Abstract
This study examined whether or not various mouthwashes have significant effects on the viability or morphology of mouse osteoblast-like cells. Mouse calvarial preosteoblast cells were cultured and prepared, then treated with a 0.12% chlorhexidine digluconate solution containing essential oils with or without alcohol, and a cetylpyridinium chloride solution, and sodium fluoride, respectively. Each well was treated with one of six mouthwashes for either 30 sec, 1.5 min, or 4.5 min. The viability of the treated cells was quantitatively analyzed by a Cell Counting Kit-8. The viability of osteogenic progenitor cells decreased significantly irrespectively of the types of mouthwashes. The changes of cell morphology were seen in all groups of mouthwashes; however, they were more noticeable on the chlorhexidine digluconate-treated group. A progressive increase in treatment time over 30 sec did not seem to deteriorate cellular viability. There was no significant difference in viability or morphological change between different formulations of the same brand. Although various mouthwashes without alcohol as an ingredient are available, nonalcoholic mouthwashes were not likely to be less harmful to the cells. Collectively, commercially available mouthwashes could inhibit cell viability and alter the morphology of osteoblastic precursor cells irrespectively of brands, treatment time, or alcohol content.
1 Introduction
Various mouthwashes are commercially available over the counter. Chlorhexidine digluconate (CHX) is the most commonly prescribed antibacterial gargling agent, a bisbiguanide that inhibits and prevents bacteria forming by binding to cell membranes and increasing permeability and leakage of intracellular components. Its effect is mainly due to substantivity in the mouth [1]. Listerine® (LIS) is phenolic essential oils combined with thymol, eucalyptol, menthol, and methylsalicylate in an alcohol vehicle. The mechanism is through protein denaturation of the bacterial membrane and inhibition of enzyme activity. LIS is a strong antimicrobial mouthwash and is frequently used in many dental fields such as orthodontic bracket disinfection [2]. Garglin® (GGN) is a popular mouthwash used for decades in the Republic of Korea. GGN consists of cetylpyridinium chloride (CPC) as an active ingredient, a cationic quaternary ammonium compound that is cytotoxic to bacterial and other microorganisms. It has been shown to be effective in preventing plaque accumulation and decreasing gingivitis [3, 4, 5].
Although these mouthwashes are effective antiseptics, there is a concern about an adverse effect on various mammalian cells. For example, previous studies have demonstrated the cytotoxicity of CHX on oral mucosal fibroblast may be increased in the concentration and time-dependent manner [6]. Clinical use of 0.12% CHX twice daily over eight days caused DNA damage on oral mucosal cells in vitro [7]. An inhibitory effect of mouthwashes has been shown in osteoblasts and osteoclasts [8,9]; however, there is a lack of thorough information on whether or not these agents can harm bone formation and hinder periodontal bone regeneration. Therefore, this study investigated the effects of the aforementioned mouthwashes on osteoblast-like cells by examining cell morphology and viability.
2 Materials and methods
2.1 Cell culture
Osteoblast-like cells (mouse calvarial preosteoblast cells, MC3T3-E1, American Type Culture Collection, Manassas, VA, USA) were plated at 96-well at a density of 6.25 x 103 cells/well and maintained in α-minimum essential medium (αMEM, Welgene, Daegu, Korea) supplemented with 10% fetal bovine serum (Thermo Scientific, Logan, UT), antibiotics (penicillin 100U/mL and streptomycin 100 μg/mL (Gibco, Invitrogen, Carlsbad, CA)). The cultures were kept in a humidified atmosphere with 5% CO2 and 95% air at 37°C for 24 hours.
2.2 Preparation with mouth rinse and evaluation of cellular morphology
Figure 1 shows the overview of the study design. Six mouthwashes were applied for this study: (1) a 0.12% chlorhexidine digluconate solution (CHX, Hexamedine, Bukwang, Seoul, Korea); (2) a solution containing essential oils (LIS Citrus, Listerine® Citrus, Johnson & Johnson, Bangkok, Thailand); (3) a solution containing essential oils without alcohol (LIS Zero, Listerine® Zero, Johnson & Johnson); (4) Garglin® Regular containing CPC (GGN, Dong-A Pharmaceutical Co., Seoul, Korea); (5) Garglin® Medical containing essential oils (GGN Med, Dong-A Pharmaceutical Co.); and (6) Garglin® Child containing sodium fluoride (GGN Child, Dong-A Pharmaceutical Co.). Each well was treated with one of the six mouthwashes for 30 sec, 1 min and 30 sec (1.5 min), or 4 min and 30 sec (4.5 min). An untreated culture well served as a control. The morphological changes were observed under an inverted microscope (Leica DM IRM, Leica Microsystems, Wetzlar, Germany) after each treatment.
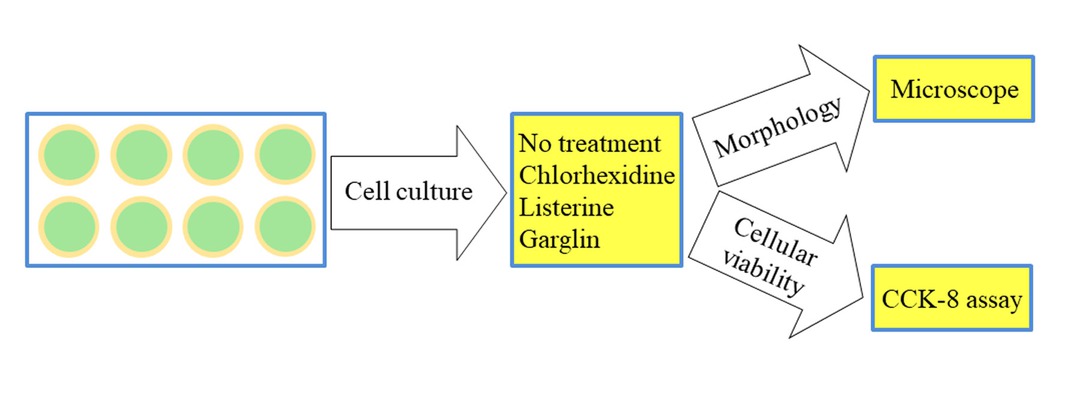
Diagram showing the overview of the study design.
2.3 Quantitative determination of cellular viability
The viability of the treated cells was quantitatively analyzed by a Cell Counting Kit-8 (Dojindo molecular technologies Inc., Rockville, MD). A water-soluble tetrazolium salt-8 (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazoium, monosodium salt) solution was added and incubated for 12 h. The amount of generated formazan was determined by reading the absorbance at a 450 nm wavelength using the microplate spectrophotometer system (BioTek, Winooski, VT).
2.4 Statistical analysis
The data are represented as the means ± standard error of the mean of the experiments. A test of normality and the equality of variances in the samples was conducted. A two-way analysis of variance (ANOVA) was used for the evaluation of the effects of application time and types of gargles. A one-way ANOVA with Tukey’s post hoc test was performed to determine the differences between the application time in each group using a commercially available program (SPSS 12 for windows, SPSS Inc., Chicago, IL, USA) with the level of significance at 0.05.
3 Results
3.1 Evaluation of cellular morphology
In the microscopic evaluation, the untreated cells attached to the culture plate showed a well-organized actin cytoskeleton. The treatment of the adult stem cells with 0.12% CHX produced a more rounded shape (Figure 2). Treatment for a longer time caused a more noticeable alteration, with 0.12% CHX. Similar trends were achieved in the LIS Citrus and LIS Zero groups. We noticed alteration in the cytoskeletal organization and observed the rounding up of the cells or progressive detachment from the substrate for the experiments.
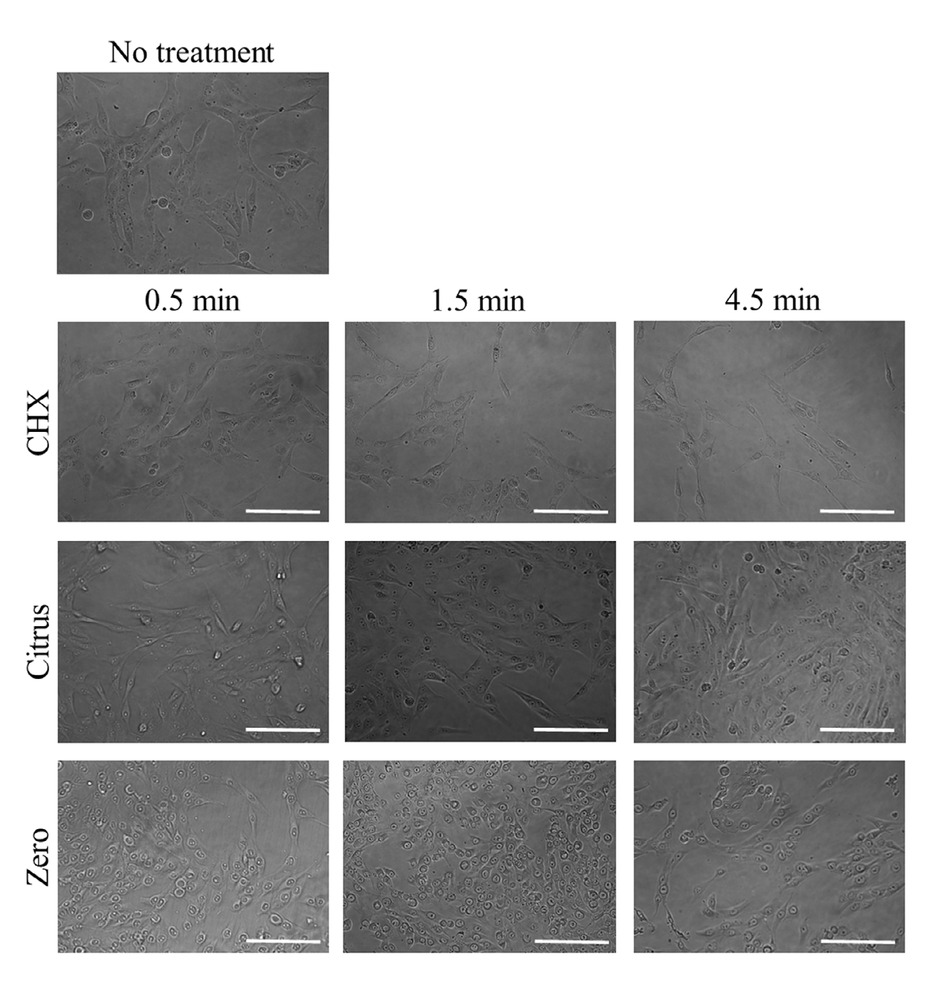
Evaluation of cellular morphology after treatment with 0.12% CHX, LIS Citrus, and LIS Zero. The scale bar indicates 200 μm.
3.2 Cellular viability
The Cell Counting Kit-8 assay showed that the treatment with CHX, LIS Citrus, and LIS Zero groups affected cell viability (Figure 3). The CHX, LIS Citrus, and LIS Zero showed cytotoxic effects on osteoblast-like cells in vitro, with a mean viability of 9.8% ± 0.2%, 11.6% ± 0.1%, and 12.5% ± 0.3%, respectively, after exposure for 30 sec when the control group was considered 100% (100.0% ± 3.0%). The progressive increase in the treatment time of CHX, LIS Citrus, and LIS Zero up to 4.5 min did not induce significant decreases of viability. The mean cell viability for CHX was 10.9% ± 0.5% and 10.3% ± 0.9% for 1.5 and 4.5 min (P=0.05). The viability of LIS Citrus for 1.5 min and 4.5 min was 11.8% ± 0.5% and 11.9% ± 0.6%, respectively (P=0.05). The mean cell viability for LIS Zero was 11.6% ± 0.3% and 11.6% ± 0.0% for 1.5 and 4.5 min (P=0.05).
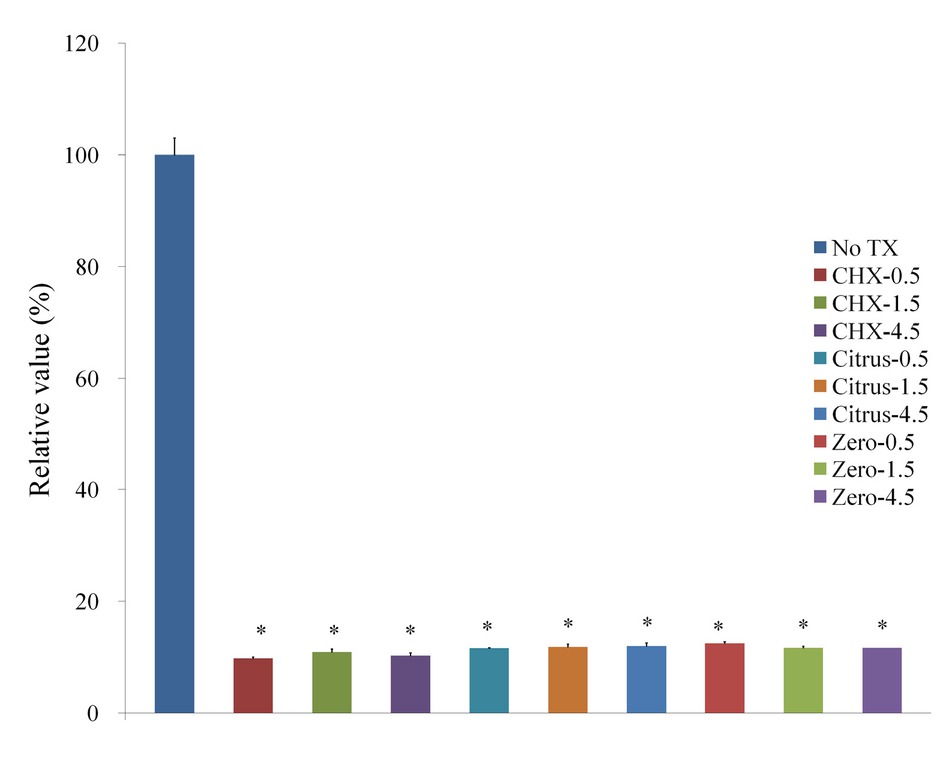
Cellular viability using Cell Counting Kit-8 after treatment with 0.12% CHX, LIS Citrus, and LIS Zero.
Cellular morphology after treatment with GGN formulations (Garglin® Regular, Garglin® Medical, and Garglin® Child) is shown in Figure 4. Alteration in the cytoskeletal organization was noted irrespective of GGN formulations. The treatment with GGN formulations affected cell viability (Figure 5).
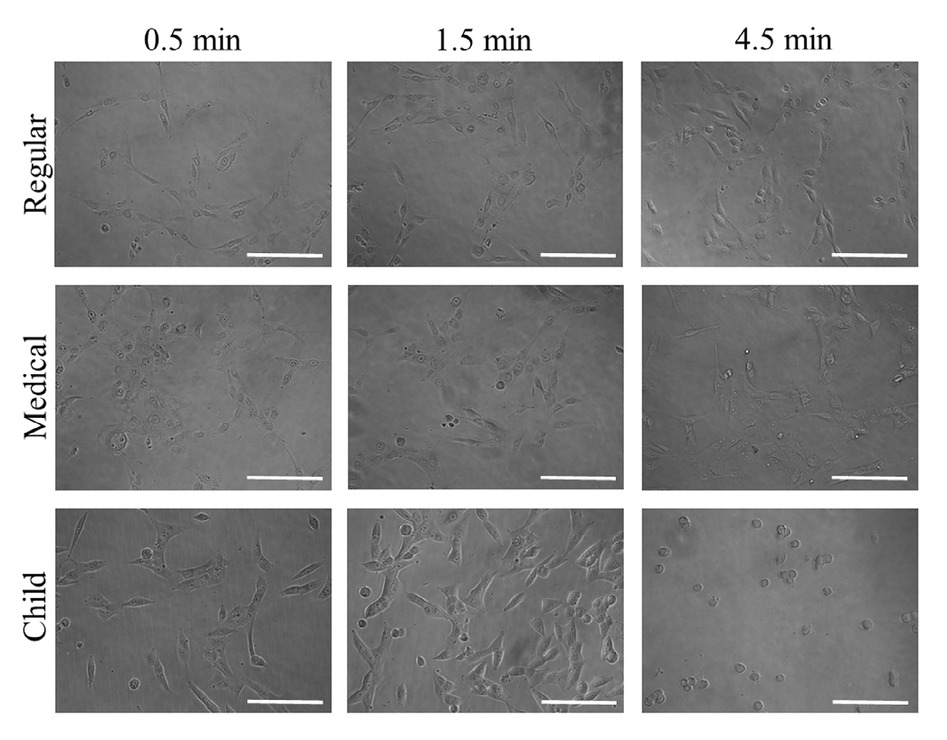
Evaluation of cellular morphology after treatment with Gargin® formulations (GGN, GGN Med, and GGN Child).
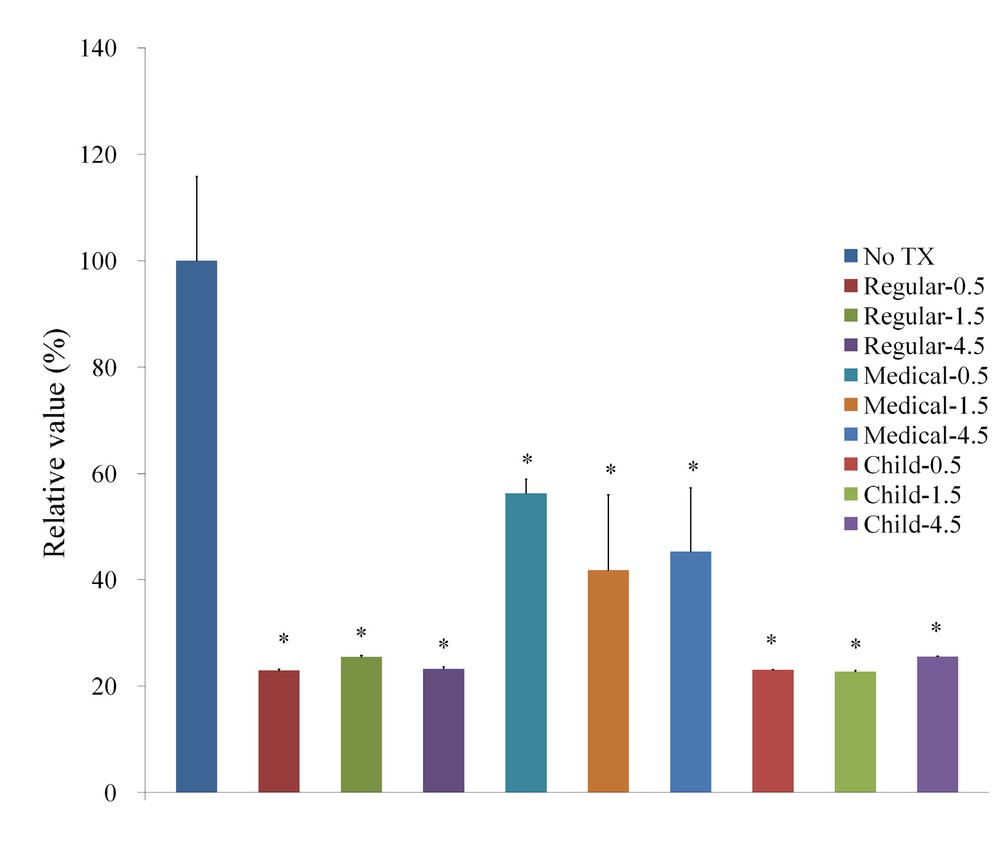
Cellular viability using Cell Counting Kit-8 after treatment with Gargin® formulations.
4 Discussion
This study showed that all of the mouthwashes—CHX, LIS, and GGN—inhibited viability and deformed the morphology of mouse calvarial preosteoblast cells. The progressive increase in the treatment time of CHX, LIS, and GGN up to 4.5 min did not induce significant decreases of viability compared to the 30 sec group. Alcohol adjuvant was not likely to affect the cytotoxicity of the mouthwashes, irrespective of brand.
A comparison between mouthwashes was performed previously. A study showed that CHX reduced the proliferation of gingival fibroblast in a dose-dependent manner [1]. The 0.12% CHX totally inhibits cell proliferation, and even 0.01% CHX inhibits cell proliferation by 50%. The dilution of essential oil (LIS), however, did not result in an antibacterial effect as much as with CHX. Also, Listerine® did not show sustained toxic effects on fibroblasts as much as CHX. Another comparative study showed that 0.2% CHX was more cytotoxic to LIS cool mint on human gingival fibroblasts [10]. Similarly, the antibacterial effect of CHX is the most powerful, followed by LIS in terms of viability of plaque bacteria [11]. A comparison of the antiseptic effects between LIS and CPC was performed previously. There was no statistically significant difference in prevention of the plaque and gingivitis benefits between the CPC mouth rinse and the EO mouthwash [3]. Another study demonstrated the superiority of daily use of LIS compared to 0.05% CPC mouthwash in decreasing plaque and gingivitis [12].
Because the cytotoxicity of mouthwashes is indiscriminate, we investigated adverse effects on various cells. Inhibition of oral fibroblastic wound healing [7,13, 14, 15] and odontoblastic tooth repair [16, 17] by CHX were noted as well as an antiseptic effect on microorganisms [18,19]. Another study reported toxic effects of CHX on human periodontal ligament cells [20]. Deletion of glutathione, which has a role in cellular protection from damage from reactive oxygen species, was proposed to be the mechanism of the CHX-induced cytotoxicity [21].
As for toxic effects on bones, CHX can have propound cytotoxic activity to osteoblast-like cells. CHX reduced the cell viability and differentiation potential in vitro on human osteoblastic cells from periodontium [22,23]. Similarly, the application of 0.12% CHX during a 2-min period immediately led to human alveolar bone cell destruction [19]. Another report for the human osteosarcoma cells revealed that CHX induced mitochondrial dysfunction, an increase in intracellular calcium ion, and oxidative stress, which resulted in apoptotic and necrotic cell deaths [24].
Thymol, one of the active ingredients of the essential oils (LIS), induced numerical chromosome abnormalities in rat bone marrow cells in a dose-dependent manner and inhibited the mitotic index irrespectively of treatment time or concentration of thymol [25]. Other studies about various cell lines, including murine melanomas [26] and human colon adenocarcinoma cells [27], showed significantly reduced cell viability or induced an antitumor effect by thymol. This study also found a cytotoxic effect of LIS Citrus and LIS Zero after treatment for 30 sec or more. Therefore, we could postulate that LIS, through active ingredients including thymol, can be a potent inhibitor for bone regeneration.
Previous clinical studies showed positive antiplaque activity and reduction of gingivitis/gingival bleeding with regular use of CPC, an ingredient of GGN, after tooth brushing for 6-month clinical trials [28,29]. A systematic review demonstrated that CPC mouth rinse, when used for adjuncts, provides a small but significant beneficial effect in reducing plaque and gingivitis [5]. The CPC is more cytotoxic to oral fungus than CHX [30]. Systematic reviews revealed that CPC may also be effective in reducing oral malodor [31, 32, 33]. However, there are few previous studies of CPC regarding its effect on osteoblast. Interestingly, one study showed CPC inhibition of osteoclast differentiation by suppressing the RANKL-induced expression of c-Fos and NFATc1 via ERK and NF-κB pathways [9]. They suggest that by modulation of RANKL/RANK signaling, and subsequent osteoclast inhibition, prevention of periodontal bone resorption would be possible.
The results of this study suggest that careful use of mouthwash is necessary when a planned intra-wound application such as guided bone or tissue regeneration because the aforementioned mouthwashes can harm the bone formation. Harvested bone soaked and cleaned with CHX showed total cell death, supporting this finding [34]. Interestingly, CHX can be applied for periodontal regeneration if it is used at very low concentrations or slow-release kinetics [19,35]. Unless the mouthwashes are diluted at a very low concentration, they should be used with caution on the bone.
This study demonstrated no significant difference of cytotoxicity between the 30-s, 1.5-min, and 4.5-min groups, irrespective of mouthwashes. Similar experiments were performed for the stem cells from buccal fat pads, which showed reduced cell viability after CHX or LIS treatment for either 30 s, 1.5 min, or 4.5 min, but no significant decrease in viability from 30 sec up to 4.5 min [36]. These results were contradictory to previous studies. CHX was cytotoxic to human periodontal ligament (PDL) cells [20], odontoblast-like cells [16], and stem cells from human exfoliated deciduous teeth in dose and time-dependent manners [37]. A report showed that although 0.12% (1.2 mg/ml) CHX has a strong cytotoxicity and DNA cell damage compared to other agents due to substantivity, this effect is not harmful to epithelial wound healing because CHX does not penetrate the basal layer of the epithelium [6]. Collectively, the responses against mouthwashes seem to differ depending on the type of cells.
Ethanol in mouthwashes can be used as a preservative, stabilizer, solubilizer, sensory cue with a distinctive taste, and antiplaque efficacy enhancer (adjuvant effect). Ethanol and methylsalicylate components in the LIS deliver strong antioxidant traits [38]. LIS can act as an antioxidant to eradicate the negative effects of oxygen-free radicals on epithelial cells and maintain epithelialization of the wound. Some formulas without alcohol have recently become available because of bad taste, mucosal irritation, fear of cancer, etc. There has been concern about whether use of mouthwashes containing alcohol increases the prevalence of oral cancer [39] because alcohol consumption is a known risk indicator for oral cancer [40]. However, a recent systematic review demonstrated that there is no association between the use of mouthwashes containing alcohol and oral cancer [41]. This study also failed to find differences of cytotoxicity to osteoblast-like cells between alcohol and nonalcoholic mouthwashes within the same brand.
This study has some limitations; First, it is confined to a laboratory environment that may be different from the real oral environment. Further, in vivo, or human clinical trials, would be required to elucidate the efficacy and safety of mouthwashes. Second, various stem or progenitor cells can involve periodontal regeneration, therefore it would be necessary to examine other precursor cells. This study also has beneficial points; We compared several types and subtypes of mouthwashes, suggesting evidence of safety, and offering guidelines for adequate treatment time.
In conclusion, commercially available mouthwashes could inhibit cell viability and alter morphology of osteoblastic precursor cells irrespectively of brand, treatment time, or alcohol content.
Ethical approval: The conducted research is not related to either human or animals use.
Conflict of interest: Authors state no conflict of interest
References
[1] Tsourounakis I, Palaiologou-Gallis AA, Stoute D et al. Effect of essential oil and chlorhexidine mouthwashes on gingival fibroblast survival and migration. J Periodontol 2013, 84, 1211-122010.1902/jop.2012.120312Search in Google Scholar PubMed
[2] Chen Y, Wong RW, Seneviratne CJ et al. Comparison of the antimicrobial activity of Listerine and Corsodyl on orthodontic brackets in vitro. Am J Orthod Dentofacial Orthop 2011, 140, 537-54210.1016/j.ajodo.2011.01.022Search in Google Scholar PubMed
[3] Albert-Kiszely A, Pjetursson BE, Salvil GE et al. Comparison of the effects of cetylpyridinium chloride with an essential oil mouth rinse on dental plaque and gingivitis - a six-month randomized controlled clinical trial. Journal of Clinical Periodontology 2007, 34, 658-66710.1111/j.1600-051X.2007.01103.xSearch in Google Scholar PubMed
[4] Costa X, Laguna E, Herrera D et al. Efficacy of a new mouth rinse formulation based on 0.07% cetylpyridinium chloride in the control of plaque and gingivitis: a 6-month randomized clinical trial. J Clin Periodontol 2013, 40, 1007-101510.1111/jcpe.12158Search in Google Scholar PubMed
[5] Haps S, Slot DE, Berchier CE et al. The effect of cetylpyridinium chloride-containing mouth rinses as adjuncts to toothbrushing on plaque and parameters of gingival inflammation: a systematic review. Int J Dent Hyg 2008, 6, 290-30310.1111/j.1601-5037.2008.00344.xSearch in Google Scholar PubMed
[6] Kozlovsky A, Artzi Z, Hirshberg A et al. Effect of local antimicrobial agents on excisional palatal wound healing: a clinical and histomorphometric study in rats. J Clin Periodontol 2007, 34, 164-17110.1111/j.1600-051X.2006.01033.xSearch in Google Scholar PubMed
[7] Ribeiro DA, Bazo AP, da Silva Franchi CA et al. Chlorhexidine induces DNA damage in rat peripheral leukocytes and oral mucosal cells. J Periodontal Res 2004, 39, 358-36110.1111/j.1600-0765.2004.00759.xSearch in Google Scholar PubMed
[8] Bhandari M, Adili A, Schemitsch EH. The efficacy of low-pressure lavage with different irrigating solutions to remove adherent bacteria from bone. J Bone Joint Surg Am 2001, 83-a, 412-41910.2106/00004623-200103000-00014Search in Google Scholar PubMed
[9] Zheng T, Chen L, Noh AL et al. Cetylpyridinium chloride inhibits receptor activator of nuclear factor-kappaB ligand-induced osteoclast formation. Biol Pharm Bull 2013, 36, 509-51410.1248/bpb.b12-00460Search in Google Scholar PubMed
[10] Flemingson, Emmadi P, Ambalavanan N et al. Effect of three commercial mouth rinses on cultured human gingival fibroblast: an in vitro study. Indian J Dent Res 2008, 19, 29-3510.4103/0970-9290.38929Search in Google Scholar PubMed
[11] Brecx M, Brownstone E, Macdonald L et al. Efficacy of Listerine, Meridol and Chlorhexidine Mouthrinses as Supplements to Regular Tooth-Cleaning Measures. J Clin Periodontol 1992, 19, 202-20710.1111/j.1600-051X.1992.tb00640.xSearch in Google Scholar PubMed
[12] Sharma NC, Araujo MW, Wu MM et al. Superiority of an essential oil mouthrinse when compared with a 0.05% cetylpyridinium chloride containing mouthrinse: a six-month study. Int Dent J 2010, 60, 175-180Search in Google Scholar
[13] Pucher JJ, Daniel JC. The effects of chlorhexidine digluconate on human fibroblasts in vitro. J Periodontol 1992, 63, 526-53210.1902/jop.1992.63.6.526Search in Google Scholar PubMed
[14] Wyganowska-Swiatkowska M, Kotwicka M, Urbaniak P et al. Clinical implications of the growth-suppressive effects of chlorhexidine at low and high concentrations on human gingival fibroblasts and changes in morphology. Int J Mol Med 2016, 37, 1594-160010.3892/ijmm.2016.2550Search in Google Scholar PubMed
[15] Verma UP, Gupta A, Yadav RK et al. Cytotoxicity of chlorhexidine and neem extract on cultured human gingival fibroblasts through fluorescence-activated cell sorting analysis : An in-vitro study. Eur J Dent 2018, 12, 344-34910.4103/ejd.ejd_149_17Search in Google Scholar PubMed PubMed Central
[16] Lessa FC, Aranha AM, Nogueira I et al. Toxicity of chlorhexidine on odontoblast-like cells. J Appl Oral Sci 2010, 18, 50-5810.1590/S1678-77572010000100010Search in Google Scholar
[17] de Souza LB, de Aquino SG, de Souza PP et al. Cytotoxic effects of different concentrations of chlorhexidine. Am J Dent 2007, 20, 400-404Search in Google Scholar
[18] Soares AF, Aquino AR, Carvalho CH et al. Frequency of oral mucositis and microbiological analysis in children with acute lymphoblastic leukemia treated with 0.12% chlorhexidine gluconate. Braz Dent J 2011, 22, 312-31610.1590/S0103-64402011000400009Search in Google Scholar PubMed
[19] Cabral CT, Fernandes MH. In vitro comparison of chlorhexidine and povidone-iodine on the long-term proliferation and functional activity of human alveolar bone cells. Clin Oral Investig 2007, 11, 155-16410.1007/s00784-006-0094-8Search in Google Scholar PubMed
[20] Chang YC, Huang FM, Tai KW et al. The effect of sodium hypochlorite and chlorhexidine on cultured human periodontal ligament cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001, 92, 446-45010.1067/moe.2001.116812Search in Google Scholar PubMed
[21] Lee TH, Hu CC, Lee SS et al. Cytotoxicity of chlorhexidine on human osteoblastic cells is related to intracellular glutathione levels. Int Endod J 2010, 43, 430-43510.1111/j.1365-2591.2010.01700.xSearch in Google Scholar PubMed
[22] Almazin SM, Dziak R, Andreana S et al. The effect of doxycycline hyclate, chlorhexidine gluconate, and minocycline hydrochloride on osteoblastic proliferation and differentiation in vitro. J Periodontol 2009, 80, 999-100510.1902/jop.2009.080574Search in Google Scholar PubMed
[23] Liu JX, Werner J, Kirsch T et al. Cytotoxicity evaluation of chlorhexidine gluconate on human fibroblasts, myoblasts, and osteoblasts. J Bone Jt Infect 2018, 3, 165-17210.7150/jbji.26355Search in Google Scholar PubMed PubMed Central
[24] Giannelli M, Chellini F, Margheri M et al. Effect of chlorhexidine digluconate on different cell types: a molecular and ultrastructural investigation. Toxicol In Vitro 2008, 22, 308-31710.1016/j.tiv.2007.09.012Search in Google Scholar PubMed
[25] Azirak S, Rencuzogullari E. The in vivo genotoxic effects of carvacrol and thymol in rat bone marrow cells. Environ Toxicol 2008, 23, 728-73510.1002/tox.20380Search in Google Scholar PubMed
[26] He L, Mo HB, Hadisusilo S et al. Isoprenoids suppress the growth of murine B16 melanomas in vitro and in vivo. J Nutr 1997, 127, 668-67410.1093/jn/127.5.668Search in Google Scholar PubMed
[27] Yousefzadi M, Riahi-Madvar A, Hadian J et al. In vitro cytotoxic and antimicrobial activity of essential oil from Satureja sahendica. Toxicol Environ Chem 2012, 94, 1735-174510.1080/02772248.2012.728606Search in Google Scholar
[28] Mankodi S, Bauroth K, Witt JJ et al. A 6-month clinical trial to study the effects of a cetylpyridinium chloride mouthrinse on gingivitis and plaque. Am J Dent 2005, 18 Spec No, 9A-14ASearch in Google Scholar
[29] Allen DR, Davies R, Bradshaw B et al. Efficacy of a mouthrinse containing 0.05% cetylpyridinium chloride for the control of plaque and gingivitis: a 6-month clinical study in adults. Compend Contin Educ Dent 1998, 19, 20-26Search in Google Scholar
[30] Fathilah AR, Himratul-Aznita WH, Fatheen AR et al. The antifungal properties of chlorhexidine digluconate and cetylpyrinidinium chloride on oral Candida. J Dent 2012, 40, 609-61510.1016/j.jdent.2012.04.003Search in Google Scholar PubMed
[31] Blom T, Slot DE, Quirynen M et al. The effect of mouthrinses on oral malodor: a systematic review. Int J Dent Hyg 2012, 10, 209-22210.1111/j.1601-5037.2012.00546.xSearch in Google Scholar PubMed
[32] Roldan S, Winkel EG, Herrera D et al. The effects of a new mouthrinse containing chlorhexidine, cetylpyridinium chloride and zinc lactate on the microflora of oral halitosis patients: a dual-centre, double-blind placebo-controlled study. J Clin Periodontol 2003, 30, 427-43410.1034/j.1600-051X.2003.20004.xSearch in Google Scholar PubMed
[33] Roldan S, Herrera D, O’Connor A et al. A combined therapeutic approach to manage oral halitosis: a 3-month prospective case series. J Periodontol 2005, 76, 1025-103310.1902/jop.2005.76.6.1025Search in Google Scholar PubMed
[34] Bauer J, Liu RW, Kean TJ et al. A comparison of five treatment protocols for contaminated bone grafts in reference to sterility and cell viability. J Bone Joint Surg Am 2011, 93, 439-44410.2106/JBJS.J.00418Search in Google Scholar PubMed
[35] Soriano-Souza CA, Rossi AL, Mavropoulos E et al. Chlorhexidine-loaded hydroxyapatite microspheres as an antimicrobial delivery system and its effect on in vivo osteo-conductive properties. J Mater Sci Mater Med 2015, 26, 16610.1007/s10856-015-5505-4Search in Google Scholar PubMed
[36] Park JB, Lee G, Yun BG et al. Comparative effects of chlorhexidine and essential oils containing mouth rinse on stem cells cultured on a titanium surface. Mol Med Rep 2014, 9, 1249-125310.3892/mmr.2014.1971Search in Google Scholar PubMed
[37] Tu YY, Yang CY, Chen RS et al. Effects of chlorhexidine on stem cells from exfoliated deciduous teeth. J Formos Med Assoc 2015, 114, 17-2210.1016/j.jfma.2012.12.008Search in Google Scholar PubMed
[38] Battino M, Ferreiro MS, Fattorini D et al. In vitro antioxidant activities of mouthrinses and their components. J Clin Periodontol 2002, 29, 462-46710.1034/j.1600-051X.2002.290512.xSearch in Google Scholar PubMed
[39] Weaver A, Fleming SM, Smith DB. Mouthwash and oral cancer: carcinogen or coincidence? J Oral Surg 1979, 37, 250-253Search in Google Scholar
[40] Chi AC, Day TA, Neville BW. Oral cavity and oropharyngeal squamous cell carcinoma--an update. CA Cancer J Clin 2015, 65, 401-42110.3322/caac.21293Search in Google Scholar PubMed
[41] Gandini S, Negri E, Boffetta P et al. Mouthwash and oral cancer risk quantitative meta-analysis of epidemiologic studies. Ann Agric Environ Med 2012, 19, 173-180Search in Google Scholar
© 2019 In-Seok Song et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Articles in the same Issue
- Plant Sciences
- Extended low temperature and cryostorage longevity of Salix seeds with desiccation control
- Genome-wide analysis of the WRKY gene family and its response to abiotic stress in buckwheat (Fagopyrum tataricum)
- Differential expression of microRNAs during root formation in Taxus chinensis var. mairei cultivars
- Metabolomics Approach for The Analysis of Resistance of Four Tomato Genotypes (Solanum lycopersicum L.) to Root-Knot Nematodes (Meloidogyne incognita)
- Beneficial Effects of Salt on Halophyte Growth: Morphology, Cells, and Genes
- Phosphate-solubilizing bacteria from safflower rhizosphere and their effect on seedling growth
- Anatomy and Histochemistry of the Roots and Shoots in the Aquatic Selenium Hyperaccumulator Cardamine hupingshanensis (Brassicaceae)
- Effects of LED light on Acacia melanoxylon bud proliferation in vitro and root growth ex vitro
- Ecology and Environmental Sciences
- Intensity of stripping and sugar content in the bark and the bast of European beech (Fagus sylvatica)
- Influence of monometallic and bimetallic phytonanoparticles on physiological status of mezquite
- Loci identification of a N-acyl homoserine lactone type quorum sensing system and a new LysR-type transcriptional regulator associated with antimicrobial activity and swarming in Burkholderia gladioli UAPS07070
- Bacillus methylotrophicus has potential applications against Monilinia fructicola
- Evaluation of Heavy Metals and Microbiological Contamination of Selected herbals from Palestine
- The effect of size of black cherry stumps on the composition of fungal communities colonising stumps
- Effect of rhamnolipids on microbial biomass content and biochemical parameters in soil contaminated with coal tar creosote
- Effects of foliar trichomes on the accumulation of atmospheric particulates in Tillandsia brachycaulos
- Isolation and characterisation of the agarolytic bacterium Pseudoalteromonas ruthenica
- Comparison of soil bioconditioners and standard fertilization in terms of the impact on yield and vitality of Lolium perenne and soil biological properties
- Biomedical Sciences
- The number of regulatory B cells is increased in mice with collagen-induced arthritis
- Lactate overload inhibits myogenic activity in C2C12 myotubes
- Diagnostic performance of serum CK-MB, TNF-α and hs-CRP in children with viral myocarditis
- Correlation between PPARGC1A gene rs8192678 G>A polymorphism and susceptibility to type-2 diabetes
- Improving the Detection of Hepatocellular Carcinoma using serum AFP expression in combination with GPC3 and micro-RNA miR-122 expression
- The ratio of neutrophil to lymphocyte is a predictor in endometrial cancer
- Expression of HER2/c-erbB-2, EGFR protein in gastric carcinoma and its clinical significance
- Clinical significance of neuropeptide Y expression in pelvic tissue in patients with pelvic floor dysfunction
- Overexpression of RASAL1 indicates poor prognosis and promotes invasion of ovarian cancer
- The effect of adrenaline on the mineral and trace element status in rats
- Effects of Ischemic Post-Conditioning on the Expressions of LC3-II and Beclin-1 in the Hippocampus of Rats after Cerebral Ischemia and Reperfusion
- Long non-coding RNA DUXAP8 regulates the cell proliferation and invasion of non-small-cell lung cancer
- Risk factors of regional lymph node metastasis in patients with cervical cancer
- Bullous prurigo pigmentosa
- Association of HIF-1α and NDRG2 expression with EMT in gastric cancer tissues
- Decrease in the level of nervonic acid and increased gamma linolenic acid in the plasma of women with polycystic ovary syndrome after a three-month low-glycaemic index and caloric reduction diet
- Depletion of VAX2 restrains the malignant progression of papillary thyroid carcinoma by modulating ERK signaling pathway
- Insulin resistance is a risk factor for mild cognitive impairment in elderly adults with T2DM
- Nurr1 promotes lung cancer apoptosis via enhancing mitochondrial stress and p53-Drp1 pathway
- Predictive significance of serum MMP-9 in papillary thyroid carcinoma
- Agmatine prevents oxidative-nitrative stress in blood leukocytes under streptozotocin-induced diabetes mellitus
- Effect of platelet-rich plasma on implant bone defects in rabbits through the FAK/PI3K/AKT signaling pathway
- The diagnostic efficacy of thrombelastography (TEG) in patients with preeclampsia and its association with blood coagulation
- Value of NSE and S100 Protein of Kawasaki Disease with aseptic meningitis in Infant
- CB2 receptor agonist JWH133 activates AMPK to inhibit growth of C6 glioma cells
- The effects of various mouthwashes on osteoblast precursor cells
- Co-downregulation of GRP78 and GRP94 induces apoptosis and inhibits migration in prostate cancer cells
- SKA3 up-regulation promotes lung adenocarcinoma growth and is a predictor of poor prognosis
- Protective effects and mechanisms of microRNA-182 on oxidative stress in RHiN
- A case of syphilis with high bone arsenic concentration from early modern cemetery (Wroclaw, Poland)
- Study of LBHD1 Expression with Invasion and Migration of Bladder Cancer
- 1-Hydroxy-8-methoxy-anthraquinon reverses cisplatin resistance by inhibiting 6PGD in cancer cells
- Andrographolide as a therapeutic agent against breast and ovarian cancers
- Accumulation of α-2,6-sialyoglycoproteins in the muscle sarcoplasm due to Trichinella sp. invasion
- Astragalus polysaccharides protects thapsigargin-induced endoplasmic reticulum stress in HT29 cells
- IGF-1 via PI3K/Akt/S6K signaling pathway protects DRG neurons with high glucose-induced toxicity
- Intra-arterial tirofiban in a male nonagenarian with acute ischemic stroke: A case report
- Effects of Huaiqihuang Granules adjuvant therapy in children with primary nephrotic syndrome
- Immune negative regulator TIPE2 inhibits cervical squamous cancer progression through Erk1/2 signaling
- Asymptomatic mediastinal extra-adrenal paraganglioma as a cause of sudden death: a case Report
- Primary mucinous adenocarcinoma of appendix invading urinary bladder with a fistula: a case report
- Minocycline attenuates experimental subarachnoid hemorrhage in rats
- Neural Remodeling of the Left Atrium in rats by Rosuvastatin following Acute Myocardial Infarction
- Protective effects of emodin on lung injuries in rat models of liver fibrosis
- RHOA and mDia1 promotes apoptosis of breast cancer cells via a high dose of doxorubicin treatment
- Bacteria co-colonizing with Clostridioides difficile in two asymptomatic patients
- A allele of ICAM-1 rs5498 and VCAM-1 rs3181092 is correlated with increased risk for periodontal disease
- Treatment of hepatic cystic echinococcosis patients with clear cell renal carcinoma: a case report
- Edaravone exerts brain protective function by reducing the expression of AQP4, APP and Aβ proteins
- Correlation between neutrophil count and prognosis in STEMI patients with chronic renal dysfunction: a retrospective cohort study
- Bioinformatic analysis reveals GSG2 as a potential target for breast cancer therapy
- Nuciferine prevents hepatic steatosis by regulating lipid metabolismin diabetic rat model
- Analysis of SEC24D gene in breast cancer based on UALCAN database
- Bioengineering and Biotechnology
- Co-cultured Bone-marrow Derived and Tendon Stem Cells: Novel Seed Cells for Bone Regeneration
- Animal Sciences
- Comparative analysis of gut microbiota among the male, female and pregnant giant pandas (Ailuropoda Melanoleuca)
- Adaptive immunity and skin wound healing in amphibian adults
- Hox genes polymorphism depicts developmental disruption of common sole eggs
- The prevalence of virulence genes and multidrug resistance in thermophilic Campylobacter spp. isolated from dogs
- Agriculture
- Effect of Lactobacillus plantarum supplementation on production performance and fecal microbial composition in laying hens
- Identification of Leaf Rust Resistance Genes in Selected Wheat Cultivars and Development of Multiplex PCR
- Determining Potential Feed Value and Silage Quality of Guar Bean (Cyamopsis tetragonoloba) Silages
- Food Science
- Effect of Thermal Processing on Antioxidant Activity and Cytotoxicity of Waste Potato Juice
Articles in the same Issue
- Plant Sciences
- Extended low temperature and cryostorage longevity of Salix seeds with desiccation control
- Genome-wide analysis of the WRKY gene family and its response to abiotic stress in buckwheat (Fagopyrum tataricum)
- Differential expression of microRNAs during root formation in Taxus chinensis var. mairei cultivars
- Metabolomics Approach for The Analysis of Resistance of Four Tomato Genotypes (Solanum lycopersicum L.) to Root-Knot Nematodes (Meloidogyne incognita)
- Beneficial Effects of Salt on Halophyte Growth: Morphology, Cells, and Genes
- Phosphate-solubilizing bacteria from safflower rhizosphere and their effect on seedling growth
- Anatomy and Histochemistry of the Roots and Shoots in the Aquatic Selenium Hyperaccumulator Cardamine hupingshanensis (Brassicaceae)
- Effects of LED light on Acacia melanoxylon bud proliferation in vitro and root growth ex vitro
- Ecology and Environmental Sciences
- Intensity of stripping and sugar content in the bark and the bast of European beech (Fagus sylvatica)
- Influence of monometallic and bimetallic phytonanoparticles on physiological status of mezquite
- Loci identification of a N-acyl homoserine lactone type quorum sensing system and a new LysR-type transcriptional regulator associated with antimicrobial activity and swarming in Burkholderia gladioli UAPS07070
- Bacillus methylotrophicus has potential applications against Monilinia fructicola
- Evaluation of Heavy Metals and Microbiological Contamination of Selected herbals from Palestine
- The effect of size of black cherry stumps on the composition of fungal communities colonising stumps
- Effect of rhamnolipids on microbial biomass content and biochemical parameters in soil contaminated with coal tar creosote
- Effects of foliar trichomes on the accumulation of atmospheric particulates in Tillandsia brachycaulos
- Isolation and characterisation of the agarolytic bacterium Pseudoalteromonas ruthenica
- Comparison of soil bioconditioners and standard fertilization in terms of the impact on yield and vitality of Lolium perenne and soil biological properties
- Biomedical Sciences
- The number of regulatory B cells is increased in mice with collagen-induced arthritis
- Lactate overload inhibits myogenic activity in C2C12 myotubes
- Diagnostic performance of serum CK-MB, TNF-α and hs-CRP in children with viral myocarditis
- Correlation between PPARGC1A gene rs8192678 G>A polymorphism and susceptibility to type-2 diabetes
- Improving the Detection of Hepatocellular Carcinoma using serum AFP expression in combination with GPC3 and micro-RNA miR-122 expression
- The ratio of neutrophil to lymphocyte is a predictor in endometrial cancer
- Expression of HER2/c-erbB-2, EGFR protein in gastric carcinoma and its clinical significance
- Clinical significance of neuropeptide Y expression in pelvic tissue in patients with pelvic floor dysfunction
- Overexpression of RASAL1 indicates poor prognosis and promotes invasion of ovarian cancer
- The effect of adrenaline on the mineral and trace element status in rats
- Effects of Ischemic Post-Conditioning on the Expressions of LC3-II and Beclin-1 in the Hippocampus of Rats after Cerebral Ischemia and Reperfusion
- Long non-coding RNA DUXAP8 regulates the cell proliferation and invasion of non-small-cell lung cancer
- Risk factors of regional lymph node metastasis in patients with cervical cancer
- Bullous prurigo pigmentosa
- Association of HIF-1α and NDRG2 expression with EMT in gastric cancer tissues
- Decrease in the level of nervonic acid and increased gamma linolenic acid in the plasma of women with polycystic ovary syndrome after a three-month low-glycaemic index and caloric reduction diet
- Depletion of VAX2 restrains the malignant progression of papillary thyroid carcinoma by modulating ERK signaling pathway
- Insulin resistance is a risk factor for mild cognitive impairment in elderly adults with T2DM
- Nurr1 promotes lung cancer apoptosis via enhancing mitochondrial stress and p53-Drp1 pathway
- Predictive significance of serum MMP-9 in papillary thyroid carcinoma
- Agmatine prevents oxidative-nitrative stress in blood leukocytes under streptozotocin-induced diabetes mellitus
- Effect of platelet-rich plasma on implant bone defects in rabbits through the FAK/PI3K/AKT signaling pathway
- The diagnostic efficacy of thrombelastography (TEG) in patients with preeclampsia and its association with blood coagulation
- Value of NSE and S100 Protein of Kawasaki Disease with aseptic meningitis in Infant
- CB2 receptor agonist JWH133 activates AMPK to inhibit growth of C6 glioma cells
- The effects of various mouthwashes on osteoblast precursor cells
- Co-downregulation of GRP78 and GRP94 induces apoptosis and inhibits migration in prostate cancer cells
- SKA3 up-regulation promotes lung adenocarcinoma growth and is a predictor of poor prognosis
- Protective effects and mechanisms of microRNA-182 on oxidative stress in RHiN
- A case of syphilis with high bone arsenic concentration from early modern cemetery (Wroclaw, Poland)
- Study of LBHD1 Expression with Invasion and Migration of Bladder Cancer
- 1-Hydroxy-8-methoxy-anthraquinon reverses cisplatin resistance by inhibiting 6PGD in cancer cells
- Andrographolide as a therapeutic agent against breast and ovarian cancers
- Accumulation of α-2,6-sialyoglycoproteins in the muscle sarcoplasm due to Trichinella sp. invasion
- Astragalus polysaccharides protects thapsigargin-induced endoplasmic reticulum stress in HT29 cells
- IGF-1 via PI3K/Akt/S6K signaling pathway protects DRG neurons with high glucose-induced toxicity
- Intra-arterial tirofiban in a male nonagenarian with acute ischemic stroke: A case report
- Effects of Huaiqihuang Granules adjuvant therapy in children with primary nephrotic syndrome
- Immune negative regulator TIPE2 inhibits cervical squamous cancer progression through Erk1/2 signaling
- Asymptomatic mediastinal extra-adrenal paraganglioma as a cause of sudden death: a case Report
- Primary mucinous adenocarcinoma of appendix invading urinary bladder with a fistula: a case report
- Minocycline attenuates experimental subarachnoid hemorrhage in rats
- Neural Remodeling of the Left Atrium in rats by Rosuvastatin following Acute Myocardial Infarction
- Protective effects of emodin on lung injuries in rat models of liver fibrosis
- RHOA and mDia1 promotes apoptosis of breast cancer cells via a high dose of doxorubicin treatment
- Bacteria co-colonizing with Clostridioides difficile in two asymptomatic patients
- A allele of ICAM-1 rs5498 and VCAM-1 rs3181092 is correlated with increased risk for periodontal disease
- Treatment of hepatic cystic echinococcosis patients with clear cell renal carcinoma: a case report
- Edaravone exerts brain protective function by reducing the expression of AQP4, APP and Aβ proteins
- Correlation between neutrophil count and prognosis in STEMI patients with chronic renal dysfunction: a retrospective cohort study
- Bioinformatic analysis reveals GSG2 as a potential target for breast cancer therapy
- Nuciferine prevents hepatic steatosis by regulating lipid metabolismin diabetic rat model
- Analysis of SEC24D gene in breast cancer based on UALCAN database
- Bioengineering and Biotechnology
- Co-cultured Bone-marrow Derived and Tendon Stem Cells: Novel Seed Cells for Bone Regeneration
- Animal Sciences
- Comparative analysis of gut microbiota among the male, female and pregnant giant pandas (Ailuropoda Melanoleuca)
- Adaptive immunity and skin wound healing in amphibian adults
- Hox genes polymorphism depicts developmental disruption of common sole eggs
- The prevalence of virulence genes and multidrug resistance in thermophilic Campylobacter spp. isolated from dogs
- Agriculture
- Effect of Lactobacillus plantarum supplementation on production performance and fecal microbial composition in laying hens
- Identification of Leaf Rust Resistance Genes in Selected Wheat Cultivars and Development of Multiplex PCR
- Determining Potential Feed Value and Silage Quality of Guar Bean (Cyamopsis tetragonoloba) Silages
- Food Science
- Effect of Thermal Processing on Antioxidant Activity and Cytotoxicity of Waste Potato Juice

