Abstract
Background and aims
Persistent postoperative pain (PPP) is a significant clinical problem. Several patient-related risk factors for PPP have been identified, including a previous chronic pain problem, young age, female gender and psychological vulnerability. Intra- and postoperative risk factors include surgical complications such as infections, haematoma, nerve damage and repeated surgery. As the length of hospital stay has been shortened, some patients may be discharged despite ongoing pain and insufficient analgesic medication. The challenge is to identify patients at high risk of developing PPP and to create a targeted care pathway to ensure effective and safe pain treatment especially in the subacute postoperative phase at home. This observational study describes the first two years of the Acute Pain Service Out-Patient Clinic (APS-OPC) at the Helsinki University Hospital.
Methods
Patient characteristics, known risk factors, and details of treatment of PPP for the first 200 patients referred to our APS-OPC were retrospectively collected from the medical records. The APS-OPC clinic functions in close collaboration with the Multidisciplinary Pain Clinic (MPC), and the number of patients in need of physiotherapist, psychologist or psychiatrist counselling was recorded, as well as the number of patients referred to the MPC for further PPP management.
Results
Patients were referred to the APS-OPC from different surgical specialities, the two most common being thoracic and orthopaedic surgery. Seventy per cent of the patients (139/200) presented symptoms indicating neuropathic postsurgical pain. The patients had, on average, five risk factors for PPP. The median time from surgery to the first contact to the APS-OPC was two months, and the median duration of follow-up was 2.8 months (0–16 months). The median number of contacts with APS-OPC was 3 (range 1–14). Every fourth patient needed only one contact to the APS-OPC. Nineteen per cent of the patients had an appointment with the physiotherapist and 20% with a psychologist or psychiatrist. At discharge after surgery, 54% of the patients were using weak opioids, 32% strong opioids and 71% gabapentinoids; at discharge from the APS-OPC, these numbers were 20%, 6% and 43%, respectively. Twenty-two per cent of the patients were referred to the MPC for further pain management.
Conclusions
The APS-OPC provides a fluent fast-track method of ensuring effective multimodal analgesia in the subacute recovery phase after surgery. Even strong opioids can be safely used after discharge and then tapered off in close supervision of the APS-OPC anaesthesiologist. As the APS-OPC was implemented in close collaboration with the MPC, the multidisciplinary resources are easily available during the course of the APS-OPC treatment.
Implications
The first two years of the APS-OPC have shown that a significant number of surgical patients benefit from continuing active pain management after discharge from hospital. This fast-track service provides physician-supervised titration of analgesics to improve pain relief in the subacute phase. An important task of the APS-OPC is to ensure that strong opioids are not inappropriately continued after recovery. Another goal of the APS-OPC is to identify patients in need of multidisciplinary pain management services to prevent chronification.
1 Introduction
The ability to control acute perioperative and postoperative pain has made modern surgery possible. Nowadays, in most cases it is possible to provide good postoperative analgesia, but some patients still experience severe pain after surgery or postoperative pain persists longer than expected. Besides, the length of postoperative hospital stay after many operations has been significantly shortened, leading to new challenges in postoperative pain management. There is great individual variation in the intensity and duration of pain after certain types of surgery. In addition to the type of operation, several factors that affect the postoperative pain experience have been identified (Fig. 1).
Persistent postoperative pain is a significant clinical problem ([1,2,3,4,5], Table 1). It is defined as pain that develops after a surgical procedure and lasts at least 2 months, with other causes for the pain excluded [6]. Continued noxious input from the postoperative injury may lead to sensitization of the central nervous system and pain spreading to a wider area (secondary hyperalgesia). This phenomenon is a dynamic reflection of neuroinflammatory changes and central neuronal plasticity, which is considered to be the basis for chronic postsurgical pain [7,8,9,10]. The progression from acute to chronic pain involves various risk factors, including surgical, psychosocial, socio-environmental and patient-related ones ([2,3,11]; Fig. 1).
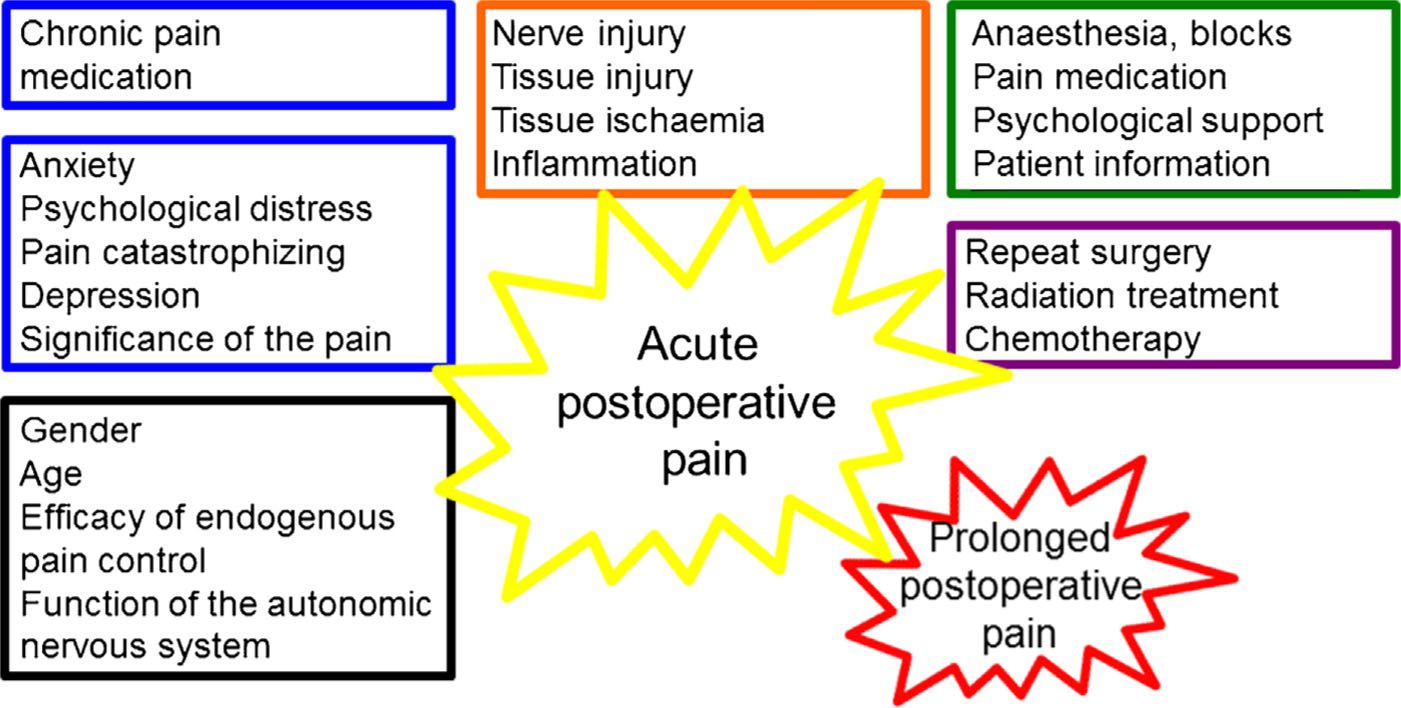
Factors that affect acute and prolonged postoperative pain. There isavery strong association between intense acute postoperative pain and prolonged chronic pain. This could indicate causality, or common risk factors. So of these factors, such as anxiety or perioperative analgesic treatment can be modified, whereas others, such as genetic construction of the patient or need for repeat surgery in practice cannot be altered, but still need to be taken into account when planning and executing the treatment of the patient.
Source: Adapted from KontinenV, Hamunen K. Leikkauksenjalkeisen kivun hoito. Duodecim 2015 Oct 13;131:1921–8.
Most of the analgesics used to alleviate postoperative pain are not very effective for secondary hyperalgesia. Also, nerve injury may be a significant factor in the development of chronic postoperative pain [12,13,14,15]. Few studies provide data on the management of pain in the sub-acute phase when patients are at home recovering and rehabilitating from surgery. Systematic pain management with individualized analgesic titration improves rehabilitation and decreases incidence of chronic post-surgical pain after knee and hip surgery [12]. Effective pain management extended to the home for the first week after discharge could be more important than any analgesic method per se in preventing chronic postsurgical pain after thoracotomy [13].
A follow-up service for persisting postoperative pain has been proposed [16,17]. This concept involves preoperative identification of patients at risk of severe acute pain and/or persisting postoperative pain, and effective and safe pain management in the subacute phase. This includes appropriate and controlled use of strong opioids and antihyperalgesic drugs (e.g. gabapentinoids), also at home. It provides a way to monitor the link between preoperative risk factors, perioperative analgesia, and persistent postoperative pain. One model for this kind of service has been recently described [18].
A follow-up service for patients with severe pain and/or need of strong opioids and large doses of neuropathic pain medication at discharge after surgery was established in 2012 at the Helsinki University Hospital. This observational study describes the two first years of our follow-up service named Acute Pain Service OutPatient Clinic (APS-OPC).
Reported incidences of chronic pain3–12 months after surgery.
| Type of operation | Incidence of chronic pain (%) | Incidence of severe (disabling) chronic pain (nRS>5/10 [2]) (%) |
|---|---|---|
| Limb amputation | 30–85 | 5–10 |
| Thoracotomy | 5–65 | 10 |
| Mastectomy | 11–57 | 5–10 |
| Major abdominal surgery | 7–14 | NA |
| Craniotomy | 7–29 | NA |
| Knee arthroplasty | 13 | NA |
| Hip arthroplasty | 12 | NA |
| Caesarean section | 4–10 | 4 |
| Inguinal hernia | 5–63 | 2–4 |
| Coronary bypass | 30–50 | 5–10 |
| Cholecystectomy | 3–50 | NA |
| Vasectomy | 0–37 | NA |
| Dental surgery | 5–13 | NA |
2 Methods
According to the Finnish regulations, formal assessment of the study protocol by the local ethical committee was not necessary or possible, as the study is descriptive, the patients were not randomized to any intervention and all received the ordinary treatment. This was confirmed by the chair of the ethical committee in a telephone conversation with VK. The committee does not assess protocols that are considered not to require assessment. Appropriate institutional permission to review patient records was obtained.
2.1 Setting
The surgical services at the Helsinki University Hospital perform some 60000 operations yearly. All units (five hospitals) performing major surgery have Acute Pain Service (APS) teams. The multidisciplinary pain clinic serves the whole University Hospital district for the treatment of chronic cancer and non-cancer pain, with 1500 out-patient referrals per year. The Acute Pain Service Out-Patient Clinic (APS-OPC) in Helsinki began in September 2012 as a collaboration combining expertise and resources of APS teams and the multidisciplinary pain clinic. The service is provided by operating room anaesthesiologists with expertise in acute postoperative pain management. Multidisciplinary pain management resources including nursing, psychology and physiotherapy are provided by the multidisciplinary pain clinic. The APS-OPC operates one day per week at the pain clinic. The patients are referred to the APS-OPC by the anaesthesiologists or surgeons, or by general practitioners whose patients are still in the sub-acute phase of their pain (less than three months postoperatively). Patients whose pain problems do not resolve within 3–4 months in the care of APS-OPC, are transferred directly to the multidisciplinary pain clinic without any referral process.
2.2 Patients
Demographic characteristics of the first 200 patients referred to our APS-OPC (from September 2012 to August 2014) were collected from medical records after appropriate institutional approval. The presence of risk factors for prolonged postoperative pain for each patient was systematically determined from the medical records using a pre-defined list compiled from earlier studies ([2,3,11]; Table 2). Details of the treatment of pain during the perioperative period, and the treatment and use of multidisciplinary resources during the APS-OPC follow-up were recorded. The criteria used for referrals to APS-OPC are shown in Table 3. Data are presented as median (range) or number of patients.
| Preoperative factors |
| Preoperative pain, moderate to severe, lasting more than 1 month in the surgical area (*) |
| Preoperative chronic pain in other locations (*) |
| Repeat surgery (e.g. cancer recurrence) (*) |
| Psychological vulnerability (e.g. pain catastrophizing, anxiety), mentioned in medical records (*) |
| Female gender (*) |
| High BMI (>30) (*) |
| Young age (adults <40 yrs) (*) |
| Workers’ compensation Genetic predisposition |
| Inefficient DNIC, diffuse noxious inhibitory control (=CPM, conditioned pain modulation) |
| Intraoperative factors |
| Nerve damage after surgery (*) |
| Tissue ischaemia Proinflammatory state |
| Postoperative factors |
| Acute pain (moderate to severe), hyperalgesia (*) |
| Radiation therapy to the surgical area (*) |
| Neurotoxic chemotherapy (*) |
| Sensory disturbances after surgery (*) |
| Surgical complications (infection, seroma, haematoma) (*) |
| Repeat surgery (*) |
| Psychological vulnerability, anxiety (mentioned in medical records) (*) |
3 Results
3.1 Patient and pain characteristics
The demographic characteristics of the patients are presented in Table 4. Patients were referred to the APS-OPC from a wide range of surgical specialities (Fig. 2). Eighty-two out of 200 patients (41%) had undergone thoracic surgery: of these, 39% were open thora-cotomies, 52% video-assisted thoracic surgeries (VATS), and the others were laparotomies, pectus excavatum operations or multiple rib fractures without surgery. Sixteen per cent of the thoracic patients were young adults (mean age 33 years) who were operated on for thoracic outlet syndrome (TOS). Twenty per cent had had reoperation, and 16% had a pre-existing chronic pain condition. Most orthopaedic operations were spine or limb surgery. The majority of the patients (139/200) presented symptoms (for instance, allodynia and hyperalgesia) suggesting neuropathic post-surgical pain: 66% of the thoracic, 72% of the orthopaedic, 35% of the gastrointestinal, 69% of the vascular, and all of the breast surgery patients. Most patients were referred to the APS-OPC by anaes the-siologists at the time of discharge from the hospital. In the early
Criteria for referral to the Acute Pain Service Out-Patient Clinic.
|

Type of surgery undergone by the first 200 patients referred to the Helsinki APS-OPC.
Demographic and treatment factors of the first 200 patients. Data are median (range) or number of patients (percentage).
| Age (years) | 46(15–83) |
| Male/female | 84/116 (42%/58%) |
| Time from surgery to first contact in the APC-OPC (months) | 2 (0, 5–24) |
| Risk factors for persistent postoperative pain/patient | 5 (0–9) |
| Duration oftreatment (months) | 2, 8 (0–16) |
| Number of contacts | 3 (1–14) |
| Total numberof physician appointments | 240 |
| Scheduled phone calls | 463 |
| Patients with only one contact | 48 (24%) |
| Physiotherapist consultations | 37 (19%) |
| Psychologist/psychiatrist consultations | 39 (20%) |
| Patients referred to the multidisciplinary pain clinic | 43 (22%) |
Pain medication of the first 200 patients.
| Weak opioids | Strong opioids | Gabapentinoids | |
|---|---|---|---|
| At discharge from the hospital after surgery | 107(54%) | 64(32%) | 142(71%) |
| At end of the Out-Patient Clinic treatment | 40(20%) | 10(6%) | 86(43%) |
4 Discussion
4.1 The Acute Pain Service Out-Patient Clinic (APS-OPC) model
APS-OPC is a novel “bridge” between acute and chronic pain management. It offers an effective and safe way to extend multimodal postoperative analgesia to home, covering also the critical sub-acute phase. Importantly, physician-supervised tapering off of medications, especially strong opioids, is achieved. This model seems to be able to provide a fast-track service with a median number of three contacts (appointments and calls), 24% of the patients requiring only one contact, and a median duration of treatment of 2.8 months.
In our model, the APS-OPC is managed by APS anaesthesiologists who also work in the operating room. They are familiar with surgical procedures and perioperative pain management. Working in APS-OPC gives them an opportunity to get feedback on acute pain management protocols and to further develop these. These physicians also acquire knowledge of the management of chronic pain and can transfer this experience to the perioperative environment and to APS work. In our APS-OPC model, the APS physicians have easy access to consultation and can discuss the management of individual patients with the multidisciplinary staff of the pain clinic. Since every fifth patient needed consultation with a physiotherapist or a psychologist/psychiatrist during APS-OPC treatment, and 22% of the patients were transferred for continued treatment by the pain clinic, this close collaboration of APS-OPC and the multidisciplinary pain clinic seems optimal, both in terms of treatment of patients and use of resources. Since the early days of the APS-OPC, the system has evolved so that patients with postsurgery pain for more than 3 months will be admitted directly to the multidisciplinary pain clinic whereas those whose pain has lasted less than 3 months will be admitted to APS-OPC.

Clinical pathway for postoperative pain with identification of risk patients
4.2 Neuropathic postsurgical pain
According to a recent review, persistent postoperative pain has neuropathic features in 6–70% of patients, depending on the type of the operation: thoracic surgery 66%, breast surgery 68%, groin hernia repair 31% and hip or knee arthroplasty 6% [15]. Persistent neuropathic pain has a significant impact on patients’ overall health, functioning and well-being, not to mention the economic burden to society [20]. Searle et al. found that 8% of patients developed acute neuropathic pain characteristics after major thoracic surgery in the immediate postoperative period at hospital, but 22% of them had features of chronic neuropathic pain three months after the operation [21]. The authors also demonstrated that acute postoperative neuropathic pain may predict persistent neuropathic pain, which may be difficult to treat.
Forty-one per cent of patients visiting our APS-OPC had undergone thoracic surgery, and 66% of these had persistent neuropathic pain (based on a clinical examination) during the sub-acute phase, in accordance with other studies [15,19,20]. Young adults operated for TOS by VATS were overrepresented among the APS-OPC patients (16% of thoracic patients). These incidences should draw attention to the need for such operations: patients should have information about the risk of prolonged pain before their operations.
4.3 Medications
One important target of the APS-OPC is to ensure the safe use of strong opioids for a restricted period of time at home after surgery [22]. The early discharge of patients after major painful surgery is possible with the tapering off of strong opioids and, for instance, gabapentinoids at home. Close scrutiny is warranted in view of the risk of inappropriate opioid use after the sub-acute pain phase. In the study of Clarke et al. [23], 49% of Canadian patients were prescribed opioids after major surgery and 3% of them continued using these for more than 3 months. In elderly patients, opioid prescription for pain after ambulatory surgery predicted long-term opioid use at one year [24]. Furthermore, left-over tablets from a short period of opioid use may be diverted [25,26,27]. Proper patient information and close follow-up during the sub-acute phase a few weeks postoperatively may prevent these problems.
4.4 The future of APS-OPC
Acute pain services should have a central role in preoperatively identifying patients at high risk of prolonged postoperative pain. An APS organization can also develop risk assessment tools to identify patients in need of multimodal or invasive interventions for their pain management during the perioperative phase [17,19]. The evaluation of patients at risk of persistent postsurgical pain is not yet systematic in our hospital. Considering the number of risk factors for persistent postsurgical pain in the patients in the present study, patient selection seems appropriate. A future challenge is to implement a risk evaluation tool for persistent postsurgical pain into clinical practice and to systematically screen all patients (Fig. 3). A further step will be to study various APS interventions in decreasing persistent postsurgical pain. We believe that it is possible to prevent or mitigate the development of persistent postoperative pain by early recognition and multidisciplinary treatment of persisting sub-acute pain, before plastic changes in the central nervous system lead to a more persistent or even permanent condition.
5 Conclusions
The APS-OPC provides a fluent fast-track method of ensuring effective multimodal analgesia in the subacute recovery phase after surgery. Even strong opioids can be safely used after discharge and then tapered off in close supervision of the APS-OPC anaesthesiologist. As the APS-OPC was implemented in close collaboration with the MPC, the multidisciplinary resources are easily available during the course of the APS-OPC treatment. Approximately every fifth of the study patients was considered to need further treatment for PPP and were easily transferred from the APS-OPC to the MPC.
6 Implications
The first two years of the APS-OPC have shown that a significant number of surgical patients benefit from continuing active pain management after discharge from hospital. This fast-track service provides physician-supervised titration of analgesics to improve pain relief in the subacute phase. An important task of the APS-OPC is to ensure that strong opioids are not inappropriately continued after recovery. Another goal of the APS-OPC is to identify patients in need of multidisciplinary pain management services to prevent chronification. In light of the Helsinki APS-OPC experience, allocating resources to maintain an APS-OPC service is well-founded.
Highlights
The APC-OPC is an outpatient clinic for subacute postoperative pain management.
Of the first 200 patients treated at the APS-OPC, 70% had neuropathic pain.
The median duration of follow-up was 2.8 months.
Pain medications, including strong opioids, were successfully tapered off.
The APS-OPC enables an easy transfer to chronic pain services if pain persists.
DOI of refers to article: http://dx.doi.org/10.1016/j.sjpain.2016.04.005.
Acknowledgements
Preparation of this paper has been partially funded from the Finnish State funding for university-level health research, grant code TYH2014305. We are grateful to Les Hearn, MSc, for proofreading the MS.
-
Conflict of interest: No external funding and no competing interests declared.
References
[1] Kehlet H, Rathmell JP. Persistent postsurgical pain: the path forward through better design of clinical studies. Anesthesiology 2010;112:514–5.Search in Google Scholar
[2] Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006;367:1618–25.Search in Google Scholar
[3] Macrae WA. Chronic post-surgical pain: 10 years on. Br J Anaesth 2008;101:77–86.Search in Google Scholar
[4] Lavand’homme P. The progression from acute to chronic pain. Curr Opin Anaes-thesiol 2011;24:545–50.Search in Google Scholar
[5] Andersen L, Gaarn-Larsen L, Kristensen BB, Husted H, Otte KS, Kehlet H. Sub-acute pain and function after fast-track hip and knee surgery. Anaesthesia 2009;64:508–13.Search in Google Scholar
[6] Merskey H, Bogduk H, editors. Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms. Seattle: IASP Press; 1994.Search in Google Scholar
[7] Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersen-sitivity by central neural plasticity.J Pain 2009;10:895–926.Search in Google Scholar
[8] Woolf CJ. Central sensitization: implications forthe diagnosis and treatment of pain. Pain 2011;152:2–15.Search in Google Scholar
[9] Shipton EA. The transition from acute to chronic post surgical pain. Anaesth Intensive Care 2011;39:824–36.Search in Google Scholar
[10] Deumens R, Steyaert A, Forget P, Schubert M, Lavand’homme P, Hermans E, De Kock M. Prevention ofchronic postoperative pain: cellular, molecular, and clinical insights for mechanism-based treatment approaches. Prog Neurobiol 2013;104:1–37.Search in Google Scholar
[11] Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain 2011;12:725–46.Search in Google Scholar
[12] Morrison RS, Flanagan S, Fischberg D, Cintron A, Siu AL. A novel interdisciplinary analgesic program reduces pain and improves function in older adults after orthopedic surgery.J Am Geriatr Soc 2009;57:1–10.Search in Google Scholar
[13] Tiippana E, Nelskyla K, Nilsson E, Sihvo E, Kataja M, Kalso E. Managing post-thoracotomy pain: epidural or systemic analgesia and extended care – a randomized study with an “as usual”control group. Scand J Pain 2014;5:240–7.Search in Google Scholar
[14] Aasvang EK, Gmaehle E, Hansen JB, Gmaehle B, Forman JL, Schwarz J, Bittner R, Kehlet H. Predictive risk factors for persistent postherniotomy pain. Anesthesiology 2010;112:957–69.Search in Google Scholar
[15] Haroutiunian S, Nikolajsen L, Finnerup NB, Jensen TS. The neuropathic component in persistent postsurgical pain: a systematic literature review. Pain 2013;154:95–102.Search in Google Scholar
[16] De Kock M. Expanding our horizons: transition of acute postoperative pain to persistent pain and establishment of chronic postsurgical pain services. Anes-thesiology 2009;111:461–3.Search in Google Scholar
[17] Kalso E. Persistent post-surgery pain: research agenda for mechanisms, prevention, and treatment. Br J Anaesth 2013;111:9–12.Search in Google Scholar
[18] Katz J, Weinrib A, Fashler SR, Katznelzon R, Shah BR, Ladak SS, Jiang J, Li Q, McMillan K, Mina DS, Wentlandt K, MaRae K, Tamir D, Lyn S, de Perrot M, Rao V, Grant D, Roche-Nagle G, Cleary SP, Hofer SO, Gilbert R, Wijeysundera D, Ritvo P, Janmohamed T, O’Leary G, Clarke H. The Toronto General Hospital Transitional Pain Service: development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res 2015;8:695–702.Search in Google Scholar
[19] Sipila R, Estlander A-M, Tasmuth T, Kataja M, Kalso E. Development of a screening instrument for risk factors of persistent pain after breast cancer surgery. Br J Cancer 2012;107:1459–66.Search in Google Scholar
[20] Parsons B, Schaefer C, Mann R, Sadosky A, Daniel S, Nalamachu S, Stacey BR, Nieshoff EC, Tuchman M, Anschel A. Economic and humanistic burden of posttrauma and post-surgical neuropathic pain among adults in the United States. J Pain Res 2013;6:459–69.Search in Google Scholar
[21] Searle RD, Simpson MP, Simpson KH, Milton R, Bennett MI. Can chronic neuropathic pain following thoracic surgery be predicted during the postoperative period? Interact Cardiovasc Thorac Surg 2009;9:999–1002.Search in Google Scholar
[22] Huang A, Katz J, Clarke H. Ensuring safe prescribing of controlled substances for pain following surgery by developing a transitional pain service. Pain Manag 2015;5:97–105.Search in Google Scholar
[23] Clarke H, Soneji N, Ko DT, Yun L, Wijeysundera DN. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ 2014;348:1–10.Search in Google Scholar
[24] Alam A, Gomes T, Zheng H, Mamdani MM, Juurlink DN, Bell CM. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med 2012;172:425–30.Search in Google Scholar
[25] Harris K, Curtis J, Larsen B, Calder S, Duffy K, Bowen G, Hadley M, Tristani-Firouzi P. Opioid pain medication use after dermatologic surgery: a prospective observational study of 212 dermatologic surgery patients. JAMA Dermatol 2013;149:317–21.Search in Google Scholar
[26] Bates C, Laciak R, Southwick A, Bishoff J. Overprescription of postoperative narcotics: a look at postoperative pain medication delivery, consumption and disposal in urological practice. J Urol 2011;185:551–5.Search in Google Scholar
[27] Rodgers J, Cunningham K, Fitzgerald K, Finnerty E. Opioid consumption follow-ing outpatient upper extremity surgery.J Hand Surg Am 2012;37:645–50.Search in Google Scholar
© 2016 Scandinavian Association for the Study of Pain
Articles in the same Issue
- Scandinavian Journal of Pain
- Editorial comment
- Depressive symptoms associated with poor outcome after lumbar spine surgery: Pain and depression impact on each other and aggravate the burden of the sufferer
- Clinical pain research
- Depressive symptoms are associated with poor outcome for lumbar spine surgery
- Editorial comment
- Chronic compartment syndrome is an under-recognized cause of leg-pain
- Observational study
- Prevalence of chronic compartment syndrome of the legs: Implications for clinical diagnostic criteria and therapy
- Editorial comment
- Genetic susceptibility to postherniotomy pain. The influence of polymorphisms in the Mu opioid receptor, TNF-α, GRIK3, GCH1, BDNF and CACNA2D2 genes
- Clinical pain research
- Genetic susceptibility to postherniotomy pain. The influence of polymorphisms in the Mu opioid receptor, TNF-α, GRIK3, GCH1, BDNF and CACNA2D2 genes
- Editorial comment
- Important development: Extended Acute Pain Service for patients at high risk of chronic pain after surgery
- Observational study
- New approach for treatment of prolonged postoperative pain: APS Out-Patient Clinic
- Editorial comment
- Working memory, optimism and pain: An elusive link
- Original experimental
- The effects of experimental pain and induced optimism on working memory task performance
- Editorial comment
- A surgical treatment for chronic neck pain after whiplash injury?
- Clinical pain research
- A small group Whiplash-Associated-Disorders (WAD) patients with central neck pain and movement induced stabbing pain, the painful segment determined by mechanical provocation: Fusion surgery was superior to multimodal rehabilitation in a randomized trial
- Editorial comment
- Social anxiety and pain-related fear impact each other and aggravate the burden of chronic pain patients: More individually tailored rehabilitation need
- Clinical pain research
- Characteristics and consequences of the co-occurrence between social anxiety and pain-related fear in chronic pain patients receiving multimodal pain rehabilitation treatment
- Editorial comment
- Transcranial magnetic stimulation, paravertebral muscles training, and postural control in chronic low back pain
- Original experimental
- Influence of paravertebral muscles training on brain plasticity and postural control in chronic low back pain
- Editorial comment
- Is there a place for pulsed radiofrequency in the treatment of chronic pain?
- Clinical pain research
- Pulsed radiofrequency in clinical practice – A retrospective analysis of 238 patients with chronic non-cancer pain treated at an academic tertiary pain centre
- Editorial comment
- More postoperative pain reported by women than by men – Again
- Observational study
- Females report higher postoperative pain scores than males after ankle surgery
- Editorial comment
- The relationship between pain and perceived stress in a population-based sample of adolescents – Is the relationship gender specific?
- Observational study
- Pain is prevalent among adolescents and equally related to stress across genders
- Editorial comment
- The Brief Pain Inventory (BPI) – Revisited and rejuvenated?
- Clinical pain research
- Confirmatory factor analysis of 2 versions of the Brief Pain Inventory in an ambulatory population indicates that sleep interference should be interpreted separately
- Editorial comment
- Pain research reported at the 40th scientific meeting of the Scandinavian Association for the Study of Pain in Reykjavik, Iceland May 26–27, 2016
- Abstracts
- Pain management strategies for effective coping with Sickle Cell Disease: The perspective of patients in Ghana
- Abstracts
- PEARL – Pain in early life. A new network for research and education
- Abstracts
- Searching for protein biomarkers in pain medicine – Mindless dredging or rational fishing?
- Abstracts
- Effectiveness of smart tablets as a distraction during needle insertion amongst children with port catheter: Pre-research with pre-post test design
- Abstracts
- Postoperative oxycodone in breast cancer surgery: What factors associate with analgesic plasma concentrations?
- Abstracts
- Sport participation and physical activity level in relation to musculoskeletal pain in a population-based sample of adolescents: The Young-HUNT Study
- Abstracts
- “Tears are also included” - women’s experience of treatment for painful endometriosis at a pain clinic
- Abstracts
- Predictors of long-term opioid use among chronic nonmalignant pain patients: A register-based national open cohort study
- Abstracts
- Coupled cell networks of astrocytes and chondrocytes are target cells of inflammation
- Abstracts
- Changes in opioid prescribing behaviour in Denmark, Sweden and Norway - 2006-2014
- Abstracts
- Opioid usage in Denmark, Norway and Sweden - 2006-2014 and regulatory factors in the society that might influence it
- Abstracts
- ADRB2, pain and opioids in mice and man
- Abstracts
- Retrospective analysis of pediatric patients with CRPS
- Abstracts
- Activation of epidermal growth factor receptors (EGFRs) following disc herniation induces hyperexcitability in the pain pathways
- Abstracts
- Pain rehabilitation with language interpreter, a multicenter development project
- Abstracts
- Trait-anxiety and pain intensity predict symptoms related to dysfunctional breathing (DB) in patients with chronic pain
- Abstracts
- Emla®-cream as pain relief during pneumococcal vaccination
- Abstracts
- Use of Complimentary/Alternative therapy for chronic pain
- Abstracts
- Effect of conditioned pain modulation on long-term potentiation-like pain amplification in humans
- Abstracts
- Biomarkers for neuropathic pain – Is the old alpha-1-antitrypsin any good?
- Abstracts
- Acute bilateral experimental neck pain: Reorganise axioscapular and trunk muscle activity during slow resisted arm movements
- Abstracts
- Mast cell proteases protect against histaminergic itch and attenuate tissue injury pain responses
- Abstracts
- The impact of opioid treatment on regional gastrointestinal transit
- Abstracts
- Genetic variation in P2RX7 and pain
- Abstracts
- Reversal of thermal and mechanical allodynia with pregabalin in a mouse model of oxaliplatin-induced peripheral neuropathy
- Clinical pain research
- Pain-related distress and clinical depression in chronic pain: A comparison between two measures
Articles in the same Issue
- Scandinavian Journal of Pain
- Editorial comment
- Depressive symptoms associated with poor outcome after lumbar spine surgery: Pain and depression impact on each other and aggravate the burden of the sufferer
- Clinical pain research
- Depressive symptoms are associated with poor outcome for lumbar spine surgery
- Editorial comment
- Chronic compartment syndrome is an under-recognized cause of leg-pain
- Observational study
- Prevalence of chronic compartment syndrome of the legs: Implications for clinical diagnostic criteria and therapy
- Editorial comment
- Genetic susceptibility to postherniotomy pain. The influence of polymorphisms in the Mu opioid receptor, TNF-α, GRIK3, GCH1, BDNF and CACNA2D2 genes
- Clinical pain research
- Genetic susceptibility to postherniotomy pain. The influence of polymorphisms in the Mu opioid receptor, TNF-α, GRIK3, GCH1, BDNF and CACNA2D2 genes
- Editorial comment
- Important development: Extended Acute Pain Service for patients at high risk of chronic pain after surgery
- Observational study
- New approach for treatment of prolonged postoperative pain: APS Out-Patient Clinic
- Editorial comment
- Working memory, optimism and pain: An elusive link
- Original experimental
- The effects of experimental pain and induced optimism on working memory task performance
- Editorial comment
- A surgical treatment for chronic neck pain after whiplash injury?
- Clinical pain research
- A small group Whiplash-Associated-Disorders (WAD) patients with central neck pain and movement induced stabbing pain, the painful segment determined by mechanical provocation: Fusion surgery was superior to multimodal rehabilitation in a randomized trial
- Editorial comment
- Social anxiety and pain-related fear impact each other and aggravate the burden of chronic pain patients: More individually tailored rehabilitation need
- Clinical pain research
- Characteristics and consequences of the co-occurrence between social anxiety and pain-related fear in chronic pain patients receiving multimodal pain rehabilitation treatment
- Editorial comment
- Transcranial magnetic stimulation, paravertebral muscles training, and postural control in chronic low back pain
- Original experimental
- Influence of paravertebral muscles training on brain plasticity and postural control in chronic low back pain
- Editorial comment
- Is there a place for pulsed radiofrequency in the treatment of chronic pain?
- Clinical pain research
- Pulsed radiofrequency in clinical practice – A retrospective analysis of 238 patients with chronic non-cancer pain treated at an academic tertiary pain centre
- Editorial comment
- More postoperative pain reported by women than by men – Again
- Observational study
- Females report higher postoperative pain scores than males after ankle surgery
- Editorial comment
- The relationship between pain and perceived stress in a population-based sample of adolescents – Is the relationship gender specific?
- Observational study
- Pain is prevalent among adolescents and equally related to stress across genders
- Editorial comment
- The Brief Pain Inventory (BPI) – Revisited and rejuvenated?
- Clinical pain research
- Confirmatory factor analysis of 2 versions of the Brief Pain Inventory in an ambulatory population indicates that sleep interference should be interpreted separately
- Editorial comment
- Pain research reported at the 40th scientific meeting of the Scandinavian Association for the Study of Pain in Reykjavik, Iceland May 26–27, 2016
- Abstracts
- Pain management strategies for effective coping with Sickle Cell Disease: The perspective of patients in Ghana
- Abstracts
- PEARL – Pain in early life. A new network for research and education
- Abstracts
- Searching for protein biomarkers in pain medicine – Mindless dredging or rational fishing?
- Abstracts
- Effectiveness of smart tablets as a distraction during needle insertion amongst children with port catheter: Pre-research with pre-post test design
- Abstracts
- Postoperative oxycodone in breast cancer surgery: What factors associate with analgesic plasma concentrations?
- Abstracts
- Sport participation and physical activity level in relation to musculoskeletal pain in a population-based sample of adolescents: The Young-HUNT Study
- Abstracts
- “Tears are also included” - women’s experience of treatment for painful endometriosis at a pain clinic
- Abstracts
- Predictors of long-term opioid use among chronic nonmalignant pain patients: A register-based national open cohort study
- Abstracts
- Coupled cell networks of astrocytes and chondrocytes are target cells of inflammation
- Abstracts
- Changes in opioid prescribing behaviour in Denmark, Sweden and Norway - 2006-2014
- Abstracts
- Opioid usage in Denmark, Norway and Sweden - 2006-2014 and regulatory factors in the society that might influence it
- Abstracts
- ADRB2, pain and opioids in mice and man
- Abstracts
- Retrospective analysis of pediatric patients with CRPS
- Abstracts
- Activation of epidermal growth factor receptors (EGFRs) following disc herniation induces hyperexcitability in the pain pathways
- Abstracts
- Pain rehabilitation with language interpreter, a multicenter development project
- Abstracts
- Trait-anxiety and pain intensity predict symptoms related to dysfunctional breathing (DB) in patients with chronic pain
- Abstracts
- Emla®-cream as pain relief during pneumococcal vaccination
- Abstracts
- Use of Complimentary/Alternative therapy for chronic pain
- Abstracts
- Effect of conditioned pain modulation on long-term potentiation-like pain amplification in humans
- Abstracts
- Biomarkers for neuropathic pain – Is the old alpha-1-antitrypsin any good?
- Abstracts
- Acute bilateral experimental neck pain: Reorganise axioscapular and trunk muscle activity during slow resisted arm movements
- Abstracts
- Mast cell proteases protect against histaminergic itch and attenuate tissue injury pain responses
- Abstracts
- The impact of opioid treatment on regional gastrointestinal transit
- Abstracts
- Genetic variation in P2RX7 and pain
- Abstracts
- Reversal of thermal and mechanical allodynia with pregabalin in a mouse model of oxaliplatin-induced peripheral neuropathy
- Clinical pain research
- Pain-related distress and clinical depression in chronic pain: A comparison between two measures

