Abstract
Tumor necrosis factor (TNF)-α-induced protein-8-like 2, or TIPE2, is a newly found immune negative regulatory molecule. This study further investigated the role of TIPE2 on proliferation and invasion of cervical squamous cancer cells. Expression of TIPE2 was compared in cervical squamous cancer tissues and adjacent normal tissues by Western blot and immunohistochemistry (IHC). Cervical squamous cancer cell lines, SiHa and C33A, were transfected with recombinant plasmid encoding TIPE2 and tested for cytologic characteristics. The impact of TIPE2 on phosphorylation of extracellular signal-regulated kinase (Erk) signaling pathway was also tested by Western blot analysis of key factors. TIPE2 expression was higher in cervical cancer tissues than that in normal tissue. IHC score of tumor tissue was negatively associated with lymphatic metastasis. Over expression of TIPE2 effectively inhibited the proliferation of cervical cancer cells. Wound healing and transwell assay showed that over expression of TIPE2 suppressed cell migration and invasion in vitro. Meanwhile, phosphorylation of Erk1/2 and upstream mitogen-activated protein kinase kinase (MEK) 1/2 was reduced by TIPE2. TIPE2 is negatively related with development of cervical squamous cancer. TIPE2 is an inhibitory factor of proliferation and invasion of cervical squamous cancer cells, probably through inhibiting Erk signaling pathway.
1 Introduction
Cervical cancer is one of the most common malignant tumors of female genital tract, and the mortality rate is higher than 1/104 in underdeveloped areas such as Africa and South-Eastern Asia [1]. The incidence and mortality of cervical in China showed a significant increasing trend in recent years [2]. Understanding the pathogenesis of cervical cancer and exploring new treatment methods are of great significance to reduce the burden of cervical cancer. The pathogenesis of cervical cancer is complex, involving abnormal expression of multiple oncogenes or tumor suppressor genes. The destruction of immune dynamic balance is an important mechanism of tumorigenesis and immune-based cancer therapies have gained more and more attentions [3].
Tumor necrosis factor (TNF)-α-induced protein-8-like 2, or TIPE2, was firstly found as an immune negative regulatory molecule in mouse model of experimental autoimmune encephalomyelitis [4, 5]. TIPE2 belongs to the TIPE family, which is a death effect domain (DED)-containing protein family [6]. TIPE2 plays a vital role in maintaining immune homeostasis and its expression is usually down-regulated in patients with infections or immune disorders [7, 8]. Murine TIPE2 is preferentially expressed in immune cells such as lymphoid or myeloid cells; while human TIPE2 can be expressed in a variety of cells, including hepatocytes, neurons, squamous epithelial cells of the cervix, and glandular epithelial cells in stomach [9].
Recently TIPE2 is gaining more attention as a novel inhibitor of tumors. The discovery of abnormal expression of TIPE2 in tumors suggests that TIPE2 may have the potential of tumor markers and therapeutic targets. TIPE2 can inhibit activity of oncogenic Ras and therefore suppress cell survival and motility of hepatocellular carcinoma [10, 11]. TIPE2 inhibits proliferation of gastric cancer cells by up-regulating p27 [12]. In renal cell carcinoma, over expression of TIPE2 is correlated with TNM stages [13]. In a recent study about intergrinαVβ6, TIPE2 expression was found to be lower in cervical cancer tissues and cervical benign lesions than in healthy cervical tissues [14]. The effect of TIPE2 on development of cervical cancer is largely unclear. Therefore, we investigated the role and possible molecular mechanism of TIPE2 on proliferation and invasion of cervical squamous cancer cells.
2 Materials and methods
2.1 Tissue samples
Cervical squamous cancer tissues and adjacent normal tissues from 40 patients were collected from the Department of Obstetrics and Gynecology, Xianning Central Hospital from January 2012 to December 2015. All the specimens were confirmed by pathological analysis. All the patients underwent operation at our hospital and none received chemotherapy or radiotherapy before sample collection. Tissue samples were surgically removed, immediately frozen in liquid nitrogen and then stored at -80°C for subsequent molecular assays.
Informed consent: Informed consent has been obtained from all individuals included in this study.
Ethical approval: The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance the tenets of the Helsinki Declaration, and has been approved by the institutional ethics committee of Xianning Central Hospital.
2.2 Immunohistochemistry (IHC)
A small piece of each tissue sample was fixed in 10% formaldehyde, embedded in paraffin, and cut into thin sections (5 μm thick). Standard IHC procedure was performed to detect TIPE2 expression using SABC-AP (human IgG) kit (Boster Biological Technology, Wuhan, China). The primary antibody used was synthesized anti-TNFAIP8L2 antibody (Boster, catalog #BA3300) at 1:100 dilution. The secondary antibody used was rabbit anti-human IgG-biotin (1:1000) provided by the IHC kit. Hybridization signals were colored by BCIP/NBT solution provided by the IHC kit and examined under light microscope (Olympus, Tokyo, Japan). The stained IHC slides were independently evaluated by two pathologists based on staining intensity and percentage area of positive stain as described [8]. For each slide, 5 randomly chosen fields in ×200 magnification were analyzed.
2.3 Cell culture
Cervical squamous cancer cell lines SiHa and C33A (American Type Culture Collection, Manassas, VA, USA) were used for in vitro assays. Cells were maintained in DMEM supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin at 37°C in 5% CO2 atmosphere. Culture medium was purchased from Gibco (Thermo Fisher Scientific Inc., Carlsbad, CA, USA).
2.4 Plasmid construction and transfection
Full-length human TIPE2 was cloned from cDNA template, sequenced, and cloned into pRK5 vector to form pRK5-TIPE2 as described [15]. The plasmid construction was finished by Sangon Biotechnology Inc. (Shanghai, China). To endogenously over-express TIPE2, cervical cancer cells (1×106) were cultured to 80% confluency and transfected with 100 nM of pRK5-TIPE2 using Lipofectamine 2000 reagent (Invitrogen by Thermo Fisher).
2.5 RNA extraction and real-time quantitative PCR
Total RNA was extracted from cells using TRIzol reagent (Invitrogen by Thermo Fisher Scientific, Carlsbad, CA, USA) and was reverse transcribed into cDNA using PrimeScript RT kit (Takara Biomedical Technology, Beijing, China). Real-time quantitative PCR was performed using SYBR Green Premix Ex Taq (Takara) on Applied Biosystem 7900 PCR systems (Applied Biosystem by Thermo Fisher Scientific, Bedford, MA, USA). The thermocycling conditions were set as: 95°C for 30 sec; 40 cycles of 95°C for 5 sec and 60°C for 30 sec. The primer sequences used were as follows: sense 5’-ACTGAGTAAGATGGCGGGTCG-3’ and anti-sense 5’-TTCTGGCGAAAGCGGGTAG-3’ for TIPE2; sense 5’-GAAGGTCGGAGTCAACGGATTT-3’ and anti-sense 5’-CCTGGAAGATGGTGATGGGATT-3’ for GAPDH. Relative TIPE2 gene level was normalized to GAPDH by the 2-ΔΔCt method [16].
2.6 Cell proliferation
Cells were harvested 48 hours after transfection, made into cell suspension (1×104/mL), and inoculated in 96-well plates (100 μL/well). During the next 5 days, the daily growth of cells was detected by CCK-8 method (Enhanced Cell Counting Kit-8, Beyotime Biotechnology, Shanghai, China). Each well was added 10 μL of CCK-8 reagent and incubated at 37°C for 1 hour. The absorbance at 450 nm was measured by microplate reader (Bio-Rad, Hercules, CA, USA).
2.7 Wound healing for cell migration
Cells were harvested 48 hours after transfection, made into cell suspension (2×105/mL), and inoculated in 6-well plates (2 mL/well) to form monolayer. The cell layer was gently scratched with a pipette tip and cultured with DMEM+FBS for 24 hours. The cells were observed under a light microscope at 0 hour and 24 hour to compare the injury width.
2.8 Transwell assay for cell invasion
Cell invasion ability was analyzed in transwell chamber (Corning by Merck, Corning, NY, USA) with 8-μm pore size polycarbonate membrane. The upper chamber was precoated with 100 μL Matrigel (1:3 dilution with serum-free DMEM). After 48 hours of transfection, cells were cultured with serum-free basic medium for 1 day before the experiment. On the day of the experiment, cells were prepared into suspension (5×105/mL) with serum-free medium, inoculated into upper chamber (200 μL), and put in 24-well plate filled with 600 μL of DMEM+FBS. After 24 hours of incubation at 37°C, cells remained in the upper chamber were gently interrupted with a wet cotton swab. The upper chamber was then fixed in methanol for 15 min, dried at room temperature, and stained with 0.1% crystal violet for 20 min. The migrating cells were counted under a light microscope at 200×magniciation. For each well, five random fields were counted.
2.9 Western blot
After 48 hours of transfection, cells were collected and suspended in 100 μL of RIPA lysis buffer with 1 mM PMSF (Beyotime). After incubation on ice for 10min, cell suspension was centrifuged (12000 rpm, 4°C) for 30 min to collect supernatant. The protein concentration of supernatant was determined by BCA reagent (Beyotime). Approximately 50 μg of proteins were mixed with SDS-PAGE loading buffer (Beyotime), boiled for 5 min, and separated by 10% SDS-polyacrylamide gel electrophoresis. Protein bands were then transferred onto PVDF membranes. After blocking with 5% non-fat milk for 2 hours, membranes were probed with appropriate primary antibodies overnight at 4°C. The following antibodies were used: rabbit polyclonal antibody to mitogen-activated protein kinase kinase (MEK) 1/2 (ab178876, 1:10000), rabbit polyclonal antibody to MEK1 (phospho S298) (ab96379, 1:2000), rabbit polyclonal antibody to MEK2 (phospho T394) (ab30622, 1:1000), rabbit polyclonal antibody to extracellular signal-regulated kinase (Erk) 1/2 (ab17942,1:1000), rabbit polyclonal antibody to Erk1 (phospho Y204)/Erk2 (phospho Y187) (ab47339, 1:1000), rabbit polyclonal antibody to TIPE2 (ab110389, 1:1000), rabbit monoclonal antibody to GAPDH (ab181602, 1:5000). The next, membranes were probed with goat anti-rabbit IgG H&L (horseradish peroxidase-conjugated) (ab6721, 1:5000) at room temperature for 1 hour. All the antibodies were purchased from Abcam (Cambridge, UK). Protein signals were visualized by enhanced chemiluminescence kit (Beyotime) and analyzed by Image J software.
2.10 Statistical analysis
Data were analyzed with SPSS software version 19.0 (SPSS Inc., Chicago, IL, USA). All cellular experiments and Western blot of tissues samples were repeated three times. Continuous variables are presented as mean ± standard deviation (SD). Classified variables between the two groups were compared by Chi-square test. Continuous variables of two groups were compared by Student’s t test; continuous variables among multiple groups were compared by single factor ANOVA followed by Tukey’s test. All statistical analyses were two-sided and P value <0.05 was considered statistically significant.
3 Results
3.1 TIPE2 is down-regulated in cervical squamous cancer tissues
As shown in Figure 1A, the protein level of TIPE2 was significantly lower in cancer tissues than in adjacent normal tissue (P<0.01). IHC analysis (typical IHC images in Figure 1B) also showed that the positive expression rate of
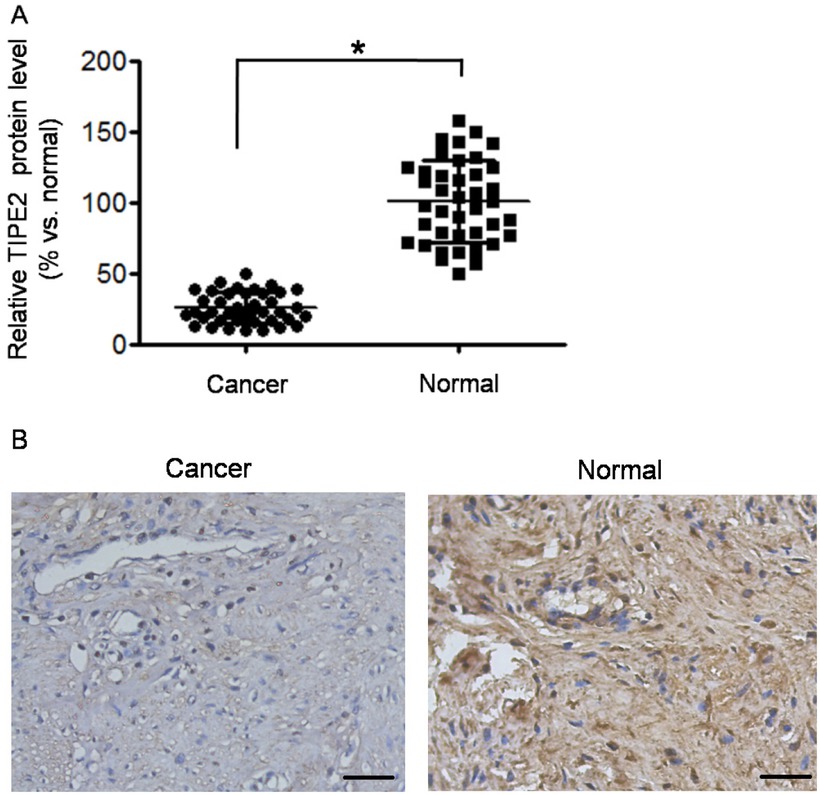
TIPE2 was down-regulated in cervical squamous cancer tissues. (A) Relative TIPE2 protein level (represented as % vs. normal) was detected by Western blot. The Western blot was repeated three times. *P<0.05 vs. normal. (B) Typical images of TIPE2 detection by immunohistochemistry (×200 magnification). The images showed medium differentiated squamous carcinoma and normal cervical tissue. Scale bar = 50 μm.
TIPE2 in cancer tissues was significantly lower than that in normal tissues (57.5% vs. 87.5%, χ2=9.028, P=0.003). Then we analyzed the relationship between IHC scores of TIPE2 and clinical characteristics in cervical cancer tissues. As shown in Table 1, there was no significant correlation of TIPE2 score to age, differentiation grade, and FIGO stage (all P>0.05). However, the TIPE2 score was associated with lymphatic metastasis (P=0.014).
Relationship between TIPE2 protein expression and clinical pathological characteristics in cervical cancer tissues
| Parameters | Pathological score of TIPE2 | χ2 | P value | |
|---|---|---|---|---|
| 0, +1 | +2, +3 | |||
| Age | ||||
| <50 | 8 | 5 | 0.333 | 0.564 |
| ≥50 | 14 | 13 | ||
| Differentiation grade | ||||
| GI+GII | 7 | 11 | 3.432 | 0.064 |
| GIII | 15 | 7 | ||
| Lymphatic metastasis | ||||
| No | 5 | 11 | 6.077 | 0.014 |
| Yes | 17 | 7 | ||
| FIGO stage | ||||
| I-II | 9 | 10 | 0.852 | 0.356 |
| III-IV | 13 | 8 | ||
3.2 TIPE2 inhibits proliferation, migration, and invasion of cervical cancer cells
The effect of TIPE2 on cell metastasis of cervical cancer was further investigated in vitro. TIPE2 expression was detected in both SiHa and C33A cells; these cell lines were then transfected with pRK5-TIPE2 to over express TIPE2. The transfection efficiently increased TIPE2 expression both on mRNA level and on protein level (Figure S1). Over expression of TIPE2 inhibited cellular viability of both SiHa and C33A cells (Figure 2A, both P<0.05). For each time point, SiHa cells over-expressing TIPE2 showed delayed growth from day 2, and C33A cells over-expressing TIPE2 showed delayed growth from day 3. Wound healing assay showed that over expressing TIPE inhibited cell migration ability (Figure 2B-2C). After 24 hours of scratch, the wound width between cells in TIPE2 transfection group was 15%-25% wider than that in control group or empty vector group. High level of TIPE2 also reduced cell invasion ability (Figure 2D-2E). The number of invasive cells was significantly reduced by about 50%-60% when over expressing TIPE2. The results suggested that TIPE2 can suppress malignant phenotypes of cervical cancer cells in vitro.
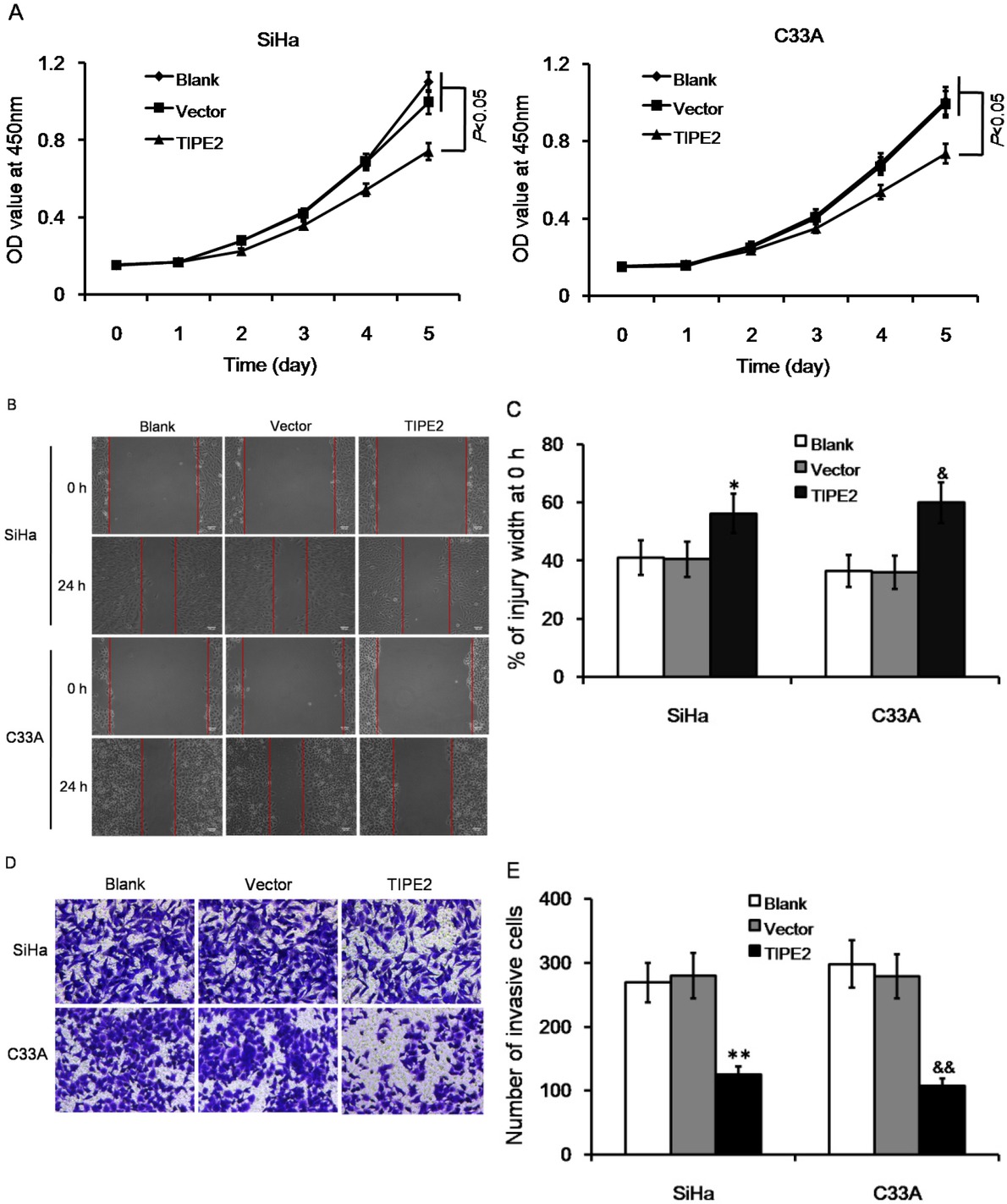
Effect of TIPE2 level on biological characteristics of cervical cancer cells without transfection (blank), transfected with empty vector (vector), or transfected with pPK5-TIPE2 (TIPE2). (A) Over expression of TIPE2 inhibited cell viability of SiHa and C33A cells. (B-C) Over expression of TIPE2 suppressed cell migration in wound healing assay. (D-E) Over expression of TIPE2 reduced number of invasive cells in transwell assay. *P<0.05 vs. blank of SiHa; **P<0.01 vs. blank of SiHa; &P<0.05 vs. blank of C33A; &&P<0.01 vs. blank of C33A.
3.3 TIPE2 inhibits phosphorylation of Erk1/2 pathway
Previous studies reported that TIPE2 could regulate immune responses through negative regulation of T cell receptor (TCR) pathway [5]. Classical Erk1/2 pathway is one of the main downstream pathways of TCR. Therefore, we tested the effect of TIPE2 on Erk1/2 expression. As shown in Figure 3, expression of total Erk1/2 was not changed in both SiHa and C33A cells. Meanwhile, Erk1/2 phosphorylation was reduced by TIPE2 at both the Erk1 Y204 and the Erk2 Y187. Erk1/2 is typically activated by MEK1/2. Western blot also showed that total expression of MEK1/2 was not changed, but phosphorylation was reduced at MEK1 S298 and MEK2 T394. The results suggested that TIPE2 affected phosphrylation of those factors rather than changing expression level.
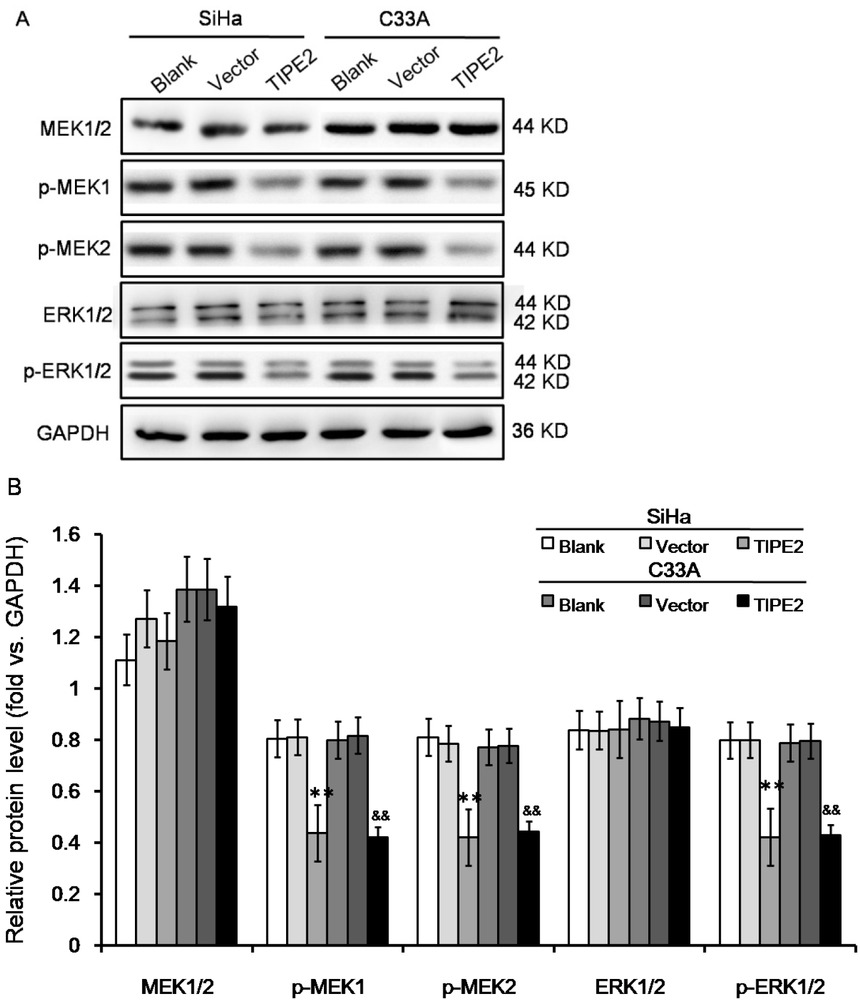
Effect of TIPE2 level on ERK signaling pathway in cervical cancer cells without transfection (blank), transfected with empty vector (vector), or transfected with pPK5-TIPE2 (TIPE2). (A-B) Western blot showed that over expression of TIPE2 inhibited phosphorylation of Erk1/2. **P<0.01 vs. blank of SiHa; &&P<0.01 vs. blank of C33A.
4 Discussion
In our study, TIPE2 expression in cervical squamous cancer tissues was down-regulated compared with that in normal adjacent tissues, and was associated with lymphatic metastasis. So far only one previous paper reported that TIPE2 was down-regulated in cervical adenocarcinoma HeLa cells [17]. Another research reported that TIPE2 expression was reduced in cervical cancer tissues and cervical benign lesions [14]. Our study also explored the molecular mechanism of TIPE2 in cervical cancer. We found that TIPE2 inhibited growth, migration, and metastasis of cervical squamous cancer cells, SiHa and C33A, via suppressing phosphorylation of Erk1/2. These findings together with our findings suggested that TIPE2 should be a tumor suppressor in cervical cancer. However, the clinical diagnostic and prognostic value of TIPE2 for cervical cancer is still unclear. In our study, only 40 tumor samples were analyzed, which is not enough for other detailed studies such as stratified analysis and survival analysis.
Existing studies have shown that the expression patterns of TIPE2 in different cancers are not consistent. In addition to cervical cancer, TIPE2 also plays a tumor suppressor role in most current studies on cancers. TIPE2 up-regulated production of CD8+ T and natural killer (NK) cells, and inhibited breast cancer development and metastasis [18]. TIPE2 expression was lost in AGS, HGC-27, and SGC-7901 gastric cancer cells; gained-expression of TIPE2 suppresses cell migration and invasion in vitro via inhibiting protein kinase B (Akt)/GSK3β/β-catenin signaling [15]. TIPE2 expression was lost or reduced in primary hepatocellular carcinoma (HCC) tissues and was significantly associated with tumor metastasis, cell growth, cell migration, and cell invasion [11]. Mechanically, TIPE2 directly inhibited endogenous Rac1 and further affected extracellular matrix synthesis and angiogenesis [11]; TIPE2 could also suppress cell migration through tumor necrosis factor-α (TNF-α)-related nuclear factor-κB (NF-κB) signaling [19]; TIPE2 suppressed cell proliferation and increased the number of cells in S phase via inhibiting phosphoinositide 3-kinase (PI3K)/Akt signaling [20]. However, TIPE2 was over expressed in kidney renal cancer tissues compared with normal kidney tissues and its expression level was positively correlated with TNM staging [13]. TIPE2 was up-regulated in the cytoplasm of colon cancer tissues and HT-20 colon cancer cells by inhibiting caspase-8 activity [21]. Therefore, if TIPE2 is used as a target for development of new drugs, it may also need to consider whether specific types of cancers can benefit from treatment.
TIPE2 regulates tumorigenesis through multiple signaling pathways. In addition to the PI3K/Akt and TNF-α/NK-κB mentioned above, TIPE2 also regulates Wnt/β-catenin cascade, signal transducer and activator of transcription 3 (STAT3), and to influence proliferation, angiogenesis, apoptosis, and metastasis (for comprehensive overview see review [22]. In this study, we found that up-regulation of TIPE2 inhibited phosphorylation of Erk1/2. Similar regulational relationship was reported in hepatocellular carcinoma and gastric cancer [19, 23]. Classical mitogen-activated protein kinase (MAPK) pathway includes three branches: p38 MAPK, c-Jun N-terminal kinase (JNK), and Erk. In TIPE2 knockout murine T cells, TIPE2 is a critical regulator of JNK and p38 pathways, but ERK is not affected [5]. In other words, the regulation mechanism of TIPE2 is different in different disease models.
In conclusion, our study further showed that down-regulation of TIPE2 was associated with the development of cervical squamous cancer. TIPE2 could suppress the proliferation and migration of cancer cells by inhibiting phosphorylation of Erk1/2. Research on the molecular mechanism of TIPE2 in cervical cancer is just beginning. More extensive studies are required to elucidate the diagnostic and therapeutic values of TIPE2.
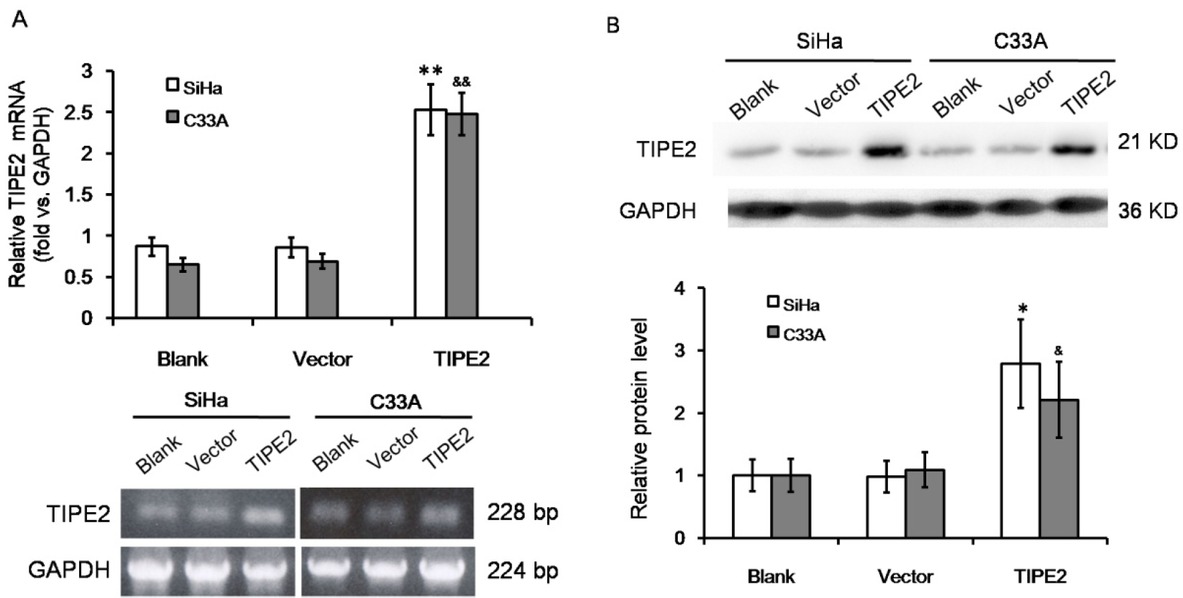
Over expression of TIPE2 in cervical squamous cancer cells by transfection. (A) RT-PCR amplification and agarose gel electrophoresis of TIPE2 mRNA level in SiHa and C33A cells without transfection (blank), transfected with empty vector (vector), or transfected with pPK5-TIPE2 (TIPE2). (B) Western blot analysis of TIPE2 protein level in SiHa and C33A cells. *P<0.05 vs. blank of SiHa; **P<0.01 vs. blank of SiHa; &P<0.05 vs. blank of C33A; &&P<0.01 vs. blank of C33A.
Conflict of interest: Authors state no conflict of interest
Reference
[1] Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424.10.3322/caac.21492Search in Google Scholar
[2] Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bary F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115-32.10.3322/caac.21338Search in Google Scholar
[3] Disis ML. Immune regulation of cancer. J Clin Oncol. 2010;28(29):4531-8.10.1200/JCO.2009.27.2146Search in Google Scholar
[4] Carmody R, Hilliard B, Maguschak K, Chodosh LA, Chen YH. Genomic scale profiling of autoimmune inflammation in the central nervous system: the nervous response to inflammation. J Neuroimmunol. 2002;133(1-2):95-107.10.1016/S0165-5728(02)00366-1Search in Google Scholar
[5] Sun H, Gong S, Carmody RJ, Hilliard A, Li L, Sun J, et al. TIPE2, a negative regulator of innate and adaptive immunity that maintains immune homeostasis. Cell. 2008;133(3):415-26.10.1016/j.cell.2008.03.026Search in Google Scholar PubMed PubMed Central
[6] Zhang X, Wang J, Fan C, Li H, Sun H, Gong S, et al. Crystal structure of TIPE2 provides insights into immune homeostasis. Nat Struct Mol Biol. 2009;16(1):89-90.10.1038/nsmb.1522Search in Google Scholar PubMed
[7] Kong L, Liu K, Zhang YZ, Jin M, Wu BR, Wang WZ, et al. Downregulation of TIPE2 mRNA expression in peripheral blood mononuclear cells from patients with chronic hepatitis C. Hepatol Int. 2013;7(3):844-9.10.1007/s12072-013-9435-2Search in Google Scholar PubMed
[8] Xi W, Hu Y, Liu Y, Zhang J, Wang L, Lou Y, et al. Roles of TIPE2 in hepatitis B virus-induced hepatic inflammation in humans and mice. Mol Immunol. 2011;48(9-10):1203-8.10.1016/j.molimm.2011.03.002Search in Google Scholar PubMed
[9] Zhang L, Shi Y, Wang Y, Zhu F, Wang Q, Ma C, et al. The unique expression profile of human TIPE2 suggests new functions beyond its role in immune regulation. Mol Immunol. 2011;48(9-10):1209-15.10.1016/j.molimm.2011.03.001Search in Google Scholar PubMed
[10] Gusbrautbar Y, Johnson DS, Zhang L, Sun H, Wang P, Zhang S, et al. The anti-inflammatory TIPE2 is an inhibitor of the oncogenic Ras. Mol Cell. 2012;45(5):610-8.10.1016/j.molcel.2012.01.006Search in Google Scholar PubMed PubMed Central
[11] Cao X, Zhang L, Shi Y, Sun Y, Dai S, Guo C, et al. Human tumor necrosis factor (TNF)-alpha-induced protein 8-like 2 suppresses hepatocellular carcinoma metastasis through inhibiting Rac1. Mol Cancer. 2013;12(1):149.10.1186/1476-4598-12-149Search in Google Scholar PubMed PubMed Central
[12] Zhao Q, Zhao M, Dong T, Zhou C, Peng Y, et al. Tumor necrosis factor‐α‐induced protein‐8 like‐2 (TIPE2) upregulates p27 to decrease gastic cancer cell proliferation. J Cell Biochem. 2015;116(6):1121-9.10.1002/jcb.25068Search in Google Scholar PubMed
[13] Zhang Z, Qi H, Hou S, Jin X. TIPE2 mRNA overexpression correlates with TNM staging in renal cell carcinoma tissues. Oncol Lett. 2013;6(2):571-5.10.3892/ol.2013.1388Search in Google Scholar PubMed PubMed Central
[14] Liang D, Xu W, Zhang Q, Tao BB. Study on the effect of Integrin αVβ6 on proliferation and apoptosis of cervical cancer cells. Eur Rev Med Pharmacol Sci. 2017;21(12):2811-5.Search in Google Scholar
[15] Wu J, Zhang H, Xu C, Xu H, Zhou X, Xie Y, et al. TIPE2 functions as a metastasis suppressor via negatively regulating β-catenin through activating GSK3β in gastric cancer. Int J Oncol. 2016;48(1):199-206.10.3892/ijo.2015.3224Search in Google Scholar PubMed
[16] Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402-8.10.1006/meth.2001.1262Search in Google Scholar PubMed
[17] Zhang G, Hao C, Lou Y, Xi W, Wang X, Wang Y, et al. Tissue-specific expression of TIPE2 provides insights into its function. Mol Immunol. 2010;47(15):2435-42.10.1016/j.molimm.2010.06.016Search in Google Scholar PubMed
[18] Zhang Z, Liu L, Cao S, Zhu Y, Mei Q. Gene delivery of TIPE2 inhibits breast cancer development and metastasis via CD8+ T and NK cell-mediated antitumor responses. Mol Immunol. 2017;85:230-7.10.1016/j.molimm.2017.03.007Search in Google Scholar PubMed
[19] Zhang YH, Yan HQ, Wang F, Wang YY, Jiang YN, Wang YN, et al. TIPE2 inhibits TNF-α-induced hepatocellular carcinoma cell metastasis via Erk1/2 downregulation and NF-κB activation. Int J Oncol. 2015;46(1):254-64.10.3892/ijo.2014.2725Search in Google Scholar PubMed
[20] Wang L, Chen C, Feng S, Tian J. TIPE‑2 suppresses growth and aggressiveness of hepatocellular carcinoma cells through downregulation of the phosphoinositide 3‑kinase/AKT signaling pathway. Mol Med Rep. 2018;17(5):7017-26.10.3892/mmr.2018.8789Search in Google Scholar PubMed PubMed Central
[21] Li X, Su J, Yan S, Cheng ZL, Yang TT, Zhu Q. A novel inflammatory regulator TIPE2 inhibits TLR4-mediated development of colon cancer via caspase-8. Cancer Biomark. 2014;14(4):233-40.10.3233/CBM-140402Search in Google Scholar PubMed
[22] Padmavathi G, Banik K, Monisha J, Bordoloi D, Shabnam B, Arfuso F, et al. Novel tumor necrosis factor-α induced protein eight (TNFAIP8/TIPE) family: Functions and downstream targets involved in cancer progression. Cancer Lett. 2018;432:260-71.10.1016/j.canlet.2018.06.017Search in Google Scholar PubMed
[23] Zhu Y, Tao M, Wu J, Meng Y, Xu C, Tian Y, et al. Adenovirus-directed expression of TIPE2 suppresses gastric cancer growth via induction of apoptosis and inhibition of AKT and ERK1/2 signaling. Cancer Gene Ther. 2016;23(4):98-106.10.1038/cgt.2016.6Search in Google Scholar PubMed
© 2019 Li-Qiong Huang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Articles in the same Issue
- Plant Sciences
- Extended low temperature and cryostorage longevity of Salix seeds with desiccation control
- Genome-wide analysis of the WRKY gene family and its response to abiotic stress in buckwheat (Fagopyrum tataricum)
- Differential expression of microRNAs during root formation in Taxus chinensis var. mairei cultivars
- Metabolomics Approach for The Analysis of Resistance of Four Tomato Genotypes (Solanum lycopersicum L.) to Root-Knot Nematodes (Meloidogyne incognita)
- Beneficial Effects of Salt on Halophyte Growth: Morphology, Cells, and Genes
- Phosphate-solubilizing bacteria from safflower rhizosphere and their effect on seedling growth
- Anatomy and Histochemistry of the Roots and Shoots in the Aquatic Selenium Hyperaccumulator Cardamine hupingshanensis (Brassicaceae)
- Effects of LED light on Acacia melanoxylon bud proliferation in vitro and root growth ex vitro
- Ecology and Environmental Sciences
- Intensity of stripping and sugar content in the bark and the bast of European beech (Fagus sylvatica)
- Influence of monometallic and bimetallic phytonanoparticles on physiological status of mezquite
- Loci identification of a N-acyl homoserine lactone type quorum sensing system and a new LysR-type transcriptional regulator associated with antimicrobial activity and swarming in Burkholderia gladioli UAPS07070
- Bacillus methylotrophicus has potential applications against Monilinia fructicola
- Evaluation of Heavy Metals and Microbiological Contamination of Selected herbals from Palestine
- The effect of size of black cherry stumps on the composition of fungal communities colonising stumps
- Effect of rhamnolipids on microbial biomass content and biochemical parameters in soil contaminated with coal tar creosote
- Effects of foliar trichomes on the accumulation of atmospheric particulates in Tillandsia brachycaulos
- Isolation and characterisation of the agarolytic bacterium Pseudoalteromonas ruthenica
- Comparison of soil bioconditioners and standard fertilization in terms of the impact on yield and vitality of Lolium perenne and soil biological properties
- Biomedical Sciences
- The number of regulatory B cells is increased in mice with collagen-induced arthritis
- Lactate overload inhibits myogenic activity in C2C12 myotubes
- Diagnostic performance of serum CK-MB, TNF-α and hs-CRP in children with viral myocarditis
- Correlation between PPARGC1A gene rs8192678 G>A polymorphism and susceptibility to type-2 diabetes
- Improving the Detection of Hepatocellular Carcinoma using serum AFP expression in combination with GPC3 and micro-RNA miR-122 expression
- The ratio of neutrophil to lymphocyte is a predictor in endometrial cancer
- Expression of HER2/c-erbB-2, EGFR protein in gastric carcinoma and its clinical significance
- Clinical significance of neuropeptide Y expression in pelvic tissue in patients with pelvic floor dysfunction
- Overexpression of RASAL1 indicates poor prognosis and promotes invasion of ovarian cancer
- The effect of adrenaline on the mineral and trace element status in rats
- Effects of Ischemic Post-Conditioning on the Expressions of LC3-II and Beclin-1 in the Hippocampus of Rats after Cerebral Ischemia and Reperfusion
- Long non-coding RNA DUXAP8 regulates the cell proliferation and invasion of non-small-cell lung cancer
- Risk factors of regional lymph node metastasis in patients with cervical cancer
- Bullous prurigo pigmentosa
- Association of HIF-1α and NDRG2 expression with EMT in gastric cancer tissues
- Decrease in the level of nervonic acid and increased gamma linolenic acid in the plasma of women with polycystic ovary syndrome after a three-month low-glycaemic index and caloric reduction diet
- Depletion of VAX2 restrains the malignant progression of papillary thyroid carcinoma by modulating ERK signaling pathway
- Insulin resistance is a risk factor for mild cognitive impairment in elderly adults with T2DM
- Nurr1 promotes lung cancer apoptosis via enhancing mitochondrial stress and p53-Drp1 pathway
- Predictive significance of serum MMP-9 in papillary thyroid carcinoma
- Agmatine prevents oxidative-nitrative stress in blood leukocytes under streptozotocin-induced diabetes mellitus
- Effect of platelet-rich plasma on implant bone defects in rabbits through the FAK/PI3K/AKT signaling pathway
- The diagnostic efficacy of thrombelastography (TEG) in patients with preeclampsia and its association with blood coagulation
- Value of NSE and S100 Protein of Kawasaki Disease with aseptic meningitis in Infant
- CB2 receptor agonist JWH133 activates AMPK to inhibit growth of C6 glioma cells
- The effects of various mouthwashes on osteoblast precursor cells
- Co-downregulation of GRP78 and GRP94 induces apoptosis and inhibits migration in prostate cancer cells
- SKA3 up-regulation promotes lung adenocarcinoma growth and is a predictor of poor prognosis
- Protective effects and mechanisms of microRNA-182 on oxidative stress in RHiN
- A case of syphilis with high bone arsenic concentration from early modern cemetery (Wroclaw, Poland)
- Study of LBHD1 Expression with Invasion and Migration of Bladder Cancer
- 1-Hydroxy-8-methoxy-anthraquinon reverses cisplatin resistance by inhibiting 6PGD in cancer cells
- Andrographolide as a therapeutic agent against breast and ovarian cancers
- Accumulation of α-2,6-sialyoglycoproteins in the muscle sarcoplasm due to Trichinella sp. invasion
- Astragalus polysaccharides protects thapsigargin-induced endoplasmic reticulum stress in HT29 cells
- IGF-1 via PI3K/Akt/S6K signaling pathway protects DRG neurons with high glucose-induced toxicity
- Intra-arterial tirofiban in a male nonagenarian with acute ischemic stroke: A case report
- Effects of Huaiqihuang Granules adjuvant therapy in children with primary nephrotic syndrome
- Immune negative regulator TIPE2 inhibits cervical squamous cancer progression through Erk1/2 signaling
- Asymptomatic mediastinal extra-adrenal paraganglioma as a cause of sudden death: a case Report
- Primary mucinous adenocarcinoma of appendix invading urinary bladder with a fistula: a case report
- Minocycline attenuates experimental subarachnoid hemorrhage in rats
- Neural Remodeling of the Left Atrium in rats by Rosuvastatin following Acute Myocardial Infarction
- Protective effects of emodin on lung injuries in rat models of liver fibrosis
- RHOA and mDia1 promotes apoptosis of breast cancer cells via a high dose of doxorubicin treatment
- Bacteria co-colonizing with Clostridioides difficile in two asymptomatic patients
- A allele of ICAM-1 rs5498 and VCAM-1 rs3181092 is correlated with increased risk for periodontal disease
- Treatment of hepatic cystic echinococcosis patients with clear cell renal carcinoma: a case report
- Edaravone exerts brain protective function by reducing the expression of AQP4, APP and Aβ proteins
- Correlation between neutrophil count and prognosis in STEMI patients with chronic renal dysfunction: a retrospective cohort study
- Bioinformatic analysis reveals GSG2 as a potential target for breast cancer therapy
- Nuciferine prevents hepatic steatosis by regulating lipid metabolismin diabetic rat model
- Analysis of SEC24D gene in breast cancer based on UALCAN database
- Bioengineering and Biotechnology
- Co-cultured Bone-marrow Derived and Tendon Stem Cells: Novel Seed Cells for Bone Regeneration
- Animal Sciences
- Comparative analysis of gut microbiota among the male, female and pregnant giant pandas (Ailuropoda Melanoleuca)
- Adaptive immunity and skin wound healing in amphibian adults
- Hox genes polymorphism depicts developmental disruption of common sole eggs
- The prevalence of virulence genes and multidrug resistance in thermophilic Campylobacter spp. isolated from dogs
- Agriculture
- Effect of Lactobacillus plantarum supplementation on production performance and fecal microbial composition in laying hens
- Identification of Leaf Rust Resistance Genes in Selected Wheat Cultivars and Development of Multiplex PCR
- Determining Potential Feed Value and Silage Quality of Guar Bean (Cyamopsis tetragonoloba) Silages
- Food Science
- Effect of Thermal Processing on Antioxidant Activity and Cytotoxicity of Waste Potato Juice
Articles in the same Issue
- Plant Sciences
- Extended low temperature and cryostorage longevity of Salix seeds with desiccation control
- Genome-wide analysis of the WRKY gene family and its response to abiotic stress in buckwheat (Fagopyrum tataricum)
- Differential expression of microRNAs during root formation in Taxus chinensis var. mairei cultivars
- Metabolomics Approach for The Analysis of Resistance of Four Tomato Genotypes (Solanum lycopersicum L.) to Root-Knot Nematodes (Meloidogyne incognita)
- Beneficial Effects of Salt on Halophyte Growth: Morphology, Cells, and Genes
- Phosphate-solubilizing bacteria from safflower rhizosphere and their effect on seedling growth
- Anatomy and Histochemistry of the Roots and Shoots in the Aquatic Selenium Hyperaccumulator Cardamine hupingshanensis (Brassicaceae)
- Effects of LED light on Acacia melanoxylon bud proliferation in vitro and root growth ex vitro
- Ecology and Environmental Sciences
- Intensity of stripping and sugar content in the bark and the bast of European beech (Fagus sylvatica)
- Influence of monometallic and bimetallic phytonanoparticles on physiological status of mezquite
- Loci identification of a N-acyl homoserine lactone type quorum sensing system and a new LysR-type transcriptional regulator associated with antimicrobial activity and swarming in Burkholderia gladioli UAPS07070
- Bacillus methylotrophicus has potential applications against Monilinia fructicola
- Evaluation of Heavy Metals and Microbiological Contamination of Selected herbals from Palestine
- The effect of size of black cherry stumps on the composition of fungal communities colonising stumps
- Effect of rhamnolipids on microbial biomass content and biochemical parameters in soil contaminated with coal tar creosote
- Effects of foliar trichomes on the accumulation of atmospheric particulates in Tillandsia brachycaulos
- Isolation and characterisation of the agarolytic bacterium Pseudoalteromonas ruthenica
- Comparison of soil bioconditioners and standard fertilization in terms of the impact on yield and vitality of Lolium perenne and soil biological properties
- Biomedical Sciences
- The number of regulatory B cells is increased in mice with collagen-induced arthritis
- Lactate overload inhibits myogenic activity in C2C12 myotubes
- Diagnostic performance of serum CK-MB, TNF-α and hs-CRP in children with viral myocarditis
- Correlation between PPARGC1A gene rs8192678 G>A polymorphism and susceptibility to type-2 diabetes
- Improving the Detection of Hepatocellular Carcinoma using serum AFP expression in combination with GPC3 and micro-RNA miR-122 expression
- The ratio of neutrophil to lymphocyte is a predictor in endometrial cancer
- Expression of HER2/c-erbB-2, EGFR protein in gastric carcinoma and its clinical significance
- Clinical significance of neuropeptide Y expression in pelvic tissue in patients with pelvic floor dysfunction
- Overexpression of RASAL1 indicates poor prognosis and promotes invasion of ovarian cancer
- The effect of adrenaline on the mineral and trace element status in rats
- Effects of Ischemic Post-Conditioning on the Expressions of LC3-II and Beclin-1 in the Hippocampus of Rats after Cerebral Ischemia and Reperfusion
- Long non-coding RNA DUXAP8 regulates the cell proliferation and invasion of non-small-cell lung cancer
- Risk factors of regional lymph node metastasis in patients with cervical cancer
- Bullous prurigo pigmentosa
- Association of HIF-1α and NDRG2 expression with EMT in gastric cancer tissues
- Decrease in the level of nervonic acid and increased gamma linolenic acid in the plasma of women with polycystic ovary syndrome after a three-month low-glycaemic index and caloric reduction diet
- Depletion of VAX2 restrains the malignant progression of papillary thyroid carcinoma by modulating ERK signaling pathway
- Insulin resistance is a risk factor for mild cognitive impairment in elderly adults with T2DM
- Nurr1 promotes lung cancer apoptosis via enhancing mitochondrial stress and p53-Drp1 pathway
- Predictive significance of serum MMP-9 in papillary thyroid carcinoma
- Agmatine prevents oxidative-nitrative stress in blood leukocytes under streptozotocin-induced diabetes mellitus
- Effect of platelet-rich plasma on implant bone defects in rabbits through the FAK/PI3K/AKT signaling pathway
- The diagnostic efficacy of thrombelastography (TEG) in patients with preeclampsia and its association with blood coagulation
- Value of NSE and S100 Protein of Kawasaki Disease with aseptic meningitis in Infant
- CB2 receptor agonist JWH133 activates AMPK to inhibit growth of C6 glioma cells
- The effects of various mouthwashes on osteoblast precursor cells
- Co-downregulation of GRP78 and GRP94 induces apoptosis and inhibits migration in prostate cancer cells
- SKA3 up-regulation promotes lung adenocarcinoma growth and is a predictor of poor prognosis
- Protective effects and mechanisms of microRNA-182 on oxidative stress in RHiN
- A case of syphilis with high bone arsenic concentration from early modern cemetery (Wroclaw, Poland)
- Study of LBHD1 Expression with Invasion and Migration of Bladder Cancer
- 1-Hydroxy-8-methoxy-anthraquinon reverses cisplatin resistance by inhibiting 6PGD in cancer cells
- Andrographolide as a therapeutic agent against breast and ovarian cancers
- Accumulation of α-2,6-sialyoglycoproteins in the muscle sarcoplasm due to Trichinella sp. invasion
- Astragalus polysaccharides protects thapsigargin-induced endoplasmic reticulum stress in HT29 cells
- IGF-1 via PI3K/Akt/S6K signaling pathway protects DRG neurons with high glucose-induced toxicity
- Intra-arterial tirofiban in a male nonagenarian with acute ischemic stroke: A case report
- Effects of Huaiqihuang Granules adjuvant therapy in children with primary nephrotic syndrome
- Immune negative regulator TIPE2 inhibits cervical squamous cancer progression through Erk1/2 signaling
- Asymptomatic mediastinal extra-adrenal paraganglioma as a cause of sudden death: a case Report
- Primary mucinous adenocarcinoma of appendix invading urinary bladder with a fistula: a case report
- Minocycline attenuates experimental subarachnoid hemorrhage in rats
- Neural Remodeling of the Left Atrium in rats by Rosuvastatin following Acute Myocardial Infarction
- Protective effects of emodin on lung injuries in rat models of liver fibrosis
- RHOA and mDia1 promotes apoptosis of breast cancer cells via a high dose of doxorubicin treatment
- Bacteria co-colonizing with Clostridioides difficile in two asymptomatic patients
- A allele of ICAM-1 rs5498 and VCAM-1 rs3181092 is correlated with increased risk for periodontal disease
- Treatment of hepatic cystic echinococcosis patients with clear cell renal carcinoma: a case report
- Edaravone exerts brain protective function by reducing the expression of AQP4, APP and Aβ proteins
- Correlation between neutrophil count and prognosis in STEMI patients with chronic renal dysfunction: a retrospective cohort study
- Bioinformatic analysis reveals GSG2 as a potential target for breast cancer therapy
- Nuciferine prevents hepatic steatosis by regulating lipid metabolismin diabetic rat model
- Analysis of SEC24D gene in breast cancer based on UALCAN database
- Bioengineering and Biotechnology
- Co-cultured Bone-marrow Derived and Tendon Stem Cells: Novel Seed Cells for Bone Regeneration
- Animal Sciences
- Comparative analysis of gut microbiota among the male, female and pregnant giant pandas (Ailuropoda Melanoleuca)
- Adaptive immunity and skin wound healing in amphibian adults
- Hox genes polymorphism depicts developmental disruption of common sole eggs
- The prevalence of virulence genes and multidrug resistance in thermophilic Campylobacter spp. isolated from dogs
- Agriculture
- Effect of Lactobacillus plantarum supplementation on production performance and fecal microbial composition in laying hens
- Identification of Leaf Rust Resistance Genes in Selected Wheat Cultivars and Development of Multiplex PCR
- Determining Potential Feed Value and Silage Quality of Guar Bean (Cyamopsis tetragonoloba) Silages
- Food Science
- Effect of Thermal Processing on Antioxidant Activity and Cytotoxicity of Waste Potato Juice

