Crystal structure and molecular docking studies of new pyrazole-4-carboxamides
-
Li Qiao
Abstract
Two pyrazol-4-carboxamides, 3-(difluoromethyl)-N-(mesitylcarbamoyl)-1-methyl-1H-pyrazole-4-carboxa-mide (7a) and 3-(difluoromethyl)-N-((3,5-dimethylphenyl) carbamoyl)-1-methyl-1H-pyrazole-4-carboxamide (7b) were synthesized and their structures were confirmed by the aid of 1H NMR and HRMS analyses. The structure of the pyrazole-4-carboxamide, 7a was also determined by X-ray diffraction. The preliminary activity results demonstrate that these two compounds exhibit good inhibitory activity against Botrytis cinerea. Further docking results indicated that the key active group is difluoromethyl pyrazole moiety.
Introduction
Heterocyclic compounds received important attention due to their wide range of biological activities [1, 2, 3, 4, 5]. Many pyrazole carboxamide compounds had been developed as commercial fungicides targeting succinate dehydrogenase inhibitors (SDHIs), including penthiopyrad, sedaxane, pydiflumetofen, bixafen, fluxapyroxad, isopyrazam and benzovindiflupyr. In addition, pyrazole derivatives displayed diversity activities, such as antimicrobial [6], DPPH radical scavenging [7], anticancer activity [8], antifungal [9, 10], anti-inflammatory [11], nematicidal [12, 13, 14, 15, 16, 17, 18, 19] and cholinesterases inhibitory activity [20]. On the other hand, urea group is always important key building block in many drugs or pesticides. The compounds with urea group exhibited diversity bioactivities, such as mosquito [21], antimicrobial [22], antiviral [23], antifungal [24, 25, 26] and antinociceptive activity [27].
In view of these facts mentioned above, and also as a part of our work on the synthesis of bioactive lead compounds for drug discovery [28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46], two new pyrazole-4-carboxamides, 3-(difluoromethyl)-N-(mesitylcarbamoyl)-1-methyl-1H-pyrazole-4-carboxamide (7a) and 3-(difluoromethyl)-N-((3,5-dimethylphenyl) carbamoyl)-1-methyl-1H-pyrazole-4-carboxamide (7b) were designed and synthesized. The structures were characterized by the aid of 1H NMR and HRMS analysis. The single crystal structure of compound 7a was determined by X-ray diffraction. The fungicidal activity of these compounds was tested and the docking studies were also carried out to study the mode of action.
Results and discussion
Synthesis
The synthetic route of 3-(difluoromethyl)-N-(mesitylcarbamoyl)-1-methyl-1H-pyrazole-4-carboxamide is outlined in Scheme 1. In this paper, ethyl-2-(ethoxymethylene)-4,4-difluoro-3-oxobutanoate and triethyl orthoformate took place smoothly in the presence of acetic anhydride resulted in the formation of intermediate 1[47]. Then the pyrazole ring was prepared by reacting intermediate 1 with methylhydrazine[48]. The pyrazole ester hydrosis under NaOH condition, then acid by HCl[49]. The pyrazole acyl chloride was given using the SOCl2 as chlorinate reagent[50]. Then the pyrazole acyl chloride reacted with NH3•H2O to give pyrazole amide[50]. The key intermediate pyrazole isocyanate was prepared by pyrazole amide and triphosgene[50]. Finally, the key intermediate pyrazole isocyanate reacted with 2,4,6-trimethylaniline and 3,5-dimethylaniline at room temperature. The reaction condition is mild.
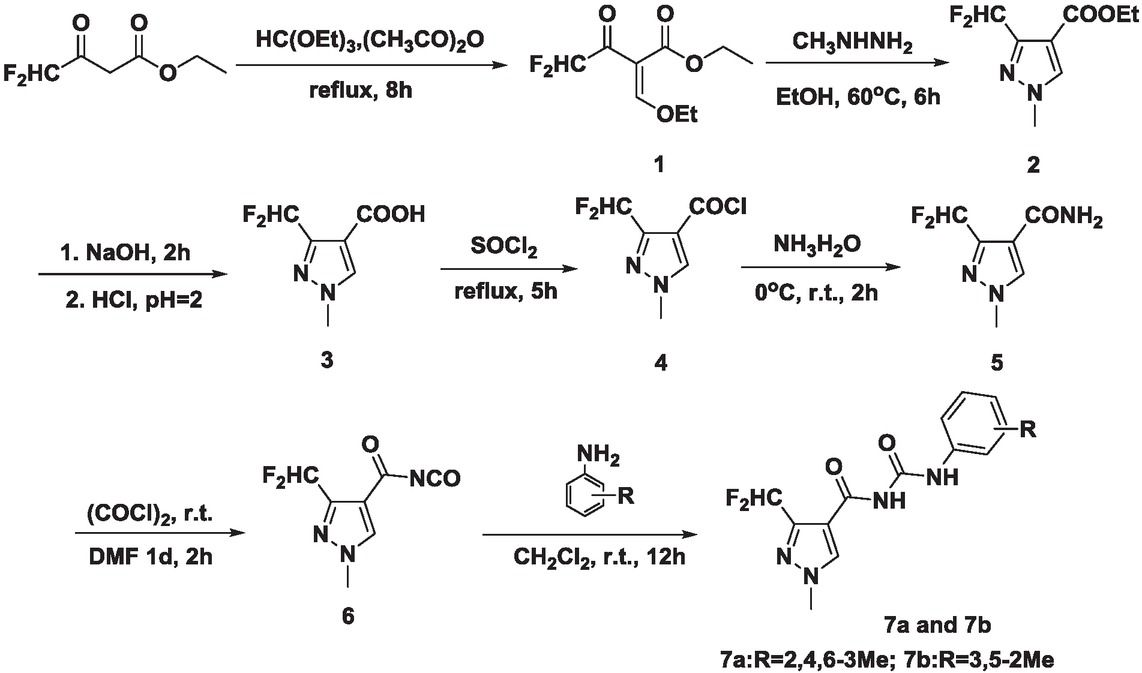
The synthetic route of title compounds
The structures of the pure compounds 7a and 7b were confirmed by 1H NMR, and HR MS. From the 1H NMR data of compound 7a, the signals at 2.28 and 2.31 ppm were recognized as three methyl group of benzene ring. The appearance of signal at 3.92 ppm belongs to the methyl protons of pyrazole ring. The two NH protons of urea bridge are found at 10.04 and 10.86 ppm as single peak. The CHF2 proton signals were triple peak with the coupling constant 54 Hz due to the influence of F atom. The high resolution mass spectroscopies of the two compounds are in agreement with their molecular formula C16H18F2N4O2 (7a) and C15H16F2N4O2 (7b).
Crystal structure
The crystal belongs to triclinic system with space group P-1. The molecular structure of compound 7a is shown in Fig. 1, and the packing diagram in Fig. 2. The selected bond lengths and torsion angles are listed in Table 1.

View of the title compound 7a, with displacement ellipsoids drawn at the 30% probability level.
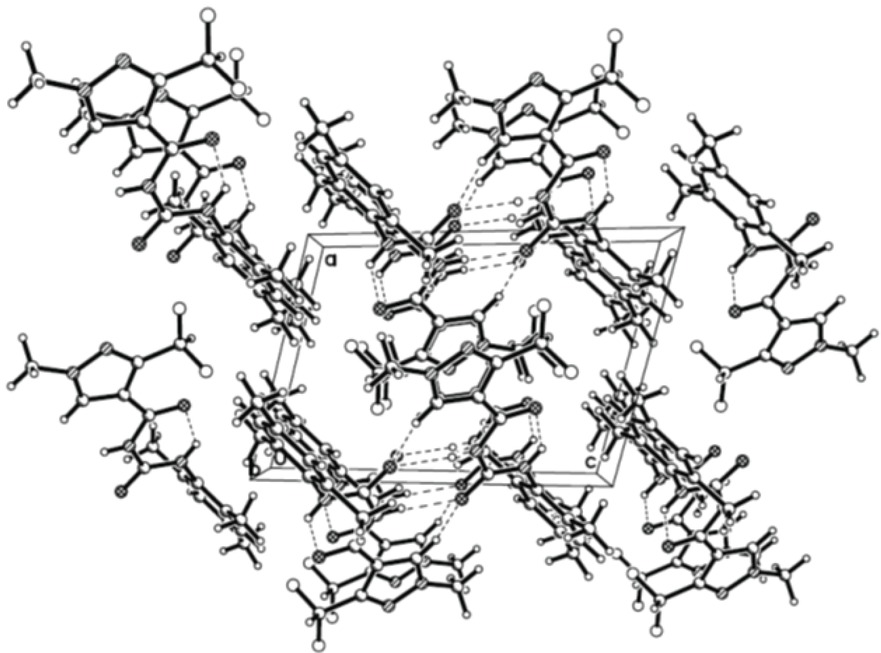
A view of pack molecule 7a.
Selected Bond lengths (Å), Selected Bond angles (°) for Compound 7a.
| Bond | Dist. | Angle | (°) |
|---|---|---|---|
| F(1)-C(1) | 1.376(3) | C(2)-N(1)-N(2) | 104.4(2) |
| F(2)-C(1) | 1.363(3) | C(4)-N(2)-N(1) | 112.6(2) |
| N(1)-N(2) | 1.360(3) | C(4)-N(2)-C(5) | 127.2(2) |
| O(1)-C(6) | 1.232(3) | N(1)-N(2)-C(5) | 120.2(2) |
| O(2)-C(7) | 1.242(3) | C(6)-N(3)-C(7) | 127.8(2) |
| N(1)-C(2) | 1.336(3) | C(7)-N(4)-C(8) | 121.7(2) |
| N(3)-C(7) | 1.391(3) | F(2)-C(1)-F(1) | 105.7(2) |
| N(2)-C(4) | 1.346(3) | N(1)-C(2)-C(1) | 120.0(2) |
| N(4)-C(7) | 1.345(3) | C(3)-C(2)-C(1) | 128.3(2) |
| C(1)-C(2) | 1.494(4) | N(3)-C(6)-C(3) | 115.2(2) |
| C(2)-C(3) | 1.424(4) | N(4)-C(7)-N(3) | 119.1(2) |
Fig.1 View of the title compound 7a, with displacement ellipsoids drawn at the 30% probability level.
Generally, the average bond lengths and bond angles of pyrazole ring and phenyl ring were normal ranges. The N2-C4 [1.346(3) Å], N1-C2 [1.336(3) Å] bond were longer than the general C=N double bond length of 1.27 Å, which indicated significant electron delocalization in the fused ring system. The torsion angle of C7-N3-C6-C3 and C8-N4-C7-N3 was -175.7(2)° and -179.4(2)° respectively, which indicated the two carbonyl groups are opposite. The angle between the pyrazole ring and benzene ring is 68.2 ⍛.
In the intermolecular edge-to-face π-π stacking pattern of the title compound, it is worth mention that the two molecules of each stacking unit are cetrosymmetric, which can be proved by the relative position of the phenyl rings (C8, C9, C10, C11, C12, C13) and methyl of pyrazole: the centroid separation of them is 3.131 Å. These interactions are estimated to play a role in stabilizing the crystal structure. The title compound 7a has an extensive network of hydrogen bonding. The parameters of intramolecular and intermolecular bonds are given in Table 2. From Fig. 1, the N(4)-H(4)…O(1) hydrogen bond formed a six member ring in the molecule. In the ac plane, they are linked together by N(3)-H(3)...O(2) hydrogen bonds. This hydrogen-bonding sequence is repeated to form a ring. The ring is shaped like a decagon and has two N(4) and two O(1) atoms at the vertices, leading to a hydrogen-bond network defining cyclic motifs denoted R22 (8). The hydrogen bonds and weak π-π interactions strengthen the integration of the 3D networks.
Hydrogen-bond Parameters (Ǻ) of Compound 7a
| D—H…A | d(D—H) | d(H…A) | d(D…A) | ∠(DHA) |
|---|---|---|---|---|
| N(3)-H(3)...O(2)# | 0.88 | 2.03 | 2.693(5) | 130.8 |
| N(4)-H(4)...O(1) | 0.88 | 1.98 | 2.830(6) | 161.6 |
Symmetry transformations used to generate equivalent atoms: #-1-X,-Y,-Z
Evaluation of fungicidal activity
Fungicidal activity of compounds 7a and 7b against Fusarium oxysporum, Corynespora mazei, Pseudomonas syringae and Botrytis cinerea was evaluated at 50 μg/mL according to our previous work [9, 42], Fluxapyroxad was used as controls and the results are listed in Table 3. The primary bioassay showed the two compounds exhibits good inhibiting activity (60.00% and 77.27%) towards Botrytis cinere, which is the same as control (67.27%). For the other three fungals Fusarium oxysporum, Corynespora mazei, Pseudomonas syringae, they exhibited weak activity with inhibitory -49.20% and -55.59%, 19.13% and 0.43%, -14.77% and 6.09% at 50 μg/mL, respectively.
The fungicidal activity of compounds 7a and 7b against four fungus at 50 mg/L
| No | R | Corynespora mazei | Pseudomonas syringae | Fusarium oxysporum | Botrytis cinerea |
|---|---|---|---|---|---|
| 7a | 2,4,6-3Me | -49.20 | 19.13 | -14.77 | 60.00 |
| 7b | 3,5-2Me | -55.59 | 0.43 | 6.09 | 77.27 |
| Control | Fluxapyroxad | 74.09 | 52.55 | 71.301 | 67.27 |
| CK | water | 0 | 0 | 0 | 0 |
Docking study
In order to study the action mode of high active compound and the target, the binding modes between SDH (PDB:2FBW) and the active compound 7a were selected as exemplified in the case of representative compound by using the Discovery studio. The compound 7a can tightly occupy binding site of SDH, the docking results are shown in Figure 3. From the docking results, the compound 7a held two weak interactions: π-cation and π-sigma. The π-cation bond was formed between the pyrazole ring and Arg 43 amino acid residue with the distance of 4.1821 Å. The other π-sigma bond was formed between the pyrazole ring and Ile 218 amino acid residue with the distance of 2.82714 Å. The results indicated that the key active group is difluoromethyl pyrazole moiety.

The docking mode of compound 7a and the SDH
Experimental
Instruments
Melting points were determined by an X-4 apparatus and uncorrected. 1H NMR spectra were measured on a Bruker AV-400 or 500 MHz instrument using TMS as an internal standard and CDCl3 or DMSO-d6 as the solvent. High resolution mass spectra were recorded on an Agilent LC-Q-TOF-MS 6520 instrument. Crystallographic data of the compound were collected on a Bruker APEX-II CCD diffractometer. All the reagents are of analytical grade or freshly prepared before use.
General procedure
The intermediates 1, 2, 3, 4, 5 and 6 were synthesized according to our previous work [42]. A 10 mL round bottom flask was charged with 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carbonyl isocyanate 6 (201 mg, 1 mmol) and 2,4,6-trimethylaniline (135 mg, 1 mmol) in CH2Cl2 (4 mL). The mixture was stirred at room temperature for overnight and the correspondence product was given. The target compounds were filtered and crude solids were recrystallized from ethanol to give the compound 7a. White solid, yield 66.7%, 224.2 mg, m.p. 247~248 °C, 1H NMR ( 500 MHz, CDCl3 ) δ: 2.28 (s, 6H, 2CH3), 2.31 (s, 3H, CH3), 3.62 (s, 3H, CH3), 6.85 (t, J = 54 Hz, 1H, CHF2), 6.97 (m, 2H, Ar), 8.35 (s, 1H, CH), 10.04 (s, 1H, NH), 10.86 (s, 1H, NH); HRMS (ESI) for C16H18F2N4O2m/z: Calculated, 337.1471, Found,
337.1470 [M+H]+. The compound 7b was synthesized according to this method. 3-(difluoromethyl)-N-((3,5-dimethylphenyl)carbamoyl)-1-methyl-1H-pyrazole-4-carboxamide 7b :White solid, yield 53.5%, 172.3 mg, m.p. 207~208 °C, 1H NMR ( 500 MHz, DMSO-d6) δ: 2.35 (s, 6H, 2CH3), 3.85 (s, 3H, CH3), 6.85 (s, 1H, Ar), 7.14 (t, J = 54 Hz, 1H, CHF2), 7.19 (s, 2H, Ar), 8.39 (s, 1H, CH), 9.89 (s, 1H, NH), 10.69 (s, 1H, NH); HRMS (ESI) for C15H16F2N4O2m/z: Calculated, 323.1314, Found, 323.1314 [M+H]+.
Structure determination
The cube-shaped single crystal of compound 7a was obtained by recrystallization from EtOH. The crystal with dimensions of 0.20mm × 0.18mm ×0.08mm was mounted on a Rigaku Saturn diffractometer with a graphite-mono-chromated MoKα radiation (λ = 0.71073Å) by using a Phi scan mode at 110(2) K in the range of 3.646≤θ≤55.758°. A total of 9797 reflections were collected, of which 3892 were independent (Rint = 0.0813) and 2075 were observed with I > 2σ(I). The calculations were performed with SHELXS-97 program [51] and the empirical absorption corrections were applied to all intensity data. The non-hydrogen atoms were refined anisotropically. The hydrogen atoms were determined with theoretical calculations and refined isotropically. Crystal data for title compound: triclinic system, P-1 space group with a = 8.426(9) Å, b = 9.149(9) Å, c = 12.406(16) Å, α = 68.374(11)°, β = 71.393(15)°, γ = 74.816(11)°, V = 831.3(16) Å3, Z = 2, T = 110(2) K, μ(MoKα) = 0.106 mm-1, Dcalc = 1.344 g/cm3, μ(MoKa) = 0.106 mm-1, GOOF = 0.973. The final full-matrix least-squares refinement gave R = 0.0654 and wR = 0.1429.
Docking studies
Molecular docking studies were done using Discovery Studio 2.5 software. The binding sites were generated from the SDH structure (PDB code: 2FBW) [12, 13, 14, 15, 16, 17, 18, 19]. At first, water molecules were removed from the complex. Then, the crystallographic disorders and unfilled valence atoms were corrected using protein report and utility and clean protein options. Protein energy was minimized by applying CHARMM and MMFF94 force fields. The rigid structure of protein was obtained by applying fixed atom constraint. The protein binding site was defined and prepared for docking process. The structure of compound 7a was drawn using Discovery Studio 2.5 and minimized by applying CHARMM force field. Then, the minimized structures were prepared for docking using prepare ligand protocol. Docking process was carried out using CDOCKER protocol. CDOCKER is a grid-based molecular docking method that employs CHARMM-based molecular dynamics (MD) scheme to dock ligands into a receptor binding site. The receptor was held rigid while the ligands were allowed to be flexible during the refinement. Each molecule was allowed to produce ten different interaction poses with the protein. Then, docking scores (-CDOCKER interaction energy) of the best-fitted poses with the active site at the SDH structure were recorded.
Conclusion
In summary, two new pyrazole-4-carboxamides, had been synthesized by multi-step reaction and characterized by 1H NMR, HRMS and single-crystal X-ray structure determination. The results show that the crystal structure exhibits intermolecular and intramolecular hydrogen bonds. The fungicidal activity results showed that it possessed moderate activity against Botrytis cinerea. The docking results indicated the key active group is difluoromethyl pyrazole moiety.
Acknowledgement
This work was funded by Zhejiang Provincial Natural Science Foundation of China (No. LY19C140002), Research Fund of Department of Education of Zhejiang Province(Y201840646) and the Opening Foundation of the Key Laboratory of Green Pesticide and Agricultural Bioengineering, Ministry of Education, Guizhou University, grant No. 2018GDGP0104.
References
[1] Liu, X.H.; Tan, C.X.; Weng, J.Q. Synthesis, dimeric crystal, and fungicidal activity of 1- (4-methylphenyl)-2-(5-((3,5-dimethyl-1H-pyrazol-1-yl)methyl)-4-phenyl-4H-1,2,4-triazol-3-ylthio) ethanone, Phosphorus Sulfur Silicon Relat. Elem. 2011, 186, 558-564.10.1080/10426507.2010.508060Search in Google Scholar
[2] Liu, X.H.; Pan, L.; Ma, Y.; Weng, J.Q.; Tan, C.X.; Li, Y.H.; Shi, Y.X.; Li, B.J.; Li, Z.M.; Zhang, Y.G. Design, synthesis, biological activities, and 3D-QSAR of new N,N’-diacylhydrazines containing 2-(2,4-dichlorophenoxy)propane moiety. Chem. Biol. Drug Des. 2011, 78, 689-694.10.1111/j.1747-0285.2011.01205.xSearch in Google Scholar PubMed
[3] Yan, S.L.; Yang, M.Y.; Sun, Z.H.; Min, L.J.; Tan, C.X.; Weng, J.Q.; Wu, H.K.; Liu, X.H. Synthesis and antifungal activity of 1,2,3-thiadiazole derivatives containing 1,3,4-thiadiazole moiety. Lett. Drug Des. Discov. 2014, 11, 940-943.10.2174/1570180811666140423222141Search in Google Scholar
[4] Zhai, Z.W.; Shi, Y.X.; Yang, M.Y.; Zhao W.; Sun Z.H.; Weng J.Q.; Tan C.X.; Liu X.H.; Li B.J.; Zhang Y.G. Microwave assisted synthesis and antifungal activity of some novel thioethers containing 1,2,4-triazolo[4,3-apyridine moiety. Lett. Drug Des. Discov. 2016, 13, 521-525.10.2174/157018081306160618181757Search in Google Scholar
[5] Liu, X.H.; Tan, C.X.; Weng, J.Q. Phase transfer-catalyzed, one-pot synthesis of some novel N-pyrimidinyl-N’-nicotinyl thiourea derivatives, Phosphorus Sulfur Silicon Relat. Elem. 2011, 186, 552-557.10.1080/10426507.2010.508059Search in Google Scholar
[6] Padalkar, V.S.; Borse, B.N.; Gupta, V.D.; Phatangare, K.R.; Patil, V.S.; Sekar, N. Synthesis and antimicrobial activities of novel 2-[substituted-1H-pyrazol-4-yl] benzothiazoles, benzoxazoles, and benzimidazoles. J. Heterocycl. Chem. 2016, 53, 1347-1355.10.1002/jhet.1506Search in Google Scholar
[7] Radi, S.; Attayibat, A.; El-Massaoudi, M.; Salhi, A.; Eddike, D.; Tillard, M.; Mabkhot, Y.N. X-ray single crystal structure, DFT calculations and biological activity of 2-(3-methyl-5-(pyridin-2 ‘-yl)-1H-pyrazol-1-yl) ethanol. Molecules 2017, 21, 1020.10.3390/molecules21081020Search in Google Scholar PubMed PubMed Central
[8] Gomha, S.M.; Abdel-aziz, H.M.; El-Reedy, A.A.M. Facile Synthesis of pyrazolo[3,4-cpyrazoles bearing coumarine ring as anticancer agents. J. Heterocycl. Chem. 2018, 55, 1960-1965.10.1002/jhet.3235Search in Google Scholar
[9] Wang, H.; Zhai, Z.W.; Shi, Y.X.; Tan, C.X.; Weng, J.Q.; Han, L.; Li, B.J.; Liu, X.H. Novel trifluoromethylpyrazole acyl thiourea derivatives: synthesis, antifungal activity and docking study. Lett Drug Des Discov 2018, published ahead of print; doi: 10.2174/1570180815666180704103047.10.2174/1570180815666180704103047Search in Google Scholar
[10] Mu, J.X.; Shi, Y.X.; Yang, M.Y.; Sun, Z.H.; Liu, X.H.; Li, B.J.; Sun, N.B. Design, synthesis, DFT study and antifungal activity of pyrazolecarboxamide derivatives. Molecules 2016, 21, 68.10.3390/molecules21010068Search in Google Scholar PubMed PubMed Central
[11] Kumbar, M.N.; Kamble, R.R.; Dasappa, J.P.; Bayannavar, P.K.; Khamees, H.A.; Mahendra, M.; Joshi, S.D.; Dodamani, S.; Rasal, V.P.; Jalalpure, S. 5-(1-Aryl-3-(thiophen-2-yl)-1H-pyrazo1-4-yl)-1H-tetrazoles: synthesis, structural characterization, hirshfeld analysis, anti-inflammatory and anti-bacterial studies. J. Mol. Struct. 2018, 1160, 63-72.10.1016/j.molstruc.2018.01.047Search in Google Scholar
[12] Zhao, W.; Shen, Z.H.; Xu, T.M.; Peng, W.L.; Liu, X.H. Synthesis and nematocidal activity of novel pyrazole carboxamide derivatives against Meloidogyne incognita. Lett. Drug Des. Discov. 2017, 14, 323-329.10.2174/1570180813666160930164327Search in Google Scholar
[13] Zhao, W.; Shen, Z.H.; Xing, J.H.; Xu, T.M.; Peng, W.L.; Liu, X.H. Synthesis, characterization, nematocidal activity and docking Study of novel pyrazole-4-carboxamide derivatives. Chin. J. Struct. Chem. 2017, 36, 423-428.Search in Google Scholar
[14] Liu, X.H.; Zhao, W.; Shen, Z.H.; Xing, J.H.; Xu, T.M.; Peng, W.L. Synthesis, nematocidal activity and SAR study of novel difluoromethylpyrazole carboxamide derivatives containing flexible alkyl chain moieties. Eur. J. Med. Chem. 2017, 125 881-889.10.1016/j.ejmech.2016.10.017Search in Google Scholar PubMed
[15] Cheng, L.; Zhao, W.; Shen, Z.H.; Xu, T.M.; Wu, H.K.; Peng, W.L.; Liu, X.H. Synthesis, nematocidal activity and docking study of novel pyrazole-4-carboxamide derivatives against meloidogyne incognita. Lett. Drug Des. Discov. 2019, 16, 29-35.10.2174/1570180815666180326150827Search in Google Scholar
[16] Cheng, L.; Shen, Z.H.; Xu, T.M.; Tan, C.X.; Weng, J.Q.; Han, L.; Peng, W.L.; Liu. X.H. Synthesis and nematocidal activity of N-substituted 3-methyl-1H-pyrazole-4-carboxamide derivatives against Meloidogyne incognitaJ. Heterocycl. Chem. 2018, 55 946-950.10.1002/jhet.3123Search in Google Scholar
[17] Zhao, W.; Xing, J.H.; Xu, T.M.; Peng, W.L.; Liu, X.H. Synthesis and in vivo nematocidal evaluation of novel 3-(trifluoromethyl)-1H-pyrazole-4-carboxamide derivatives. Front. Chem. Sci. Eng. 2017, 11, 363-368.10.1007/s11705-016-1595-xSearch in Google Scholar
[18] Zhao, W.; Shen, Z.H.; Xing, J.H.; Yang, G.; Xu, T.M.; Peng, W.L.; Liu, X.H. Synthesis and nematocidal activity of novel 1-(3-chloropyridin-2-yl)-3-(trifluoromethyl)-1H-pyrazole-4-carboxamide derivatives. Chem. Pap. 2017, 71, 921-928.10.1007/s11696-016-0012-8Search in Google Scholar
[19] Liu, X.H.; Zhao, W.; Shen, Z.H.; Xing, J.H.; Yuan, J.; Yang, G.; Xu, T.M.; Peng, W.L. Synthesis, nematocidal activity and docking study of novel chiral 1-(3-chloropyridin-2-yl)-3-(trifluoromethyl)-1H-pyrazole-4-carboxamide derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 3626-3628.10.1016/j.bmcl.2016.06.004Search in Google Scholar PubMed
[20] Derabli, C.; Boualia, I.; Abdelwahab, A.B.; Boulcina, R.; Bensouici, C.; Kirsch, G.; Debache, A. A cascade synthesis, in vitro cholinesterases inhibitory activity and docking studies of novel Tacrine-pyranopyrazole derivatives. Bioorg. Med. Chem. Lett. 2018, 28, 2481-2489.10.1016/j.bmcl.2018.05.063Search in Google Scholar PubMed
[21] Fang, Y.M.; Zhang, R.R.; Shen, Z.H.; Tan, C.X.; Weng, J.Q.; Xu, T.M.; Liu, X.H. Huang, H.Y.; Wu, H.K. Synthesis and antifungal activity of some 6-tert-butyl-8-chloro-2, 3-dimethylquinolin-4-ol derivatives against Pyricularia oryaeLett. Drug Des. Discov. 2018, 15 1314-1318.10.2174/1570180815666180313121735Search in Google Scholar
[22] Venkatesh, T.; Bodke, Y.D.; Joy, M.N.; Dhananjaya, B.L.; Venkataraman, S. Synthesis of some benzofuran derivatives containing pyrimidine moiety as potent antimicrobial agents. Iran J. Pharm. Res. 2018, 17, 75-86.Search in Google Scholar
[23] Huang, Y.Q.; Guo, Z.L.; Song, H.J.; Liu, Y.X.; Wang, L.Z.; Wang, Q.M. Design, Synthesis, and biological activity of beta-carboline analogues containing hydantoin, thiohydantoin, and urea moieties. J. Agr. Food Chem. 2018, 66, 8253-8261.10.1021/acs.jafc.8b03087Search in Google Scholar PubMed
[24] Zhang, Z.J.; Zeng, Y.; Jiang, Z.Y.; Shu, B.S.; Sethuraman, V.; Zhong, G.H. Design, synthesis, fungicidal property and QSAR studies of novel-carbolines containing urea, benzoylthiourea and benzoylurea for the control of rice sheath blight. Pest Manag. Sci. 2018, 74, 1736-1746.10.1002/ps.4873Search in Google Scholar PubMed
[25] Liu, X.H.; Wang, Q.; Sun, Z.H.; Wedge, D.E.; Becnel, J.J.; Estep, A.S.; Tan, C.X.; Weng, J.Q. Synthesis and insecticidal activity of novel pyrimidine derivatives containing urea pharmacophore against Aedes aegypti. Pest Manag. Sci. 2017, 73, 953-959.10.1002/ps.4370Search in Google Scholar PubMed
[26] Jin, T.; Zhai, Z.W.; Han, L.; Weng, J.Q.;Tan, C.X.; Liu, X.H. Synthesis, crystal structure, docking and antifungal activity of a new pyrazole acylurea compound. Chin. J. Struct. Chem. 2018, 37, 1259-1264.Search in Google Scholar
[27] Lopes, R.D.; Alves, M.A.; Pinheiro, R.O.; Freitas, C.S.; Cunha, F.Q.; Barreiro, E.J.; Lima, L.M. Synthesis, aqueous solubility, metabolic stability and pharmacological profile of simplified urea derivatives. Lett. Drug Des. Discov. 2018, 15, 766-777.10.2174/1570180814666171012155204Search in Google Scholar
[28] Zhang, L.J.; Yang, M.Y.; Sun, Z.H.; Tan, C.X.; Weng, J.Q.; Wu, H.K.; Liu X.H. Synthesis and antifungal activity of 1,3,4-thiadiazole derivatives containing pyridine group. Lett. Drug Des. Discov. 2014, 11, 1107-1111.10.2174/1570180811666140610212731Search in Google Scholar
[29] Shen, Z.H.; Sun, Z.H.; Becnel, J.J.; Estep, A.; Wedge, D.E.; Tan, C.X.; Weng, J.Q.; Han, L, Liu. X.H. Synthesis and mosquiticidal activity of novel hydrazone containing pyrimidine derivatives against Aedes aegyptiLett. Drug Des. Discov. 2018, 15, 951-956.10.2174/1570180815666180102141640Search in Google Scholar
[30] Liu, X.H.; Fang, Y.M.; Xie, F.; Zhang, R.R.; Shen, Z.H.; Tan, C.X.; Weng, J.Q.; Xu, T.M.; Huang, H.Y., Synthesis and in vivo fungicidal activity of some new quinoline derivatives against rice blast. Pest Manag. Sci. 2017, 73, 1900-1907.10.1002/ps.4556Search in Google Scholar PubMed
[31] Cheng, L.; Cai, P.P.; Zhang, R.R.; Han, L.; Tan, C.X.; Weng, J.Q. Xu, T.M.; Liu, X.H. Synthesis and biological activity of some new 6-perfluoropropanyl quinoline derivatives. J. Heterocycl. Chem. 2018, 55, 2585-2589.10.1002/jhet.3316Search in Google Scholar
[32] Cheng, L.; Cai, P.P.; Zhang, R.R.; Han, L.; Tan, C. X.; Weng, J. Q.; Xu, T. M.; Liu, X. H. Synthesis and Insecticidal Activity of New Quinoline Derivatives Containing Perfluoropropanyl Moiety. J. Heterocycl. Chem. 2019, 56, 1312-1317.10.1002/jhet.3502Search in Google Scholar
[33] Cheng, L.; Zhang, R.R.; Wu, H.K.; Xu, T.M.; Liu, X.H. The synthesis of 6-tert-butyl)-8-fluoro-2,3-dimethylquinoline carbonate derivatives and their antifungal activity against pyricularia oryzae. Front. Chem. Sci. Eng. 2018, published ahead of print; doi:10.1007/s11705-018-1734-7.10.1007/s11705-018-1734-7Search in Google Scholar
[34] Liu, X.H.; Sun, Z.H.; Yang, M.Y.; Tan, C.X.; Weng, J.Q.; Zhang, Y.G.; Ma, Y. Microwave assistant one pot synthesis, crystal structure, antifungal activities and 3D-QSAR of novel 1,2,4-triazolo[4,3-apyridines. Chem. Biol. Drug Des. 2014, 84, 342-34710.1111/cbdd.12323Search in Google Scholar PubMed
[35] Liu, X.H.; Xu, X.Y.; Tan, C.X.; Weng, J.Q.; Xin, J.H.; Chen, J. Synthesis, crystal structure, herbicidal activities and 3D-QSAR study of some novel 1,2,4-triazolo[4,3-apyridine derivatives, Pest Manag. Sci. 2015, 71 292-301.10.1002/ps.3804Search in Google Scholar PubMed
[36] Liu, X.H.; Zhai, Z.W.; Xu, X.Y.; Yang, M.Y.; Sun, Z.H.; Weng, J.Q.; Tan, C.X.; Chen, J. Facile and efficient synthesis and biological activity determination of novel 1,2,4-triazolo[4,3-apyridin-3(2H-one derivatives via microwave irradiation. Bioorg. Med. Chem. Lett. 2015, 25 5524-5528.10.1016/j.bmcl.2015.10.064Search in Google Scholar PubMed
[37] Fang, Y.M.; Zhang, R.R.; Shen, Z.H.; Tan, C.X.; Weng, J.Q.; Xu, T.M.; Liu, X.H. Huang, H.Y.; Wu, H.K. Synthesis and antifungal activity of some 6-tert-butyl-8-chloro-2, 3-dimethylquinolin-4-ol derivatives against Pyricularia oryaeLett. Drug Des. Discov. 2018, 15, 1314-1318.10.2174/1570180815666180313121735Search in Google Scholar
[38] Sun, N.B.; Zhai, Z.W.; Tong, J.Y.; Cai, P.P.; He, F.Y.; Han, L.; Liu, X.H. Synthesis, Crystal Structure and fungicidal activity of 3-(difluoromethyl)-1-methyl-N-((2-(trifluoromethyl)phenyl) carbamoyl)-1H-pyrazole-4-carboxamide. Chin. J. Struct. Chem. 2018, published ahead of print; doi: 10.14102/j.cnki.0254-5861.2011-2176.10.14102/j.cnki.0254-5861.2011-2176Search in Google Scholar
[39] Sun, N.B.; Zhai, Z.W.; Shen, Z.H.; Weng, J.Q.; Tan, C.X.; Liu, X.H.; Han, L. Synthesis, crystal structure and antifungal activity of N-((2,6-difluorophenyl)carbamoyl)1,3-dimethyl -1H-pyrazole-4-carboxamide. Chin. J. Struct. Chem. 2017, 36, 1667-1672.Search in Google Scholar
[40] Shen, Z.H.; Zhai, Z.W.; Sun, Z.H.; Weng, J.Q.; Tan, C.X.; Liu, X.H. Synthesis, crystal structure and biological activity of 2-chloro-5-(((5-(1-methyl-3-(trifluoromethyl) -1H-pyrazol-4-yl)-4-phenyl-4H-1,2,4-triazol-3-yl)thio)methyl)thiazole. Chin. J. Struct. Chem. 2017, 36, 1137-1141.Search in Google Scholar
[41] Yang, M.Y.; Zhai, Z.W.; Sun, Z.H.; Yu, S.J.; Liu, X.H.; Weng, J.Q.; Tan, C.X.; Zhao, W.G. A facile one-pot synthesis of novel 1,2,4-triazolo[4,3-apyridine derivatives containing the trifluoromethyl moiety using microwave irradiation. J. Chem. Res. 2015, 9, 521-523.10.3184/174751915X14400874295745Search in Google Scholar
[42] Zhai, Z.W.; Yang, M.Y.; Sun, Z.H.; Liu, X.H.; Weng, J.Q.; Tan, C.X. Facile and efficient synthesis of novel 1,2,3-thiadiazole derivatives using microwave irradiation. J. Chem. Res. 2015, 9, 340-342.10.3184/174751915X14326308763971Search in Google Scholar
[43] Liu, X.H.; Pan, L.; Weng, J.Q.; Tan, C.X.; Li, Y.H.; Wang, B.L.; Li, Z.M. Synthesis, structure, and biological activity of novel (oxdi/ tri)azoles derivatives containing 1,2,3-thiadiazole or methyl moiety. Mol. Divers. 2012, 16, 251-260.10.1007/s11030-011-9352-zSearch in Google Scholar PubMed
[44] Liu, X.H.; Pan, L.; Tan, C.X.; Weng, J.Q.; Wang, B.L.; Li, Z.M. Synthesis, crystal structure, bioactivity and DFT calculation of new oxime ester derivatives containing cyclopropane moiety. Pestic. Biochem. Physiol. 2011, 101, 143-147.10.1016/j.pestbp.2011.08.006Search in Google Scholar
[45] Weng, J.Q.; Wang, L.; Liu, X.H. Synthesis, Crystal Structure and Herbicidal Activity of a 1, 2, 4-triazol-5(4H)-one Derivative. J. Chem. Soc. Pak. 2012, 34, 1248-1252.Search in Google Scholar
[46] Liu, X.H.; Zhao, W.G.; Wang, B.L.; Li, Z.M. Synthesis, bioactivity and DFT structure-activity relationship study of novel 1,2,3-thiadiazole derivatives. Res. Chem. Intermed. 2012, 38, 1999-2008.10.1007/s11164-012-0521-1Search in Google Scholar
[47] Sun, N.B.; Shen, Z.H.; Zhai, Z.W.; Han, L.; Weng, J.Q.; Tan, C.X.; Liu, X.H. Design, Synthesis, Fungicidal Activity and Docking Study of Acyl Thiourea Derivatives Containing Pyrazole Moiety. Chin. J. Org. Chem. 2017, 37, 2705-2710.10.6023/cjoc201704032Search in Google Scholar
[48] Ding, X.M.; Zhai, Z.W.; Lv, L.P.; Sun, Z.H.; Liu, X.H. Design, synthesis, biological activity and density function theory study of pyrazole derivatives containing 1,3,4-thiadiazole moiety. Front. Chem. Sci. Eng. 2017, 11, 379-386.10.1007/s11705-017-1634-2Search in Google Scholar
[49] Wang, H.; Zhai, Z.W.; Shi, Y.X.; Tan, C.X.; Weng, J.Q.; Han, L.; Li, B.J.; Liu, X.H. Novel trifluoromethylpyrazole acyl urea derivatives: Synthesis, crystal structure, fungicidal activity and docking study. J Mol Struct 2018, 1171, 631-638.10.1016/j.molstruc.2018.06.050Search in Google Scholar
[50] Sun, N.B.; Shen, Z.H.; Zhai, Z.W.; Wu, H.K.; Weng, J.Q.; Tan, C.X.; Liu, X.H. Design, Synthesis, Fungicidal Activity and Docking Study of Acyl Urea Derivatives Containing Pyrazole Moiety. Chin. J. Org. Chem. 2017, 37, 2044-2049.10.6023/cjoc201702003Search in Google Scholar
[51] Sheldrick, G. M. SHELXS97 and SHELXL97 University of Göttingen, Germany, 1997.Search in Google Scholar
© 2019 Li Qiao et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Research Article
- Magnesium porphyrazine with peripheral methyl (3,5-dibromophenylmethyl)amino groups – synthesis and optical properties
- Synthesis and fungicidal activity of novel imidazo[4, 5-b]pyridine derivatives
- Synthesis of indazolo[5,4-b][1,6]naphthyridine and indazolo[6,7-b][1,6]naphthyridine derivatives
- Zinc Chloride Catalyzed Amino Claisen Rearrangement of 1-N-Allylindolines: An Expedient Protocol for the Synthesis of Functionalized 7-Allylindolines
- Synthesis and Biological Evaluation of (E)-N’-Benzylidene-7-methyl-2-propyl-1H-benzo[d] imidazole-5-carbohydrazides as Antioxidant, Anti-inflammatory and Analgesic agents
- Efficient synthesis, reactions and spectral characterization of pyrazolo[4’,3’:4,5]thieno[3,2-d] pyrimidines and related heterocycles
- Asymmetric Mannich Reaction: Synthesis of Novel Chiral 5-(substituted aryl)-1,3,4-Thiadiazole Derivatives with Anti-Plant-Virus Potency
- Synthesis and antitubercular activity of new N-[5-(4-chlorophenyl)-1,3,4-oxadiazol-2-yl]-(nitroheteroaryl)carboxamides
- Remarkable electronic effect on the total stereoselectivity of the cycloaddition reaction of arylnitrile oxides with pyrrol-2-one derivatives
- Preliminary Communications
- Crystal structure and molecular docking studies of new pyrazole-4-carboxamides
- Research Article
- Synthesis of polycyclic phosphonates via an intramolecular Diels-Alder reaction of 2-benzoylbenzalaldehyde and alkenyl phosphites
- Asymmetric total synthesis of filamentous fungi related resorcylic acid lactones 7-epi-zeaenol and zeaenol
- The first in situ synthesis of 1,3-dioxan-5-one derivatives and their direct use in Claisen-Schmidt reactions
- Synthesis and fungicidal activities of perfluoropropan-2-yl-based novel quinoline derivatives
- Combined XRD-paramagnetic 13C NMR spectroscopy of 1,2,3-triazoles for revealing copper traces in a Huisgen click-chemistry cycloaddition. A model case
- Cytotoxic and antimicrobial activities of some novel heterocycles employing 6-(1,3-diphenyl-1H-pyrazol-4-yl)-4-oxo-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile
- Substrate-controlled Diastereoselective Michael Addition of Alkylidene Malonates by Grignard Reagents
- Synthesis of 1,2,3 triazole-linked benzimidazole through a copper-catalyzed click reaction
- Synthesis and spectral characteristics of N-(1-([1,2,4]triazolo[3,4-b][1,3,4]thiadiazol-6-ylamino)-2,2,2-trichloroethyl)carboxamides
- Facile One-pot Protocol of Derivatization Nitropyridines: Access to 3-Acetamidopyridin-2-yl 4-methylbenzenesulfonate Derivatives
- Naphthalene substituted benzo[c]coumarins: Synthesis, characterization and evaluation of antibacterial activity and cytotoxicity
- A Green Synthesis and Antibacterial Activity of N-Arylsulfonylhydrazone Compounds
- Preliminary Communications
- Facile Synthesis of Spiro[cyclohexane-1,3’-indoline]-2,2’-diones
- Research Article
- Synthesis and AChE inhibitory activity of N-glycosyl benzofuran derivatives
- [DMImd-DMP]: A highly efficient and reusable catalyst for the synthesis of 4H-benzo[b]pyran derivatives
Articles in the same Issue
- Research Article
- Magnesium porphyrazine with peripheral methyl (3,5-dibromophenylmethyl)amino groups – synthesis and optical properties
- Synthesis and fungicidal activity of novel imidazo[4, 5-b]pyridine derivatives
- Synthesis of indazolo[5,4-b][1,6]naphthyridine and indazolo[6,7-b][1,6]naphthyridine derivatives
- Zinc Chloride Catalyzed Amino Claisen Rearrangement of 1-N-Allylindolines: An Expedient Protocol for the Synthesis of Functionalized 7-Allylindolines
- Synthesis and Biological Evaluation of (E)-N’-Benzylidene-7-methyl-2-propyl-1H-benzo[d] imidazole-5-carbohydrazides as Antioxidant, Anti-inflammatory and Analgesic agents
- Efficient synthesis, reactions and spectral characterization of pyrazolo[4’,3’:4,5]thieno[3,2-d] pyrimidines and related heterocycles
- Asymmetric Mannich Reaction: Synthesis of Novel Chiral 5-(substituted aryl)-1,3,4-Thiadiazole Derivatives with Anti-Plant-Virus Potency
- Synthesis and antitubercular activity of new N-[5-(4-chlorophenyl)-1,3,4-oxadiazol-2-yl]-(nitroheteroaryl)carboxamides
- Remarkable electronic effect on the total stereoselectivity of the cycloaddition reaction of arylnitrile oxides with pyrrol-2-one derivatives
- Preliminary Communications
- Crystal structure and molecular docking studies of new pyrazole-4-carboxamides
- Research Article
- Synthesis of polycyclic phosphonates via an intramolecular Diels-Alder reaction of 2-benzoylbenzalaldehyde and alkenyl phosphites
- Asymmetric total synthesis of filamentous fungi related resorcylic acid lactones 7-epi-zeaenol and zeaenol
- The first in situ synthesis of 1,3-dioxan-5-one derivatives and their direct use in Claisen-Schmidt reactions
- Synthesis and fungicidal activities of perfluoropropan-2-yl-based novel quinoline derivatives
- Combined XRD-paramagnetic 13C NMR spectroscopy of 1,2,3-triazoles for revealing copper traces in a Huisgen click-chemistry cycloaddition. A model case
- Cytotoxic and antimicrobial activities of some novel heterocycles employing 6-(1,3-diphenyl-1H-pyrazol-4-yl)-4-oxo-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile
- Substrate-controlled Diastereoselective Michael Addition of Alkylidene Malonates by Grignard Reagents
- Synthesis of 1,2,3 triazole-linked benzimidazole through a copper-catalyzed click reaction
- Synthesis and spectral characteristics of N-(1-([1,2,4]triazolo[3,4-b][1,3,4]thiadiazol-6-ylamino)-2,2,2-trichloroethyl)carboxamides
- Facile One-pot Protocol of Derivatization Nitropyridines: Access to 3-Acetamidopyridin-2-yl 4-methylbenzenesulfonate Derivatives
- Naphthalene substituted benzo[c]coumarins: Synthesis, characterization and evaluation of antibacterial activity and cytotoxicity
- A Green Synthesis and Antibacterial Activity of N-Arylsulfonylhydrazone Compounds
- Preliminary Communications
- Facile Synthesis of Spiro[cyclohexane-1,3’-indoline]-2,2’-diones
- Research Article
- Synthesis and AChE inhibitory activity of N-glycosyl benzofuran derivatives
- [DMImd-DMP]: A highly efficient and reusable catalyst for the synthesis of 4H-benzo[b]pyran derivatives

