Magnetic nanoparticle-supported sulfonic acid as a green catalyst for the one-pot synthesis of 2,4,5-trisubstituted imidazoles and 1,2,4,5-tetrasubstituted imidazoles under solvent-free conditions
Abstract
In this work, magnetic nanoparticle-supported sulfonic acid (γ-Fe2O3-SO3H) is used as an efficient catalyst in the synthesis of 2,4,5-trisubstituted imidazoles and 1,2,4,5-tetrasubstituted imidazoles in a short time (40–70 min for trisubstituted imidazoles and 30–40 min for tetrasubstituted imidazoles) and high-purity products were obtained (92–98% for trisubstituted imidazoles and 94–98% for tetrasubstituted imidazoles) in simple multicomponent reactions. The structure of these products was confirmed via FT-IR and NMR. Green and recyclable catalysts, eco-friendly and solvent-free conditions, high catalytic activity, shorter reaction time, easy recovery by an external magnet, high purity, and excellent yields are some features of these reactions.
Graphical abstract

1 Introduction
Due to the effects of the chemical industry on the environment since the 1940s, fundamental changes have been carried out to increase the adoption of green chemistry principles [1,2]. Therefore, due to the significance of green chemistry goals, the use of green catalysts, especially nanocatalysts, has strongly attracted scientific interest. Owing to the properties of magnetic nanocatalysts such as large special surface area and simple separation with external magnets, [3,4,5,6,7] they play a crucial role in the removal of hazardous and toxic compounds from the environment.
Multicomponent reactions (MCRs) have applications in organic and medicinal chemistry that produce complex products from simple substances in one-pot processes. These reactions, which follow green chemistry concepts, are recognised as one of the most effective and economical methods for synthesising complex molecules in recent decades [8]. Imidazoles, one of the important heterocycles [9], can be synthesised by this method. Imidazoles have pharmaceutical and medicinal properties and are used in pesticides, dyeing, agent for resins, polymers, anion sensors, and ionic liquids [10,11]. Moreover, imidazole derivatives have been found to be anti-fungal, anti-bacterial, anti-malarial, anti-nociceptive, anti-inflammatory, anti-hypertensive, hypoxic cell therapy, imaging agents, anti-cancer, and anti-plasmodium [10,11,12]. In addition, imidazoles are an important component of natural compounds such as vitamin B12, histamine, histidine, and biotin [11].
According to the importance of green chemistry goals, in this project, we use magnetic particle-supported sulfonic acid (γ-Fe2O3-SO3H) [13] as a recyclable catalyst in the synthesis of 2,4,5-trisubstituted imidazoles and 1,2,4,5-tetrasubstituted imidazoles, which are valuable biologically active compounds [14], in a simple one-pot procedure from which substituted imidazoles were obtained with high efficiency and purity.
2 Results and discussion
2.1 Optimisation of reaction conditions for the preparation of substituted imidazoles
Firstly, to optimise the reaction conditions for the preparation of 2,4,5-trisubstituted imidazoles, condensation of benzil (1 mmol), ammonium acetate (5 mmol) and benzaldehyde (1 mmol) in the presence of the γ-Fe2O3-SO3H catalyst (10 mol%) under solvent-free conditions was selected as a model reaction (Scheme 1). Then, different amounts of the catalyst were studied. As shown in Table 1 entry 2a, the use of 10 mol% of the γ-Fe2O3-SO3H catalyst afforded the best efficiency (94% for trisubstituted imidazoles). Further increasing the amount of catalyst did not improve the efficiency (Table 1, entry 3a).
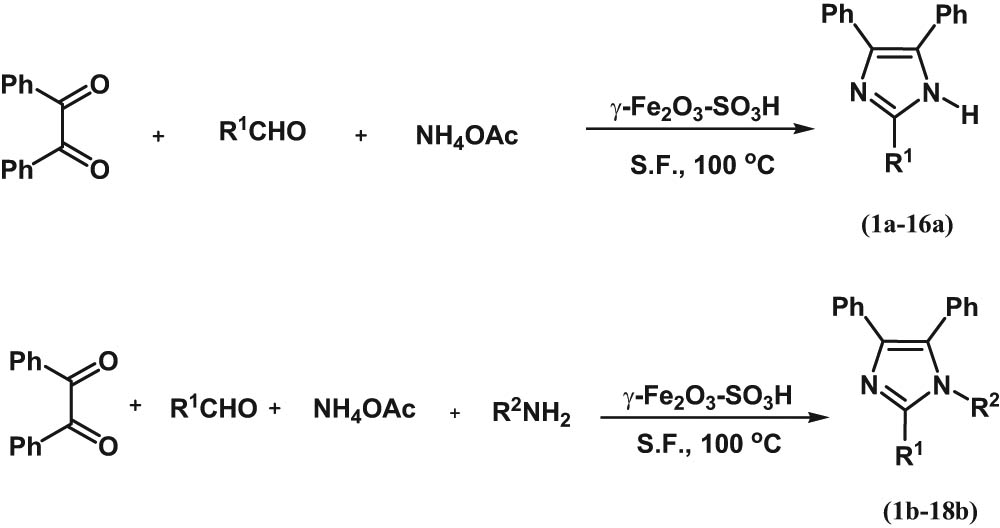
One-pot synthesis of polysubstituted imidazoles in the presence of the γ-Fe2O3-SO3H catalyst (a: trisubstituted imidazole, b: tetrasubstituted imidazole).
Optimisation of catalyst amount for model reactions (a: trisubstituted imidazole, b: tetrasubstituted imidazole)
| Entry | Catalyst amount (mol%) | Time (min) | Yield (%) * | Time (min) | Yield (%) * |
|---|---|---|---|---|---|
| 1a | 1a | 1b | 1b | ||
| 1 | 5 | 50 | 90 | 40 | 92 |
| 2 | 10 | 50 | 94 | 40 | 97 |
| 3 | 20 | 50 | 92 | 40 | 94 |
- *
Yields refer to isolated products.
Secondly, we examined the effect of different temperatures on the yield, under solvent-free conditions. The highest efficiency was achieved at 100°C (94%) for the synthesis of 2,4,5-trisubstituted imidazoles (Table 2, entry 3a). As indicated in Table 2, increasing the reaction temperature from 80 to 100°C resulted in an improvement in yield from 85 to 94% (Table 2, entries 1a and 3a), but upon increasing the temperature to 120°C the reaction yield decreased to 88% due to the formation of by-products (Table 2, entry 4a). All of the reactions were monitored by TLC. Further reactions were performed at 100°C.
Optimisation of temperature for model reactions (a: trisubstituted imidazole, b: tetrasubstituted imidazole)
| Entry | Temperature (°C) | Time (min) | Yield (%)* | Time (min) | Yield (%)* |
|---|---|---|---|---|---|
| 1a | 1a | 1b | 1b | ||
| 1 | 80 | 50 | 85 | 40 | 85 |
| 2 | 90 | 50 | 90 | 40 | 87 |
| 3 | 100 | 50 | 94 | 40 | 97 |
| 4 | 120 | 50 | 88 | 40 | 91 |
- *
Yields refer to isolated products.
Thirdly, the reaction was examined in various solvents. As shown in Table 3, solvent-free conditions resulted in higher yields than those obtained in any of the solvents investigated (Table 3, entry 6a).
Solvent effect on the model reactions (a: trisubstituted imidazole, b: tetrasubstituted imidazole)
| Entry | Solvent | Condition | Yield (%)* | Condition | Yield (%)* |
|---|---|---|---|---|---|
| 1a | 1a | 1b | 1b | ||
| 1 | EtOH | 10 mL/50 min/60°C | 85 | 10 mL/40 min/50oC | 80 |
| 2 | MeOH | 10 mL/50 min/60°C | 74 | 10 mL/40 min/40°C | 68 |
| 3 | THF | 10 mL/50 min/60°C | 40 | 10 mL/40 min/60°C | 55 |
| 4 | DMSO | 10 mL/50 min/100°C | 30 | 10 mL/40 min/100°C | 45 |
| 5 | CH3CN | 10 mL/50 min/60°C | 50 | 10 mL/40 min/60°C | 50 |
| 6 | Solvent-free | 50 min/100°C | 94 | 40 min/100°C | 97 |
- *
Yields refer to isolated products.
For 1,2,4,5-tetrasubstituted imidazoles, a mixture of benzil (1 mmol), ammonium acetate (2 mmol), benzaldehyde (1 mmol), and aniline (2 mmol) in the presence of the γ-Fe2O3-SO3H catalyst (10 mol%) under solvent-free conditions was selected as the model reaction (Scheme 1). The catalyst loading, temperature and solvent condition optimisation steps were repeated. The best yield (97%) was obtained in the presence of 10 mol% catalyst (Table 1, entry 2b) at 100°C (Table 2, entry 3b) under solvent-free conditions (Table 3, entry 6b).
Inspired by previous reports [15,16,17], a possible mechanism for the synthesis of 2,4,5-trisubstituted imidazoles using a γ-Fe2O3-SO3H catalyst is illustrated in Scheme 2. In the process, γ-Fe2O3-SO3H as an acidic catalyst can activate the aldehyde and the 1,2-diketone, which react with ammonium acetate to form imine intermediates. Then, the imine nitrogen of imine intermediate (1) reacts with the positive carbonyl carbon of 1,2-diketone imine intermediate (2) to form carbocation (3). Finally, dehydration and the sigmatropic shift results in the 2,4,5-trisubstituted imidazole. In addition, a similar mechanism is suggested for the synthesis of 1,2,4,5-tetrasubstituted imidazoles, except that at the beginning of the reaction amine derivatives participate instead of ammonium acetate [18].
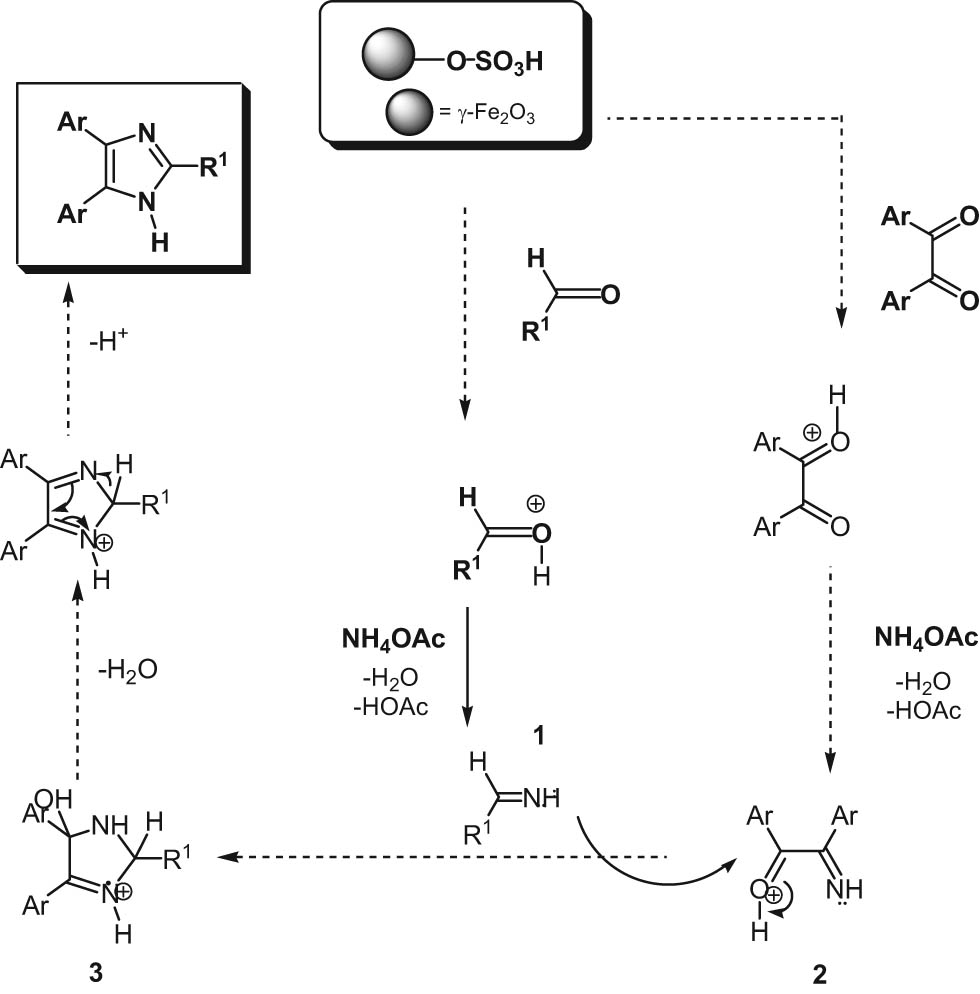
Mechanism for the synthesis of trisubstituted imidazoles.
An investigation was also performed to optimise the amount of ammonium acetate used in this reaction under solvent-free conditions, and the best result was achieved with 5 mmol ammonium acetate (Table 4, entry 3).
Optimisation of the ammonium acetate amount for model reactions (a: trisubstituted imidazole, b: tetrasubstituted imidazole)
| Entry | NH4OAc amount (mmol) | Condition | Yield (%) * | Condition | Yield (%) * |
|---|---|---|---|---|---|
| 1a | 1a | 1b | 1b | ||
| 1 | 1.5 | 50 min/100°C | 70 | 40 min/100°C | 72 |
| 2 | 3 | 50 min/100°C | 79 | 40 min/100°C | 83 |
| 3 | 5 | 50 min/100°C | 94 | 40 min/100°C | 97 |
- *
Yields refer to isolated products.
In the synthesis of trisubstituted imidazoles, several aromatic aldehydes with electron-withdrawing or electron-donating substituent groups were evaluated. High efficiency was achieved for substituent groups in ortho, meta or para positions of the aromatic ring (Table 5). Similar results were obtained for the synthesis of tetrasubstituted imidazoles from combinations of differently substituted aromatic aldehydes and amine derivatives (Table 6). The structure of polysubstituted imidazoles was characterized by IR and NMR.
One-pot synthesis of trisubstituted imidazoles catalysed by γ-Fe2O3-SO3H
| Compound | R1 | Time (min) | Yield (%) * | M.p. (°C) |
|---|---|---|---|---|
| 1a | C6H5 | 50 | 94 | 277–279 [19] |
| 2a | 4-ClC6H4 | 55 | 97 | 266–268 [20] |
| 3a | 2-ClC6H4 | 40 | 98 | 194–196 [19] |
| 4a | 4-MeOC6H4 | 50 | 98 | 231–233 [9] |
| 5a | 2-MeOC6H4 | 40 | 95 | 208–210 [20] |
| 6a | 4-FC6H4 | 40 | 95 | 253–255 [21] |
| 7a | 4-BrC6H4 | 50 | 96 | 260–262 [9] |
| 8a | 2-HOC6H4 | 55 | 90 | 212–214 [9] |
| 9a | 3-O2NC6H4 | 60 | 93 | 321–323 [22] |
| 10a | 4-O2NC6H4 | 60 | 92 | 241–243 [9] |
| 11a | 4-MeC6H4 | 70 | 93 | 232–235 [20] |
| 12a | 2,4-Cl2C6H3 | 65 | 97 | 173–175 [9] |
| 13a | 3-HOC6H4 | 50 | 94 | 261–263 [23] |
| 14a | 3-BrC6H4 | 50 | 95 | 303–305 [23] |
| 15a | 3,4-(MeO)2C6H3 | 40 | 96 | 220–222 [9] |
| 16a | 4-(Me)2NC6H4 | 60 | 92 | 256–258 [20] |
- *
Yields refer to isolated products.
One-pot synthesis of tetrasubstituted imidazoles catalysed by γ-Fe2O3-SO3H
| Compound | R1 | R2 | Time (min) | Yield (%) * | M.p. (°C) |
|---|---|---|---|---|---|
| 1b | C6H5 | C6H5 | 40 | 97 | 219–221 [9] |
| 2b | 2-HOC6H4 | C6H5 | 30 | 98 | 252–254 [24] |
| 3b | 4-ClC6H4 | C6H5 | 35 | 98 | 171–173 [9] |
| 4b | 4-MeOC6H4 | C6H5 | 40 | 97 | 180–182 [25] |
| 5b | 4-MeC6H4 | C6H5 | 35 | 96 | 185–187 [26] |
| 6b | 4-Br C6H4 | C6H5 | 30 | 95 | 193–195 [27] |
| 7b | 3-HOC6H4 | C6H5 | 25 | 96 | 186–188 [27] |
| 8b | 3-O2NC6H4 | C6H5 | 35 | 95 | 242–245 [28] |
| 9b | 4-O2NC6H4 | C6H5 | 40 | 96 | 196–198 [28] |
| 10b | 4-HOC6H4 | C6H5 | 20 | 97 | 285–287 [9] |
| 11b | 3-BrC6H4 | C6H5 | 35 | 95 | 148–150 [29] |
| 12b | 2-HOC6H4 | 4-MeC6H4 | 40 | 97 | 222–225 [9] |
| 13b | 4-MeC6H4 | 4-MeC6H4 | 40 | 96 | 196–198 [9] |
| 14b | 3-O2NC6H4 | 4-MeC6H4 | 25 | 97 | 155–157 [9] |
| 15b | 4-O2NC6H4 | 4-MeC6H4 | 30 | 94 | 220–222 [9] |
| 16b | 3-HOC6H4 | 4-MeC6H4 | 35 | 94 | 229–231 [9] |
| 17b | 4-ClC6H4 | 4-ClC6H4 | 35 | 95 | 182–185 [26] |
| 18b | 4-MeC6H4 | 4-ClC6H4 | 30 | 97 | 169–170 [26] |
- *
Yields refer to isolated products.
Table 7 compares the performance of γ-Fe2O3-SO3H with several other reported catalysts for the synthesis of substituted imidazoles. As one can see, high efficiency and a short time for the reaction are the features of this catalyst.
Comparison of the γ-Fe2O3-SO3H catalyst with several catalysts for the synthesis of imidazoles
| Entry | Catalyst | Condition for trisubstituted imidazoles | Yield (%) | Condition for tetrasubstituted imidazoles | Yield (%) |
|---|---|---|---|---|---|
| 1 | [(CH2)4SO3HMIM][HSO4] [30] | — | — | SF/140°C/150 min | 88 |
| 2 | SASPSPE [29] | — | — | SF/140°C/70 min | 89 |
| 3 | PTSA [31] | Ethanol/80°C/60 min | 90 | Ethanol/80°C/60 min | 84 |
| 4 | Y(NO3)3.6H2O [32] | SF/140°C/50 min | 90 | — | — |
| 5 | ZnO nanorods [20] | H2O/reflux/150 min | 80 | — | — |
| 6 | I 2 [12] | DMSO/130°C, EtOH/100°C/1440-120 | 86 | — | — |
| 7 | This work | SF/100°C/50 min | 94 | SF/100°C/40 min | 97 |
After the reaction completion, the γ-Fe2O3-SO3H catalyst used can be easily separated from the products by an external magnet and reused. Therefore, the catalyst was recycled and reused several times in subsequent reactions. At the end of these reactions, the products were still obtained with satisfactory yields (Figure 1).

The reusability of the catalyst for model reactions (a: Trisubstituted imidazole and b: tetrasubstituted imidazole).
3 Conclusion
Here, we introduce a simple method for producing 2,4,5-trisubstituted imidazoles and 1,2,4,5-tetrasubstituted imidazoles, valuable heterocyclic compounds with pharmaceutical properties and medical applications, in a simple one-pot procedure with a recyclable catalyst in a green process. This project has various advantages such as a recyclable catalyst, high catalytic activity, eco-friendly solvent-free conditions, short reaction time, easy separation of catalyst by an external magnet, high-purity products, and excellent yields.
4 Experimental section
4.1 General
All chemicals used in this study were commercially available, synthesis-grade compounds, purchased from Merck or Fluka and used without any further purification. The products were identified by comparison of their melting points and spectral data with those reported in the literature. Melting points were recorded on the Electrothermal 9100 apparatus and are uncorrected. The reaction progress was monitored by TLC on commercial aluminum-backed plates of silica gel 60 F254, using ultraviolet light. FT-IR spectra were obtained with a Shimadzu 8400 spectrometer in the range 400–4000 cm−1 and the 1H NMR (500 or 300 MHz) and 13C NMR (125 or 75 MHz) spectra were recorded on a Bruker Avance 300 spectrometer in pure deuterated chloroform, with tetramethylsilane (TMS) as the internal standard.
4.2 General procedure for the synthesis of the γ-Fe2O3-SO3H catalyst
According to the reported method [13,33,34,35] for the synthesis of the desired γ-Fe2O3-SO3H catalyst, 3 g of γ-Fe2O3 nanoparticles and chlorosulfonic acid (1.163 g, 9.98 mmol) was added over a period of 30 min (Scheme 3). For comparison with the reported literature [13], the γ-Fe2O3-SO3H catalyst particles were characterized via FT-IR, XRD, TGA, XPS, TEM, titration, VSM, and DLS.
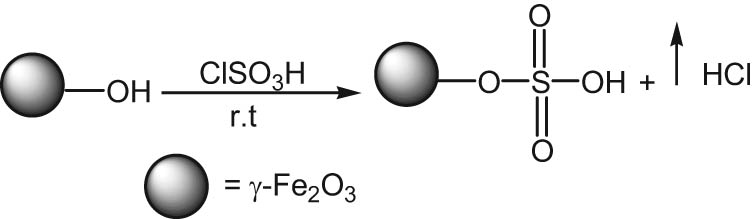
Synthesis of the γ-Fe2O3-SO3H catalyst.
4.3 General procedure for the synthesis of 2,4,5-trisubstituted imidazoles
A mixture of benzil 1 (1 mmol), ammonium acetate (5 mmol), aromatic aldehyde (1 mmol), and the γ-Fe2O3-SO3H catalyst (10 mol%) was stirred at 100°C under solvent-free conditions for an appropriate time. The progression of the reaction was monitored by TLC. After completion of the reaction, acetone was added to the contents of the reaction vessel, the product was completely dissolved in acetone, and the catalyst was separated by an external magnet. For further purification, the solid product was recrystallised from acetone/water 9:1 (v/v) to obtain the pure product.
4.3.1 2-(4-Chlorophenyl)-4,5-diphenyl-1H-imidazole (2a)
M.p.: 266–268°C; FT-IR (KBr) ν max/cm–1: 690, 750, 830, 1,475, 1,600, 3,100, 3,400. 1H NMR (500.13 MHz, DMSO-d 6), δ/ppm: 7.25–7.82 (m, 14H, H-Ar), 12.60 (s, 1H, −NH).
4.3.2 2-(2-Methoxyphenyl)-4,5-diphenyl-1H-imidazole (5a)
M.p.: 208–210°C; FT-IR (KBr) ν max/cm–1: 690, 750, 900, 1,475, 1,600, 1,650, 3,100, 3,300. 1H NMR (500.13 MHz, DMSO-d 6), δ/ppm: 3.882 (s, 3H, OCH3), 6.862–8.025 (m, 14H, H-Ar), 11.855 (s, 1H, NH).
4.3.3 2-(2,4-Dichlorophenyl)-4,5-diphenyl-1H-imidazole (12a)
M.p.: 173–175°C; FT-IR (KBr) ν max/cm–1: 690, 900, 1,475, 1,560, 1,600, 3,100, 3,300. 1 H NMR (500.13 MHz, DMSO-d 6), δ/ppm: 7.175–7.808 (m, 13H, H-Ar), 12.658 (s, 1H, NH).
4.3.4 2-(3-Bromophenyl)-4,5-diphenyl-1H-imidazole (14a)
M.p.: 303–305°C; FT-IR (KBr) ν max/cm–1: 690, 900, 1,075, 1,475, 1,600, 1,650, 3,100, 3,300. 1H NMR (500.13 MHz, DMSO-d 6), δ/ppm: 7.234–8.314 (m, 14H, H-Ar), 12.99 (s, 1H, NH).
4.3.5 2-(3,4-Dimethoxyphenyl)-4,5-diphenyl-1H-imidazole (15a)
M.p.: 220–222°C; FT-IR (KBr) ν max/cm–1: 1475, 1,600, 1,650, 3,100, 3,400. 1 H NMR (500.13 MHz, DMSO-d 6), δ/ppm: 3.137 (s, 3H, OCH3), 3.814 (s, 3H, OCH3) 7.014–7.636 (m, 13H, H-Ar), 12.477(s, 1H, NH).
4.4 General procedure for the synthesis of 1,2,4,5-tetrasubstituted imidazoles
A mixture of benzil (1 mmol), ammonium acetate (2 mmol), aromatic aldehyde (1 mmol), amine derivative (2 mmol), and the γ-Fe2O3-SO3H catalyst (10 mol%) was stirred at 100°C under solvent-free conditions for an appropriate time. The progression of the reaction was monitored by TLC. After the completion of the reaction, acetone was added to the reaction vessel, the product was completely dissolved in the acetone, and the catalyst was separated by an external magnet. For further purification, the solid product was recrystallised from acetone/water 9:1 (v/v) to obtain the pure product.
4.4.1 1,2,4,5-Tetraphenyl-1H-imidazole (1b)
M.p.: 219–221°C; FT-IR (KBr) ν max/cm–1: 690, 900, 1,475, 1,520, 1,600, 3,100. 1H NMR (500.13 MHz, DMSO-d 6), δ/ppm: 7.23–7.61 (m, 20H, H-Ar). 13C NMR (125.76 MHz, DMSO-d 6), δ (ppm): 127.136, 129.95, 132.17, 135.87, 138.26.
4.4.2 2-(4-Methylphenyl)-1,4,5-triphenyl-1H-imidazole (5b)
M.p.: 185–187°C; FT-IR (KBr) ν max/cm–1: 690, 770, 820, 1,475, 1,500, 1,600, 2,920, 3,100. 1H NMR (500.13 MHz, DMSO-d 6), δ/ppm: 2.28 (s, 3H, CH3), 7.23–7.61 (m, 19H, H-Ar). 13C NMR (125.76 MHz, DMSO-d 6), δ (ppm): 21.22, 138.26, 138.36, 138.75, 147.29.
4.4.3 3-(1,4,5-Triphenyl-1H-imidazol-2-yl)phenol (7b)
M.p.: 186–188°C; FT-IR (KBr) ν max/cm–1: 694, 1,274, 1,494, 1,606, 3,060, 3,466. 1H NMR (500.13 MHz, DMSO-d 6), δ/ppm: 4.675 (s, 1H, OH), 6.475-7.556 (m, 19H, H-Ar).
Acknowledgements
The authors gratefully acknowledge the Semnan University Research Council for supporting this work.
-
Funding information: The authors state no funding involved.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Vaidya N. Toxicity focus is essential for green chemistry adoption and sustainable product development. Sustain Chem Pharm. 2019;11:12–6.10.1016/j.scp.2018.11.005Search in Google Scholar
[2] Marco BAd, Rechelo BS, Tótol EG, Kogawa AC, Salgado HRN. Evolution of green chemistry and its multidimensional impacts: a review. Saudi Pharm J. 2019;27(1):1–8.10.1016/j.jsps.2018.07.011Search in Google Scholar PubMed PubMed Central
[3] Akbarzadeh P, Koukabi N. Fibroin-functionalized magnetic carbon nanotube as a green support for anchoring silver nanoparticles as a biocatalyst for A3 coupling reaction. Appl Organometal Chem. 2019;34(3). 10.1002/aoc.5395.Search in Google Scholar
[4] Albadi J, Samimi HA, Momeni AR. Alumina-supported cobalt nanoparticles efficiently catalyzed the synthesis of chromene derivatives under solvent-free condition. Chem Methodolog. 2020;4:565–71.Search in Google Scholar
[5] Maria A, Vincent MV, Mookkaiah R, Subramani R, Nadesan K. Catharanthus roseus leaf extract mediated facile green synthesis of copper oxide nanoparticles and its photocatalytic activity. Chem Methodolog. 2020;4(4):424–36.10.33945/SAMI/CHEMM.2020.4.5Search in Google Scholar
[6] Rohaniyan M, Davoodnia A, Khojastehnezhad A, Beyramabadi SA. Catalytic evaluation of newly prepared GO-SB-H2PMo as an efficient and reusable nanocatalyst for the neat synthesis of amidoalkyl naphthols. Eurasian Chem Commun. 2019;2(3):329–39.10.33945/SAMI/ECC.2020.3.4Search in Google Scholar
[7] Sajjadifara S, Amini I, Jabbari H, Pouralimardan O, Fekri MH, Pal K. An efficient facile and one-pot synthesis of 2-arylsubstituted benzimidazole derivatives using 1-methyl-3-(2-oxyethyl)-1Himidazol-3-ium-borate sulfonic acid as a recyclable and highly efficient ionic liquid catalyst at green condition. Eurasian. Chem Commun. 2019;1(2):191–9.10.33945/SAMI/ECC.2019.2.7Search in Google Scholar
[8] Chen M-N, Mo L-P, Cui Z-S, Zhang Z-H. Magnetic nanocatalysts: synthesis and application in multicomponent reactions. Curr OpGreen SustaChem. 2019;15:27–37.10.1016/j.cogsc.2018.08.009Search in Google Scholar
[9] Shaterian HR, Ranjbar M. An environmental friendly approach for the synthesis of highly substituted imidazoles using bronsted acidic ionic liquid, N-methyl-2-pyrrolidonium hydrogen sulphate, as reusable catalyst. J Mol Liq. 2011;160:40–9.10.1016/j.molliq.2011.02.012Search in Google Scholar
[10] Siciliano A, Russo D, Spasiano D, Marotta R, Race M, Fabbricino M, et al. Chronic toxicity of treated and untreated aqueous solutions containing imidazole-based ionic liquids and their oxidized by-products. Ecotoxicol Environ Saf. 2019;180:466–72.10.1016/j.ecoenv.2019.05.048Search in Google Scholar PubMed
[11] Ramaiah MM, Shubha PB, Prasada H, Shivananju NS. Novel synthesis of N-unsubstituted imidazoles via the cycloaddition of N-(tertbutylsulfinyl)imines and TosMIC. Tetrahedron Lett. 2020;38:3235–45.10.1016/j.tetlet.2020.151705Search in Google Scholar
[12] Naidoo S, Jeena V. Molecular iodine/DMSO mediated oxidation of internal alkynes and primary alcohols using a one-pot, two step approach towards 2,4,5-trisubstituted imidazoles: substrate scope and mechanistic studies. Tetrahedron. 2020;76:131028.10.1016/j.tet.2020.131028Search in Google Scholar
[13] Koukabi N, Kolvari E, Zolfigol MA, Khazaei A, Shaghasemi BS, Fasahati B. A magnetic particle-supported sulfonic acid catalyst: tuning catalytic activity between homogeneous and heterogeneous catalysis. Adv Synth Catal. 2012;354(10):2001–8.10.1002/adsc.201100352Search in Google Scholar
[14] Safari J, Akbari Z, Naseh S. Nanocrystalline MgAl2O4 as an efficient catalyst for one-pot synthesis of multisubstituted imidazoles rnal of Saudi Chemical Society (2012)under solvent-free conditions. J Saudi Chem Soc. 2016;20:S250–5.10.1016/j.jscs.2012.10.012Search in Google Scholar
[15] Khalifeh R, Naseri V, Rajabzadeh M. Synthesis of imidazolium-based ionic liquid on modified magnetic nanoparticles for application in one-pot synthesis of trisubstituted imidazoles. ChemistrySelect. 2020;5:11453–62.10.1002/slct.202003133Search in Google Scholar
[16] Mardani HR, Forouzani M, Emami R. Efficient and green synthesis of trisubstituted imidazoles by magnetically nanocatalyst and microwave assisted. Asian J Green Chem. 2019;3:525–35.Search in Google Scholar
[17] Safari J, Zarnegar Z. A highly efficient magnetic solid acid catalyst for synthesis of 2,4,5-trisubstituted imidazoles under ultrasound irradiation. Ultrason Sonochemistr. 2013;20(2):740–6.10.1016/j.ultsonch.2012.10.004Search in Google Scholar PubMed
[18] Zolfagharinia S, Kolvari E, Koukabi N, Hosseini MM. Core-shell zirconia-coated magnetic nanoparticles offering a strong option to prepare a novel and magnetized heteropolyacid based heterogeneous nanocatalyst for three- and four-component reactions. Arab J Chem. 2020;13(1):227–41.10.1016/j.arabjc.2017.04.004Search in Google Scholar
[19] Jourshari MS, Mamaghani M, Shirini F, Tabatabaeian K, Rassa M, Langari H. An expedient one-pot synthesis of highly substituted imidazoles using supported ionic liquid-like phase (SILLP) as a green and efficient catalyst and evaluation of their anti-microbial activity. Chin Chem Lett. 2013;24:993–6.10.1016/j.cclet.2013.06.005Search in Google Scholar
[20] Nikoofar K, Haghighi M, Lashanizadegan M, Ahmadvand Z. ZnO nanorods: efficient and reusable catalysts for the synthesis of substituted imidazoles in water. J Taibah Univ Sci. 2015;9(4):570–8.10.1016/j.jtusci.2014.12.007Search in Google Scholar
[21] Xu F, Wang N, Tian Y, Li G. Simple and efficient method for the synthesis of highly substituted imidazoles catalyzed by benzotriazole. J Heterocycl Chem. 2013;50(3):668–75.10.1002/jhet.1818Search in Google Scholar
[22] Mohammadi A, Keshvari H, Sandaroos R, Rouhi H, Sepehr Z. A novel polymeric catalyst for the one-pot synthesis of 2,4,5-triaryl-1H-imidazoles. J Chem Sci. 2012;124:717–22.10.1007/s12039-012-0248-ySearch in Google Scholar
[23] Safari J, Zarnega Z. A highly efficient magnetic solid acid catalyst for synthesis of 2,4,5-trisubstituted imidazoles under ultrasound irradiation. Ultrason Sonochemistr. 2013;20(2):740–6.10.1016/j.ultsonch.2012.10.004Search in Google Scholar
[24] Safari J, Gandomi-Ravandi S, Akbari Z. Sonochemical synthesis of 1,2,4,5-tetrasubstituted imidazoles using nanocrystalline MgAl2O4 as an effective catalyst. J Adv Res. 2012;4:509–14.10.1016/j.jare.2012.09.001Search in Google Scholar PubMed PubMed Central
[25] Wang XB, He L, Jian TY, Ye S. Cyclic phosphoric acid catalyzed one-pot, four-component synthesis of 1,2,4,5-tetrasubstituted imidazoles. Chin Chem Lett. 2012;23:13–6.10.1016/j.cclet.2011.09.018Search in Google Scholar
[26] Karimi AR, Alimohammadi Z, Azizian J, Mohammadi AA, Mohammadizadeh MR. Solvent-free synthesis of tetrasubstituted imidazoles on silica gel/NaHSO4 support. Catal Commun. 2006;7:728–32.10.1016/j.catcom.2006.04.004Search in Google Scholar
[27] Das B, Kashanna J, Kumar RA, Jangili P. Synthesis of 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazoles in water using p-dodecylbenzenesulfonic acid as catalyst. Monatsh Chem. 2013;144:223–6.10.1007/s00706-012-0770-0Search in Google Scholar
[28] Hekmatshoar R, Kargar M, Mostashari A, Hashemi Z, Goli F, Mousavizadeh F. A practical and highly efficient synthesis of 1,2,4,5 tetrasubstituted imidazoles using 2-ethylhexanoic acid as a reusable organocatalyst and reaction medium Gazi University. J Sci. 2015;28(1):21–6.Search in Google Scholar
[29] Tavakoli Z, Bagherneghad M, Niknam K. Synthesis of 1,2,4,5‐tetrasubstituted imidazoles using sulfuric acid ([3‐(3‐silicapropyl)sulfanyl]propyl]ester as a recyclable solid acid. J Heterocycl Chem. 2012;49:634–9.10.1002/jhet.847Search in Google Scholar
[30] Davoodnia A, Heravi MM, Safavi-Rad Z, Tavakoli-Hoseini N. Green, one-pot, solvent-free synthesis of 1,2,4,5-tetrasubstituted imidazoles using a brønsted acidic ionic liquid as novel and reusable catalyst. Synth Commun. 2010;40:2588–97.10.1080/00397910903289271Search in Google Scholar
[31] Vikrant K, Ritu M, Neha S. Synthesis of substituted imidazoles via a multi-component condensation catalyzed by p-toluene sulfonic acid, PTSA. Res J Chem Sci. 2012;2(4):18–23.Search in Google Scholar
[32] Karami B, Dehghani FM, Eskandari K. Facile and rapid synthesis of polysubstituted imidazoles by employing Y(NO3)3 × 6H2O as catalyst. Croat Chem Acta. 2012;85(2):147–53.10.5562/cca1979Search in Google Scholar
[33] Kolvari E, Koukabi N, Armandpour O. A simple and efficient synthesis of 3,4-dihydropyrimidin-2-(1H)- ones via Biginelli reaction catalyzed by nanomagnetic-supported sulfonic acid. Tetrahedron. 2014;70(6):1383–6.10.1016/j.tet.2013.10.085Search in Google Scholar
[34] Koukabi N, Kolvari E, Khazaei A, Zolfigol MA, Shirmardi-Shaghasemi B, Khavasi HR. Hantzsch reaction on free nano-Fe2O3 catalyst: excellent reactivity combined with facile catalyst recovery and recyclability. Chem Commun. 2011;47:9230–2.10.1039/c1cc12693hSearch in Google Scholar PubMed
[35] Otokesh S, Koukabi N, Kolvari E, Amoozadeh A, Malmir M, Azhari S. A solvent-free synthesis of polyhydroquinolines via hantzsch multicomponent condensation catalyzed by nanomagnetic-supported sulfonic acid. S Afr J Chem. 2015;68:15–20.10.17159/0379-4350/2015/v68a3Search in Google Scholar
© 2021 Mahnaz Sakhdari et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Molecular, Electronic, Nonlinear Optical and Spectroscopic Analysis of Heterocyclic 3-Substituted-4-(3-methyl-2-thienylmethyleneamino)-4,5-dihydro-1H-1,2,4-triazol-5-ones: Experiment and DFT Calculations
- Lewis acid / Base-free Strategy for the Synthesis of 2-Arylthio and Selenyl Benzothiazole / Thiazole and Imidazole
- Synergistic promoting effect of ball milling and Fe(ii) catalysis for cross-dehydrogenative-coupling of 1,4-benzoxazinones with indoles
- Magnetic nanoparticle-supported sulfonic acid as a green catalyst for the one-pot synthesis of 2,4,5-trisubstituted imidazoles and 1,2,4,5-tetrasubstituted imidazoles under solvent-free conditions
- Synthesis, characteristic fragmentation patterns, and antibacterial activity of new azo compounds from the coupling reaction of diazobenzothiazole ions and acetaminophen
- Para toluenesulfonic acid-catalyzed one-pot, three-component synthesis of benzo[5,6]chromeno[3,2-c]quinoline compounds in aqueous medium
- Design and microwave-assisted synthesis of a novel Mannich base and conazole derivatives and their biological assessment
- Quantum chemical studies on structural, spectroscopic, nonlinear optical, and thermodynamic properties of the 1,2,4-triazole compound
- Design, synthesis, and biological evaluation of phenyl-isoxazole-carboxamide derivatives as anticancer agents
- Quantum chemistry study on the promoted reactivity of substituted cyclooctynes in bioorthogonal cycloaddition reactions
- Quantum chemical calculations of phenazine-based organic dyes in dye-sensitized solar cells
- Rapid Communications
- One-pot synthesis of benzofurans via heteroannulation of benzoquinones
- Design and development of novel thiazole-sulfonamide derivatives as a protective agent against diabetic cataract in Wistar rats via inhibition of aldose reductase
- Review Articles
- Organic electrochemistry: Synthesis and functionalization of β-lactams in the twenty-first century
- Some applications of deep eutectic solvents in alkylation of heterocyclic compounds: A review of the past 10 years
Articles in the same Issue
- Research Articles
- Molecular, Electronic, Nonlinear Optical and Spectroscopic Analysis of Heterocyclic 3-Substituted-4-(3-methyl-2-thienylmethyleneamino)-4,5-dihydro-1H-1,2,4-triazol-5-ones: Experiment and DFT Calculations
- Lewis acid / Base-free Strategy for the Synthesis of 2-Arylthio and Selenyl Benzothiazole / Thiazole and Imidazole
- Synergistic promoting effect of ball milling and Fe(ii) catalysis for cross-dehydrogenative-coupling of 1,4-benzoxazinones with indoles
- Magnetic nanoparticle-supported sulfonic acid as a green catalyst for the one-pot synthesis of 2,4,5-trisubstituted imidazoles and 1,2,4,5-tetrasubstituted imidazoles under solvent-free conditions
- Synthesis, characteristic fragmentation patterns, and antibacterial activity of new azo compounds from the coupling reaction of diazobenzothiazole ions and acetaminophen
- Para toluenesulfonic acid-catalyzed one-pot, three-component synthesis of benzo[5,6]chromeno[3,2-c]quinoline compounds in aqueous medium
- Design and microwave-assisted synthesis of a novel Mannich base and conazole derivatives and their biological assessment
- Quantum chemical studies on structural, spectroscopic, nonlinear optical, and thermodynamic properties of the 1,2,4-triazole compound
- Design, synthesis, and biological evaluation of phenyl-isoxazole-carboxamide derivatives as anticancer agents
- Quantum chemistry study on the promoted reactivity of substituted cyclooctynes in bioorthogonal cycloaddition reactions
- Quantum chemical calculations of phenazine-based organic dyes in dye-sensitized solar cells
- Rapid Communications
- One-pot synthesis of benzofurans via heteroannulation of benzoquinones
- Design and development of novel thiazole-sulfonamide derivatives as a protective agent against diabetic cataract in Wistar rats via inhibition of aldose reductase
- Review Articles
- Organic electrochemistry: Synthesis and functionalization of β-lactams in the twenty-first century
- Some applications of deep eutectic solvents in alkylation of heterocyclic compounds: A review of the past 10 years

