Synthesis of polycyclic phosphonates via an intramolecular Diels-Alder reaction of 2-benzoylbenzalaldehyde and alkenyl phosphites
-
Kenji Yamana
Abstract
In this paper, we present a Lewis-acid-promoted reaction of 2-benzoylbenzaldehyde and trialkenyl phosphites, which resulted in the formation of polycyclic phosphonates. The reaction proceeded via nucleophilic attack of trialkenyl phosphite on the carbonyl carbon of 2-benzoylbenzaldehyde. The subsequent intramolecular Diels-Alder reaction led to the formation of the cyclic phosphonate.
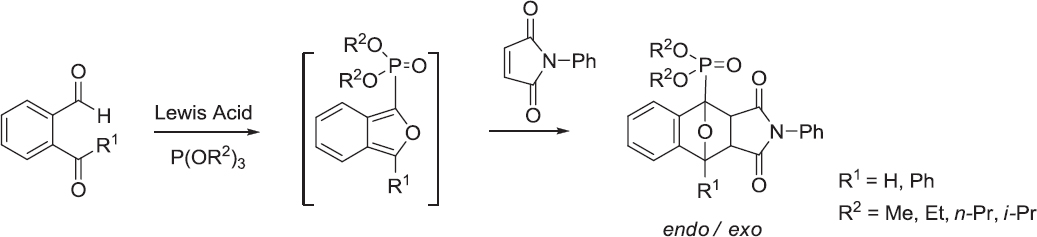
Cyclic phosphonates are often utilized as key intermediate reagents (synthetic intermediates) in the preparation of synthetically useful products and biologically active compounds [1,2]. Therefore, the synthesis of these compounds has attracted a great deal of research attention in the fields of synthetic organic, bioorganic, and medicinal chemistry [3, 4, 5, 6]. Moreover, the development of new methods for the preparation of cyclic phosphonates has become very important in organic chemistry. For this purpose, the chemistry of isobenzofuran [7,8,9] and the intramolecular Diels-Alder reaction [10,11] are extremely interesting from a theoretical point of view. They represent a possible way to synthesize pharmaceutical candidate compounds, such as natural products and biologically active compounds with complicated structures in only a few short steps [3,12, 13, 14, 15, 16, 17, 18, 19, 20,]. For example, the synthesis of alkaloid derivatives using Lewis acids has been reported by Yilin et al. [21,22]. Previously, we reported the formation of dialkyl isobenzofuran-1-ylphosphonates by the reaction between o-phthalalde-hyde and trialkyl phosphites in the presence of a Lewis acid (Scheme 1) [23,24].
In this study, we attempted to apply a Lewis-acid-promoted reaction of aromatic aldehydes and alkenyl phosphites to establish a new method for the one-step synthesis of polycyclic phosphonates.
Unfortunately, the reaction of o-phthalaldehyde 1 with triallyl phosphite 2a or tributenyl phosphite 2b failed to produce the intramolecular Diels-Alder adducts 4a and 4b (Scheme 2). We considered the possibility that isobenzofuran derivatives 3a and 3b were formed as intermediates, but decomposed due to their instability under these reaction conditions. Because of the low dienophilicity of the C=C bond of allyl and butenyl groups, the intramolecular Diels-Alder reaction of 3 did not proceed smoothly. On the other hand, adducts 5a and 5b were formed from the reaction of 1 with trialkenyl phosphite and N-phenylmal-eimide via intermediates 3a and 3b. However, we considered the possibility to obtain the intramolecular adducts by stabilizing the intermediates 3a and 3b.
Considering the previously reported stabilization effect of a phenyl group on the intermediate isobenzofuran-1-ylphosphonate [25], a similar approach was employed in this study. It was expected that the replacement of 1 by 2-benzoylbenzaldehyde 6 would stabilize the intermediates, isobenzofuran derivatives 7a and 7b, due to a resonance effect derived from the phenyl group. The intramolecular adduct 9b (Scheme 3, 31% yield) was generated from the intramolecular Diels-Alder reaction of isobenzofuran derivative 7b and the subsequent aromatization of the product 8b. However, these reaction conditions did not yield the intramolecular adduct 9a. This suggested that the strain of the 5-membered ring in 8a is stronger than that of the 6-membered ring in 8b.
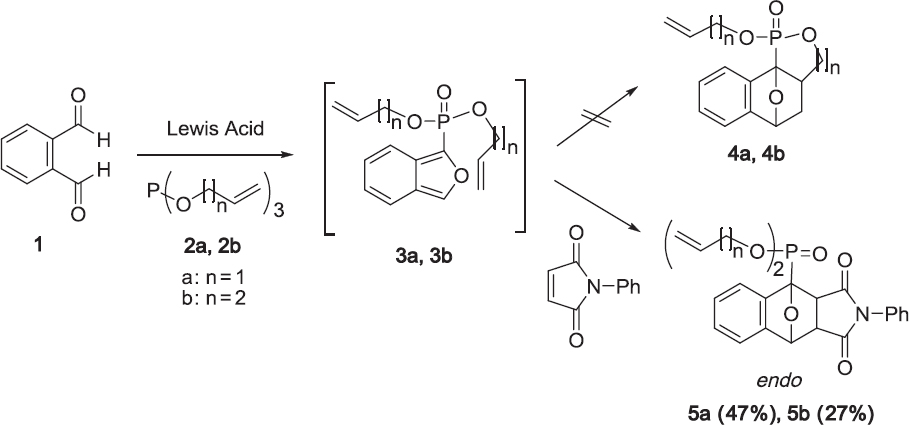
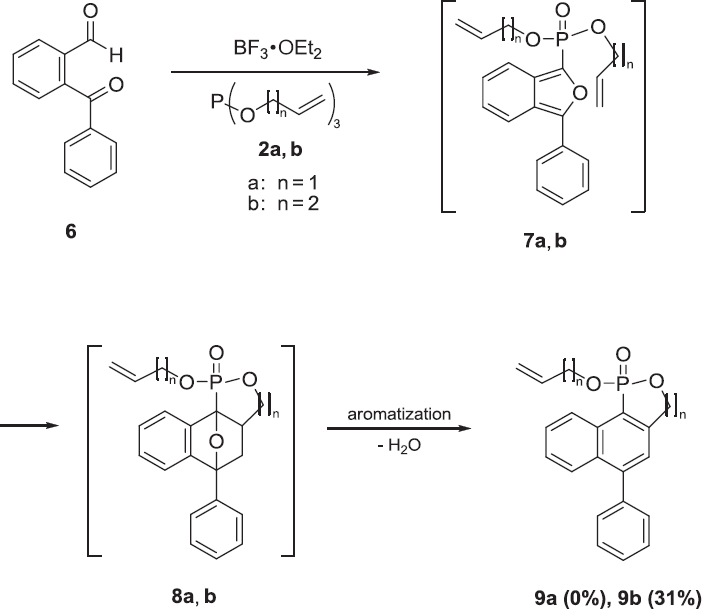
The fact that the yield of the intermolecular adduct 5a (47%) from triallyl phosphite 2a was better than that of 5b (27%) from tributenyl phosphite 2b (Scheme 2) indicated that the reaction of 2a proceeded more smoothly than that of 2b because the allyl cation is more stable than the butenyl cation.
As previously reported, the rate limiting step in the formation of isobenzofuran-1-ylphosphonates is the alkyl group elimination from the trialkyl phosphite [25]. Moreover, the reaction proceeds more easily and the yield is increased when the alkyl group is smaller [Scheme 1, R1 = H, R2 = Me (80%), Et (71%), Pr (67%), and i-Pr (55%); endo/exo total yield). Similarly, the yield of polycyclic phosphonates should improve when the butenyl group of the trialkenyl phosphite is converted to a methyl group.
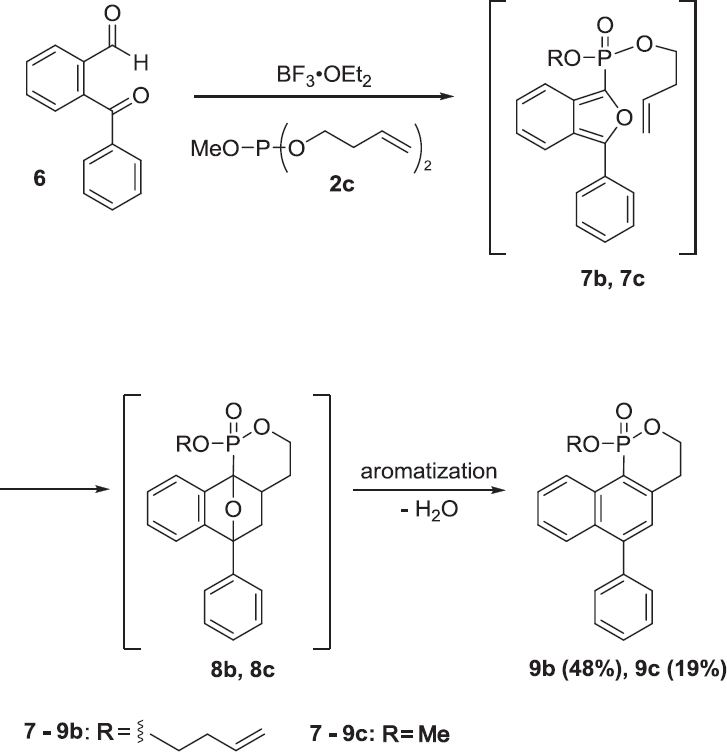
Therefore, dibutenyl methyl phosphite 2c, where one of the butenyl groups is replaced by a methyl group, was employed in the reaction. This led to an increase in the total yield of the reaction to 67%, with 9b (48%) being the major product after elimination of the methyl group (Scheme 4).
In summary, a new method for the synthesis of polycyclic phosphonates was developed, involving the treatment of 2-benzoylbenzaldehyde 6 with alkenyl phosphites, such as 2b and 2c. Polycyclic phosphonates 9b and 9c were obtained via an intramolecular cycloaddition in only one step. These reactions provide a new approach to the generation of cyclic phosphonates.
Experimental Details
1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were recorded in CDCl3 on a Bruker AVANCE III instrument. Tetramethylsilane was employed as an internal standard. The melting points were determined using a Yanako micro melting point apparatus and were uncorrected. High-resolution mass spectra were obtained using a JEOL JMS-T100GCV (EI) and microOTOF-QII (ESI).
General Procedure for the Preparation of Intramolecular Diels-Alder Adducts
BF3•OEt2 (1 mmol) was added to a solution of 2-benzoylbenzaldehyde 6 (1 mmol) in acetonitrile (3 mL) at
Yields of intramolecular Diels-Alder adducts (9a-c) by the reaction of 2-benzoylbenzaldehyde (6) and phosphites (2a-c)a
| Entry | Phosphites | Adducts | Yieldsb ( % ) |
|---|---|---|---|
| 1 | 2a | 9a | - |
| 2 | 2b | 9b | 31 |
| 3 | 2c | 9b, 9c | 48, 19 |
aReaction conditions: BF3 ∙ OEt2 and 2-benzoylbenzaldehyde (1 equiv), MeCN, 0 °C, 0.5 h, followed by phosphites (1 equiv), 25 °C, 48 h. bIsolated.
0 °C. After stirring at this temperature for 0.5 h, alkenyl phosphite 2b or 2c (1 mmol) was added and the mixture was stirred at 25 °C for 48 h. HCl solution was added to quench the reaction, and the organic layer was extracted with CH2Cl2, washed with NaHCO3, dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was chromatographed on silica gel (AcOEt:Hexane = 1:1) to give 9b or 9c.
1-(But-3-en-1-yloxy)-6-phenyl-1H,3H,4H-1l5-naphtho[1,2-c][1,2]oxaphosphinin-1-one (9b)
The compound was obtained as a colorless oil; 1H NMR (400 MHz, CDCl3): δ 8.62 (d, 1H, J = 8.5 Hz), 7.87 (d, 1H, J = 8.5 Hz), 7.62 (t, 1H, J = 7.4 Hz), 7.43−7.52 (m, 6H), 7.23 (d, 1H, J = 5.2 Hz), 5.81 (ddt, 1H, J = 17.1, 10.3, 6.8 Hz), 5.13 (d, 1H, J = 17.2 Hz), 5.08 (d, 1H, J = 10.2 Hz), 4.62-4.68 (m, 2H), 4.29−4.37 (m, 1H), 4.23 (ddd, 1H, J = 13.9, 10.0, 6.9 Hz), 3.35−3.43 (m, 1H), 3.14 (ddd, 1H, J = 17.2, 7.3, 4.0 Hz), 2.48−2.53 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 145.3 (d, 4JC,P =3.2 Hz), 142.0 (d, 2JC,P = 6.3 Hz), 139.5, 133.6, 133.4 (d, 2JC,P = 9.8 Hz), 130.9 (d, 3JC,P = 12.2 Hz), 129.7, 128.4, 128.0, 127.8, 127.2 (d, 3JC,P = 14.9 Hz), 127.1 (d, 3JC,P = 5.8 Hz, C-5), 126.8 (C-7), 126.4 (C-8), 120.1 (d, 1JC,P = 174.7 Hz, C-10a), 117.7 (CH2=CH-), 65.9 (d, 2JC,P = 6.0 Hz, CH2), 65.3 (d, 2JC,P = 7.0 Hz, CH2), 34.9 (d, 3JC,P = 6.3 Hz, CH2), 32.3 (d, 3JC,P = 7.1 Hz, C-4). HRMS (EI) Calcd for C19H17NO3P (M+): 364.1228. Found: 364.1226.
1-Methoxy-6-phenyl-1H,3H,4H-1l5-naphtho[1,2-c][1,2] oxaphosphinin-1-one (9c)
The compound was obtained as a colorless oil; 1H NMR (400 MHz, CDCl3): δ 8.61 (d, 1H, J = 8.5 Hz, ArH), 7.87 (d, 1H, J = 8.4 Hz, ArH), 7.63 (t, 1H, J = 7.7 Hz, ArH), 7.44−7.53 (m, 6H, ArH), 7.24 (d, J = 5.2 Hz, 1H, ArH), 4.62 (m, 2H, CH2), 3.89 (d, 3H, 3JP-O-C-H = 11.4 Hz, CH3), 3.34−3.41 (m, 1H, CH2), 3.14−3.21(m, 1H, CH2); 13C NMR (100 MHz, CDCl3): δ 145.4 (d, 3JC-P =3.6 Hz, ArC), 142.1 (d, 2JC-P =6.4 Hz, ArC), 139.5 (ArC), 133.4 (d, 2JC-P =9.9 Hz, ArC), 130.9 (d, 3JC-P =12.1 Hz, ArC), 129.7 (ArC), 128.4, 128.0, 127.8, 127.2 (d, 3JC-P =14.7 Hz, ArC), 126.9 (d, 3JC-P=5.7 Hz, ArC), 126.8 (ArC), 126.4, 120.0 (d, JC- P = 174.1 Hz, ArC-P), 66.0 (d, 2JC-O-P = 5.9 Hz, CH2), 52.7 (d, 3JC-O-P = 6.8 Hz, CH2), 32.3 (d, 3JC-O-P = 7.1 Hz, CH2). HRMS (EI) Calcd for C19H17NO3P (M+): 324.0915. Found: 324.0907.
Bis(but-3-enyl) methyl phosphite (2c)
The compound was obtained as a colorless oil; 1H NMR (400 MHz, CDCl3): δ 5.76−5.85 (m, 2H), 5.06−5.14 (m, 4H), 3.51 (dt, 4H, J =7.8, 6.9 Hz), 3.51 (d, 3H, J = 10.4 Hz), 2.36−2.45 (m, 4H); 13C NMR (100 MHz, CDCl3): δ 134.5, 117.7, 61.7 (d, 2JC,P = 11.7 Hz), 49.0 (d, 2JC,P = 9.3 Hz), 35.6 (d, 4JC,P = 5.0 Hz). HRMS (ESI Negative) Calcd for C19H17NO3P (M+H-): 205.0994. Found: 205.0637.
Bis(prop-2-enyl) [(3aR*,4S*,9S*,9aR*)-1,3-dioxo-2-phenyl-1,2,3,3a,9,9a-hexahydro-4H-4,9-epoxybenzo[f] isoindol-4-yl]phosphonate (endo) (5a)
The compound was obtained as a colorless oil; 1H NMR (400 MHz, CDCl3): δ 7.52–7.61 (m, 1H), 7.34–7.40 (m, 3H), 7.25–7.28 (m, 3H), 6.38–6.42 (m, 2H), 6.02–6.11 (m, 1H), 5.87–5.96 (m, 2H), 5.44–5.49 (m, 1H), 5.28–5.35 (m, 2H), 5.20–5.22 (m, 1H), 4.87–4.98 (m, 2H), 4.64–4.76 (m, 2H), 4.27 (dd, 1H, J = 9.0, 8.6 Hz), 4.07 (dd, 1H, J = 8.5, 5.8 Hz); 13C NMR (67.80 MHz, CDCl3): δ 172.6, 171.5, 140.7, 140.7, 139.7, 139.6, 132.8, 132.7, 132.4, 132.4, 130.8, 129.0, 128.9, 128.7, 128.6, 126.3, 125.8, 122.1, 121.3, 118.7, 118.5, 86.2 (d, JC,P = 191.3 Hz), 81.6 (d, 3JC,P = 15.4 Hz), 68.5 (d, 2JC,P = 6.0 Hz), 68.0 (d, 2JC,P = 6.2 Hz), 50.7 (d, 3JC,P = 6.6 Hz), 49.7 (d, 3JC,P = 4.4 Hz). HRMS (ESI Negative) Calcd for C24H21NO6P (M-H+): 450.1106. Found: 450.1169.
Bis(but-3-enyl) [(3aR*,4S*,9S*,9aR*)-1,3-dioxo-2-phenyl-1,2,3,3a,9,9a-hexahydro-4H-4,9-epoxybenzo[f] isoindol-4-yl]phosphonate (endo) (5b)
The compound was obtained as a colorless oil; 1H NMR (400 MHz, CDCl3): δ 7.55–7.58 (m, 1H), 7.33–7.39 (m, 3H), 7.24–7.26 (m, 3H), 6.38–6.41 (m, 2H), 5.81–5.91 (m, 2H), 5.66–5.76 (m, 1H), 5.01–5.20 (m, 4H), 4.47 (dt, 2H, J = 6.8, 8.0 Hz, 2H), 4.18–4.29 (m, 3H), 4.05 (dd, 1H, J = 5.8, 5.8 Hz), 2.56–2.61 (m, 2H), 2.41 (dt, 2H, J = 6.7, 6.7 Hz); 13C NMR (67.80 MHz, CDCl3): δ 172.6, 171.5, 140.8, 140.7, 139.8, 139.8, 133.5, 133.2, 130.8, 129.1, 129.0, 128.8, 128.7, 128.5, 126.3, 122.1, 121.2, 117.9, 117.7, 86.2 (d, JC,P = 191.1 Hz), 81.6 (d, 3JC,P = 15.3 Hz), 67.0 (d, 2JC,P = 6.5 Hz ), 66.7 (d, 2JC,P = 6.5 Hz), 50.7 (d, 3JC,P = 6.6 Hz), 49.7 (d, 3JC,P = 4.3 Hz), 35.1 (d, 3JC-P = 5.5 Hz), 34.8 (d, 3JC,P = 5.8 Hz). HRMS (ESI Negative) Calcd for C26H25NO6P (M-H+): 478.1419. Found: 478.1476.
Acknowledgements
We would like to thank Editage (www. editage.jp) for English language editing
References
[1] Zhang, H.; Tsukuhara, R.; Tigyi, G.; Prestwich, G. D. Synthesis of cyclic phosphonate analogues of (lyso)phosphatidic acid using a ring-closing metathesis reaction. J. Org. Chem 2006, 71 6061-6066.10.1021/jo0607919Suche in Google Scholar PubMed
[2] Stoianova, D. S.; Whitehead, A.; Hanson, P. R. P-Heterocyclic building blocks: Application to the stereoselective synthesis of P-sugars. J. Org. Chem 2005, 70 5880-5889.10.1021/jo0505122Suche in Google Scholar PubMed
[3] Unoh, Y.; Hashimoto, Y.; Takeda, D.; Hirano, K.; Satoh, T.; Miura, M. Rhodium(III)-catalyzed oxidative coupling through C–H bond cleavage directed by phosphinoxy groups. Org. Lett 2013, 15 3258-3261.10.1021/ol4012794Suche in Google Scholar PubMed
[4] López-Cortina, S.; Basiulis, D. I.; Marsi, K. L.; Muñoz-Hernández, M. A.; Ordoñez, M.; Fernández-Zertuche, M. Synthesis of new 1,3-oxaphosphorinanium salts. Stereo-chemistry of hydroxide-induced displacement of methoxide ion. J. Org. Chem 2005, 70 7473-7478.10.1021/jo050901wSuche in Google Scholar PubMed
[5] Li, Z.; Han, J.; Jiang, Y.; Browne, P.; Knox, R. J.; Hu, L. Nitrobenzocyclophosphamides as potential prodrugs for bioreductive activation: synthesis, stability, enzymatic reduction, and antiproliferative activity in cell culture. Bioorg. Med. Chem 2003, 11 4171-4178.10.1016/S0968-0896(03)00459-0Suche in Google Scholar
[6] Pirat, J.-L.; Monbrun, J.; Virieux, D.; Volle, J.-N.; Tillard, M.; Cristau, H.-J. Diastereoselective addition of 2H-2-oxo-1,4,2-oxazaphosphinanes to aldehydes and imines. J. Org. Chem 2005, 70 7035-7041.10.1021/jo050201rSuche in Google Scholar PubMed
[7] Haddadin, M. J. Isobenzofuran. Heterocycles 1978, 9 865-901.10.3987/R-1978-07-0865Suche in Google Scholar
[8] Haddadin, M. J.; Agha, B. J.; Tabri, R. F. Syntheses of some furans and naphtho[2,3-c] derivatives of furan, pyrrole, and thiophene. J. Org. Chem 1979, 44 494-497.10.1021/jo01318a004Suche in Google Scholar
[9] Ohmura, H.; Mikami, K. Novel isobenzofuran generation from silylated lactol leading to desilylated or silylated adducts depending on the choice of metal fluorides. Synlett 2002, 1868-1870.10.1002/chin.200308103Suche in Google Scholar
[10] Ohba, M.; Izuta, R. Effect of copper(II) triflate on intramolecular Diels-Alder reaction of oxazole-olefins. Heterocycles 2001, 55 823-826.10.3987/COM-01-9159Suche in Google Scholar
[11] Padwa, A.; Eidell, C. K.; Lynch, S. M. A new construct of the cis-3a-aryloctahydroindole skeleton via the [4+2] cycloaddition of furanyl carbamates. Heterocycles 2002, 58 227-242.10.3987/COM-02-S(M)12Suche in Google Scholar
[12] Zhang, L.; Koreeda, M. Radical deoxygenation of hydroxyl groups via phosphites. J. Am. Chem. Soc 2004, 126 13190-13191.10.1021/ja0462777Suche in Google Scholar PubMed
[13] Peng, A.-Y.; Ding, Y.-X. Synthesis of 2H-1,2-oxaphosphorin 2-oxides via Ag2CO3-catalyzed cyclization of Z-2-alken-4-ynylphosphonic monoesters. Org. Lett 2005 7 3299-3301.10.1021/ol051126+Suche in Google Scholar PubMed
[14] Mo, J.; Kang, D.; Eom, D.; Kim, S. H.; Lee, P. H. Gold-catalyzed sequential alkyne activation for the synthesis of 4,6-disubstituted phosphorus 2-pyrones. Org. Lett 2013, 15 26-29.10.1021/ol3029274Suche in Google Scholar PubMed
[15] Li, B.; Zhou, B.; Lu, H.; Ma, L.; Peng, A.-Y. Phosphaisocoumarins as a new class of potent inhibitors for pancreatic cholesterol esterase. Eur. J. Med. Chem 2010, 45 1955-1963.10.1016/j.ejmech.2010.01.038Suche in Google Scholar PubMed
[16] Li, X.; Zhang, D.; Pang, H.; Shen, F.; Fu, H.; Jiang, Y.; Zhao, Y. Synthesis of a diverse series of phosphacoumarins with biological activity. Org. Lett 2005, 7 4919-4922.10.1021/ol051871mSuche in Google Scholar PubMed
[17] Peng, A.-Y.; Ding, Y.-X. Synthesis of phosphaisocoumarins via iodocyclization. Org. Lett 2004, 6 1119-1121.10.1021/ol0499506Suche in Google Scholar PubMed
[18] Seo, J.; Park, Y.; Jeon, I.; Ryu, T.; Park, S.; Lee, P. H. Synthesis of phosphaisocoumarins through rhodium-catalyzed cyclization using alkynes and arylphosphonic acid monoesters. Org. Lett 2013, 15 3358-3361.10.1021/ol401407vSuche in Google Scholar PubMed
[19] Park, Y.; Jeon, I.; Shin, S.; Min, J.; Lee, P. H. Rutheniumcatalyzed C–H activation/cyclization for the synthesis of phosphaisocoumarins. J. Org. Chem 2013, 78 10209-10220.10.1021/jo401608vSuche in Google Scholar PubMed
[20] Jeon, W. H.; Son, J.-Y.; Kim, S.-E.; Lee, P. H. Phosphaannulation of aryl- and benzylphosphonic acids with unactivated alkenes via palladium-catalyzed C-H activation/oxidative cyclization reaction. Adv. Synth. Catal 2015, 357 811-817.10.1002/chin.201530232Suche in Google Scholar
[21] Zhang, Y.; Hubbard, J. W.; Akhmedov, N. G.; Petersen, J. L.; Söderberg, B. C. G. Total synthesis of the tetracyclic indole alkaloid Ht-13-B. J. Org. Chem 2015, 80 4783–4790.10.1021/acs.joc.5b00433Suche in Google Scholar PubMed
[22] Zhang, Y.; McArdle, I. W.; Hubbard, J. W.; Akhmedov, N. G.; Söderberg, B. C. G. Total synthesis of the tetracyclic indole alkaloid Ht-13-A. Tetrahedron Lett 2016, 57 2865-2867.10.1016/j.tetlet.2016.05.060Suche in Google Scholar
[23] Yamana, K.; Nakano, H. The Lewis acid-promoted reactions of o-phthaladehyde with trialkyl phosphites: Formation of 1-dialkoxyphosphorylisobenzofuran. Tetrahedron Lett 1996, 37 5963-5966.10.1016/0040-4039(96)01288-9Suche in Google Scholar
[24] Yamana, K.; Ibata, T.; Nakano, H. The formation of dialkyl isobenzofuran-1-ylphosphonates by Lewis acid promoted reaction of o-phthalaldehyde with trialkyl phosphites. Synthesis 2006, 4124-4130.10.1002/chin.200717167Suche in Google Scholar
© 2019 Kenji Yamana and Hirofumi Nakano, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Artikel in diesem Heft
- Research Article
- Magnesium porphyrazine with peripheral methyl (3,5-dibromophenylmethyl)amino groups – synthesis and optical properties
- Synthesis and fungicidal activity of novel imidazo[4, 5-b]pyridine derivatives
- Synthesis of indazolo[5,4-b][1,6]naphthyridine and indazolo[6,7-b][1,6]naphthyridine derivatives
- Zinc Chloride Catalyzed Amino Claisen Rearrangement of 1-N-Allylindolines: An Expedient Protocol for the Synthesis of Functionalized 7-Allylindolines
- Synthesis and Biological Evaluation of (E)-N’-Benzylidene-7-methyl-2-propyl-1H-benzo[d] imidazole-5-carbohydrazides as Antioxidant, Anti-inflammatory and Analgesic agents
- Efficient synthesis, reactions and spectral characterization of pyrazolo[4’,3’:4,5]thieno[3,2-d] pyrimidines and related heterocycles
- Asymmetric Mannich Reaction: Synthesis of Novel Chiral 5-(substituted aryl)-1,3,4-Thiadiazole Derivatives with Anti-Plant-Virus Potency
- Synthesis and antitubercular activity of new N-[5-(4-chlorophenyl)-1,3,4-oxadiazol-2-yl]-(nitroheteroaryl)carboxamides
- Remarkable electronic effect on the total stereoselectivity of the cycloaddition reaction of arylnitrile oxides with pyrrol-2-one derivatives
- Preliminary Communications
- Crystal structure and molecular docking studies of new pyrazole-4-carboxamides
- Research Article
- Synthesis of polycyclic phosphonates via an intramolecular Diels-Alder reaction of 2-benzoylbenzalaldehyde and alkenyl phosphites
- Asymmetric total synthesis of filamentous fungi related resorcylic acid lactones 7-epi-zeaenol and zeaenol
- The first in situ synthesis of 1,3-dioxan-5-one derivatives and their direct use in Claisen-Schmidt reactions
- Synthesis and fungicidal activities of perfluoropropan-2-yl-based novel quinoline derivatives
- Combined XRD-paramagnetic 13C NMR spectroscopy of 1,2,3-triazoles for revealing copper traces in a Huisgen click-chemistry cycloaddition. A model case
- Cytotoxic and antimicrobial activities of some novel heterocycles employing 6-(1,3-diphenyl-1H-pyrazol-4-yl)-4-oxo-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile
- Substrate-controlled Diastereoselective Michael Addition of Alkylidene Malonates by Grignard Reagents
- Synthesis of 1,2,3 triazole-linked benzimidazole through a copper-catalyzed click reaction
- Synthesis and spectral characteristics of N-(1-([1,2,4]triazolo[3,4-b][1,3,4]thiadiazol-6-ylamino)-2,2,2-trichloroethyl)carboxamides
- Facile One-pot Protocol of Derivatization Nitropyridines: Access to 3-Acetamidopyridin-2-yl 4-methylbenzenesulfonate Derivatives
- Naphthalene substituted benzo[c]coumarins: Synthesis, characterization and evaluation of antibacterial activity and cytotoxicity
- A Green Synthesis and Antibacterial Activity of N-Arylsulfonylhydrazone Compounds
- Preliminary Communications
- Facile Synthesis of Spiro[cyclohexane-1,3’-indoline]-2,2’-diones
- Research Article
- Synthesis and AChE inhibitory activity of N-glycosyl benzofuran derivatives
- [DMImd-DMP]: A highly efficient and reusable catalyst for the synthesis of 4H-benzo[b]pyran derivatives
Artikel in diesem Heft
- Research Article
- Magnesium porphyrazine with peripheral methyl (3,5-dibromophenylmethyl)amino groups – synthesis and optical properties
- Synthesis and fungicidal activity of novel imidazo[4, 5-b]pyridine derivatives
- Synthesis of indazolo[5,4-b][1,6]naphthyridine and indazolo[6,7-b][1,6]naphthyridine derivatives
- Zinc Chloride Catalyzed Amino Claisen Rearrangement of 1-N-Allylindolines: An Expedient Protocol for the Synthesis of Functionalized 7-Allylindolines
- Synthesis and Biological Evaluation of (E)-N’-Benzylidene-7-methyl-2-propyl-1H-benzo[d] imidazole-5-carbohydrazides as Antioxidant, Anti-inflammatory and Analgesic agents
- Efficient synthesis, reactions and spectral characterization of pyrazolo[4’,3’:4,5]thieno[3,2-d] pyrimidines and related heterocycles
- Asymmetric Mannich Reaction: Synthesis of Novel Chiral 5-(substituted aryl)-1,3,4-Thiadiazole Derivatives with Anti-Plant-Virus Potency
- Synthesis and antitubercular activity of new N-[5-(4-chlorophenyl)-1,3,4-oxadiazol-2-yl]-(nitroheteroaryl)carboxamides
- Remarkable electronic effect on the total stereoselectivity of the cycloaddition reaction of arylnitrile oxides with pyrrol-2-one derivatives
- Preliminary Communications
- Crystal structure and molecular docking studies of new pyrazole-4-carboxamides
- Research Article
- Synthesis of polycyclic phosphonates via an intramolecular Diels-Alder reaction of 2-benzoylbenzalaldehyde and alkenyl phosphites
- Asymmetric total synthesis of filamentous fungi related resorcylic acid lactones 7-epi-zeaenol and zeaenol
- The first in situ synthesis of 1,3-dioxan-5-one derivatives and their direct use in Claisen-Schmidt reactions
- Synthesis and fungicidal activities of perfluoropropan-2-yl-based novel quinoline derivatives
- Combined XRD-paramagnetic 13C NMR spectroscopy of 1,2,3-triazoles for revealing copper traces in a Huisgen click-chemistry cycloaddition. A model case
- Cytotoxic and antimicrobial activities of some novel heterocycles employing 6-(1,3-diphenyl-1H-pyrazol-4-yl)-4-oxo-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile
- Substrate-controlled Diastereoselective Michael Addition of Alkylidene Malonates by Grignard Reagents
- Synthesis of 1,2,3 triazole-linked benzimidazole through a copper-catalyzed click reaction
- Synthesis and spectral characteristics of N-(1-([1,2,4]triazolo[3,4-b][1,3,4]thiadiazol-6-ylamino)-2,2,2-trichloroethyl)carboxamides
- Facile One-pot Protocol of Derivatization Nitropyridines: Access to 3-Acetamidopyridin-2-yl 4-methylbenzenesulfonate Derivatives
- Naphthalene substituted benzo[c]coumarins: Synthesis, characterization and evaluation of antibacterial activity and cytotoxicity
- A Green Synthesis and Antibacterial Activity of N-Arylsulfonylhydrazone Compounds
- Preliminary Communications
- Facile Synthesis of Spiro[cyclohexane-1,3’-indoline]-2,2’-diones
- Research Article
- Synthesis and AChE inhibitory activity of N-glycosyl benzofuran derivatives
- [DMImd-DMP]: A highly efficient and reusable catalyst for the synthesis of 4H-benzo[b]pyran derivatives


