Abstract
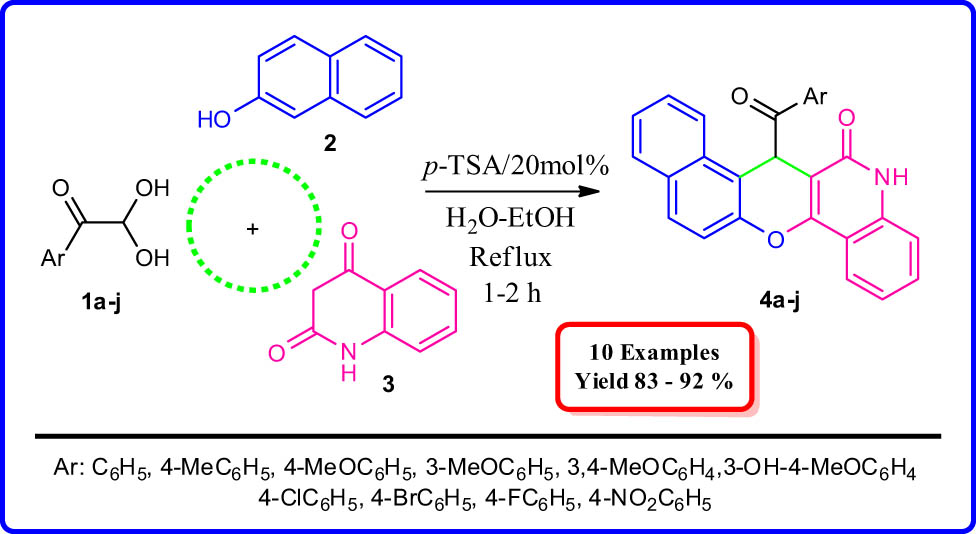
A new series of benzo[5,6]chromeno[3,2-c]quinoline derivatives were successfully synthesized using various arylglyoxal monohydrates, quinoline-2,4-dione, and β-naphthol in H2O:EtOH (2:1) as a green solvent in the presence of catalytic amounts p-toluenesulfonic acid as a mild catalyst under reflux conditions with high yields (83–92%). The reaction conditions were optimized in different solvents at variable thermal conditions, and the optimized reaction condition for this synthesis has been reported. The structures of all new products were defined by 1H-NMR, 13C-NMR, FT-IR, mass spectral data, and HRMS.
Graphical abstract
1 Introduction
In this research, ten new analogs of 4H-pyran compounds were successfully synthesized using a one-pot, multicomponent reaction approach, which may have medicinal and pharmacological properties. Polyfunctionalized 4H-pyran nucleus is a fertile source of important biological molecules with a wide range of interestingly biological and pharmaceutical activities [1]. 4H-pyran and chromene structures have had an effective role in the pharmaceutical research in recent years and have been used as precursors in synthesizing pharmaceutically active compounds [2].
Some reported crucial features of these therapeutic compounds are as follows: anti-tumor activities [3,4], antibacterial [3,5], anti-viral [3,6], anti-oxidant [7], anti-diabetic [8], anti-allergenic [9], anti-rheumatic [10], antispasmodics [11], anti-cancer [12,13,14], anti-HIV protease inhibitors [15], anticonvulsants [16], antimicrobials [17], anti-Schizophrenia [18], psychotropic, anti-inflammatory [19], cardiotonic activities [20], anti-malaria [21], and treatment of neurodegenerative disorders including Alzheimer and Parkinson [22]. Due to the widespread and useful applications of heterocyclic derivatives incorporating the 4H-pyrans and chromenes scaffold [23], the development of this method to synthesize other new derivatives has been considered by chemists and pharmacists in recent years; hence, it can be emphasized that heterocyclic compounds play a significant and fundamental role in the development of modern organic chemistry [24,25]. As mentioned, many pyrans and chromenes have some effects in the field of pharmacy, and some of them are currently used as potent drugs in some clinical treatments [26,27,28,29,30,31,32].
For example: 4-(allyloxy)-2H-chromen-2-one (Figure 1a) [33] and N-(3-cyano-5-oxo-4,7-diphenyl-5,6,7,8-tetrahydro-4H-chromen-2-yl)acetamide (Figure 1b) [34] nowadays are being developed as anti-cancer agents. Moreover, 2-amino-3-cyano-4,7-dihydro-4-(3,4,5-trimethoxyphenyl)-pyrano[2,3-e]indole (Figure 1c) [35] is also used as an antibacterial agent. Additionally, 2-(3,4-dihydroxyphenyl)3,5-dihydroxy-7-methoxy chromen-4-one (Figure 1d) [36] exhibits an effect of anti-rheumatism usage.
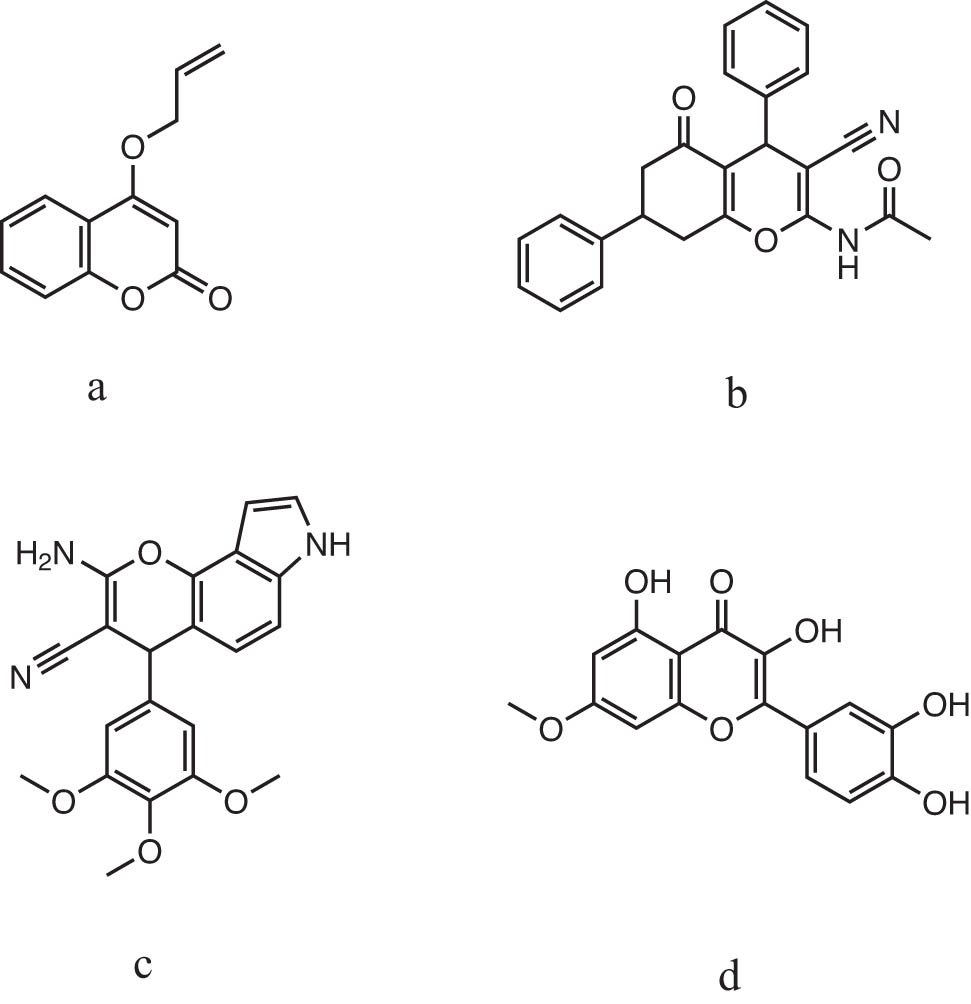
Chromene-based active molecules.
In addition, the selected method has been one of the best available techniques for the synthesis of biologically active heterocycles in recent years because of some remarkable targeted advantages such as simplicity of isolation and purification steps, use of green solvent, more productivity with chemo- and regioselectivity, having environmentally sustainable conditions, green strategy, avoiding unwanted and undesirable by-products and economic cost-effectiveness and easy handling [37,38,39,40,41,42,43,44]. As mentioned earlier, a one-pot three-component reaction protocol is used in this research to synthesize the new series of benzo[5,6]chromeno[3,2-c]quinoline derivatives.
2 Results and discussion
In continuation of our work on arylglyoxal-based synthesis of heterocyclic compounds, using one-pot, multicomponent strategies, herein, it is reported as a convenient one-pot, three-component process for the synthesis of chromeno derivatives, using arylglyoxals, quinoline-2,4(1H,3H)-dione, and naphthalen-2-ol in the presence of p-TSA in EtOH:H2O (1:2). This synthetic strategy is promising for the synthesis of novel chromene structures that may have pharmaceutical and biological activities (Figure 2).
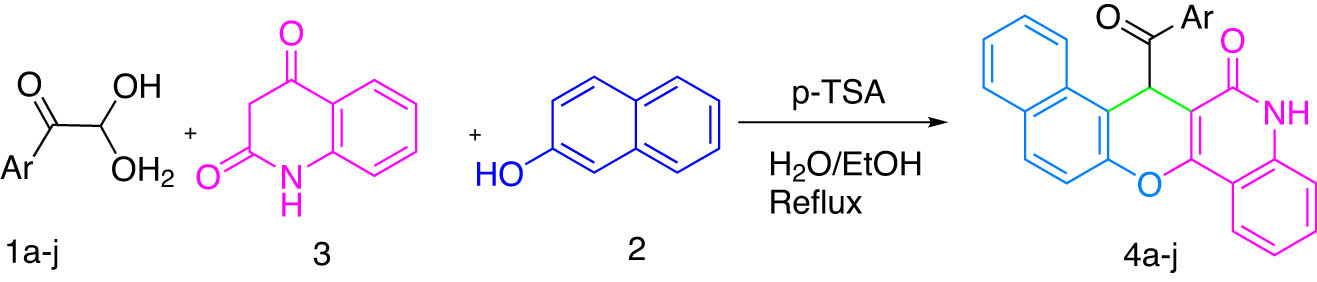
Synthesis of chromeno derivatives using arylglyoxals, quinoline-2,4(1H,3H)-dione, and naphthalen-2-ol in the presence of p-TSA in EtOH:H2O (1:2).
To find the optimal reaction conditions, this investigation started with the synthesis of benzo[5,6]chromeno[3,2-c]quinoline derivatives by a systematic study on the model reaction of arylglyoxals, quinoline-2,4(1H,3H)-dione, and naphthalen-2-ol (molar ratio 1:1:1) using various solvents, catalysts, times, and temperatures to evaluate the rate and the yield of reactions.
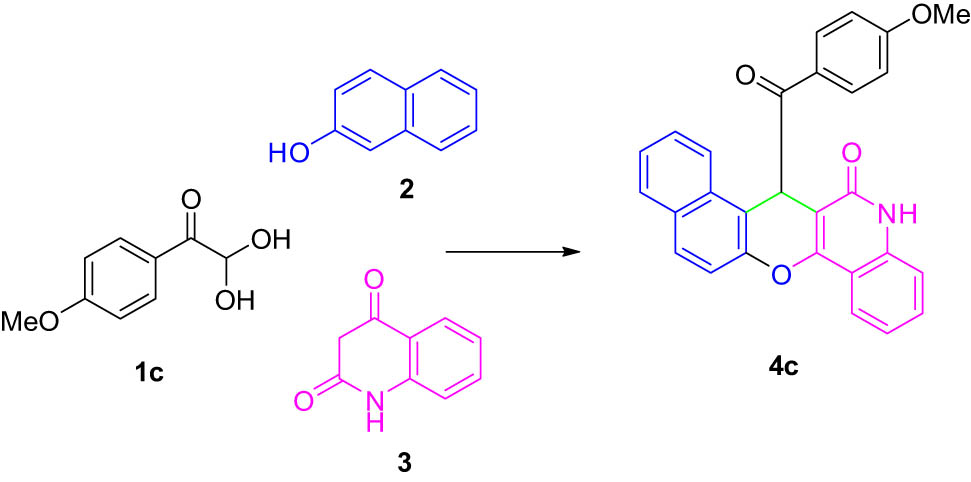
To find the best catalyst for these reactions, the reactions were performed using p-TSA, l-cysteine, DABCO, l-proline, β-alanine, and Et3N as selected catalysts, and the best result was obtained in terms of yield (92%) and reaction time (1 h) when the reaction was performed using 20 mol% of p-TSA (Table 1, entry 5).
Optimization of the reaction conditions for the synthesis of compound 4c
|
|||||
|---|---|---|---|---|---|
| Entry | Catalyst | Temperature (°C) | Solvent | Time (h) | Yield (%) |
| 1 | No catalyst | 50 | EtOH/H2O | 5 | No reaction |
| 2 | No catalyst | Reflux | EtOH/H2O | 3 | 47 |
| 3 | p-TSA (20 mol%) | Reflux | H2O | 5 | No reaction |
| 4 | p-TSA (20 mol%) | Reflux | EtOH | 5 | 50 |
| 5 | p-TSA (20 mol%) | Reflux | EtOH/H2O | 1 | 92 |
| 6 | P-TSA (20 mol%) | Reflux | THF | 1 | 66 |
| 7 | P-TSA (20 mol%) | Reflux | CH2Cl2 | 1 | 63 |
| 8 | l-Cysteine (20 mol%) | Reflux | EtOH/H2O | 1 | 61 |
| 9 | DABCO (20 mol%) | Reflux | EtOH/H2O | 1 | 62 |
| 10 | l-Proline (20 mol%) | Reflux | EtOH/H2O | 1 | 63 |
| 11 | β-Alanine (20 mol%) | Reflux | EtOH/H2O | 1 | 63 |
| 12 | Et3N (20 mol%) | Reflux | EtOH/H2O | 1 | 47 |
| 13 | K2CO3 (20 mol%) | Reflux | EtOH/H2O | 1 | 48 |
DABCO: 1,4-diazabicyclo[2,2,2]octane; p-TSA: p-toluene sulfonic acid.
To determine the best solvent, the reactions were repeated in various solvents such as water (H2O), ethanol (EtOH), EtOH:H2O (1:2), tetrahydrofuran (THF), and dichloromethane (CH2Cl2). The use of EtOH:H2O (1:2) proved to be the best in terms of yield and reaction time (Table 1, entry 5). The products were fully characterized by their FT-IR, 1H-NMR, and 13C-NMR spectral data and mass analysis.
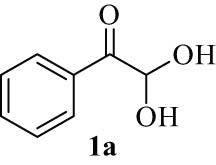
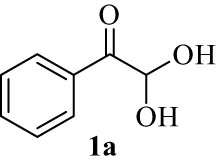
We explored the optimized condition to different arylglyoxal derivatives 1a–j (monohydrate form), naphthalen-2-ol (2), and quinoline-2,4(1H,3H)-dione (3) to form the desired products 4a–j in high yields. The results with various arylglyoxals, product, melting points, and yields are summarized in Table 2.
Synthesis of chromeno derivatives 4a–j via the one-pot, three-component reaction
| Entry | Arylglyoxal monohydrates | Products | Time (h) | M.P. (oC) | Yield (%) | Color |
|---|---|---|---|---|---|---|
| 1(a) |
|

|
2 | 299–300 | 87 | White |
| 2(b) |
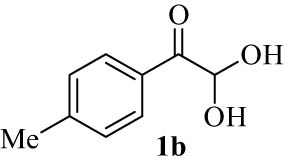
|
|
1 | 297–298 | 90 | White |
| 3(c) |
|
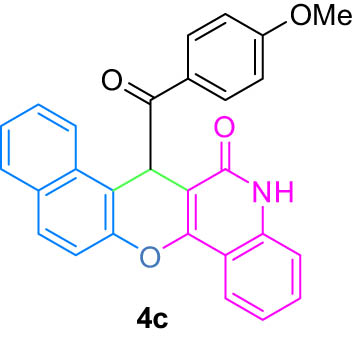
|
1 | 281–282 | 92 | White |
| 4(d) |
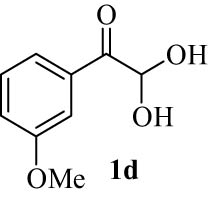
|
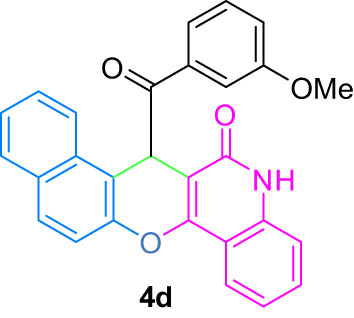
|
2 | 294 (Dec.) | 86 | White |
| 5(e) |
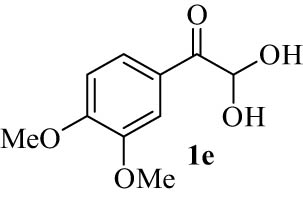
|

|
1 | 274–275 | 84 | White |
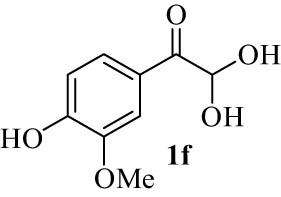
| 6(f) |
|
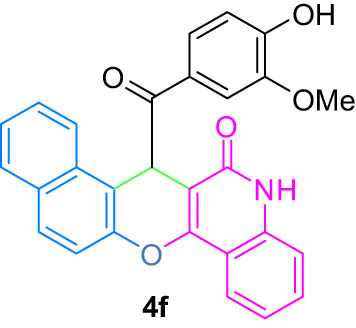
|
1 | 288–289 | 85 | White |
| 7(g) |
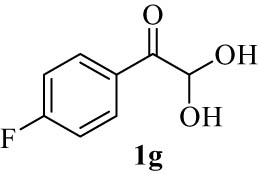
|
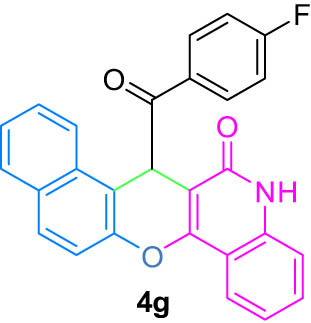
|
1 | 294 (Dec.) | 83 | White |
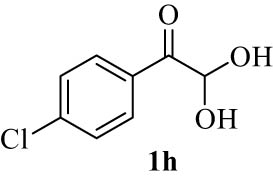
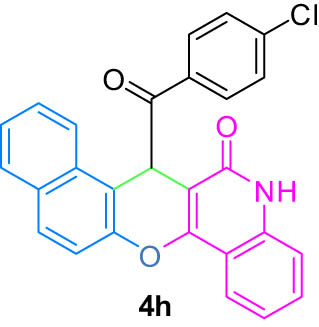
| 8(h) |
|
|
1 | 211–212 | 91 | Light yellow |
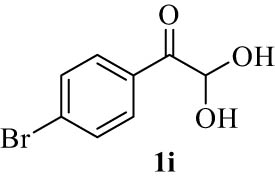
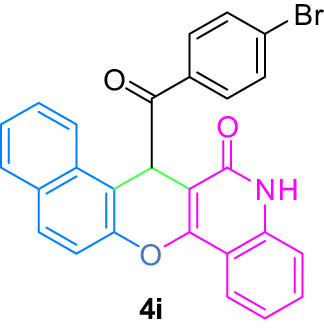
| 9(i) |
|
|
1 | 293 (Dec.) | 86 | White |
| 10(j) |
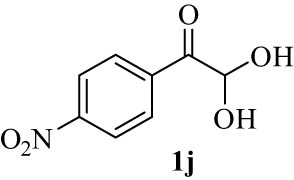
|

|
2 | 224–225 | 89 | Light yellow |
In the first stage, arylglyoxal monohydrates 1a–j are converted to free arylglyoxal in the presence of para-toluenesulfonic acid catalyst by removing a water molecule. This catalyst also converts quinoline-2,4-dione (3) to its enol form (enolate ion). Then Knoevenagel condensation between free glyoxal and quinoline enol form leads to the production of intermediate A, which is converted to intermediate B by the loss of a water molecule with the help of a catalyst.
Adding Michael β-naphthol (2) to intermediate B in the presence of para-toluene catalyst produces intermediate sulfonic acid C, which has a keto-enol totomerization. In the next step, the intermediate C in the presence of para-toluene sulfonic acid catalyst produces D through intramolecular density. By removing a water molecule from compound D in the presence of an acid catalyst, new heterocyclic derivatives 4a–j are synthesized. The possible mechanism is shown in Figure 3.
![Figure 3
A plausible mechanism for the synthesis of benzo[5,6]chromeno[2,3-d]pyrimidine derivatives catalyzed by p-TSA.](/document/doi/10.1515/hc-2020-0128/asset/graphic/j_hc-2020-0128_fig_003.jpg)
A plausible mechanism for the synthesis of benzo[5,6]chromeno[2,3-d]pyrimidine derivatives catalyzed by p-TSA.
All of the synthesized products, except 4h and 4j, are white powder and have a stable keto form. In 4j and 4h products, a stable enol form of eno l-keto tautomerization can be seen. It can be considered that the presence of the nitro group in 4j product and chloro group in h product on para position caused to form a stable enol form. The synthesized enol and keto form products have light yellow and white colors, respectively.
3 Conclusion
We have synthesized the 4H-pyran derivatives 4a–j by one-pot, three-component reaction of various arylglyoxals, quinoline-2,4-dione, and β-naphthol in H2O:EtOH (2:1) as a green solvent in the presence of catalytic amounts para toluenesulfonic acid as a mild and efficient catalyst under reflux conditions with high yields (83–92%). The simplicity of operation, high yields, environmentally benign, regioselectivity, and green solvents are the key advantages of this method.
4 Experimental
The chemicals and reagents used for the synthesis were obtained from Merck and Sigma Aldrich. Melting points were measured on an Electrothermal 9,200 apparatus and are uncorrected. Infrared spectra were measured on a Spectrum Tensor 27, Bruker, Equinox 55 FT-IR instrument using KBr disks. 1H (500 MHz) and 13C (125 MHz) NMR spectra were recorded on a Varian-Inova spectrometer in DMSO-d 6 with TMS as an internal reference. Analytical thin-layer chromatography (TLC) was carried out on pre-coated aluminum sheet with silica gel 60 F 254 obtained from Merck, and the detection was made with the help of a UV lamp (λ 254 nm). Mass analysis was performed on an Agilent Technology (HP) 5,973 Network Mass Selective Detector, and high-resolution mass spectra were recorded on a Kratos mass spectrometry (MS) 25RF spectrometer.
4.1 General procedure for the synthesis of benzo[5,6]chromeno[3,2-c]quinoline derivatives (4a–j)
To a suspension of aryl glyoxal monohydrates 1a–j (1 mmol) in H2O:EtOH (2:1) (5 mL), p-TSA (20 mol%) was added. The reaction mixture was heated and stirred under reflux conditions for 15 min to dissolve the reactant. Then, 4-hydroxyquinolin-2(1H)-one (2, 1 mmol, 161 mg) and β-naphthalene (3, 1 mmol, 144 mg) were added to the reaction mixture, which were stirred at the above-mentioned temperature for appropriate times as listed in Table 2. The development of the reaction was controlled by TLC (MeOH:CHCl3/1:10 as eluent). After completion of the reaction, the precipitate was filtered and washed with water to give the desired products 4a-j in high yield (83–92%).
4.2 7-Benzoyl-5,7-dihydro-6H-benzo[5,6]chromeno[3,2-c]quinolin-6-one (4a)
White powder; mp: 299–300°C; FT-IR ν
max: 2,947, 1,696, 1,633, 1,602, 1,444, 1,400, 818, 755, 673 cm−1; 1H NMR (500 MHz, DMSO-d
6) δ 12.56 (s, 1H, OH, D2O exch.), 12.17 (s, 1H, OH, D2O exch.), 7.98 (d, J = 7.7 Hz, 2H, Ar), 7.70 (d, J = 7.4 Hz, 2H, Ar), 7.59 (t, J = 7.3 Hz, 2H, Ar), 7.44 (t, J = 7.3 Hz, 2H, Ar), 7.39–7.34 (m, 3H, Ar), 7.33–7.28 (m, 3H, Ar), 6.35 (s, 1H, Ar); 13C NMR (125 MHz, DMSO-d
6) δ 183.8, 183.4, 179.4, 175.0, 174.5, 165.7, 161.9, 159.3, 149.5, 147.9, 144.7, 137.5, 132.5, 131.8, 128.48, 127.9, 123.7, 123.1, 116.4, 112.0, 109.8, 106.3, 102.4, 99.2, 88.1; LRMS (EI, 70 eV) m/z (%): 403 (M+, 33), 333 (100), 315 (26), 277 (40), 248 (47), 220 (10), 174 (19), 161 (31), 146 (13), 120 (33), 105 (74), 92 (28), 77 (68), 51 (12); HRMS (ESI): m/z (M)+ calcd for C27H17
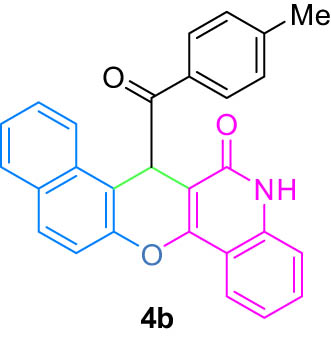
4.3 (E)-7-(Hydroxy(p-tolyl)methylene)-7H-benzo[5,6]chromeno[3,2-c]quinolin-6-ol (4b)
White powder; mp: 297–298°C; FT-IR ν
max: 2,952, 1,730, 1,694, 1,634, 1,604, 1,547, 1,401, 1,305, 807, 761, 669 cm−1; 1H NMR (500 MHz, DMSO-d
6) δ 12.53 (s, 1H, OH, D2O exch.), 12.16 (s, 1H, OH, D2O exch.), 7.98 (d, J = 8.1 Hz, 2H, Ar), 7.59 (t, J = 8.3 Hz, 4H, Ar), 7.37 (d, J = 8.2 Hz, 2H, Ar), 7.30 (t, J = 7.5 Hz, 3H, Ar), 7.14 (d, J = 7.9 Hz, 2H, Ar), 6.31 (s, 1H, Ar), 2.25 (s, 3H, Me); 13C NMR (125 MHz, DMSO-d
6) δ 194.9, 192.1, 186.3, 185.5, 184.4, 180.0, 177.1, 174.2, 169.8, 165.8, 164.5, 162.1, 142.8, 137.5, 135.2, 134.6, 131.8, 129.9, 129.1, 128.1, 123.7, 123.1, 116.4, 115.8, 109.9, 21.5; LRMS (EI, 70 eV) m/z (%): 417 (M+, 11), 333 (100), 315 (21), 240 (34), 119 (51), 91 (34), 65 (11); HRMS (ESI): m/z (M)+ calcd for C28H19
4.4 7-(4-Methoxybenzoyl)-5,7-dihydro-6H-benzo[5,6]chromeno[3,2-c]quinolin-6-one (4c)
White powder; mp: 281–282°C; FT-IR ν
max: 2,947, 1,696, 1,638, 1,603, 1,394, 1,313, 827, 758 cm−1; 1H NMR (500 MHz, DMSO-d
6) δ 12.51 (s, 1H, OH, D2O exch.), 12.15 (s, 1H, OH, D2O exch.), 7.98 (d, J = 8.0 Hz, 2H, Ar), 7.68 (d, J = 8.6 Hz, 2H, Ar), 7.59 (t, J = 7.4 Hz, 2H, Ar), 7.38 (d, J = 8.0 Hz, 2H, Ar), 7.30 (t, J = 7.4 Hz, 3H, Ar), 6.89 (d, J = 8.7 Hz, 2H, Ar), 6.28 (s, 1H, Ar), 3.74 (s, 3H, OMe); 13C NMR (125 MHz, DMSO-d
6) δ 182.7, 181.9, 177.8, 175.2, 172.1, 168.2, 165.8, 162.0, 159.2, 151.6, 149.2, 145.3, 142.8, 137.5, 134.3, 131.8, 130.1, 123.9, 123.1, 120.7, 116.4, 113.9, 103.8, 99.2, 52.4; LRMS (EI, 70 eV) m/z (%): 433 (M+, 49), 412 (18), 396 (15), 368 (12), 340 (42), 313 (85), 285 (41), 264 (85), 239 (100), 211 (47), 171 (19), 135 (51), 98 (40), 83 (35), 57 (41); HRMS (ESI): m/z (M)+ calcd for C28H19
4.5 (E)-7-(Hydroxy(3-methoxyphenyl)methylene)-7H-benzo[5,6]chromeno[3,2-c]quinolin-6-ol (4d)
White powder; mp: 294°C (Dec.); FT-IR ν
max: 2,874, 1,736, 1,683, 1,618, 1,435, 828, 756 cm−1; 1H NMR (500 MHz, DMSO-d
6) δ 12.52 (s, 1H, OH, D2O exch.), 12.16 (s, 1H, OH, D2O exch.), 7.96 (d, J = 8.2 Hz, 2H, Ar), 7.57 (t, J = 7.5 Hz, 2H, Ar), 7.35 (d, J = 8.1 Hz, 2H, Ar), 7.28 (t, J = 9.5 Hz, 2H, Ar), 7.23 (m, 4H, Ar), 7.00 (bs, 1H, Ar), 6.32 (s, 1H, Ar), 3.64 (s, 3H, OMe); 13C NMR (125 MHz, DMSO-d
6) δ 187.0, 182.9, 176.6, 172.8, 169.7, 166.6, 160.6, 158.9, 153.8, 150.6, 149.0, 145.8, 144.4, 141.2, 132.8, 128.8, 126.7, 123.2, 118.3, 114.5, 110.0, 103.2, 100.8, 96.0, 94.0, 84.4, 81.6, 50.2; LRMS (EI, 70 eV) m/z (%): 433 (M+, 8), 333 (39), 309 (72), 278 (30), 264 (14), 248 (33), 174 (26), 161 (38), 146 (10), 135 (100), 119 (22), 107 (24), 92 (50), 77 (31), 64 (14); HRMS (ESI): m/z (M)+ calcd for C28H19
4.6 (E)-7-((3,4-Dimethoxyphenyl)(hydroxy)methylene)-7H-benzo[5,6]chromeno[3,2-c]quinolin-6-ol (4e)
White powder; mp: 274–275; FT-IR ν
max: 3,366, 3,002, 2,837, 1,640, 1,603, 1,458, 1,399, 1,260, 1,231, 1,147, 1,027, 798, 762; 1H NMR (500 MHz, DMSO-d
6) δ 11.72 (s, 1H, OH, D2O exch.), 10.59 (s, 1H, OH, D2O exch.), 8.04 (d, J = 7.6 Hz, 1H, Ar), 7.96 (d, J = 7.8 Hz, 1H, Ar), 7.93–7.85 (m, 3H, Ar), 7.64 (s, 1H, Ar), 7.42 (d, J = 33.3 Hz, 3H, Ar), 7.33–7.24 (m, 3H, Ar), 7.03 (d, J = 8.4 Hz, 1H, Ar), 3.76 (s, 3H, OMe), 3.58 (s, 3H, OMe); 13C NMR spectrum could not be recorded due to the low solubility of the sample; LRMS (EI, 70 eV) m/z (%): 463 (M+, 100), 448 (8), 388 (3), 360 (3), 298 (3), 232 (6), 216 (4), 200 (6), 186 (4), 146 (5), 120 (5), 92 (4); HRMS (ESI): m/z (M)+ calcd for C29H21
4.7 (E)-7-(Hydroxy(4-hydroxy-3-methoxyphenyl)methylene)-7H-benzo[5,6]chromeno[3,2 c]quinolin-6-ol (4f)
White powder; mp: 288–289°C; FT-IR ν
max: 3,078, 2,617, 1,685, 1,637, 1,602, 1,389, 1,316, 1,270, 1,184, 817, 769 cm−1; 1H NMR (500 MHz, DMSO-d
6) δ 12.54 (s, 1H, OH, D2O exch.), 12.16 (s, 1H, OH, D2O exch.), 9.80 (s, 1H, OH, D2O exch.), 7.98 (d, J = 7.8 Hz, 2H, Ar), 7.59 (t, J = 7.7 Hz, 2H, Ar), 7.38 (d, J = 8.1 Hz, 2H, Ar), 7.33–7.27 (m, 4H, Ar), 7.20 (d, J = 8.0 Hz, 1H, Ar), 6.68 (d, J = 8.3 Hz, 1H, Ar), 6.26 (s, 1H, Ar), 3.60 (s, 3H, OMe); 13C NMR (125 MHz, DMSO-d
6) δ 195.1, 179.8, 174.1, 173.1, 171.7, 168.3, 165.8, 161.9, 159.0, 151.1, 147.2, 142.6, 137.4, 131.8, 128.3, 123.7, 123.1, 122.3, 116.4, 115.1, 112.0, 109.1, 108.5, 107.3, 102.3, 99.6, 55.6, 42.0; LRMS (EI, 70 eV) m/z (%): 449 (M+, 1), 325 (26), 174 (11), 161 (41), 151 (100), 119 (28), 108 (7), 92 (21), 77 (10), 65 (9), 52 (7); HRMS (ESI): m/z (M)+ calcd for C28H19
4.8 (E)-7-((4-Fluorophenyl)(hydroxy)methylene)-7H-benzo[5,6]chromeno[3,2-c]quinolin-6-ol (4g)
White powder; mp: 294°C (Dec.) °C; FT-IR ν
max: 2,948, 1,696, 1,634, 1,602, 1,439, 1,401, 1,304, 1,230, 1,154, 834, 751 cm−1; 1H NMR (500 MHz, DMSO-d
6) δ 12.53 (s, 1H, OH, D2O exch.), 12.18 (s, 1H, OH, D2O exch.), 7.98 (d, J = 8.1 Hz, 2H, Ar), 7.78–7.75 (m, 2H, Ar), 7.59 (t, J = 7.7 Hz, 2H, Ar), 7.37 (d, J = 8.2 Hz, 2H, Ar), 7.30 (t, J = 7.7 Hz, 3H, Ar), 7.19 (t, J = 8.6 Hz, 2H, Ar), 6.36 (s, 1H, Ar); 13C NMR (125 MHz, DMSO-d
6) δ 198.2, 173.4, 171.9, 171.3, 165.6, 163.6, 162.1, 155.0, 143.9, 142.0, 137.5, 133.9, 131.9, 130.7, 123.8, 123.1, 119.6, 116.35, 115.4, 109.5, 107.9, 102.6, 101.6, 96.7, 42.3; LRMS (EI, 70 eV) m/z (%): 421 (M+, 100), 404 (28), 298 (7), 273 (6), 244 (23), 120 (6), 92 (7). HRMS (ESI): m/z (M)+ calcd for C27H16F
4.9 7-(4-Chlorobenzoyl)-5,7-dihydro-6H-benzo[5,6]chromeno[3,2-c]quinolin-6-one (4h)
Light yellow powder; mp: 211v212°C; FT-IR ν
max: 2,947, 2,860, 1,659, 1,607, 1,578, 1,493, 1,443, 1,293, 1,259, 994, 882, 828, 749, 652 cm−1; 1H NMR (500 MHz, DMSO-d
6) δ 11.71 (s, 1H, OH, D2O exch.), 8.94 (d, J = 8.3 Hz, 1H, Ar), 7.97 (d, J = 8.7 Hz, 1H, Ar), 7.93 (d, J = 8.2 Hz, 1H, Ar), 7.80 (d, J = 8.0 Hz, 1H, Ar), 7.61 (t, J = 8.4 Hz, 3H, Ar), 7.56 (t, J = 8.7 Hz, 3H, Ar), 7.46 (d, J = 8.8 Hz, 1H, Ar), 7.41 (t, J = 8.5 Hz, 2H, Ar), 7.25 (t, J = 7.6 Hz, 1H, Ar), 5.54 (s, 1H, CH); 13C NMR (125 MHz, DMSO-d
6) δ 192.6, 171.3, 162.6, 160.5, 155.3, 140.4, 137.1, 135.1, 132.4, 131.4, 130.5, 129.5, 128.7, 127.4, 126.1, 125.5, 124.5, 122.9, 122.5, 119.6, 116.1, 112.3, 110.1, 110.0, 55.5; LRMS (EI, 70 eV) m/z (%): 439 ([M + 2]+, 37), 437 (M+, 100), 420 (28), 298 (11), 226 (25), 201 (10), 187 (23), 120 (12), 92 (10); HRMS (ESI): m/z (M)+ calcd for C27H16Cl
4.10 (E)-7-((4-Bromophenyl)(hydroxy)methylene)-7H-benzo[5,6]chromeno[3,2-c]quinolin-6-ol (4i)
White powder; mp: 293°C (Dec.); FT-IR ν
max: 2,957, 1,695, 1,634, 1,602, 1,441, 1,303, 1,008, 949, 806, 752, 701, 648 cm−1; 1H NMR (500 MHz, DMSO-d
6) δ 12.52 (s, 1H, OH, D2O exch.), 12.18 (s, 1H, OH, D2O exch.), 7.97 (d, J = 7.9 Hz, 2H, Ar), 7.64–7.55 (m, 7H, Ar), 7.37 (d, J = 8.2 Hz, 2H, Ar), 7.30 (t, J = 7.6 Hz, 2H, Ar), 6.36 (s, 1H, Ar); 13C NMR (125 MHz, DMSO) δ 165.7, 162.2, 137.5, 136.5, 132.4, 131.9, 131.5, 129.8, 127.6, 126.3, 123.7, 123.2, 116.4, 42.3; LRMS (EI, 70 eV) m/z (%): 483 ([M + 2]+, 96), 481 (M+, 100), 464 (22), 385 (15), 357 (46), 333 (84), 315 (21), 298 (18), 240 (37), 226 (38), 201 (19), 183 (75), 161 (59), 146 (23), 120 (71), 92 (69), 76 (35), 65 (23); HRMS (ESI): m/z (M)+ calcd for C27H16Br
4.11 (6-Hydroxy-7H-benzo[5,6]chromeno[3,2-c]quinolin-7-yl)(4-nitrophenyl)methanone (4j)
Light yellow powder; mp: 224–225°C; FT-IR ν max: 2,865, 1,662, 1,631, 1,608, 1,524, 1,349, 1,254, 1,010, 853, 807, 702 cm−1; 1H NMR (500 MHz, DMSO-d 6) δ 11.72 (s, 1H, OH, D2O exch.), 8.93 (d, J = 8.4 Hz, 1H, Ar), 8.30 (d, J = 8.7 Hz, 2H, Ar), 7.97 (d, J = 8.9 Hz, 1H, Ar), 7.92 (d, J = 8.3 Hz, 1H, Ar), 7.86 (d, J = 8.7 Hz, 2H, Ar), 7.79 (d, J = 7.8 Hz, 1H, Ar), 7.61–7.52 (m, 2H, Ar), 7.46 (d, J = 8.9 Hz, 1H, Ar), 7.40 (t, J = 7.7 Hz, 2H, Ar), 7.24 (t, J = 7.4 Hz, 1H, Ar), 5.62 (s, 1H, CH); 13C NMR (125 MHz, DMSO-d 6) δ 189.5, 179.6, 174.0, 169.1, 162.6, 160.4, 155.2, 144.4, 140.4, 138.6, 132.5, 131.5, 130.6, 128.8, 127.1, 126.2, 124.7, 122.6, 119.5, 116.1, 112.3, 109.9, 107.7, 99.9, 55.7; LRMS (EI, 70 eV) m/z (%): 448 (M+, 100), 431 (21), 385 (10), 372 (6), 298 (5), 226 (13), 187 (7), 120 (7), 92 (8); HRMS (ESI): m/z (M)+ calcd for C27H16N2O5 +: 448.1059; found: 448.1056.
Acknowledgment
The authors gratefully acknowledge the financial assistance from Islamic Azad University, Ahar branch.
-
Funding information: The authors state no funding involved.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Shehab WS, Ghoneim AA. Synthesis and biological activities of some fused pyran derivatives. Arab J Chem. 2011;9:S966–70. 10.1016/j.arabjc.2011.10.008.Suche in Google Scholar
[2] Sari E, Aslan H, Dadi S, Oktemer A, Logoglu E. Biological activity studies of some synthesized novel Furan and Pyran derivatives. J Sci. 2017;30(4):49–55.Suche in Google Scholar
[3] Mohammadi Ziarani G, Hosseini Mohtasham N, Lashkari N, Badiei A, Amanlou MR. Convenient one-pot synthesis of spirooxindole-4H-pyrans in the presence of SBA-Pr-NH2 and evaluation of their urease inhibitory activities. J Nanostruct. 2013;2:489–500.Suche in Google Scholar
[4] Keiko NA, Stepanova LG, Voronkov MG, Potapova GI, Gudratov NO, Treshchalina EM. Synthesis, DNA-inhibiting activity, and antitumor activity of 2-formyl-2,5-dimethoxy-2,3-dihydro-4H-pyran Thiosemicarbazone, a related ethyl analog, and a copper complex. Pharm Chem J. 2002;36:407–9.10.1023/A:1021246107534Suche in Google Scholar
[5] Us D, Gurdal E, Berk B, Oktem S, Kocagoz T, Caglayan B, et al. 4H-Pyran-4-one derivatives: leading molecule for preparation of compounds with antimycobacterial potential. Turk J Chem. 2009;33:803–12.10.3906/kim-0905-15Suche in Google Scholar
[6] Aytemir MD, Özçelik B. A study of cytotoxicity of novel chlorokojic acid derivatives with their antimicrobial and antiviral activities. Eur J Med Chem. 2010;45:4089–95.10.1016/j.ejmech.2010.05.069Suche in Google Scholar
[7] Shanthi G, Perumal PT, Rao U, Sehgal PK. Synthesis and antioxidant activity of indolyl chromenes. Indian J Chem. 2009;48:1319.10.1002/chin.201004144Suche in Google Scholar
[8] Soni R, Durgapal SD, Soman SS, Georrge JJ. Synthesis of 4H-Pyran derivatives under solvent-free and grinding conditions. Arab J Chem. 2016;12:701.10.1016/j.arabjc.2016.11.011Suche in Google Scholar
[9] Coudert P, Couquelet JM, Bastide J, Marion Y, Fialip J. Synthesis and anti-allergic properties of N-arylnitrones with furo-pyran structure. Ann Pharm Fr. 1988;46:91.Suche in Google Scholar
[10] Smith CW, Bailey JM, Billingham ME, Chandrasekhar C, Dell P, Harvey AK, et al. The anti-rheumatic potential of a series of 2,4-di-substituted-4H-naphtho[1,2-b]pyran-3-carbonitriles. Bioorg Med Chem Lett. 1995;5:2783.10.1016/0960-894X(95)00487-ESuche in Google Scholar
[11] Domarle O, Blampain G, Agnaniet H, Nzadiyabi T, Lebibi J, Brocard J, et al. In vitro antimalarial activity of a new organometallic analog, ferrocene-chloroquine. J Med Chem. 1997;40:3715.10.1128/AAC.42.3.540Suche in Google Scholar
[12] Wang Ch. Synthesis and biological evaluation of tetrahydrobenzo[b]pyran derivatives as potential anti-ovarian cancer agents. Biomed Res-India. 2016;27:S322–5.Suche in Google Scholar
[13] Wang Z, Shi XH, Wang J. Synthesis, structure–activity relationships and preliminary antitumor evaluation of benzothiazole-2-thiol derivatives as novel apoptosis inducers. Bioorg Med Chem Lett. 2011;21:1097–101.10.1016/j.bmcl.2010.12.124Suche in Google Scholar
[14] Jain Sh, Chandra V, Jain PK, Pathak K, Pathak D, Vaidya A. Comprehensive review on current developments of quinoline-based anticancer agents. Arab J Chem. 2019;12(8):4920–46.10.1016/j.arabjc.2016.10.009Suche in Google Scholar
[15] Douglas CJ, Sklenicka HM, Shen HC, Mathias DS, Degen SJ, Golding GM, et al. Synthesis and UV studies of a small library of 6-aryl-4-hydroxy-2-pyrones. A relevant structural feature for the inhibitory property of arisugacin against acetylcholinesterase. Tetrahedron. 1999;55(48):13683–96.10.1016/S0040-4020(99)00847-9Suche in Google Scholar
[16] Aytemir MD, Calis U, Ozalp M. Synthesis and evaluation of anticonvulsant and antimicrobial activities of 3-hydroxy-6-methyl-2-substituted 4H-pyran-4-one derivatives. Arch Pharm Weinh. 2004;337(5):281–8.10.1002/ardp.200200754Suche in Google Scholar PubMed
[17] Fairlamb IJ, Marrison LR, Dickinson JM, Lu FJ, Schmidt JP. 2-Pyrones possessing antimicrobial and cytotoxic activities. Bioorg Med Chem. 2004;12(15):4285–99.10.1016/j.bmc.2004.01.051Suche in Google Scholar
[18] Konkoy CS, Fick DB, Cai SX, Lan NC, Keana JFW. PCT Int. Appl. WO 0075123, 2000. Chem Abstr. 2001;134:29313a.Suche in Google Scholar
[19] Magesh CJ, Makesh SV, Perumal PT. Highly diastereoselective inverse electron demand (IED) Diels–Alder reaction mediated by chiral salen–AlCl complex: the first, target-oriented synthesis of pyranoquinolines as potential antibacterial agents. Bioorg Med Chem Lett. 2004;14:2035–40.10.1016/j.bmcl.2004.02.057Suche in Google Scholar
[20] Liu Z, Meinwald J. 5-(Trimethylstannyl)-2H-pyran-2-one and 3-(Trimethylstannyl)-2H-pyran-2-one: New 2H-Pyran-2-one Synthons. J Org Chem. 1996;61(19):6693–9.10.1021/jo951394tSuche in Google Scholar
[21] Bonsignore L, Loy G, Secci D, Calignano A. Synthesis and pharmacological activity of 2-oxo-(2H) 1-benzopyran-3-carboxamide derivatives. Eur J Med Chem. 1993;28:517.10.1016/0223-5234(93)90020-FSuche in Google Scholar
[22] (a) Andreani LL, Lapi E. On some new esters of coumarin-3-carboxylic acid wit balsamic and bronchodilator action. Bull Chim Farm. 1960;99:583–6; (b) Bonsignore L, Loy G, Secci D, Calignano A. Synthesis and pharmacological activity of 2-oxo-(2H) 1-benzopyran-3-carboxamide derivatives. Eur J Med Chem. 1993;28:517–20.Suche in Google Scholar
[23] Costa M, Dias TA, Brito A, Proenca F. Biological importance of structurally diversified chromens. Eur J Med Chem. 2016;123:487–507.10.1016/j.ejmech.2016.07.057Suche in Google Scholar
[24] Dig NC, Mahajan PG, Raza H, Hassan M, Vanjare BD, Hong H, et al. Synthesis and characterization of new 4H-chromene-3-carboxylates ensuring potent elastase inhibition activity along with their molecular docking and chemoinformatics properties. Bioorg Chem. 2020;100:103906. 10.1016/j.bioorg.2020.103906.Suche in Google Scholar
[25] (a) Lidstrom P, Tierney J, Wathey B. Westman, microwave assisted organic synthesis – a reviw. J Tetrahedron. 2001;57:9225–83; (b) Cabrele C, Reiser O. The modern face of synthetic heterocyclic chemistry. J Org Chem. 2016;81:10109–25; (c) Taylor AP, Robinson RP, Fobian Y M, Blakemore DC, Jones LH, Fadeyi O. Editorial for the special issue on heterocycles. Org Biomol Chem. 2016;14:6611–37.10.1016/S0040-4020(01)00906-1Suche in Google Scholar
[26] Mladenovic M, Vukovic N, Niciforovic N, Sukdolak S, Solujic S. Synthesis and molecular descriptor characterization of novel 4-hydroxy-chromene-2-one derivatives as antimicrobial agents. Molecules. 2009;14:1495–512.10.3390/molecules14041495Suche in Google Scholar PubMed PubMed Central
[27] Kasabe A, Mohite V, Ghodake J, Vidhate J. Synthesis, characterization and primary antimicrobial, antifungal activity evaluation of Schiff bases of 4-Chloro-(3-substitutedphenylimino)-methyl-[2H]chromene-2-one. E J Chem. 2010;7(2):377–82.10.1155/2007/237645Suche in Google Scholar
[28] Gandhi D, Agarwal DKr, Kalal P, Bhargava A, Jangid D, Agarwal Sh. Synthesis, characterization and evaluation of novel benzothiazole clubbed chromene derivatives for their anti-inflammatory potential. Phosphorus Sulfur Silicon Relat Elem. 2018;193(12):840–7. 10.1080/10426507.2018.1514502.Suche in Google Scholar
[29] Assirey E, Alsaggaf A, Naqvi A, Moussa Z, Okasha RM, Afifi TH, et al. Synthesis, biological assessment, and structure activity relationship studies of new flavanones embodying chromene moieties. Molecules. 2020;25:544.10.3390/molecules25030544Suche in Google Scholar PubMed PubMed Central
[30] Youssef MSK, Abdou A, Abeed O, El-Emary TI. Synthesis and evaluation of chromene-based compounds containing pyrazole moiety as antimicrobial agents. Heterocycl Commun. 2017;23(1):55–64.10.1515/hc-2016-0136Suche in Google Scholar
[31] Amr AGE, Mohamed AM, Mohamed SF, Abdel-Hafez NA, Hammam AE. Anticancer activities of some newly synthesized pyridine, pyrane and pyrimidine derivatives. Bioorg Med Chem. 2006;14:5481–8.10.1016/j.bmc.2006.04.045Suche in Google Scholar PubMed
[32] Paliwal PK, Jetti SR, Jain S. Green approach towards the facile synthesis of dihydropyrano(c)chromene and pyrano[2,3-d]pyrimidine derivatives and their biological evaluation. Med Chem Res. 2013;22:2984–90.10.1007/s00044-012-0288-3Suche in Google Scholar
[33] Zghab I, Trimeche B, Ben Mansour M, Hassine M, Touboul D, Ben Jannet B. Regiospecific synthesis, antibacterial and anticoagulant activities of novel isoxazoline chromene derivatives. Arab J Chem. 2017;10:s2651–58.10.1016/j.arabjc.2013.10.008Suche in Google Scholar
[34] Erichsen MN, Huynh TH, Abrahamsen B, Bastlund JF, Bundgaard C, Monrad O, et al. Structure-activity relationship study of first selective inhibitor of excitatory amino acid transporter subtype 1: 2-Amino-4-(4-methoxyphenyl)-7-(naphthalen-1-yl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (UCPH-101). J Med Chem. 2010;53:7180–91.10.1021/jm1009154Suche in Google Scholar PubMed
[35] Kemnitzer W, Drewe J, Jiang S, Zhang H, Grundy CC, Labreque D, et al. Discovery of 4-Aryl-4H-chromenes as a new series of apoptosis inducers using a cell- and caspase-based high-throughput screening assay. 3. Structure-activity relationships of fused rings at the 7,8-positions. J Med Chem. 2008;51:417–23.10.1016/j.bmcl.2005.07.066Suche in Google Scholar PubMed
[36] Mondal A, Rajalingam D, Maity T, K. Anti-inflammatory effect of O-methylated flavonol 2-(3,4-dihydroxy-phenyl)-3,5-dihydroxy-7-methoxy-chromen-4-one obtained from Cassia sophera Linn in rats. Ethnopharmacol Commun. 2013;147:525–9.10.1016/j.jep.2013.01.021Suche in Google Scholar PubMed
[37] Romdhane A, Jannet HB. Synthesis of new Pyran and Pyranoquinoline derivatives. Arab J Chem. 2017;10:S3128–34.10.1016/j.arabjc.2013.12.002Suche in Google Scholar
[38] Etivand N, Khalafy J, Dekamin MG. Fast and efficient green procedure for the synthesis of Benzo[5,6]chromene derivatives and their sulfur analogues in water by organocatalyst potassium phthalimide-N-oxyl. Synthesis. 2020;52:A–L.10.1055/s-0037-1610755Suche in Google Scholar
[39] Smits R, Belyakov S, Plotniece A, Duburs G. Synthesis of 4H-Pyran derivatives under solvent-free and grinding conditions. Synth Commun. 2013;43:465–75.10.1080/00397911.2012.716484Suche in Google Scholar
[40] Poursattar Marjani A, Khalafy J, Eslamipour P, Ahmadi Sabegh M. Synthesis of a new series of 4H-benzo[h]chromenes by a multicomponent reaction under solvent-free microwave conditions. Iran J Chem Chem Eng. 2019;38:51–7.Suche in Google Scholar
[41] Ahmadi Sabegh M, Khalafy J. The regioselective and catalyst-free synthesis of bis-quinoxalines and bis-pyrido[2,3-b]pyrazines by double condensation of 1,4-phenylenebis-glyoxal with 1,2-diamines. Heterocycl Commun. 2018;24(4):193–6. 10.1515/hc-2018-0039.Suche in Google Scholar
[42] Ahmadi Sabegh M, Khalafy J, Etivand N. One-pot, three-component synthesis of a series of new bis-pyrrolo[2,3-d]pyrimidines in the presence of TPAB under reflux conditions. J Heterocycl Chem. 2018;55:2610.10.1002/jhet.3320Suche in Google Scholar
[43] Gunaganti N, Ruchir K, Tadigoppula N. Copper(II) catalyzed expeditious synthesis of furoquinoxalines through a one-pot three-component coupling strategy. Org Lett. 2014;16(17):4528–31.10.1021/ol502072kSuche in Google Scholar PubMed
[44] Biswanath D, Nisith B, Maram L. A simple and efficient metal-free synthesis of tetrasubstituted pyrroles by iodine-catalyzed four-component coupling reaction of aldehydes, amines, dialkyl acetylenedicarboxylates, and nitromethane. Synthesis. 2011;21:3471–4.10.1055/s-0030-1260228Suche in Google Scholar
© 2021 Naser Sadeghpour Orang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Molecular, Electronic, Nonlinear Optical and Spectroscopic Analysis of Heterocyclic 3-Substituted-4-(3-methyl-2-thienylmethyleneamino)-4,5-dihydro-1H-1,2,4-triazol-5-ones: Experiment and DFT Calculations
- Lewis acid / Base-free Strategy for the Synthesis of 2-Arylthio and Selenyl Benzothiazole / Thiazole and Imidazole
- Synergistic promoting effect of ball milling and Fe(ii) catalysis for cross-dehydrogenative-coupling of 1,4-benzoxazinones with indoles
- Magnetic nanoparticle-supported sulfonic acid as a green catalyst for the one-pot synthesis of 2,4,5-trisubstituted imidazoles and 1,2,4,5-tetrasubstituted imidazoles under solvent-free conditions
- Synthesis, characteristic fragmentation patterns, and antibacterial activity of new azo compounds from the coupling reaction of diazobenzothiazole ions and acetaminophen
- Para toluenesulfonic acid-catalyzed one-pot, three-component synthesis of benzo[5,6]chromeno[3,2-c]quinoline compounds in aqueous medium
- Design and microwave-assisted synthesis of a novel Mannich base and conazole derivatives and their biological assessment
- Quantum chemical studies on structural, spectroscopic, nonlinear optical, and thermodynamic properties of the 1,2,4-triazole compound
- Design, synthesis, and biological evaluation of phenyl-isoxazole-carboxamide derivatives as anticancer agents
- Quantum chemistry study on the promoted reactivity of substituted cyclooctynes in bioorthogonal cycloaddition reactions
- Quantum chemical calculations of phenazine-based organic dyes in dye-sensitized solar cells
- Rapid Communications
- One-pot synthesis of benzofurans via heteroannulation of benzoquinones
- Design and development of novel thiazole-sulfonamide derivatives as a protective agent against diabetic cataract in Wistar rats via inhibition of aldose reductase
- Review Articles
- Organic electrochemistry: Synthesis and functionalization of β-lactams in the twenty-first century
- Some applications of deep eutectic solvents in alkylation of heterocyclic compounds: A review of the past 10 years
Artikel in diesem Heft
- Research Articles
- Molecular, Electronic, Nonlinear Optical and Spectroscopic Analysis of Heterocyclic 3-Substituted-4-(3-methyl-2-thienylmethyleneamino)-4,5-dihydro-1H-1,2,4-triazol-5-ones: Experiment and DFT Calculations
- Lewis acid / Base-free Strategy for the Synthesis of 2-Arylthio and Selenyl Benzothiazole / Thiazole and Imidazole
- Synergistic promoting effect of ball milling and Fe(ii) catalysis for cross-dehydrogenative-coupling of 1,4-benzoxazinones with indoles
- Magnetic nanoparticle-supported sulfonic acid as a green catalyst for the one-pot synthesis of 2,4,5-trisubstituted imidazoles and 1,2,4,5-tetrasubstituted imidazoles under solvent-free conditions
- Synthesis, characteristic fragmentation patterns, and antibacterial activity of new azo compounds from the coupling reaction of diazobenzothiazole ions and acetaminophen
- Para toluenesulfonic acid-catalyzed one-pot, three-component synthesis of benzo[5,6]chromeno[3,2-c]quinoline compounds in aqueous medium
- Design and microwave-assisted synthesis of a novel Mannich base and conazole derivatives and their biological assessment
- Quantum chemical studies on structural, spectroscopic, nonlinear optical, and thermodynamic properties of the 1,2,4-triazole compound
- Design, synthesis, and biological evaluation of phenyl-isoxazole-carboxamide derivatives as anticancer agents
- Quantum chemistry study on the promoted reactivity of substituted cyclooctynes in bioorthogonal cycloaddition reactions
- Quantum chemical calculations of phenazine-based organic dyes in dye-sensitized solar cells
- Rapid Communications
- One-pot synthesis of benzofurans via heteroannulation of benzoquinones
- Design and development of novel thiazole-sulfonamide derivatives as a protective agent against diabetic cataract in Wistar rats via inhibition of aldose reductase
- Review Articles
- Organic electrochemistry: Synthesis and functionalization of β-lactams in the twenty-first century
- Some applications of deep eutectic solvents in alkylation of heterocyclic compounds: A review of the past 10 years

