Abstract
Background
The deposition of Aβ42 has been regarded as one of the important pathological features of Alzheimer’s disease (AD). However, drug development for Aβ42 toxicity has been progressed slowly.
Objective
Our aim was to introduce the effect and related mechanism of trehalose on an Aβarc (arctic mutant Aβ42) Drosophila AD model.
Methods
The human Aβarc was expressed in Drosophila to construct the AD model. Trehalose was added to the culture vial. The movement ability was determined by detecting climbing ability and flight ability. Enzyme-linked immunosorbent assay was used to detect the levels of Aβarc, ATP, and lactate. Electron microscopy assay, mitochondrial membrane potential assay, and mitochondrial respiration assay were used to assess the mitochondrial structure and function.
Results
Trehalose strongly improved the movement ability of Aβarc Drosophila in a concentration gradient-dependent manner. Furthermore, trehalose increased the content of ATP and decreased the content of Aβarc and lactate both in the brain and thorax of Aβarc Drosophila. More importantly, the mitochondrial structure and function were greatly improved by trehalose treatment in Aβarc Drosophila.
Conclusion
Trehalose improves movement ability at least partly by reducing the Aβarc level and restoring the mitochondrial structure and function in Aβarc Drosophila.
1 Introduction
Alzheimer’s disease (AD) is the most common form of senile dementia [1]. The accumulation and deposition of β amyloid (Aβ) is one of the most important pathological features of AD [2]. Recently, a widely accepted hypothesis suggests that the main pathological features in AD, including Tau protein hyperphosphorylation, glial cell activation, inflammation, synaptic damage, oxidative stress, and energy metabolism damage, are all attributed to the accumulation of soluble Aβ [3]. Therefore, developing drugs targeting Aβ would be useful for anti-AD.
Due to its small size, easy reproduction, and ease of genetic manipulation, Drosophila has been widely used to construct AD models [4]. Drosophila AD models were almost constructed by expressing human Aβ, APP, BACE, and presenilin [5]. These AD models could effectively imitate the pathological state of AD patients [5]. Arctic mutant Aβ42 (Aβarc) is an important mutation of Aβ42 in familial Alzheimer’s disease [6]. The expression of Aβarc exhibited stronger neurotoxicity than the expression of Aβ40 and Aβ42 in Drosophila [7,8]. In other words, Aβarc Drosophila AD model is more suitable to explore drugs for AD.
Trehalose is a safe and reliable natural non-reducing disaccharide, widely present in non-mammalian animals, plants, and microorganisms, with excellent capabilities of anti-inflammatory and antioxidant stress [9]. Currently, trehalose has been reported in various neurodegenerative diseases, mainly by activating autophagy to improve related pathological features [10]. In AD, most studies have focused on evaluating the effects of trehalose on AD cell models in vitro [11,12,13,14,15], which makes the progress of trehalose as a therapeutic drug for AD very slow. Only several studies simply reported that trehalose could improve cognition in several types of transgenic mice, such as Tg2576, APP23, and APP/PS1 mice [16,17,18]. It has still been unclear whether trehalose could reduce the toxicity of Aβ and its mechanism in vivo.
In this study, we first reported that trehalose strongly improves the climbing ability and flight ability of Aβarc Drosophila. We also found that trehalose significantly reduces the content of Aβarc both in the brain and thorax of Aβarc Drosophila. Energy is directly linked to the movement ability of Aβarc Drosophila [19]. Therefore, we detected the levels of ATP and lactate both in the brain and thorax of Aβarc Drosophila. The results showed that trehalose significantly increases the ATP level and decreases the lactate level both in the brain and thorax of Aβarc Drosophila. Mitochondria are the main organelles for ATP production [20]. We further detected the structure and function of mitochondria. Excitedly, the results showed that trehalose greatly restores the mitochondrial structure and function damaged by Aβarc toxicity. These results indicated that Aβarc consumption and mitochondrial repairment may be the key mechanism for trehalose to rescue Aβarc Drosophila.
In summary, our study implied that trehalose could greatly improve the movement ability of Aβarc Drosophila. This improvement was realized at least partially through reducing the content of Aβarc and restoring the damaged mitochondria in Aβarc Drosophila. In other words, trehalose could be a potential therapeutic drug for the treatment of AD.
2 Materials and methods
2.1 Drosophila stocks
The cultured conditions for Drosophila stocks were described as follows. First, the culture medium is composed of multiple components, such as ddH2O 0.65 L/L, yeast 15 g/L, corn flour 38.85 g/L, sucrose 15.81 g/L, glucose 31.6 g/L, methyl p-hydroxybenzoate 0.75 g/L (soluble in alcohol 7.5 mL), and agar 5.6 g/L. Second, Drosophila was placed into an incubator with 12 h/12 h light/dark cycle, 25°C, and 50–70% relative humidity. W1118 Drosophila (Bloomington stock, #5905) was obtained from Drosophila Bloomington Stock Center (University of Indiana, Bloomington, IN). The upstream activating sequence transgenic line used for expressing Aβarc (P{UAS-Aβarc}) and [Gal4]A307 transgenic line used for driving the expression of Aβarc in giant fiber (GF) system and other components of the nervous system were generous gifts from Dr. Fu-De Huang (Institute of Neuroscience and State Key Laboratory of Neuroscience, Shanghai, China) [7]. P{UAS-Aβarc} was crossed to [GAL4]A307 (virgin flies) line to generate heterozygous flies expressing Aβarc. [Gal4]A307 (virgin flies) was crossed to W1118 to generate control flies containing one copy of [Gal4]A307. Before use, all these transgenic flies were separately backcrossed to an isogenic control line W1118 for at least five generations. Male flies were collected and used in this study.
2.2 Drug intervention
Trehalose (Yuanye, Shanghai, China, # S11051) was dissolved and diluted to the final concentrations with ddH2O in this study. The administration method was completely based on a previous study [19]. Briefly, 80 μl of trehalose solution with the final concentrations (0, 50, 100, and 200 mM) was daily added to the culture vial (with sufficient food) containing 20 newly eclosed subject flies (1–2 days old) until 25 days. The flies were transferred into fresh food every 7 days. The groups were designed as follows: wild-type group: A307 > W1118, Aβarc expression group: A307 > Aβarc, Aβarc expression plus low-dose trehalose treatment group (TRE50): A307 > Aβarc + TRE 50 mM, Aβarc expression plus middle-dose trehalose treatment group (TRE100): A307 > Aβarc + TRE 100 mM, and Aβarc expression plus high-dose trehalose treatment group (TRE200): A307 > Aβarc + TRE 200 mM.
2.3 Climbing assay
The climbing assay was performed according to the negative geotaxis climbing assay as previously described [19]. Briefly, flies (n = 30, 25 days after eclosion) were placed into a transparent testing vial (a diameter of 2.1 cm and height of 19.0 cm) and tapped down to the bottom of the vial. So the flies were allowed to climb upwards the walls of the vial due to negative geotaxis. A digital video recorder was used to record the climbing process. The flies were appraised in 3–5 consecutive trials separated by 40 s intervals. The height of each fly in each vial at 20 s was measured by software “RflyDetection” to evaluate the fly’s climbing ability. All behavioral recording was done at 25°C.
2.4 Flight ability
The flight ability detection was performed as the previous study described [19]. Briefly, the single fly was tapped down into a glass cylinder (inner diameter of 10 cm and length of 39 cm), which was divided into 13 zones of 3 cm each. The zone in which the fly landed was recorded and used to evaluate the landing height. Ten flies were used in each group.
2.5 Detection of Aβarc, ATP, and lactate
Brains and thoraces of the flies (n = 50, 25 days after eclosion) in each group were homogenized (Tissue homogenizer, Next Advance) thoroughly in cold RIPA buffer (Solarbio, # R0020) supplemented with cocktail protease inhibitor (bimake, # B14001). The samples were incubated on ice for 30 min and then centrifuged at 12,000g for 10 min at 4°C. Supernatants were collected for enzyme-linked immunosorbent assay (ELISA) analysis. The content of Aβarc (Invitrogen, # KHB3441), ATP (Beyotime, # S0026), and lactate (Sigma-Aldrich, MAK064) was determined according to the manufacturer’s instructions respectively.
2.6 Immunostaining
Brains of the flies were dissected in pre-cooling PBS and fixed with 4% paraformaldehyde for 1 h, washed with 0.3% Triton X-100 for 5 min (five times), and then treated with 70% formic acid for 45 min to re-expose the epitope. The brains were then washed with 0.3% Triton X-100 for 5 min (three times) and blocked with 5% normal goat serum (Solarbio, # SL038) at room temperature for 30 min. The brains were then incubated with primary antibody (Beta Amyloid, 1:100, Covance, # SIG-39300) overnight at 4°C, washed with 0.3% Triton X-100 for 10 min (five times), and then incubated with FITC-conjugated secondary goat anti-mouse antibody (ZSGB-BIO, 1:100, # ZF-0312) at room temperature for 2 h in dark. A laser scanning confocal microscope (Olympus FV3000) at the step of 1 μm was used to acquire the projection of Z-stack images. The standard images of Aβ were taken.
2.7 Quantitative real-time PCR (qRT-PCR)
Brains of the flies (n = 20, 25 days after eclosion) in each group were homogenized thoroughly with 1 mL of TRI reagent (MRC, # TR118). Total RNAs were extracted according to the manufacturer’s instructions. cDNAs were obtained with PrimeScript™ RT Reagent Kit with gDNA Eraser (Takara, # RR047A). qRT-PCR was performed with MonAmp™ SYBR® Green qPCR Mix (Monad, # MQ10101S) according to the manufacturer’s instructions. The primers were described as follows: forward primer of Aβarc: ATGGCGAGCAAAGTCTCGATC, reverse primer of Aβarc: CGCAATCACCACGCCGCCCAC; forward primer of 18S: TCTAGCAATATGAGATTGAGCAATAAG, reverse primer of 18S: AATACACGTTGATACTTTCATTGTAGC. The expression level of Aβarc was normalized to 18S.
2.8 Electron microscopy
Thoraces of the flies were dissected and fixed in 2.5% glutaraldehyde and 1% osmium tetroxide and embedded in Epon resin as previously described (standard procedures optimized for Drosophila tissue) [19]. Ultra-thin sections were stained with uranyl acetate and lead citrate. HT7700 TEM (HITACHI, Japan) was used for imaging. Broken mitochondria were defined as previously described [21]. Broken mitochondria were quantified in each group by manual counting.
2.9 Mitochondrial respiration assay
Oxygraph-2 K high-resolution respirometry (Oxygraph-2K 10000-1, Oroboros, AT) was used to detect mitochondrial respiration. Brains of the flies (n = 10, 25 days after eclosion) in each group were homogenized thoroughly on ice using a pestle in MiR05 respiration buffer (10 mM KH2PO4, 20 mM HEPES, 110 mM sucrose, 60 mM K-lactobionate, 20 mM taurine, 0.5 mM EGTA, 3 mM MgCl2, and 1 g/l fatty acid-free BSA). Substrates, uncoupler, and inhibitors of mitochondrial respiratory chain complexes were used as follows: substrates including 2 M pyruvate, 0.8 M malate, 2 M glutamate, 1 M succinate, 0.5 M ADP + Mg2+, and 4 mM cytochrome C; uncoupler including 1 mM carbonyl cyanide m-chlorophenyl hydrazine; and inhibitors including 1 mM rotenone and 5 mM Antimycin A. Complex І respiration was measured in MiR05 respiration buffer in the presence of pyruvate, malate, glutamate, and ADP + Mg2+. Complex II was assayed in respiration buffer supplemented with rotenone and succinate. Oxygen concentration and oxygen flux indicating the function of complex І and complex Ⅱ were recorded using DatLab software (Oroboros Instruments) as previously described [19].
2.10 Determination of mitochondrial membrane potential
Brains of the flies (n = 50, 25 days after eclosion) in each group were used to extract mitochondria with the Tissue Mitochondria Isolation Kit (Beyotime, # C3606). Mitochondrial membrane potential was determined by Enhanced Mitochondrial Membrane Potential Assay Kit with JC-1 (Beyotime, # C2003S) according to the manufacturer’s instructions.
2.11 Statistical analysis
Data analysis was conducted by GraphPad Prism software. Multiple groups’ comparison used one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. Two groups’ comparison used a two-tailed unpaired Student’s t test. Significance was considered at *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.005, or ****P ≤ 0.001 in this study. The data were represented as mean ± SD. All experiments were run at least in triplicate.
3 Results
3.1 The construction of Aβarc Drosophila AD model
Aβarc Drosophila was used as an AD model in this study. The qRT-PCR results showed that the gene expression of Aβarc was confirmed in Aβarc Drosophila (Figure 1a). The immunostaining also showed that the deposition of Aβarc exists in the brain of Aβarc Drosophila (Figure 1b). The analysis of Figure 1b found that both the plaque quantity (area >4 μm2) and the plaque average area significantly increased in Aβarc Drosophila (Figure 1c and d). The ELISA also found that soluble Aβarc significantly increases in Aβarc Drosophila (Figure 1e).

The construction of Aβarc Drosophila AD model. (a) Determining the Ct value of 18S and Aβarc by qRT-PCR. (b) Immunostaining of Aβarc (whole brain; n = 10). (c) Quantitative analysis of plaque quantity (staining with Aβarc) in (b). (d) Quantitative analysis of plaque average area (staining with Aβarc) in (b). (e) Determining the content of soluble Aβarc. WT: wild-type group; AD: Aβarc expression group. Error bars represent the SD of at least three independent experiments. NS represents not significant. Scale bars, 50 μm. ****P ≤ 0.001 use an unpaired two-tailed Student’s t-test.
3.2 Trehalose improves the movement ability of Aβarc Drosophila
The climbing ability and the flight ability are always used to evaluate the movement ability of Aβarc Drosophila. We found that trehalose rescues the climbing ability of Aβarc Drosophila in a concentration gradient-dependent manner (Figure 2a). We also found that trehalose rescues the flight ability of Aβarc Drosophila in the same concentration gradient-dependent manner (Figure 2b). These results indicated that trehalose successfully rescues the behaviors of Aβarc Drosophila.

Trehalose restores the climbing ability and flight ability of Aβarc Drosophila. Determination of the climbing ability (n = 30 in each vial) and flight ability (n = 10) in each group and the concentrations of trehalose used are 50, 100, and 200 mM. Determination of (a) climbing ability (n = 30 in each vial) and (b) flight ability (n = 10). WT: wild-type group; AD: Aβarc expression group; TRE50: Aβarc expression plus trehalose 50 mM treatment group; TRE100: Aβarc expression plus trehalose 100 mM treatment group; TRE200: Aβarc expression plus trehalose 200 mM treatment group. Error bars represent the SD of at least three independent experiments. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.005, and ****P ≤ 0.001 use a one-way ANOVA followed by Tukey’s post hoc test.
3.3 Trehalose reduces the content of Aβarc in Aβarc Drosophila
Aβarc toxicity is directly related to the content of Aβarc. We detected the level of Aβarc in Aβarc Drosophila. We found that trehalose reduces the content of Aβarc both in the brain and thorax of Aβarc Drosophila with a concentration gradient-dependent manner (Figure 3a and b). These results indicated that trehalose could significantly reduce the Aβarc toxicity.

Trehalose reduces the content of Aβarc in Aβarc Drosophila. (a) Measuring the content of Aβarc in the brain. (b) Determining the content of Aβarc in the thorax. WT: wild-type group; AD: Aβarc expression group; TRE50: Aβarc expression plus trehalose 50 mM treatment group; TRE100: Aβarc expression plus trehalose 100 mM treatment group; TRE200: Aβarc expression plus trehalose 200 mM treatment group. Error bars represent the SD of at ten independent experiments. ****P ≤ 0.001 use a one-way ANOVA followed by Tukey’s post hoc test.
3.4 Trehalose significantly restores the ATP and lactate levels
The improvement of energy metabolism is an important prerequisite for enhancing movement ability. We found that trehalose greatly increases the ATP production both in the brain and thorax of Aβarc Drosophila (Figure 4a and b). The improvements exhibited a gradient dependence of trehalose concentration (Figure 4a and b). We also found that trehalose attenuates the accumulation of lactate both in the brain and thorax of Aβarc Drosophila in the same concentration-dependent manner (Figure 4c and d). These results demonstrated that trehalose could enhance the movement ability of Aβarc Drosophila by improving damaged energy metabolism.
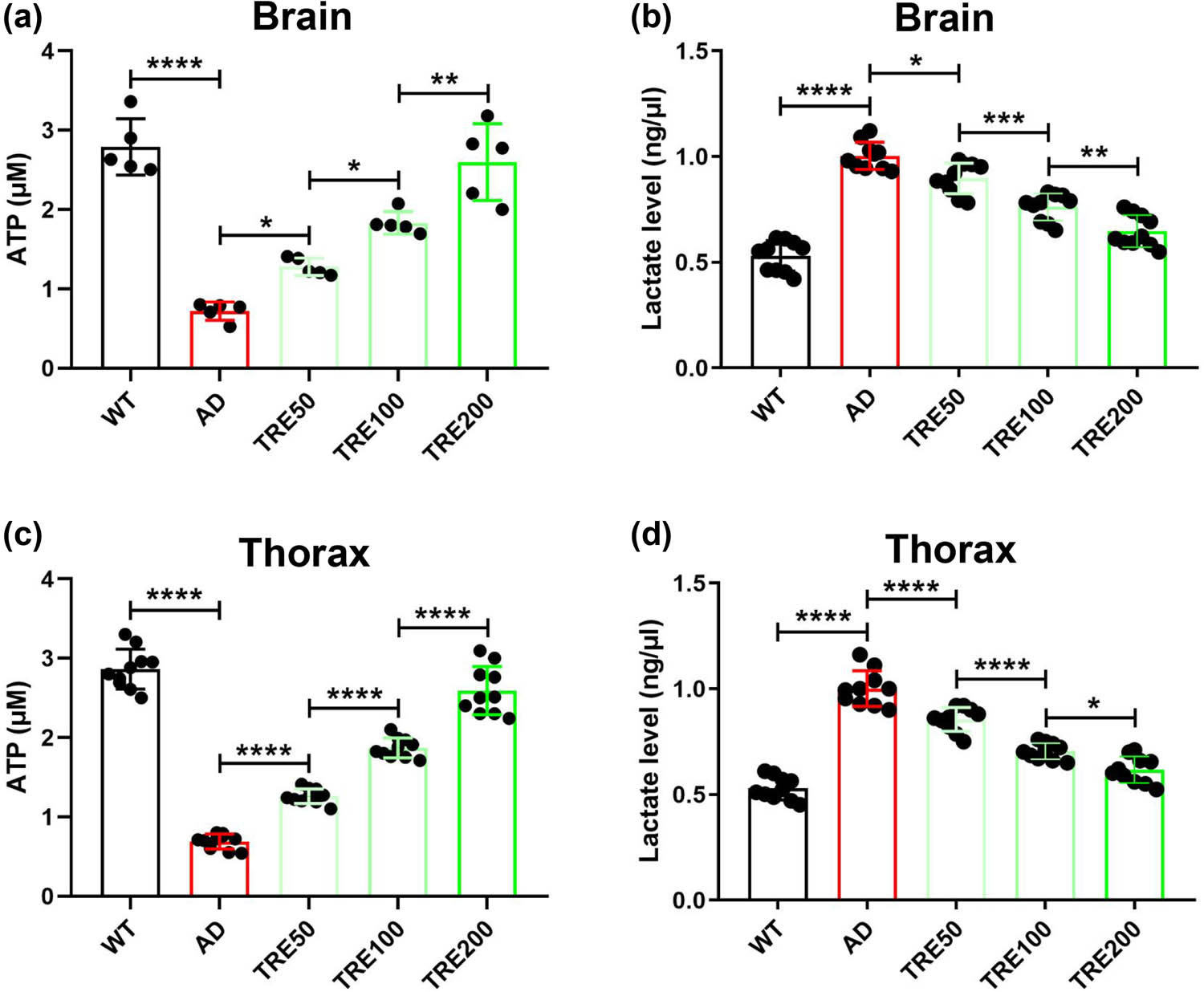
Trehalose rescues the production of ATP and lactate in Aβarc Drosophila. (a) Measuring the content of ATP in the brain. (b) Determining the content of lactate in the brain. (c) Measuring the content of ATP in the thorax. (d) Determining the content of lactate in the thorax. WT: wild-type group; AD: Aβarc expression group; TRE50: Aβarc expression plus trehalose 50 mM treatment group; TRE100: Aβarc expression plus trehalose 100 mM treatment group; TRE200: Aβarc expression plus trehalose 200 mM treatment group. Error bars represent the SD of at least five independent experiments. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.005, and ****P ≤ 0.001 use a one-way ANOVA followed by Tukey’s post hoc test.
3.5 Trehalose strongly restores the damaged mitochondria
Mitochondria are the most important organelles for ATP production. Electron microscopy analysis showed that trehalose restores the mitochondrial structure damaged by Aβarc toxicity (Figure 5a and b). We also found that trehalose greatly ameliorates the mitochondrial function damaged by Aβarc toxicity (Figure 5c). In detail, mitochondria functional analysis showed that trehalose dramatically restores the function of complex Ⅰ and complex Ⅱ disrupted by Aβarc toxicity (Figure 5d and e). Moreover, mitochondrial membrane potential analysis showed that trehalose could repair the mitochondrial structure damaged in Aβarc Drosophila (Figure 5f). The above results indicated that mitochondria may be the targeted organelles for trehalose to rescue Aβarc Drosophila.

Trehalose restores the damaged mitochondria in Aβarc Drosophila. (a) The red dashed line represents mitochondria in each group, and the red asterisk represents the location of mitochondrial structural disruption. (b) Determining the number of mitochondria without intact structure in (a). (c) Mitochondrial respiration in each group. (d) Analyzing the Complex І respiration in (c). (e) Analyzing the Complex Ⅱ respiration in (c). (f) Determining the mitochondrial membrane potential. WT: wild-type group; AD: Aβarc expression group; TRE200: Aβarc expression plus trehalose 200 mM treatment group. Error bars represent the SD of at least three independent experiments. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.005, and ****P ≤ 0.001 use a one-way ANOVA followed by Tukey’s post hoc test. Scale bars, 2 μm.
4 Discussion
Aβ has always played a very important role in the definition of pathological features in AD [1]. It is a consensus that the accumulation and deposition of Aβ is the main cause of the cliff-like decline in motor function in AD patients [3]. Therefore, the development of drugs targeting the toxicity of Aβ is crucial.
Trehalose has played a therapeutic role in various neurodegenerative diseases, especially amyotrophic lateral sclerosis [10,22,23,24,25,26]. However, the effects of trehalose on AD mostly focused on AD cell models just in vitro. Liu et al. found that trehalose differentially inhibits aggregation and neurotoxicity of Aβ40 and Aβ42 in human neuroblastoma cells (SH-SY5Y) [11]. Reddy et al. found that trehalose promotes the insertion of α-helical Aβ into biological membranes in vitro [12]. Krüger et al. found that trehalose suppresses Tau aggregation by activating autophagy in mouse neuroblastoma cell line N2a [13]. Tien et al. found that trehalose decreases the lysosomal metabolism of APP by altering its endocytic vesicular transport in SH-SY5Y [14]. Benito-Cuesta et al. found that the neuroprotective effect of trehalose is mediated by a reduced colocalization of APP and BACE1 in primary neurons [15]. There has not been clear enough whether trehalose could exert the expected anti-AD effects in vivo. Only several studies simply reported that trehalose could improve cognition in several types of transgenic mice, such as Tg2576, APP23, and APP/PS1 mice [16,17,18]. There still has been no study to evaluate the effect of trehalose on Aβ toxicity and its mechanism in vivo. We found for the first time that trehalose significantly improves the movement ability damaged by Aβ toxicity in Aβarc Drosophila. This has taken a big step forward in its treatment of AD, greatly improving our understanding of trehalose therapy.
The damage of energy metabolism has been widely recognized in AD [27]. ATP is the main carrier of energy for the body [28]. The declined ATP production is closely related to the damaged movement ability in AD [29]. Excitingly, we investigated that trehalose significantly elevates the ATP levels both in the brain and thorax of Aβarc Drosophila. Similarly, the accumulation of lactate is always a hallmark of energy metabolism damage [30]. We also found that trehalose decreases the lactate levels both in the brain and thorax of Aβarc Drosophila. These results implied that trehalose could improve energy metabolism to combat the damaged movement ability of AD in vivo.
ATP is mainly produced by mitochondria [31]. Previous studies showed that therapeutic drugs and strategies targeting mitochondria can effectively rescue AD [32]. And the strength of AD resistance is closely related to the supply of ATP [33]. Therefore, we focused on the effects of trehalose on the damaged mitochondria in Aβarc Drosophila. Mitochondrial function analysis found that trehalose effectively repairs the damaged mitochondrial function in Aβarc Drosophila. And mitochondrial structure analysis also found that trehalose effectively repairs the damaged mitochondrial structure in Aβarc Drosophila. These results suggested that mitochondria should be the targeted organelles of trehalose for anti-AD.
Moreover, mitochondrial cascade hypothesis proposes that mitochondrial dysfunction drive the pathogenesis of AD [34]. Toxin-induced mitochondrial dysfunction drives Aβ production [32]. Aβ also drives mitochondrial dysfunction [32]. It means that mitochondrial dysfunction and Aβ production may form a vicious cycle, leading to a rapid deterioration of AD. We also found that trehalose reduces the level of Aβarc in Aβarc Drosophila. It implied that trehalose may be a candidate drug to break this vicious cycle. Otherwise, mitochondria can directly uptake Aβ via the TOM import machinery [35]. Subsequently, the mitochondrial peptidase, named PreP peptidasome, can degrade the Aβ, which is uptake by mitochondria [36]. This may be the reason for the decrease in Aβarc.
In addition, since Aβ tends to bind to a wide range of molecules, trehalose may directly interact with Aβarc thereby preventing the formation of toxic Aβarc. In future, this is a very interesting research direction for further exploring the mechanism of trehalose against Aβ toxicity.
5 Conclusions
In summary, our results suggested that Aβarc-mitochondria-ATP-movement ability is a potential axis for trehalose to rescue Aβarc Drosophila AD model. In other words, trehalose could improve the movement ability of AD at least partially through the Aβarc-mitochondria-ATP-movement ability signal axis (Figure 6).

Schematic diagram of the mechanism of trehalose against Aβarc toxicity. The red parallel lines represent that Aβarc disrupts the mitochondria. The light green rectangles represent that trehalose restores the disruptions by Aβarc toxicity. AD: Aβarc expression group; TRE: Aβarc expression plus trehalose treatment group.
Acknowledgments
We would like to thank Dr. Fu-De Huang (Institute of Neuroscience and State Key Laboratory of Neuroscience, Shanghai, China) for supplying P{UAS-Aβarc} and [Gal4]A307 transgenic lines used for driving the expression of Aβarc in the GF system and other components of the nervous system.
-
Funding information: This work was supported by the National Natural Science Foundation of China (82360010), the Open Project Program of Guangxi Key Laboratory of Brain and Cognitive Neuroscience (GKLBCN-20190103, GKLBCN-20190105-04, and GKLBCN-202106-02), the Young and Middle-aged Teachers’ Basic Research Ability Improvement Project of Universities in Guangxi (2023KY0522 and 2023KY0541), the Open Fund of Guangxi Key Laboratory of Glucose and Lipid Metabolic Diseases, Second Affiliated Hospital of Guilin Medical University (No. KFKT2022001), and the Innovation Project of Guangxi Graduate Education (No. YCSW2023429).
-
Author contributions: Conceptualization, B.X. and B.M.; methodology, L.L. and Z.H; software, L.L.; validation, B.X., L.L., Z.H., M.W., and X.L.; formal analysis, M.W. and X.L.; investigation, L.L.; resources, Z.H. and M.W.; data curation, B.X., L.L., Z.H., M.W., and X.L.; writing – original draft preparation, D.Y. and L.L.; writing – review and editing, B.X., D.Y., and B.M.; supervision, D.Y. and B.M.; project administration, L.L. and Z.H.; funding acquisition, B.X. and L.L. All authors have read and agreed to the published version of the manuscript.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Scheltens P, Blennow K, Breteler MMB, de Strooper B, Frisoni GB, Salloway S, et al. Alzheimer’s disease. Lancet. 2016;388:505–17.10.1016/S0140-6736(15)01124-1Search in Google Scholar PubMed
[2] Yamanishi K, Hata M, Gamachi N, Watanabe Y, Yamanishi C, Okamura H, et al. Molecular mechanisms of IL18 in disease. Int J Mol Sci. 2023;24(24):17170.10.3390/ijms242417170Search in Google Scholar PubMed PubMed Central
[3] Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, et al. Alzheimer’s disease. Lancet. 2021;397:1577–90.10.1016/S0140-6736(20)32205-4Search in Google Scholar PubMed PubMed Central
[4] Lee B, Choi B, Park Y, Jang S, Yuan C, Lim C, et al. Roles of ZnT86D in neurodevelopment and pathogenesis of Alzheimer disease in a Drosophila melanogaster model. Int J Mol Sci. 2022;23(19):11832.10.3390/ijms231911832Search in Google Scholar PubMed PubMed Central
[5] Bolus H, Crocker K, Boekhoff-Falk G, Chtarbanova S. Modeling neurodegenerative disorders in Drosophila melanogaster. Int J Mol Sci. 2020;21(9):3055.10.3390/ijms21093055Search in Google Scholar PubMed PubMed Central
[6] Crowther DC, Kinghorn KJ, Miranda E, Page R, Curry JA, Duthie FAI, et al. Intraneuronal Aβ, non-amyloid aggregates and neurodegeneration in a Drosophila model of Alzheimer’s disease. Neuroscience. 2005;132:123–35.10.1016/j.neuroscience.2004.12.025Search in Google Scholar PubMed
[7] Zhao X-L, Wang W-A, Tan J-X, Huang J-K, Zhang X, Zhang B-Z, et al. Expression of β-amyloid induced age-dependent presynaptic and axonal changes in Drosophila. J Neurosci. 2010;30:1512–22.10.1523/JNEUROSCI.3699-09.2010Search in Google Scholar PubMed PubMed Central
[8] Huang J-K, Ma P-L, Ji S-Y, Zhao X-L, Tan J-X, Sun X-J, et al. Age-dependent alterations in the presynaptic active zone in a Drosophila model of Alzheimer’s disease. Neurobiol Dis. 2013;51:161–7.10.1016/j.nbd.2012.11.006Search in Google Scholar PubMed
[9] Elbein AD. New insights on trehalose: A multifunctional molecule. Glycobiology. 2003;13:17R–27.10.1093/glycob/cwg047Search in Google Scholar PubMed
[10] Zhang X, Chen S, Song L, Tang Y, Shen Y, Jia L, et al. MTOR-independent, autophagic enhancer trehalose prolongs motor neuron survival and ameliorates the autophagic flux defect in a mouse model of amyotrophic lateral sclerosis. Autophagy. 2014;10:588–602.10.4161/auto.27710Search in Google Scholar PubMed PubMed Central
[11] Liu R, Barkhordarian H, Emadi S, Park C, Sierks M. Trehalose differentially inhibits aggregation and neurotoxicity of beta-amyloid 40 and 42. Neurobiol Dis. 2005;20:74–81.10.1016/j.nbd.2005.02.003Search in Google Scholar PubMed
[12] Reddy AS, Izmitli A, de Pablo JJ. Effect of trehalose on amyloid β (29–40)-membrane interaction. J Chem Phys. 2009;131:085101.10.1063/1.3193726Search in Google Scholar PubMed
[13] Krüger U, Wang Y, Kumar S, Mandelkow EM. Autophagic degradation of tau in primary neurons and its enhancement by trehalose. Neurobiol Aging. 2012;33:2291–305.10.1016/j.neurobiolaging.2011.11.009Search in Google Scholar PubMed
[14] Tien NT, Karaca I, Tamboli IY, Walter J. Trehalose alters subcellular trafficking and the metabolism of the Alzheimer-associated amyloid precursor protein. J Biol Chem. 2016;291:10528–40.10.1074/jbc.M116.719286Search in Google Scholar PubMed PubMed Central
[15] Benito-Cuesta I, Ordoñez-Gutierrez L, Wandosell F. Trehalose reduces the secreted beta-amyloid levels in primary neurons independently of autophagy induction. Metabolites. 2021;11(7):421.10.3390/metabo11070421Search in Google Scholar PubMed PubMed Central
[16] Portbury SD, Hare DJ, Sgambelloni C, Perronnes K, Portbury AJ, Finkelstein DI, et al. Trehalose improves cognition in the transgenic Tg2576 mouse model of Alzheimer’s disease. J Alzheimer’s Dis. 2017;60:549–60.10.3233/JAD-170322Search in Google Scholar PubMed PubMed Central
[17] Liu Y, Wang J, Hsiung G-YR, Song W. Trehalose inhibits Aβ generation and plaque formation in Alzheimer’s disease. Mol Neurobiol. 2020;57:3150–7.10.1007/s12035-020-01942-1Search in Google Scholar PubMed
[18] Zhou HH, Luo L, Zhai XD, Chen L, Wang G, Qin LQ, et al. Sex‐specific neurotoxicity of dietary advanced glycation end products in APP/PS1 mice and protective roles of trehalose by inhibiting tau phosphorylation via GSK‐3β‐TFEB. Mol Nutr Food Res. 2021;65(23):2100464.10.1002/mnfr.202100464Search in Google Scholar PubMed
[19] Li L, Wei Z, Tang Y, Jin M, Yao H, Li X, et al. Icaritin greatly attenuates β‐amyloid‐induced toxicity in vivo. CNS Neurosci Ther. 2023.10.1111/cns.14527Search in Google Scholar PubMed
[20] Nunnari J, Suomalainen A. Mitochondria: In sickness and in health. Cell. 2012;148:1145–59.10.1016/j.cell.2012.02.035Search in Google Scholar PubMed PubMed Central
[21] Guo S, Zhang S, Zhuang Y, Xie F, Wang R, Kong X, et al. Muscle PARP1 inhibition extends lifespan through AMPKα PARylation and activation in Drosophila. Proc Natl Acad Sci U S A. 2023;120:e2213857120.10.1073/pnas.2213857120Search in Google Scholar PubMed PubMed Central
[22] Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and α-synuclein. J Biol Chem. 2007;282:5641–52.10.1074/jbc.M609532200Search in Google Scholar PubMed
[23] Castillo K, Nassif M, Valenzuela V, Rojas F, Matus S, Mercado G, et al. Trehalose delays the progression of amyotrophic lateral sclerosis by enhancing autophagy in motoneurons. Autophagy. 2014;9:1308–20.10.4161/auto.25188Search in Google Scholar PubMed
[24] Li Y, Guo Y, Wang X, Yu X, Duan W, Hong K, et al. Trehalose decreases mutant SOD1 expression and alleviates motor deficiency in early but not end-stage amyotrophic lateral sclerosis in a SOD1-G93A mouse model. Neuroscience. 2015;298:12–25.10.1016/j.neuroscience.2015.03.061Search in Google Scholar PubMed
[25] He Q, Koprich JB, Wang Y, Yu W-B, Xiao B-G, Brotchie JM, et al. Treatment with trehalose prevents behavioral and neurochemical deficits produced in an AAV α-synuclein rat model of Parkinson’s disease. Mol Neurobiol. 2015;53:2258–68.10.1007/s12035-015-9173-7Search in Google Scholar PubMed
[26] Holler CJ, Taylor G, McEachin ZT, Deng Q, Watkins WJ, Hudson K, et al. Trehalose upregulates progranulin expression in human and mouse models of GRN haploinsufficiency: A novel therapeutic lead to treat frontotemporal dementia. Mol Neurodegener. 2016;11:1–17.10.1186/s13024-016-0114-3Search in Google Scholar PubMed PubMed Central
[27] Butterfield DA, Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci. 2019;20:148–60.10.1038/s41583-019-0132-6Search in Google Scholar PubMed PubMed Central
[28] Deng J, Walther A. ATP‐responsive and ATP‐fueled self‐assembling systems and materials. Adv Mater. 2020;32(42):2002629.10.1002/adma.202002629Search in Google Scholar PubMed
[29] Patro S, Ratna S, Yamamoto HA, Ebenezer AT, Ferguson DS, Kaur A, et al. ATP synthase and mitochondrial bioenergetics dysfunction in Alzheimer’s disease. Int J Mol Sci. 2021;22(20):11185.10.3390/ijms222011185Search in Google Scholar PubMed PubMed Central
[30] Rabinowitz JD, Enerbäck S. Lactate: The ugly duckling of energy metabolism. Nat Metab. 2020;2:566–71.10.1038/s42255-020-0243-4Search in Google Scholar PubMed PubMed Central
[31] Jonckheere AI, Smeitink JAM, Rodenburg RJT. Mitochondrial ATP synthase: architecture, function and pathology. J Inherit Metab Dis. 2011;35:211–25.10.1007/s10545-011-9382-9Search in Google Scholar PubMed PubMed Central
[32] Ashleigh T, Swerdlow RH, Beal MF. The role of mitochondrial dysfunction in Alzheimer’s disease pathogenesis. Alzheimer’s Dement. 2022;19:333–42.10.1002/alz.12683Search in Google Scholar PubMed
[33] Li Q, Peng J, Luo Y, Zhou J, Li T, Cao L, et al. Far infrared light irradiation enhances Aβ clearance via increased exocytotic microglial ATP and ameliorates cognitive deficit in Alzheimer’s disease-like mice. J Neuroinflammation. 2022;19(1):145.10.1186/s12974-022-02521-ySearch in Google Scholar PubMed PubMed Central
[34] Swerdlow RH, Perry G, Avila J, Tabaton M, Zhu X. Mitochondria and mitochondrial cascades in Alzheimer’s disease. J Alzheimer’s Dis. 2018;62:1403–16.10.3233/JAD-170585Search in Google Scholar PubMed PubMed Central
[35] Hansson Petersen CA, Alikhani N, Behbahani H, Wiehager B, Pavlov PF, Alafuzoff I, et al. The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc Natl Acad Sci U S A. 2008;105:13145–50.10.1073/pnas.0806192105Search in Google Scholar PubMed PubMed Central
[36] Falkevall A, Alikhani N, Bhushan S, Pavlov PF, Busch K, Johnson KA, et al. Degradation of the amyloid β-protein by the novel mitochondrial peptidasome, PreP. J Biol Chem. 2006;281:29096–104.10.1074/jbc.M602532200Search in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Brain expression profiles of two SCN1A antisense RNAs in children and adolescents with epilepsy
- Silibinin suppresses glioblastoma cell growth, invasion, stemness, and glutamine metabolism by YY1/SLC1A5 pathway
- Early exercise intervention promotes myelin repair in the brains of ischemic rats by inhibiting the MEK/ERK pathway
- Comparative analysis of CRASH and IMPACT in predicting the outcome of 340 patients with traumatic brain injury
- Association between FOXP3 polymorphisms and expression and neuromyelitis optica spectrum disorder risk in the Northern Chinese Han population
- Trehalose improves the movement ability of Aβarc Drosophila by restoring the damaged mitochondria
- The ACE2/Ang-(1-7)/MasR axis alleviates brain injury after cardiopulmonary resuscitation in rabbits by activating PI3K/Akt signaling
- Single cocaine exposure attenuates the intrinsic excitability of CRH neurons in the ventral BNST via Sigma-1 receptors
- Effect of dopamine on limbic network connectivity at rest in Parkinson’s disease patients with freezing of gait
- FT4-to-FT3 ratio is a novel prognostic marker in subacute combined spinal cord degeneration patients
- Suanzaoren decoction exerts its antidepressant effect via the CaMK signaling pathway
- Acute ischemic STROKE – from laboratory to the Patient’s BED (STROKELABED): A translational approach to reperfusion injury. Study Protocol
- Thyroid hormone T3 induces Fyn modification and modulates palmitoyltransferase gene expression through αvβ3 integrin receptor in PC12 cells during hypoxia
- Activating α7nAChR suppresses systemic inflammation by mitigating neuroinflammation of the medullary visceral zone in sepsis in a rat model
- Amelioration of behavioral and histological impairments in somatosensory cortex injury rats by limbal mesenchymal stem cell transplantation
- TTBK2 T3290C mutation in spinocerebellar ataxia 11 interferes with ciliogenesis
- In a rodent model of autism, probiotics decrease gut leakiness in relation to gene expression of GABA receptors: Emphasize how crucial the gut–brain axis
- A data science approach to optimize ADHD assessment with the BRIEF-2 questionnaire
- Cystatin C alleviates unconjugated bilirubin-induced neurotoxicity by promoting bilirubin clearance from neurocytes via exosomes, dependent on hepatocyte UGT1A1 activity
- Macrophage accumulation in dorsal root ganglion is associated with neuropathic pain in experimental autoimmune neuritis
- Identifying key biomarkers and therapeutic candidates for post-COVID-19 depression through integrated omics and bioinformatics approaches
- The hidden link: Investigating functional connectivity of rarely explored sub-regions of thalamus and superior temporal gyrus in Schizophrenia
- A pilot evaluation of the diagnostic accuracy of ChatGPT-3.5 for multiple sclerosis from case reports
- Review Articles
- Adaptation of the layer V supraspinal motor corticofugal projections from the primary (M1) and premotor (PM) cortices after CNS motor disorders in non-human primates: A survey
- Comorbidity in spinal cord injury in Iran: A narrative review
- Lipid-based nanoparticles for drug delivery in Parkinson’s disease
- Disgust sensitivity and psychopathic behavior: A narrative review
- Rapid Communications
- Long COVID elevated MMP-9 and release from microglia by SARS-CoV-2 Spike protein
- Internal consistency of the Mental Health Professional Culture Inventory: A pilot study in Romanian population
- Retraction
- Retraction of “Effect of C-phycocyanin on HDAC3 and miRNA-335 in Alzheimer’s disease”
- Corrigendum
- Corrigendum to “The ACE2/Ang-(1-7)/MasR axis alleviates brain injury after cardiopulmonary resuscitation in rabbits by activating PI3K/Akt signaling”
- Corrigendum to “Tongxinluo promotes axonal plasticity and functional recovery after stroke”
Articles in the same Issue
- Research Articles
- Brain expression profiles of two SCN1A antisense RNAs in children and adolescents with epilepsy
- Silibinin suppresses glioblastoma cell growth, invasion, stemness, and glutamine metabolism by YY1/SLC1A5 pathway
- Early exercise intervention promotes myelin repair in the brains of ischemic rats by inhibiting the MEK/ERK pathway
- Comparative analysis of CRASH and IMPACT in predicting the outcome of 340 patients with traumatic brain injury
- Association between FOXP3 polymorphisms and expression and neuromyelitis optica spectrum disorder risk in the Northern Chinese Han population
- Trehalose improves the movement ability of Aβarc Drosophila by restoring the damaged mitochondria
- The ACE2/Ang-(1-7)/MasR axis alleviates brain injury after cardiopulmonary resuscitation in rabbits by activating PI3K/Akt signaling
- Single cocaine exposure attenuates the intrinsic excitability of CRH neurons in the ventral BNST via Sigma-1 receptors
- Effect of dopamine on limbic network connectivity at rest in Parkinson’s disease patients with freezing of gait
- FT4-to-FT3 ratio is a novel prognostic marker in subacute combined spinal cord degeneration patients
- Suanzaoren decoction exerts its antidepressant effect via the CaMK signaling pathway
- Acute ischemic STROKE – from laboratory to the Patient’s BED (STROKELABED): A translational approach to reperfusion injury. Study Protocol
- Thyroid hormone T3 induces Fyn modification and modulates palmitoyltransferase gene expression through αvβ3 integrin receptor in PC12 cells during hypoxia
- Activating α7nAChR suppresses systemic inflammation by mitigating neuroinflammation of the medullary visceral zone in sepsis in a rat model
- Amelioration of behavioral and histological impairments in somatosensory cortex injury rats by limbal mesenchymal stem cell transplantation
- TTBK2 T3290C mutation in spinocerebellar ataxia 11 interferes with ciliogenesis
- In a rodent model of autism, probiotics decrease gut leakiness in relation to gene expression of GABA receptors: Emphasize how crucial the gut–brain axis
- A data science approach to optimize ADHD assessment with the BRIEF-2 questionnaire
- Cystatin C alleviates unconjugated bilirubin-induced neurotoxicity by promoting bilirubin clearance from neurocytes via exosomes, dependent on hepatocyte UGT1A1 activity
- Macrophage accumulation in dorsal root ganglion is associated with neuropathic pain in experimental autoimmune neuritis
- Identifying key biomarkers and therapeutic candidates for post-COVID-19 depression through integrated omics and bioinformatics approaches
- The hidden link: Investigating functional connectivity of rarely explored sub-regions of thalamus and superior temporal gyrus in Schizophrenia
- A pilot evaluation of the diagnostic accuracy of ChatGPT-3.5 for multiple sclerosis from case reports
- Review Articles
- Adaptation of the layer V supraspinal motor corticofugal projections from the primary (M1) and premotor (PM) cortices after CNS motor disorders in non-human primates: A survey
- Comorbidity in spinal cord injury in Iran: A narrative review
- Lipid-based nanoparticles for drug delivery in Parkinson’s disease
- Disgust sensitivity and psychopathic behavior: A narrative review
- Rapid Communications
- Long COVID elevated MMP-9 and release from microglia by SARS-CoV-2 Spike protein
- Internal consistency of the Mental Health Professional Culture Inventory: A pilot study in Romanian population
- Retraction
- Retraction of “Effect of C-phycocyanin on HDAC3 and miRNA-335 in Alzheimer’s disease”
- Corrigendum
- Corrigendum to “The ACE2/Ang-(1-7)/MasR axis alleviates brain injury after cardiopulmonary resuscitation in rabbits by activating PI3K/Akt signaling”
- Corrigendum to “Tongxinluo promotes axonal plasticity and functional recovery after stroke”

