Abstract
Parkinson’s disease (PD) is a neurodegenerative disorder that predominantly affects dopaminergic neurons in the substantia nigra and ventral tegmental area, resulting in symptoms such as tremors, muscle rigidity, bradykinesia, and potential cognitive and affective disturbances. The effective delivery of pharmacological agents to the central nervous system is hindered by various factors, including the restrictive properties of the blood‒brain barrier and blood‒spinal cord barrier, as well as the physicochemical characteristics of the drugs. Traditional drug delivery methods may not provide the therapeutic concentrations necessary for functional restoration in PD patients. However, lipid-based nanoparticles (NPs) offer new possibilities for enhancing the bioavailability of established treatment regimens and developing innovative therapies that can modify the course of the disease. This review provides a concise overview of recent advances in lipid-based NP strategies aimed at mitigating specific pathological mechanisms relevant to PD progression. This study also explores the potential applications of nanotechnological innovations in the development of advanced treatment modalities for individuals with PD.
1 Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder and affects 7–10 million people worldwide. The incidence rate of PD is 41 per 100,000 individuals, increasing to 1,900 per 100,000 individuals older than 80 years. It is projected that by 2040, the incidence rate will reach 12.9 million cases [1]. PD is a debilitating disease that significantly reduces patients’ quality of life, increases medical costs, and poses a social burden. PD can be characterized by motor and nonmotor symptoms. Motor symptoms include movement disorders, postural instability, and tremors [2], while nonmotor symptoms encompass sleep disorders, depression, anxiety, and loss of smell [3]. A pathological hallmark of PD is the progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNpC), which leads to a reduction in dopamine (DA) levels in the brain; this neurotransmitter plays a vital role in regulating movement, emotion, and cognitive function [4]. Another characteristic feature of PD is the presence of Lewy bodies (LBs), which are intracellular inclusions consisting of abnormal α-syn [5]. Although α-syn typically participates in neuronal signal transmission, its abnormal aggregation in PD leads to impaired neuronal function.
The exact etiology of PD has not been determined, but it is believed to be influenced by both genetic and environmental factors [6]. Approximately 5–10% of PD patients have a family history associated with certain gene mutations (LRRK2, PARK7, PINK1, Parkin RBR E3 Ubiquitin Protein Ligase [PRKN], and SNCA), while the majority of cases (90–95%) are sporadic and potentially linked to environmental toxin exposure (pesticides, heavy metals, organic solvents). Sex also plays a role in PD susceptibility, with men being more prone to PD than women, particularly before menopause, due to the neuroprotective effects of estrogen [7,8]. Both genetic factors in familial PD and environmental factors in sporadic PD affect specific pathways, such as mitochondrial dysfunction, oxidative stress, neuroinflammation, and abnormal protein degradation pathways, contributing to the clinical manifestations of PD [9,10]. Currently, there is no cure for PD, and treatment primarily focuses on symptom relief. Drugs such as levodopa, DA agonists, monoamine oxidase B (MAO-B) inhibitors, and catechol-O-methyltransferase inhibitors are used to restore DA levels and alleviate associated symptoms [11,12,13]. However, these drugs are limited by their ability to cross the blood‒brain barrier (BBB), susceptibility to peripheral metabolism, drug resistance, and side effects. Surgical treatments, such as deep brain stimulation (DBS), focused ultrasound (FUS), and cell replacement therapy [14], are options when drug therapy fails, but they provide only temporary improvement in motor symptoms without preventing neuronal death.
To overcome the limitations of PD treatment, novel strategies are needed. Nanotechnology has emerged as a promising approach in recent years, offering improvements in drug bioavailability/stability, overcoming biological barriers (including the BBB), reducing side effects, targeting disease sites precisely, and enabling real-time tracking. Nanotechnology can revitalize potentially valuable PD treatments with limited efficacy. This review provides an overview of PD mechanisms, current treatments, and ongoing clinical trials. The study also explores the challenges associated with delivering PD drugs to the central nervous system (CNS) and discusses how nanotechnology can address these obstacles. Furthermore, the review highlights the latest advancements in utilizing nanotechnology-based strategies to target specific pathophysiological aspects related to PD progression.
2 Proposed mechanisms of PD
Although the exact mechanisms of PD are not fully understood, they are believed to be mediated by complex interactions between cells, molecules, and genetic pathways. The currently proposed main mechanisms of PD include the following: (1) Loss of DA neurons: the primary feature of PD is the loss of dopaminergic neurons, which are responsible for motor regulation. The loss of dopaminergic neurons leads to a decrease in DA levels, resulting in symptoms such as motor disorders. (2) Protein misfolding and aggregation: Abnormal aggregation of α-syn occurs inside DA neurons, forming inclusions called LBs. These aggregates may cause neuronal dysfunction and cell death. (3) Oxidative stress: An imbalance between oxidants and antioxidants in the body leads to excess production of reactive oxygen species (ROS) or reactive nitrogen species (RNS) or insufficient clearance, resulting in cell damage. PD patients exhibit significant oxidative stress responses in the brain, manifested by increased lipid peroxidation, protein carbonylation, DNA damage, etc. Oxidative stress may promote PD development through various pathways, including increasing α-syn misfolding and aggregation, impairing mitochondrial function and structure, and activating neuroinflammatory responses. (4) Mitochondrial dysfunction: Mitochondrial dysfunction related to energy metabolism, cell apoptosis, calcium ion balance, etc. leads to insufficient cellular energy and oxidative stress. PD patients exhibit significant mitochondrial dysfunction in the brain, manifested as decreased mitochondrial respiratory chain enzyme activity, mitochondrial DNA (mtDNA) mutations or deletions, reduced mitochondrial membrane potential, and impaired mitochondrial autophagy. Mitochondrial dysfunction may affect PD pathogenesis through various pathways, including increasing α-syn misfolding and aggregation, releasing cytochrome c and proapoptotic factors, and inducing neuroinflammatory responses. (5) Neuroinflammation: Sustained or excessive inflammatory responses in the nervous system lead to neuronal damage or death. PD patients exhibit significant neuroinflammation, manifested by the activation of glial cells (such as astrocytes and microglia), the release of proinflammatory cytokines (such as TNF-α, IL-1β, and IL-6), and the infiltration of immune cells (such as T cells and B cells). Neuroinflammation may exacerbate PD progression through various mechanisms, including inducing mitochondrial dysfunction, increasing the production of ROS or RNS, and promoting α-syn transfer and diffusion. (6) Genetic factors: Some PD cases are associated with genetic mutations, such as LRRK2, PINK1, Parkin and Wnt/β-catenin (WβC) [15] gene mutations. These mutations may affect mitochondrial function, α-syn metabolism, and other pathways related to PD. Currently, treatment strategies targeting these mechanisms have made some progress in PD clinical trials and research. In Section 3, a brief introduction of approved PD treatments and ongoing clinical trials is given.
3 Current treatments and clinical trials for PD
Table 1 shows the various therapeutic drugs employed in the management of PD, with a primary focus on reinstating dopaminergic neurotransmission in neurons. Additional drugs targeting the glutamatergic, noradrenergic, serotonergic, and cholinergic systems are also utilized. Directly supplementing exogenous DA to counteract the depletion caused by the loss of dopaminergic neurons is the most effective approach. However, the hydrophilicity of DA poses challenges in crossing the BBB, and its short half-life and potential long-term adverse reactions limit its use. Hence, the current practice involves administering levodopa, a DA precursor that is converted into DA in the brain, which has therapeutic effects.
Ongoing phase III interventional trials on PD
| Compound | Target/mechanism | Recruiting | Identifier |
|---|---|---|---|
| Apomorphine | DA receptor agonist | Not yet | NCT02339064 |
| Exenatide | Neuroprotective | Not yet | NCT04232969 |
| Apomorphine | DA receptor agonist | Yes | NCT02864004 |
| Carbidopa and levodopa | Increase DA levels | Not yet | NCT04006210 |
| Pimavanserin tartrate | Modulating the activity of 5-HT2A receptors | Yes | NCT06068465 |
| ABBV-951 | Supplement DA and inhibit DA degradation | Not yet | NCT04750226 |
| ABBV-951 | Supplement DA and inhibit DA degradation | Not yet | NCT04379050 |
| Buntanetap | Anti-inflammation, neuroprotective, regulation of protein metabolism | Not yet | NCT05357989 |
| Tavapadon | Partial agonist of DA D1/D5 receptors | Enrolling by invitation | NCT04760769 |
| Apomorphine | DA receptor agonist | Yes | NCT04879134 |
| Tavapadon | Partial agonist of DA D1/D5 receptors | Yes | NCT04223193 |
| Tavapadon | Partial agonist of DA | Yes | NCT04201093 |
| Conventional medication/Chinese herbal medicine | Neuroprotective | Yes | NCT05001217 |
| Botulinum toxin type A | Neuromuscular blocker | Yes | NCT04277247 |
| Rasagiline | MAO-B inhibitor | Not yet | NCT05611372 |
| Lactobacillus acidophilus | Anti-oxidative effect, immune modulation, regulation of gut microbiota, and modulation of neurotransmitters | Yes | NCT05576818 |
| Tavapadon | Partial agonist of DA D1/D5 receptors | Not yet | NCT04542499 |
| Dipraglurant | Modulating the glutamatergic neurotransmitter system | Yes | NCT04857359 |
| Dipraglurant | Modulating the glutamatergic neurotransmitter system | Yes | NCT05116813 |
| Opicapone | Catechol-O-methyltransferase (COMT) inhibitor | Not yet | NCT04978597 |
| Nicergoline | Vasodilation, activation of α-adrenergic receptors, and improvement of cerebral metabolism | Not yet | NCT05551182 |
DA receptor agonists such as ropinirole (Requip), pramipexole (Mirapex), piribedil (Trivastal), and rotigotine (Neupro) directly stimulate DA receptors, improving both motor and nonmotor functions [16]. MAO-B inhibitors such as selegiline, rasagiline, and safinamide effectively prevent DA level reductions and the production of toxic intermediates by inhibiting MAO-B. These inhibitors can be used alone or in combination with other medications to prolong levodopa’s action and reduce “off” periods in early- or mid-to-late-stage patients. COMT inhibitors such as entacapone (Comtan) and tolcapone (Tasmar) inhibit the peripheral metabolism of levodopa, increasing its concentration in the brain [17]. These agents are combined with levodopa for mid-to-late-stage patients who experience motor fluctuations, particularly “wearing-off” phenomena, to extend the duration of action and reduce the dosage.
Anticholinergic drugs such as trihexyphenidyl and benztropine rebalance DA and acetylcholine by inhibiting cholinergic neuron activity [18]. In PD, elevated levels of glutamate, a neurotransmitter, lead to neuronal loss and disease progression. Along with associated complications, anti-glutamatergic drugs such as amantadine (a glutamate/N-methyl-D-aspartic acid [NMDA] receptor antagonist) partially alleviate motor and nonmotor symptoms in PD patients.
Surgical treatment, which is primarily suitable for mid-to-late-stage patients who have an inadequate response to medications or severe complications, serves as an alternative therapeutic approach for PD. DBS, involving the implantation of microelectrodes into the patient’s brain, is a widely used surgical technique. It utilizes high-frequency electrical stimulation to suppress abnormal neural activity and improve motor impairments. DBS preserves brain tissue, resulting in minimal complications while enabling adjustments to stimulation parameters for long-term therapeutic effects [14]. Internationally, DBS has been recognized as the gold standard for surgical treatment of mid- to late-stage PD.
Research efforts to develop additional drugs and treatment strategies for PD through clinical trials have been ongoing in recent years. These include drugs targeting gene mutations, such as LRRK2, PINK1, and Parkin; excitotoxicity inhibitors; mitochondrial protectants; antiapoptotic agents; anti-inflammatory drugs; and neurotrophic factors (NTFs). Table 1 provides a list of ongoing phase III intervention trials based on data from the United States National Library of Medicine. The future holds promise for an increasing number of available drugs for PD treatment. However, considering the complex nature of PD etiology, drugs targeting individual mechanisms may not yield significant therapeutic effects. Hence, combination therapy may improve clinical outcomes. Furthermore, challenges persist in effectively delivering therapeutic drugs to the CNS to slow the clinical progression of PD due to biological barriers (e.g., the BBB and blood–spinal cord barrier [BSCB]) and inherent limitations of drugs (e.g., low solubility, susceptibility to degradation, and off-target effects). Section 4 provides a detailed discussion of the challenges involved in delivering therapeutic drugs to the CNS for PD treatment.
4 Challenges in delivering therapeutic PD drugs to the CNS
4.1 BBB
Several anatomical and metabolic barriers, including the cerebrospinal fluid (CSF), the choroid plexus, and the BBB, exist between the peripheral blood circulation and the brain. The BBB is one of the most important and highly selective barriers that regulates the movement of various substances between the bloodstream and the CNS, thereby playing a crucial role in maintaining brain homeostasis. The BBB is composed of endothelial cells (ECs), vascular smooth muscle cells, neurons, astrocytes, and perivascular macrophages (Figure 1) [19]. The ability of the BBB to act as a selectively permeable filter is achieved through the presence of tight junctions and adherens junctions in the endothelial lining of the BBB. The tight junctions seal the gaps between ECs, creating a continuous lumen within the blood vessels. On the other hand, adherens junctions facilitate cell‒cell contact among ECs [20,21].

Schematic illustration of the BBB. General structure of the BBB. Figure 1a and b shows different cross-sectional views of brain blood vessels, respectively. The BBB is formed by ECs with tight junctions, surrounded by pericytes and astrocyte end feet. The image shows the direction of cross-section of (a) (cut in round slices) and (b) (cut into squares) when the brain blood vessels are viewed as a cylinder.
The molecular mechanisms of the transport of substances across the BBB include efflux and influx mechanisms. Transcellular pathways are crucial for transporting lipophilic agents, whereas paracellular pathways are responsible for transporting hydrophilic molecules [22]. Transport across the BBB occurs through endogenous carrier-mediated transport (CMT) or a receptor-mediated transport (RMT) system [23]; through adsorptive-mediated transcytosis (AMT); or through active efflux, facilitated by various major transporters, receptors, and channels present in ECs and pericytes. CMT is accountable for transporting vitamins, hormones, organic anions/cations, and nucleotides across the BBB. This process entails the spontaneous passive transport of these molecules through transmembrane integral proteins along a concentration gradient. The RMT pathway is the primary pathway for transporting essential macromolecules for brain function across the BBB. The ECs that form the BBB are equipped with receptors that specifically recognize certain ligands. When these receptors engage with their corresponding ligands, a process is triggered within the cell membrane that causes them to fold inwards, creating specialized intracellular compartments called coated vesicles. These vesicles undergo intricate processing mechanisms, allowing for the efficient recycling of both the receptor and the ligand. Ultimately, recycled receptor‒ligand complexes are released at the basolateral surface of ECs, where they contribute to the dynamic regulation of BBB function [24]. Large molecules such as antibodies, transferrin, insulin, and low-density lipoprotein (LDL) are transported through RMT. This process involves specific transporters, including insulin receptor, insulin-like growth factor receptor, type 1 transferrin receptor, low-density lipoprotein receptor (LDLR), leptin receptor, and neonatal Fc receptor.
The concept of AMT emerged from the observation that polycationic peptides not only have the ability to bind to the surface of ECs but also possess the remarkable capability to penetrate the BBB [25]. AMT involves the interaction of positively charged (cationic) and negatively charged (anionic) molecules on the EC membrane, facilitating the transport of positively charged proteins and basic short peptides, such as cell-penetrating peptides.
Efflux pumps, including P-glycoprotein (P-gP), ATP-binding cassette transporters (ABCs), and multidrug resistance proteins, establish a formidable barrier that hinders the passage of drugs across the BBB. P-gP, identified as ATP-binding cassette subfamily B member 1 (ABCB1), is a prominent ABC efflux transporter located on the luminal membrane of brain capillary ECs [26]. In cancer cells, the active efflux of drugs by P-gP contributes to the emergence of multidrug resistance, whereby a wide range of drugs, such as paclitaxel, digoxin, and dexamethasone, can function as substrates for this transporter and are actively expelled into the bloodstream [27]. Administration of paclitaxel in combination with a potent P-gP inhibitor in a mouse model resulted in a notable increase in the brain accumulation of paclitaxel [28].
Although numerous drugs are utilized for the treatment of neurological disorders, the specialized microvasculature of the BBB limits the use of only a few medications during systemic administration, leading to inadequate efficacy. Research endeavors have been directed toward the advancement of innovative approaches to transport drugs to the CNS. These approaches include the utilization of in-deliver drug-conjugated nanoparticles (NPs), liposomes, albumin NPs, and solid lipid nanoparticles (SLNs). By leveraging the intrinsic transport systems within the ECs of cerebral capillaries, small pharmaceutical drugs capable of traversing the BBB can be designed.
4.2 Biostability and bioavailability
In addition to the selective restrictions imposed by the BBB, the efficacy of potential therapeutic agents for PD faces significant challenges due to inadequate bioavailability and biostability of the drugs. Notably, many neuroprotective agents developed by the pharmaceutical industry have problems associated with low aqueous solubility. For instance, coenzyme Q10, an antioxidant, exhibits poor solubility in water, which hampers its therapeutic potential in PD treatment [29]. Similarly, a compound known as a Nurr1 agonist has shown promise in combating PD in animal models but is also limited by poor water solubility, necessitating improvements in pharmacological formulations to enhance its effectiveness [30]. In clinical practice, the majority of drugs employed for treating CNS disorders are small molecules that typically possess high lipophilicity and can traverse the BBB via active or passive mechanisms. However, drug molecules that are too lipophilic might accumulate in the ECs of brain vessels, preventing effective delivery to the deep brain regions where DA neurons – targeted in PD – are located. Therefore, designing drug molecules with a proper balance of lipophilicity and aqueous solubility is essential for ensuring successful accumulation and distribution of these drugs within the CNS for PD treatment. Moreover, emerging biologic therapies, such as gene-editing tools, NTF therapy, and antibody therapy, have demonstrated potential in PD treatment. For example, the use of adeno-associated virus vectors to deliver specific genes can restore the function of impaired DA neurons in PD models [30,31]. However, these large biomolecular drugs typically struggle to cross the BBB and are susceptible to degradation by enzymes in the body, which limits their clinical utility. To surmount these barriers, the application of nanotechnology provides a promising approach. Encapsulating drug molecules in nanocarriers can improve their stability in the bloodstream, enhance their capacity to cross the BBB, and facilitate targeted release in specific brain regions. This methodology has the potential to increase the bioavailability of PD therapeutics, thereby offering more effective treatment modalities for patients with PD.
4.3 Systemic distribution and clearance
Due to the lipophilic properties of many drugs, they exhibit extensive distribution in the body, potentially compromising their specificity. This may necessitate an increase in drug dosage to achieve the desired therapeutic concentration. However, this nonspecific systemic distribution can lead to heightened systemic toxicity and adverse effects on patient quality of life. For instance, upon oral administration, levodopa requires absorption through the intestine into the bloodstream and entry into the brain via the amino acid transport system [32]. However, it also undergoes widespread distribution in other bodily tissues, resulting in a spectrum of side effects, including cardiovascular and gastrointestinal discomfort. Additionally, the rapid systemic clearance of numerous anti-Parkinson’s drugs significantly impacts the concentration of drugs that reach the brain. For instance, l-3,4-dihydroxyphenylalanine (l-DOPA) may undergo enzymatic and nonenzymatic degradation in the gastrointestinal tract after oral administration. While combining carbidopa or benserazide can reduce levodopa metabolism in the body, the primary issue lies in the rapid clearance of hydrophilic drug molecules by the kidneys due to poor reabsorption, whereas lipophilic drugs are typically converted into hydrophilic metabolites in the liver before excretion via the kidneys or bile. Consequently, the therapeutic efficacy of drugs for PD may be influenced by their systemic distribution and rapid systemic clearance.
5 Lipid-based NPs for PD
5.1 Classification of lipid-based NPs
Lipid-based nanocarriers are commonly preferred over other types of nanocarriers due to their ability to mimic the natural lipid environment of biomembranes and their lower toxicity when administered in vivo [33]. Additionally, they can effectively utilize physiological transport mechanisms, such as the LDL receptor pathway, to facilitate delivery to the brain. Lipid-based NPs can be classified into several main types, including liposomes, lipid nanoparticles (LNPs), SLNs, and second-generation LNPs known as nanostructured lipid carriers (NLCs) (Figure 2).
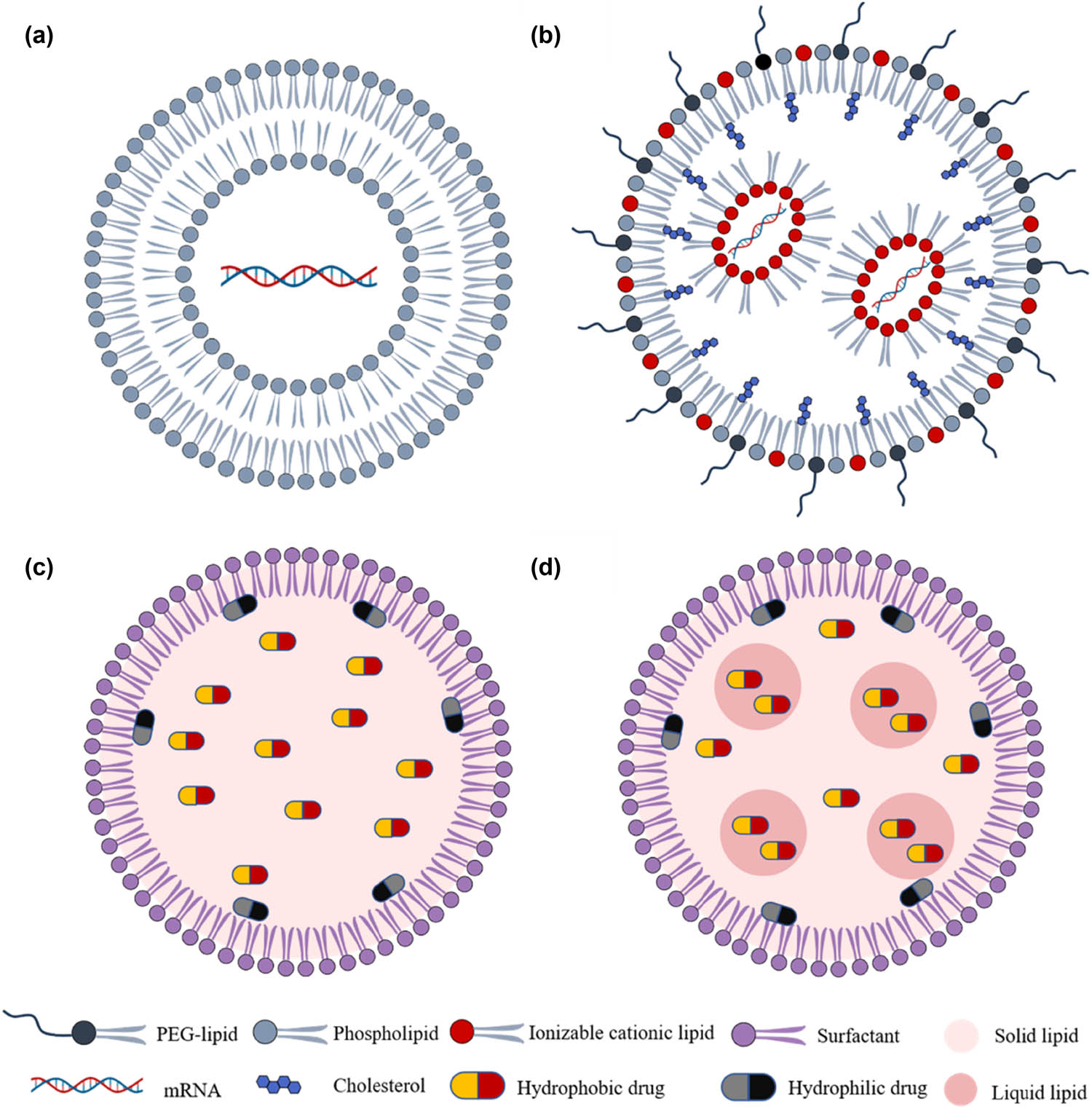
Schematic representation of lipid-based NPs. They are classified according to the physical state of their lipidic component in NPs, which include (a) liposomes, (b) LNPs, (c) SLNs, and (d) NLCs.
5.1.1 Liposomes
Liposomes, as the first generation of colloidal nanocarriers, have been widely recognized as effective drug delivery systems (DDSs) since the 1970s [34]. Owing to the hydrophilic core enveloped by one or multiple phospholipid bilayers resembling cell membranes, liposomes are strategically utilized for the targeted delivery of drugs, proteins, and peptides [35]. Based on their size and number of bilayers, liposomes can be categorized into three types: small unilamellar (10–50 nm), large unilamellar (50–1,000 nm), and multilamellar (20–100 nm) liposomes. These reversible structures are amenable to noncovalent interactions, such as van der Waals forces and hydrogen bonding among molecules [36].
The phospholipid bilayer of liposomes holds promise for transporting medications across the BBB. However, BBB crossing is strictly regulated. To enhance liposomal carrier transport through the BBB, various surface modifications have been investigated [37]. The BBB surface contains diverse proteins, peptides, antibodies, and ligand receptors. Surface-active ligands derived from these compounds show potential for facilitating transcytosis and cationic liposome absorption into the BBB. Nutrient coatings, such as glucose, are commonly applied to liposomes for optimal systemic distribution. Upon successful BBB traversal, passive diffusion mechanisms driven by brain efflux occur [38].
5.1.2 LNPs
Lipid nanoparticles (LNPs) are an advanced nanoscale drug delivery system, typically with sizes controlled between 20 and 200 nanometers, which facilitates their circulation within biological systems and penetration of biological barriers. LNPs are spherical vesicles composed of cationic or ionizable lipids, cholesterol, phospholipids, and polyethylene glycol (PEG) lipids, capable of stably encapsulating and delivering drug molecules. These nanoparticles possess low cytotoxicity, exceptional drug encapsulation capacity, high biocompatibility, and minimal immunogenicity potential, making them optimal candidates for targeted therapies in the treatment of central nervous system (CNS) diseases [39]. Below is a detailed introduction to the main components of LNPs.
Initially, cationic lipids were used because they not only bind anionic RNA but also fuse with the membrane to promote cellular uptake and endosomal escape. Although cationic lipids have shown promising efficacy for successful delivery, the permanent charge they carry results in high cytotoxicity and limits their potential applications. Ionizable lipids have been used instead of permanent cationic lipids to avoid hemolysis and to enable rapid clearance from the circulation because of their positive charge. Ionizable lipids can change charge based on their environment, portraying a neutral charge at physiological pH with low toxicity and a positive charge at low pH that permits nucleic acid complexation. Several parameters, such as tail length, unsaturation, branching, and pKa, significantly influence the properties of ionizable lipids. The unsaturation of ionizable lipids affects their pKa and fusion capability [40]. Reduced unsaturation increases pKa values, facilitating the ionization of ionizable lipids in alkaline environments. Appropriate unsaturation enhances fusion with cell membranes, promoting efficient gene or drug delivery. Similarly, longer lipid tails increase hydrophilicity and solubility, improving stability and manipulability in vitro and in vivo. An appropriate tail length allows for effective targeting and delivery to specific cells or tissues.
Phospholipids and cholesterol serve as helper lipids to enhance cellular internalization, facilitate membrane fusion, and promote escape from the endosome [41]. Specifically, cholesterol fills interstitial gaps between lipids in the liposomal membrane, thereby stabilizing the LNP structure.
PEGylation, the covalent attachment of PEG to lipids, is a widely used strategy to prolong the circulation half-life of lipids [42]. This approach effectively reduces macrophage-mediated clearance and inhibits apolipoprotein adhesion [43]. Additionally, PEG modification enhances steric stability, thereby extending the storage time of lipid-based formulations [44].
5.1.3 SLNs
SLNs are lipid-based biocompatible nanocarrier systems that primarily consist of lipids or modified lipid derivatives (e.g., triglycerides, fatty acids, and waxes) that exhibit nanostructures ranging in diameter from 10 to 1,000 nm. SLNs have a solid hydrophobic lipid core in which both hydrophilic and lipophilic drugs can be dispersed [45]. The physicochemical properties of SLNs, such as size, polydispersity, surface charge, stability, drug loading, and release profile, are greatly influenced by the careful selection of lipids, surfactants, and formulation composition. SLNs have demonstrated their remarkable ability to effectively transport both lipophilic and hydrophilic drugs to various diseased tissues. Moreover, they offer a versatile delivery system for not only drugs but also therapeutic molecules such as oligonucleotides, peptides, genes, and even smaller NPs such as superparamagnetic iron oxide NPs. This versatility positions SLNs as a highly promising platform for targeted drug delivery in various biomedical applications.
There are various patterns of SLN formulations according to the distribution of the drug components within them. According to the Drug-Enriched Shell Model, the core of the SLN is drug free, and the main active drug is distributed around the outer shell of the SLN. A hot homogenization process is utilized, causing the lipid content to precipitate at the core while leaving the drugs at the outer shell upon cooling. This model enables faster drug release due to the large surface area of the outer layer, with burst release controlled through larger drugs or adjusted surfactant formulations [46,47]. According to the drug-enriched core model, the active drug is concentrated within the SLN and is encased by the lipid outer shell. The drug is first liquified in the lipid, leading to supersaturation, and then concentrated at the center upon cooling. Drug release is controlled by the nature of the lipid membrane and follows Fick’s law of diffusion [48]. According to the homogeneous matrix model, the active drug is distributed within the lipid matrix of the SLN. The drug may exist in dispersion or in amorphous clusters within the lipid. Lipophilic drugs can be encapsulated in the lipid matrix without the use of surfactants. The drug release profile is extended in this model due to the strong molecular dispersion of drug particles in the colloidal matrix [46].
SLNs possess high biocompatibility and reduced systemic toxicity due to their physiological solid lipid emulsion system and minimal organic solvent usage [49]. This, in turn, allows for controlled and sustained drug release through their solid lipid composition, resulting in extended diffusion pathways and enhanced therapeutic efficacy [50]. Moreover, the lipid matrix of SLNs offers effective protection against biochemical degradation, ensuring improved drug stability [51]. With their ability to be tailored for active targeting of specific tissues and crossing the BBB, SLNs facilitate drug accumulation at desired sites of action [52]. Extensive studies have demonstrated that SLNs not only improve the pharmacokinetics, tissue distribution, and bioavailability but also lead to improved therapeutic outcomes. The scalability of SLN production utilizing easily obtainable raw materials enables cost-effective large-scale industrial manufacturing [53]. Additionally, the affordability of the sterilization and lyophilization processes further supports the wide application of SLNs. Furthermore, SLNs exhibit versatility in delivering a diverse range of drugs, including both hydrophilic and lipophilic compounds, while also accommodating traditional pharmaceutical formulations and biomolecules.
Although SLNs have many advantages, they also have several disadvantages, such as therapeutic expulsion, gelation tendency, and low encapsulation efficiency (EE) [54]. The low EE is primarily due to the internal structure of the lipid core, which lacks empty spaces during crystallization, making it challenging for the encapsulated substance to remain within the solid phase. To address this issue and enhance the loading efficiency of SLNs, Müller et al. [55] proposed modified SLNs with structural defects in the solid lipid core, increasing the internal free space for larger payloads. This advanced generation of SLNs not only improved the loading efficiency but also exhibited enhanced stability and prevented drug expulsion during storage [56].
5.1.4 NLCs
In the late 1990s, as the second generation of lipid nanocarriers, NLCs were developed to overcome the limitations associated with SLNs. These limitations include poor drug-loading capacity due to the highly organized crystalline structure and the tendency toward drug expulsion during storage caused by lipid crystallization [57]. In contrast, NLCs consist of a combination of solid and liquid lipids at a 70:30 to 99.9:0.1 ratio, along with an aqueous phase consisting of a surfactant [58]. This composition allows for the formation of a less ordered crystalline structure, which provides additional space for drug loading. Moreover, it reduces the crystallinity of the lipid matrix and prevents drug expulsion [59].
NLCs can be classified into three types based on their preparation methods, lipid matrix structure, and drug location [59]. The first type is the imperfect NLCs, which result from blending different lipids and yield a disorganized lipid matrix. This type of oil typically consists of a small amount of liquid oil mixed with a larger quantity of solid lipids. The lipids used may vary in terms of fatty acid origin, carbon chain length, or degree of saturation. Altering the type of fatty acid triglyceride used allows for modifications in the NP structure and imperfections. Increasing the lipid concentration enhances drug incorporation, as lipophilic drugs have greater solubility in liquid lipids. The second type is amorphous NLCs, which incorporate specific lipids such as hydroxypropyl behenate and isopropyl myristate through a specific blending method of solid and liquid lipids. These lipids facilitate the formation of a noncrystalline matrix, preventing crystallization of the solid lipid core and minimizing drug leakage [60]. The third type is multiple-type NLCs, which consist of a solid lipid matrix and liquid oil. These NLCs can be further subdivided into two subtypes: oil-in-solid, lipid-in-water type; and oil-in-water, lipid-in-water type. In oil-in-solid, lipid-in-water-type NLCs, numerous nanocompartments of liquid oil are embedded within a solid lipid matrix. This unique structure enhances drug bioavailability and enables controlled drug release [61]. On the other hand, oil-in-water, lipid-in-water-type NLCs comprise multiple oil droplets surrounded by lipids and dispersed in an aqueous phase. They exhibit a similar mechanism of drug release to oil-in-solid, lipid-in-water-type NLCs.
NLCs are influenced by various factors, shaping their properties. The lipid composition is crucial because different components affect the stability, drug entrapment efficiency, and release performance of a material. A high content of saturated fatty acids enhances physical stability, while unsaturated fatty acids or solid oils improve drug entrapment. Surfactant selection impacts the stability, particle size, and drug release rate, with hydrophilic and lipophilic properties influencing the interfacial properties. Processing methods, such as high-pressure homogenization, ultrasonic emulsification, and solvent precipitation, affect the microstructure, drug entrapment, and dispersibility. Environmental factors, including temperature, humidity, and pH, influence stability and drug release.
5.2 Designing advanced therapeutic lipid-based NPs specifically targeting PD pathophysiology
Overcoming the hurdle of effectively delivering therapeutic drugs, trophic factors, and biomacromolecules through the BBB and BSCB to access the CNS remains a formidable challenge in treating PD. However, there is promise in the rapid development of nanotechnologies, which offer potential solutions to the multifaceted barriers currently impeding the effectiveness of PD treatments, as previously discussed. This section revisits the various mechanisms underlying PD pathophysiology and delves into the detailed exploration of the design and utilization of nanomaterials to address these challenges (Table 2). These discussions provide an impetus to transform nanomedicine from a theoretical concept into practical clinical trials for PD treatment.
LNP-based drug delivery for PD
| Biological target | Nanocarrier | Encapsulated drug/genes | Reference | Biological target | Nanocarrier | Encapsulate drug/genes | Reference |
|---|---|---|---|---|---|---|---|
| DA replacement | Rabies virus glycoprotein (RVG29)-liposomes | N 3, 4bis(pivaloyloxy)-DA | [63] | Liposome | Resveratrol (RES) | [95] | |
| CITX-Liposomes | l-DOPA | [64] | Chitosan-SLNs | Curcumin | [158] | ||
| Transferrin-liposomes | DA | [131] | SLN | Trehalose | [159] | ||
| Chitosan-liposomes | l-DOPA | [65] | SLN | Quercetin | [160] | ||
| Amyloid precursor protein (APP)-liposome | DA | [66] | SLN | Curcumin | [161] | ||
| Liposomes | L-DOPA | [134] | SLN | GER-UDCA | [162] | ||
| Liposomes | DOPH | [135] | Polysorbate 80-SLN | Formoterol | [100] | ||
| OX26 monoclonal antibody (Mab)-PEG-Liposomes | DA | [136] | SLN | Bacopa monnieri | [163] | ||
| Maltodextrin-Liposomes | Levodopa | [137] | SLN | Citicoline | [164] | ||
| Fe3O4-NMD-Liposomes | Nimodipine | [138] | Nanoemulsion | Vitamin E and RES | [91] | ||
| Liposomes | DA | [139] | Lipid- poly(lactic-co-glycolic acid) (PLGA) bubbles | Curcumin | [97] | ||
| Liposomes | L-DOPA prodrugs | [140] | LNPs | Curcumin and piperine | [165] | ||
| SLN | L-DOPA | [141] | PUFA-PL-LCLNs | NPs themselves served therapeutic purpose | [166] | ||
| SLN | Ropinirole hydrochloride | [142] | Neurotrophic factor | Liposome | Glial cell line-derived neurotrophic factor (GDNF) | [108] | |
| SLN | Pyrrolobenzodiazepine | [143] | Liposome | Recombinant human fibroblast growth factor-20 | [111] | ||
| SLN | Bromocriptine mesylate | [68] | Liposome | GDNF | [93] | ||
| SLN | Apomorphine | [70] | TAT-NLC | GDNF | [95] | ||
| SLN | Piribedil | [144] | NLC | Basic fibroblast growth factor (bFGF) | [110] | ||
| SLN | DA | [145] | Chitosan-NLC | GDNF | [119] | ||
| SLN/liposomes | DA | [146] | Lipid nanomicro-bubble | GDNF | [120] | ||
| NLC | Apomorphine diester prodrugs | [147] | Lipid nanomicro-bubble | Nrf2 | [167] | ||
| NLC | L-DOPA codrugs | [148] | RVG-liposomes | α-syn small interfering RNA (siRNA) | [114] | ||
| NLC | Bromocriptine | [69] | Gene therapy | Mab-liposome | GDNF plasmid | [107] | |
| SLN and NLC | Ropinirole | [67] | Liposome | GDNF plasmid | [120,121] | ||
| LNP | Apomorphine | [149] | PEG-immunoliposomes | Tyrosine hydroxylase (TH) plasmid | [116,168,169] | ||
| LNP | L-DOPA precursor | [150] | LNP | Csf2 mRNA | [117] | ||
| SLN | DA | [151] | LNP | GRIN1 siRNA | [115] | ||
| Lipid-polymer hybrid NP | L-dopa | [152] | LNP | L-PGDS ASO | [118] | ||
| Lipid-polymer hybrid NP | Ropinirole hydrochloride | [153] | LNP | Albumin-GM-CSF mRNA | [170] | ||
| SLN | Bromocriptine and RES | [154] | SLN | NPs themselves served therapeutic purpose | [128] | ||
| Polysorbate80-liposomes | Curcumin | [79] | Stem cell therapy | Heparinized cationic SLN | NGF-hcsln | [129] | |
| Alpha-Synuclein Aggregates | Leptin (Lep)-liposome | RES and epigallocatechin gallate (EGCG) | [78] | Ln5-P4-ASLNs | NGF | [78] | |
| Mab-Liposomes | SynO4 | [80] | Lipid-based NPs | miRNA | [131] | ||
| PEG-cholesterol nanoliposomes (NLPs) | Baicalein | [155] | LNPs | mRNA | [132] | ||
| Chol-PEG-Zwitterionic NLPs | NPs themselves served therapeutic purpose | [81] | |||||
| Antitransferrin receptor antibody-liposomes | pomace seed extract | [156] | |||||
| Oxidative stress, mitochondrial dysfunction and inflammation | Fe3O4-liposome | RES | [157] | ||||
5.2.1 DA replacement
DA is synthesized from tyrosine through the action of an enzyme called TH, which converts tyrosine into l-DOPA. l-DOPA is then decarboxylated by aromatic l-amino acid decarboxylase to form DA. Once synthesized, DA is stored and protected within small synaptic vesicles by vesicular monoamine transporter 2 until it is ready for release into the synaptic cleft. This storage mechanism ensures the maintenance of low levels of free cytoplasmic DA, as DA is prone to oxidation and the generation of potentially neurotoxic substances under intracellular conditions. In the striatum, a brain region, DA exerts its effects by interacting with five distinct types of DA receptors. Notably, D1R and D2R (receptors 1 and 2, respectively) play crucial roles in the regulation of motor control signaling pathways. D1R facilitates movement via direct pathways, whereas D2R inhibits movement through indirect pathways. Upon DA binding to these receptors, a cascade of signal transduction pathways is initiated, modulating the excitatory or inhibitory state of neurons and leading to specific physiological effects.
After being released by dopaminergic neurons, DA quickly dissociates from its receptors and is taken back into dopaminergic neurons through the DA transporter (DAT) to clear the extracellular space of DA. DA, which is not reuptaken, is decomposed by enzymes called catechol-O-methyltransferases and monoamine oxidases in the presynaptic terminal. The breakdown process can generate hydrogen peroxide (H2O2), which can cause oxidative stress and neurotoxicity. After damage to the nigrostriatal pathway occurs, surviving dopaminergic neurons undergo adaptive changes to offset this loss. These changes involve heightened DA metabolism, elevated D2 DA receptor density, and diminished expression of DAT, all aimed at preserving appropriate levels of extracellular DA. These compensatory mechanisms serve to impede the progression of early-stage PD symptoms. Nevertheless, as the neurodegenerative process persists, DA depletion transpires, resulting in disruptions in motor control and the manifestation of symptomatic conditions.
Current therapies for PD focus on relieving motor symptoms by administering levodopa, a DA precursor or DA agonist (e.g., pramipexole, ropinirole, or rotigotine). Despite providing a temporary solution for controlling motor symptoms, the therapeutic efficacy of these drugs is constrained by various factors, including adverse effects, short half-lives in the systemic circulation and extracellular compartments, instability, poor bioavailability, and challenges in traversing the BBB. These shortcomings can be overcome through the delivery of drugs using different types of nanomaterials.
In the context of PD, liposomes loaded with DA have been engineered to facilitate prolonged and controlled release of this neurotransmitter. Klionsky et al. effectively encapsulated DA within liposomes and demonstrated its controlled release capabilities in vivo. The sustained release of DA from these liposomes resulted in increased DA levels within the striatum, leading to partial behavioral restoration [62]. With the confirmation of the feasibility of DA encapsulation and transport within liposomes, scientific investigations have shifted their attention toward surface modification to enhance the EE, targeting specificity, and controlled release in specific regions of the brain. For instance, DA-loaded RVG-29-conjugated liposomes effectively permeated the BBB when administered intravenously, leading to increased levels of DA- and TH-positive cells in the striatum [63]. ClTx-modified stealth liposomes loaded with levodopa showed promising therapeutic effects in a mouse model of PD, reducing behavioral disorders and protecting dopaminergic neurons, indicating their potential as a targeted DDS for improved PD therapy [64]. Cao et al. demonstrated that chitosan-coated levodopa liposomes could mitigate the induction of dyskinesias in PD patients. The mechanism may be associated with the signaling pathway involving phosphorylated ERK1/2, phosphorylated Thr34 DARPP-32, and ΔFosB within the striatum [65]. The surface modification of these liposomes with APP facilitates the penetration of DA through the BBB and leads to an increase in the striatal DA concentration [66].
Despite being nanosystems with great potential for controlled release, phospholipid-based liposomes have low stability and high costs, which has prompted the recent development of SLN and NLC. Dudhipala et al. developed SLNs and NLCs to encapsulate ropinirole. Pharmacokinetic and pharmacodynamic studies demonstrated a significant improvement in the oral and topical bioavailability of ropinirole, resulting in effective treatment of PD [67]. Similarly, Esposito et al. developed NLCs and SLNs as delivery systems for bromocriptine, an ergoline agonist of DA receptors. The encapsulation of bromocriptine in NLC/SLN can yield sustained therapeutic effects in a rat model of PD and prolong the half-life of bromocriptine in vivo [68,69]. The oral bioavailability and brain regional distribution of apomorphine, a DA receptor agonist used to treat PD, can be enhanced by utilizing SLNs as carriers, thereby improving the therapeutic efficacy of PD [70].
5.2.2 α-Syn aggregation
α-Syn is a highly expressed neuronal protein in presynaptic terminals that, under normal physiological conditions, plays a role in regulating signal transmission and synaptic plasticity at the synaptic terminal. α-Syn is naturally unfolded in aqueous solution, but when bound to negatively charged lipids (such as phospholipids on cell membranes), it can form α-helical structures or be rich in β-folded structures [71]. When α-syn is overexpressed or misfolded due to genetic mutations or protein modifications, α-syn fibers may accumulate and aggregate into soluble oligomers and protofibrils, which may eventually stabilize into insoluble amyloid-like fibrillar structures. This misfolding increases the tendency of these proteins to aggregate with other misfolded proteins, leading to fibrillation, aggregate formation, and protein and organelle isolation, ultimately leading to the formation of LBs. Moreover, α-Syn fibers have been shown to be infectious pathogenic factors that spread in a prion-like manner, leading to enhanced aggregation [72].
The main mechanisms responsible for the degradation of misfolded α-syn at synapses are the ubiquitin‒proteasome pathway (UPP) and the autophagy‒lysosome pathway (ALP). UPP is essential for degrading misfolded or damaged proteins within cells. Proteins are tagged with ubiquitin, which targets them for destruction by the proteasome, thus sustaining protein balance. ALP is vital for cellular waste management. It engulfs and degrades cellular debris and damaged organelles through autophagosomes that merge with lysosomes, where they are broken down by lysosomal enzymes, ensuring cell health and functionality. However, the gradual accumulation of α-syn at synapses leads to the breakdown of these systems. Consequently, proteostasis dysregulation in dopaminergic neurons precipitates the accumulation of α-syn, which subsequently impairs protein degradation pathways, thereby establishing a deleterious feedback loop [73,74]. In addition, α-syn aggregation triggers endoplasmic reticulum stress and unfolded protein response (UPR) activation, further driving the development of neurodegenerative diseases [75,76]. Currently, therapeutic strategies for α-syn mainly include two aspects: one is to reduce its abnormal aggregation by regulating the expression and metabolism of α-syn, and the other is to prevent the damage of α-syn to the nervous system by intervening in the formation and stability of α-syn aggregates.
EGCG has been shown to inhibit α-syn aggregation in vitro. However, its therapeutic efficacy in PD is hindered by the BBB and strong binding affinities between EGCG and membrane proteins, impeding its accumulation within dopaminergic neurons. To overcome these challenges, researchers have developed multifunctional lipid micelles that contain EGCG. Micelles have been engineered to enhance the transport of EGCG across the blood–brain barrier by utilizing B6, a peptide with a high affinity for the TfR. Additionally, ligands such as mazindol have been employed to selectively target dopaminergic neurons by interacting with the DAT, facilitating its internalization. The results demonstrated a significant increase in the accumulation of EGCG within PD lesions. Furthermore, a remarkable inhibition of α-syn aggregation was observed, accompanied by a notable increase in the population of dopaminergic neurons [77]. Moreover, Kuo et al. conducted a study in which liposomes were surface-modified with Lep and loaded with RES and EGCG. This formulation exhibited neuroprotective effects by downregulating the expression of apoptotic proteins, such as the Bcl-2-associated X protein and α-syn, while upregulating the expression of the antiapoptotic protein B-cell lymphoma 2, TH, and DAT [78]. Similarly, in addition to EGCG, curcumin has the potential to clear α-syn aggregates and treat PD, but it is limited by the BBB, resulting in low bioavailability and poor penetration. Zhang et al. [79] developed a curcumin-based NP system named Curcumin Polyethylene Glycol 8000 Oleate (CPC NPs) modified with polyethylene glycol 8000 oleate, which utilizes the ultrasound-targeted microbubble disruption technique to increase the permeability of the BBB and achieve localized delivery of curcumin to target nuclei in the brains of PD patients. Furthermore, Sela et al. employed transferrin-modified brain-targeted liposomes to accurately deliver the monoclonal antibody Syno4 to dopaminergic neurons in the brains of mice with PD. This approach successfully reduced α-syn aggregation and neuroinflammation, resulting in improved motor function [80]. In addition to being nanocarriers, NPs themselves can be used as therapeutic agents. Aliakbari et al. [81] showed that zwitterionic NLPs with cholesterol and PEG (NLP-Chol-PEG) can effectively inhibit α-syn fibrillization and reduce neurotoxicity and ROS production without altering neuronal intracellular calcium. The results showed that the clearance of α-syn led to the restoration of normal motor behavior, DA levels, and TH expression.
5.2.3 Oxidative stress, mitochondrial dysfunction, and neuroinflammation
The pathogenesis of PD involves the complex interplay of oxidative stress, mitochondrial dysfunction, and neuroinflammation. Oxidative stress refers to the imbalance between oxidation and antioxidant processes within the body and plays an important role in the pathogenesis of PD [82,83]. Under normal circumstances, the body’s oxidation system and antioxidant system maintain a dynamic balance, and free radicals can be promptly eliminated by substances such as catalase, superoxide dismutase (SOD), and glutathione peroxidase. However, oxidative stress occurs when external factors induce a large accumulation of free radicals or a decrease in antioxidant levels. Research on early-stage PD patients has shown significant increases in oxidative damage markers such as malondialdehyde and lipid peroxidation in the substantia nigra. SOD activity and zinc levels are elevated, while glutathione (GSH) is reduced by 40%. The activities of catalase and GSH reductase are also decreased, indicating the occurrence of oxidative stress in PD patients [84]. As a result, the imbalance between oxidation and antioxidant processes in PD leads to various damaging effects, including DNA breakage, lipid autooxidation, protein inactivation, calcium imbalance, and mitochondrial dysfunction. ROS directly damage DA, which is released into the extracellular space by dopaminergic neurons and promotes the production of a substantial number of intracellular oxygen radicals. This leads to the activation of NF-κB and the subsequent release of significant amounts of inflammatory factors. The accumulation of these factors activates nitric oxide synthase, culminating in the production of a large quantity of nitric oxide (NO), which exerts potent toxic effects on dopaminergic neurons.
In PD, oxygen radicals interact with calcium ions, disrupting cellular calcium homeostasis and leading to elevated intracellular calcium concentrations. This disruption initiates a cascade of enzymatic activations involving nicotinamide adenine dinucleotide phosphate oxidase, fatty acid oxidase, and mitochondrial enzymes, which in turn increase the production of oxygen radicals [85]. This interaction establishes a detrimental feedback loop that accelerates dopaminergic neuron loss. Moreover, elevated levels of calcium and oxygen radicals within cells trigger various cell death pathways, including the mitochondrial pathway and apoptosis receptor pathway, further contributing to the rapid degeneration of dopaminergic neurons. Iron ions can react with ROS to produce highly reactive free radicals, leading to oxidative damage and cell death. Additionally, iron ions can increase mitochondrial ROS levels by promoting the production of mitochondrial complex I. Furthermore, iron ions can suppress the cellular antioxidant system, further increasing ROS accumulation [86]. These interactions may exacerbate oxidative stress in PD patients, ultimately leading to neuronal cell death and disease progression.
Mitochondria, essential organelles within cells, primarily function to generate ATP, serving as the primary energy source for cellular activities. This process involves the transfer of electrons from Complex I to Complex IV of the electron transport chain (ETC) and is associated with oxidative phosphorylation. A significant byproduct of mitochondrial ETC activity is ROS, and mitochondrial dysfunction leads to an overproduction of ROS [87]. Importantly, ROS can exert deleterious effects on the ETC itself. In the context of PD, mitochondrial dysfunction plays a pivotal role. It has been demonstrated that, upon metabolism in the brain to 1-methyl-4-phenylpyridinium (MPP+), MPTP selectively inhibits mitochondrial complex I, resulting in the death of dopaminergic neurons in the substantia nigra [88]. Notably, PD patients exhibit a decrease in mitochondrial complex I activity and a decrease in the expression of genes encoding mitochondrial proteins. Certain gene mutations, including those in LRRK2, PRKN, DJ-1, PINK1, FBXO7, and ATP13A2, may precipitate mitochondrial impairment. Furthermore, mitochondrial dysfunction is implicated in processes such as mitochondrial biogenesis, fusion/fission, and mitophagy [89]. In addition to their role as energy suppliers, mitochondria have emerged as central signaling hubs in the regulation of networks associated with innate immunity and inflammation. In response to diverse stressors, mtDNA can translocate to intra- or extracellular compartments. Released mtDNA from mitochondria can serve as damage-associated molecular patterns, stimulating inflammation through interactions with pattern recognition receptors.
Microglia, the resident macrophages of the CNS, maintain cerebral homeostasis and respond to injury or pathogens. In PD, microglia can be activated by a myriad of factors, encompassing abnormal α-syn aggregation, cell damage signals, and environmental factors. Upon activation, microglia release inflammatory mediators, including cytokines, chemokines, and ROS, promoting neurodegeneration. Concurrently, microglia-mediated inflammatory responses can exacerbate oxidative stress. In PD patients, there is evidence of microglial activation in the substantia nigra and elevated proinflammatory factor levels in the brain and CSF [90]. Moreover, dying neurons release oxidized lipids, proteins, and DNA, which in turn activate microglia, perpetuating a vicious cycle of neurotoxicity [91].
Research has shown that enhancing the activity of the antioxidant system and boosting intracellular antioxidant capacity can mitigate oxidative stress caused by ROS. This alleviation of oxidative stress can provide protection against PD. Consequently, antioxidants are viewed as potential therapeutic agents for treating this disease. At present, natural antioxidant compounds from plants are widely utilized to mitigate toxicity, repair the CNS, and prevent the onset of PD. Commonly used natural antioxidants, including curcumin, RES, ginsenosides, quercetin, catechins, Mucuna pruriens, Withania somnifera, and ursolic acid, play important roles in the prevention and treatment of PD [92,93,94]. However, the effectiveness of these agents is limited by their ability to cross the BBB, which affects their utilization, stability, and solubility at target sites in the brain. To overcome these limitations, the nanosization of plant bioactive compounds for the treatment of PD can maximize therapeutic efficacy, improve the ability of these compounds to penetrate the brain, and enhance stability.
Nanodelivery technologies, such as SLNs, NLCs, NLPs, and nanobubbles, enable the controlled delivery of nanosized bioactive compounds to the brain. For instance, the oral administration of RES encapsulated in liposomes significantly mitigates abnormal rotational behavior, curtails the loss and apoptosis of cells in the substantia nigra, diminishes the levels of ROS, and bolsters the total antioxidant capacity within the substantia nigra tissue. In vivo studies have demonstrated that RES encapsulated in liposomes has stronger effects than free RES [95]. Furthermore, incorporating RES into vitamin E nanoemulsions has been found to amplify its pharmacological activity. Pharmacokinetic studies revealed that drug concentrations in nanoemulsions are elevated, consistent with increased antioxidant activity [96]. Additionally, liposomes can codeliver drugs encapsulating RES and EGCG in Lep-modified liposomes and adding SH-SY5Y cells treated with MPP+ can reduce apoptosis-promoting protein Bcl-2-associated X protein and α-syn, and increase apoptosis-inhibiting protein B-cell lymphoma 2, TH, and DAT, thereby enhancing the neuroprotective effect of neurons [78].
Researchers have prepared nanobubbles loaded with curcumin using a mixed nanostructure based on lipids and PLGA via double emulsion evaporation. These nanobubbles can open the BBB and accumulate in the brain when stimulated by low-intensity FUS. Furthermore, they can release curcumin into the CNS, thereby improving motor function in animal models of PD [97]. In addition to natural antioxidants, coenzyme Q10 and vitamin E are commonly used antioxidant molecules in PD treatment. However, coenzyme Q10 has poor solubility, resulting in low oral bioavailability. To address this issue, a nanoemulsion loaded with coenzyme Q10 was developed. When orally administered to rats with PD, this nanoemulsion effectively reduced DA depletion, increased GSH content, and decreased sulfo-barbituric acid reactive substances [98]. In PD rats, the intranasal delivery of a grapefruit flavonoid nanoemulsion loaded with vitamin E can increase the levels of SOD and GSH induced by 6-hydroxydopamine (6-OHDA) while improving motor ability [99]. Furthermore, several drugs are also used to alleviate oxidative stress and neuroinflammation. Polysorbate 80 surface-modified SLNs from formoterol can suppress the expression of the SNCA gene, mitochondrial membrane damage, and oxidative stress markers in a mouse model of PD [100].
In addition to the liposomal delivery of natural antioxidants, other nanomaterials, such as polymer NPs, exosomes, and metal NPs, are widely used to deliver various antioxidants. Some metal NPs can directly alleviate oxidative stress by acting as scavengers for ROS and NO, mimicking the function of major antioxidant enzymes involved in oxidative stress, including metal oxides and nanozymes that mimic peroxidase, SOD, and other antioxidant enzymes. In summary, the abovementioned research indicates that nanosization techniques can enhance the penetration, stability, and efficacy of natural antioxidants in the brain, suggesting that they are promising therapeutic strategies for PD. However, further research and clinical trials are required to validate the safety and effectiveness of these agents.
5.2.4 NTFs
NTFs are a group of small extracellular proteins that regulate the survival, development, and function of neurons in the nervous system. These genes belong to four families: the nerve growth factor (NGF) family (also known as neurotrophins), the glial cell line-derived neurotrophic factor (GDNF) family, the cerebral dopamine neurotrophic factor (CDNF)/mesencephalic astrocyte-derived NTF family, and other NTFs. Through binding to specific receptors, NTFs regulate multiple aspects of neuronal survival, development, function, and synaptic connectivity, playing crucial roles in normal development and maintenance of the nervous system. Additionally, some NTFs have anti-inflammatory, antiapoptotic, remyelination, and axonal regeneration properties, and they promote tissue repair and regeneration by facilitating the involvement of adult stem cells in tissue repair after neural damage [101,102,103].
In the context of PD, changes in NTFs have been observed in both clinical and experimental studies. Research has shown a significant decrease in NTFs, such as those in the SNpC and basal ganglia, in regions associated with dopaminergic neurodegeneration in PD patients [104]. One of the well-studied NTFs in PD is brain-derived neurotrophic factor (BDNF), which is highly expressed in motor-related areas of the brain. Reductions in BDNF levels, which are closely related to dopaminergic neurodegeneration, have been observed in the substantia nigra and putamen of PD patients. Moreover, the overexpression of α-syn in certain brain regions of PD patients is associated with the downregulation of BDNF [105]. Another important NTF in the maintenance of the dopaminergic system is GDNF. GDNF is highly expressed in brain regions involved in motor control in the human brain [106]. However, the decreases in GDNF in the putamen, substantia nigra, and motor control areas of the brain in PD patients are more significant than those in BDNF patients.
NTFs play a critical role in the nervous system, especially in the treatment of PD. However, different methods of administering NTFs in clinical trials have not yielded the expected results and have resulted in severe side effects. The delivery and selectivity of NTFs play crucial roles in clinical cases of PD. Systemic administration of NTFs has poor pharmacokinetic effects and results in very low permeability through the BBB; thus, expensive and risky intracranial surgery is needed to directly deliver NTFs into the brains of PD patients. Only patients in the middle and late stages of the disease can undergo surgical treatment. In recent years, NP-based strategies have received widespread attention for their ability to improve the stability and delivery of NTFs to the brain. This strategy can enhance the bioavailability and stability of NTFs, reduce dosage and side effects and is expected to become a new approach for future PD treatments.
Xia et al. developed a Trojan horse liposome (THL) by intravenous injection of GDNF plasmid DNA targeting the TfR in rats. They observed an increase in the GDNF concentration in the substantia nigra, which led to a reduction in apomorphine-induced rotation at different doses and an increase in TH activity in the striatum [107]. Similarly, the intranasal delivery of GDNF via liposomes to PD rats increases brain GDNF levels and has a neuroprotective effect [108]. In addition to liposomes, NLCs have also been investigated as useful DDSs for the gold standard, NTF-GDNF, in PD treatment. In fact, chitosan-modified GDNF-loaded NLCs were nasally administered to a PD rat model. Chronic administration of these chitosan-modified lipid NPs successfully delivered GDNF to 6-OHDA-lesioned rats for 2 weeks, resulting in the restoration of motor symptoms [109]. Although GDNF is one of the most studied NTFs for the treatment of PD, other similar molecules have also been encapsulated to improve their bioavailability. Collagen nanocrystal carriers encapsulating bFGF have been studied as a novel method of nasal administration. Nasal administration of NLCs improved rotational behavior, monoamine neurotransmitter levels, and TH expression in in vitro and in vivo studies without adverse effects on nasal mucosal integrity [110]. Niu et al. [111] discovered that the use of FUS and liposome delivery of small ubiquitin-related modifier fused with fibroblast growth factor 20 (FGF20) improved motor behavior and protected dopaminergic neurons in a rat model of PD.
In conclusion, the delivery of NTFs using NP systems has emerged as a new research hotspot in the treatment of PD. By utilizing NPs for NTF delivery, their bioavailability and stability can be enhanced while reducing their dosage and side effects. Although further research is still needed to improve the effectiveness of this treatment approach, it has already demonstrated significant potential and is expected to become a key technology in future PD therapy.
5.2.5 Gene therapy
Recent gene engineering technologies have led to the emergence of gene therapy as a promising approach for treating CNS diseases. Compared to traditional small molecule-based therapies, gene therapy offers several advantages, such as the modulation of protein-coding genes beyond the reach of conventional small molecules, high target specificity, reversible effects, and potential reprogramming without altering drug pharmacokinetics [112,113]. Genotype analysis of various CNS diseases has revealed novel therapeutic targets, and several promising treatment strategies aimed at activating or silencing these targets, including plasmid DNA, small interfering RNA (siRNA), antisense oligonucleotides, miRNAs, and mRNAs, have been identified.
Gene therapy for CNS diseases is limited by the restrictive nature of the BBB and blood-cerebrospinal fluid barrier (BCSFB), which play crucial roles in maintaining CNS homeostasis and preventing the entry of harmful compounds. Various approaches have been explored to enhance drug delivery into the brain, including direct CNS administration, BBB disruption, and carrier-mediated delivery. However, these methods have limitations and potential adverse effects. To address these challenges, nanomaterials have emerged as promising vehicles for nucleic acid delivery, offering improved stability, bioavailability, and penetration across the BBB and BCSFB. Nanomaterials such as liposomes, polymers, inorganic NPs, and extracellular vesicles can be tailored for precise targeting of CNS disease sites.
Several nonviral gene delivery systems, such as liposomes, have been reported for use in PD treatment. The RVG-decorated liposomes exhibited suitable properties for potential in vivo applications and successfully induced α-syn gene silencing in primary neurons without affecting cell viability [114]. The authors employed liposomal delivery of siRNA targeting the GluN1 subunit of NMDA receptors and demonstrated its effective and selective reduction of synaptic NMDA receptor currents compared to synaptic α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor currents [115]. TH is an important enzyme involved in the synthesis of DA in the body. Therefore, increasing the expression or activity of TH can enhance DA synthesis and potentially alleviate symptoms of PD. A study demonstrated that in the 6-OHDA rat model of PD, targeted immunoliposomes loaded with plasmids expressing TH and the TfR Mab resulted in complete normalization of TH activity in the striatum [116].
Lipid NPs have changed the history of COVID-19 vaccines through the use of mRNAs. Restoring the numbers and functions of regulatory T cells (Tregs) represents a novel therapeutic strategy for treating neurodegenerative disorders. The authors developed a novel LNP containing mRNA encoding granulocyte-macrophage colony-stimulating factor (Gm-csf mRNA), which can increase plasma GM-CSF levels and peripheral CD4+CD25+FoxP3+ Treg cell populations in PD rats [117]. Neuroinflammation has been noted in PD, resulting in neuronal damage and death that accelerate the progression of PD. Several studies have shown that the level of l-PGDS in the CSF of PD patients is associated with clinical symptoms and disease progression. The use of mannosylated LNPs for the targeted delivery of l-PGDS ASO to brain border-associated macrophages in rats leads to the silencing of neuroinflammation-related genes and represents a significant step forward in the development of gene therapy platforms for the advanced treatment of neuroinflammation-related pathologies using ASO@LNP nanovectors [118]. Glial cell line-a (GDNF) is a potent agent for PD therapy due to its neuroprotective and neurotrophic effects. Xia et al. utilized THLs targeted with a Mab to rat TfR to deliver intravenous GDNF plasmid DNA. Elevated concentrations of GDNF in the substantia nigra led to a dose-dependent reduction in apomorphine-induced rotations and an increase in striatal TH enzyme activity [70]. Similarly, the delivery of the GDNF plasmid using THLs targeted to TfRs can eliminate neurotoxic effects through multiple intravenous administrations [119]. FUS induced by microbubbles is a promising technique for noninvasive opening of the BBB to allow targeted delivery of therapeutic substances into the brain and thus noninvasive delivery of genetic vectors for CNS treatment. The use of FUS to deliver liposome-coupled microbubbles to mediate the gene transfer of the GDNF plasmid into the brain mitigates behavioral deficits and neuron loss in a rat model of PD [120,121].
5.2.6 Stem cell therapy
In the twenty-first century, the significance of stem cells in medical research and therapeutic applications has dramatically increased. Stem cell therapy represents a therapeutic approach wherein appropriate cells or tissue groups are transplanted into the host brain to substitute for neurons and brain tissues damaged by neurodegenerative diseases, thereby reconstructing or restoring neural function. Stem cells are characterized by unique features, including the capacity for self-renewal and differentiation into various cell types [122]. They maintain an undifferentiated state (pluripotency) and can potentially differentiate into multiple mature cell types [123]. Depending on their origin, stem cells can be categorized as embryonic stem cells, mesenchymal stem cells (MSCs), adult stem cells, or induced pluripotent stem cells (iPSCs).
Stem cell therapy offers a promising treatment approach for neurodegenerative diseases, including PD. In PD, stem cell therapy aims to promote the differentiation of stem cells into DA-producing neurons, which are subsequently delivered to damaged areas of the patient’s brain to restore neural function and alleviate the progression of the disease. Injection of MSCs from different sources into PD rats effectively improved neurological function, promoted the survival of damaged neurons, and increased DA levels [124,125,126]. In clinical trials, MSCs from different sources, administered through intracerebral transplantation, arterial injection, or venous injection, have been found to improve unified parkinson’s disease rating scale scores in PD patients [127]. Moreover, both human embryonic stem cells (hESCs) and iPSCs have distinctive characteristics that render them suitable for investigating the progression of PD. These cells have been implanted into model organisms under established PD conditions, yielding a combination of results that are both varied and encouraging.
Although stem cell therapy holds great promise for treating neurodegenerative diseases, there are still several limitations. For instance, the selection of appropriate cell types is crucial for treating CNS diseases. The presence of the BBB makes it difficult for cells to be delivered into the brain. Additionally, transplanted cells may lead to immune reactions, tumor formation, or inappropriate cell proliferation, thereby increasing treatment risk. The efficiency of stem cell differentiation and the functional expression of differentiated cells are also challenging. The survival rate of transplanted cells in the body is an important concern. Furthermore, monitoring and tracking transplanted stem cells in the human body are difficult. However, nanotechnology combined with stem cell therapy can overcome the abovementioned difficulties to a certain extent. For example, functionalized nanocarriers can target the delivery of stem cells and drugs to the lesion area, improving treatment efficacy and reducing the impact on healthy tissues. These carriers can protect stem cells, facilitate their passage through the BBB, and increase their survival rate after transplantation. Nanotechnology-delivered biomolecules can precisely control stem cell differentiation and reduce immune rejection. Nanocarriers can track and monitor the behavior of stem cells to assess treatment effects. Furthermore, nanoscaffolds provide an ideal microenvironment for stem cells, aiding in the repair of neural tissues. Nanocarriers also increase the bioavailability of drugs, reducing dosage and side effects, thus opening up new avenues for the treatment of CNS diseases.
In recent years, researchers have been continuously exploring the use of lipid-based nanocarriers for targeted differentiation and treatment of stem cells. Chabra et al. reported that SLNs can maintain the characteristics of mouse MSCs by serving as a scaffold for both stem cell attachment and stemness while also inducing stem cell differentiation [128]. Similarly, Kuo et al. designed a heparinized cationic SLN loaded with NGF that can direct the differentiation of stem cells into neurons [129]. The same team also prepared amphiphilic solid lipid nanoparticles (ASLNs) with the Ln5-P4 peptide for loading NGF and retinoic acid. This nanocarrier can bind to α3β1 integrin on iPSCs and promote their differentiation into neurons. These findings indicate that Ln5-P4/NGF-RA-ASLNs represent a potential system for inducing iPSC-derived neuron differentiation, providing a new approach for treating neurodegenerative diseases [130]. Takeda et al. successfully delivered miRNA to human mesenchymal stem cells (hMSCs) using synthetic bioreducible lipids, and miRNA-9 promoted their neural differentiation, demonstrating the potential of these lipids in gene delivery and stem cell programming [131]. Similarly, Zhao et al. developed a novel cationic lipidoid-based nanocarrier to deliver Cas9 mRNA and sgRNA targeting the neuronal restrictive silencing factor to induce neural-like cell differentiation in hMSCs [132]. These studies demonstrate the potential application value of lipid-based nanocarriers in stem cell therapy and differentiation, providing a solid foundation for the treatment of neurodegenerative diseases, including PD.
6 Discussion
The occurrence of PD is associated with complex pathogenic mechanisms, and aging is one of the primary reasons for the global increase in PD cases. Current treatment strategies rely on NTFs, antioxidants, and DA replacement medications conducted in various cellular or animal models [133]. However, these molecules often struggle to cross the BBB, which limits their effectiveness and clinical application. To overcome these obstacles, lipid-based nanocarrier DDSs have shown promise. These systems exhibit minimal toxicity, enhance drug loading, provide sustained release, and safely traverse the BBB. Lipid carriers can also be conjugated with genes, peptides, and ligands to facilitate the active penetration of drugs through the BBB and to target therapeutic agents to the affected sites. The FDA’s approval of the Spikevax® and Comirnaty vaccines for COVID-19 immunization marks a milestone that may propel research into lipid-based nanomedicines, promoting additional clinical trials and the development of commercial products. For the mechanisms of oxidative stress, mitochondrial damage, inflammation, and misfolding of the α-syn protein in PD, lipid NPs have been developed to deliver various molecules and drugs to alleviate oxidative stress, reduce neuroinflammation, eliminate neurotoxic α-syn, and promote the regeneration of dopaminergic neurons as therapeutic strategies.
Despite the significant potential of lipid-based NPs for PD demonstrated in preclinical studies, their clinical application remains limited. Challenges include the potential accumulation of NPs in the liver, the formation of a protein corona through binding with serum proteins, and the possibility of triggering immune responses. To address these challenges, the development of nanotherapeutics that are both selective and biocompatible is possible through the optimization of NP surfaces. However, ongoing research into the molecular neuropathology of PD, as well as the advancement of nanocomposite delivery systems and the refinement of surface properties, is needed to ensure precise targeting and biocompatibility. All abbreviations are listed in Table 3.
Abbreviations
| Abbreviations | Full Names | Abbreviations | Full Names |
|---|---|---|---|
| 6-OHDA | 6-hydroxydopamine | LDLR | Low-density Lipoprotein Receptor |
| AAV | Adeno-associated Virus | L-DOPA | L-3,4-dihydroxyphenylalanine |
| ABCB1 | ATP-binding Cassette sub-family B member 1 | LEPR | Leptin Receptor |
| ALP | Autophagy-Lysosome Pathway | LNP | Lipid Nanoparticle |
| AMT | Adsorptive-Mediated Transcytosis | LRRK2 | Leucine-Rich Repeat Kinase 2 |
| APP | Amyloid Precursor Protein | MANF | Mesencephalic Astrocyte-derived Neurotrophic Factor |
| BBB | Blood Brain Barrier | MAO-B | Monoamine Oxidase-B |
| BDNF | Brain-derived Neurotrophic Factor | MPP+ | 1-methyl-4-phenypyridinium |
| BSCB | Blood-Spinal Cord Barrier | MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| CDNF | Cerebral Dopamine Neurotrophic Factor | MSC | Mesenchymal Stem Cell |
| CMT | Endogenous-Carrier-Mediated Transport | mtDNA | Mitochondrial DNA |
| CNS | Central Nervous System | NGF | Nerve Growth Factor |
| COMT | Catechol-O-methyltransferase | NLC | nanostructured lipid carriers |
| CSF | Cerebrospinal Fluid | NO | Nitric Oxide |
| DA | Dopamine | NP | Nanoparticles |
| DAT | Dopamine Transporter | NTF | Neurotrophic Factor |
| DBS | Deep Brain Stimulation | PARK7 | Parkinson Disease Protein 7 |
| EC | Endothelial Cell | PD | Parkinson’s Disease |
| EGCG | Epigallocatechin Gallate | PEG | Polyethylene Glycol |
| ER | Endoplasmic Reticulum | P-gp | P-glycoprotein |
| ETC | Electron Transport Chain | PINK1 | Putative Kinase Protein 1 |
| FcRN | Neonatal Fc Receptor | PINK1 | PTEN-Induced Putative Kinase 1 |
| GDNF | Glial Cell Line-derived Neurotrophic Factor | PRKN | Parkin RBR E3 Ubiquitin Protein Ligase |
| GSH | Glutathione | RMT | Receptor-Mediated Transport |
| H2O2 | Hydrogen Peroxide | RNS | Reactive Nitrogen Species |
| hESCs | Human Embryonic Stem Cells | LDLR | Low-density Lipoprotein Receptor |
| IGFR | Insulin-like Growth Factor Receptor | L-DOPA | L-3,4-dihydroxyphenylalanine |
| iPSCs | Induced Pluripotent Stem Cells | LEPR | Leptin Receptor |
| IR | Insulin Receptor | LNP | Lipid Nanoparticle |
| LB | Lewy Body | LRRK2 | Leucine-Rich Repeat Kinase 2 |
| LDL | Low-Density Lipoprotein | MANF | Mesencephalic Astrocyte-derived Neurotrophic Factor |
-
Funding information: This review article was supported by Guangdong Celconta Biotechnology Co., Ltd, China.
-
Author contributions: H.C.: writing – original draft. D.L.: writing – original draft. W-W.X.: writing – original draft. L.M.: writing – original draft. H-T.X.: writing – original draft. K.N.: data curation, supervision, visualization, writing – review and editing.
-
Conflict of interest: Authors state no conflicts of interest.
-
Data availability statement: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
[1] Dorsey ER, Bloem BR. The Parkinson pandemic-A call to action. JAMA Neurol. 2018;75:9–10.10.1001/jamaneurol.2017.3299Suche in Google Scholar PubMed
[2] Abeliovich A, Gitler AD. Defects in trafficking bridge Parkinson’s disease pathology and genetics. Nature. 2016;539:207–16.10.1038/nature20414Suche in Google Scholar PubMed
[3] Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, et al. Parkinson disease. Nat Rev Dis Primers. 2017;3:17013.10.1038/nrdp.2017.13Suche in Google Scholar PubMed
[4] Benazzouz A, Mamad O, Abedi P, Bouali-Benazzouz R, Chetrit J. Involvement of dopamine loss in extrastriatal basal ganglia nuclei in the pathophysiology of Parkinson’s disease. Front Aging Neurosci. 2014;6:87.10.3389/fnagi.2014.00087Suche in Google Scholar PubMed PubMed Central
[5] Ebanks K, Lewis PA, Bandopadhyay R. Vesicular dysfunction and the pathogenesis of Parkinson’s disease: Clues From genetic studies. Front Neurosci. 2019;13:1381.10.3389/fnins.2019.01381Suche in Google Scholar PubMed PubMed Central
[6] Simon DK, Tanner CM, Brundin P. Parkinson disease epidemiology, pathology, genetics, and pathophysiology. Clin Geriatr Med. 2020;36:1–12.10.1016/j.cger.2019.08.002Suche in Google Scholar PubMed PubMed Central
[7] Georgiev D, Hamberg K, Hariz M, Forsgren L, Hariz GM. Gender differences in Parkinson’s disease: A clinical perspective. Acta Neurol Scand. 2017;136:570–84.10.1111/ane.12796Suche in Google Scholar PubMed
[8] Wooten GF, Currie LJ, Bovbjerg VE, Lee JK, Patrie J. Are men at greater risk for Parkinson’s disease than women? J Neurol Neurosurg Psychiatry. 2004;75:637–9.10.1136/jnnp.2003.020982Suche in Google Scholar PubMed PubMed Central
[9] Chen Z, Li G, Liu J. Autonomic dysfunction in Parkinson’s disease: Implications for pathophysiology, diagnosis, and treatment. Neurobiol Dis. 2020;134:104700.10.1016/j.nbd.2019.104700Suche in Google Scholar PubMed
[10] Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy. 2016;12:1–222.10.1080/15548627.2015.1100356Suche in Google Scholar PubMed PubMed Central
[11] Woitalla D, Buhmann C, Hilker-Roggendorf R, Höglinger G, Koschel J, Müller T, et al. Role of dopamine agonists in Parkinson’s disease therapy. J Neural Transm (Vienna). 2023;130:863–73.10.1007/s00702-023-02647-0Suche in Google Scholar PubMed
[12] Schapira AHV. Monoamine oxidase B inhibitors for the treatment of Parkinson’s disease: a review of symptomatic and potential disease-modifying effects. CNS Drugs. 2011;25:1061–71.10.2165/11596310-000000000-00000Suche in Google Scholar PubMed
[13] Müller T. Catechol-O-methyltransferase inhibitors in Parkinson’s disease. Drugs. 2015;75:157–74.10.1007/s40265-014-0343-0Suche in Google Scholar PubMed
[14] Strauss I, Kalia SK, Lozano AM. Where are we with surgical therapies for Parkinson’s disease? Parkinsonism Relat Disord. 2014;20:S187–91.10.1016/S1353-8020(13)70044-0Suche in Google Scholar PubMed
[15] Ramakrishna K, Nalla LV, Naresh D, Venkateswarlu K, Viswanadh MK, Nalluri BN, et al. WNT-β catenin signaling as a potential therapeutic target for neurodegenerative diseases: Current status and future perspective. Diseases. 2023;11:89.10.3390/diseases11030089Suche in Google Scholar PubMed PubMed Central
[16] Latt MD, Lewis S, Zekry O, Fung VSC. Factors to consider in the selection of dopamine agonists for older persons with Parkinson’s disease. Drugs Aging. 2019;36:189–202.10.1007/s40266-018-0629-0Suche in Google Scholar PubMed
[17] Karthivashan G, Ganesan P, Park SY, Lee HW, Choi DK. Lipid-based nanodelivery approaches for dopamine-replacement therapies in Parkinson’s disease: From preclinical to translational studies. Biomaterials. 2020;232:119704.10.1016/j.biomaterials.2019.119704Suche in Google Scholar PubMed
[18] Jankovic J, Tan EK. Parkinson’s disease: etiopathogenesis and treatment. J Neurol Neurosurg Psychiatry. 2020;91:795–808.10.1136/jnnp-2019-322338Suche in Google Scholar PubMed
[19] Cardoso FL, Brites D, Brito MA. Looking at the blood-brain barrier: molecular anatomy and possible investigation approaches. Brain Res Rev. 2010;64:328–63.10.1016/j.brainresrev.2010.05.003Suche in Google Scholar PubMed
[20] González-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog Biophys Mol Biol. 2003;81:1–44.10.1016/S0079-6107(02)00037-8Suche in Google Scholar
[21] Rubin LL, Staddon JM. The cell biology of the blood-brain barrier. Annu Rev Neurosci. 1999;22:11–28.10.1146/annurev.neuro.22.1.11Suche in Google Scholar PubMed
[22] Lee CS, Leong KW. Advances in microphysiological blood-brain barrier (BBB) models towards drug delivery. Curr Opin Biotechnol. 2020;66:78–87.10.1016/j.copbio.2020.06.009Suche in Google Scholar PubMed PubMed Central
[23] Pardridge WM. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32:1959–72.10.1038/jcbfm.2012.126Suche in Google Scholar PubMed PubMed Central
[24] Jones AR, Shusta EV. Blood-brain barrier transport of therapeutics via receptor-mediation. Pharm Res. 2007;24:1759–71.10.1007/s11095-007-9379-0Suche in Google Scholar PubMed PubMed Central
[25] Bickel U. Antibody delivery through the blood-brain barrier. Adv Drug Deliv Rev. 1995;15:53–72.10.1016/0169-409X(95)00005-RSuche in Google Scholar
[26] Sugawara I, Hamada H, Tsuruo T, Mori S. Specialized localization of P-glycoprotein recognized by MRK 16 monoclonal antibody in endothelial cells of the brain and the spinal cord. Jpn J Cancer Res. 1990;81:727–30.10.1111/j.1349-7006.1990.tb02636.xSuche in Google Scholar PubMed PubMed Central
[27] Lee G, Dallas S, Hong M, Bendayan R. Drug transporters in the central nervous system: brain barriers and brain parenchyma considerations. Pharmacol Rev. 2001;53:569–96.10.1146/annurev.pharmtox.41.1.569Suche in Google Scholar PubMed
[28] Kemper EM, van Zandbergen AE, Cleypool C, Mos HA, Boogerd W, Beijnen JH, et al. Increased penetration of paclitaxel into the brain by inhibition of P-Glycoprotein. Clin Cancer Res. 2003;9:2849–55.Suche in Google Scholar
[29] Bhagavan HN, Chopra RK. Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations. Mitochondrion. 2007;7(Suppl):S78–88.10.1016/j.mito.2007.03.003Suche in Google Scholar PubMed
[30] Kim W, Tripathi M, Kim C, Vardhineni S, Cha Y, Kandi SK, et al. An optimized Nurr1 agonist provides disease-modifying effects in Parkinson’s disease models. Nat Commun. 2023;14:4283.10.1038/s41467-023-39970-9Suche in Google Scholar PubMed PubMed Central
[31] Shalaby KE, El-Agnaf OMA. Gene-based therapeutics for Parkinson’s disease. Biomedicines. 2022;10:1790.10.3390/biomedicines10081790Suche in Google Scholar PubMed PubMed Central
[32] Camargo SM, Vuille-dit-Bille RN, Mariotta L, Ramadan T, Huggel K, Singer D, et al. The molecular mechanism of intestinal levodopa absorption and its possible implications for the treatment of Parkinson’s disease. J Pharmacol Exp Ther. 2014;351:114–23.10.1124/jpet.114.216317Suche in Google Scholar PubMed
[33] Patel SN, Patel DM. Self emulsifying drug delivery system. J Glob Pharma Technol. 2010;2:29–37.10.4103/2231-4040.79799Suche in Google Scholar PubMed PubMed Central
[34] Bawarski WE, Chidlowsky E, Bharali DJ, Mousa SA. Emerging nanopharmaceuticals. Nanomedicine. 2008;4:273–82.10.1016/j.nano.2008.06.002Suche in Google Scholar PubMed
[35] Wong HL, Wu XY, Bendayan R. Nanotechnological advances for the delivery of CNS therapeutics. Adv Drug Deliv Rev. 2012;64:686–700.10.1016/j.addr.2011.10.007Suche in Google Scholar PubMed
[36] Ramos-Cabrer P, Campos F. Liposomes and nanotechnology in drug development: focus on neurological targets. Int J Nanomed. 2013;8:951–60.10.2147/IJN.S30721Suche in Google Scholar PubMed PubMed Central
[37] Spuch C, Navarro C. Liposomes for targeted delivery of active agents against neurodegenerative diseases (Alzheimer’s disease and Parkinson’s disease). J Drug Deliv. 2011;2011:469679.10.1155/2011/469679Suche in Google Scholar PubMed PubMed Central
[38] Noble GT, Stefanick JF, Ashley JD, Kiziltepe T, Bilgicer B. Ligand-targeted liposome design: challenges and fundamental considerations. Trends Biotechnol. 2014;32:32–45.10.1016/j.tibtech.2013.09.007Suche in Google Scholar PubMed
[39] Cano A, Espina M, García ML. Recent advances on antitumor agents-loaded polymeric and lipid-based nanocarriers for the treatment of brain cancer. Curr Pharm Des. 2020;26:1316–30.10.2174/1381612826666200116142922Suche in Google Scholar PubMed
[40] Heyes J, Palmer L, Bremner K, MacLachlan I. Cationic lipid saturation influences intracellular delivery of encapsulated nucleic acids. J Control Rel. 2005;107:276–87.10.1016/j.jconrel.2005.06.014Suche in Google Scholar PubMed
[41] Witzigmann D, Kulkarni JA, Leung J, Chen S, Cullis PR, van der Meel R. Lipid nanoparticle technology for therapeutic gene regulation in the liver. Adv Drug Deliv Rev. 2020;159:344–63.10.1016/j.addr.2020.06.026Suche in Google Scholar PubMed PubMed Central
[42] Kulkarni JA, Witzigmann D, Leung J, Tam YYC, Cullis PR. On the role of helper lipids in lipid nanoparticle formulations of siRNA. Nanoscale. 2019;11:21733–39.10.1039/C9NR09347HSuche in Google Scholar
[43] Miao L, Zhang Y, Huang L. mRNA vaccine for cancer immunotherapy. Mol Cancer. 2021;20:41.10.1186/s12943-021-01335-5Suche in Google Scholar PubMed PubMed Central
[44] Ambegia E, Ansell S, Cullis P, Heyes J, Palmer L, MacLachlan I. Stabilized plasmid-lipid particles containing PEG-diacylglycerols exhibit extended circulation lifetimes and tumor selective gene expression. Biochim Biophys Acta. 2005;1669:155–63.10.1016/j.bbamem.2005.02.001Suche in Google Scholar PubMed
[45] Chen DB, Yang TZ, Lu WL, Zhang Q. In vitro and in vivo study of two types of long-circulating solid lipid nanoparticles containing paclitaxel. Chem Pharm Bull (Tokyo). 2001;49:1444–7.10.1248/cpb.49.1444Suche in Google Scholar PubMed
[46] Muller RH, Schwarz C, Muhlen AZ, Mehnert W. Incorporation of lipophilic drugs and drug release profiles of solid lipid nanoparticles (SLN); 1994.Suche in Google Scholar
[47] Mühlen AZ, Mehnert WJP. Drug release and release mechanism of prednisolone loaded solid lipid nanoparticles. Pharmazie. 1998;53:552–5.Suche in Google Scholar
[48] Müller RH, Mäder K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery - a review of the state of the art. Eur J Pharm Biopharm. 2000;50:161–77.10.1016/S0939-6411(00)00087-4Suche in Google Scholar
[49] Yadav N, Khatak S, Sara UVS. Solid lipid nanoparticles- A review. Int J Appl Pharm. 2013;5:8–18.Suche in Google Scholar
[50] Schwarz C, Mehnert W. Solid lipid nanoparticles (SLN) for controlled drug delivery. II. Drug incorporation and physicochemical characterization. J Microencapsul. 1999;16:205–13.10.1080/026520499289185Suche in Google Scholar PubMed
[51] Baek JS, Cho CW. Surface modification of solid lipid nanoparticles for oral delivery of curcumin: Improvement of bioavailability through enhanced cellular uptake, and lymphatic uptake. Eur J Pharm Biopharm. 2017;117:132–40.10.1016/j.ejpb.2017.04.013Suche in Google Scholar PubMed
[52] Lockman PR, Oyewumi MO, Koziara JM, Roder KE, Mumper RJ, Allen DD. Brain uptake of thiamine-coated nanoparticles. J Control Rel. 2003;93:271–82.10.1016/j.jconrel.2003.08.006Suche in Google Scholar PubMed
[53] Gohla SH, Dingler A. Scaling up feasibility of the production of solid lipid nanoparticles (SLN). Pharmazie. 2001;56:61–3.Suche in Google Scholar
[54] Naseri N, Valizadeh H, Zakeri-Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application. Adv Pharm Bull. 2015;5:305–13.10.15171/apb.2015.043Suche in Google Scholar PubMed PubMed Central
[55] Müller RH, Radtke M, Wissing SA. Nanostructured lipid matrices for improved microencapsulation of drugs. Int J Pharm. 2002;242:121–8.10.1016/S0378-5173(02)00180-1Suche in Google Scholar
[56] Das S, Ng WK, Tan RB. Are nanostructured lipid carriers (NLCs) better than solid lipid nanoparticles (SLNs): development, characterizations and comparative evaluations of clotrimazole-loaded SLNs and NLCs? Eur J Pharm Sci. 2012;47:139–51.10.1016/j.ejps.2012.05.010Suche in Google Scholar PubMed
[57] Ghasemiyeh P, Mohammadi-Samani S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages. Res Pharm Sci. 2018;13:288–303.10.4103/1735-5362.235156Suche in Google Scholar PubMed PubMed Central
[58] Beloqui A, Solinís M, Rodríguez-Gascón A, Almeida AJ, Préat V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomedicine. 2016;12:143–61.10.1016/j.nano.2015.09.004Suche in Google Scholar PubMed
[59] Scioli Montoto S, Muraca G, Ruiz ME. Solid lipid nanoparticles for drug delivery: Pharmacological and biopharmaceutical aspects. Front Mol Biosci. 2020;7:587997.10.3389/fmolb.2020.587997Suche in Google Scholar PubMed PubMed Central
[60] Dhiman N, Awasthi R, Sharma B, Kharkwal H, Kulkarni GT. Lipid nanoparticles as carriers for bioactive delivery. Front Chem. 2021;9:580118.10.3389/fchem.2021.580118Suche in Google Scholar PubMed PubMed Central
[61] Haider M, Abdin SM, Kamal L, Orive G. Nanostructured lipid carriers for delivery of chemotherapeutics: A review. Pharmaceutics. 2020;12:288.10.3390/pharmaceutics12030288Suche in Google Scholar PubMed PubMed Central
[62] During MJ, Freese A, Deutch AY, Kibat PG, Sabel BA, Langer R, et al. Biochemical and behavioral recovery in a rodent model of Parkinson’s disease following stereotactic implantation of dopamine-containing liposomes. Exp Neurol. 1992;115:193–9.10.1016/0014-4886(92)90053-SSuche in Google Scholar PubMed
[63] Qu M, Lin Q, He S, Wang L, Fu Y, Zhang Z, et al. A brain targeting functionalized liposomes of the dopamine derivative N-3,4-bis(pivaloyloxy)-dopamine for treatment of Parkinson’s disease. J Control Rel. 2018;277:173–82.10.1016/j.jconrel.2018.03.019Suche in Google Scholar PubMed
[64] Xiang Y, Wu Q, Liang L, Wang X, Wang J, Zhang X, et al. Chlorotoxin-modified stealth liposomes encapsulating levodopa for the targeting delivery against Parkinson’s disease in the MPTP-induced mice model. J Drug Target. 2012;20:67–75.10.3109/1061186X.2011.595490Suche in Google Scholar PubMed
[65] Cao X, Hou D, Wang L, Li S, Sun S, Ping Q, et al. Effects and molecular mechanism of chitosan-coated levodopa nanoliposomes on behavior of dyskinesia rats. Biol Res. 2016;49:32.10.1186/s40659-016-0093-4Suche in Google Scholar PubMed PubMed Central
[66] Kahana M, Weizman A, Gabay M, Loboda Y, Segal-Gavish H, Gavish A, et al. Liposome-based targeting of dopamine to the brain: a novel approach for the treatment of Parkinson’s disease. Mol Psychiatry. 2021;26:2626–32.10.1038/s41380-020-0742-4Suche in Google Scholar PubMed
[67] Dudhipala N, Gorre T. Neuroprotective effect of ropinirole lipid nanoparticles enriched hydrogel for Parkinson’s disease: In vitro, ex vivo, pharmacokinetic and pharmacodynamic evaluation. Pharmaceutics. 2020;12:448.10.3390/pharmaceutics12050448Suche in Google Scholar PubMed PubMed Central
[68] Esposito E, Fantin M, Marti M, Drechsler M, Paccamiccio L, Mariani P, et al. Solid lipid nanoparticles as delivery systems for bromocriptine. Pharm Res. 2008;25:1521–30.10.1007/s11095-007-9514-ySuche in Google Scholar PubMed
[69] Esposito E, Mariani P, Ravani L, Contado C, Volta M, Bido S, et al. Nanoparticulate lipid dispersions for bromocriptine delivery: characterization and in vivo study. Eur J Pharm Biopharm. 2012;80:306–14.10.1016/j.ejpb.2011.10.015Suche in Google Scholar PubMed
[70] Tsai MJ, Huang YB, Wu PC, Fu YS, Kao YR, Fang JY, et al. Oral apomorphine delivery from solid lipid nanoparticles with different monostearate emulsifiers: pharmacokinetic and behavioral evaluations. J Pharm Sci. 2011;100:547–57.10.1002/jps.22285Suche in Google Scholar PubMed
[71] Stefanis L. α-Synuclein in Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2:a009399.10.1101/cshperspect.a009399Suche in Google Scholar PubMed PubMed Central
[72] Fields CR, Bengoa-Vergniory N, Wade-Martins R. Targeting alpha-synuclein as a therapy for Parkinson’s disease. Front Mol Neurosci. 2019;12:299.10.3389/fnmol.2019.00299Suche in Google Scholar PubMed PubMed Central
[73] Bentea E, Van der Perren A, Van Liefferinge J, El Arfani A, Albertini G, Demuyser T, et al. Nigral proteasome inhibition in mice leads to motor and non-motor deficits and increased expression of Ser129 phosphorylated α-synuclein. Front Behav Neurosci. 2015;9:68.10.3389/fnbeh.2015.00068Suche in Google Scholar PubMed PubMed Central
[74] Jinn S, Drolet RE, Cramer PE, Wong AH, Toolan DM, Gretzula CA, et al. TMEM175 deficiency impairs lysosomal and mitochondrial function and increases α-synuclein aggregation. Proc Natl Acad Sci U S A. 2017;114:2389–94.10.1073/pnas.1616332114Suche in Google Scholar PubMed PubMed Central
[75] Padilla-Godínez FJ, Ramos-Acevedo R, Martínez-Becerril HA, Bernal-Conde LD, Garrido-Figueroa JF, Hiriart M, et al. Protein misfolding and aggregation: The relatedness between Parkinson’s disease and hepatic endoplasmic reticulum storage disorders. Int J Mol Sci. 2021;22:12467.10.3390/ijms222212467Suche in Google Scholar PubMed PubMed Central
[76] Heman-Ackah SM, Manzano R, Hoozemans JJM, Scheper W, Flynn R, Haerty W, et al. Alpha-synuclein induces the unfolded protein response in Parkinson’s disease SNCA triplication iPSC-derived neurons. Hum Mol Genet. 2017;26:4441–50.10.1093/hmg/ddx331Suche in Google Scholar PubMed PubMed Central
[77] Li Y, Chen Z, Lu Z, Yang Q, Liu L, Jiang Z, et al. “Cell-addictive” dual-target traceable nanodrug for Parkinson’s disease treatment via flotillins pathway. Theranostics. 2018;8:5469–81.10.7150/thno.28295Suche in Google Scholar PubMed PubMed Central
[78] Kuo YC, Wang IH, Rajesh R. Use of leptin-conjugated phosphatidic acid liposomes with resveratrol and epigallocatechin gallate to protect dopaminergic neurons against apoptosis for Parkinson’s disease therapy. Acta Biomater. 2021;119:360–74.10.1016/j.actbio.2020.11.015Suche in Google Scholar PubMed
[79] Zhang N, Yan F, Liang X, Wu M, Shen Y, Chen M, et al. Localized delivery of curcumin into brain with polysorbate 80-modified cerasomes by ultrasound-targeted microbubble destruction for improved Parkinson’s disease therapy. Theranostics. 2018;8:2264–77.10.7150/thno.23734Suche in Google Scholar PubMed PubMed Central
[80] Sela M, Poley M, Mora-Raimundo P, Kagan S, Avital A, Kaduri M, et al. Brain-targeted liposomes loaded with monoclonal antibodies reduce alpha-synuclein aggregation and improve behavioral symptoms in Parkinson’s disease. Adv Mater. 2023;35:e2304654.10.1002/adma.202370367Suche in Google Scholar
[81] Aliakbari F, Mohammad-Beigi H, Rezaei-Ghaleh N, Becker S, Dehghani Esmatabad F, Eslampanah Seyedi HA, et al. The potential of zwitterionic nanoliposomes against neurotoxic alpha-synuclein aggregates in Parkinson’s disease. Nanoscale. 2018;10:9174–85.10.1039/C8NR00632FSuche in Google Scholar PubMed
[82] Dias V, Junn E, Mouradian MM. The role of oxidative stress in Parkinson’s disease. J Parkinsons Dis. 2013;3:461–91.10.3233/JPD-130230Suche in Google Scholar PubMed PubMed Central
[83] Liu C, Fang J, Liu W. Superoxide dismutase coding of gene polymorphisms associated with susceptibility to Parkinson’s disease. J Integr Neurosci. 2019;18:299–303.10.31083/j.jin.2019.03.127Suche in Google Scholar PubMed
[84] Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson’s disease. Annu Rev Neurosci. 1999;22:123–44.10.1146/annurev.neuro.22.1.123Suche in Google Scholar PubMed
[85] Madreiter-Sokolowski CT, Thomas C, Ristow M. Interrelation between ROS and Ca(2+) in aging and age-related diseases. Redox Biol. 2020;36:101678.10.1016/j.redox.2020.101678Suche in Google Scholar PubMed PubMed Central
[86] Wang ZL, Yuan L, Li W, Li JY. Ferroptosis in Parkinson’s disease: glia-neuron crosstalk. Trends Mol Med. 2022;28:258–69.10.1016/j.molmed.2022.02.003Suche in Google Scholar PubMed
[87] Barja G. Updating the mitochondrial free radical theory of aging: An integrated view, key aspects, and confounding concepts. Antioxid Redox Signal. 2013;19:1420–45.10.1089/ars.2012.5148Suche in Google Scholar PubMed PubMed Central
[88] Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–80.10.1126/science.6823561Suche in Google Scholar PubMed
[89] Park JS, Davis RL, Sue CM. Mitochondrial dysfunction in Parkinson’s disease: New mechanistic insights and therapeutic perspectives. Curr Neurol Neurosci Rep. 2018;18:21.10.1007/s11910-018-0829-3Suche in Google Scholar PubMed PubMed Central
[90] Mogi M, Harada M, Narabayashi H, Inagaki H, Minami M, Nagatsu T. Interleukin (IL)-1 beta, IL-2, IL-4, IL-6 and transforming growth factor-alpha levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson’s disease. Neurosci Lett. 1996;211:13–6.10.1016/0304-3940(96)12706-3Suche in Google Scholar PubMed
[91] Wang Q, Liu Y, Zhou J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl Neurodegener. 2015;4:19.10.1186/s40035-015-0042-0Suche in Google Scholar PubMed PubMed Central
[92] Rai SN, Yadav SK, Singh D, Singh SP. Ursolic acid attenuates oxidative stress in nigrostriatal tissue and improves neurobehavioral activity in MPTP-induced Parkinsonian mouse model. J Chem Neuroanat. 2016;71:41–9.10.1016/j.jchemneu.2015.12.002Suche in Google Scholar PubMed
[93] Yadav SK, Rai SN, Singh SP. Mucuna pruriens reduces inducible nitric oxide synthase expression in Parkinsonian mice model. J Chem Neuroanat. 2017;80:1–10.10.1016/j.jchemneu.2016.11.009Suche in Google Scholar PubMed
[94] Prakash J, Chouhan S, Yadav SK, Westfall S, Rai SN, Singh SP. Withania somnifera alleviates parkinsonian phenotypes by inhibiting apoptotic pathways in dopaminergic neurons. Neurochem Res. 2014;39:2527–36.10.1007/s11064-014-1443-7Suche in Google Scholar PubMed
[95] Wang Y, Xu H, Fu Q, Ma R, Xiang J. Protective effect of resveratrol derived from Polygonum cuspidatum and its liposomal form on nigral cells in parkinsonian rats. J Neurol Sci. 2011;304:29–34.10.1016/j.jns.2011.02.025Suche in Google Scholar PubMed
[96] Pangeni R, Sharma S, Mustafa G, Ali J, Baboota S. Vitamin E loaded resveratrol nanoemulsion for brain targeting for the treatment of Parkinson’s disease by reducing oxidative stress. Nanotechnology. 2014;25:485102.10.1088/0957-4484/25/48/485102Suche in Google Scholar PubMed
[97] Yan Y, Chen Y, Liu Z, Cai F, Niu W, Song L, et al. Brain delivery of curcumin through low-intensity ultrasound-induced blood-brain barrier opening via lipid-PLGA nanobubbles. Int J Nanomed. 2021;16:7433–47.10.2147/IJN.S327737Suche in Google Scholar PubMed PubMed Central
[98] Gupta BK, Kumar S, Kaur H, Ali J, Baboota S. Attenuation of oxidative damage by coenzyme Q(10) loaded nanoemulsion through oral route for the management of Parkinson’s disease. Rejuvenation Res. 2018;21:232–48.10.1089/rej.2017.1959Suche in Google Scholar PubMed
[99] Gaba B, Khan T, Haider MF, Alam T, Baboota S, Parvez S, et al. Vitamin E loaded naringenin nanoemulsion via intranasal delivery for the management of oxidative stress in a 6-OHDA Parkinson’s disease model. Biomed Res Int. 2019;2019:2382563.10.1155/2019/2382563Suche in Google Scholar PubMed PubMed Central
[100] Sola P, Garikapati KK, Krishnamurthy PT, Kumari M. Polysorbate 80 surface modified SLNs of formoterol suppress SNCA gene and mitochondrial oxidative stress in mice model of Parkinson’s disease. Sci Rep. 2023;13:19942.10.1038/s41598-023-46511-3Suche in Google Scholar PubMed PubMed Central
[101] Yuan J, Huang G, Xiao Z, Lin L, Han T. Overexpression of β-NGF promotes differentiation of bone marrow mesenchymal stem cells into neurons through regulation of AKT and MAPK pathway. Mol Cell Biochem. 2013;383:201–11.10.1007/s11010-013-1768-6Suche in Google Scholar PubMed
[102] Elliott Donaghue I, Tator CH, Shoichet MS. Sustained delivery of bioactive neurotrophin-3 to the injured spinal cord. Biomater Sci. 2015;3:65–72.10.1039/C4BM00311JSuche in Google Scholar
[103] Zhang ZJ, Li YJ, Liu XG, Huang FX, Liu TJ, Jiang DM, et al. Human umbilical cord blood stem cells and brain-derived neurotrophic factor for optic nerve injury: a biomechanical evaluation. Neural Regen Res. 2015;10:1134–8.10.4103/1673-5374.160110Suche in Google Scholar PubMed PubMed Central
[104] Goes ATR, Jesse CR, Antunes MS, Lobo Ladd FV, Lobo Ladd AAB, Luchese C, et al. Protective role of chrysin on 6-hydroxydopamine-induced neurodegeneration a mouse model of Parkinson’s disease: Involvement of neuroinflammation and neurotrophins. Chem Biol Interact. 2018;279:111–20.10.1016/j.cbi.2017.10.019Suche in Google Scholar PubMed
[105] Bradshaw RA, Mobley W, Rush RA. Nerve growth factor and related substances: A brief history and an introduction to the international NGF meeting series. Int J Mol Sci. 2017;18:1143.10.3390/ijms18061143Suche in Google Scholar PubMed PubMed Central
[106] Bothwell M. NGF, BDNF, NT3, and NT4. Handb Exp Pharmacol. 2014;220:3–15.10.1007/978-3-642-45106-5_1Suche in Google Scholar PubMed
[107] Xia CF, Boado RJ, Zhang Y, Chu C, Pardridge WM. Intravenous glial-derived neurotrophic factor gene therapy of experimental Parkinson’s disease with Trojan horse liposomes and a tyrosine hydroxylase promoter. J Gene Med. 2008;10:306–15.10.1002/jgm.1152Suche in Google Scholar PubMed
[108] Bender TS, Migliore MM, Campbell RB, John Gatley S, Waszczak BL. Intranasal administration of glial-derived neurotrophic factor (GDNF) rapidly and significantly increases whole-brain GDNF level in rats. Neuroscience. 2015;303:569–76.10.1016/j.neuroscience.2015.07.016Suche in Google Scholar PubMed
[109] Gartziandia O, Herrán E, Ruiz-Ortega JA, Miguelez C, Igartua M, Lafuente JV, et al. Intranasal administration of chitosan-coated nanostructured lipid carriers loaded with GDNF improves behavioral and histological recovery in a partial lesion model of Parkinson’s disease. J Biomed Nanotechnol. 2016;12:2220–30.10.1166/jbn.2016.2313Suche in Google Scholar PubMed
[110] Zhao YZ, Li X, Lu CT, Lin M, Chen LJ, Xiang Q, et al. Gelatin nanostructured lipid carriers-mediated intranasal delivery of basic fibroblast growth factor enhances functional recovery in hemiparkinsonian rats. Nanomedicine. 2014;10:755–64.10.1016/j.nano.2013.10.009Suche in Google Scholar PubMed
[111] Niu J, Xie J, Guo K, Zhang X, Xia F, Zhao X, et al. Efficient treatment of Parkinson’s disease using ultrasonography-guided rhFGF20 proteoliposomes. Drug Deliv. 2018;25:1560–9.10.1080/10717544.2018.1482972Suche in Google Scholar PubMed PubMed Central
[112] Setten RL, Rossi JJ, Han SP. The current state and future directions of RNAi-based therapeutics. Nat Rev Drug Discov. 2019;18:421–46.10.1038/s41573-019-0017-4Suche in Google Scholar PubMed
[113] Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1:727–30.10.1038/nrd892Suche in Google Scholar PubMed
[114] Schlich M, Longhena F, Faustini G, O’Driscoll CM, Sinico C, Fadda AM, et al. Anionic liposomes for small interfering ribonucleic acid (siRNA) delivery to primary neuronal cells: Evaluation of alpha-synuclein knockdown efficacy. Nano Res. 2017;10:3496–3508.10.1007/s12274-017-1561-zSuche in Google Scholar
[115] Rungta RL, Choi HB, Lin PJ, Ko RW, Ashby D, Nair J, et al. Lipid nanoparticle delivery of siRNA to silence neuronal gene expression in the brain. Mol Ther Nucleic Acids. 2013;2:e136.10.1038/mtna.2013.65Suche in Google Scholar PubMed PubMed Central
[116] Pardridge WM. Tyrosine hydroxylase replacement in experimental Parkinson’s disease with transvascular gene therapy. NeuroRx. 2005;2:129–38.10.1602/neurorx.2.1.129Suche in Google Scholar PubMed PubMed Central
[117] Olson KE, Namminga KL, Lu Y, Thurston MJ, Schwab AD, de Picciotto S, et al. Granulocyte-macrophage colony-stimulating factor mRNA and Neuroprotective Immunity in Parkinson’s disease. Biomaterials. 2021;272:120786.10.1016/j.biomaterials.2021.120786Suche in Google Scholar PubMed PubMed Central
[118] Calero M, Moleiro LH, Sayd A, Dorca Y, Miquel-Rio L, Paz V, et al. Lipid nanoparticles for antisense oligonucleotide gene interference into brain border-associated macrophages. Front Mol Biosci. 2022;9:887678.10.3389/fmolb.2022.887678Suche in Google Scholar PubMed PubMed Central
[119] Zhang Y, Pardridge WM. Near complete rescue of experimental Parkinson’s disease with intravenous, non-viral GDNF gene therapy. Pharm Res. 2009;26:1059–63.10.1007/s11095-008-9815-9Suche in Google Scholar PubMed
[120] Lin CY, Hsieh HY, Chen CM, Wu SR, Tsai CH, Huang CY, et al. Non-invasive, neuron-specific gene therapy by focused ultrasound-induced blood-brain barrier opening in Parkinson’s disease mouse model. J Control Rel. 2016;235:72–81.10.1016/j.jconrel.2016.05.052Suche in Google Scholar PubMed
[121] Yue P, Gao L, Wang X, Ding X, Teng J. Ultrasound-triggered effects of the microbubbles coupled to GDNF- and Nurr1-loaded PEGylated liposomes in a rat model of Parkinson’s disease. J Cell Biochem. 2018;119:4581–91.10.1002/jcb.26608Suche in Google Scholar PubMed
[122] Hui H, Tang Y, Hu M, Zhao X. Stem cells: General features and characteristics. Stem Cell ClRes. 201110.5772/23755Suche in Google Scholar
[123] Mitalipov S, Wolf D. Totipotency, pluripotency and nuclear reprogramming. Adv Biochem Eng Biotechnol. 2009;114:185–99.10.1007/10_2008_45Suche in Google Scholar PubMed PubMed Central
[124] Forouzandeh M, Bigdeli MR, Mostafavi H, Nadri S, Eskandari M. Therapeutic potentials of human microfluidic encapsulated conjunctival mesenchymal stem cells on the rat model of Parkinson’s disease. Exp Mol Pathol. 2021;123:104703.10.1016/j.yexmp.2021.104703Suche in Google Scholar PubMed
[125] Salama M, Sobh M, Emam M, Abdalla A, Sabry D, El-Gamal M, et al. Effect of intranasal stem cell administration on the nigrostriatal system in a mouse model of Parkinson’s disease. Exp Ther Med. 2017;13:976–82.10.3892/etm.2017.4073Suche in Google Scholar PubMed PubMed Central
[126] Schwerk A, Altschüler J, Roch M, Gossen M, Winter C, Berg J, et al. Human adipose-derived mesenchymal stromal cells increase endogenous neurogenesis in the rat subventricular zone acutely after 6-hydroxydopamine lesioning. Cytotherapy. 2015;17:199–214.10.1016/j.jcyt.2014.09.005Suche in Google Scholar PubMed
[127] Boika A, Aleinikava N, Chyzhyk V, Zafranskaya M, Nizheharodava D, Ponomarev V. Mesenchymal stem cells in Parkinson’s disease: Motor and nonmotor symptoms in the early posttransplant period. Surg Neurol Int. 2020;11:380.10.25259/SNI_233_2020Suche in Google Scholar PubMed PubMed Central
[128] Chabra S, Ranjan M, Bhandari R, Kaur T, Aggrawal M, Puri V, et al. Solid lipid nanoparticles regulate functional assortment of mouse mesenchymal stem cells. J Stem Cell Regen Med. 2011;7:75–9.10.46582/jsrm.0702012Suche in Google Scholar PubMed PubMed Central
[129] Kuo YC, Rajesh R. Nerve growth factor-loaded heparinized cationic solid lipid nanoparticles for regulating membrane charge of induced pluripotent stem cells during differentiation. Mater Sci Eng C Mater Biol Appl. 2017;77:680–9.10.1016/j.msec.2017.03.303Suche in Google Scholar PubMed
[130] Kuo YC, Shih-Huang CY, Rajesh R. Enhanced integrin affinity and neural differentiation of induced pluripotent stem cells using Ln5-P4-grafted amphiphilic solid lipid nanoparticles. Mater Sci Eng C Mater Biol Appl. 2021;118:111339.10.1016/j.msec.2020.111339Suche in Google Scholar PubMed
[131] Takeda YS, Wang M, Deng P, Xu Q. Synthetic bioreducible lipid-based nanoparticles for miRNA delivery to mesenchymal stem cells to induce neuronal differentiation. Bioeng Transl Med. 2016;1:160–7.10.1002/btm2.10021Suche in Google Scholar PubMed PubMed Central
[132] Zhao X, Glass Z, Chen J, Yang L, Kaplan DL, Xu Q. mRNA delivery using bioreducible lipidoid nanoparticles facilitates neural differentiation of human mesenchymal stem cells. Adv Healthc Mater. 2021;10:e2000938.10.1002/adhm.202000938Suche in Google Scholar PubMed
[133] Rai SN, Singh P. Advancement in the modelling and therapeutics of Parkinson’s disease. J Chem Neuroanat. 2020;104:101752.10.1016/j.jchemneu.2020.101752Suche in Google Scholar PubMed
[134] Stefano DA, Sozio P, Cocco A, Lannitelli A, Santucci E, Costa M, et al. L-dopa- and dopamine-(R)-alpha-lipoic acid conjugates as multifunctional codrugs with antioxidant properties. J Med Chem. 2006;49:1486–93.10.1021/jm051145pSuche in Google Scholar PubMed
[135] Carafa M, Marianecci C, Di Marzio L, De Caro V, Giandalia G, Giannola LI, et al. Potential dopamine prodrug-loaded liposomes: preparation, characterization, and in vitro stability studies. J Liposome Res. 2010;20:250–7.10.3109/08982100903384129Suche in Google Scholar PubMed
[136] Kang YS, Jung HJ, Oh JS, Song DY. Use of PEGylated immunoliposomes to deliver dopamine across the blood–brain barrier in a rat model of Parkinson’s disease. CNS Neurosci Ther. 2016;22:817–23.10.1111/cns.12580Suche in Google Scholar PubMed PubMed Central
[137] Gurturk Z, Tezcaner A, Dalgic AD, Korkmaz S, Keskin D. Maltodextrin modified liposomes for drug delivery through the blood-brain barrier. Medchemcomm. 2017;8:1337–45.10.1039/C7MD00045FSuche in Google Scholar
[138] Ji B, Wang M, Gao D, Xing S, Li L, Liu L, et al. Combining nanoscale magnetic nimodipine liposomes with magnetic resonance image for Parkinson’s disease targeting therapy. Nanomed (Lond). 2017;12:237–53.10.2217/nnm-2016-0267Suche in Google Scholar PubMed
[139] Zhigaltsev IV, Kaplun AP, Kucheryanu VG, Kryzhanivsky GN, Kolomeichuk SN, Shvets VI, et al. Liposomes containing dopamine entrapped in response to transmembrane ammonium sulfate gradient as carrier system for dopamine delivery into the brain of parkinsonian mice. J Liposome Res. 2001;11:55–71.10.1081/LPR-100103170Suche in Google Scholar PubMed
[140] Di Stefano A, Carafa M, Sozio P, Pinnen F, Braghiroli D, Orlando G, et al. Evaluation of rat striatal L-dopa and DA concentration after intraperitoneal administration of L-dopa prodrugs in liposomal formulations. J Control Rel. 2004;99:293–300.10.1016/j.jconrel.2004.07.010Suche in Google Scholar PubMed
[141] Zhan SM, Hou DZ, Ping QN, Xu Y. Preparation and entrapment efficiency determination of solid lipid nanoparticles loaded levodopa. Chin J Hosp Pharm. 2010;14:1171–5.Suche in Google Scholar
[142] Pardeshi CV, Rajput PV, Belgamwar VS, Tekade AR, Surana SJ. Novel surface modified solid lipid nanoparticles as intranasal carriers for ropinirole hydrochloride: application of factorial design approach. Drug Deliv. 2013;20:47–56.10.3109/10717544.2012.752421Suche in Google Scholar PubMed
[143] Uppuluri CT, Ravi PR, Dalvi AV. Design, optimization and pharmacokinetic evaluation of Piribedil loaded solid lipid nanoparticles dispersed in nasal in situ gelling system for effective management of Parkinson’s disease. Int J Pharm. 2021;606:120881.10.1016/j.ijpharm.2021.120881Suche in Google Scholar PubMed
[144] Demirel M, Yazan Y, Müller RH, Kiliç F, Bozan B. Formulation and in vitro-in vivo evaluation of piribedil solid lipid micro- and nanoparticles. J Microencapsul. 2001;18:359–71.10.1080/02652040010018119Suche in Google Scholar PubMed
[145] Cometa S, Bonifacio MA, Trapani G, Di Gioia S, Dazzi L, De Giglio E, et al. In vitro investigations on dopamine loaded Solid Lipid Nanoparticles. J Pharm Biomed Anal. 2020;185:113257.10.1016/j.jpba.2020.113257Suche in Google Scholar PubMed
[146] Trapani A, De Giglio E, Cometa S, Bonifacio MA, Dazzi L, Di Gioia S, et al. Dopamine-loaded lipid based nanocarriers for intranasal administration of the neurotransmitter: A comparative study. Eur J Pharm Biopharm. 2021;167:189–200.10.1016/j.ejpb.2021.07.015Suche in Google Scholar PubMed
[147] Liu KS, Wen CJ, Yen TC, Sung KC, Ku MC, Wang JJ, et al. Combined strategies of apomorphine diester prodrugs and nanostructured lipid carriers for efficient brain targeting. Nanotechnology. 2012;23:095103.10.1088/0957-4484/23/9/095103Suche in Google Scholar PubMed
[148] Cortesi R, Esposito E, Drechsler M, Pavoni G, Cacciatore I, Sguizzato M, et al. L-dopa co-drugs in nanostructured lipid carriers: A comparative study. Mater Sci Eng C Mater Biol Appl. 2017;72:168–76.10.1016/j.msec.2016.11.060Suche in Google Scholar PubMed
[149] Itin C, Komargodski R, Domb AJ, Hoffman A. Controlled delivery of apomorphine through buccal mucosa, towards a noninvasive administration method in Parkinson’s disease: A preclinical mechanistic study. J Pharm Sci. 2020;109:2729–34.10.1016/j.xphs.2020.05.017Suche in Google Scholar PubMed
[150] Ravani L, Sarpietro MG, Esposito E, Di Stefano A, Sozio P, Calcagno M, et al. Lipid nanocarriers containing a levodopa prodrug with potential antiparkinsonian activity. Mater Sci Eng C Mater Biol Appl. 2015;48:294–300.10.1016/j.msec.2014.12.014Suche in Google Scholar PubMed
[151] Ortega E, Blanco S, Ruiz A, Peinado MÁ, Peralta S, Morales ME. Lipid nanoparticles for the transport of drugs like dopamine through the blood-brain barrier. J Nanopart Res. 2021;23:1–11.10.1007/s11051-021-05218-0Suche in Google Scholar
[152] Ngwuluka NC, Pillay V, Choonara YE, Modi G, Naidoo D, Toit L, et al. Fabrication, modeling and characterization of multi-crosslinked methacrylate copolymeric nanoparticles for oral drug delivery. Int J Mol Sci. 2011;12:6194–225.10.3390/ijms12096194Suche in Google Scholar PubMed PubMed Central
[153] Pardeshi CV, Belgamwar VS, Tekade AR, Surana SJ. Novel surface modified polymer-lipid hybrid nanoparticles as intranasal carriers for ropinirole hydrochloride: in vitro, ex vivo and in vivo pharmacodynamic evaluation. J Mater Sci Mater Med. 2013;24:2101–15.10.1007/s10856-013-4965-7Suche in Google Scholar PubMed
[154] Sp A. Dual drug-loaded lipid nanoparticles for the treatment of Parkinson’s disease. 2018Suche in Google Scholar
[155] Otzen DE, Morshedi D, Mohammad-Beigi H, Aliakbari F. A triple role for a Bilayer: Using nanoliposomes to cross and protect cellular membranes. J Membr Biol. 2021;254:29–39.10.1007/s00232-020-00159-6Suche in Google Scholar PubMed
[156] Marino A, Battaglini M, Desii A, Lavarello C, Genchi G, Petretto A, et al. Liposomes loaded with polyphenol-rich grape pomace extracts protect from neurodegeneration in a rotenone-based in vitro model of Parkinson’s disease. Biomater Sci. 2021;9:8171–88.10.1039/D1BM01202ASuche in Google Scholar PubMed
[157] Wang M, Zhang L, Liu X, Zhu Y, Liu R, Fang L, et al. Magnetic resveratrol liposomes as a new theranostic platform for magnetic resonance imaging guided Parkinson’s Disease targeting therapy. ACS Sustain Chem Eng. 2018;6:17124–33.10.1021/acssuschemeng.8b04507Suche in Google Scholar
[158] Ramalingam P, Ko YT. Enhanced oral delivery of curcumin from N-trimethyl chitosan surface-modified solid lipid nanoparticles: pharmacokinetic and brain distribution evaluations. Pharm Res. 2015;32:389–402.10.1007/s11095-014-1469-1Suche in Google Scholar PubMed
[159] Cunha A, Gaubert A, Verget J, Thiolat ML, Barthélémy P, Latxague L, et al. Trehalose-based nucleolipids as nanocarriers for autophagy modulation: An in vitro study. Pharmaceutics. 2022;14:857.10.3390/pharmaceutics14040857Suche in Google Scholar PubMed PubMed Central
[160] Dhawan S, Kapil R, Singh B. Formulation development and systematic optimization of solid lipid nanoparticles of quercetin for improved brain delivery. J Pharm Pharmacol. 2011;63:342–51.10.1111/j.2042-7158.2010.01225.xSuche in Google Scholar PubMed
[161] Prabhu A, Jose J, Kumar L, Salwa S, Kumar MV, Nabavi SM. Transdermal delivery of curcumin-loaded solid lipid nanoparticles as microneedle patch: an in vitro and in vivo study. AAPS PharmSciTech. 2022;23:49.10.1208/s12249-021-02186-5Suche in Google Scholar PubMed
[162] de Oliveira Junior ER, Truzzi E, Ferraro L, Fogagnolo M, Pavan B, Beggiato S, et al. Nasal administration of nanoencapsulated geraniol/ursodeoxycholic acid conjugate: Towards a new approach for the management of Parkinson’s disease. J Control Rel. 2020;321:540–52.10.1016/j.jconrel.2020.02.033Suche in Google Scholar PubMed
[163] Joy D, Jose O, Bibi S, Bandiwadekar A, Gopan G, Lima CMG, et al. Development of microneedle patch loaded with Bacopa monnieri solid lipid nanoparticles for the effective management of Parkinson’s disease. Bioinorg Chem Appl. 2022;2022:9150205.10.1155/2022/9150205Suche in Google Scholar PubMed PubMed Central
[164] Margari A, Monteduro AG, Rizzato S, Capobianco L, Crestini A, Rivabene R, et al. The encapsulation of citicoline within solid lipid nanoparticles enhances its capability to counteract the 6-hydroxydopamine-induced cytotoxicity in human neuroblastoma SH-SY5Y cells. Pharmaceutics. 2022;14:1827.10.3390/pharmaceutics14091827Suche in Google Scholar PubMed PubMed Central
[165] Wu S, Li G, Li X, Lin C, Yu D, Luan S, et al. Transport of glial cell line-derived neurotrophic factor into liposomes across the blood-brain barrier: in vitro and in vivo studies. Int J Mol Sci. 2014;15:3612–23.10.3390/ijms15033612Suche in Google Scholar PubMed PubMed Central
[166] Hernando S, Herran E, Figueiro-Silva J, Pedraz JJ, Manoli Igartua M, Carro M, et al. Intranasal administration of TAT-conjugated lipid nanocarriers loading GDNF for Parkinson’s disease. Mol Neurobiol. 2018;55:145–55.10.1007/s12035-017-0728-7Suche in Google Scholar PubMed
[167] Long L, Cai X, Guo R, Wang P, Wu L, Yin T, et al. Treatment of Parkinson’s disease in rats by Nrf2 transfection using MRI-guided focused ultrasound delivery of nanomicrobubbles. Biochem Biophys Res Commun. 2017;482:75–80.10.1016/j.bbrc.2016.10.141Suche in Google Scholar PubMed
[168] Zhang Y, Schlachetzki F, Zhang YF, Boado RJ, Pardridge WM. Intravenous nonviral gene therapy causes normalization of striatal tyrosine hydroxylase and reversal of motor impairment in experimental parkinsonism. Hum Gene Ther. 2003;14:1–12.10.1089/10430340360464660Suche in Google Scholar PubMed
[169] Zhang Y, Schlachetzki F, Zhang YF, Boado RJ, Pardridge WM. Normalization of striatal tyrosine hydroxylase and reversal of motor impairment in experimental parkinsonism with intravenous nonviral gene therapy and a brain-specific promoter. Hum Gene Ther. 2004;15:339–50.10.1089/104303404322959498Suche in Google Scholar PubMed
[170] Yeapuri P, Olson KE, Lu Y, Abdelmoaty MM, Namminga KL, Markovic M, et al. Development of an extended half-life GM-CSF fusion protein for Parkinson’s disease. J Control Rel. 2022;348:951–65.10.1016/j.jconrel.2022.06.024Suche in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Brain expression profiles of two SCN1A antisense RNAs in children and adolescents with epilepsy
- Silibinin suppresses glioblastoma cell growth, invasion, stemness, and glutamine metabolism by YY1/SLC1A5 pathway
- Early exercise intervention promotes myelin repair in the brains of ischemic rats by inhibiting the MEK/ERK pathway
- Comparative analysis of CRASH and IMPACT in predicting the outcome of 340 patients with traumatic brain injury
- Association between FOXP3 polymorphisms and expression and neuromyelitis optica spectrum disorder risk in the Northern Chinese Han population
- Trehalose improves the movement ability of Aβarc Drosophila by restoring the damaged mitochondria
- The ACE2/Ang-(1-7)/MasR axis alleviates brain injury after cardiopulmonary resuscitation in rabbits by activating PI3K/Akt signaling
- Single cocaine exposure attenuates the intrinsic excitability of CRH neurons in the ventral BNST via Sigma-1 receptors
- Effect of dopamine on limbic network connectivity at rest in Parkinson’s disease patients with freezing of gait
- FT4-to-FT3 ratio is a novel prognostic marker in subacute combined spinal cord degeneration patients
- Suanzaoren decoction exerts its antidepressant effect via the CaMK signaling pathway
- Acute ischemic STROKE – from laboratory to the Patient’s BED (STROKELABED): A translational approach to reperfusion injury. Study Protocol
- Thyroid hormone T3 induces Fyn modification and modulates palmitoyltransferase gene expression through αvβ3 integrin receptor in PC12 cells during hypoxia
- Activating α7nAChR suppresses systemic inflammation by mitigating neuroinflammation of the medullary visceral zone in sepsis in a rat model
- Amelioration of behavioral and histological impairments in somatosensory cortex injury rats by limbal mesenchymal stem cell transplantation
- TTBK2 T3290C mutation in spinocerebellar ataxia 11 interferes with ciliogenesis
- In a rodent model of autism, probiotics decrease gut leakiness in relation to gene expression of GABA receptors: Emphasize how crucial the gut–brain axis
- A data science approach to optimize ADHD assessment with the BRIEF-2 questionnaire
- Cystatin C alleviates unconjugated bilirubin-induced neurotoxicity by promoting bilirubin clearance from neurocytes via exosomes, dependent on hepatocyte UGT1A1 activity
- Macrophage accumulation in dorsal root ganglion is associated with neuropathic pain in experimental autoimmune neuritis
- Identifying key biomarkers and therapeutic candidates for post-COVID-19 depression through integrated omics and bioinformatics approaches
- The hidden link: Investigating functional connectivity of rarely explored sub-regions of thalamus and superior temporal gyrus in Schizophrenia
- A pilot evaluation of the diagnostic accuracy of ChatGPT-3.5 for multiple sclerosis from case reports
- Review Articles
- Adaptation of the layer V supraspinal motor corticofugal projections from the primary (M1) and premotor (PM) cortices after CNS motor disorders in non-human primates: A survey
- Comorbidity in spinal cord injury in Iran: A narrative review
- Lipid-based nanoparticles for drug delivery in Parkinson’s disease
- Disgust sensitivity and psychopathic behavior: A narrative review
- Rapid Communications
- Long COVID elevated MMP-9 and release from microglia by SARS-CoV-2 Spike protein
- Internal consistency of the Mental Health Professional Culture Inventory: A pilot study in Romanian population
- Retraction
- Retraction of “Effect of C-phycocyanin on HDAC3 and miRNA-335 in Alzheimer’s disease”
- Corrigendum
- Corrigendum to “The ACE2/Ang-(1-7)/MasR axis alleviates brain injury after cardiopulmonary resuscitation in rabbits by activating PI3K/Akt signaling”
- Corrigendum to “Tongxinluo promotes axonal plasticity and functional recovery after stroke”
Artikel in diesem Heft
- Research Articles
- Brain expression profiles of two SCN1A antisense RNAs in children and adolescents with epilepsy
- Silibinin suppresses glioblastoma cell growth, invasion, stemness, and glutamine metabolism by YY1/SLC1A5 pathway
- Early exercise intervention promotes myelin repair in the brains of ischemic rats by inhibiting the MEK/ERK pathway
- Comparative analysis of CRASH and IMPACT in predicting the outcome of 340 patients with traumatic brain injury
- Association between FOXP3 polymorphisms and expression and neuromyelitis optica spectrum disorder risk in the Northern Chinese Han population
- Trehalose improves the movement ability of Aβarc Drosophila by restoring the damaged mitochondria
- The ACE2/Ang-(1-7)/MasR axis alleviates brain injury after cardiopulmonary resuscitation in rabbits by activating PI3K/Akt signaling
- Single cocaine exposure attenuates the intrinsic excitability of CRH neurons in the ventral BNST via Sigma-1 receptors
- Effect of dopamine on limbic network connectivity at rest in Parkinson’s disease patients with freezing of gait
- FT4-to-FT3 ratio is a novel prognostic marker in subacute combined spinal cord degeneration patients
- Suanzaoren decoction exerts its antidepressant effect via the CaMK signaling pathway
- Acute ischemic STROKE – from laboratory to the Patient’s BED (STROKELABED): A translational approach to reperfusion injury. Study Protocol
- Thyroid hormone T3 induces Fyn modification and modulates palmitoyltransferase gene expression through αvβ3 integrin receptor in PC12 cells during hypoxia
- Activating α7nAChR suppresses systemic inflammation by mitigating neuroinflammation of the medullary visceral zone in sepsis in a rat model
- Amelioration of behavioral and histological impairments in somatosensory cortex injury rats by limbal mesenchymal stem cell transplantation
- TTBK2 T3290C mutation in spinocerebellar ataxia 11 interferes with ciliogenesis
- In a rodent model of autism, probiotics decrease gut leakiness in relation to gene expression of GABA receptors: Emphasize how crucial the gut–brain axis
- A data science approach to optimize ADHD assessment with the BRIEF-2 questionnaire
- Cystatin C alleviates unconjugated bilirubin-induced neurotoxicity by promoting bilirubin clearance from neurocytes via exosomes, dependent on hepatocyte UGT1A1 activity
- Macrophage accumulation in dorsal root ganglion is associated with neuropathic pain in experimental autoimmune neuritis
- Identifying key biomarkers and therapeutic candidates for post-COVID-19 depression through integrated omics and bioinformatics approaches
- The hidden link: Investigating functional connectivity of rarely explored sub-regions of thalamus and superior temporal gyrus in Schizophrenia
- A pilot evaluation of the diagnostic accuracy of ChatGPT-3.5 for multiple sclerosis from case reports
- Review Articles
- Adaptation of the layer V supraspinal motor corticofugal projections from the primary (M1) and premotor (PM) cortices after CNS motor disorders in non-human primates: A survey
- Comorbidity in spinal cord injury in Iran: A narrative review
- Lipid-based nanoparticles for drug delivery in Parkinson’s disease
- Disgust sensitivity and psychopathic behavior: A narrative review
- Rapid Communications
- Long COVID elevated MMP-9 and release from microglia by SARS-CoV-2 Spike protein
- Internal consistency of the Mental Health Professional Culture Inventory: A pilot study in Romanian population
- Retraction
- Retraction of “Effect of C-phycocyanin on HDAC3 and miRNA-335 in Alzheimer’s disease”
- Corrigendum
- Corrigendum to “The ACE2/Ang-(1-7)/MasR axis alleviates brain injury after cardiopulmonary resuscitation in rabbits by activating PI3K/Akt signaling”
- Corrigendum to “Tongxinluo promotes axonal plasticity and functional recovery after stroke”


