Abstract
Stainless steel (SS) is a very corrosion-resistant alloy used in different industrial plants because of its chemical and mechanical properties. However, the high chloride concentration in sulfuric acid (H2SO4) may promote both general corrosion and pitting corrosion. The pitting corrosion susceptibility in SS in chlorinated H2SO4 and the effect of Euphorbia echinus extract (EEE) on both general corrosion and pitting corrosion have been studied using potentiodynamic polarization, electrochemical impedance spectroscopy, chronoamperometry, cyclic voltammetry, and scanning electron microscopy (SEM). The pitting potential has been found to shift slightly in the presence of chloride ions (Cl−) in H2SO4. Also, pitting corrosion initiation has been demonstrated in the recorded chronoamperograms as a linear straight line having a positive slope. EEE has reduced the general corrosion and the inhibitor adsorption was found to follow the Langmuir isotherm. SEM micrographs showed that the tested inhibitor has efficiently acted on pitting corrosion for different concentrations of Cl−. Also, the kinetic findings were in good agreement with the surface analysis data. Fourier transform infrared spectroscopy and ultraviolet-visible absorption spectrophotometric measurements provided more insights on the interaction between the chemical functional groups of the inhibitor and the SS surface.
1 Introduction
Corrosion is a natural phenomenon that presents the reactivity of most materials toward the environments to which they are placed, leading the materials to lose their properties and subsequently change their main functionality. This creates a financial burden too important for industrial engineering applications; it costs approximately 5% GDP in most industrial countries (Martinez et al., 2009). Austenitic stainless steels (SS) are a family of unique engineering materials because of their applications in a variety of industries, i.e. petroleum refining, chemical processes, and desalination (Loto et al., 2012a,b; Oguike, 2014). They are the most used materials in industrial processes involving higher temperature and resistance to pitting corrosion. The corrosion resistance of austenitic SS is attributed to the formation of a thin, adherent passive film developed on the surface in most environments (Oguike, 2014). It is generally believed that passivity is due to the formation of a 3D film (usually an oxide film) that impedes the flow of current across the metal-solution interface (MacDonald, 2012). The passive film exists only because of a judicious relationship between the rate of formation and the rate of dissolution of oxide. If any circumstances upset this balance, the passivity is lost and the passive film will be destroyed at some localized areas with a dangerous manifestation of localized corrosion that could lead to unexpected serious failures (MacDonald, 2011). The resistance of SS is generally limited and depends on its elemental composition under exposure to aggressive environments. Despite all its encouraging properties, the resistance of the passive film is often limited in some specific environments (Ait Albrimi et al., 2015). The susceptibility to localized corrosion such as pitting or stress corrosion cracking (SCC) is usually assessed based on chloride content pH and temperature.
Pitting corrosion has an insidious character, where the material can be pitted rapidly in a few days. The difficulty of detection rises from the cover of the pits by the corrosion products, which leads to the failure of the whole engineering systems, although the material presents a minimal weight loss (Ehsani et al., 2011). The creation of pits in most SS structures derives from the breakdown of the passive film created in the presence of a corrosive medium, particularly those containing aggressive ions, namely, halide ions, i.e. chloride, bromide, hypochlorite, and iodide, but fluorides are much less aggressive (Loto, 2013). The chloride ion (Cl−) effect is generally restricted to preventing repassivation rather than promoting breakdown, and the pitting occurs at potentials at which the rate of passivity breakdown is greater than that of passivation (Soltis, 2015). Among the ways of corrosion protection published in the literature, the use of organic inhibitors has gained wide interest among corrosion investigators as an effective alternative for reducing the aggressiveness of acidic media. Organic inhibitors that have polar functions (such as N, O, and S) and heterocyclic compounds with polar groups and π electrons have shown a satisfactory corrosion inhibition (Loto et al., 2012a,b), 2015). This inhibition efficiency is generally linked to the interaction between inhibitor functional groups and the metal surface. Besides that, those organic compounds have gained great interest as an ecofriendly inhibitor and biodegradable (Fouda et al., 2014).
The inhibition of SS localized corrosion using corrosion inhibitors is rarely discussed compared to the prevention by developing new resistant alloys with improved and reinforced metallurgical structure. It has been reported that pitting corrosion could be inhibited with the same technique used to control general corrosion (Loto, 2015). The penetration of aggressive ions through the passive film, also known as penetration mechanism, is the first main step for the passive film breakdown (Soltis, 2015). Typically, the efficient inhibitors acting by adsorption onto the metal surface prevent the migration of the aggressive anions through the oxide to the metal-oxide interface. Refaey et al. (2004) investigated the effect of 2-mercaptobenzoxazole (MBO) on the SS pitting corrosion in acidic medium. The MBO compound has been proven to be an efficient inhibitor for general corrosion and pitting corrosion of 316L SS in HCl solution. Finšgar et al. (2009) have found that pit or crevice corrosive damage of AISI 430 SS in near-neutral chloride media can be best prevented using branched polyethyleneimines molecules. Wei et al. (2004) examined the inhibitory effect of anionic sodium dodecyl sulfate, cationic dodecyl trimethylammonium chloride, and nonionic Triton X-100 MBO as pitting corrosion inhibitors of type 304 SS in neutral sodium medium. Zucchi et al. (1978) assessed the effect of N-N′-diphenylthiourea, di-o-tolylthiourea, benzimidazole-2-thiol, and benzothiazole-2-thiol in inhibiting the SCC of SS in chloride solutions. Observations showed that the tested inhibitors have completely reduced the SCC by blocking the fissuring attack. Huang et al. (1993) examined the effect of some iodides I− and iodine I2 in inhibiting the SCC of AISI 321 SS in 0.5 m l−1 sodium chloride (NaCl)+0.5 m hydrochloric acid (HCl). Results showed that the SCC of AISI 321 SS was inhibited by I− and I2 alone; also, I− was more effective than I2.
There are few studies that focused on the corrosion inhibition of SS in seawater environments where SS undergoes a very serious damage due to the localized corrosion, i.e. pitting and SCC. However, there is no particular interest manifested in preventing the localized corrosion of SS using natural ecofriendly product such plant extracts as corrosion inhibitors. Shabani-Nooshabadi and Ghandchi (2015) examined the inhibitive action of Santolina chamaecyparissus extract as a natural inhibitor on the corrosion of 304 SS in 3.5% NaCl solution. The results revealed that the inhibition efficiency increases with increasing concentration of Santolina and attains the highest inhibition efficiency of 86.9% for 1.0 g l−1. It was reported by Iyasara and Ovri (2013) that molasses could best serve as a corrosion inhibitor for 304L SS in freshwater, seawater, brine, and sulfuric acid (H2SO4) solutions and perform optimally at 0.5 g l−1 concentration.
The literature abounds with studies of SS material failure caused by chloride-induced crevice corrosion in seawater environments. For instance, the majority of failures in seawater desalination plants, i.e. multistage flash and seawater reverse osmosis, have been attributed to the localized attack such as crevice corrosion. The particular emphasis on the need of applying new materials (i.e. UNS S32974 and UNS S31803) that would likely restrict the damage in such environments has gained a wide interest. No studies have examined the corrosion inhibition of chloride-induced crevice corrosion of SS in chlorinated environments using corrosion inhibitors. Instead, the focus has been put on the right selection of the materials and the proper control of environmental conditions that could create an ideal situation for plant operation. Some authors discussed the improvement of crevice resistance of SS cerium ion implantation. Cerium ion implantation has improved the crevice corrosion resistance of UNS S31603 SS by inhibiting the cathodic kinetics of the reduction of oxygen and protons.
The present work deals with the inhibitory effect of Euphorbia echinus extract (EEE) on the corrosion behavior of SS in H2SO4 (1 m H2SO4) containing Cl− (400 ppm Cl−). The corrosion behavior of SS in chlorinated acid was investigated using potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) and the surface morphology was also characterized using scanning electron microscopy (SEM). First, we evaluated the effect of the Cl− on the pitting corrosion of SS by determining the pitting potential shift. Subsequently, the effect of the inhibitor on both general corrosion and pitting corrosion has been studied.
2 Materials and methods
The SS specimens employed in this study were AISI 321 SS having the chemical compositions of 69.10% Fe, 17.58% Cr, 9.01% Ni, 2.19% C, 0.54% Si, and 0.39% Ti. Samples were cut into a rectangular shape with dimensions of 1.2×0.8 cm, and then specimens were sealed by epoxy resin leaving an exposed area of 0.96 cm2. The pretreatment of samples consisted of mechanical polishing of exposed area with silicon carbide SiC abrasion papers of 500, 1200, 1500, and 2000 grits, and the samples were then washed with distilled water, rinsed with acetone, and dried in air room temperature for use in electrochemical cells.
The aggressive solution (1 m H2SO4) was prepared by diluting an analytical 97% H2SO4 with distilled water. Chlorinated acid solutions were prepared by the dissolution of NaCl in H2SO4 (1 m) with a concentration range of 100–10,000 ppm Cl−. Euphorbia echinus plant was collected from the anti-Atlas region of Arbâa Sahel-Tiznit, Morocco. The voucher specimen number (10050) was deposited at the herbarium of Cadi Ayyad University. Euphorbia plant is cleaned and then chopped into small pieces and dried in open air without sunlight and powdered. The mixture of 10 g of the plant powder and 200 ml ethanol was stirred for 24 h and then filtered and put in a vacuum rotary evaporator for use as the concentrated inhibitor.
A three-electrode conventional cell consisting of an SS working electrode, a platinum counter electrode, and a saturated calomel electrode (SCE) as a reference electrode was used for measurements. All experiments were performed under stirring at constant temperature by using a bath thermostat. The electrochemical measurements were carried out using laboratory potentiostat (voltalab PGZ 301). Polarization curves were recorded at a constant sweep rate of 1 mV s−1 and the scanning range was from −600 mV vs. SCE to −100 mV vs. SCE. Before running each experiment, the open circuit potential (OCP) of SS was studied versus time until a steady state was attained. Impedance measurements were performed from 100 kHz to 0.01 Hz with a sinusoidal perturbation of 10 mV using AC signals at OCP. The measurements were repeated three times for each condition for reproducibility.
3 Results and discussion
3.1 Effect of Cl− on the corrosion behavior of SS in H2SO4
For insights into evaluating the effect of Cl− on the pitting corrosion susceptibility, potentiodynamic polarization measurements of SS behavior in H2SO4 were performed for different concentrations of Cl− in the potential range of −0.6 V vs. SCE to 1.8 V vs. SCE at 323 K. All curves presented in Figure 1 display the same characteristics of dissolution and passive and transpassive regions followed by oxygen evolution. The identical shape of the potentiodynamic polarization curves over the potential scan suggests the same reaction kinetics. The broad peak at 1.5 V vs. SCE is most likely attributed to the Cr(III) oxidation to Cr(VI) responsible for the Cr2O3 dissolution in the passive film. The presence of Cr(III) oxide being oxidized to Cr(VI) leads most likely to the pit initiation due to the increase in the local acidity as described by this equation:
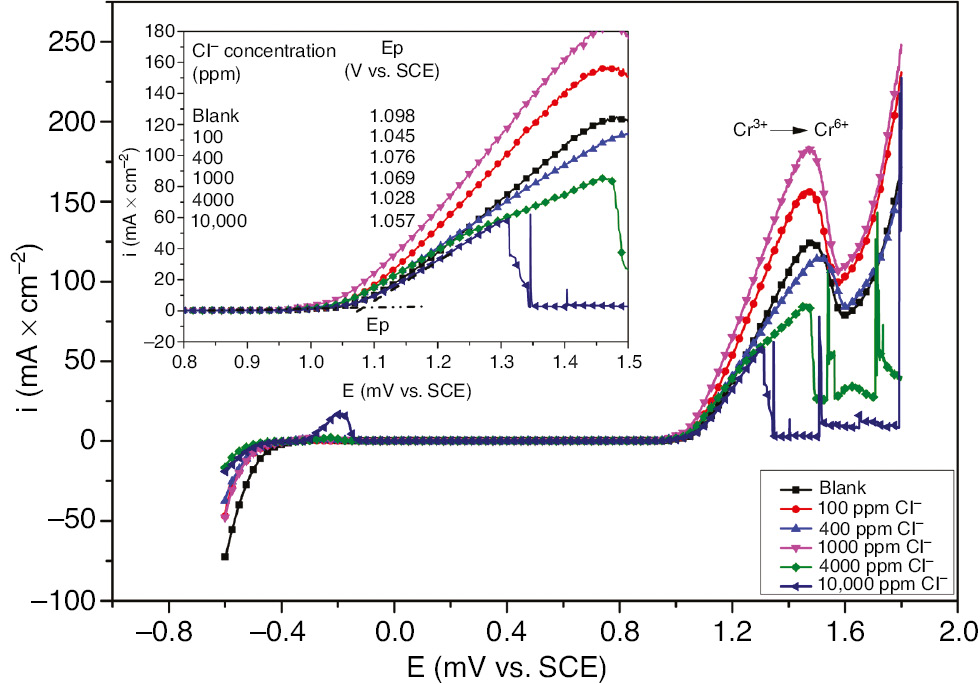
Potentiodynamic polarization curves for 321 SS in 1 m H2SO4 containing different concentrations of Cl− at 323 K.
From this fact, the pitting potential of SS could be determined as the onset of the abrupt increase in current density denoting the oxidation of Cr3+ to Cr6+. It is clearly seen in Figure 1 that the presence of Cl− slightly decreases the pitting potential. The maximum potential shift has been observed in the case of 4000 ppm Cl−. Even for the slight decrease in pitting potential, the presence of Cl− apparently promotes the susceptibility of the passive film breakdown and pitting corrosion. Thompson and Syrett (1992) pointed out the different factors of pit initiation, including inhomogeneity in the metal, acidity levels in microscopic, and chloride incorporation into localized areas of the passive film.
In general, the pitting corrosion occurs in two stages: the initiation and the propagation of the pits. In most cases, the initiation of the pit is mostly due to the material defects or the presence of the aggressive anions such as halide ions. Many authors have focused on the formation of an intermediate complex between the metal surface and the Cl−; subsequently, the adsorption of Cl− ions on the intermediate complex stimulates the pit initiation. The mechanism of Zhang and Zhang (1993) describes the process of the metal local dissolution in the presence of the sulfate and Cl− by considering that only H2O-M-OH2 and HO-M-OH form the passive film. The following reactions will take place on the passive film:
3.2 Effect of different concentrations of EEE on the corrosion behavior of SS in H2SO4
3.2.1 Potentiodynamic polarization measurements
The anodic and cathodic polarization curves of the electrochemical influence of EEE on the SS dissolution in H2SO4 (H2SO4+400 ppm Cl−) are shown in Figure 2. The evaluated parameters of Tafel polarization curves including corrosion potential (Ecorr), corrosion current density (icorr), cathodic Tafel constant (βc), and inhibition efficiency (η) are presented in Table 1. The corrosion current density (icorr) was obtained by extrapolating the linear segments of the cathodic Tafel curve to the corrosion potential (Ecorr). The inhibition efficiency η (%) was calculated by
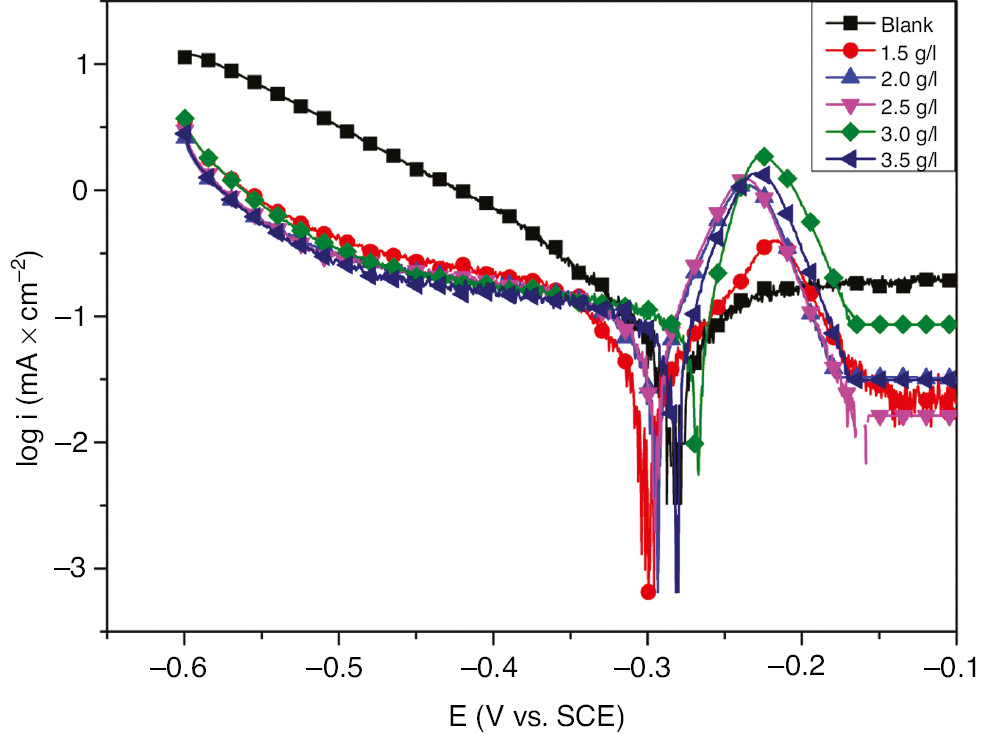
Tafel polarization curves for 321 SS in (1 m H2SO4+ 400 ppm Cl−) in the absence and presence of different concentrations of EEE at 293 K.
Potentiodynamic polarization parameters and corresponding inhibition efficiency for the corrosion of 321 SS at 293 K.
| Concentration (g l−1) | E corr (mV vs. SCE) | i corr (μA cm−2) | βc (mV dec−1) | η (%) |
|---|---|---|---|---|
| Blank | −355 | 269 | −143 | – |
| 1.5 | −300 | 106 | −359 | 60 |
| 2 | −295 | 91 | −329 | 66 |
| 2.5 | −296 | 91 | −305 | 66.20 |
| 3 | −269 | 86 | −360 | 67 |
| 3.5 | −282 | 70 | −226 | 74 |
where icorr and
From the results shown in Figure 2 and Table 1, it is evident that EEE acted significantly on both anodic and cathodic processes by decreasing their current densities. The addition of the EEE reduced significantly the corrosion current density. It is clearly observed that the inhibition efficiency of EEE increases by the increase of inhibitor concentration, and the maximum inhibition efficiency was observed at 3.5 g l−1 EEE. Furthermore, the decreased value of icorr in the presence of inhibitors particularly at higher inhibitor concentration is mainly attributed to the adsorption on inhibitor molecules over the SS surface. Also, it is clearly seen that the corrosion potential shows a positive shift with the addition of EEE. Generally, the inhibitor type is determined by its Ecorr displacement; if the value of Ecorr for inhibited sample is displaced more than 85 mV with respect to the blank, then the inhibitor can be categorized as anodic or cathodic type (Loto et al., 2015). However, if the shift in the Ecorr value is less than 85 mV, then the inhibitor can be categorized as mixed-type inhibition; thereby, EEE acts as a mixed-type inhibitor with an anodic character. The Tafel slopes of the cathodic reaction βc vary slightly with the change in the concentration of the inhibitor.
3.2.2 EIS measurements
EIS is used to investigate the corrosion behavior of SS in aggressive acid (1 m H2SO4+400 ppm Cl−) in the absence and presence of the EEE and to explore even more the metal-solution processes. All experiments were conducted at OCP and at 293 K.
The inhibition efficiency was calculated by the equation as follows:
where Rct and
The Nyquist plots of SS in H2SO4 with and without various concentrations of EEE are given in Figure 3. The parameters derived from EIS measurements and the corresponding inhibition efficiencies are listed in Table 2. As shown in Figure 3, all EIS graphs had two time constants; a well-defined capacitance loop at higher frequencies arises from the charge-transfer resistance and the electric double layer followed by a Warburg tail at lower frequencies. The presence of the Warburg time constant can be linked to ion diffusion through the passive film. Such semi-infinite Warburg diffusion process may also confirm that the mass transport is limited by the surface passive film (Li et al., 2014) and thereby indicates that both charge transfer and diffusion processes are controlling the corrosion mechanism. It is observed that Warburg tail tends to curve over the real axis with intersection at sufficiently lower frequencies. These diffusion tails and arcs were found to bear a close relationship with anomalous diffusion of the electroactive species (Ehsani et al., 2011). EIS spectra presented in Figure 3 are described by a simple equivalent circuit as shown in Figure 4, and all the circuit components were determined by the impedance fitting procedure. Rs is the solution resistance, Rct is the charge transfer resistance, Cdl is the double layer capacitance, and W is the Warburg impedance.
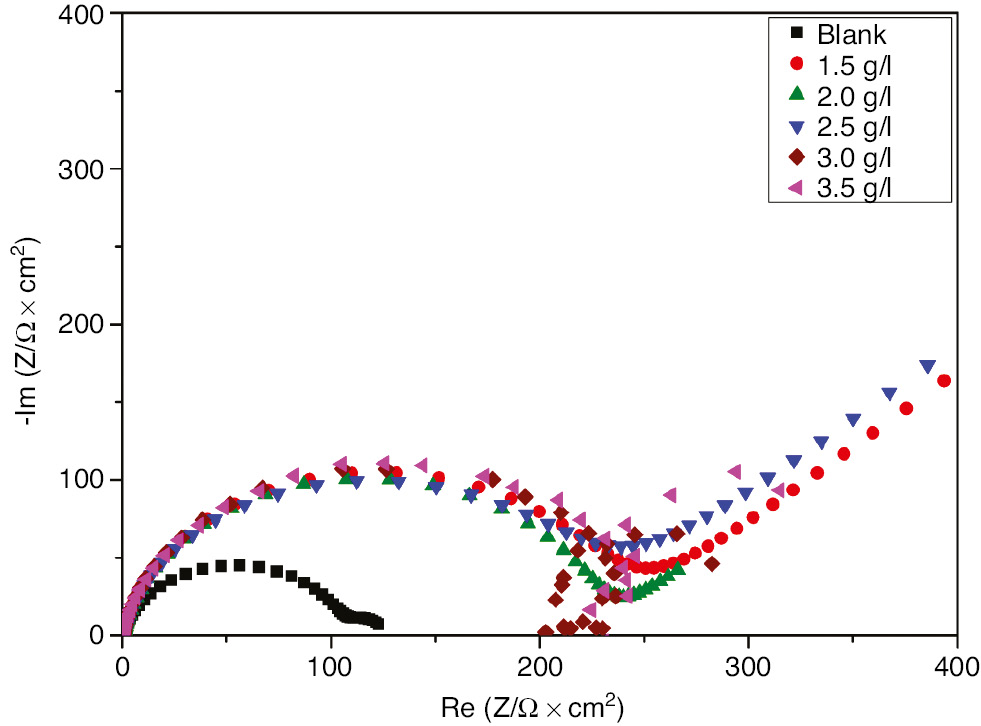
Nyquist plots for 321 SS in 1 m H2SO4+400 ppm Cl− in the absence and presence of different concentrations of EEE at 293 K.
EIS parameters and corresponding inhibition efficiency for the corrosion of 321 SS at 293 K.
| Concentration (g l−1) | R ct (Ω cm2) | C dl (μF cm−2) | η (%) |
|---|---|---|---|
| Blank | 106 | 297 | – |
| 1.5 | 222 | 113 | 51.84 |
| 2 | 247 | 101 | 56.77 |
| 2.5 | 252 | 126 | 57.64 |
| 3 | 254 | 125 | 58.00 |
| 3.5 | 256 | 98 | 58.30 |
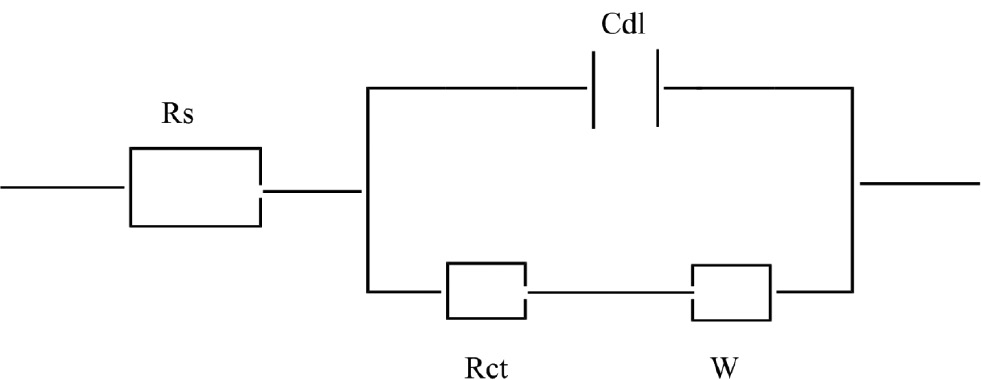
Equivalent circuit used to model impedance data for 321 SS.
Compared to the blank, the larger capacitive loop in the inhibited solution indicates the adsorption of inhibitor compounds on the SS surface. From the results depicted in Table 2 and Figure 3, it is evident that the charge transfer resistance increases as the concentration of EEE increases, whereas the double layer capacitance was found to decrease as the inhibitor concentration increases. The lower Cdl values in the inhibited solution confirm the enhancement of the adsorption of the inhibitor on the SS surface (Seifzadeh, 2014). The decrease in Cdl is attributed to an increase in the thickness of the electronic double layer due to the adsorption of EEE organic compounds. Furthermore, Bode diagrams presented in Figure 5 show clearly the presence of two time constants as well as the impedance increases with increasing inhibitor concentrations at low frequencies. All the electrochemical parameters clearly propose that the corrosion control depends on the concentration of the inhibitor and its addition increases the impedance but does not change the other aspects of the SS behavior in aggressive medium. It is clearly observed that both EIS and polarization measurements show the same trend, only the difference in the inhibition efficiency may be due to the surface status, and impedance tests were run at the last reached OCP value, whereas in potentiodynamic measurements the electrode was polarized at higher potential.
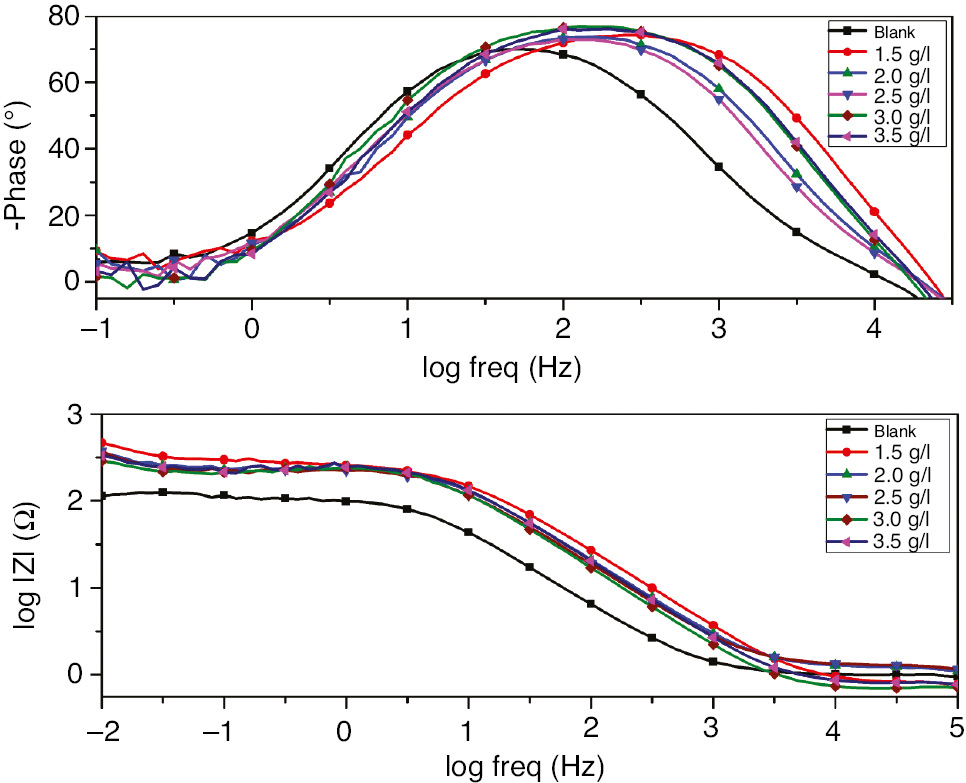
Bode diagrams for 321 SS in 1 m H2SO4+400 ppm Cl− in the absence and presence of different concentrations of EEE at 293 K.
3.2.3 Cyclic voltammetry
To study the effect of EEE on the pitting corrosion of SS, cyclic voltammograms for SS in 1 m H2SO4+400 ppm Cl− have been recorded in the absence and presence of different concentrations of EEE. Figure 6 shows that the pitting potential has been obtained as the onset of the peak observed at 1 V corresponding the anodic oxidation of Cr(III) to Cr(VI). The obtained data depicted in Figure 6 demonstrates that the pitting potential remains almost the same regardless of the tested concentration of the inhibitor. Therefore, the cyclic voltammetry method shows that EEE has almost no effect on the pitting corrosion.
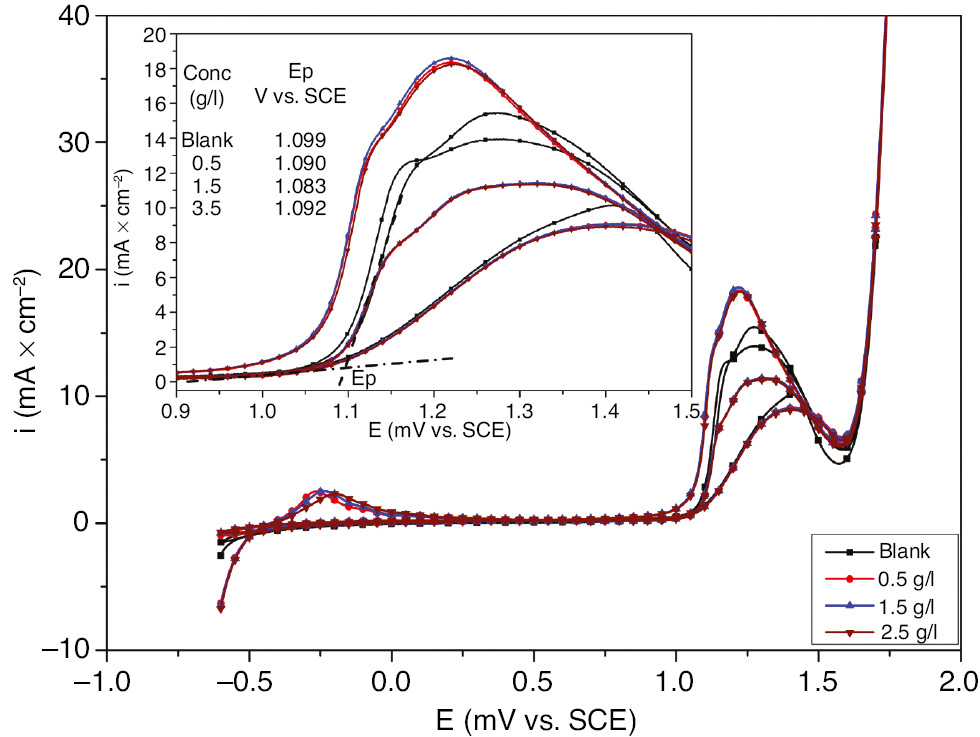
Cyclic voltammograms for 321 SS in 1 m H2SO4+400 ppm Cl− in the absence and presence of different concentrations of EEE at 293 K.
3.2.4 Current time potentiostatic transients
To describe the kinetic behavior of SS passivation and pitting, chronoamperometric transients were recorded by polarizing the working electrode at 1000 mV vs. SCE in 1 m H2SO4+400 ppm Cl− in the absence and presence of 3.5 g l−1 inhibitor. To study the early stages of passive film growth and pitting, the current time curves were plotted in logarithmic scale as presented in Figure 7 (inset).
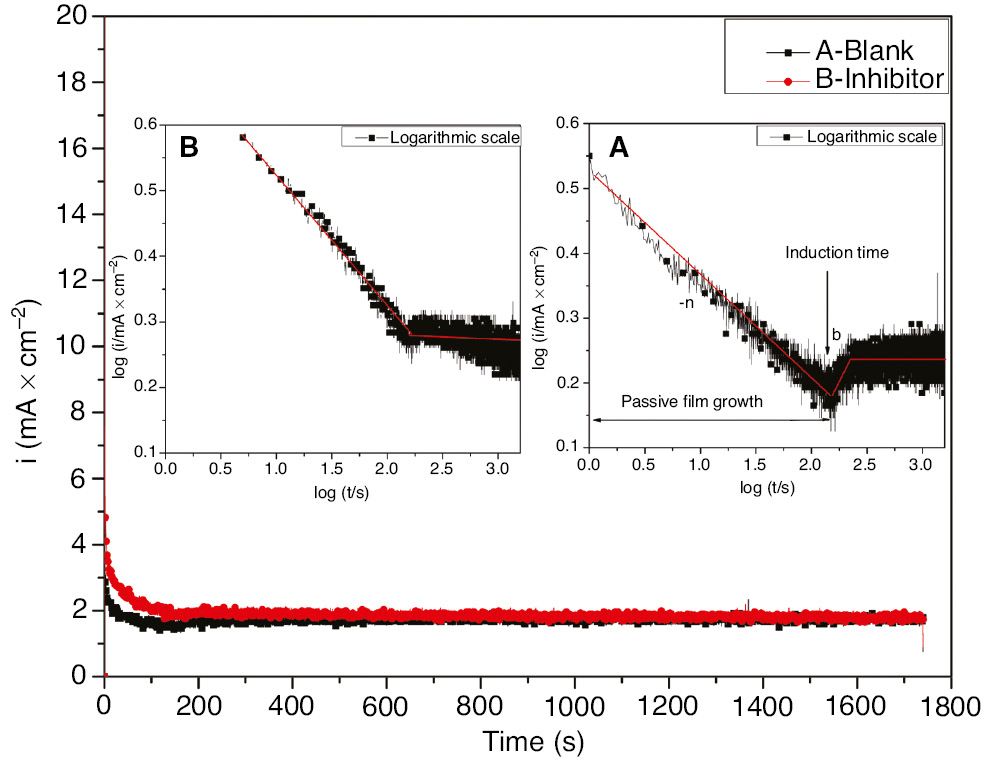
Current time transients for 321 SS polarized for 1 h at 1000 mV vs. SCE in 1 m H2SO4+400 ppm Cl− in the absence and presence of 3.5 g l−1 EEE at 293 K.
For both cases, the current density decrease with time is related to the passive film growth as given by the MacDonald model [Eq. (19; Chen et al., 2009]. In the absence of the inhibitor, the current density decreases rapidly to a minimal current density value corresponding to the induction time at which the initiation of pitting corrosion starts. The continuous increase in current density taking place after the induction time is indicative of the pitting initiation as described by the Engell stollica model [Eq. (18; Albrimi et al., 2011]:
where B and b are constants that depend on the anodic applied potential of polarization and the solution concentration. b corresponds to the rate of pitting initiation.
where A and n are constants that depend on the constant anodic potential and the electrolyte concentration. The n constant yields the rate of the passive film growth and is given by the first slope of the descending current density as depicted in Figure 7. However, in the presence of the inhibitor, the pitting initiation domain has been reduced and no clear current density increase has been observed. From this fact, it could be stated that the tested inhibitor acts on the pitting initiation. A possible explanation for this observation is that the adsorption of the inhibitor on the metal surface blocks the penetration process that is the main step responsible for passivity loss induced by the pitting corrosion. As further inspection of Figure 7, for both cases, the steady value attained after the initiation of the metastable pits corresponds to the repassivation of the passive film. This fact indicates that no pitting growth could be seen as no second linear straight line with a different slope has been observed after the initiation stage.
3.2.5 Adsorption isotherm
The inhibition efficiency of a corrosion inhibitor is related mainly to its adsorption nature on the metal surface; hence, the study of the adsorption isotherms can provide further data on the nature of the interaction between the inhibitor polar functions and the metal surface and could therefore give precious information on the inhibition mechanism (Idouhli et al., 2018a,b). The graphical study of adsorption process was done by fitting the surface coverage and the inhibitor concentration to different adsorption isotherms, i.e. Langmuir, Freundlich, and Temkin. The plot of C/θ against C gives a linear plot as shown in Figure 8 with a correlation coefficient close to unity (R2=0.997), suggesting that the adsorption of E. echinus compounds on the SS surface is consistent with the Langmuir isotherm. Moreover, the Langmuir isotherm is based on the assumptions that the inhibitor adsorption is a monolayer with no lateral interaction between the adsorbed species (Karthik et al., 2014).
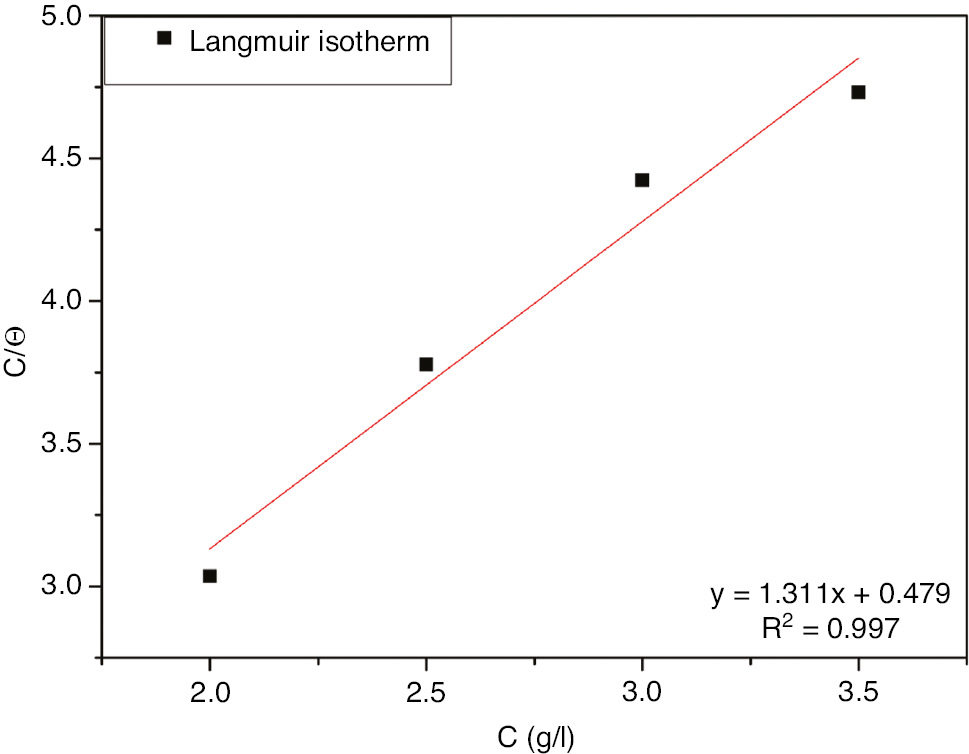
Langmuir adsorption isotherm plots for 321 SS in 1 m H2SO4+400 ppm Cl− with different concentrations of EEE at 293 K.
The adsorption process of the inhibitor on the metal surface is considered as a substitution between water molecules and the organic molecules of the inhibitor (Lopes-Sesenes et al., 2012).
where Orgsol and Orgads are the organic molecules of the inhibitor dissolved in the aqueous solution and adsorbed onto the metallic surface, respectively. H2Oads is the water molecules adsorbed on the metal surface and n is the ratio that represents the number of water molecules replaced by a single inhibitor organic molecule (Umoren et al., 2013).
The surface coverage (θ) is calculated using the following relation:
The Langmuir isotherm is given by the following relation:
Temkin isotherm:
Freundlich isotherm:
where θ is the surface coverage, Cinh is the concentration of the inhibitor, and Kads is the standard adsorption equilibrium constant that related to the standard free energy of adsorption
where 55.5 is the molar concentration of water in the solution, R is the gas equilibrium constant, and temperature T is 293 K.
To describe in more detail the adsorption process, the standard free energy (ΔG°) was determined according to Eq. (26) and presented in Table 3. The negative sign of ΔG° values suggest the spontaneous adsorption of EEE on SS surface (Loto, 2013). In contrast, the calculated ΔG° values are more than −20 kJ mol−1, indicating the physical interaction between the charged molecules and the charged metal (Idouhli et al., 2016).
Adsorption isotherms parameters for 321 SS in 1 m H2SO4+400 ppm Cl− with different concentrations of EEE at 293 K.
| Isotherm | R 2 | Slope | Intercept | K ads |
|
|
|---|---|---|---|---|---|---|
| Langmuir | 0.997 | 1.311 | 0.479 | 2.0876 | – | −11.573 |
| Temkin | bT | |||||
| 0.873 | 0.101 | 0.571 | 1.0056 | 24.107 | −9.794 | |
| Freundlich | n | |||||
| 0.866 | 0.158 | −0.240 | 0.5754 | 0.158 | −7.435 |
3.2.6 Thermodynamic parameters for the corrosion process
The temperature factor has a tremendous effect on the inhibitor-metal interaction; it is well known that the variation in the medium temperature may considerably promote the change in the inhibition mechanism such as the adsorption of the inhibitor and its stability in the aggressive medium (Idouhli et al., 2018a,b). For this reason, polarization measurements were performed at different temperatures. It is clearly shown in Table 4 that current density increases significantly with the rise of temperature for both inhibited and uninhibited solutions. We also note that the inhibition efficiency depends on the temperature and decreases with the rise of temperature from 293 to 323 K, indicating that a higher temperature dissolution of SS predominates on the adsorption of EEE at the metal surface. This can be explained by the decrease of the strength of the adsorption process at high temperature and can suggest a physical adsorption mode (Daoud et al., 2015).
Temperature effect on the polarization measurements parameters for 321 SS in 1 m H2SO4+400 ppm Cl− in the absence and presence of 3.5 g l−1 EEE at 293 K.
| Inhibitor | T (K) | E corr (mV vs. SCE) | i corr (μA cm−2) | η (%) |
|---|---|---|---|---|
| Blank | 293 | −355 | 269 | – |
| 303 | −345 | 212 | – | |
| 313 | −336 | 534 | – | |
| 323 | −360 | 706 | – | |
| Inhibitor | 293 | −282 | 70 | 74.00 |
| 303 | −349 | 90 | 57.36 | |
| 313 | −344 | 332 | 37.68 | |
| 323 | −341 | 359 | 49.17 |
For a better understanding of the inhibition process, the activation energy parameter was determined from Arrhenius equation:
where Ea is the apparent activation energy of the corrosion reaction, R is the gas constant, T is the absolute temperature, and A is the Arrhenius pre-exponential factor. Figure 9 presents the evolution of ln(icorr) against 1/T in the absence and presence of inhibitor. The linear regression allows us to determine the slope (−Ea/R) of the straight line, which enables to determine the apparent activation energy for both cases.
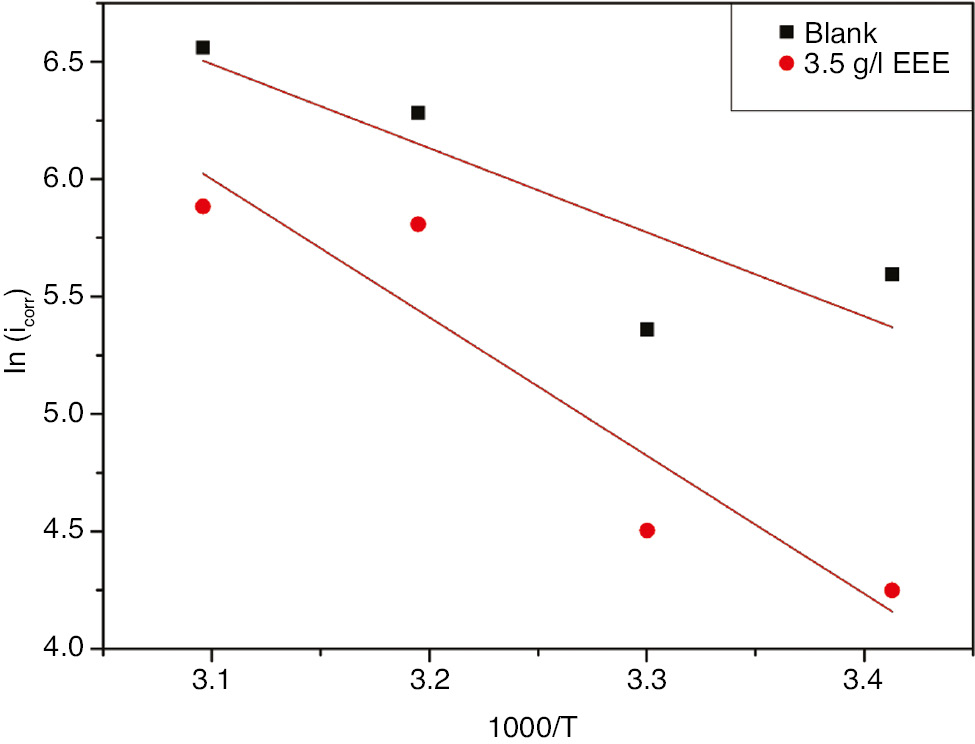
Arrhenius plots for 321 SS in 1 m H2SO4+400 ppm Cl− in the absence and presence of 3.5 g l−1 EEE at 293 K.
The analysis of Table 5 shows that the magnitude of apparent activation energy for the inhibited medium (46.32 kJ mol−1) is much greater than its value in the absence of the inhibitor. The increase value of Ea in the presence of the inhibitor with respect to the blank may be due to a physical adsorption.
Corrosion kinetic parameters for 321 SS in 1 m H2SO4+400 ppm Cl− in the absence and presence of 3.5 g l−1 EEE at 293 K.
| E a (kJ mol−1) |
|
|
|
|
|---|---|---|---|---|
| Blank | 29.73 | 27.18 | −107.24 | 2.55 |
| Inhibitor | 48.87 | 46.32 | −51.98 | 2.55 |
According to Szauer and Brandt (1981), the activation energy value increase could be assigned to an appreciable decrease in the adsorption of the inhibitor on the metal surface that occurs with the increase of temperature. Due to the fact that the desorption process is in equilibrium with the adsorption of molecules on the metal surface, as adsorption decreases, more desorption process of inhibitor molecules happens, subsequently leading to more dissolution with the increase in temperature. Furthermore, the whole process was controlled by the surface reaction as the energy of the activation corrosion process in both absence and presence of inhibitor was greater than 20 kJ mol−1 (Herrag et al., 2010).
The enthalpy and entropy of activation
where h is Planck’s constant and N is Avogadro’s number.
Figure 10 shows a plot of ln(icorr/T) versus 1/T. Straight lines are obtained with a slope of
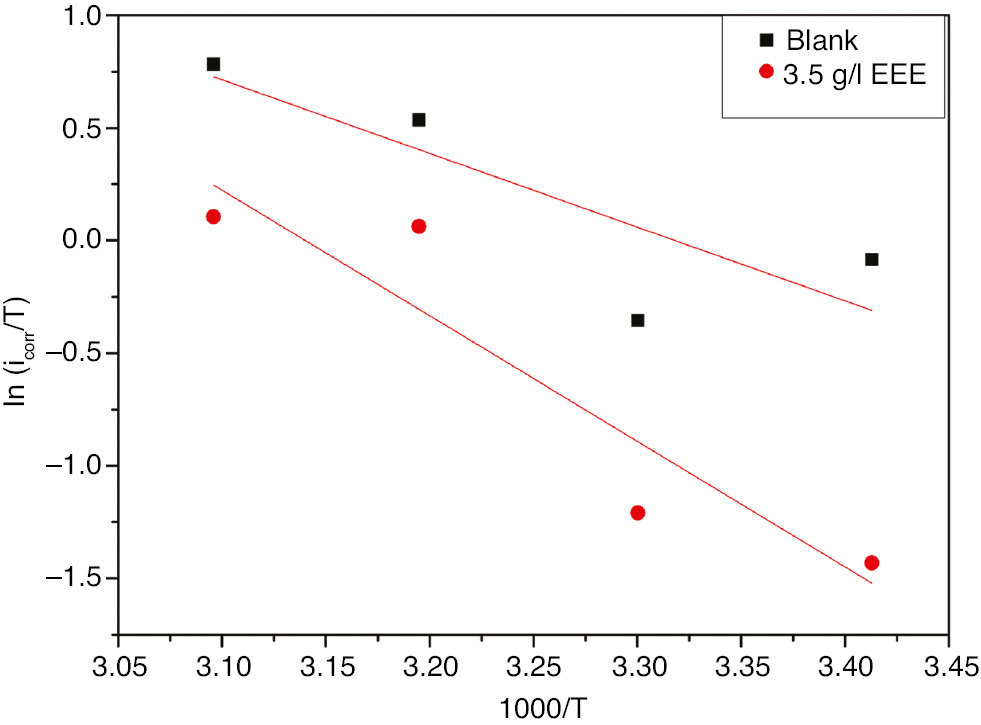
Transition-state plots for 321 SS in 1 m H2SO4+400 ppm Cl− in the absence and presence of 3.5 g l−1 EEE at 293 K.
3.2.7 Fourier transform infrared spectroscopy (FTIR) analysis
To improve our knowledge about the inhibition provided by the crude extract constituents of E. echinus plant, spectral analyses were made using a vertex 70/70v FTIR spectrometer. Infrared spectroscopy is a valuable method for the detection and identification of chemical functional groups; the FTIR spectra were recorded in the range of 400–4000 cm−1. Figure 11 shows the FTIR spectrum of the EEE powder. The assignments of vibrational bands are achieved by comparing the band positions observed in IR spectra to the wave numbers of the studied known pure compounds in the literature. The spectra can be assigned generally as in the following:
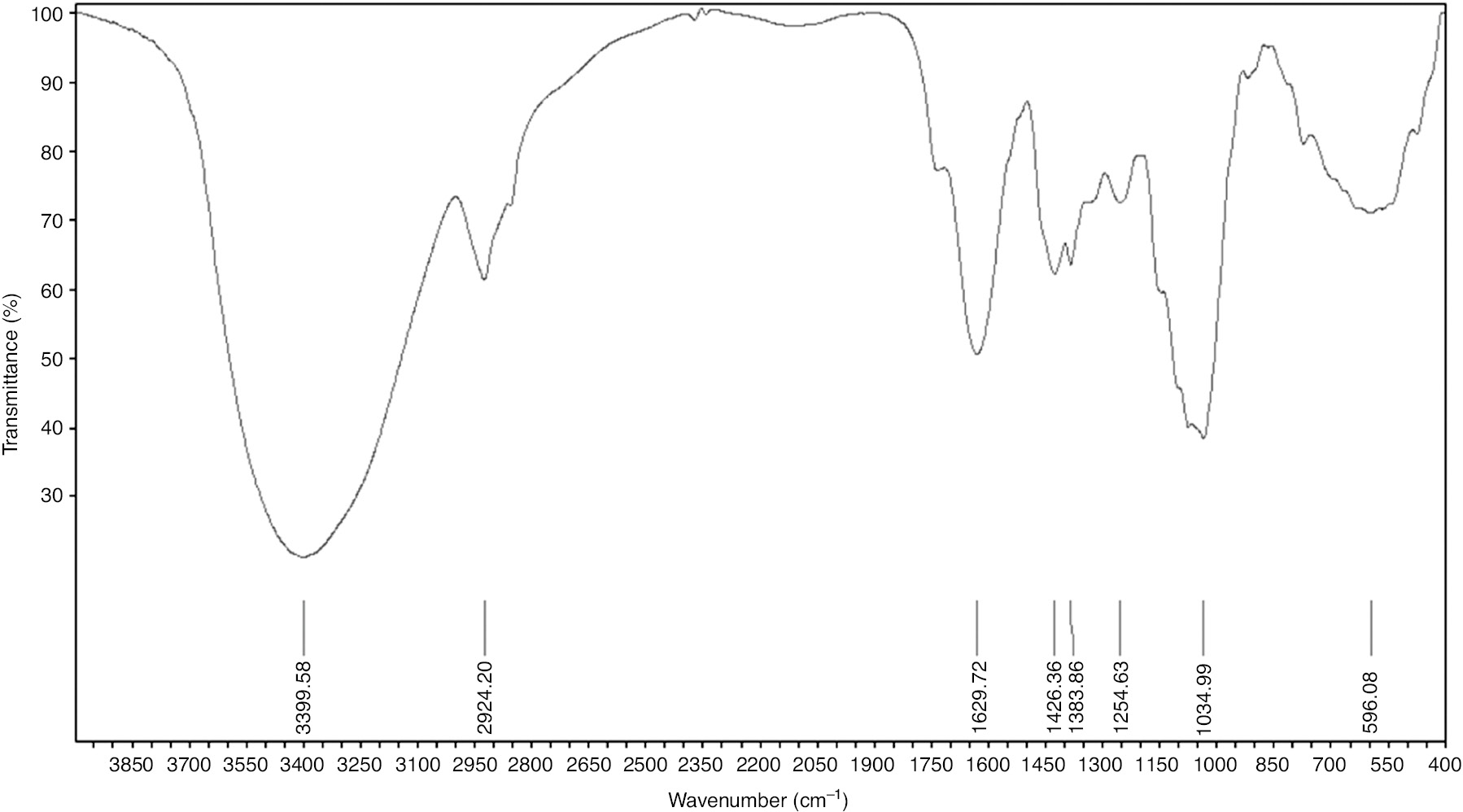
FTIR spectra of E. echinus powder.
The strong band at 3399 cm−1 corresponds to N-H and O-H stretching, the band at 2924 cm−1 is attributed to an -CH2 asymmetrical stretching vibration, and the strong band at 1926 cm−1 is related to C=O stretching vibration. The peaks at 1426 and 1383 cm−1 correspond to C=C and N-O stretch, respectively. The band at 1254 cm−1 is the C-O stretch and the vibrational bands at about 1034 and 596.08 cm−1 correspond to C-N and C=C-H stretching. Based on the assignment of the FTIR spectra, it is observed that the extract tested meets the structural criteria of green corrosion inhibitors that have been widely reported and justifies the inhibition provided.
3.2.8 Analysis of ultraviolet (UV)-visible absorption spectra
To study the metal-inhibitor interaction, UV-visible analysis was conducted. The UV-visible spectra of EEE solution without and with the exposed SS are shown in Figure 12. As can be observed, the absorption spectra exhibit a single peak at 280 nm with a higher change in the absorbance maximum for the EEE solution resulting from SS immersion. The increase in absorption intensity indicates the formation of the Fe2+-inhibitor complex (Abuthahir et al., 2014) and subsequently corroborates the efficiency of the inhibitor.
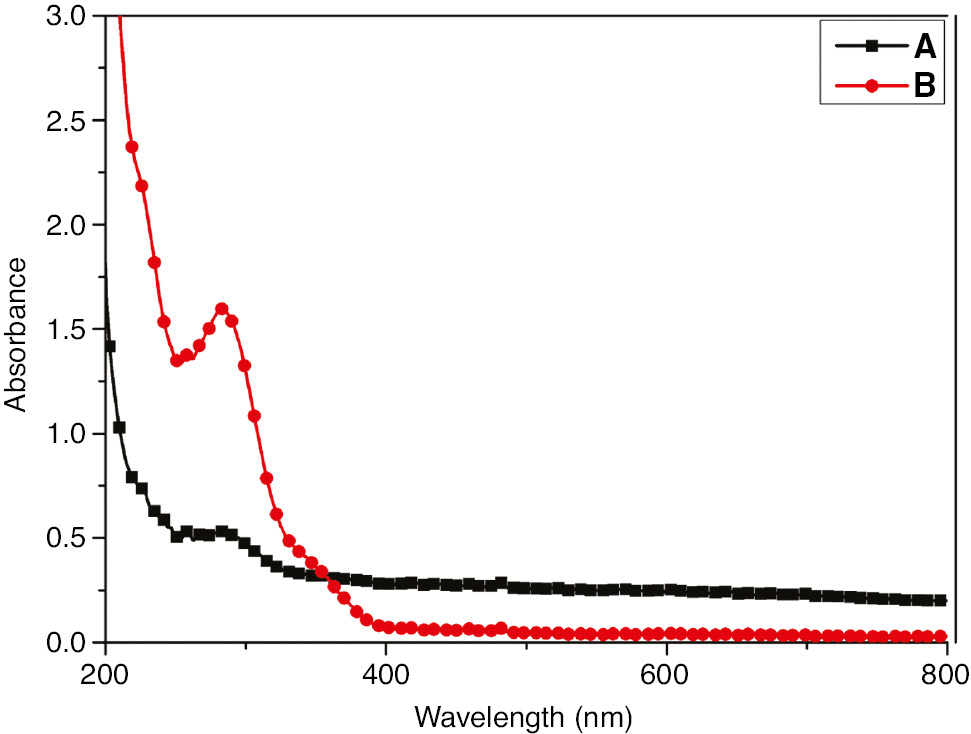
UV-visible absorption spectra of (A) solution containing EEE and (B) solution containing EEE-SS.
3.2.9 SEM-energy-dispersive X-ray (EDX) analysis
Using SEM-EDX analyses, the chemical composition and morphology of the SS surface have been studied for the electrode polarization at the critical value of 1000 mV vs. SCE in H2SO4 containing different concentrations of Cl− in the absence and presence of inhibitor. The EDX elemental analysis of the weight percentage of the SS alloying elements obtained in different condition is summarized in Table 6. It is clearly observed from the SEM micrographs (Figure 13) that pitting frequency increases with the increase in Cl− concentration. In the presence of 10,000 ppm Cl−, the SS surface appears to be highly pitted with the maximum number of pits. It is worthwhile noting that even for lower concentrations some localized pits could be observed on SEM micrographs. It should be added that for lower concentration most nucleated pits die immediately and do not apparently produce metastable pits. After the addition of 3.5 g l−1 inhibitor, the SS surface is shown to be less pitted for all Cl− concentrations. Table 6 shows that the carbon content increases with the addition of inhibitor. These facts could be justified by the inhibitor adsorption onto the metal surface. These findings substantiate the claim that pitting initiation could be blocked in the presence of EEE as described earlier using current time transients.
Weight percentage of 321 SS alloying elements for 24 h immersion in H2SO4 without and with inhibitor and different concentrations of Cl− at 293 K.
| Element | Freshly polished SS | 400 ppm Cl− | 1000 ppm Cl− | 10,000 ppm Cl− | 400 ppm Cl−+3.5 g l−1 EEE | 1000 ppm Cl−+3.5 g l−1 EEE | 1000 ppm Cl−+3.5 g l−1 EEE |
|---|---|---|---|---|---|---|---|
| C | 2.19 | 3.36 | – | – | 6.88 | 13.62 | 13.60 |
| O | 1.19 | 1.72 | – | 2.95 | – | – | – |
| Cr | 17.58 | 16.81 | 20.45 | 19.68 | 18.70 | 17.63 | 18.01 |
| Fe | 69.10 | 66.34 | 69.87 | 68.19 | 65.47 | 60.27 | 60.12 |
| Ni | 9.01 | 9.76 | 9.68 | 9.17 | 8.95 | 8.48 | 8.50 |
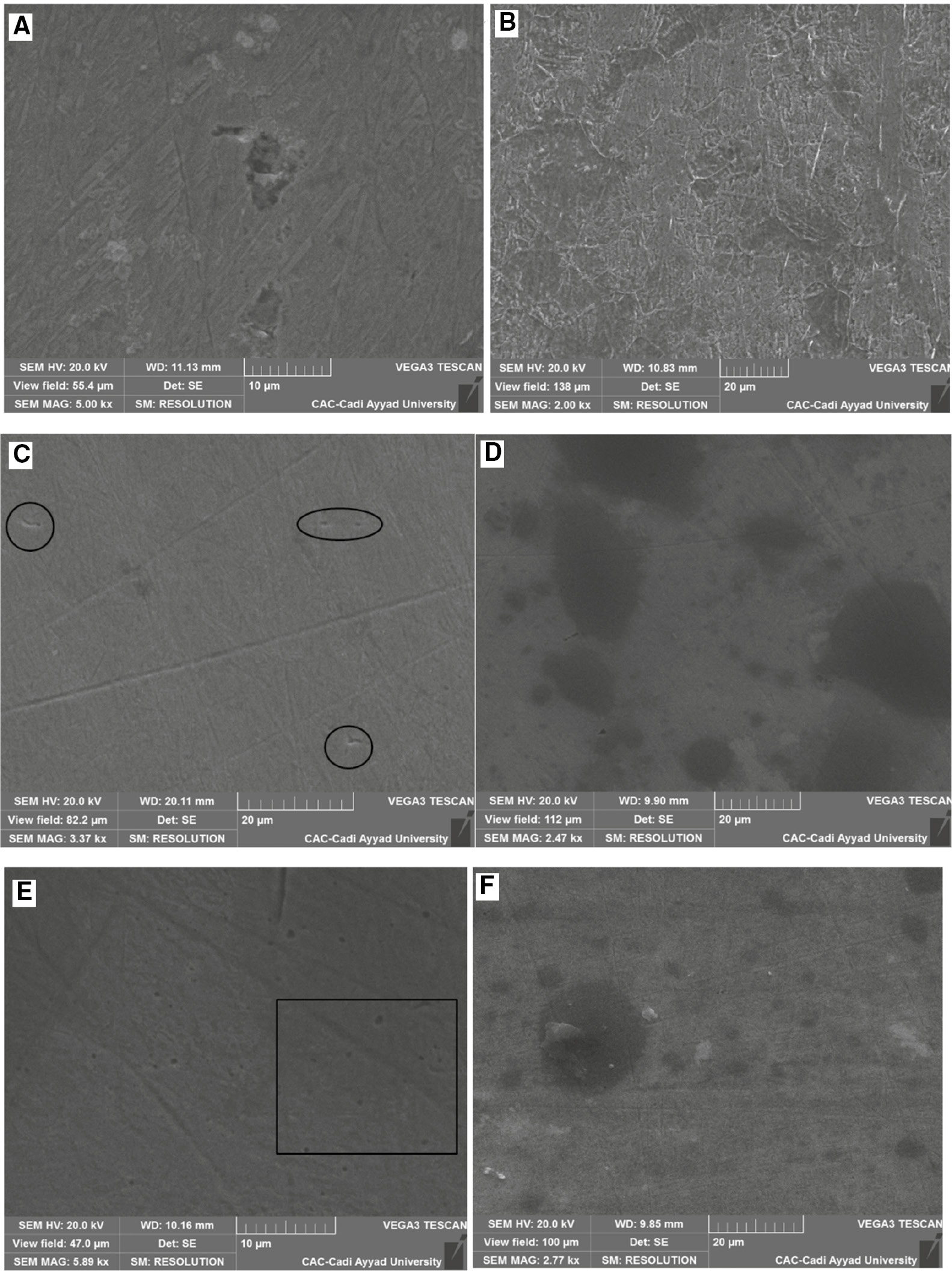
SEM images of polarized 321 SS in H2SO4 containing (A) 400 ppm Cl−, (B) 400 ppm Cl−+3.5 g l−1 EEE, (C) 1000 ppm Cl−, (D) 1000 ppm Cl−+3.5 g l−1 EEE, (E) 10,000 ppm Cl−, and (F) 10,000 ppm Cl−+3.5 g l−1 EEE at 293 K.
4 Conclusion
The general corrosion and pitting corrosion of SS in chlorinated H2SO4 were studied by combining the stationary and transient electrochemical techniques as well as the surface examination. Potentiodynamic polarization shows that the presence of Cl− has slightly decreased the pitting potential. Further, the tested inhibitor has been found to reduce the general corrosion in chlorinated H2SO4 and the inhibition efficiency increases as the inhibitor concentration increases to attain about 74% at 3.5 g l−1. Chronoamperometric measurements have shown the initiation of pitting corrosion and its blocked kinetic in the presence of the inhibitor. In contrast, the inhibitor adsorption on the SS surface was found to follow the Langmuir isotherm. FTIR and UV-visible analysis have confirmed the interaction between the inhibitor organic molecules and the SS surface. Surface analysis revealed the SS pitting in the presence of Cl− and the increased pitting frequency at higher chloride concentrations. The chronoamperometry findings correlate favorably with SEM observations and substantiate the inhibitor effect in reducing the pitting corrosion in 321 SS.
Acknowledgments
The authors gratefully acknowledge the help provided by the Center of Analysis and Characterization (CAC) at the Cady Ayyad University (Marrakech, Morocco).
References
Abuthahir SSS, Nasser AJA, Rajendran S. Inhibition of mild steel corrosion by 1-(8-hydr-oxyquinolin-2-ylmethyl)urea. Eur Chem Bull 2014; 3: 40–45.Search in Google Scholar
Ait Albrimi Y, Eddib A, Douch J, Berghoute Y, Hamdani M, Souto RM. Electrochemical behavior of AISI 316 austenitic stainless steel in acidic media containing chloride ions. Int J Chem Sci 2011; 6: 4614–4627.10.1016/S1452-3981(23)18352-0Search in Google Scholar
Ait Albrimi Y, Ait Addi A, Douch J, Souto RM, Hamdani M. Inhibition of the pitting corrosion of 304 stainless steel in 0.5 M hydrochloric acid solution by heptamolybdate ions. Corros Sci 2015; 90: 522–528.10.1016/j.corsci.2014.10.023Search in Google Scholar
Chen Y, Lobo RFM, Santos DMF, Sequiera CAC. Kinetic measurements during transient film growth on zinc. Quim Nova 2009; 32: 387–390.10.1590/S0100-40422009000200022Search in Google Scholar
Daoud D, Douadi T, Hamani H, Chafaa S, Al-Noaimi M. Corrosion inhibition of mild steel by two new heterocyclic compounds in 1 M HCl: experimental and computational study. Corros Sci 2015; 94: 21–37.10.1016/j.corsci.2015.01.025Search in Google Scholar
Ehsani A, Mahjani MG, Jafarian M. Electrochemical impedance spectroscopy study on intercalation and anomalous diffusion of AlCl−4 ions into graphite in basic molten salt. Turk J Chem 2011; 35: 735–743.10.3906/kim-1103-50Search in Google Scholar
Finšgar M, Fassbender S, Hirth S, Milošev I. Electrochemical and XPS study of polyethyleneimines of different molecular sizes as corrosion inhibitors for AISI 430 stainless steel in near-neutral chloride media. Mater Chem Phys 2009; 116: 198–206.10.1016/j.matchemphys.2009.03.010Search in Google Scholar
Fouda AS, Elewady GY, Habouba S. Anise extract as green corrosion inhibitor for carbon steel in hydrochloric acid solutions. Int J Innov Res Sci Eng Technol 2014; 3: 11210–11228.Search in Google Scholar
Heakal FE, Fouda AS, Radwan MS. Inhibitive effect of some thiadiazole derivatives on C-steel corrosion in neutral sodium chloride solution. Mater Chem Phys 2011; 125: 26–36.10.1016/j.matchemphys.2010.08.067Search in Google Scholar
Huang YL, Cao CN, Lu M, Lin HC. Inhibition effects of I− and I2 on stress-corrosion cracking of stainless-steel in acidic chloride solutions. Corrosion 1993; 49: 644–649.10.5006/1.3316095Search in Google Scholar
Idouhli R, Abouelfida A, Benyaich A, Aityoub A. Cuminum cyminum extract – a green corrosion inhibitor of S300 steel in 1 M HCl. Chem Process Eng Res 2016; 44: 16–25.Search in Google Scholar
Idouhli R, N’Ait Ousidi A, Koumya Y, Abouelfida A, Benyaich A, Auhmani A, Ait Itto MY. Electrochemical studies of monoterpenic thiosemicarbazones as corrosion inhibitor for steel in 1 M HCl. Int J Corros 2018a; 44: 1–15.10.1155/2018/9212705Search in Google Scholar
Idouhli R, Oukhrib A, Koumya Y, Abouelfida A, Benyaich A, Benharref A. Inhibitory effect of Atlas cedar essential oil on the corrosion of steel in 1 m HCl. Corros Rev 2018b; 4: 323–412.10.1515/corrrev-2017-0076Search in Google Scholar
Iyasara AC, Ovri JEO. Corrosion inhibition of stainless steel (314L) using molasses. Int J Eng 2013; 2: 346–352.Search in Google Scholar
Karthik R, Muthukrishnan P, Chen SM, Jeyaprabha B, Prakash P. Anti-corrosion inhibition of mild steel in 1M hydrochloric acid solution by using Tiliacora accuminata leaves extract. Int J Electrochem Sci 2014; 10: 3707–3725.10.1016/S1452-3981(23)06573-2Search in Google Scholar
Herrag L, Hammouti B, Elkadiri S, Aouniti A, Jamab C, Vezin H, Bentiss F. Adsorption properties and inhibition of mild steel corrosion in hydrochloric solution by some newly synthesized diamine derivatives: experimental and theoretical investigations. Corros Sci 2010; 52: 3042–3051.10.1016/j.corsci.2010.05.024Search in Google Scholar
Li DG, Wang JD, Chen DR, Liang P. Influences of pH value, temperature, chloride ions and sulfide ions on the corrosion behaviors of 316L stainless steel in the simulated cathodic environment of proton exchange membrane fuel cell. J Power Sources 2014; 272: 448–456.10.1016/j.jpowsour.2014.06.121Search in Google Scholar
Lopes-Sesenes R, Dominguez-Patino GF, Rodriguez GGJ, Marinez-Villafane A. Corrosion inhibition of carbon steel by extract of Buddleja perfoliata. J Electrochem Sci Eng 2012; 2: 77–90.10.5599/jese.2012.0013Search in Google Scholar
Loto RT. Pitting corrosion evaluation of austenitic stainless steel type 304 in acid chloride media. J Mater Environ Sci 2013; 4: 448–459.Search in Google Scholar
Loto RT. Pitting corrosion evaluation and inhibition of stainless steels: a review. J Mater Environ Sci 2015; 6: 2750–2762.Search in Google Scholar
Loto RT, Loto CA, Popoola API. Corrosion inhibition of thiourea and thiadiazole derivatives: a review. J Mater Environ Sci 2012a; 3: 885–894.Search in Google Scholar
Loto CA, Popoola API, Fayomi OS, Loto RT. Corrosion polarization behaviour of type 316 stainless steel in strong acids and acid chlorides. Int J Electrochem Sci 2012b; 7: 3787–3797.10.1016/S1452-3981(23)13997-6Search in Google Scholar
Loto RT, Loto CA, Popoola API, Fedotova T. Inhibition effect of butan-1-ol on the corrosion behavior of austenitic stainless steel (type 304) in dilute sulfuric acid. Arab J Chem 2015 (in press). https://doi.org/10.1016/j.arabjc.2014.12.024.10.5755/j01.ms.21.4.8977Search in Google Scholar
MacDonald DD. The history of the point defect model for the passive state: a brief review of film growth aspects. Electrochim Acta 2011; 56: 1761–1772.10.1016/j.electacta.2010.11.005Search in Google Scholar
MacDonald DD. Some personal adventures in passivity – a review of the point defect model for film growth. Russ J Electrochem 2012; 48: 235–258.10.1134/S1023193512030068Search in Google Scholar
Martinez C, Sancy M, Zagal JH, Rabagliati FM, Tribollet B, Torres H, Paez MA. A zirconia-polyester glycol coating on differently pretreated AISI 316L stainless steel: corrosion behavior in chloride solution. J Solid State Electrochem 2009; 13: 1327–1337.10.1007/s10008-008-0674-4Search in Google Scholar
Obi-egbedi NO, Obot IB. Adsorption behavior and corrosion inhibitive potential of xanthene on mild steel/sulphuric acid interface. Arab J Chem 2012; 5: 121–133.10.1016/j.arabjc.2010.08.004Search in Google Scholar
Oguike RS. Corrosion studies on stainless steel (FE6956) in hydrochloric acid solution. Adv Mater Phys Chem 2014; 4: 153–163.10.4236/ampc.2014.48018Search in Google Scholar
Refaey SA, Taha F, Abd El-Malak A. Inhibition of stainless steel pitting corrosion in acidic medium by 2-mercaptobenzoxazole. Appl Surf Sci 2004; 236: 175–185.10.1016/j.apsusc.2004.04.016Search in Google Scholar
Seifzadeh D, Bezaatpour A, Joghani RA. Corrosion inhibition effect of N,N′-bis(2-pyridylmethylidene)-1,2-diiminoethane on AZ91D magnesium alloy in acidic media. Trans Nonferrous Met Soc Chin 2014; 24: 3441–3451.10.1016/S1003-6326(14)63487-7Search in Google Scholar
Shabani-Nooshabadi M, Ghandchi MS. Santolina chamaecyparissus extract as a natural source inhibitor for 304 stainless steel corrosion in 3.5% NaCl. J Ind Eng Chem 2015; 31: 231–237.10.1016/j.jiec.2015.06.028Search in Google Scholar
Soltis J. Passivity breakdown, pit initiation and propagation of pits in metallic materials – review. Corros Sci 2015; 90: 5–22.10.1016/j.corsci.2014.10.006Search in Google Scholar
Szauer T, Brandt A. Adsorption of oleates of various amines on iron in acidic solution. Electrochim Acta 1981; 26: 1253–1256.10.1016/0013-4686(81)85107-9Search in Google Scholar
Thompson NG, Syrett B. Relationship betwwen conventional pitting and protection potentials and a new, unique pitting potential. Corrosion 1992; 48: 649–659.10.5006/1.3315985Search in Google Scholar
Umoren SA, Banera MJ, Alonso-Garcia T, Gervasi CA, Mirífico MV. Inhibition of mild steel corrosion in HCl solution using chitosan. Cellulose 2013; 20: 2529–2545.10.1007/s10570-013-0021-5Search in Google Scholar
Wei Z, Somasundaran P, Duby P. Pitting inhibition by surfactants. J Electrochem Soc 2004; 151: B304–B308.10.1149/1.1710517Search in Google Scholar
Zhang PQ, Zhang WQ. A pitting mechanism for passive 304 stainless steel in sulphuric acid media containing chloride ions. Corros Sci 1993; 34: 1343–1354.10.1016/0010-938X(93)90091-TSearch in Google Scholar
Zucchi F, Trabanelli G, Frignani A, Zucchini M. The inhibition of stress corrosion cracking of stainless steels in chloride solutions. Corros Sci 1978; 18: 87–95.10.1016/S0010-938X(78)80078-XSearch in Google Scholar
©2019 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- In this issue
- Review
- Supramolecular concepts and approaches in corrosion and biofouling prevention
- Mini review
- Sulfate-reducing bacteria-assisted cracking
- Original articles
- Corrosion characteristics of an automotive coolant formulation dispersed with nanomaterials
- Pitting corrosion and effect of Euphorbia echinus extract on the corrosion behavior of AISI 321 stainless steel in chlorinated acid
- Study on interference and protection of pipeline due to high-voltage direct current electrode
Articles in the same Issue
- Frontmatter
- In this issue
- Review
- Supramolecular concepts and approaches in corrosion and biofouling prevention
- Mini review
- Sulfate-reducing bacteria-assisted cracking
- Original articles
- Corrosion characteristics of an automotive coolant formulation dispersed with nanomaterials
- Pitting corrosion and effect of Euphorbia echinus extract on the corrosion behavior of AISI 321 stainless steel in chlorinated acid
- Study on interference and protection of pipeline due to high-voltage direct current electrode

