Abstract
In this study, quantum chemical calculations of phenazine-based organic molecules applied in organic dye-sensitized solar cells (DSSCs) have been made and interpreted. Since DSSC molecules work with the electron push–pull system, the sequence of other compounds (2–8) from compound 1 is designed as a donor–π bridge (weak acceptor)–acceptor (D–π–A). Later, the studied molecules were expanded from 2a to 8c by lengthening the conjugation with phenyl, thiophene, and furan to the acceptor parts. Density functional theory (DFT) and time-dependent density functional theory (TD-DFT) calculations are practiced to investigate all structures and absorption spectra of molecules, respectively. It has been observed that the phenazine-based molecule series are good candidates for DSSCs, both with their band gap and their absorption spectrum results. It can be assumed that changing the HOMO and LUMO energy values of all designed structures according to compound 1 can absorb light in the organic dye-sensitized solar cells and transfer electrons to the conductivity band of TiO2. As a result, it has been resolved that various dyes can be designed for dye-sensitizing solar cells by calculating electronic energies, HOMO–LUMO energies, and absorption wavelengths.
1 Introduction
Wide band-gap semiconductors can be made sensitive to the visible area by adsorbing organic dyes on their surface. Previously, Fujihira [1] reported the sensitization of wide band-gap semiconductors to the visible region using dyes. The organic dye-sensitized solar cell, in other words, the Grätzel battery, is a structure that combines three separate layers such as semiconductor, dye, and electrolyte, which transform photon energy into electrical energy without the need for any chemical transformation [2]. Dye-sensitized solar cells (DSSCs), which are being researched as promising candidates for renewable energy systems, are of great interest in academic and industrial fields [3,4,5,6,7,8,9,10,11].
A dye-sensitized solar cell consists of the semiconductor film (working electrode) formed by sensitizing the nanocrystalline structure (usually TiO2) coated on the conductive glass surface with organic dye, platinum-coated conductive glass (counter electrode), and the space that connects the working electrode and the counter electrode and fills the pores of the TiO2 layer. In liquid electrolyte batteries, the space conducting material consists of iodide/triiodide (I–/I3–) redox couple in an organic solvent (usually nitriles). Good insulation is required for highly volatile solvent electrolytes. The semiconductor that will be used is directly related to the dye used. Still, TiO2 has given the best result so far in terms of performance. The reduction (LUMO) and oxidation (HOMO) energy levels of the materials that produce organic DSSCs are selected in a way that the electron migration takes place in the desired direction and voluntarily.
Various dyes are synthesized for use in organic dye-sensitized solar cell production. For these dyes to be efficient in organic solar cells, they must have some basic properties [12]. The absorption spectrum of the dye should cover the entire visible region and the near-infrared (NIR) region. To reduce energy losses and keep the photovoltage at the highest possible level, the excited state energy (LUMO) of the dye should be slightly above the conductivity band of TiO2 (more negative), and the energy difference should be sufficient to enable electron transfer [13]. Likewise, for the dye to be regenerated, the ground-state energy level (HOMO) must be more positive than the redox potential of the electrolyte [14]. Electron transfer from the excited state of the dye to the conductivity band of TiO2 must be fast as not to allow quenching reactions such as fluorescence, phosphorescence, or dark process. The dye adsorbed on the TiO2 surface must be stable for 20 years under operating conditions (at the semiconductor–electrolyte interface). It must be capable of solid adsorption to the semiconductor surface. The dye must have high solubility and include a binder group that can adhere to the semiconductor surface. For dye-sensitizing solar cells, there are many parameters to consider in dye designing: The dye should contain a broad absorption spectrum and, if possible, absorb in the IR region. The designed dye should have a high damping coefficient (the dye with this feature provides high photon absorption even in the use of thin semiconductor films). The designed dye must be able to bond strongly to the surface of the metal oxide semiconductor. Therefore, it is necessary to have binding groups such as –COOH, –H2PO3, –SO3H in the designed dye. The energy levels of the dye must be compatible with the conductivity band of the inorganic semiconductor and the redox pair, which additionally acts as a space conduction material. It should possess an easy and understandable synthesis procedure for possible large-scale production. It should be easy to recycle and have low toxicity. The designed dye should not settle on the semiconductor surface. The dye should be electrochemically stable. In order for the dye to be long-lasting, it must be resistant to light and heat.
Ruthenium dyes included in organometallic complexes are the dyes with the highest efficiency in organic solar cells so far. As is known, the absorption band expands with the increase of the conjugated system [15]. Although ruthenium polypyridyl dyes are the highest-yielding dyes to date, they are not ideal dyes. The limitations of these dyes are that they are challenging to synthesize, expensive starting materials, low molar absorption coefficients, and absorption in a very narrow range of the solar spectrum. Due to the limitations mentioned regarding metal complexes, interest in metal-free organic chromophores has increased in recent years. Organic dyes have some advantages over ruthenium-based chromophores. Fundamentally, they have a higher molar absorption coefficient and can be modified more easily due to the short synthesis methods (Figure 1).

Structure of the parent compound (phenazine).
2 Materials and methods
All calculations were achieved via the Gaussian 09W package [16]. While the density functional theory (DFT) method is used for the ground-state gas-phase optimization, the excited-state calculations are made using the time-dependent DFT (TD-DFT) method. DFT was calculated in hybrid functional B3LYP (Becke3-Lee Yang-Parr hybrid functional) with a 6-311++G(d,p) base set [17]. Excitation energies, oscillator powers, and orbital contribution for the lowest 10 singlet–singlet transitions in the optimized geometry in the ground state were obtained with TD-DFT calculations using the same basis set as geometry minimization.
Calculations were performed on an HP420WS desktop computer with Intel Xeon®CPU E5–1650. Calculations are carried out with high-performance server systems (workstations) over the WINDOWS operating system.
3 Conclusion and discussion
It is possible to synthesize several different chromophores using various donor binding acceptor groups for dye-sensitizing solar cells. Within the scope of the study, it was predicted that the phenazine (weak acceptor) π-bridge-based material will carry out electron transmission with the push–pull system with donor groups attached to one side. First, the pulling force was increased by using cyanoacrylic acid in the acceptor part; studies were conducted to determine the performance parameters of DSSCs of 2–8 molecules. Later, phenyl, thiophene, and furan were modified by cyanoacrylic acid (2a–8c). The push–pull system of the synthesized compounds from the donor to the acceptor is a concept that enables electron transmission in molecular dimensions.
Geometry optimizations of all structures (Figure 2) were performed first using the MM2 method, followed by the semi-empirical PM3 coherent molecular orbital (SCFMO) method [18]. Then, further geometry optimizations were achieved using RHF and B3LYP/6-311++G(d,p) levels. Average mode analysis for each structure did not result in any negative frequency in all three calculation methods. The results of the analysis are presented in Table 1.

Chemical structures of paints designed on the basis of phenazine.
Quantum chemical calculation results of dyes designed on the basis of phenazine
| E (B3LYP) (au) | E HOMO (eV) | E LUMO (eV) | E gap (eV) | μ (Debye) | Wavelength (nm) | ||
|---|---|---|---|---|---|---|---|
| EST1 | EST2 | ||||||
| 1 | −879.1134 | −6.36 | −2.59 | 3.77 | 0.3487 | 386.33 | 380.42 |
| 2 | −1468.5436 | −6.39 | −3.08 | 3.31 | 6.4683 | 416.60 | 394.01 |
| 2a | −1699.6535 | −6,29 | −3.18 | 3.11 | 7.5680 | 442.86 | 421.00 |
| 2b | −2020.4218 | −6.26 | −3.27 | 2.99 | 8.4398 | 458.61 | 433.00 |
| 2c | −1697.4459 | −6.18 | −3.21 | 2.97 | 7.9350 | 456.55 | 434.23 |
| 3 | −1775.8642 | −5.58 | −2.94 | 2.64 | 5.7510 | 540.99 | 473.57 |
| 3a | −2006.9720 | −5.57 | −3.04 | 2.53 | 6.7191 | 542.56 | 499.29 |
| 3b | −2327.7391 | −5.59 | −3.13 | 2.46 | 7.4852 | 561.37 | 506.07 |
| 3c | −2004.7633 | −5.59 | −3.07 | 2.52 | 6.9881 | 550.87 | 496.79 |
| 4 | −1852.1264 | −5.76 | −2.94 | 2.82 | 6.2643 | 489.42 | 432.35 |
| 4a | −2083.2341 | −5.75 | −3.03 | 2.72 | 7.2572 | 494.23 | 455.06 |
| 4b | −2404.0011 | −5.78 | −3.12 | 2.66 | 8.1106 | 509.12 | 460.19 |
| 4c | −2081.0255 | −5.77 | −3.06 | 2.71 | 7.5782 | 500.73 | 457.56 |
| 5 | −1753.8007 | −5.75 | −3.02 | 2.73 | 4.6738 | 518.97 | 466.58 |
| 5a | −1984.9085 | −5.71 | −3.09 | 2.62 | 5.0692 | 527.71 | 494.66 |
| 5b | −2305.6754 | −5.74 | −3.18 | 2.56 | 5.8156 | 541.05 | 501.77 |
| 5c | −1982.6997 | −5.73 | −3.12 | 2.61 | 5.4221 | 534.34 | 494.40 |
| 6 | −1829.0136 | −5.01 | −3.09 | 1.92 | 4.0095 | 801.02 | 657.09 |
| 6a | −2060.1217 | −4.99 | −3.13 | 1.86 | 3.8323 | 779.90 | 698.68 |
| 6b | −2380.8925 | −5.01 | −3.11 | 1.89 | 5.2960 | 786.98 | 697.88 |
| 6c | −2057.9171 | −5.01 | −3.07 | 1.94 | 4.8185 | 775.89 | 679.80 |
| 7 | −1753.7627 | −4.19 | −2.99 | 1.20 | 5.7289 | 1484.38 | 1012.83 |
| 7a | −1984.8705 | −4.17 | −3.06 | 1.11 | 6.6205 | 1433.03 | 1128.01 |
| 7b | −2305.6438 | −4.17 | −3.02 | 1.15 | 5.3487 | 1436.35 | 1110.70 |
| 7c | −1982.6683 | −4.17 | −2.97 | 1.20 | 5.2072 | 1396.37 | 1070.53 |
| 8 | −1986.0946 | −5.33 | −2.89 | 2.44 | 7.1119 | 566.72 | 500.87 |
| 8a | −2217.2022 | −5.31 | −3.01 | 2.30 | 8.1615 | 589.63 | 536.07 |
| 8b | −2537.9692 | −5.34 | −3.09 | 2.25 | 9.0984 | 608.02 | 542.53 |
| 8c | −2214.9936 | −5.33 | −3.03 | 2.30 | 8.5296 | 593.83 | 533.75 |
The ground-state geometry (GSG), HOMO, and LUMO diagrams of phenazine-based and dyes calculated in DFT/B3LYP/6-3++g(d,p) in the gas phase are given in Table 2.
GSG, HOMO, and LUMO diagrams of phenazine-based designed dyes calculated in DFT/B3LYP/6-311++g(d,p) in the gas phase
| GSG | HOMO | LUMO | |
|---|---|---|---|
| 1 |
 |
 |
 |
| 2a |
 |
 |
 |
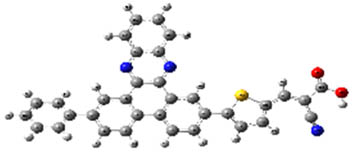
| 2b |
 |
 |
 |
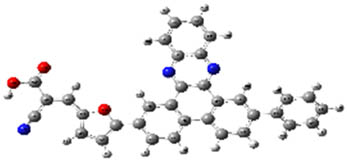
| 2c |
 |
 |
 |
| 3a |
 |
 |
 |
| 3b |
 |
 |
 |
| 3c |
 |
 |
 |
| 4a |
 |
 |
 |
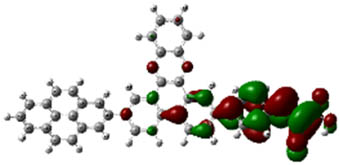
| 4b |
 |
 |
 |
| 4c |
 |
 |
 |
| 5a |
 |
 |
 |
| 5b |
 |
 |
 |
| 5c |
 |
 |
 |
| 6a |
 |
 |
 |
| 6b |
 |
 |
 |
| 6c |
 |
 |
 |
| 7a |
 |
 |
 |
| 7b |
 |
 |
 |
| 7c |
 |
 |
 |
| 8a |
 |
 |
 |
| 8b |
 |
 |
 |
| 8c |
 |
 |
 |
When examined in Table 2, good separation of HOMO–LUMO did not occur in structures 2a, 2b, and 2c formed by benzene substitution due to the low donor ability of benzene. Also, the HOMO–LUMO energy levels have not changed enough for the structures to have a better semiconducting capability. Deviations from planarity in donor–acceptor dihedral angles allowed good separation of HOMO–LUMO in the system for compounds (3a–8c). From Table 2, for compounds 3a–8c, HOMOs appear to be embedded in the donor part; LUMOs, on the other hand, appear to be embedded in receiver parts, so they can be said to be good semiconductor materials with potential use in devices. These results suggest that the designed compounds are combinations that might work well.
The frontier MO energies of the optimized compounds were calculated at the DFT/B3LYP/6-311++(d,p) level. Calculation results are shown in Table 1. As is known, the electronic structure of organic semiconductors is based on the conjugation of the electrons they have in their structure. Electrochemical parameters are interpreted with HOMO–LUMO values. From cyanoacrylic acid, which acts as the electron-withdrawing group, in which the LUMO orbitals are delocalized, phenazine in which the electron-donating group HOMO orbitals are delocalized is calculated. In general, as the number of electron-donating groups increases, HOMO and LUMO energy levels also change. The results obtained in Table 1 also support this. The energy band gap is calculated by subtracting the LUMO energy from the HOMO energy. It can be seen from the table that the calculated band gap decreases with increasing conjugation. The band gap of semiconductor molecules ranges from 0.5 to 4.0 eV [19]. From Table 1, it is seen that the ∆E values, including the main compound (1), are below 4 eV. This band gap has narrowed with the addition of donor and acceptor units. The most significant reason for this is that the conjugation in the structure is prolonged with the combination made precisely as expected. As a result, it can be assumed that all compounds have the potential to be semiconductor materials. The three molecules with the narrowest band gap among all structures are 7, 6, and 8 and their derivatives.
Regarding the addition of donor groups to one side of the parent compound (1) and the acceptor group on the other side, considering the compounds numbered 2–8 obtained by keeping the acceptor group constant and changing the donor groups, it was discovered that the three molecules with the lowest band energy were 7, 6, and 8. From Table 1, it can be observed that the HOMO–LUMO energy levels did not change enough for the structures to have better semiconductivities due to the two structures formed by benzene substitution and the low donor ability of benzene. Anthracene (3) [20], pyrene (4) [21], carbazole (5) [22], pheoxazine (6) [23], quinolizine (7) [24], and triphenylamine (8) [25] based systems have been well researched in the literature. Therefore, combining each of these donors with the parent compound resulted in potential candidate compounds working well for semiconductivity. When examining the 2a–8c structures in terms of energy (Table 1), ∆E of the HOMO–LUMO ranges from 3.11 to 1.11 eV. These results reveal that the designed compounds are combinations that can work well. The potential utilization of compound 1 as a semiconductor material has been successfully increased by modification with strong donor groups and strong acceptor groups.
Substituents added to the structure in the synthesis of phenazine-based dyes cause changes in the absorption spectrum. The photophysical properties of the gas phase were calculated using TD-DFT calculations to obtain and interpret phenazine derivatives with high absorption in a wide range in the red/NIR region. From Table 1, EST1 gives the first transition state wavelength and EST2 gives the second single pass wavelength. The incoming peaks are due to the π–π* transitions and the second peaks are due to the intramolecular charge transfer (ICT) between the donor and acceptor.
As seen in Table 1, the 2–8 molecules gave absorption spectra at 416.60, 540.99, 489.42, 518.97, 801.02, 1484.38, and 566.72 nm, respectively; thus, a redshift, that is, bathochromic effect, was observed by changing the 386.33 nm wavelength donor groups of compound 1. Similarly, a bathochromic effect was observed in the absorption spectra due to increased conjugation with the addition of phenyl (a), thiophene (b), and furan (c) groups to the acceptor site of the phenazine-based molecule. Again, a considerable increase in redshift is observed with the effect of substances added to phenazine. It can be assumed that it can be applied in photovoltaic applications as it absorbs the solar spectrum in the visible region and the NIR region, among the other molecules studied. The best candidates are molecule 7.
The molecule with the lowest bandgap (7a) gave a longer wavelength in the gas phase than other structures. The provided structures made it even more stable by adding π-bridge, donor, and acceptor groups. As a result, it can be assumed that both increasing the conjugation and heteroatoms have made the structure more stable by shifting the oxidation potential to the right; oxidation potential means the HOMO energy of the molecule. To provide effective regeneration in the solar cell, the HOMO potential of the dye should be lower than that of the electrolyte. The energy level in the I−/I3− system is −4.8 eV [26]. Increased conjugation and the addition of different substitution groups also affect the reduction potential in a molecule. With the increase of conjugation, the reduction potential, namely the LUMO value, shifts to a more negative region. The LUMO value must be higher than the conductivity band of TiO2 (−4.0 eV for TiO2) to ensure electron transfer from the organic molecule to the conductivity band (CB) of TiO2. Thus, the dyes synthesized can absorb light and transfer electrons to the conductivity band of TiO2 in organic DSSCs [27]. When looking at the result table, the HOMO values of all molecules, including molecule number 1, are lower than −4.8 eV, and all LUMO values are higher than −4.0 eV. This reveals that the energy levels and band gaps of the designed and studied molecules can be applied in DSSCs. As a result, many different dyes can be designed for dye-sensitizing solar cells by calculating electronic energy, HOMO–LUMO energies, and absorption wavelengths.
4 Results
In this study, the group is the highly soluble derivative of the structure known in the literature as phenazine or quinoxaline. This group has a weak acceptor feature and attracts the electrons given to the structure by the donor at a specific rate and provides transmission to the strong acceptor group on the other side. In this study, the properties of organic dyes that contain different donor groups to phenazine-based molecules and that can be used in organic solar cells by adding other π-linker groups to the system on the acceptor side have been quantum chemically studied. Therefore, it was assumed that various dyes could be a guide for a rational design. Using cyanoacrylic acid and then the derivatives of cyanoacrylic acid with phenyl, thiophene, and furan as the acceptor, the transmission of the electron along the molecule has been tried to be achieved most efficiently.
The phenazine derivative π-bridge group in the structure has a very high conjugation. Molar absorption coefficients that increase with conjugation are a factor that increases light absorption and charge separation. The π-bridge-group length decreases the possibility of reunifying the loads with its structure, providing structural rigidity and high conjugation and all these effects. Thiophene has lower transition energy and lower equilibration energy than those of phenyl and furan by adding the free electron pair in its structure to conjugation. As a result, the absorption redshifts, and the molar absorption coefficient is increased, with a relatively narrow bandgap and more photons have been absorbed.
One of the most critical parameters in dye-sensitizing solar cells is the movement of the electron from the dye to the anode in the opposite direction, namely the cathode, which is called recombination. Although there are multiple factors such as the use of materials that do not have suitable band values, the use of unsuitable electrolytes, and the conductivity band values of the selected electrodes, the synthesis and the use of materials with appropriate band gaps are the most important. The HOMO value has increased in all of the designed molecules and the LUMO value has also been brought to a more negative value than the vacuum.
Phenazine-based molecule series are good candidates for DSSCs, both with band gap and absorption spectrum results. It can be assumed that by changing the HOMO and LUMO energy values of all designed structures according to compound 1, it can absorb light in the organic dye-sensitized solar cell and transfer electrons to the conductivity band of TiO2. As a result, many different dyes can be designed for dye-sensitizing solar cells by calculating electronic energy, HOMO–LUMO energies, and absorption wavelengths.
-
Funding information: The authors state no funding involved.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Fujihira M, Ohishi N, Osa T. Photocell using covalently-bound dyes on semiconductor surfaces. Nature. 1977;268(5617):226–8.10.1038/268226a0Suche in Google Scholar
[2] Grätzel M. Solar energy conversion by dye-sensitized photovoltaic cells. Inorg Chem. 2005;44(20):6841–51.10.1021/ic0508371Suche in Google Scholar PubMed
[3] Armaroli N, Balzani V. The future of energy supply: challenges and opportunities. Angew Chem Int Ed. 2007;46(1–2):52–66.10.1002/anie.200602373Suche in Google Scholar PubMed
[4] Listorti A, O’regan B, Durrant JR. Electron transfer dynamics in dye-sensitized solar cells. Chem Mater. 2011;23(15):3381–99.10.1021/cm200651eSuche in Google Scholar
[5] Hagfeldt A, Boschloo G, Sun L, Kloo L, Pettersson H. Dye-sensitized solar cells. Chem Rev. 2010;110(11):6595–663.10.1016/B978-0-12-811165-9.00006-5Suche in Google Scholar
[6] O’regan B, Grätzel M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature. 1991;353(6346):737–40.10.4324/9781315793245-57Suche in Google Scholar
[7] Ning Z, Tian H. Triarylamine: a promising core unit for efficient photovoltaic materials. Chem Commun. 2009;37:5483–95.10.1039/b908802dSuche in Google Scholar PubMed
[8] Yum JH, Baranoff E, Wenger S, Nazeeruddin MK, Grätzel M. Panchromatic engineering for dye-sensitized solar cells. Energ Environ Sci. 2011;4(3):842–57.10.1039/C0EE00536CSuche in Google Scholar
[9] Calogero G, Di Marco G, Caramori S, Cazzanti S, Argazzi R, Bignozzi CA. Natural dye senstizers for photoelectrochemical cells. Energ Environ Sci. 2009;2(11):1162–72.10.1039/b913248cSuche in Google Scholar
[10] Ray JK, Singha R, Ray D, Ray P, Rao DY, Anoop A. Palladium-catalyzed expedient Heck annulations in 1-bromo-1, 5-dien-3-ols: exceptional formation of fused bicycles. Tetrahedron Lett. 2019;60(13):931–5.10.1016/j.tetlet.2019.02.043Suche in Google Scholar
[11] Ray JK, Paul S, Ray P, Singha R, Rao DY, Nandi S, et al. Pd-catalyzed intramolecular sequential Heck cyclization and oxidation reactions: a facile pathway for the synthesis of substituted cycloheptenone evaluated using computational studies. New J Chem. 2017;41(1):278–84.10.1039/C6NJ02694JSuche in Google Scholar
[12] Kim S, Lee JK, Kang SO, Ko J, Yum JH, Fantacci S, et al. Molecular engineering of organic sensitizers for solar cell applications. J Am Chem Soc. 2006;128(51):16701–7.10.1021/ja066376fSuche in Google Scholar PubMed
[13] Bignozzi CA, Argazzi R, Kleverlaan CJ. Molecular and supramolecular sensitization of nanocrystalline wide band-gap semiconductors with mononuclear and polynuclear metal complexes. Chem Soc Rev. 2000;29(2):87–96.10.1039/a803991gSuche in Google Scholar
[14] Qin P, Yang X, Chen R, Sun L, Marinado T, Edvinsson T, et al. Influence of π-conjugation units in organic dyes for dye-sensitized solar cells. J Phys Chem C. 2007;111(4):1853–60.10.1021/jp065550jSuche in Google Scholar
[15] Chatterjee T, Sarma M, Das SK. Synthesis and photo-physical properties of methoxy-substituted π-conjugated-2, 2′-bipyridines. Tetrahedron Lett. 2010;51(15):1985–8.10.1016/j.tetlet.2010.02.027Suche in Google Scholar
[16] Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR et al. Gaussian 09, Revision D.01. Wallingford CT: Gaussian Inc.; 2009.Suche in Google Scholar
[17] Becke AD. A new mixing of Hartree–Fock and local density-functional theories. J Chem Phys. 1993;98(2):1372–7.10.1063/1.464304Suche in Google Scholar
[18] Irak ZT, Gümüş S. Heterotricyclic compounds via click reaction: a computational study. Noble Int J Sci Res. 2017;7:80–9.Suche in Google Scholar
[19] Atkins P, Overton T, Rourke J, Weller M. Armstrong F. Shriver and Atkins. Inorganic Chemistry. Fourth edn. Oxford: Oxford University Press; 2006.Suche in Google Scholar
[20] Aydemir M, Haykır G, Battal A, Jankus V, Sugunan SK, Dias FB, et al. High efficiency OLEDs based on anthracene derivatives: The impact of electron donating and withdrawing group on the performance of OLED. Org Electron. 2016;30:149–57.10.1016/j.orgel.2015.11.026Suche in Google Scholar
[21] Shan T, Gao Z, Tang X, He X, Gao Y, Li J, et al. Highly efficient and stable pure blue nondoped organic light-emitting diodes at high luminance based on phenanthroimidazole-pyrene derivative enabled by triplet-triplet annihilation. Dyes Pigments. 2017;142:189–97.10.1016/j.dyepig.2017.03.032Suche in Google Scholar
[22] Çiçek B, Çalışır Ü, Tavaslı M, Tülek R, Teke A. Synthesis and optical characterization of novel carbazole Schiff bases. J Mol Struct. 2018;1153:42–7.10.1016/j.molstruc.2017.09.109Suche in Google Scholar
[23] Li P, Cui Y, Song C, Zhang H. A systematic study of phenoxazine-based organic sensitizers for solar cells. Dyes Pigments. 2017;137:12–23.10.1016/j.dyepig.2016.09.060Suche in Google Scholar
[24] Zhang D, Zhao C, Zhang Y, Song X, Wei P, Cai M, et al. Highly efficient full-color thermally activated delayed fluorescent organic light-emitting diodes: extremely low efficiency roll-off utilizing a host with small singlet–triplet splitting. ACS App Mater Inter. 2017;9(5):4769–77.10.1021/acsami.6b15272Suche in Google Scholar PubMed
[25] Gümüş S, Gümüş A. A computational study on a series of phenanthrene and phenanthroline based potential organic photovoltaics. Maced J Chem Chem En. 2017;36(2):239–49.10.20450/mjcce.2017.1199Suche in Google Scholar
[26] Hagfeldt A, Grätzel M. Light-induced redox reactions in nanocrystalline systems. Chem Rev. 1995;95(1):49–68.10.1021/cr00033a003Suche in Google Scholar
[27] Asbury JB, Wang YQ, Hao E, Ghosh HN, Lian T. Evidences of hot excited state electron injection from sensitizer molecules to TiO2 nanocrystalline thin films. Res Chem Intermediat. 2001;27(4):393–406.10.1163/156856701104202255Suche in Google Scholar
© 2021 Nesim Yigit and Zeynep Şilan Turhan, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Molecular, Electronic, Nonlinear Optical and Spectroscopic Analysis of Heterocyclic 3-Substituted-4-(3-methyl-2-thienylmethyleneamino)-4,5-dihydro-1H-1,2,4-triazol-5-ones: Experiment and DFT Calculations
- Lewis acid / Base-free Strategy for the Synthesis of 2-Arylthio and Selenyl Benzothiazole / Thiazole and Imidazole
- Synergistic promoting effect of ball milling and Fe(ii) catalysis for cross-dehydrogenative-coupling of 1,4-benzoxazinones with indoles
- Magnetic nanoparticle-supported sulfonic acid as a green catalyst for the one-pot synthesis of 2,4,5-trisubstituted imidazoles and 1,2,4,5-tetrasubstituted imidazoles under solvent-free conditions
- Synthesis, characteristic fragmentation patterns, and antibacterial activity of new azo compounds from the coupling reaction of diazobenzothiazole ions and acetaminophen
- Para toluenesulfonic acid-catalyzed one-pot, three-component synthesis of benzo[5,6]chromeno[3,2-c]quinoline compounds in aqueous medium
- Design and microwave-assisted synthesis of a novel Mannich base and conazole derivatives and their biological assessment
- Quantum chemical studies on structural, spectroscopic, nonlinear optical, and thermodynamic properties of the 1,2,4-triazole compound
- Design, synthesis, and biological evaluation of phenyl-isoxazole-carboxamide derivatives as anticancer agents
- Quantum chemistry study on the promoted reactivity of substituted cyclooctynes in bioorthogonal cycloaddition reactions
- Quantum chemical calculations of phenazine-based organic dyes in dye-sensitized solar cells
- Rapid Communications
- One-pot synthesis of benzofurans via heteroannulation of benzoquinones
- Design and development of novel thiazole-sulfonamide derivatives as a protective agent against diabetic cataract in Wistar rats via inhibition of aldose reductase
- Review Articles
- Organic electrochemistry: Synthesis and functionalization of β-lactams in the twenty-first century
- Some applications of deep eutectic solvents in alkylation of heterocyclic compounds: A review of the past 10 years
Artikel in diesem Heft
- Research Articles
- Molecular, Electronic, Nonlinear Optical and Spectroscopic Analysis of Heterocyclic 3-Substituted-4-(3-methyl-2-thienylmethyleneamino)-4,5-dihydro-1H-1,2,4-triazol-5-ones: Experiment and DFT Calculations
- Lewis acid / Base-free Strategy for the Synthesis of 2-Arylthio and Selenyl Benzothiazole / Thiazole and Imidazole
- Synergistic promoting effect of ball milling and Fe(ii) catalysis for cross-dehydrogenative-coupling of 1,4-benzoxazinones with indoles
- Magnetic nanoparticle-supported sulfonic acid as a green catalyst for the one-pot synthesis of 2,4,5-trisubstituted imidazoles and 1,2,4,5-tetrasubstituted imidazoles under solvent-free conditions
- Synthesis, characteristic fragmentation patterns, and antibacterial activity of new azo compounds from the coupling reaction of diazobenzothiazole ions and acetaminophen
- Para toluenesulfonic acid-catalyzed one-pot, three-component synthesis of benzo[5,6]chromeno[3,2-c]quinoline compounds in aqueous medium
- Design and microwave-assisted synthesis of a novel Mannich base and conazole derivatives and their biological assessment
- Quantum chemical studies on structural, spectroscopic, nonlinear optical, and thermodynamic properties of the 1,2,4-triazole compound
- Design, synthesis, and biological evaluation of phenyl-isoxazole-carboxamide derivatives as anticancer agents
- Quantum chemistry study on the promoted reactivity of substituted cyclooctynes in bioorthogonal cycloaddition reactions
- Quantum chemical calculations of phenazine-based organic dyes in dye-sensitized solar cells
- Rapid Communications
- One-pot synthesis of benzofurans via heteroannulation of benzoquinones
- Design and development of novel thiazole-sulfonamide derivatives as a protective agent against diabetic cataract in Wistar rats via inhibition of aldose reductase
- Review Articles
- Organic electrochemistry: Synthesis and functionalization of β-lactams in the twenty-first century
- Some applications of deep eutectic solvents in alkylation of heterocyclic compounds: A review of the past 10 years

