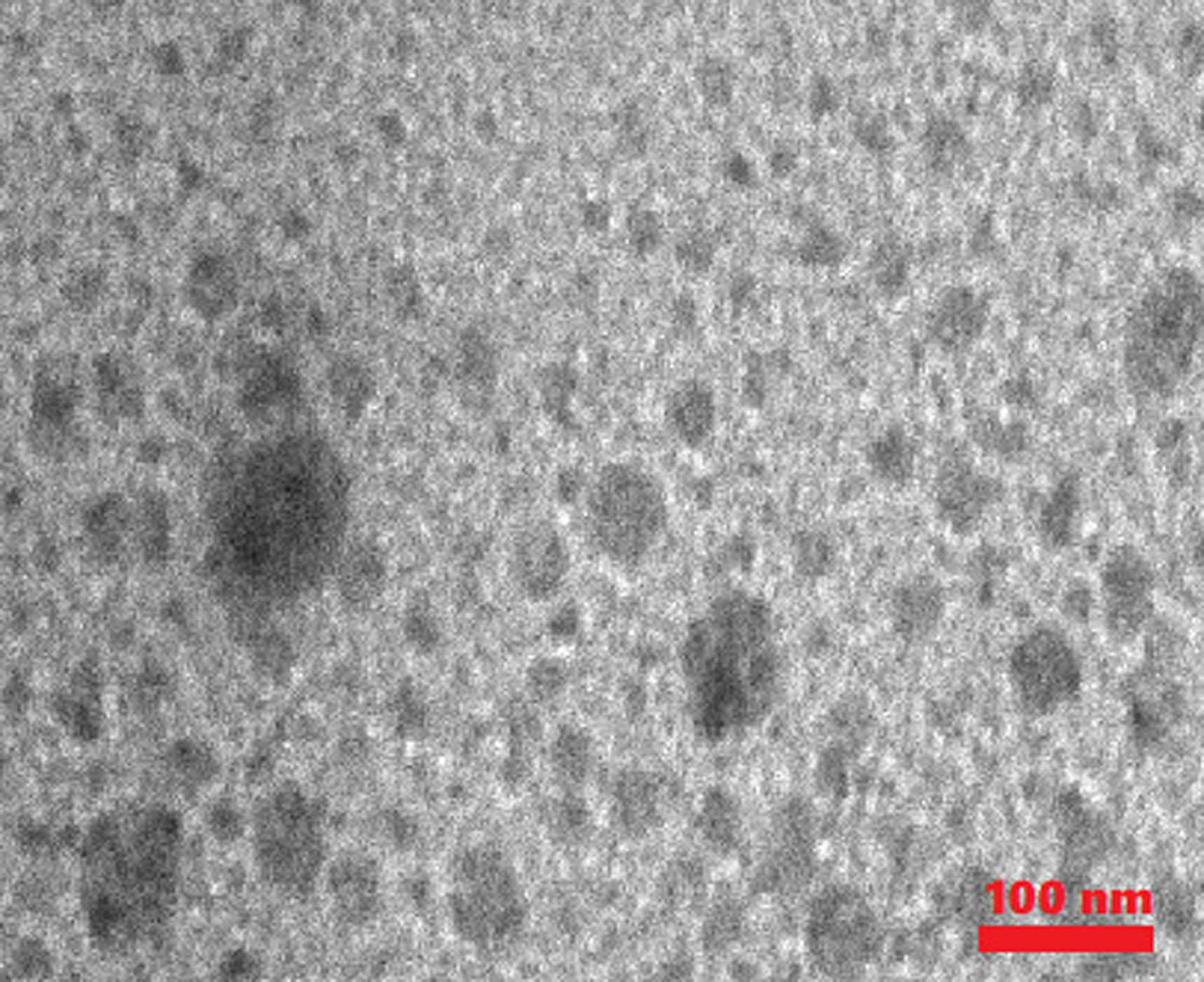
TEM image of synthesized Fe3O4@SP-vanillin-TGA MNCs.
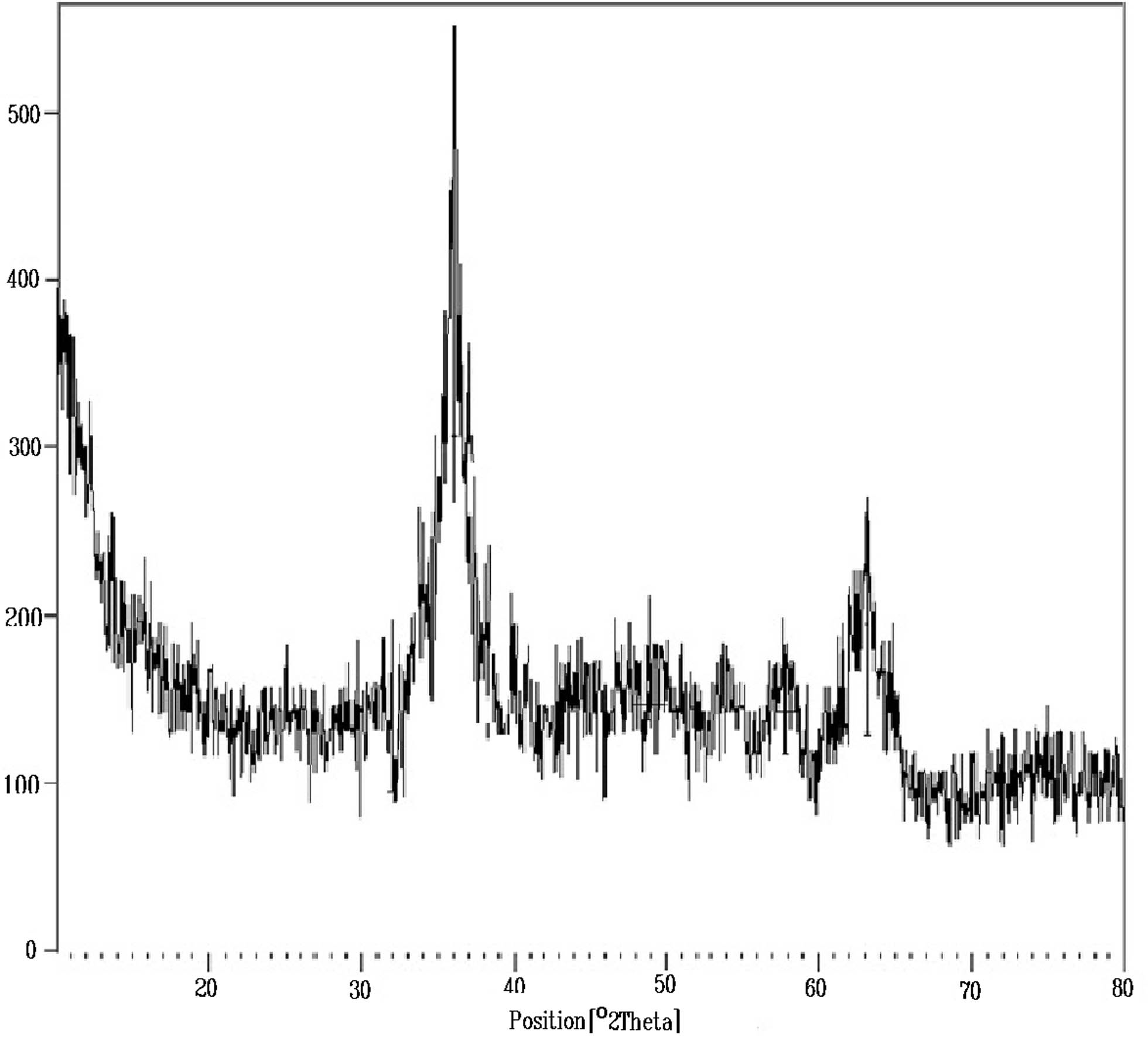
XRD image of synthesized Fe3O4@SP-vanillin-TGA MNCs.

EDX image of synthesized Fe3O4@SP-vanillin-TGA MNCs.

VSM image of synthesized Fe3O4@SP-vanillin-TGA MNCs.
Figure 2 shows the FT-IR spectra of Fe3O4 MNCs, Fe3O4@SP, Fe3O4@SP-vanillin, and Fe3O4@SP-vanillin-TGA MNCs with the aim of identifying functional groups on the synthesized nanoparticles. In the spectrum, peaks 3,503 and 3,472 cm−1 correspond to hydrogen bonds at –OH strength of Fe3O4@SP-vanillin and –COOH group of TGA in Fe3O4@SP-vanillin-TGA MNCs, respectively (Figure 2c and d). The peaks related to Fe–O bending are visible at 632–655 cm−1 (Figure 2a–d) that confirms the presence of Fe3O4 in the structure of the composites. The bands at 1,701 and 1,622 cm−1 confirm the carbonyl group stretching of vanillin in Fe3O4@SP-vanillin and Fe3O4@SP-vanillin-TGA, respectively, that is not discerned in the FT-IR spectra of precursor nanoparticles Fe3O4 and Fe3O4@SP (Figure 2c and d). Also, two peaks in ∼911–1,041 cm−1 belonging to Si–O in SiO2 shell appear (Figure 2b–d). The C–S bending of TGA in the nanoparticle can be seen at 692 cm−1 (Figure 2d). The C═C stretching of vanillin in Fe3O4@SP-vanillin and Fe3O4@SP-vanillin-TGA are observed at 1,439 and 1,537 cm−1, respectively (Figure 2c and d).
The morphology and size of synthesized MNCs were determined by TEM (Figure 3). Figure 3 shows the TEM images of the sample at different scales of 75 and 100 nm wherein two different areas with dark and light colors represent different elements of this sample. TEM image of the Fe3O4@SP-vanillin-TGA catalyst reveals that nanoparticles are formed with nearly spherical morphology having a molecule measure of 35–49 nm (Figure 3).
To confirm the synthesized nanocatalyst, its XRD patterns were compared with pure magnetite, and as a result, the formation of MNCs was confirmed. This pattern is visible for all peaks at 2θ = 24.1, 30.2, 31.2, 35.8, 38.0, 48.8, 57.8, and 63.0 and are in a good agreement with the standard XRD pattern for the fcc structure of Fe3O4. Thus, a broad peak at 10–20° is related to SiO2 (Figure 4).
The EDX spectrum is shown in Figure 5. Each peak corresponds to an element, and of course, in some elements, due to the collision of X-rays different diffraction angles may have more than one spectral line. The more the relative value of an element in the composition studied, the height of its peaks is higher. Quantitative results of elemental analysis according to the EDX spectrum of synthesized Fe3O4@SP-vanillin-TGA MNCs proved the existence of element (w/w%; Fe: 42.11, S: 20.14, Si: 0.24, O: 18.76, and C: 15.12) particles within the structure that affirms the presence of Fe3O4 within the structure of MNCs.
The magnetic properties of Fe3O4, Fe3O4@SP, Fe3O4@SP-vanillin MNCs, and Fe3O4@SP-vanillin-TGA MNCs were measured by VSM at room temperature. Accordingly, it can be seen that with the increase in the strength of the external magnetic field, the magnetic property in the material has increased. At the saturation limit, the magnetization of the material has reached about 30 emu/g, which indicates that the material is intrinsically magnetic. On the other hand, by reducing the magnetic field to negative values, the magnetic property is greatly reduced and the magnetic behavior similar to the positive phase is repeated. Therefore, the synthesized Fe3O4@SP-vanillin-TGA MNCs are considered as a type of ferromagnetic material (Figure 6).
2.3 General procedure for the preparation of 5-aryldiazenyl-1,2,4-triazol-3-ones 5a–j using Fe3O4@SP-vanillin-TGA nanocomposite
A mixture of synthesized salicylaldehydes (1 mmol), Fe3O4@SP-vanillin-TGA MNCs (0.1 g), ammonium acetate (1 mmol), and distillated water (5.0 mL) was added in a Teflon-lined stainless steel reactor (22 mL). The materials were stirred in this reactor equipped with a magnetic stirrer for 10 min at room temperature. Then phenylhydrazine (1 mmol) was added and pure carbon dioxide was charged to the desired pressure (1 MPa) in the reactor and stirring was continued again. After the completion of the reaction, the catalyst was separated by a 1.4 T external magnet and washed twice with hot distilled water (10.0 mL) and ethanol (15.0 mL). As a result, 5-aryldiazenyl-1,2,4-triazol-3-ones were incubated in the furnace. The pure products were recrystallized from ethanol. The structures of the synthesized 5-aryldiazenyl-1,2,4-triazol-3-ones 5a–j were determined by analyzing and interpreting their spectroscopic data (1H NMR, 13C NMR, FT-IR, and elemental analyses).
2.3.1 5-(2-Hydroxy-5-(phenyldiazenyl)phenyl)-1-phenyl-1H-1,2,4-triazol-3(2H)-one (5a)
White solid, m.p. 231–233°C; FT-IR (KBr) (υ max cm−1): 3,421 (O–H), 3,057 (CH–Ar), 2,981 (CH–aliphatic), 1,738 (C═O), 1,582 and 1,535 (C═C, Ar); 1H NMR (400 MHz, DMSO-d 6) δ: 12.30 (s, 1H, OH), 8.63 (t, J = 2.8 Hz, 2H), 8.21 (d, J = 8.2 Hz, 1H), 8.11 (d, J = 8.2 Hz, 1H), 8.04 (s, 1H), 7.94 (d, J = 2.8 Hz, 2H), 7.72 (s, 3H), 7.57–7.63 (m, 3H) ppm; 13C NMR (100 Hz, DMSO-d 6) δ: 200.2, 168.1, 160.0, 150.3, 137.6, 136.8, 135.5, 132.5, 131.6, 131.4, 131.0, 130.3, 129.3, 128.8, 126.0, 122.2 ppm. HRMS (m/z 357.12). Anal calc. for C20H15N5O2: C, 67.22; H, 4.23; N, 19.60. Found: C, 67.20; H, 4.22; N, 19.63.
2.3.2 5-(2-Hydroxy-5-(phenyldiazenyl)phenyl)-1-(p-tolyl)-1H-1,2,4-triazol-3(2H)-one (5b)
White solid, m.p. 206–208°C; FT-IR (KBr) (υ max cm−1): 3,041 (CH–Ar), 2,985 (CH–aliphatic), 1,635 (C═O), 1,573 and 1,554 (C═C, Ar); 1H NMR (400 MHz, CDCl3) δ: 12.01 (s, 1H, OH), 8.51 (d, J = 2.2 Hz, 1H), 8.32 (d, J = 8.2 Hz, 1H), 7.80 (d, J = 8.2 Hz, 4H), 7.30 (d, J = 7.8 Hz, 4H), 7.11 (d, J = 8.2 Hz, 2H), 2.44 (s, 3H, CH3) ppm; 13C NMR (100 Hz, DMSO-d 6) δ: 201.1, 165.7, 159.3, 157.2, 146.1, 136.0, 133.6, 133.3, 131.9, 131.0, 129.4, 129.6, 129.1, 125.2, 123.5, 121.4, 21.1 (CH3) ppm. HRMS (m/z 371.14). Anal calc. for C21H17N5O2: C, 67.91; H, 4.61; N, 18.86. Found: C, 67.90; H, 4.63; N, 18.88.
2.3.3 5-(2-Hydroxy-5-((2-nitrophenyl)diazenyl)phenyl)-1-(p-tolyl)-1H-1,2,4-triazol-3(2H)-one (5c)
Off-white solid, m.p. 212–214°C; FT-IR (KBr) (υ max cm−1): 3,027 (CH–Ar), 2,963 (CH–aliphatic), 1,605 (C═O), 1,581 and 1,556 (C═C, Ar), 1,351 (NO2); 1H NMR (400 MHz, CDCl3) δ: 12.40 (s, 1H, OH), 9.11 (s, 1H), 8.50 (d, J = 2.6 Hz, 2H), 8.30 (d, J = 2.8 Hz, 1H), 8.31 (d, J = 7.8 Hz, 1H), 8.06 (d, J = 7.8 Hz, 1H), 7.82 (d, J = 8.2 Hz, 1H), 7.80 (d, J = 8.2 Hz, 2H), 7.33 (d, J = 8.2 Hz, 2H), 7.11 (d, J = 8.2 Hz, 1H), 2.45 (s, 3H, CH3) ppm; 13C NMR (100 Hz, DMSO-d 6) δ: 201.2, 164.3, 157.2, 149.4, 138.4, 135.7, 133.8, 133.6, 131.2, 131.1, 128.1, 127.1, 125.3, 123.4, 121.4, 21.2 (CH3) ppm. HRMS (m/z 416.12). Anal calc. for C21H16N6O4: C, 60.57; H, 3.87; N, 20.18. Found: C, 60.59; H, 3.85; N, 20.16.
2.3.4 5-(5-((2-Bromophenyl)diazenyl)-2-hydroxyphenyl)-1-Phenyl-1H-1,2,4-triazol-3(2H)-one (5d)
White solid, m.p. 221–223°C; FT-IR (KBr) (υ max cm−1): 3,402 (O–H), 3,055 (CH–Ar), 2,922 (CH–aliphatic), 1,638 (C═O), 1,566 and 1,520 (C═C, Ar), 1,228 (C–O); 1H NMR (400 MHz, DMSO-d 6) δ: 12.01 (s, 1H, OH), 8.15 (d, J = 8.2 Hz, 2H), 8.07–8.03 (m, 3H), 7.82 (s, br., 3H), 7.53–7.62 (m, 3H), 7.42–7.50 (m, 2H) ppm; 13C NMR (100 Hz, DMSO-d 6) δ: 211.3, 164.5, 163.1, 152.2, 148.3, 142.4, 138.3, 134.9, 133.3, 133.0, 131.8, 131.6, 130.5, 129.6, 129.1, 127.0, 125.4, 121.9 ppm. HRMS (m/z 435.03). Anal calc. for C20H14BrN5O2: C, 55.06; H, 3.23; N, 16.05. Found: C, 55.09; H, 3.26; N, 16.02.
2.3.5 5-(5-((3-bromophenyl)diazenyl)-2-hydroxyphenyl)-1-Phenyl-1H-1,2,4-triazol-3(2H)-one (5e)
White solid, m.p. 219–221°C; FT-IR (KBr) (υ max cm−1): 3,402 (O–H), 3,056 (CH–Ar), 1,637 (C═O), 1,596 and 1,566 (C═C, Ar), 949 (C–Br); 1H NMR (400 MHz, DMSO-d 6) δ: 12.15 (s, 1H, OH), 8.72 (s, 1H), 8.62 (d, J = 7.8 Hz, 1H), 8.22 (d, J = 8.2 Hz, 1H), 8.14 (d, J = 8.2 Hz, 1H), 8.05 (d, J = 7.8 Hz, 1H), 7.92 (s, 2H), 7.78–7.82 (m, 1H), 7.72 (d, J = 7.8 Hz, 2H), 7.59 (t, J = 7.8 Hz, 2H) ppm; 13C NMR (100 Hz, DMSO-d 6) δ: 212.4, 163.9, 161.4, 149.2, 138.7, 136.8, 135.6, 133.3, 133.5, 132.1, 131.5, 131.4, 130.7, 130.4, 129.7, 128.4, 125.2, 121.5 ppm. HRMS (m/z 435.03). Anal calc. for C20H14BrN5O2: C, 55.06; H, 3.23; N, 16.05. Found: C, 55.03; H, 3.25; N, 16.03.
2.3.6 5-(2-Hydroxy-5-((3-nitrophenyl)diazenyl)phenyl)-1-Phenyl-1H-1,2,4-triazol-3(2H)-one (5f)
Off-white solid, m.p. 232–234°C; FT-IR (KBr) (υ max cm−1): 3,402 (O–H), 3,068 (CH–Ar), 1,639 (C═O), 1,596 and 1,568 (C═C, Ar), 1,531 and 1,319 (NO2); 1H NMR (400 MHz, DMSO-d 6) δ: 12.33 (s, 1H, OH), 8.64 (s, 2H), 8.34–8.48 (m, 6H), 7.92 (d, J = 8.4 Hz, 2H), 7.67 (t, J = 8.2 Hz, 2H) ppm; 13C NMR (100 Hz, DMSO-d 6) δ: 197.4, 167.4, 161.7, 149.4, 147.4, 145.2, 133.2, 131.3, 130.6, 129.4, 129.0, 119.4, 115.2 ppm. HRMS (m/z 402.11). Anal calc. for C20H14N6O4: C, 59.70; H, 3.51; N, 20.89. Found: C, 59.68; H, 3.49; N, 20.91.
2.3.7 5-(2-Hydroxy-5-((4-nitrophenyl)diazenyl)phenyl)-1-Phenyl-1H-1,2,4-triazol-3(2H)-one (5g)
Off-white solid, m.p. 231–233°C; FT-IR (KBr) (υ max cm−1): 3,372 (O–H), 3,070 (CH–Ar), 1,663 (C═O), 1,614 and 1,561 (C═C, Ar), 1,533 and 1,361 (NO2); 1H NMR (400 MHz, DMSO-d 6) δ: 12.33 (s, 1H, OH), 8.40–8.44 (m, 5H), 8.07 (d, J = 8.2 Hz, 2H), 8.04–8.06 (m, 4H), 7.20 (t, J = 7.8 Hz, 2H) ppm; 13C NMR (100 Hz, DMSO-d 6) δ: 205.3, 170.7, 155.4, 148.3, 144.4, 128.4, 126.8, 124.6, 122.0, 121.2, 118.4, 113.9 ppm. HRMS (m/z 402.11). Anal calc. for C20H14N6O4: C, 59.70; H, 3.51; N, 20.89. Found: C, 59.72; H, 3.48; N, 20.91.
2.3.8 5-(2-Hydroxy-5-((4-methoxyphenyl)diazenyl)phenyl)-1-Phenyl-1H-1,2,4-triazol-3(2H)-one (5h)
White solid, m.p. 211–213°C; FT-IR (KBr) (υ max cm−1): 3,422 (O–H), 3,029 (CH–Ar), 2,926 (CH–aliphatic), 1,729 (C═O), 1,534 and 1,475 (C═C, Ar), 1,284 (C–O); 1H NMR (400 MHz, DMSO-d 6) δ: 12.22 (s, 1H, OH), 8.50 (d, J = 8.2 Hz, 2H), 8.18 (d, J = 7.8 Hz, 1H), 8.07 (t, J = 8.2 Hz, 1H), 7.72 (s, br., 2H), 7.42 (s, br., 4H), 7.40 (d, J = 7.8 Hz, 2H), 3.71 (s, 3H, OCH3) ppm; 13C NMR (100 Hz, DMSO-d 6) δ: 202.2, 150.4, 141.8, 135.7, 132.5, 131.3, 130.7, 130.3, 129.7, 129.2, 128.5, 125.9, 59.1 ppm. HRMS (m/z 387.13). Anal calc. for C21H17N5O3: C, 65.11; H, 4.42; N, 18.08. Found: C, 65.09; H, 4.45; N, 18.10.
2.3.9 5-(5-((3-Chlorophenyl)diazenyl)-2-hydroxyphenyl)-1-Phenyl-1H-1,2,4-triazol-3(2H)-one (5i)
White solid, m.p. 189–191°C; FT-IR (KBr) (υ max cm−1): 3,284 (O–H), 3,035 (CH–Ar), 1,603 (C═O), 1,565 and 1,546 (C═C, Ar); 1H NMR (400 MHz, CDCl3) δ: 11.68 (s, 1H, OH), 8.61 (s, 1H), 8.51–8.48 (m, 6H), 8.27–8.33 (m, 3H), 7.90 (t, J = 7.8 Hz, 2H) ppm; 13C NMR (100 Hz, CDCl3) δ: 196.5, 163.8, 159.4, 155.3, 150.8, 138.6, 133.7, 130.9, 130.3, 129.3, 129.1, 123.0, 118.4 ppm. HRMS (m/z 391.08). Anal calc. for C20H14ClN5O2: C, 61.31; H, 3.60; N, 17.87. Found: C, 61.33; H, 3.59; N, 17.85.
2.3.10 5-(5-((4-chlorophenyl)diazenyl)-2-hydroxyphenyl)-1-Phenyl-1H-1,2,4-triazol-3(2H)-one (5j)
White solid, m.p. 199–201°C; FT-IR (KBr) (υ max cm−1): 3,376 (O–H), 3,035 (CH–Ar), 1,605 (C═O), 1,543 and 1,525 (C═C, Ar); 1H NMR (400 MHz, CDCl3) δ: 10.14 (s, 1H, OH), 8.51–8.43 (m, 4H), 8.21 (d, J = 7.8 Hz, 2H), 8.09–7.85 (m, 4H), 7.80 (d, J = 7.8 Hz, 2H) ppm; 13C NMR (100 Hz, CDCl3) δ: 197.9, 168.7, 161.4, 154.2, 148.6, 143.4, 133.3, 131.4, 130.5, 112.5, 118.6, 116.0 ppm. HRMS (m/z 391.08). Anal calc. for C20H14ClN5O2: C, 61.31; H, 3.60; N, 17.87. Found: C, 61.29; H, 3.63; N, 17.89.
3 Results and discussion
To identify the optimal conditions deploying a model reaction, and to find the most suitable solvent, common solvents such as EtOAc, H2O, EtOH, Et2O, CH2Cl2, and DMF were studied. In these experiments, 2-hydroxy-5-(phenyldiazenyl)benzaldehyde 1a (1.0 mmol), ammonium acetate (1.0 mmol), phenylhydrazine (1.0 mmol), CO2 (1 MPa), and Fe3O4@SP-vanillin-TGA nanocomposite (0.1 g) were mixed with 5 mL solvent (Table 1). According to the data presented in Table 1, distilled water is found to be the most suitable solvent for this reaction (95% yield in 120 min).
Effect of various solvents on the synthesis of 5-aryldiazenyl-1,2,4-triazol-3-one 5a
| Entry | Solvent | Time (min) | Yield (%) |
|---|---|---|---|
| 1 | EtOAc | 180 | 68 |
| 2 | H 2 O | 120 | 95 |
| 3 | EtOH | 180 | 90 |
| 4 | Et2O | 240 | 78 |
| 5 | CH2Cl2 | 360 | 83 |
| 6 | DMF | 360 | 86 |
The optimum carbon dioxide pressure used to synthesize 5-aryldiazenyl-1,2,4-triazol-3-one 5a in H2O (5 mL) was investigated at room temperature. The results of the experimental study showed that increasing the gas pressure of CO2 has no significant effect on the reaction time and yields. The pressure of 1 MPa of CO2 for the synthesis of 5-(2-hydroxy-5-(phenyldiazenyl)phenyl)-1-phenyl-1H-1,2,4-triazol-3(2H)-one 5a is found to be optimal (Table 2).
Efficiency of various CO2 pressure in synthesis of 5-aryldiazenyl-1,2,4-triazol-3-one 5a
| Entry | CO2 pressure (MPa) | Time (min) | Yield (%) |
|---|---|---|---|
| 1 | 1 | 120 | 95 |
| 2 | 2 | 120 | 94 |
| 3 | 5 | 120 | 95 |
In order to optimize different reaction conditions, especially to improve the reaction time and yield, one-pot reaction of 2-hydroxy-5-(phenyldiazenyl)benzaldehyde 1a (1.0 mmol), ammonium acetate (1.0 mmol), phenylhydrazine (1.0 mmol), CO2 (1 MPa), and Fe3O4@SP@vanillin@TGA nanocomposite (0.1 g) in H2O (5 mL) was selected as a model reaction. First, among the available catalysts, such as montmorillonite K-10, KSF clay, ZnCl2, silica gel, nano-Fe3O4, nano-SiO2, nano-RHA/MCM-41, CuO@RHA/MCM-41, and Fe3O4@SP-vanillin-TGA nanocomposite were deployed as catalysts for the synthesis of 5-(2-hydroxy-5-(phenyldiazenyl)phenyl)-1-phenyl-1H-1,2,4-triazol-3(2H)-one 5a. According to the results presented in Table 3, reaction performed in the presence of Fe3O4@SP-vanillin-TGA nanocomposite in aqueous media, afforded excellent yields of products in short reaction times. Also, the model reaction did not show a significant difference at reflux temperature (100°C) and at room temperature (25°C) (Table 3; entries 10 and 11). Consequently, the optimal value of the Fe3O4@SP-vanillin-TGA nanocomposite was investigated at room temperature (Table 3; entries 11–13). The results showed that the reaction in the presence of Fe3O4@SP-vanillin-TGA nanocomposite (0.1 g) in H2O (5 mL) demonstrated the best result with 95% isolated yield for 2 h (Table 3; entry 11).
Optimization of reaction conditions for the synthesis of 5-(2-hydroxy-5-(phenyldiazenyl)phenyl)-1-phenyl-1H-1,2,4-triazol-3(2H)-one 5a
| Entry | Catalyst | Catalyst amount/1 mmol of aldehyde | Temperature | Time (h) | Yield (%) |
|---|---|---|---|---|---|
| 1 | Silica gel | 0.1 g | Reflux | 24 | — |
| 2 | ZnCl2 | 0.1 g | Reflux | 24 | — |
| 3 | K-10 | 0.1 g | Reflux | 24 | — |
| 4 | KSF | 0.1 g | Reflux | 24 | 63 |
| 5 | Nano-SiO2 | 0.1 g | Reflux | 24 | 59 |
| 6 | Nano-Fe3O4 | 0.1 g | Reflux | 12 | 72 |
| 7 | MCM-41 | 0.1 g | Reflux | 12 | 79 |
| 8 | Nano-RHA/MCM-41 [23] | 0.1 g | Reflux | 6 | 82 |
| 9 | CuO@RHA/MCM-41 [23] | 0.1 g | Reflux | 6 | 74 |
| 10 | Fe3O4@SP-vanillin-TGA | 0.1 g | Reflux | 2 | 94 |
| 11 | Fe 3 O 4 @SP-vanillin-TGA | 0.1 g | r.t. | 2 | 95 |
| 12 | Fe3O4@SP-vanillin-TGA | 0.2 g | r.t. | 2 | 94 |
| 13 | Fe3O4@SP-vanillin-TGA | 0.05 g | r.t. | 4 | 90 |
Investigation of the range of diversity of products showed that various 4-aryldiazenyl-salicylaldehydes could be easily accessed deploying this protocol. It was shown that different synthesized salicylaldehydes reacted faster for electron-withdrawing groups than electron-releasing groups. In the interim, it has been observed that superior yields are attained with substrates bearing electron-withdrawing groups (Table 4).
Synthesis of 5-aryldiazenyl-1,2,4-triazol-3-ones using Fe3O4@SP-vanillin-TGA nanocomposite
| Entry | Product | Ar1 | Ar2 | Time (h) | Yield (%)a |
|---|---|---|---|---|---|
| 1 | 5a | C6H5 | C6H5 | 2 | 95 |
| 2 | 5b | C6H5 | 4-CH3C6H4 | 1 | 90 |
| 3 | 5c | 2-NO2C6H4 | 4-CH3C6H4 | 1 | 94 |
| 4 | 5d | 2-BrC6H4 | C6H5 | 1 | 92 |
| 5 | 5e | 3-BrC6H4 | C6H5 | 1 | 95 |
| 6 | 5f | 3-NO2C6H4 | C6H5 | 1.5 | 93 |
| 7 | 5g | 4-NO2C6H4 | C6H5 | 1.5 | 94 |
| 8 | 5h | 4-CH3OC6H4 | C6H5 | 1 | 91 |
| 9 | 5i | 3-ClC6H4 | C6H5 | 1.5 | 88 |
| 10 | 5j | 4-ClC6H4 | C6H5 | 1.5 | 87 |
aIsolated yield.
Next, in view of establishing the sturdiness of the catalyst for recycling of the catalyst and its reuse was evaluated for Fe3O4@SP-vanillin-TGA. The data revealed that the catalyst could be recycled and reused up to the sixth time without significant loss of activity (Table 5).
Recycling of Fe3O4@SP-vanillin-TGA nanocomposite
| Run of reusability | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Yield (%) | 95 | 94 | 95 | 94 | 93 | 94 | 83 |
After study of recycling of Fe3O4@SP-vanillin-TGA nanocomposite, finally turn over number and turn over frequency of the present catalyst were also calculated for the model reaction based on the synthesis of 5a; they were found to be 661.32 and 330.66 h−1, respectively.
In the proposed mechanism for this reaction, it appears that Fe3O4@SP-vanillin-TGA nanocomposite serves as a Bronsted acid activates carbonyl group of aldehyde, then amino group of substrate 2 attacks active intermediate 2, thus producing imine B. In the next step, NH group as a nucleophile from imine compound B attacks CO2 and produces intermediate C. Finally, nucleophilic attack of arylhydrazine 4, followed by ring closure and oxidation, generates the final product 5 (Scheme 2).

Proposed mechanism for synthesis of 5-aryldiazenyl-1,2,4-triazol-3-ones.
4 Conclusion
In conclusion, an efficient, green, and novel procedure is developed for the synthesis of new 5-aryldiazenyl-1,2,4-triazol-3-ones using Fe3O4@SP-vanillin-TGA. The expeditious and eco-friendly protocols with the ease of work-up, along with the use of inexpensive and earth-abundant materials are the notable features of this unprecedented procedure. To the best of our knowledge, this is the first report for the synthesis of novel 5-aryldiazenyl-1,2,4-triazol-3-ones using carbon dioxide as an available substrate under greener conditions. The presented procedure may stimulate further studies for the assembly of new heterocyclic entities in future.
Acknowledgements
Financial support from the Research Council of Rasht Branch, Islamic Azad University, Rasht, Iran is sincerely acknowledged.
-
Funding information: This research was funded by Research Council of Rasht Branch, Islamic Azad University, Rasht, Iran.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Batchelor DV, Beal DM, Brown TB, Ellis D, Gordon DW, Johnson PS, et al. A convenient synthesis of highly substituted 3-N,N-dialkylamino-1,2,4-triazoles. Synlett. 2008;16:2421–4.10.1055/s-2008-1078208Search in Google Scholar
[2] Ueda S, Nagasawa H. Facile synthesis of 1,2,4-triazoles via a copper-catalyzed tandem addition-oxidative cyclization. J Am Chem Soc. 2009;131:15080–1.10.1021/ja905056zSearch in Google Scholar PubMed
[3] Koparir M, Cansiz A, Demirdag A. Synthesis of some new 4,5-substituted-4H-1,2,4-triazole-3-thiol derivatives. Molecules. 2004;9:204–12.10.3390/90400204Search in Google Scholar PubMed PubMed Central
[4] Foroumadi A, Mansouri S, Kiani Z, Rahmani A. Synthesis and in vitro antibacterial evaluation of N-[5-(5-nitro-2-thienyl)-1,3,4-thiadiazole-2-yl] piperazinyl quinolones. Eur J Med Chem. 2003;38:851–4.10.1016/S0223-5234(03)00148-XSearch in Google Scholar PubMed
[5] Mekuskiene G, Gaidelis P, Vainilavicius P. Synthesis and properties of 5(4,6-diphenyl-2-pyrimidin-2-yl)-1, 2,4-triazolin-3-thione and derivatives. Pharmazie. 1998;53:94–8.10.1002/chin.199821145Search in Google Scholar
[6] Noto R, Meo PL, Gruttadauria M, Werber G. A quantitative study of substituent effects on oxidative cyclization of some 2-aryl-substituted aldehyde thiosemicarbazones induced by ferric chloride and cupric perchlorate. J Heterocycl Chem. 1999;36:667–74.10.1002/jhet.5570360315Search in Google Scholar
[7] Varma RS. Greenness of things. Clean Technol Environ Policy. 2021;23:2497–8.10.1007/s10098-021-02201-0Search in Google Scholar
[8] Varma RS. Journey on greener pathways: from the use of alternate energy inputs and benign reaction media to sustainable applications of nano-catalysts in synthesis and environmental remediation. Green Chem. 2014;16:2027–41.10.1039/c3gc42640hSearch in Google Scholar
[9] Polshettiwar V, Varma RS. Green chemistry by nano-catalysis. Green Chem. 2010;12:743–54.10.1039/b921171cSearch in Google Scholar
[10] Nasir Baig RB, Varma RS. Book Chapter in “Metal catalysis in water”. In Dixneuf, Cadierno, editors. Chapter 9. Wiley-VCH Book; 2013. p. 337–94.Search in Google Scholar
[11] Vasconcelos SC, Marchini L, Lima CGS, Madriaga VG, Ribeiro RSA, Rossa V, et al. Single-atom catalysts for the upgrading of biomass-derived molecules: an overview on their preparation, properties and applications. Green Chem. 2022;24:2722–51. 10.1039/D1GC03809E.Search in Google Scholar
[12] Singh B, Gawande MB, Kute AT, Varma RS, Fornasiero P, McNeice P, et al. Single-atom (iron-based) catalysts: synthesis and applications. Chem Rev. 2021;121:13620–97. 10.1021/acs.chemrev.1c00158.Search in Google Scholar PubMed
[13] Sharma P, Kumar S, Tomanec O, Petr M, Chen JZ, Miller JT, et al. Carbon nitride-based ruthenium single atom photocatalyst for CO2 reduction to methanol. Small. 2021;17:2006478. 10.1002/smll.202006478.Search in Google Scholar PubMed
[14] Fardood ST, Ramazani A, Moradi S. Green synthesis of Ni–Cu–Mg ferrite nanoparticles using tragacanth gum and their use as an efficient catalyst for the synthesis of polyhydroquinoline derivatives. J Sol–Gel Sci. 2017;82:432–9.10.1007/s10971-017-4310-6Search in Google Scholar
[15] Zare Fekri L, Nikpassand M, Pourmirzajani S, Aghazadeh B. Synthesis and characterization of amino glucose-functionalized silica-coated NiFe2O4 nanoparticles: a heterogeneous, new and magnetically separable catalyst for the solvent-free synthesis of pyrano[3,2-c]chromen-5(4H)-ones. RSC Adv. 2018;8:22313–20.10.1039/C8RA02572JSearch in Google Scholar
[16] Molla RA, Iqubal MA, Ghosh K, Islam SM. A route for direct transformation of aryl halides to benzyl alcohols via carbon dioxide fixation reaction catalyzed by a (Pd@N-GMC) palladium nanoparticle encapsulated nitrogen doped mesoporous carbon material. Green Chem. 2016;18:4649–56.10.1039/C6GC01038ESearch in Google Scholar
[17] Tani Y, Kuga K, Fujihara T, Terao J, Tsuji Y. Copper-catalyzed C–C bond-forming transformation of CO2 to alcohol oxidation level: selective synthesis of homoallylic alcohols from allenes, CO2, and hydrosilanes. Chem Commun. 2015;51:13020–3.10.1039/C5CC03932KSearch in Google Scholar
[18] Farshbaf S, Zare Fekri L, Nikpassand M, Mohammadi R, Vessally E. Dehydrative condensation of β-aminoalcohols with CO2: an environmentally benign access to 2-oxazolidinone derivatives. J CO2 Util. 2018;25:194–204.10.1016/j.jcou.2018.03.020Search in Google Scholar
[19] Jafarian Z, Nikpassand M, Pourahmad A, Zare Fekri L. Synthesis of novel fused Azo-linked acridine derivatives using GO-ZnO nanocomposite. J Mol Struc. 2021;1245:131081.10.1016/j.molstruc.2021.131081Search in Google Scholar
[20] Nikpassand M. NiFe2O4@SiO2@glucose amine nanoparticle catalyzed reaction of azo-linked thiosalicylic acid with CO2: access to azo-linked benzo [d]oxathiine-2,4-diones. Dye Pigm. 2020;173:107936.10.1016/j.dyepig.2019.107936Search in Google Scholar
[21] Nikpassand M, Farshami MJ. One-pot synthesis of novel 3-pyrazolyl-4H-1,2,4-triazoles using amino glucose‐functionalized silica-coated NiFe2O4 nanoparticles as a magnetically separable catalyst. J Clust Sci. 2021;32:975–82.10.1007/s10876-020-01855-ySearch in Google Scholar
[22] Nikpassand M, Keyhani A, Zare Fekri L, Varma RS. Mechanochemical synthesis of azo-linked 2-amino-4H-chromene derivatives using Fe3O4@SiO2@KIT-6-NH2@Schiff-base complex nanoparticles. J Mol Struct. 2022;251:132065.10.1016/j.molstruc.2021.132065Search in Google Scholar
[23] Nikpassand M, Zare Fekri L, Farokhian P. An efficient and green synthesis of novel benzoxazole under ultrasound irradiation. Ultrason Sonochem. 2016;28:341–5.10.1016/j.ultsonch.2015.08.014Search in Google Scholar PubMed
[24] Nikpassand M, Zare Fekri L, Nabatzadeh M. Fe3O4@SiO2@KIT-6 as an efficient and reusable catalyst for the synthesis of novel derivatives of 3,3’-((aryl-1-phenyl-1H-pyrazol-4-yl) methylene) bis (1H-indole). Comb Chem High Throughput Screen. 2017;20:533–8.10.2174/1386207320666170425123248Search in Google Scholar PubMed
[25] Nikpassand M, Zare Fekri L, Pourahmad A. One-pot synthesis of new azo-linked 4H-benzo [d][1,3]oxazine-2,4-diones from carbon dioxide using CuO@RHA/MCM-41 nanocomposite in green media. J CO2 Util. 2018;27:320–5.10.1016/j.jcou.2018.08.011Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Synthesis, characterization, and antibacterial activity of a new poly azo compound containing N-arylsuccinimid and dibenzobarrelene moieties
- Design, synthesis, and antiviral activities evaluation of novel quinazoline derivatives containing sulfonamide moiety
- Design, synthesis, and anticancer activity of novel 4,6-dimorpholinyl-1,3,5-triazine compounds
- Design, synthesis, biological evaluation, and bio-computational modeling of imidazo, thieno, pyrimidopyrimidine, pyrimidodiazepene, and motifs as antimicrobial agents
- Synthesis of a novel phosphate-containing ligand rhodium catalyst and exploration of its optimal reaction conditions and mechanism for the polymerization of phenylacetylene
- Design, synthesis, and antiproliferative activity of novel 1,2,4-triazole-chalcone compounds
- Synthesis of metal–organic nanofiber/rGO nanocomposite as the sensing element for electrochemical determination of hypoxanthine
- Design and synthesis of various 1,3,4-oxadiazoles as AChE and LOX enzyme inhibitors
- Bis(2-cyanoacetohydrazide) as precursors for synthesis of novel azoles/azines and their biological evaluation
- Synthesis, characterization, and biological target prediction of novel 1,3-dithiolo[4,5-b]quinoxaline and thiazolo[4,5-b]quinoxaline derivatives
- Sustainable conversion of carbon dioxide into novel 5-aryldiazenyl-1,2,4-triazol-3-ones using Fe3O4@SP-vanillin-TGA nanocomposite
- Erratum
- Erratum to “Design, synthesis and study of antibacterial and antitubercular activity of quinoline hydrazone hybrids”
- SI: Undergraduate Research in the Synthesis of Biologically Active Small Molecules and Their Applications
- Preparation of novel acyl pyrazoles and triazoles by means of oxidative functionalization reactions
- Synthesis and conformational analysis of N-BOC-protected-3,5-bis(arylidene)-4-piperidone EF-24 analogs as anti-cancer agents
- SI: Development of Heterocycles for Biomedical and Bioanalytical Applications
- Influence of octreotide on apoptosis and metabolome expression in lipopolysaccharide-induced A549 cells
- Crude extract of J1 fermentation promotes apoptosis of cervical cancer cells
Articles in the same Issue
- Research Articles
- Synthesis, characterization, and antibacterial activity of a new poly azo compound containing N-arylsuccinimid and dibenzobarrelene moieties
- Design, synthesis, and antiviral activities evaluation of novel quinazoline derivatives containing sulfonamide moiety
- Design, synthesis, and anticancer activity of novel 4,6-dimorpholinyl-1,3,5-triazine compounds
- Design, synthesis, biological evaluation, and bio-computational modeling of imidazo, thieno, pyrimidopyrimidine, pyrimidodiazepene, and motifs as antimicrobial agents
- Synthesis of a novel phosphate-containing ligand rhodium catalyst and exploration of its optimal reaction conditions and mechanism for the polymerization of phenylacetylene
- Design, synthesis, and antiproliferative activity of novel 1,2,4-triazole-chalcone compounds
- Synthesis of metal–organic nanofiber/rGO nanocomposite as the sensing element for electrochemical determination of hypoxanthine
- Design and synthesis of various 1,3,4-oxadiazoles as AChE and LOX enzyme inhibitors
- Bis(2-cyanoacetohydrazide) as precursors for synthesis of novel azoles/azines and their biological evaluation
- Synthesis, characterization, and biological target prediction of novel 1,3-dithiolo[4,5-b]quinoxaline and thiazolo[4,5-b]quinoxaline derivatives
- Sustainable conversion of carbon dioxide into novel 5-aryldiazenyl-1,2,4-triazol-3-ones using Fe3O4@SP-vanillin-TGA nanocomposite
- Erratum
- Erratum to “Design, synthesis and study of antibacterial and antitubercular activity of quinoline hydrazone hybrids”
- SI: Undergraduate Research in the Synthesis of Biologically Active Small Molecules and Their Applications
- Preparation of novel acyl pyrazoles and triazoles by means of oxidative functionalization reactions
- Synthesis and conformational analysis of N-BOC-protected-3,5-bis(arylidene)-4-piperidone EF-24 analogs as anti-cancer agents
- SI: Development of Heterocycles for Biomedical and Bioanalytical Applications
- Influence of octreotide on apoptosis and metabolome expression in lipopolysaccharide-induced A549 cells
- Crude extract of J1 fermentation promotes apoptosis of cervical cancer cells

