Chemical Speciation of Environmentally Significant Metals: An IUPAC contribution to reliable and rigorous computer modelling
-
Kipton J. Powell
The mobility and bioavailability of metal ions in natural waters depend on their chemical speciation, which involves a distribution of the metal ions between different complex (metal-ligand) species, colloid-adsorbed species and insoluble phases, each of which may be kinetically labile or inert. For example, in fresh water the metal ions are distributed among organic complexes (e.g., humates), colloids (e.g., as surface-adsorbed species on colloidal phases such as FeOOH), solid phases (e.g., hydroxide, oxide, carbonate mineral phases), and labile complexes with the simple inorganic anionic ligands commonly present in natural waters (e.g., for ZnII, the aqueous species, Zn2+, ZnOH+, Zn(OH)2(aq), Zn2OH3+, ZnSO4(aq), ZnCO3(aq)…).
The question to be answered is: how does one select the most reliable values?
In contrast to Na+, K+, Ca2+ and Mg2+, which are the common cations present in most natural waters, most metal ions of environmental interest occur at ‘trace’ levels: at very low concentrations (mg dm−3 (ppb) range) in pristine waters and in low concentrations (mg dm−3 (ppm) range) in polluted waters. With the availability of very sensitive analytical techniques such as ASV, ICP and ICP-MS, measurement of the total concentrations of these ‘trace’ inorganic metal ions is easily accomplished. However, these techniques provide little, if any, information about the distribution of metal ions amongst their labile complex species.

pH measurement of natural water is a routine procedure but conclusive interpretation can be challenging (photo courtesy of Lars Lovgren, Umeå University)
The speciation of metal ions, viz., their distribution between soluble labile complexes and inorganic solid phases, can be calculated conveniently by computer simulation. However, the worth of such calculations is critically dependent on the equilibrium model used to define the system, the rigor of the computer modelling program and the reliability of the equilibrium constants used in the calculations. As there are a number of good, well-tested speciation modelling programs now available, reliable simulations depend on the completeness of the equilibrium model and the reliability of the equilibrium constants. The equilibrium model must include all labile species, both soluble and solid phase, that make a measureable contribution to the distribution of the metal ion. In the case of natural waters, this model will include ions such as Ca2+ and Mg2+ that will be in competition with trace metal ions for inorganic ligands such as CO32−, SO42− and PO43−.
The equilibrium constants reported in the literature will often be derived from experiments conducted at different ionic strengths, often significantly higher than those applicable to natural waters. For a speciation simulation to be numerically reliable, the equilibrium constants must be valid for the ionic strength of the natural water of interest. This may require extrapolation or interpolation from a set of experimental values determined at different ionic strengths (typically in a 0.05 to 3.0 mol dm−3 medium of a supposedly non-labile electrolyte) to the value applicable to pristine fresh water (ca. 0.0015 mol dm−3) or a saline (seawater) medium (ca. 0.67 mol dm−3). Choosing the most reliable constants is a serious challenge even for experienced solution chemists. Constants will have been determined by a range of experimental methods, some less appropriate than others, by laboratories with diverse levels of expertise. Even when the experimental measurements are on a simple binary system, such as Pb2+ + OH−, the choice of different experimental pH ranges, total metal ion concentrations, or ionic strengths, or the nature of the medium (e.g., LiClO4 vs NaClO4 or KNO3) [1], will lead to different species models and numerically different results. The question to be answered is: how does one select the most reliable values?
Critical evaluations of equilibrium data - the role of IUPAC
One responsibility of IUPAC is the critical evaluation of published experimental data. For equilibrium constants this includes the identification and recommendation of the most reliable values for each metal-ligand system. This is a very time-consuming process which involves the critical evaluation of experimental methods, numerical treatment of data in each publication and at all ionic strengths reported, applying clearly stated criteria to identify reliable publications and reject others, as well as the test of data reliability by establishing correlations between reliable (“accepted”) data for different ionic strengths and rejecting statistical outliers.
This process of critical evaluation of metal + ligand equilibrium constants has been applied to the complexes formed between the natural-water-dominant inorganic ligands OH−, Cl−, CO32−, SO42− and PO43−, the proton H+, and the environmentally-significant heavy-metal ions Hg2+, Cu2+, Pb2+, Cd2+ and Zn2+, which all occur at trace levels in fresh water and sea water. The results are the outcome of IUPAC project 1999-050-1-500 and are published in a recently-completed series of IUPAC Technical Reports. [2-6]
This review of the IUPAC Technical Reports presents examples of speciation diagrams, viz., plots of metal ion distribution as a function of pH. For brevity the examples and tables are limited to the speciation of ZnII in natural waters. Comprehensive examples for each metal ion for binary systems and natural waters are given in the respective Technical Reports. These diagrams are calculated from the stability constants Kn or βp,q,r for each ligand-metal combination. In addition to the experimental equilibrium constants, values of the standard state (zero ionic strength) constants, Kn° or βp,q,r°, are also reported. These standard state constants are obtained by extrapolation of the accepted experimental values at finite ionic strengths using the Brønsted–Guggenheim–Scatchard Specific Ion-Interaction Theory (SIT), [7] which represents a reasonable trade-off between theoretical rigor and numerical simplicity.
For the general reaction of a metal ion M with a ligand L (omitting charges except for H+):
pM + qL + rH2O ⇌ MpLq(OH)r + rH+
the SIT relationship between the standard equilibrium constant βp,q,r° and that determined in an ionic
medium of ionic strength Im (molality scale), βp,q,r is:
log10βp,q,r − Δz2D − rlog10a(H2O) =
log10βp,q,r° − ΔεIm (1)
in which Δz2 = (pzM + qzL – r)2 + r – p(zM)2 – q(zL)2. The value of the constant D is defined by the SIT activity coefficient relationship on the molality scale for a single ion i:
log10 γm(i) = − zi2A√Im (1 + ajB√ Im)⁻1 + Σk ε(i,k) mk
= − zi2D + Σk ε(i,k) mk(2)
in which k represents the electrolyte ions N+ or X⁻, ε(i,k) is the aqueous SIT coefficient for short-range interactions between ions i and k, and Δε is the reaction SIT coefficient given by:
Δε = ε(complex, N+ or X−) + rε (H+,X−) − pε (Mn+,X−)
− qε (Lm−, N+)
A is the Debye–Hückel limiting slope (0.509 mol−1/2 kg1/2 at 25 °C) and ajB = 1.5. [7] Equation 1 is used to determine log10βp,q,r° values as the intercept of the extrapolation of a suite of accepted log10βp,q,r values. To view all of these extrapolations and a more rigorous treatment, the reader is referred to the IUPAC Technical Reports. [2-6] These reports include all log10βp,q,r° or log10Kn° and Δε values determined in this IUPAC project.
| Table 1. Stability constants for selected species critical in modelling the speciation of ZnII in fresh water (Ic = 0.0015 mol dm-3) and seawater (Ic = 0.67 mol dm-3) at 25 °C.* | |||
| Reaction | log10K0 (Ic = 0) | log10K (Ic = 0.0015) | log10K (Ic = 0.67) |
| Zn2+ + H2O ⇌ ZnOH+ + H+ | −8.96 | –9.00 | –9.35 |
| Zn2+ + 2H2O ⇌ Zn(OH)2(aq) + 2H+ | −17.82 | –17.86 | –18.31 |
| Zn2+ + H2CO3 ⇌ ZnCO3(aq) + 2H+ | –11.94 | –11.98 | –12.3 |
| H2CO3 ⇌ HCO3− + H+ | −6.35 | −6.32 | −5.98 |
| H2CO3 ⇌ CO32− + 2H+ | −16.69 | −16.58 | −15.52 |
| Zn2+ + SO42− ⇌ ZnSO4(aq) | 2.30 | 2.15 | 0.84 |
| Zn2+ + Cl− ⇌ ZnCl+ | 0.40 | 0.32 | −0.25 |
| Zn2+ + 2Cl− ⇌ ZnCl2(aq) | 0.69 | 0.58 | −0.30 |
| Zn2+ + 3Clˉ ⇌ ZnCl3ˉ | 0.48 | 0.37 | −0.40 |
| * The constant log10K (CO2(g) ⇌ CO2(aq)) = –1.5 applies in all calculations. [8] | |||
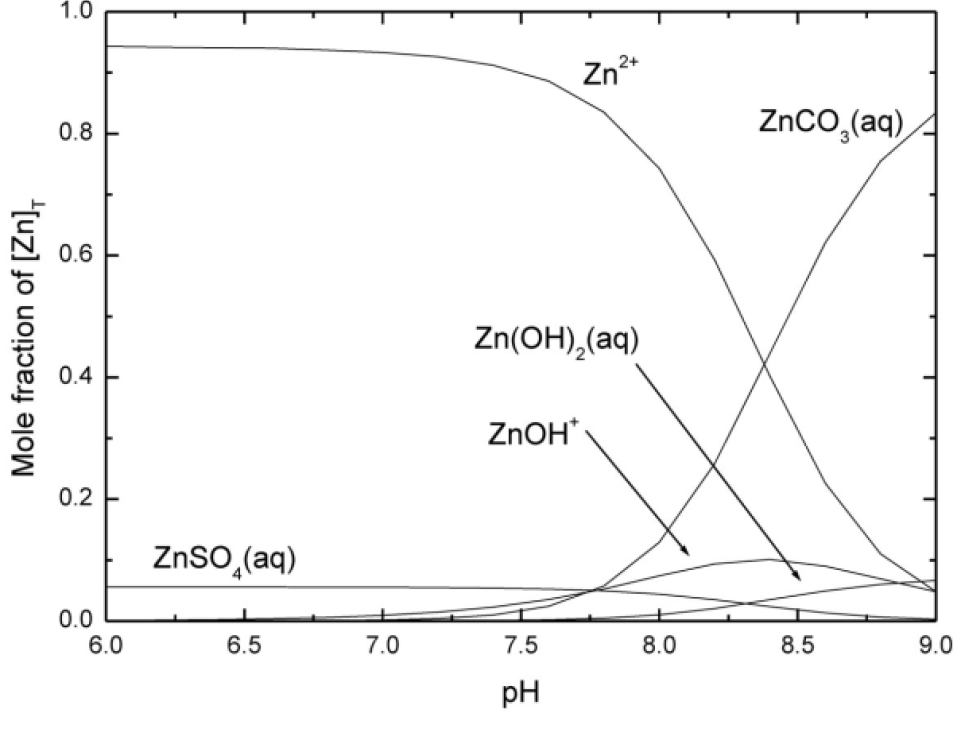
Figure 1: Speciation diagram for the system Zn2+ + H+ + Cl¯ + CO32¯ + SO42¯ + HPO42¯ in a simulated fresh water medium, 25 °C, including carbonato- and sulfato- complexes of Mg2+ and Ca2+ (not shown). [8] It was assumed that [ZnII]T = 1 nmol dm¯3 and that the system is in equilibrium with air having a CO2 fugacity of 10¯1.5 kPa. Formation constants for the major species are given in Table 1. (Ic = 0.0015 mol dm¯3).
| Table 2. Equilibrium (stability) constants for the different Zn2+ systems at 298.15 K, p = 102 kPa, and Im = 0 mol kg−1 . Δε values are for ClO4¯ medium. Values presented are either Recommended, Provisional or Indicative; see ref. 2 for full details. | ||
| Reaction | Constant | Δε(kg mol−1) |
| Zn2+ + H2O ⇌ ZnOH+ + H+ | log10 *K1° = −8.96 ± 0.05 | 0.03 ± 0.02 |
| ΔrH° = (56.8 ± 0.9) kJ mol−1 | ||
| Zn2+ + 2H2O ⇌ Zn(OH)2(aq) + 2H+ | log10 *β2° = −17.82 ± 0.08 | −0.07 ± 0.04 |
| ΔrH° = (109 ± 4) kJ mol−1 | ||
| Zn2+ + 3H2O ⇌ Zn(OH)3− + 3H+ | log10 *β3° = −28.05 ± 0.05 | 0.19 ± 0.06 |
| ΔrH° = (151 ± 3) kJ mol−1 | ||
| Zn2+ + 4H2O ⇌ Zn(OH)42− + 4H+ | log10 *β4° = −40.41 ± 0.12 | 0.46 ± 0.04 |
| ΔrH° = (188 ± 6) kJ mol−1 | ||
| 2Zn2+ + H2O ⇌ Zn2OH3+ + H+ | log10 * β2,1° = −7.9 ± 0.2 | 0.3 ± 0.1 |
| Zn2+ + Cl− ⇌ ZnCl+ | log10K1° = 0.40 ± 0.09 | −0.14 ± 0.02 |
| Zn2+ + 2Cl− ⇌ ZnCl2(aq) | log10β2° = 0.69 ± 0.15 | −0.20 ± 0.04 |
| Zn2+ + 3Cl− ⇌ ZnCl3− | log10β3° = 0.48 ± 0.54 | −0.27 ± 0.13 |
| Zn2+ + CO32− ⇌ ZnCO3(aq) | log10K1° = 4.75 ± 0.10 | |
| Zn2+ + 2CO32− ⇌ Zn(CO3)22− | log10β2 = 5.4 ± 0.6 (a) | |
| Zn2+ + HCO3− ⇌ ZnHCO3+ | log10K° = 1.62 ± 0.10 | 0.10 ± 0.06 |
| Zn2+ + SO42− ⇌ ZnSO4(aq) | log10K1° = 2.30 ± 0.04 | −0.05 ± 0.03 |
| ΔrH° = 6.0 ± 0.5 kJ mol−1 | ||
| Zn2+ + 2SO42− ⇌ Zn(SO4)22− | log10β2° = 3.2 ± 0.2 | 0.09 ± 0.08 |
| Zn2+ + HPO42− ⇌ ZnHPO4(aq) | log10K = 2.44 ± 0.20 (b) | |
| Note (a) Im = 0.68 mol kg−1 ; (b) Im = 0.10 mol kg−1 | ||
Speciation calculations
The availability of equilibrium constants and their ionic strength dependencies allows the modelling of metal ion speciation in natural water systems. Figures 1 and 2 present examples for ZnII in natural waters. In fresh water the ionic strength is low, which will cause only small changes in the formation constants from values at Ic (or Im) = 0. However, in a sea water system the changes become more pronounced. This is evident from the data presented in Table 1, which contains calculated log10Kn or log10βp,q,r values for species found to contribute significantly to the speciation of ZnII in fresh and sea water systems. The speciation calculations were achieved using WinSGW (www.WinSGW.se). This program incorporates the SIT functions and generates the ionic-strength-corrected values of log10βp,q,r for each datum in the calculation.
Example: a multicomponent freshwater system
Figure 1 illustrates the speciation diagram for ZnII in fresh water (Ic = 0.0015 mol dm⁻3) in equilibrium with air having a CO2 fugacity of 10⁻1.5 kPa. The total concentration of ZnII was set to 1 nmol dm⁻3 and the total concentrations of the inorganic anions were those typically found in fresh water [8]: [Cl⁻]T = 0.23 mmol dm⁻3, [SO42⁻]T = 0.42 mmol dm⁻3 and [HPO42⁻]T = 0.7 µmol dm⁻3. The pH was allowed to vary between 6.0 and 9.0. In this range the ionic strength varies from ca. Ic = 0.0015 mol dm⁻3 at pH = 7, to 0.007 mol dm⁻3 at pH = 9 due to the increase in [HCO3⁻] and [CO32⁻] at constant f(CO2). The impact on the stability constant values caused by this change in Ic was accounted for in the calculation. This calculation also includes the competing reactions between Mg2+ and Ca2+ and the ligands (not shown). [9] Figure 1 shows that the predominant species are Zn2+ (pH ≤ 8.4) and ZnCO3(aq) (pH ≥ 8.4), and that hydroxido- complexes
(Znp(OH)q(2p – q)+) make only a minor contribution (< 10 %).
Illustrating chemical speciation in distribution diagrams can be very informative (courtesy of Anneli Sundman, Umeå University)
Example: a multicomponent seawater system
In a sea water system high concentrations of chloride (545 mmol dm⁻3) and sulphate (28 mmol dm⁻3) significantly affect the speciation. [8] This is illustrated in Figure 2, which shows the speciation for ZnII in a sea water medium (Ic = 0.67 mol dm⁻3), a calculation including carbonato- and sulfato- complexes of Mg2+ and Ca2+. It was assumed that [ZnII]T = 1 nmol dm⁻3. Similar to the fresh water system, the species Zn2+ and ZnCO3(aq) predominate. However, due to the competing formation of chlorido- and sulphato- complexes, the concentration of Zn2+ is now ca. 55% of [Zn]T at sea water pH (ca. 8.2), in contrast to ca. 94 % of [Zn]T at the pH of fresh water (ca. 6.5). Similarly, the formation of ZnCO3(aq) is also suppressed and this does not become the predominant species until pH ≥ 8.7.
IUPAC Recommended values of critical equilibrium constants
For a full set of the critically evaluated Recommended or Provisional equilibrium data the reader is referred to the published Technical Reports. [2-6] To illustrate the scope of the data currently available, Table 2 presents the evaluated stability constants, reaction ion interaction coefficients (Δε) and, where available, the reaction enthalpy change for formation of soluble ZnII complexes with the common environmental inorganic ligands. For the sake of brevity the reactions for nine heterogeneous reactions are omitted from this Table. The reader is referred to the Technical Report. [6]
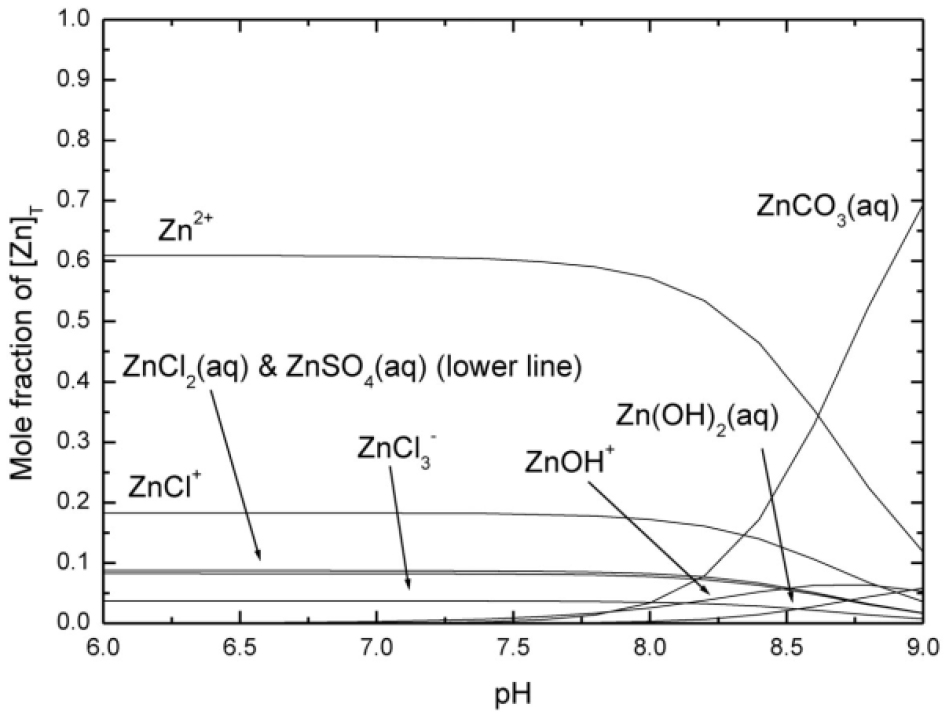
Figure 2: Speciation diagram for the ZnII system in a simulated seawater medium, 25 °C, including carbonato- and sulfato- complexes of Mg2+ and Ca2+ (not shown).[7] It was assumed that [ZnII]T = 1 nmol dm−3 and that the system is in equilibrium with air having a CO2 fugacity of 10−1.5 kPa. Formation constants for the major species are given in Table 1 (Ic = 0.67 mol dm-3).
Comments
As a result of this project, the environmental chemist wishing to model inorganic complexation of the important trace metal ions in natural waters now has easy access to the most reliable stability constant values currently available for the relevant equilibria and applicable ionic strengths. An outcome from the critical evaluations [2-6] is that a lack of detailed high-quality data for many of the metal + ligand systems has been identified. This is particularly true for the M2+ + PO43⁻ systems for which no values could be recommended at Im = 0 mol dm⁻3. Reliable data in several of the systems M2+ + CO32⁻ (e.g. M = Cd, Pb, Zn) were also missing at finite ionic strengths, as too were data for some of the systems M2+ + SO42⁻. It has been possible to evaluate very few enthalpy values, let alone recommend them, which means that a rigorous thermodynamic modelling of the chemical speciation at temperatures other than 25 °C is still not possible. Further, the absence of reliable thermodynamic data for metal complexes with relevant organic ligands (e.g. humic substances) remains the major challenge to the modelling of complexation in natural water systems. In this domain there is an urgent need for additional critical evaluations. 
Kipton J. Powell is an Emeritus Professor at University of Canterbury (Christchurch). Paul L. Brown is Principal Advisor, Mineral Waste Management at Rio Tinto Technology and Innovation (Bundoora). Robert H. Byrne is Distinguished University Professor in Seawater Physical Chemistry at the University of South Florida (St. Petersburg). Tamas Gajda is at University of Szeged (Szeged). Glenn Hefter is Professor of Chemistry at Murdoch University. Ann-Kathrin Leuz is Section Head at the Swiss Federal Nuclear Safety Inspectorate (Brugg). Staffan Sjöberg is Professor Emeritus in Chemistry at Umeå University. Hans Wanner is Director General of the Swiss Federal Nuclear Safety Inspectorate (Brugg).
References
1. G. T. Hefter, J. Solution Chem.13, 179 (1984).Suche in Google Scholar
2. K. J. Powell, P. L. Brown, R. H. Byrne, T. Gajda, G. Hefter, S Sjöberg, H. Wanner. Pure Appl. Chem.77, 739 (2005). [Evaluations for HgII, and H+ + CO32−, H+ + PO43−]Suche in Google Scholar
3. K. J. Powell, P. L. Brown, R. H. Byrne, T. Gajda, G. Hefter, S Sjöberg, H. Wanner. Pure Appl. Chem. 79, 895 (2007). [Evaluations for CuII]Suche in Google Scholar
4. K. J. Powell, P. L. Brown, R. H. Byrne, T. Gajda, G. Hefter, A-K. Leuz, S. Sjöberg, H. Wanner. Pure Appl. Chem.81, 2425 (2009). [Evaluations for PbII]Suche in Google Scholar
5. Ibid.. Pure Appl. Chem.83, 1163 (2011). [Evaluations for CdII]Suche in Google Scholar
6. Ibid.. Pure Appl. Chem.85, 2249 (2013). [Evaluations for ZnII]Suche in Google Scholar
7. I. Grenthe, A. V. Plyasunov, K. Spahiu. In Modelling in Aquatic Chemistry (I. Grenthe, I. Puigdomenech, eds.), pp. 325-426. Organisation for Economic Co-operation and Development, Paris (France) (1997).Suche in Google Scholar
8. F. M. M. Morel and J. G. Hering, Principles and Applications of Aquatic Chemistry, John Wiley, New York (1993).Suche in Google Scholar
9. L. D. Pettit and K. J. Powell. SC-Database, IUPAC Stability Constants Database. Release 5.8. IUPAC; Academic Software, Otley, UK (2010); for availability see http://www.iupac.org/index.php?id=410Suche in Google Scholar
(Ed): Critical evaluation of stability constants is an ongoing activity of IUPAC Analytical Chemistry Division (Division V) through its Sub-Committee on Solubility and Equilibrium Data (SSED) www.iupac.org/body/502

©2015 by Walter de Gruyter Berlin/Boston
Artikel in diesem Heft
- Masthead - Full issue pdf
- From the Editor
- President’s Column
- Refocus on Contacts
- Features
- Chemistry: Meeting the World’s Needs?
- Materials and Technologies for Energy Conversion, Saving and Storage: A global network enabling capacity-building for sustainable energy in developing countries
- Concepts in Toxicology: Development of Online Instructional Modules
- Chemical Speciation of Environmentally Significant Metals: An IUPAC contribution to reliable and rigorous computer modelling
- IUPAC Wire
- IUPAC Elections for the 2016–2017 Term
- IUPAC 2015 Distinguished Women in Chemistry or Chemical Engineering — Call for Nominations
- PhosAgro/UNESCO/IUPAC Research Grants in Green Chemistry
- 2015 IUPAC-SOLVAY International Award for Young Chemists
- Thieme Chemistry Website Relaunched
- Strengthening the ties between IUPAC and the Chinese Chemical Society
- Chemistry International Survey
- No Price Increase in 2015 for Pure and Applied Chemistry
- UNESCO Partners with Nature Education and Roche to Launch a Free Online Science Education Resource
- Cefic Sustainability Report 2013-2014
- Project Place
- The Emerging Problem of Novel Psychoactive Substances
- Nomenclature of Carbon Nanotubes and Related Substances
- Terminology for Modeling and Simulation of Polymers
- Chemistry Beyond Chlorine
- Stamps International
- Let There Be Light!
- Conference Call
- Boron Chemistry
- Isoprenoids
- 100 volumes of IUPAC’s Solubility Data Series
- FloHet-2014
- Nanomaterials and Human Health: The Trends and Future
- Chemistry Education
- Green Chemistry
- Where 2B & Y
- Mark Your Calendar
Artikel in diesem Heft
- Masthead - Full issue pdf
- From the Editor
- President’s Column
- Refocus on Contacts
- Features
- Chemistry: Meeting the World’s Needs?
- Materials and Technologies for Energy Conversion, Saving and Storage: A global network enabling capacity-building for sustainable energy in developing countries
- Concepts in Toxicology: Development of Online Instructional Modules
- Chemical Speciation of Environmentally Significant Metals: An IUPAC contribution to reliable and rigorous computer modelling
- IUPAC Wire
- IUPAC Elections for the 2016–2017 Term
- IUPAC 2015 Distinguished Women in Chemistry or Chemical Engineering — Call for Nominations
- PhosAgro/UNESCO/IUPAC Research Grants in Green Chemistry
- 2015 IUPAC-SOLVAY International Award for Young Chemists
- Thieme Chemistry Website Relaunched
- Strengthening the ties between IUPAC and the Chinese Chemical Society
- Chemistry International Survey
- No Price Increase in 2015 for Pure and Applied Chemistry
- UNESCO Partners with Nature Education and Roche to Launch a Free Online Science Education Resource
- Cefic Sustainability Report 2013-2014
- Project Place
- The Emerging Problem of Novel Psychoactive Substances
- Nomenclature of Carbon Nanotubes and Related Substances
- Terminology for Modeling and Simulation of Polymers
- Chemistry Beyond Chlorine
- Stamps International
- Let There Be Light!
- Conference Call
- Boron Chemistry
- Isoprenoids
- 100 volumes of IUPAC’s Solubility Data Series
- FloHet-2014
- Nanomaterials and Human Health: The Trends and Future
- Chemistry Education
- Green Chemistry
- Where 2B & Y
- Mark Your Calendar

