Synthesis of [1,3]thiazolo[3,2-b][1,2,4]triazol-7-ium and [1,2,4]triazolo[5,1-b][1,3]thiazin-4-ium salts via regioselective electrophilic cyclization of 3-[(2-alken-1-yl)sulfanyl]-4H-1,2,4-triazoles
Abstract
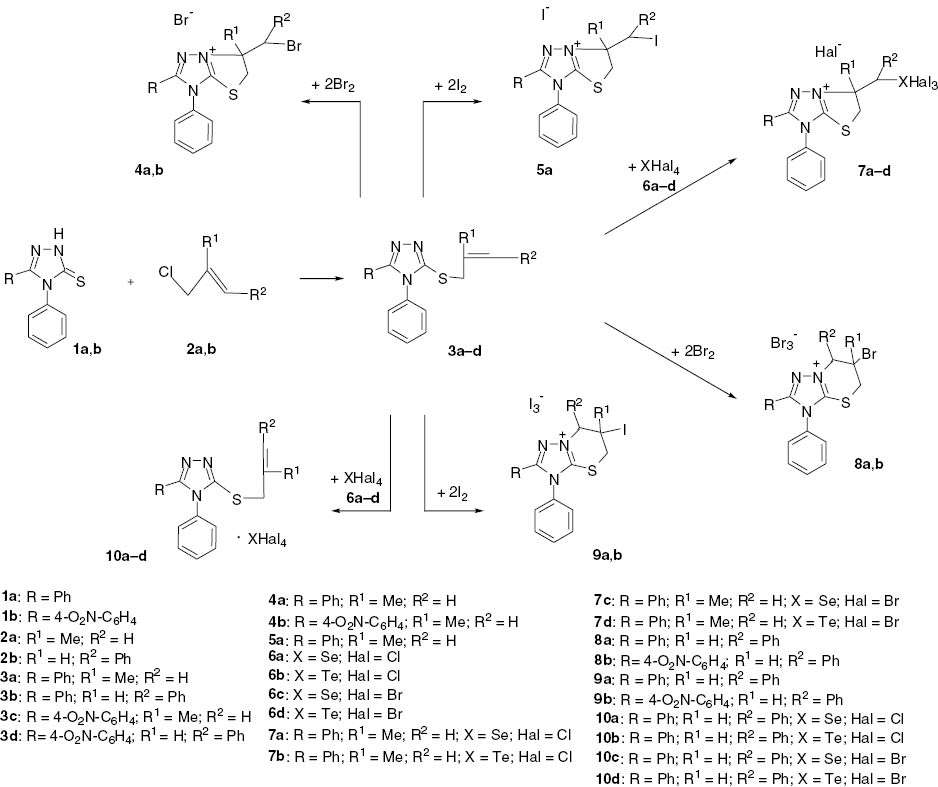
A convenient procedure for the regioselective preparation of [1,3]thiazolo[3,2-b][1,2,4]triazol-7-ium 10 and [1,2,4]triazolo[5,1-b][1,3]thiazin-4-ium salts 9 via regioselective electrophilic cyclization of 3-[(2-alken-1-yl)sulfanyl]-4H-1,2,4-triazoles 3 is reported. Direction of electrophilic heterocyclization strongly depends on nature of the alkenyl substitutent.
Introduction
Among the large number of condensed derivatives of the symmetric triazoles many compounds show a wide range of biological activities: antimicrobial and antifungal [1–5], anti-inflammatory [6–8], and anti-convulsive properties [5, 9], among others [10]. Considering these observations, it was envisaged to search effective ways for synthesis of new condensed derivatives of symmetrical triazoles, which can possess valuable properties. In recent years, hetero-annelation processes based on electrophilic halocyclization have proved to be useful in producing various heterocycles including furan [11–14], pyrrole [11, 15], quinoline [16, 17], and a large number of complex heterocyclic compounds [18–30]. The regioselectivity of electrophilic halocyclization of 1,2,4-triazole derivatives was investigated as a function of the reaction conditions [25–27], nature of electrophilic agents [25, 27, 29, 30] and nature of the heterocyclic nucleophilic center in the molecule [26, 29]. On this basis, it was reasoned that introduction of various groups into an allyl substituent of 1,2,4-triazole system can also influence regioselectivity of the annelation reactions.
Results and discussion
Synthesis of [1,3]thiazolo[2,3-c][1,2,4]triazoles upon halogenation of 3-allylsulfanyl-4,5-diphenyl-3H-1,2,4-triazole was discussed in our previous work [25]. In this work the regioselectivity of electrophilic cyclization of the alkenyl derivatives of 3-sulfanyl-1,2,4-triazole 3 was investigated. Compounds 3 were obtained by the reaction of an excess of the corresponding alkenyl chloride 2 with alkaline alcoholic solutions of triazoles 1. Methylallyl chloride 2a and cinnamyl chloride 2b were used in the reaction conducted in boiling ethanol. Target sulfanyl ethers 3 precipitated after cooling the reaction mixture.
The structure of compounds 3a–d was confirmed by using spectral methods.
Bromination of (methylallyl)sulfanyl compounds 3a,b furnished condensed monobromides 4a,b. The reactions were carried out at 15°C for 3 h with a ratio of Br2/3 of 2:1. It should be noted that the nature of solvent (chloroform, glacial acetic acid) did not influence the yields of salts 4a,b. A similar pattern was observed in the reaction of iodine with (methylallyl)sulfanyl derivative 3a. This treatment gave iodide 5a as the cyclization product. On the other hand, condensed salts 7a–d were obtained upon electrophilic cyclization of compound 3a using selenium and tellurium tetrahalogenides 6a–d. Solutions of the reagents 6a–d in acetic acid were prepared from a chalcogen oxide in the presence of an excess amount of hydrochloric or hydrobromic acid.
In comparison to the outcome of the reaction of methylallyl derivatives, the regioselectivity process of halocyclization of the cinnamyl analogs 3b,d is different as the cyclization is accompanied by an annelation to a six-membered thiazine system to form [1,2,4]triazolo[5,1-b][1,3]thiazin-8-ium bromides 8a,b and [1,2,4]triazolo[5,1-b][1,3]thiazin-8-ium iodides 9a,b. The difference in halocyclization selectivity can be explained by a powerful steric effect of the aromatic substitutient in the cinnamyl fragment.
In contrast to the treatment with halogens, the reaction of tetrahalides of selenium and tellurium 6a–d with cinnamyl derivatives does not cause annelation to form an additional heterocyclic system. This reaction yields stoichiometric yellowish adducts 10a–d. The spectral characteristics of these adducts are almost identical to those of the substrates 3b,d. This outcome can be explained in terms of the deactivating influence of steric factors of the cinnamyl substituent and selenium/tellurium tetrahalides. It should be noted that heating of compounds 10a–d for 7 h did not lead to their cyclization, and the starting complexes 10a–d were isolated in high yields.
All compounds 4–10 were characterized by means of NMR spectroscopy including 1H, 13C, 79Se, NOE and COSY experiments, mass spectrometry and elemental analysis. The proposed structures are fully consistent with the experimental data.
Conclusions
Methylallylsulfanyl- and cinnamylsulfanyl-substituted 1,2,4-triazoles 3a–d were synthesized and their diverse electrophilic transformations were studied. The nature of the alkenyl fragment exerts a strong influence on the product structure.
Experimental
1H NMR (300 MHz or 400 MHz) and 13C NMR (75 MHz or 100 MHz) spectra were recorded in DMSO-d6 on a Varian VXR-300 or Bruker DPX-400 instruments; the 79Se NMR spectrum of 7c was recorded on a Bruker DPX-400 instrument. 2D-NOESY and COSY experiments were carried out for compounds 7d, 8b in CDCl3 on a Varian Mercury-400 instrument. Microanalyses were performed by the microanalytical unit of the Institute of Organic Chemistry of the National Academy of Sciences (Kyiv, Ukraine). The EI mass spectrum of compound 7d was obtained using an Agilent 1100 LCMSD SL instrument. Melting points were determined on a Koefler block instrument and were not corrected.
General procedure for the preparation of substituted 4H-1,2,4-triazoles 3a–d
A substituted 2,4-dihydro-3H-1,2,4-triazole-3-thione 1a or 1b (10.0 mmol) was dissolved in ethanol (20 mL) with heating and the solution was cooled and treated with an alkenyl halogenide 2a or 2b (12.0 mmol) in ethanol (5 mL). The mixture was heated under reflux for 1 h. The precipitated product was filtered, washed with ether and dried.
3-[(2-Methylprop-2-en-1-yl)sulfanyl]-4,5-diphenyl-4H-1,2,4-triazole (3a)
This compound was obtained from 1a and 2a; yield 80% of colorless crystals; mp 140oC; 1H NMR (300 MHz): δ 1.70 (s, 3H), 3.76 (s, 2H), 4.83 (s, 1H), 4.91 (s, 1H), 7.35–7.55 (m, 10H). Anal. Calcd for C18H17N3S: C, 70.33; H, 5.57; N, 13.67; S, 10.43. Found: C, 70.70; H, 5.61; N, 13.52; S, 10.29.
3,4-Diphenyl-5-[(3-phenylprop-2-en-1-yl)sulfanyl]-4H-1,2,4-triazole (3b)
This compound was obtained from 1a and 2b; yield 52% of colorless crystals; mp 152–154oC; 1H NMR (300 MHz): δ 3.97 (d, J = 4.2 Hz, 2H), 6.36 (m, 1H), 6.58 (d, J = 9.3 Hz, 1H), 7.23–7.52 (m, 15H). Anal. Calcd for C23H19N3S: C, 74.77; H, 5.18; N, 11.37; S, 8.68. Found: C, 75.01; H, 5.11; N, 11.21; S, 8.50.
3-[(2-Methylprop-2-en-1-yl)sulfanyl]-5-(4-nitrophenyl)-4-phenyl-4H-1,2,4-triazole (3c)
This compound was obtained from 1b and 2a; yield 57% of yellowish crystals; mp 168–170oC; 1H NMR (300 MHz): δ 1.72 (s, 3H), 3.82 (s, 2H), 4.85 (s, 1H), 4.94 (s, 1H), 7.42–7.68 (m, 7H), 8.21 (d, J = 8.1 Hz, 2H). Anal. Calcd for C18H16N4O2S: C, 61.35; H, 4.58; N, 15.90; S, 9.10. Found: C, 61.48; H, 4.49; N, 15.72; S, 9.14.
3-(4-Nitrophenyl)-4-phenyl-5-[(3-phenylprop-2-en-1-yl)sulfanyl]-4H-1,2,4-triazole (3d)
This compound was obtained from 1b and 2b; yield 64% of yellowish crystals; mp 170–172oC; 1H NMR (300 MHz): δ 3.98 (d, J = 4.5 Hz, 2H), 6.32 (m, 1H), 6.58 (d, J = 9.9 Hz, 1H), 7.20–7.57 (m, 12H), 8.15 (d, J = 5.4 Hz, 2H); 13C NMR (75 MHz): δ 153.2, 153.0, 148.1, 136.5, 133.9, 133.6, 133.0, 130.7, 130.5, 129.3, 129.0, 128.2, 128.0, 126.7, 124.8, 124.1, 35.2. Anal. Calcd for C23H18N4O2S: C, 66.65; H, 4.38; N, 13.52; S, 7.74. Found: C, 66.79; H, 4.16; N, 13.32; S, 7.70.
General procedure for the preparation of fused salts 4, 5, 7–9 and complexes 10
A solution of bromine or iodine (20.0 mmol) or a solution of a tetrahalogenide of selenium or tellurium 6a–d (10.0 mmol) in acetic acid was added dropwise to the solution of a triazole 3a–d (10.0 mmol) in acetic acid with constant stirring at room temperature. The resultant solid product was filtered, washed with acetone (4, 5, 8, 9) or ether (7, 10) and dried.
6-(Bromomethyl)-6-methyl-2,3-diphenyl-5,6-dihydro-3H-[1,3]thiazolo[3,2-b][1,2,4]triazol-7-ium bromide (4a)
This compound was obtained by bromination of 3a; yield 78% of a white powder; mp 285–287°C; 1H NMR (300 MHz): δ 2.01 (s, 3H), 4.28–4.34 (m, 2H), 4.48 (dd, J = 31.5, 12.0 Hz, 2H), 7.46–7.75 (m, 10H); 13C NMR (75 MHz): δ 160.1, 157.5, 132.6, 131.4, 129.6, 126.9, 123.9, 69.1, 48.2, 37.9, 23.3. Anal. Calcd for C18H17Br2N3S: C, 46.27; H, 3.67; Br, 34.20; N, 8.99; S, 6.86. Found: C, 45.98; H, 3.52; Br, 34.52; N, 8.84; S, 6.79.
6-(Bromomethyl)-6-methyl-2-(4-nitrophenyl)-3-phenyl-5,6-dihydro-3H-[1,3]thiazolo[3,2-b][1,2,4]triazol-7-ium bromide (4b)
This compound was obtained by bromination of 3c; yield 56% of yellowish powder; mp 259–261°C; 1H NMR (400 MHz): δ 1.96 (s, 3H), 4.21–4.25 (m, 2H), 4.45 (dd, J = 30.0, 11.6 Hz, 2H), 7.65–7.73 (m, 7H), 8.35 (d, J = 9.2 Hz, 2H). Anal. Calcd for C18H16Br2N4O2S: C, 42.21; H, 3.15; Br, 31.20; N, 10.94; S, 6.26. Found: C, 42.02; H, 3.11; Br, 31.55; N, 10.78; S, 6.17.
6-(Iodomethyl)-6-methyl-2,3-diphenyl-5,6-dihydro-3H-[1,3]thiazolo[3,2-b][1,2,4]triazol-7-ium iodide (5a)
This compound was obtained by iodination of 3a; yield 61% of a brown powder; mp 248–250°C; 1H NMR (300 MHz): δ 1.98 (s, 3H), 3.95–4.04 (m, 2H), 4.41 (dd, J = 27.3, 12.0 Hz, 2H), 7.39–7.72 (m, 10H). Anal. Calcd for C18H17I2N3S: C, 38.52; H, 3.05; I, 45.22; N, 7.49; S, 5.71. Found: C, 38.79; H, 2.99; I, 45.56; N, 7.33; S, 5.68.
6-Methyl-2,3-diphenyl-6-[(trichloro-λ4-selenanyl)methyl]-5,6-dihydro-3H-[1,3]thiazolo[3,2-b][1,2,4]triazol-7-ium chloride (7a)
This compound was obtained from 3a and 6a; yield 39% of a white powder; mp 220°C (dec); 1H NMR (400 MHz): δ 2.01 (s, 3H), 3.96 (d, J = 9.0 Hz, 1H), 4.07 (d, J = 8.7 Hz, 1H), 4.41 (d, J = 11.1 Hz, 1H), 4.97 (d, J = 11.1 Hz, 1H), 7.22–7.41 (m, 10H). Anal. Calcd for C18H17Cl4N3SSe: C, 40.93; H, 3.24; Cl, 26.85; N, 7.96. Found: C, 41.20; H, 3.29; Cl, 26.68; N, 8.02.
6-Methyl-2,3-diphenyl-6-[(trichloro-λ4-telluranyl)methyl]-5,6-dihydro-3H-[1,3]thiazolo[3,2-b][1,2,4]triazol-7-ium chloride (7b)
This compound was obtained from 3a and 6b; yield 45% of a white powder; mp 255°C (dec); 1H NMR (400 MHz): δ 2.21 (s, 3H), 4.06 (d, J = 9.0 Hz, 1H), 4.35 (d, J = 12.0 Hz, 1H), 4.49 (d, J = 9.0 Hz, 1H), 5.17 (d, J = 12.0 Hz, 1H), 7.34–7.91 (m, 10H). Anal. Calcd for C18H17N3S: C, 37.48; H, 2.97; Cl, 24.58; N, 7.28. Found: C, 37.72; H, 3.03; Cl, 24.28; N, 7.39.
6-Methyl-2,3-diphenyl-6-[(tribromo-λ4-selenanyl)methyl]-5,6-dihydro-3H-[1,3]thiazolo[3,2-b][1,2,4]triazol-7-ium bromide (7c)
This compound was obtained from 3a and 6c; yield 44% of a yellowish powder; mp 210°C (dec); 1H NMR (400 MHz): δ 2.02 (s, 3H), 4.35 (d, J = 11.1 Hz, 1H), 4.48 (d, J = 9.0 Hz, 1H), 4.59 (d, J = 9.0 Hz, 1H), 4.90 (d, J = 11.1 Hz, 1H), 7.24–7.50 (m, 10H); 13C NMR (100 MHz): δ 159.5, 156.7, 132.4, 129.7, 126.5, 123.9, 70.9, 49.3, 45.4, 25.2; 79Se NMR (DMSO-d6): δ 660. Anal. Calcd for C18H17Br4N3SSe: C, 30.62; H, 2.43; Br, 45.27; N, 5.95. Found: C, 30.68; H, 2.42; Br, 45.36; N, 5.84.
6-Methyl-2,3-diphenyl-6-[(tribromo-λ4-telluranyl)methyl]-5,6-dihydro-3H-[1,3]thiazolo[3,2-b][1,2,4]triazol-7-ium bromide (7d)
This compound was obtained from 3a and 6d; yield 59% of an orange powder; mp 267°C (dec); 1H NMR (400 MHz): δ 2.21 (s, 3H), 3.94 (d, J = 11.1 Hz, 1H), 4.14 (d, J = 9.0 Hz, 1H), 4.24 (d, J = 11.1 Hz, 1H), 4.91 (d, J = 9.0 Hz, 1H), 7.46–7.67 (m, 10H); 13C NMR (100 MHz): δ 158.2, 157.6, 132.5, 123.3, 131.2, 129.8, 129.7, 126.7, 123.8, 67.8, 50.1, 40.0, 25.0; MS: m/z 354.0 (60), 326.0 (70), 309.8 (50), 308.0 (100), 253.8 (25), 238.2 (25). Anal. Calcd for C18H17Br4N3STe: C, 28.65; H, 2.27; Br, 42.35; N, 5.57. Found: C, 28.69; H, 2.25; Br, 42.22; N, 5.61.
6-Bromo-2,3,7-triphenyl-3,5,6,7-tetrahydro[1,2,4]triazolo[5,1-b][1,3]thiazin-8-ium tribromide (8a)
This compound was obtained by bromination of 3b; yield 44% of a white powder; mp 181–183°C; 1H NMR (300 MHz): δ 3.72 (d, J = 12.0 Hz, 1H), 3.87 (dd, J = 13.6, 7.2 Hz, 1H), 5.45 (m, 1H), 6.33 (d, J = 4.0 Hz, 1H), 7.37–7.81 (m, 15H); 13C NMR (75 MHz): δ 153.7, 153.6, 136.1, 133.0, 131.6, 130.3, 129.8, 129.6, 128.4, 128.2, 123.1, 69.0, 45.2, 32.1. Anal. Calcd for C23H19Br4N3S: C, 40.09; H, 2.78; Br, 46.38; N, 6.10; S, 4.65. Found: C, 40.21; H, 2.84; Br, 46.02; N, 6.18; S, 4.70.
6-Bromo-2-(4-nitrophenyl)-3,7-diphenyl-3,5,6,7-tetrahydro[1,2,4]triazolo[5,1-b][1,3]thiazin-8-ium tribromide (8b)
This compound was obtained by bromination of 3d; yield 90% of a yellowish powder; mp 115–120°C; 1H NMR (400 MHz): δ 3.80 (d, J = 11.8 Hz, 1H), 3.97 (dd, J = 13.6, 7.1 Hz, 1H), 5.50 (m, 1H), 6.39 (d, J = 5.2 Hz, 1H), 7.42–7.98 (m, 12H), 8.29 (d, J = 8.8 Hz, 2H); 13C NMR (100 MHz, DMSO-d6): δ 153.8, 151.6, 149.7, 135.4, 132.7, 131.2, 131.1, 130.9, 129.9, 129.3, 128.7, 128.3, 127.8, 124.4, 69.0, 45.0, 32.4. Anal. Calcd for C23H18Br4N4O2S: C, 37.63; H, 2.47; Br, 43.54; N, 7.63; S, 4.37. Found: C, 37.72; H, 2.51; Br, 43.22; N, 7.69; S, 4.40.
6-Iodo-2,3,7-triphenyl-3,5,6,7-tetrahydro[1,2,4]triazolo[5,1-b][1,3]thiazin-8-ium triiodide (9a)
This compound was obtained by iodination of 3b; yield 57% of a brown powder; mp 190–192°C; 1H NMR (300 MHz): δ 3.76 (d, J = 14.9 Hz, 1H), 3.92 (dd, J = 13.5, 6.9 Hz, 1H), 5.48 (m, 1H), 6.35 (d, J = 5.2 Hz, 1H), 7.33–7.91 (m, 15H). Anal. Calcd for C23H19I4N3S: C, 31.50; H, 2.18; I, 57.87; N, 4.79; S, 3.66. Found: C, 31.67; H, 2.21; I, 57.72; N, 4.84; S, 3.68.
6-Iodo-2-(4-nitrophenyl)-3,7-diphenyl-3,5,6,7-tetrahydro[1,2,4]triazolo[5,1-b][1,3]-thiazin-8-ium triiodide (9b)
This compound was obtained by iodination of 3d; yield 67% of a brown powder; mp 132–134°C; 1H NMR (300 MHz): δ 3.79–3.90 (m, 1H), 3.97 (dd, J = 15.2, 7.0 Hz, 1H), 5.25 (m, 1H), 6.23 (d, J = 7.0 Hz, 1H), 7.46–7.67 (m, 7H), 7.77 (s, 5H), 8.28 (d, J = 8.9 Hz, 2H). Anal. Calcd for C23H18I4N4O2S: C, 29.96; H, 1.97; I, 55.05; N, 6.08; S, 3.48. Found: C, 30.16; H, 2.01; I, 54.90; N, 6.12; S, 3.44.
Molecular complex 10a
This compound was obtained from 3b and 6a; yield 32% of colorless crystals; mp 220°C (dec); 1H NMR (300 MHz): δ 3.98 (d, J = 4.2 Hz, 2H), 6.38 (m, 1H), 6.61 (d, J = 9.3 Hz, 1H), 7.18–7.53 (m, 15H). Anal. Calcd for C23H19Cl4N3SSe: C, 46.80; H, 3.24; Cl, 24.03; N, 7.12. Found: C, 47.06; H, 3.20; Cl, 24.34; N, 7.26.
Molecular complex 10b
This compound was obtained from 3b and 6b; yield 45% (colorless crystals); mp 255°C (dec); 1H NMR (300 MHz): δ 3.98 (d, J = 4.2 Hz, 2H), 6.38 (m, 1H), 6.60 (d, J = 9.3 Hz, 1H), 7.22–7.56 (m, 15H). Anal. Calcd for C23H19Cl4N3STe: C, 43.24; H, 3.00; Cl, 22.20; N, 6.58. Found: C, 43.52; H, 3.08; Cl, 22.43; N, 6.50.
Molecular complex 10c
This compound was obtained from 3b and 6c; yield 27% of a colorless crystals; mp 222°C (dec); 1H NMR (300 MHz): δ 3.98 (d, J = 4.2 Hz, 2H), 6.36 (m, 1H), 6.61 (d, J = 9.3 Hz, 1H), 7.19–7.53 (m, 15H). Anal. Calcd for C23H19Br4N3SSe: C, 35.97; H, 2.49; Br, 41.61; N, 5.47. Found: C, 36.18; H, 2.45; Br, 41.94; N, 5.33.
Molecular complex 10d
This compound was obtained from 3b and 6d; yield 39% of a colorless crystals; mp 267°C (dec); 1H NMR (300 MHz): δ 3.98 (d, J = 4.2 Hz, 2H), 6.38 (m, 1H), 6.58 (d, J = 9.3 Hz, 1H), 7.26–7.58 (m, 15H). Anal. Calcd for C23H19Br4N3STe: C, 33.82; H, 2.34; Br, 39.14; N, 5.15. Found: C, 33.98; H, 2.29; Br, 39.46; N, 5.11.
Acknowledgments
This work was supported by the Department of Education and Science (DES) of Ukraine (Project GR-0113U002360).
References
[1] Mohan, J.; Kumar, A. Condensed bridgehead nitrogen heterocyclic systems: synthesis, bioactivity and stereochemistry of pyrazolo[3′,4′:4,5]thiazolo[3,2-b]-s-triazoles. Ind. J. Heterocycl. Chem.2003, 13, 97–100.Suche in Google Scholar
[2] Mohan, J. Heterocyclic system containing bridgehead nitrogen atom: synthesis and bioactivity 3-(2-thienyl)-s-triazolo [3,4-b] [1,3,4] thiadiazole, 2-(2-thienyl)triazolo [3,2-b]-s-triazolo and isomeric 3-(2-thienyl)thiazolo [2,3-c]-s-triazole. Ind. J. Chem.2003, 42B, 401–404.10.1002/chin.200322122Suche in Google Scholar
[3] Mohan, J. Novel bridgehead nitrogen heterocyclic-systems – synthesis, stereochemistry and biological-activity of pyrazolo[3′,4′4,5]thiazolo[3,2-b]-s-triazoles. Ind. J. Chem.1998, 37B, 953–955.Suche in Google Scholar
[4] Singh, A.; Handa, R. N.; Pujari, H. K. Heterocyclic systems containing bridgehead nitrogen atom: part XXXIII–syntheses of s-triazolo[I,3,4] thiadiazine, s-triazolo[3,4,b][I,3,4]thiadiazino[6,7-b]quinoxaline and as-triazino [1,3,4]- thiadiazines. Indian J. Chem.1978, 16B, 481–483.Suche in Google Scholar
[5] Erol, D. D.; Calis, U.; Demirdamar, R.; Yulug, N.; Ertan, M. J. Synthesis and biological activities of some 3,6-disubstituted thiazolo (3,2-b) (1,2,4) triazoles. Pharm. Sci.1995, 84, 462–465.10.1002/jps.2600840414Suche in Google Scholar PubMed
[6] Berk, B.; Aktay, G.; Yesilada, E.; Ertan, M. Synthesis and pharmacological activities of some new 2-[1-(6-methoxy-2-naphthyl)ethyl]-6-(substituted)-benzylidene thiazolo[3,2-b]-1,2,4-triazole-5(6H)-one derivatives. Pharmazie2001, 56, 613–616.Suche in Google Scholar
[7] Crisan, O.; Bojita, M.; Munoz, T. V.; Terencio, M. C.; Aguilar, G. A.; Zaharia, V. Synthesis and pharmacological activity of some thiazolo[3,2-b]triazolederivatives. Farmacia2001, 49, 15–22.Suche in Google Scholar
[8] Zaharia, M.; Bogdan, M.; Chirtoc, I.; Matinca, D. Synthesis and evaluation of the antibacterial and antifungic potential of some 2-aryl-5-(1-R-3-aryl-δ-2-pyrazolin-5-yl)-thiazolo[3,2-b][1,2,4]triazoles and 2-aryl-5-(3-aryl-δ-2-isoxazolin-5-yl)-thiazolo[3,2-b]-[1,2,4]triazoles. Farmacia2001, 49, 32–39.Suche in Google Scholar
[9] Vijaya Raj, K. K.; Narayana, B. The one step synthesis of 2-(2-bromo-5-methoxyphenyl)-5-(3-arylidene)-1,3-thiazolo[3,2-b]1,2,4-triazol-6-(5H)-ones and the evaluation of anticonvulsant activity. Phosphorus, Sulfur, and Silicon2006, 181, 1971–1981.10.1080/10426500500544170Suche in Google Scholar
[10] Milczarska, B.; Foks, H.; Dobrzycka, U. Synthesis and tuberculostatic activity of 1,3-thiazacycloalkyl[3,2-b]-1,2,4-triazoles. Phosphorus, Sulfur, and Silicon2005, 180, 2793–2799.10.1080/104265090968244Suche in Google Scholar
[11] Godoi, B.; Schumacher, R. F.; Zeni, G. Synthesis of heterocycles via electrophilic cyclization of alkynes containing heteroatom. Chem. Rev.2011, 111, 2937–2980.10.1021/cr100214dSuche in Google Scholar PubMed
[12] Windmon, N.; Dragojlovic, V; Beilstein J. Phase-vanishing halolactonization of neat substrates. Org. Chem.2008, 4, 29.10.3762/bjoc.4.29Suche in Google Scholar PubMed PubMed Central
[13] Raffa, G.; Balme, G.; Monterio, N. Direct access to fully substituted 3-formyl-4-iodofurans through iodocyclization of β-alkynyl-β-alkoxy enones. Eur. J. Org. Chem.2013, 1, 105–110.10.1002/ejoc.201201203Suche in Google Scholar
[14] He, Y.; Pu, Y.; Shao, B.; Yan, J. Novel catalytic bromolactonization of alkenoic acids using iodobenzene and oxone. J. Heterocycl. Chem.2011, 48, 695–698.10.1002/jhet.617Suche in Google Scholar
[15] Spina, R.; Colacino, E.; Gabriele, B.; Salerno, G.; Martinez, J.; Lamaty, F. Preparation of enantioenriched iodinated pyrrolinones by iodocyclization of a-amino-ynones. Org. Biomol. Chem.2012, 10, 9085–9089.10.1039/c2ob26427gSuche in Google Scholar PubMed
[16] Zhang, X.; Yao, T.; Campo, M. A.; Larock, R. C. Synthesis of substituted quinolines by the electrophilic cyclization of N-(2-alkynyl)anilines. Tetrahedron2010, 66, 1177–1187.10.1016/j.tet.2009.12.012Suche in Google Scholar PubMed PubMed Central
[17] Onysko, M. Yu.; Lendel, V. G. Haloheterocyclization of 2-methallyl(propargyl)-thioquinoline-3-carbaldehydes. Chem. Heterocycl. Comp. 2009, 45, 853–855.10.1007/s10593-009-0349-9Suche in Google Scholar
[18] Nesterenko, A. M.; Vas’kevich, R. I.; Zborovskii, Yu. L.; Staninets, V. I. Reactions of 3-allyl-4-oxothieno[2,3-d]pyrimidin-2-yl disulfides with iodine. Russ. Chem. Bull.2005, 54, 2582–2585.10.1007/s11172-006-0159-5Suche in Google Scholar
[19] Khripak, S. M.; Plesha, M. V.; Slivka, M. V.; Yakubets, V. I.; Krivovyaz, A. A. Syntesis and reactivity of 1-bromomethyl-5-oxo-4-phenyl-1,2,4,5,6,7,8,9-octahydrobenzo[4,5]thieno[3,2-e][1,3]oxazolo[3,2-a]-pyrimidin-11-ium bromides. Russ. J. Org. Chem. 2004, 40, 1705–1706.10.1007/s11178-005-0086-1Suche in Google Scholar
[20] Wippich, P.; Gutschow, M.; Leistner, S. Regioselective preparation of 1-(bromomethyl)-5H-thiazolo[3,2-a]quinazolin-5-ones and analogous 5H-thieno[3,2-e]thiazolo[3,2-a]pyrimidin-5-ones from fused 2-(alkenylthio)pyrimidin-4-ones. Synthesis2000, 5, 714–720.10.1055/s-2000-6390Suche in Google Scholar
[21] Svaljavyn, O. V.; Onysko, M. Yu.; Turov, A. V.; Vlasenko, Yu. G.; Lendel, V. G. Peculiar electrophilic heterocyclization of 5-allyl-6-thioxopyrazolo[3,4-d]pyrimidin-4-one. Chem. Heterocycl. Comp.2013, 49, 491–495.10.1007/s10593-013-1273-6Suche in Google Scholar
[22] Slivka, Mar. V.; Krivovjaz, A. A.; Slivka, M. V.; Lendel, V. G. Stereoselective Synthesis of (E)-Halogenmethylidene[1,3]thiazolo[3,2-a]-thieno[3,2-e]-pyrimidinium and Analogous [1,3]oxazolo[3,2-a]thieno[3,2-e]pyrimidinium Halogenides from 3-N-Substituted 2-Propargylthio(oxy-)thieno-[2,3-d]pyrimidin-4-ones. Heterocycl. Commun. 2013, 19, 189–193.10.1515/hc-2013-0036Suche in Google Scholar
[23] Fizer, M. M.; Slivka, M. V.; Rusanov, E.; Turov, A.; Lendel, V. G. [1,3]Thiazolo[2′,3′:3,4][1,2,4]triazolo[1,5-a]pyrimidines – a new heterocyclic system accessed via bromocyclization. J. Heterocyclic Chem.2015, 52, 949–952.10.1002/jhet.2073Suche in Google Scholar
[24] Fizer, M. M.; Slivka, M. V.; Lendel V. G. New method of synthesis of 3,5,6,7-tetrahydro[1,2,4]triazolo[1,5-a]pyrimidine-2(1H)-thione. Chem. Heterocycl. Comp.2013, 49, 1243–1245.10.1007/s10593-013-1369-zSuche in Google Scholar
[25] Usenko, R. M.; Slivka, M. V.; Lendel, V. G. Electrophilic heterocyclization of 4,5-disubstituted 3-allylthio-4H-1,2,4-triazoles by the action of halogens. Chem. Heterocycl. Comp.2011, 47, 1029–1036.10.1007/s10593-011-0870-5Suche in Google Scholar
[26] Khripak, S.; Slivka, M.; Vilkov, R.; Usenko, R.; Lendel, V. Regioselectivity of the monohalogenation of 4-allyl-3-allylamino-1,2,4-triazole-5-thione. Chem. Heterocycl. Comp.2007, 43, 781–785.10.1007/s10593-007-0126-6Suche in Google Scholar
[27] Shmygarev, V.; Kim, D. Study of the products of iodocyclization of 4-allyl-5-phenyl-1,2,4-triazole-3-thione. Chem. Heterocycl. Comp.2004, 40, 1077–1082.10.1023/B:COHC.0000046700.89050.90Suche in Google Scholar
[28] Slivka, M.; Khripak, S.; Britsun, V.; Staninets, V. Stereoselective synthesis of (E)-halomethylidene[1,3]thiazolo[3,2-a]thieno[3,2-e]pyrimidinium and analogous [1,3]oxazolo[3,2-a]thieno[3,2-e]pyrimidinium halides starting from 3-N-substituted 2-propargylthio(oxy)thieno[2,3-d]pyrimidin-4-ones. Russ. J. Org. Chem.2000, 36, 1033–1038.Suche in Google Scholar
[29] Ernst, S.; Jelonek, S.; Sieler, J.; Schulze, K. 4-methallyl substituted 1,2,4-triazoline-3-thiones as a source of N-bridgehead heterocycles. Tetrahedron1996, 52, 791–798.10.1016/0040-4020(95)01038-6Suche in Google Scholar
[30] Strzemecka, L. Synthesis of 3,6-disubstituted 5,6-dihydrothiazolo-[2,3-c][1,2,4]triazole system. Polish, J. Chem.1983, 57, 567.Suche in Google Scholar
©2015 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- Preliminary Communications
- Triphenylphosphine catalyzed domino reaction of dialkyl acetylenedicarboxylate with 3-aryl- 2-benzoylcyclopropane-1,1-dicarbonitrile
- Synthesis of colletotrichumine A
- Research Articles
- Synthesis of the spiroacetal fragments of spirofungins A and B, antibiotics isolated from Streptomyces violaceusniger Tü 4113
- Efficient synthesis and fungicidal activities of strobilurin analogues containing benzofuro [3,2-d]-1,2,4-triazolo[1,5-a]pyrimidinone side chains
- Synthesis of 2-amino-6,7,8,9-tetrahydro-6-phenethyl-3H-pyrimido[4,5-e][1,4]diazepin-4(5H)-one: a model for a potential pyrimido[4,5-e][1,4]diazepine-based folate anti-tumor agent
- Cascade assembling of pyrazolin-5-ones and benzylidenemalononitriles: the facile and efficient approach to medicinally relevant spirocyclopropylpyrazolone scaffold
- Synthesis, characterization and bioactivity of novel 5,6-dihydropyrrolo[3,4-c]pyrazol-4- (1H)one derivatives
- Molecular modeling and synthesis of new 1,5-diphenylpyrazoles as breast cancer cell growth inhibitors
- An efficient synthesis of 11-aryl-10-oxo-7,8,10,11-tetrahydro-1H-[1,2,3]triazolo [4′,5′:3,4]benzo[1,2-b][1,6]naphthyridine derivatives under catalyst-free conditions
- Mechanochemical synthesis of 2,2-difluoro-4, 6-bis(β-styryl)-1,3,2-dioxaborines and their use in cyanide ion sensing
- Visible-light-mediated radical aryltrichloromethylation of N-arylacrylamides for the synthesis of trichloromethyl-containing oxindoles
- A stereolibrary of conformationally restricted amino acids based on pyrrolidinyl/piperidinyloxazole motifs
- Synthesis of [1,3]thiazolo[3,2-b][1,2,4]triazol-7-ium and [1,2,4]triazolo[5,1-b][1,3]thiazin-4-ium salts via regioselective electrophilic cyclization of 3-[(2-alken-1-yl)sulfanyl]-4H-1,2,4-triazoles
Artikel in diesem Heft
- Frontmatter
- Preliminary Communications
- Triphenylphosphine catalyzed domino reaction of dialkyl acetylenedicarboxylate with 3-aryl- 2-benzoylcyclopropane-1,1-dicarbonitrile
- Synthesis of colletotrichumine A
- Research Articles
- Synthesis of the spiroacetal fragments of spirofungins A and B, antibiotics isolated from Streptomyces violaceusniger Tü 4113
- Efficient synthesis and fungicidal activities of strobilurin analogues containing benzofuro [3,2-d]-1,2,4-triazolo[1,5-a]pyrimidinone side chains
- Synthesis of 2-amino-6,7,8,9-tetrahydro-6-phenethyl-3H-pyrimido[4,5-e][1,4]diazepin-4(5H)-one: a model for a potential pyrimido[4,5-e][1,4]diazepine-based folate anti-tumor agent
- Cascade assembling of pyrazolin-5-ones and benzylidenemalononitriles: the facile and efficient approach to medicinally relevant spirocyclopropylpyrazolone scaffold
- Synthesis, characterization and bioactivity of novel 5,6-dihydropyrrolo[3,4-c]pyrazol-4- (1H)one derivatives
- Molecular modeling and synthesis of new 1,5-diphenylpyrazoles as breast cancer cell growth inhibitors
- An efficient synthesis of 11-aryl-10-oxo-7,8,10,11-tetrahydro-1H-[1,2,3]triazolo [4′,5′:3,4]benzo[1,2-b][1,6]naphthyridine derivatives under catalyst-free conditions
- Mechanochemical synthesis of 2,2-difluoro-4, 6-bis(β-styryl)-1,3,2-dioxaborines and their use in cyanide ion sensing
- Visible-light-mediated radical aryltrichloromethylation of N-arylacrylamides for the synthesis of trichloromethyl-containing oxindoles
- A stereolibrary of conformationally restricted amino acids based on pyrrolidinyl/piperidinyloxazole motifs
- Synthesis of [1,3]thiazolo[3,2-b][1,2,4]triazol-7-ium and [1,2,4]triazolo[5,1-b][1,3]thiazin-4-ium salts via regioselective electrophilic cyclization of 3-[(2-alken-1-yl)sulfanyl]-4H-1,2,4-triazoles

