Research progress of low dielectric constant polymer materials
-
Zhendong Hu
Abstract
The advent of high frequency communication era presents new challenges for further development of dielectric polymer materials. In the field of communication, efficient signal transmission is critical. The lower the dielectric constant of the dielectric material used, the lower the signal delay and the higher the signal fidelity. The preparation of polymer materials with low dielectric constant or reduce the dielectric constant of polymer materials becomes a key research topic. Summarizing past progress and providing perspective, this paper primarily discusses the intrinsic low dielectric polymers, fluorine doped low dielectric polymers, and microporous low dielectric polymers, while predicting the research trend of low dielectric materials.
1 Introduction
In recent years, with the rapid development of electronic information industry and stimulation from demands of wireless technology revolution, low dielectric constant materials have become one of the most important topics in the current semiconductor industry [1], [2], [3], [4]. In the process of signal transmission, there were signal delay and circuit loss. The relationship between signal delay and dielectric constant of dielectric material is shown in the following equation (1) [5].
where Td represents the signal delay, K is a coefficient, and DK represents the dielectric constant of the dielectric material. It can be seen that the lower the dielectric constant of the material, the lower the signal delay and the higher the signal fidelity. Therefore, under the background of in-depth development of the fifth-generation communication technology, the use of low-k materials has become an effective approach to reduce the signal hysteresis time.
Generally, dielectrics are commonly used in the field of microelectronics with relatively low dielectric materials. Low dielectric materials mean that the dielectric constant is higher than that of air (1) and lower than that of silica (3.9), of which the value range is between 1 and 3.9. Low dielectric polymer materials were widely used in electronic and electrical engineering, electronic integration, printed circuit board, communication materials and other fields, due to their several advantages including processability, thermal stability and electrical insulation. As of known, polytetrafluoroethylene (PTFE) [6, 7], liquid crystal polymer (LCP) [8], [9], [10], and polyimide (PI) [11], [12], [13], [14] have already been widely used in circuit board substrates. Epoxy resin and cyanate ester resin were also widely used as an excellent adhesive in packaging materials of electronic devices [15], [16], [17]. Figure 1 shows the dielectric properties of some polymers related to circuit substrates for electronic device packaging. It can be seen that there are few types of polymers with low dielectric constant, on the other hand, the dielectric constant of some polymers was relatively large, such as epoxy resin (DK = 3–4) [18], [19], [20]. The preparation of polymer materials with low dielectric constant or reduce the dielectric constant of polymer materials is an important research topic. This paper describes the research progress of low dielectric polymer materials from the aspects of intrinsic low dielectric polymers, fluorine doped low dielectric polymers, and microporous low dielectric polymers.
![Figure 1:
Dielectric properties of polymer materials [15].](/document/doi/10.1515/polyeng-2021-0338/asset/graphic/j_polyeng-2021-0338_fig_001.jpg)
Dielectric properties of polymer materials [15].
The dielectric constant of polymer material conformed to Clausius Mosotti equation (2) [21]:
where N is the number of polarized molecules per unit volume, ɑ is molecular polarizability, and ε0 is vacuum permittivity (or vacuum dielectric constant). It can be seen that from the above formula, reducing the number of polarized molecules per unit volume or reducing the density of polar groups per unit volume is useful for preparing polymers with low dielectric constant or reducing the dielectric constant of polymers. To reduce the dielectric constant of polymers, there were some methods, including (a) introducing hyperbranched molecular chains to increase the free volume of polymers, which can reduce the number of polarization groups per unit volume [3, 22], [23], [24], [25], (b) synthesizing polymers with fluorine-containing monomers or doping fluorine on the molecular chains of polymers, due to C–F has smaller dipole moment and lower polarizability than C–H bond. At the same time, fluorine atoms can also increase the free volume [26], [27], [28], [29], [30], (c) nanoporous polymer materials as air have a very low dielectric constant (Dk = 1) [31]. In this paper, intrinsic low dielectric polymers, fluorine doped low dielectric polymers, and nano-microporous low dielectric polymers are described and discussed.
2 Intrinsic low dielectric polymer
For dielectric constant (DK) relationship of polymer materials, the dielectric constant can be expressed by the following formula (3) [32]:
where P is the molar polarization of polymer functional group (cm3/mol); V is the molar volume of polymer functional groups (cm3/mol). In order to obtain a polymer with low dielectric constant (DK), it is necessary to reduce the ratio of P/V by increasing the molar volume V or reducing the molar polarization P. However, Table 1 below showed the P and V values and P/V ratio of some common functional groups [33]. It can be seen from that the following principles should be followed to obtain polymers with intrinsically low dielectric constant: (a) minizine the presence of polar groups such as hydroxyl (–OH), carboxyl (–COOH) and amide bond (–CONH–). On one hand, these groups themselves have high P/V value, and on the other hand, these polar groups easily absorbing moisture resulted in the dielectric constant of polymer materials will be further increased due to the high dielectric constant of water being close to 80. (b) The introduction of fluorine-containing groups (–F), methylene (–CH2–) and alicyclic groups (such as cyclohexyl) can effectively reduce the dielectric constant of polymer materials because these groups have small P/V values. (c) The introduction of phenyl, naphthyl, and fluorene groups can also effectively reduce the dielectric constant of polymer materials, because these groups have high V value. Several intrinsic low dielectric polymers were introduced from the perspective of molecular chain structure.
P, V and P/V values of some functional groups [33].
| Functional groups | Molar polarization (P) | Molar volume (V) | P/V |
|---|---|---|---|
| Fluorine (–F) | 1.8 | 10.9 | 0.16 |
| Methylene (–CH2–) | 4.7 | 15.9 | 0.30 |
| Ether bond (–O–) | 5.2 | 10.0 | 0.52 |
| Methyl (–CH3) | 5.6 | 23.9 | 0.23 |
| Carbonyl (–CO–) | 10.0 | 13.4 | 0.78 |
| Ester bond (–COO–) | 15.0 | 23.0 | 0.65 |
| Hydroxyl (–OH) | 20.0 | 9.7 | 2.06 |
| Phenyl (–C6H5) | 25.0 | 65.5 | 0.38 |
2.1 Polytetrafluoroethylene (PTFE)
Polytetrafluoroethylene (PTFE) contains fluorine atoms and the chain structure is symmetrical. The general structural formula is shown in Figure 2. The highly symmetrical molecular chain structure and the presence of carbon fluorine bonds make polytetrafluoroethylene have low dielectric constant (2.0), and the dielectric properties were less affected by temperature and frequency. At the same time, it had the advantages of temperature resistance (−230 to 260 °C), heat resistance, good chemical stability, and nonhygroscopicity [34, 35]. Therefore, Teflon PCB was used in the circuit board industry. However, the disadvantages such as difficult processing, low mechanical strength, poor adhesion, and large linear expansion coefficient, limited its application in ultrathin circuit boards [36, 37].
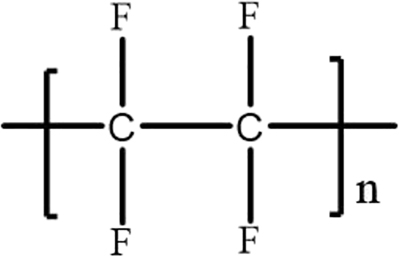
Teflon structure general formula.
2.2 Polyimide (PI)
Polyimide is a kind of polymer synthesized by polycondensation of diamine and diacid, with the backbone containing imide groups (–CO–N–CO–). It was usually obtained by heat treatment, cyclization, and dehydration from polyamide acid which synthesized from reacting aromatic diamine and aromatic anhydride [38, 39]. The general structural formula is shown in Figure 3. In 1970s, polyimide has been used as an intermetallic dielectric layer, passivation layer material, buffer protective layer material, and dielectric insulating layer material in microelectronics industry. Polyimide had been used for a long time at 200–250 °C with excellent heat resistance, superior mechanical properties, insulation, excellent molding processing and dimensional stability. The dielectric constant of polyimide was 2.9–3.5, and the dielectric loss was only 0.004–0.007. The main reason for low dielectric constant of PI was the availability of a large number of aromatic rings in the molecular chain [40], [41], [42], [43]. Wang et al. [44] synthesized a polyimide containing aromatic ether groups and naphthalene side groups with a low dielectric constant (DK = 2.7), and glass transition temperature was 294 °C. The thermogravimetric temperature was 564 °C when the mass loss was 10%. This polyimide can also be dissolved in organic solvents such as chloroform, which was conducive to subsequent processing and applications. Yang et al. [45] synthesized polyimide with excellent properties from aromatic diamine and fluorinated anhydride, of which the dielectric constant was between 2.71–2.79, the glass transition temperature was 245–283 °C, and the tensile strength was between 8717 and 10,217 MPa. Goto et al. [46] successfully prepared low dielectric constant polyimide with an excellent performance by introducing diphenylfluorene group into the main chain, in which the dielectric constant was 2.77. It is worth noting that Fujiwara et al. [47] prepared a side chain poly (norbornenimide) containing huge fluorinated aromatic groups, and confirmed that the macromolecular main chain structure of fluorinated poly (norbornenimide) was a helical structure by molecular simulation. The results showed that the glass transition temperature of poly (norbornene imide) was higher than 400 °C, and the heat resistance was also excellent. More importantly, due to its helical structure, the free volume of the molecular chain increased, resulting in a low dielectric constant (DK = 2.31). Although polyimide has good dielectric properties, its disadvantages, such as high cost and not resistant to water and alkali, cannot be neglected.
![Figure 3:
Polyimide structure diagram [43].](/document/doi/10.1515/polyeng-2021-0338/asset/graphic/j_polyeng-2021-0338_fig_003.jpg)
Polyimide structure diagram [43].
2.3 Polybenzoxazine
Benzoxazine compounds are a kind of intermediates with heterocyclic structure. Generally, they were synthesized by condensation reaction of phenolic compounds, primary amines and formaldehyde, and then ring opening polymerization under the action of heating or catalyst to form a network structure similar to phenolic resin, which was called polybenzoxazine [48]. The curing reaction of benzoxazine is shown in Figure 4. In terms of electrical properties, azine resin was used as an adhesive for printed circuit boards with low dielectric constant at room temperature to 150 °C, such as benzoxazine prepolymer synthesized from bisphenol A and aniline [49], [50], [51]. Zhang et al. [52] synthesized a new benzoxazine monomer containing benzoxazole group via a nonsolvent method, and then the monomer was cured to obtain a new benzoxazolyl polybenzoxazine polymer with low dielectric constant. These results showed that the polymer has a high glass transition temperature of 402 °C, and the dielectric constant was relative resist to the temperature. Su et al. [53] synthesized fluorinated benzoxazine materials with low dielectric constant, the results showed that the dielectric constant of the fluorinated benzoxazine can be as low as 2.36. Benzoxazine resin has the advantages of almost zero curing shrinkage, excellent mechanical properties, low water absorption, and no release of small molecules in the process of ring opening polymerization. In addition, the concentration, toxicity and corrosiveness of polybenzoxazine combustion smoke were relatively low as well.
![Figure 4:
Curing reaction of benzoxazine [48].](/document/doi/10.1515/polyeng-2021-0338/asset/graphic/j_polyeng-2021-0338_fig_004.jpg)
Curing reaction of benzoxazine [48].
2.4 Poly ether etherketone (PEEK)
PEEK is a type of phenylene ring-containing polymer connected by oxygen bridge (ether bond) and carbonyl (ketone) [54]. Many different poly aryl ether ketone polymers can be formed by the connection order and proportion of ether bond, ketone group and benzene ring in the molecular chain, such as mainly polyether ether ketone (PEEK), polyether ketone (PEK), polyether ketone (PEKK), polyether ether ketone (PEEKK), and polyether ketone ether ketone (PEKEKK) [55]. The ether bond in the molecular structure of poly (aryl ether ketone) made it flexible, so it can be formed by the processing method of engineering thermoplastic. The lower the ratio of ether bond to ketone group was in the molecular chain, the higher was the melting point and glass transition temperature. However, due to the regularity and rigidity of the main chain, it was insoluble in most organic solvents. Therefore, the development of polyaryl ether ketones with good solubility and low dielectric constant becomes an important research topic. It was reported that Wang et al. [56] synthesized a novel poly aryl ether ketone POP-PEEK (Figure 5) by introducing phenoxy benzene side groups. The POP-PEEK is very soluble in organic solvents such as chloroform and tetrahydrofuran, and has low dielectric constant (DK = 2.9). PEEK own excellent high temperature properties, mechanical properties, electrical insulation, radiation resistance, and chemical resistance due to their rigid benzene ring. However, high glass transition temperature (Tg = 143 °C) and melting point (Tm = 343 °C) of PEEK increase the difficulty of manufacturing.
![Figure 5:
POP-PEEK structure diagram [56].](/document/doi/10.1515/polyeng-2021-0338/asset/graphic/j_polyeng-2021-0338_fig_005.jpg)
POP-PEEK structure diagram [56].
2.5 Benzocyclobutene resin (BCB)
Benzocyclobutene was obtained through Diels–Alder cycloaddition reaction and free radical addition [57], [58], [59]. The reaction process is shown in Figure 6. As early as the 1990s, it was found that benzocyclobutene resin has been applied in electronic high-tech fields because of its excellent electrical insulation [60]. In 1985, Kirchhoff applied for the first patent on benzocyclobutene polymer [61]. In the early 1990s, Dow Chemical Company of the United States launched a photosensitive negative polymer named cyclotene based on benzocyclobutene. In recent years, cyclotene had been officially adopted in Japan [62]. Under the test conditions at frequency 100 Hz to 1 MHz and at temperature 20–200 °C, the dielectric constant of divinylsiloxane dibenzocyclobutylene resin remained almost unchanged at about 2.65. Additionally, there was no need to use catalyst during resin curing, and small molecules would not volatilize, and the curing time could be adjusted and controlled by curing temperature [63], [64], [65]. It was worth noting that benzocyclobutene was very easy to react with metals (copper, gold, aluminum, etc.) and nonmetals (silicon and silicon dioxide, etc.) Shimoto et al. [66] found that compared with polyimide, the resistivity of CPU module made of Cu/BCB decreased by 30% and the delay time under high frequency transmission decreased by 10%. However, the synthesis and purification of benzocyclobutene were complex and the yield was not high [67, 68]. Benzocyclobutene resin had excellent comprehensive properties, low dielectric constant, small dielectric loss tangent, low moisture absorption, high chemical stability, thermal stability, and film flatness. However, the prepolymerization and film formation of BCB resin are difficult to control, resulting in the brittle property of the cured resin film.
![Figure 6:
Synthesis process of BCB [57].](/document/doi/10.1515/polyeng-2021-0338/asset/graphic/j_polyeng-2021-0338_fig_006.jpg)
Synthesis process of BCB [57].
2.6 Other intrinsic low dielectric polymers
In recent years, the depletion of global fossil resources and environmental problems had attracted great attention to the use of renewable raw materials from biomass [69, 70]. Compared with some traditional petroleum based low dielectric polymers mentioned above, biomass based low dielectric polymer materials had an important prospect in the sustainability of environmental protection materials in the future [71, 72]. Researchers have performed a lot of work on the conversion of biological compounds into materials with high-performance, because the renewability and richness of these compounds can greatly reduce the demand for fossil oil resources. Particularly, these regenerated raw materials had special chemical structures which were very difficult to synthesize and design [73].
Chen et al. [74] synthesized a new dielectric polysiloxane with low dielectric constant formed a renewable biomass of eugenol. The polymer had a low dielectric constant which was 2.77 at the frequency of 0.1–30 MHz. After thermal curing, polysiloxane showed high thermal stability that the degradation initiation temperature and glass transition temperature were 400 and 201 °C, respectively. The coefficient of thermal expansion was 64 ppm/°C when immersed in deionized water at room temperature for 144 h, it showed low water absorption (<0.15%). Therefore, eugenol can be considered as a sustainable raw material for the preparation of high-performance dielectric polymers. The reaction process and corresponding results are shown in Figure 7a.
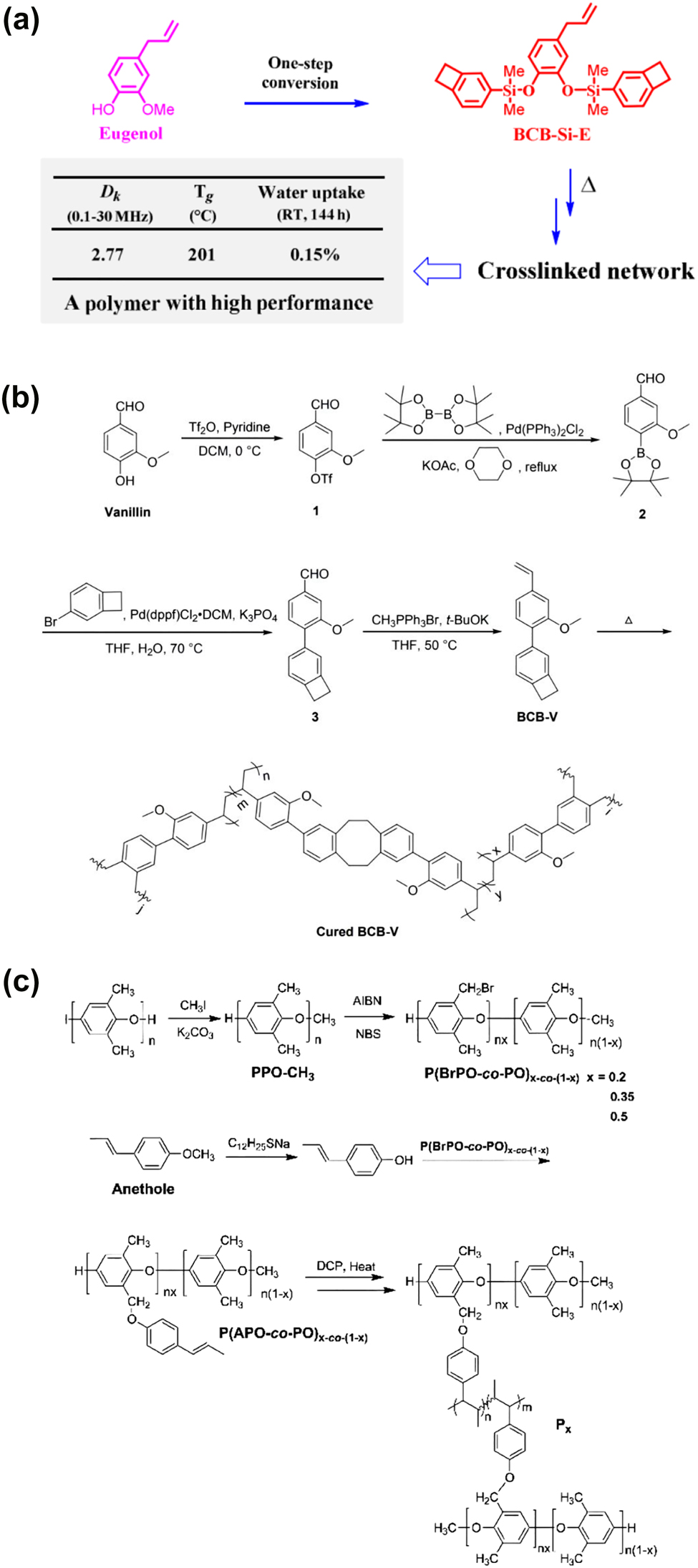
(a) Synthesis diagram of new polysiloxane with dielectric properties, (b) synthesis process of BCB-V and (c) synthesis process of PPO.
Vanillin had become the second largest renewable resource. Vanillin was considered to be one of the ideal raw materials for the synthesis of high-performance polymers, because of its natural aromatic ring and easily modified functional groups [75]. On this basis, Dai et al. [76] conducted research on vanillin derived low dielectric polymers, and designed and synthesized a monomer (BCB-V) containing vinyl and benzocyclobutene units fromed vanillin molecules. The synthesis of process is shown in Figure 7b. The monomer can be easily thermally modified to form a crosslinked network with good thermal stability. The 5% weight loss temperature could reach 436 °C and the coefficient of thermal expansion (CTE) was 60.9 ppm/°C. The crosslinked polymer (BCB-V) had good dielectric properties that dielectric constant was less than 2.84 and dissipation factor was less than 4.9 × 10−3 at frequency of range 0.15–20 MHz. Meanwhile, the dielectric constant and dissipation factor were 2.81 and 6.79 × 10−3, respectively, at the high frequency of 5 GHz. In addition, when the crosslinked polymer was immersed in water at room temperature for 96 h, it showed low water absorption 0.44%. These results showed that the new biomass based thermosetting resin had potential application prospects in microelectronic industry as matrix resin or packaging material.
Recently, Wang et al. [77] synthesized a new type of modified polyphenylene oxide (PPOs) polymer with low dielectric constant, high thermal stability, and good dimensional stability after processing by treating brominated polyphenylene oxide (PPO) with biomass based anethole. The synthesis method was shown in Figure 7c. It was reported that when the molar ratios of anisole in PPO were 0.2, 0.35, and 0.5, the crosslinked PPO had lower dielectric constant (<2.74) and higher Tg (greater than 220 °C). In particular, PPO showed a coefficient of thermal expansion (CTE, 23.4 ppm/°C) similar to thermal expansion of copper foil (about 18 ppm/°C). The results showed that the modified PPOs were suitable for the production of copper-clad laminates in the electronic industry, and the modified PPO with anethole unit had higher thermal stability. Therefore, PPO had a good prospect in the electronic industry, and this contribution provided a new method for preparing high-performance materials from biomass based raw materials.
3 Fluorine doped low dielectric constant polymer
There are two reasons for introducing fluorine atom energy into polymers to reduce dielectric constant: one is that C–F bond has lower polarizability than C–H bond; the other is that fluorine atom can also increases free volume, and the polarization molar degree P of fluorine group is extremely low. Dong et al. [78] synthesized a series of polyimides containing pyridine and trifluoromethyl groups. Polyimides contained trifluoromethyl groups in the main chain showed low dielectric constant and water absorption. The dielectric constant was 2.36–2.52 (1 MHz) and the water absorption was 0.64–0.79%. Fang et al. [79] designed and synthesized two resveratrol based monomers (TFVE) with thermally crosslinked trifluoroethylether group (–OCF=CF2–) via resveratrol molecule. The synthesis process is shown in Figure 8a. It was found that the dielectric constant was 2.45–2.52 and the dielectric loss was 2.2 × 10−3 to 2.6 × 10−3. In addition, the two monomers have high thermal stability with the weight loss temperature of 5% exceeding 450 °C. When they were immersed in boiling water for 3 days, the absorbed water was also less than 0.08%. These data showed that resveratrol-based monomer was suitable for packaging resin in microelectronic industry. Chen et al. [80] synthesized a fluoropolymer (PNBE-TFVE) with high thermal stability and low dielectric constant by ring opening polymerization using two monomers of trifluoroethylene ether (HSi-TFVE) and arylhydrosilane (NBE-OEt). The reaction process is shown in Figure 8b. The produced membrane was treated at high temperature to form a cross-linked network, transmittance was 92% and a water contact angle was more than 100°. The dielectric constant of the crosslinked network was 2.39 and the dielectric loss factor was 3.7 × 10−3 at high frequency of 5 GHz. Even after soaking in water for 3 days, the cured sample maintained a dielectric constant of 2.49 and a dielectric loss of 3.8 × 10−3. These excellent properties indicate that this type polymer had potential application prospects as matrix or packaging resin in microelectronic industry. However, fluorine doped polymers decompose at high temperature and produce hydrogen fluoride gas, which causes corrosion problems to the device.
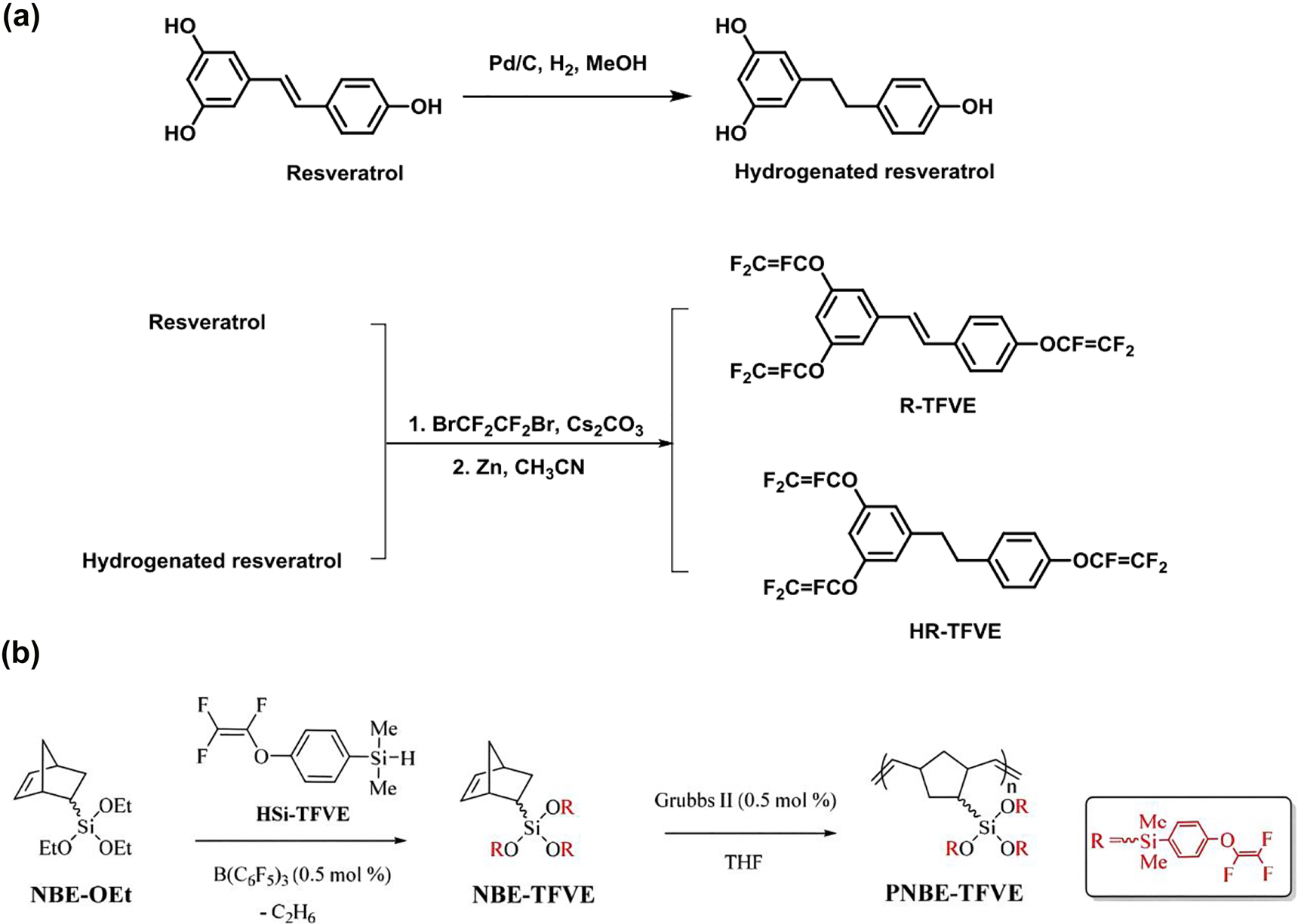
Synthesis process of (a) TFVE and (b) PNBE-TFVE.
4 Nano microporous low dielectric constant polymer
In recent years, the preparation of ultralow dielectric constant polymer materials had been attracted a lot of attention. Ultralow dielectric constant polymer materials were mainly prepared by adding nanoporous nanoparticles [81], sol-gel [82], phase separation technology [83, 84] and foam [85, 86]. Because air has a low dielectric constant close to 1, the introduction of nano-micropores was an effective method to reduce the dielectric constant of materials. Zhao et al. [87] controlled the pore diameter and porosity of foamed polyvinylidene fluoride (PVDF) by controlling the temperature in range of 166–172 °C and the CO2 pressure of 13.8 MPa. The porosity range of PVDF foaming material was 63–96.4%. When the saturation temperature was 169.5 °C, the porosity of PVDF foaming material was 96.4% and the dielectric constant was low to 1.1. In addition, Chen et al. [88] pretreated fluorinated polyimide (FPI) with ozone, and then thermally initiated free radical graft polymerization with poly ethylene glycol methyl methacrylate. Finally, the nanopore structure was obtained by the thermal decomposition of unstable poly ethylene glycol methyl methacrylate in the component. Fluorinated polyimide with nanopore structure has ultralow dielectric constant material, and the dielectric constant is about 2.0. The pore size was 20–50 nm and the porosity was in the range of 2–10%. Recently, Zhang et al. [89] synthesized a new type of polyethersulfone (PES) resin containing bisphenol cyclohexyl group, and then foamed the polyethersulfone (PES) resin. The process is shown in Figure 9a. It was reported that the PES density after foaming decreased from 1.23 to 0.012 g/cm3, the dielectric constant and dielectric loss of the foamed samples were 1.9–2.7 and 4.22 × 10−3 to 7.99 × 10−3, respectively. Polyethersulfone (PES) resins provided a controllable process for the preparation of porous, ultralight and low dielectric constant materials by foaming. It can be potentially used in the fields of microelectronics and high-speed communication systems. In recent years, nanoporous polysiloxane (NPS) had become a research hotspot. Liu et al. [90] grafted cubic oligomeric silsesquioxane monomers onto the silicon surface by reacting octameric silsesquioxane with terminated hydroxyl groups on the silicon surface under the catalysis of SnCl2, and the POSS cage layer as a nanoporous interlayer could reduce the dielectric constant of polyimide film on the silicon surface to about 2.47, due to the air between silicon and polyimide layer. This method could be used to manufacture ultralow dielectric constant materials. It was reported that Li et al. [91] successfully synthesized benzoxazine modified polyhedral oligomeric silsesquioxane (BZPOSS) and used it to prepare bisphenol a nanocomposite epoxy resin. The preparation process was shown in Figure 9b. It was shown that the dielectric property test confirmed that the introduction of BZPOSS could reduce the dielectric constant, which was attributed to the nanopores in the POSS cage structure. When 20 wt% BZPOSS was added, the dielectric constant decreased to 2.28 at 1 MHz frequency. At the same time, dynamic mechanical analysis (DMA) and thermogravimetric analysis (TGA) results showed that the material has good thermal stability and heat resistance. The content of E51/BZPOSS increases with the increase of temperature, which was due to the change of crosslinking density and crosslinking structure of copolymer. It could be potentially used in the fields of microelectronics and high-speed communication systems. However, the porous structure leads to the moisture absorption of the material and decrease of mechanical properties.
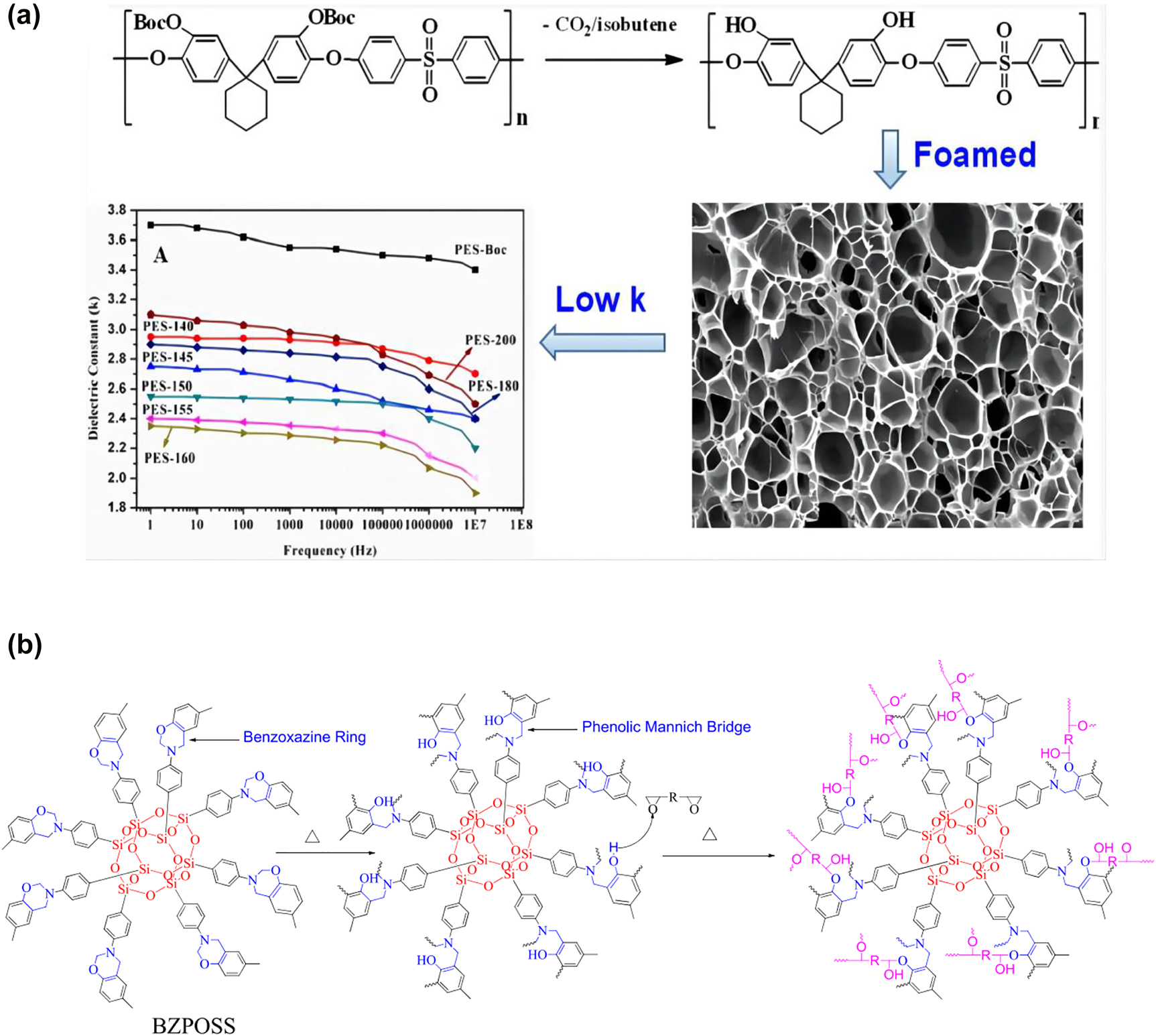
(a) Foaming process and dielectric constant of PES and (b) synthesis process of BZPOSS.
5 Summary and outlook
Low dielectric polymers have become one of the promising research directions in the field of basic and applied research of polymer materials. These low dielectric polymer materials need to have the following properties: in terms of electrical properties, they should have low loss and low leakage current; in terms of mechanical properties, it was necessary to have high adhesion and high strength; in terms of chemical properties, it should be resistant to corrosion and low water absorption; in terms of thermal properties, it was necessary to have high stability and low shrinkage. However, at present, low dielectric polymers also had several bottlenecks, such as poor thermal stability and low mechanical strength of intrinsic polymer dielectric materials, for example, high cost and complex preparation process of fluoropolymer dielectric materials and fluoropolymer materials will produce substances harmful to the environment in the process of thermal degradation. For example, the presence of microporous dielectric polymers further increases the risk of moisture absorption of polymer materials, resulting in the deterioration of their dielectric properties.
Looking forward, the development of low dielectric polymer materials may show the following trends: (1) the development of composite dielectric materials. Dielectric composites can effectively simultaneously meet the requirements of comprehensive properties such as dielectric properties, mechanical properties, and processability. (2) The structure-function integration development of dielectric materials, the development of functional materials with energy storage, intelligent sensing, biocompatibility and so on. (3) Environmental protection and development of green dielectric materials, development and utilization of biomass materials. In brief, the research and development of new dielectric polymer materials with low dielectric constant and dielectric loss is of far-reaching significance.
Funding source: Open Fund of Key Laboratory of Rubber Plastics, Ministry of Education/Shandong Provincial Key Laboratory of Rubber-plastics
Award Identifier / Grant number: KF2020002
Funding source: National Key Laboratory on Ship Vibration and Noise
Award Identifier / Grant number: 6142204200608
Funding source: Opening Project of Key Laboratory of Optoelectronic Chemical Materials and Devices of Ministry of Education, Jianghan University
Award Identifier / Grant number: JDGD-202001
Funding source: Team Innovation Foundation of Hubei province
Award Identifier / Grant number: T201935
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 51803103
Acknowledgments
The authors would like to acknowledge Prof. Chuncheng Hao, Men Xin, their inspiring our research in dielectric materials.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work is supported by the Open Fund of Key Laboratory of Rubber Plastics, Ministry of Education/Shandong Provincial Key Laboratory of Rubber-plastics (KF2020002). The authors would also like to acknowledge financial support from National Key Laboratory on Ship Vibration and Noise (6142204200608), Team Innovation Foundation of Hubei province (T201935), Opening Project of Key Laboratory of Optoelectronic Chemical Materials and Devices of Ministry of Education, Jianghan University (JDGD-202001), and the National Natural Science Foundation of China (51803103).
-
Conflict of interest statement: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Hou, J., Fang, L., Huang, G., Dai, M., Liu, F., Wang, C., Li, M., Zhang, H., Sun, J., Fang, Q. Low-dielectric polymers derived from biomass. ACS Appl. Polym. Mater. 2021, 3, 2835–2848. https://doi.org/10.1021/acsapm.1c00043.Suche in Google Scholar
2. Andrews, J. G., Buzzi, S., Choi, W., Hanly, S. V., Lozano, A., Soong, A. C. K., Zhang, J. C. What will 5G be? IEEE J. Sel. Area.Commun. 2014, 32, 1065–1082. https://doi.org/10.1109/jsac.2014.2328098.Suche in Google Scholar
3. Qian, C., Bei, R., Zhu, T., Zheng, W., Liu, S., Chi, Z., Aldred, M. P., Chen, X., Zhang, Y., Xu, J. Facile strategy for intrinsic low-k dielectric polymers: molecular design based on secondary relaxation behavior. Macromolecules 2019, 52, 4601–4609. https://doi.org/10.1021/acs.macromol.9b00136.Suche in Google Scholar
4. Shi, H., Liu, X., Lou, Y. Materials and micro drilling of highfrequency and high speed printed circuit board: a review. Int. J. Adv. Manuf. Technol. 2019, 100, 827–841. https://doi.org/10.1007/s00170-018-2711-5.Suche in Google Scholar
5. Maier, G. Low dielectric constant polymers for microelec-tronics. Prog. Polym. Sci. 2001, 26, 3–65. https://doi.org/10.1016/s0079-6700(00)00043-5.Suche in Google Scholar
6. Puts, G. J., Crouse, P., Ameduri, B. M. Polytetrafluoroethylene: synthesis and characterization of the original extreme polymer. Chem. Rev. 2019, 119, 1763–1805. https://doi.org/10.1021/acs.chemrev.8b00458.Suche in Google Scholar PubMed
7. Light, D. N., Wilcox, J. R. Process considerations in the fabrication of fluoropolymer printed circuit boards. IEEE Trans. Compon. Packag. Manuf. Technol. Part A 1995, 18, 118–126. https://doi.org/10.1109/95.370745.Suche in Google Scholar
8. Carius, H. E., Schönhals, A., Guigner, D., Sterzynski, T., Brostow, W. Dielectric and mechanical relaxation in the blends of a polymer liquid crystal with polycarbonate. Macromolecules 1996, 29, 5017–5025. https://doi.org/10.1021/ma951706u.Suche in Google Scholar
9. Raveendran, R., Nagaraj, M., Manoj, A. G., Namboothiry, M. A. G. High-performance, transparent solution-processed organic field-effect transistor with low-k elastomeric gate dielectric and liquid crystalline semiconductor: promises and challenges. ACS Appl. Electron. Mater. 2020, 2, 3336–3345. https://doi.org/10.1021/acsaelm.0c00635.Suche in Google Scholar
10. Bapat, P. N., Rao, D. S. S., Prasad, S. K., Yelamaggad, C. V. High-pressure dielectric investigations of nanocolloidal aerosil-nematic liquid crystal composites. J. Phys. Chem. B 2010, 114, 12825–12832. https://doi.org/10.1021/jp106318e.Suche in Google Scholar PubMed
11. Liaw, D. J., WangK, L., Huang, Y. C., Lee, K. R., Lai, J. Y., Ha, C. S. Advanced polyimide materials: syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. https://doi.org/10.1016/j.progpolymsci.2012.02.005.Suche in Google Scholar
12. Liu, Y., Zhang, Y., Lan, Q., Liu, S., Qin, Z., Chen, L., Zhao, C., Chi, Z., Xu, J., Economy, J. High-performance functional polyimides containing rigid nonplanar conjugated triphenylethylene moieties. Chem. Mater. 2012, 24, 1212–1222. https://doi.org/10.1021/cm3003172.Suche in Google Scholar
13. Liu, Y., Qian, C., Qu, L., Wu, Y., Zhang, Y., Wu, X., Zou, B., Chen, W., Chen, Z., Chi, Z., Liu, S., Chen, X., Xu, J. A bulk dielectric polymer film with intrinsic ultralow dielectric constant and outstanding comprehensive properties. Chem. Mater. 2015, 27, 6543–6549. https://doi.org/10.1021/acs.chemmater.5b01798.Suche in Google Scholar
14. Sydlik, S. A., Chen, Z., Swager, T. M. Triptycene polyimides: soluble polymers with high thermal stability and low refractive indices. Macromolecules 2011, 44, 976–980. https://doi.org/10.1021/ma101333p.Suche in Google Scholar
15. Tasaki, T., Shiotani, A., Yamguchi, T. Low Dk/Df polyimide adhesives for low transmission loss substrates. J. Jpn. Inst. Electron. Packag. 2018, 11. E17-006-1–E17-006-7. https://doi.org/10.5104/jiepeng.11.e17-006-1.Suche in Google Scholar
16. Wang, Z., Zhang, X., Weng, L., Liu, L. Low dielectric constant and high toughness epoxy resin based on hyperbranched polyester grafted by flexible chain modified. J. Mater. Sci. Mater. Electron. 2019, 30, 5936–5946. https://doi.org/10.1007/s10854-019-00893-1.Suche in Google Scholar
17. Rakotomalala, M., Wagner, S., Döring, M. Recent developments in halogen free flame retardants for epoxy resins for electrical and electronic applications. Materials 2010, 3, 4300–4327. https://doi.org/10.3390/ma3084300.Suche in Google Scholar
18. Vidil, T., Tournilhac, F., Musso, S., Robisson, A., Leibler, L. Control of reactions and network structures of epoxy thermosets. Prog. Polym. Sci. 2016, 62, 126–179. https://doi.org/10.1016/j.progpolymsci.2016.06.003.Suche in Google Scholar
19. Carfagna, C., Amendola, E., Giamberini, M. Liquid crystalline epoxy based thermosetting polymers. Prog. Polym. Sci. 1997, 22, 1607–1647. https://doi.org/10.1016/s0079-6700(97)00010-5.Suche in Google Scholar
20. Tian, Y., Wang, Q., Shen, L., Cui, Z., Kou, L., Cheng, J., Zhang, J. A renewable resveratrol-based epoxy resin with high Tg, excellent mechanical properties and low flammability. Chem. Eng. J. 2020, 383, 123124. https://doi.org/10.1016/j.cej.2019.123124.Suche in Google Scholar
21. Maex, K., Baklanov, M. R., Shamiryan, D. Low dielectric constant materials for microelectronics. J. Appl. Phys. 2003, 93, 8793–8841. https://doi.org/10.1063/1.1567460.Suche in Google Scholar
22. Mi, Z., Liu, Z., Tian, C., Zhao, X., Zhou, H., Wang, D., Chen, C. Soluble polyimides containing 1,4:3,6-dianhydro-d-glucidol and fluorinated units: preparation, characterization, optical, and dielectric Properties. J. Polym. Sci., Part A: Polym. Chem. 2017, 55, 3253. https://doi.org/10.1002/pola.28700.Suche in Google Scholar
23. Han, M., Zhao, K. Effect of volume fraction and temperature on dielectric relaxation spectroscopy of suspensions of PS/PANI composite microspheres. J. Phys. Chem. C 2008, 112, 19412–19422. https://doi.org/10.1021/jp803530m.Suche in Google Scholar
24. Yang, M., Zhao, K. Anomalous volume phase transition temperature of thermosensitive semi-interpenetrating polymer network microgel suspension by dielectric spectroscopy. J. Phys. Chem. B 2015, 119, 13198–13207. https://doi.org/10.1021/acs.jpcb.5b05491.Suche in Google Scholar PubMed
25. Ramani, R., Das, V., Singh, A., Ramachandran, R., Amarendra, G., Alam, S. Free volume study on the origin of dielectric constant in a fluorine-containing polyimide blend: poly(vinylidene fluoride-co-hexafluoro propylene)/poly(ether imide). J. Phys. Chem. B 2014, 118, 12282–12296. https://doi.org/10.1021/jp506039y.Suche in Google Scholar PubMed
26. Song, N., Yao, H., Ma, T., Wang, T., Shi, K., Tian, Y., Zhang, B., Zhu, S., Zhang, Y., Guan, S. Decreasing the dielectric constant and water uptake by introducing hydrophobic cross-linked networks into co-polyimide films. Appl. Surf. Sci. 2019, 480, 990–997. https://doi.org/10.1016/j.apsusc.2019.02.141.Suche in Google Scholar
27. Yin, X., Feng, Y., Zhao, Q., Li, Y., Li, S., Dong, H., Hu, W., Feng, W. Highly transparent, strong, and flexible fluorographene/fluorinated polyimide nanocomposite films with low dielectric constant. J. Mater. Chem. C 2018, 6, 6378. https://doi.org/10.1039/c8tc00998h.Suche in Google Scholar
28. Wang, X., Dai, Y., Wang, W., Ren, M., Li, B., Fan, C., Liu, X. Fluorographene with high fluorine/carbon ratio: a nanofiller for preparing low-k polyimide hybrid films. ACS Appl. Mater. Interfaces 2014, 6, 16182–16188. https://doi.org/10.1021/am5042516.Suche in Google Scholar PubMed
29. Bei, R., Qian, C., Zhang, Y., Chi, Z., Liu, S., Chen, X., Xu, J., Aldred, M. P. Intrinsic low dielectric constant polyimides: relationship between molecular structure and dielectric properties. J. Mater. Chem. C 2017, 5, 12807. https://doi.org/10.1039/c7tc04220e.Suche in Google Scholar
30. Liu, Y., Tang, L., Qu, L., Liu, S., Chi, Z., Zhang, Y., Xu, J. Synthesis and properties of high performance functional polyimides containing rigid nonplanar conjugated fluorene moieties. Chin. J. Polym. Sci. 2019, 37, 416–427. https://doi.org/10.1007/s10118-019-2225-0.Suche in Google Scholar
31. Ma, Y., Xu, L., He, Z., Xie, J., Shi, L., Zhang, M., Zhang, W., Cui, W. Tunable dielectric and other properties in high performance sandwich-type polyimide films achieved by adjusting the porous structure. J. Mater. Chem. C 2019, 7, 7360. https://doi.org/10.1039/c9tc02017a.Suche in Google Scholar
32. Hougham, G., Tesoro, G., ViehbeckA, Chapple-Sokol, J. D. Polarization effects of fluorine on the relative permittivity in polyimides. Macromolecules 1994, 27, 5964–5971. https://doi.org/10.1021/ma00099a006.Suche in Google Scholar
33. Van Krevelen, D. W., Nijenhuis, K. Properties of Polymers; Elsevier: Amsterdam, The Netherlands, 2009.10.1016/B978-0-08-054819-7.00001-7Suche in Google Scholar
34. Sparnacci, K., Antonioli, D., Deregibus, S., Laus, M., Poggio, T., Kapeliouchko, V., Palamone, G., Zuccheri, G., Passeri, R. PTFE based core-soft shell nanospheres and soft matrix nanocomposites. Macromolecules 2009, 42, 3518–3524. https://doi.org/10.1021/ma802871y.Suche in Google Scholar
35. Zhao, P., Soin, N., Prashanthi, K., Chen, J., Dong, S., Zhou, E., Zhu, Z., Narasimulu, A. A., Montemagno, C. D., Yu, L., Luo, J. Emulsion electrospinning of polytetrafluoroethylene (PTFE) nanofibrous membranes for high-performance triboelectric nanogenerators. ACS Appl. Mater. Interfaces 2018, 10, 5880–5891. https://doi.org/10.1021/acsami.7b18442.Suche in Google Scholar PubMed
36. Suzuki, S., Whittaker, M. R., Wentrup-Byrne, E., Monteiro, M. J., Grøndahl, L. Adsorption of well-defined fluorine-containing polymers onto poly(tetrafluoroethylene). Langmuir 2008, 24, 13075–13083. https://doi.org/10.1021/la802300q.Suche in Google Scholar PubMed
37. Qiang, Q., Qin, J., Ma, Y., Wang, Z., Zhao, C. Robust Conductive micropatterns on PTFE achieved via selective UV-induced graft copolymerization for flexible electronic applications. ACS Appl. Mater. Interfaces 2019, 11, 5517–5525. https://doi.org/10.1021/acsami.8b18209.Suche in Google Scholar PubMed
38. Chen, D., Zhu, H., Liu, T. In situ thermal preparation of polyimide nanocomposite films containing functionalized graphene sheets. ACS Appl. Mater. Interfaces 2010, 2, 3702–3708. https://doi.org/10.1021/am1008437.Suche in Google Scholar PubMed
39. Jia, M., Li, Y., He, C., Huang, X. Soluble perfluorocyclobutyl aryl ether-based polyimide for high-performance dielectric material. ACS Appl. Mater. Interfaces 2016, 8, 26352–26358. https://doi.org/10.1021/acsami.6b09383.Suche in Google Scholar PubMed
40. Chen, Z., Huang, H., Yan, S., Zheng, Z., Liu, S., Yuan, Y., Zhao, J., Fu, Y. New synthetic approach of fluorine-containing graphene oxide for improving dielectric and mechanical properties of polyimide composites. Ind. Eng. Chem. Res. 2017, 56, 9926–9932. https://doi.org/10.1021/acs.iecr.7b02183.Suche in Google Scholar
41. Chen, Z., Zhu, D., Tong, F., Lu, X., Lu, Q. Low dielectric constant polyimide hybrid films prepared by in situ blow-balloon method. ACS Appl. Polym. Mater. 2019, 1, 2189–2196. https://doi.org/10.1021/acsapm.9b00448.Suche in Google Scholar
42. Miyane, S., Chen, C., Lin, Y., Ueda, M., Chen, W. Thermally stable colorless copolyimides with a low dielectric constant and dissipation factor and their organic field-effect transistor applications. ACS Appl. Polym. Mater. 2021, 3, 3153–3163. https://doi.org/10.1021/acsapm.1c00351.Suche in Google Scholar
43. Lv, P., Dong, Z., Dai, X., Qiu, X. Flexible polydimethylsiloxane-based porous polyimide films with an ultralow dielectric constant and remarkable water resistance. ACS Appl. Polym. Mater. 2019, 1, 2597–2605. https://doi.org/10.1021/acsapm.9b00484.Suche in Google Scholar
44. Wang, C., Leu, T. Synthesis and characterization of polyimides containing naphthalene pendant group and flexible ether linkages. Polymer 2000, 41, 3581–3591. https://doi.org/10.1016/s0032-3861(99)00613-8.Suche in Google Scholar
45. Yang, S., Ge, Z., Yin, D., Liu, J., Li, Y., Fan, L. Synthesis and characterization of novel fluorinated polyimides derived from 4,4′-[2,2,2-trifluoro-1-(3-trifluoromethylphenyl)ethylidene] diphthalic anhydride and aromatic diamines. J. Polym. Sci. Polym. Chem. 2004, 42, 4143–4152. https://doi.org/10.1002/pola.20252.Suche in Google Scholar
46. Goto, K., Akiike, T., Inoue, Y. Polymer design for thermally stable polyimides with low dielectric constant. Macromol. Symp. 2003, 199, 321–332. https://doi.org/10.1002/masy.200350927.Suche in Google Scholar
47. Fujiwara, T., Shinba, Y., Sugimoto, K., Mori, Y., Tomikawa, M. Novel high Tg low dielectric constant coil-shaped polymer. J. Photopolym. Sci. Technol. 2005, 18, 289–295. https://doi.org/10.2494/photopolymer.18.289.Suche in Google Scholar
48. Wang, M., Jeng, R., Lin, C. Study on the ring-opening polymerization of benzoxazine through multisubstituted polybenzoxazine precursors. Macromolecules 2015, 48, 530–535. https://doi.org/10.1021/ma502336j.Suche in Google Scholar
49. Zhang, K., Han, L., Froimowicz, P., Ishida, H. A smart latent catalyst containing o-trifluoroacetamide functional benzoxazine: precursor for low temperature formation of very high performance polybenzoxazole with low dielectric constant and high thermal stability. Macromolecules 2017, 50, 6552–6560. https://doi.org/10.1021/acs.macromol.7b00887.Suche in Google Scholar
50. Ma, Q., Wang, H., Zhan, G., Liu, X., Yang, Y., Zhuang, Q., Qian, J. Preparing multifunctional high-performance cross-linked polybenzoxazole aerogels from polybenzoxazine. ACS Appl. Polym. Mater. 2021, 3, 2352–2362. https://doi.org/10.1021/acsapm.0c01309.Suche in Google Scholar
51. Chen, J., Zeng, M., Feng, Z., Pang, T., Huang, Y., Xu, Q. Design and preparation of benzoxazine resin with high-frequency low dielectric constants and ultralow dielectric losses. ACS Appl. Polym. Mater. 2019, 1, 625–630. https://doi.org/10.1021/acsapm.8b00083.Suche in Google Scholar
52. Zhang, K., Zhuang, Q., Zhou, Y., Liu, X., Yang, G., Han, Z. Preparation and properties of novel low dielectric constant benzoxazole-based polybenzoxazine. J. Polym. Sci. Polym. Chem. 2012, 50, 5115–5123. https://doi.org/10.1002/pola.26344.Suche in Google Scholar
53. Su, Y., Chang, F. Synthesis and characterization of fluorinated polybenzoxazine material with low dielectric constant. Polymer 2003, 44, 7989–7996. https://doi.org/10.1016/j.polymer.2003.10.026.Suche in Google Scholar
54. Dupont, O., Jonas, A. M., Nysten, B., Legras, R., Adriaensens, P., Gelan, J. PEEK oligomers as physical model compounds for the Polymer. 4. lamellar microstructure and chain dynamics. Macromolecules 2000, 33, 562–568. https://doi.org/10.1021/ma991116m.Suche in Google Scholar
55. David, L., Girard, C., Dolmazon, R., Albrand, M., Etienne, S. Molecular mobility in para-substituted polyaryls.3. low-temperature dynamics. Macromolecules 1996, 29, 8343–8348. https://doi.org/10.1021/ma960181i.Suche in Google Scholar
56. Wang, Y., Mou, J., Zhu, C., Jian, Z. Synthesis and properties of a novel kind of PEAK(poly aryl ether ketone) with lower dielectric constant. Chem. J. Chin. Univ. 2005, 26, 586–588. in Chinese.Suche in Google Scholar
57. So, Y., Garrou, P. E., Im, J., Ohba, K. Benzocyclobutene-based polymers for microelectronic applications. Polym. Microelectron. Nanoelectron. 2004, 21, 279–293.10.1021/bk-2004-0874.ch021Suche in Google Scholar
58. Cheng, Y., Yang, J., Jin, Y., Deng, D., Xiao, F. Synthesis and properties of highly cross-linked thermosetting resins of benzocyclobutene-functionalized benzoxazine. Macromolecules 2012, 45, 4085–4091. https://doi.org/10.1021/ma3004218.Suche in Google Scholar
59. Zhou, J., Wang, J., Tao, Y., Fang, L., Sun, J., Fang, Q. New Triazine-based polymers with low dielectric constants and high thermostability derived from biorenewable anethole and thermocrosslinkable benzocyclobutene. ACS Sustain. Chem. Eng. 2018, 6, 5620–5626. https://doi.org/10.1021/acssuschemeng.8b00655.Suche in Google Scholar
60. Burdeaux, D., Townsend, P., Carr, J., Garrou, P. Benzocyclobutene (BCB) dielectrics for the fabrication of high density, thin film multichip modules. J. Electron. Mater. 1990, 19, 1357–1366. https://doi.org/10.1007/bf02662825.Suche in Google Scholar
61. Kirchhof, R. A. Polymers Derived from Poly(arylcyclobutenes). U.S. Patent 4,540,763, September 10, 1985.Suche in Google Scholar
62. Hallani, R. K., Moser, M., Bristow, H., Jenart, M. V. C., Faber, H., Neophytou, M., Yarali, E., Paterson, A. F., Anthopoulos, T. D., McCulloch, I. Low-temperature cross-linking benzocyclobutene based polymer dielectric for organic thin film transistors on plastic substrates. J. Org. Chem. 2020, 85, 277–283. https://doi.org/10.1021/acs.joc.9b02981.Suche in Google Scholar PubMed
63. Hayes, C. O., Chen, P., Thedford, R. P., Ellison, C. J., Dong, G., Willson, C. G. Effect of ring functionalization on the reaction temperature of benzocyclobutene thermoset polymers. Macromolecules 2016, 49, 3706–3715. https://doi.org/10.1021/acs.macromol.6b00316.Suche in Google Scholar
64. Dobish, J. N., Hamilton, S. K., Harth, E. Synthesis of lowtemperature benzocyclobutene cross-linker and utilization. Polym. Chem. 2012, 3, 857–860. https://doi.org/10.1039/c2py00606e.Suche in Google Scholar
65. Pugh, C., Baker, J. S., Storms, W. K. Synthesis of a polymerizable benzocyclobutene that undergoes ring-opening isomerization at reduced temperature. Synlett 2013, 25, 148–152. https://doi.org/10.1055/s-0033-1339925.Suche in Google Scholar
66. Shimoto, T., Matsui, K., Utsumi, K. Cu/photosensitive-BCB thin-film muhilayer technology for high-performance muhichip module. In Proceedings of the International Conference on Multichip Modules, Denver, CO, USA, April 13–15, 1994.Suche in Google Scholar
67. Marks, M. J., Sekinger, J. K. Synthesis, crosslinking, and properties of benzocyclobutene-terminated bisphenol A polycarbonates. Macromolecules 1994, 27, 4106–4113. https://doi.org/10.1021/ma00093a012.Suche in Google Scholar
68. Tan, L. S., Soloski, E. J., Arnold, F. E. Benzocyclobutene in polymer synthesis. Cross-Linked Polym. 1988, 24, 349–365. https://doi.org/10.1021/bk-1988-0367.ch024.Suche in Google Scholar
69. Zhu, Y., Romain, C., Williams, C. K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. https://doi.org/10.1038/nature21001.Suche in Google Scholar PubMed
70. Hepbasli, A. A key review on exergetic analysis and assessment of renewable energy resources for a sustainable future. Renew. Sustain. Energy Rev. 2008, 12, 593–661. https://doi.org/10.1016/j.rser.2006.10.001.Suche in Google Scholar
71. Tao, Y., Zhou, J., Wang, J., Wang, Y., Sun, J., Fang, Q. High performance low dielectric constant polymer with good film forming ability developed from renewable plant oil (anethole). Macromol. Chem. Phys. 2018, 219, 1800133. https://doi.org/10.1002/macp.201800133.Suche in Google Scholar
72. Fang, L., Zhou, J., Tao, Y., Wang, Y., Chen, X., Chen, X., Hou, J., Sun, J., Fang, Q. Low dielectric fluorinated polynorbornene with good thermostability and transparency derived from a biobased allylphenol (eugenol). ACS Sustain. Chem. Eng. 2019, 7, 4078–4086. https://doi.org/10.1021/acssuschemeng.8b05527.Suche in Google Scholar
73. Wan, J., Gan, B., Li, C., Molina-Aldareguia, J., Kalali, E. N., Wang, X., Wang, D. Y. A sustainable, eugenol-derived epoxy resinwith high bio-based content, modulus, hardness and low flammability:synthesis, curing kinetics and structure-property relationship. Chem. Eng. J. 2016, 284, 1080–1093. https://doi.org/10.1016/j.cej.2015.09.031.Suche in Google Scholar
74. Chen, X., Fang, L., Chen, X., Zhou, J., Wang, J., Sun, J., Fang, Q. A low dielectric polymer derived from a bio-renewable phenol(eugenol). ACS Sustain. Chem. Eng. 2018, 6, 13518–13523. https://doi.org/10.1021/acssuschemeng.8b03594.Suche in Google Scholar
75. Laurichesse, S., Averous, L. Chemical modification of lignins: towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. https://doi.org/10.1016/j.progpolymsci.2013.11.004.Suche in Google Scholar
76. Dai, M., Tao, Y., Fang, L., Wang, C., Sun, J., Fang, Q. Low dielectric polymers with high thermostability derived from biobased vanillin. ACS Sustain. Chem. Eng. 2020, 8, 15013–15019. https://doi.org/10.1021/acssuschemeng.0c05503.Suche in Google Scholar
77. Wang, Y., Tao, Y., Zhou, J., Sun, J., Fang, Q. Bio-based anethole-functionalized poly(phenylene oxides) (PPOs): new low dielectric materials with high Tg and good dimensional stability. ACS Sustain. Chem. Eng. 2018, 6, 9277–9282. https://doi.org/10.1021/acssuschemeng.8b01596.Suche in Google Scholar
78. Dong, W., Guan, Y., Shang, D. Novel soluble polyimides containing pyridine and fluorinated units: preparation, characterization, and optical and dielectric properties. RSC Adv. 2016, 6, 21662–21671. https://doi.org/10.1039/c6ra00322b.Suche in Google Scholar
79. Fang, L., Tao, Y., Wang, C., Dai, M., Huang, G., Sun, J., Fang, Q. Resveratrol-based fluorinated materials with high thermostability and good dielectric properties at high frequency. ACS Sustain. Chem. Eng. 2020, 8, 16905–16911. https://doi.org/10.1021/acssuschemeng.0c06061.Suche in Google Scholar
80. Chen, X., Sun, J., Fang, L., Tao, Y., Chen, X., Zhou, J., Fang, Q. Cross-linkable fluorinated polynorbornene with high thermostability and low dielectric constant at high frequency. ACS Appl. Polym. Mater. 2020, 2, 768–774. https://doi.org/10.1021/acsapm.9b01070.Suche in Google Scholar
81. Zhang, S., Yan, Y., Li, X., Fan, H., Ran, Q., Fu, Q., Gu, Y. A novel ultra low-k nanocomposites of benzoxazinyl modified polyhedral oligomeric silsesquioxane and cyanate ester. Eur. Polym. J. 2018, 103, 124–132. https://doi.org/10.1016/j.eurpolymj.2018.03.013.Suche in Google Scholar
82. Tkachenko, I., Kononevich, Y., Kobzar, Y., Purikova, O., Yakovlev, Y., Khalakhan, I., Shevchenko, V. Low dielectric constant silica-containing cross-linked organic-inorganic materials based on fluorinated poly(arylene ether)s. Polymer 2018, 157, 131–138. https://doi.org/10.1016/j.polymer.2018.10.035.Suche in Google Scholar
83. Zhang, P., Zhao, J., Zhang, K., Wu, Y., Li, Y. Effect of co-solvent on the structure and dielectric properties of porous polyimide membranes. J. Phys. Appl. Phys. 2018, 51, 215305. https://doi.org/10.1088/1361-6463/aabe19.Suche in Google Scholar
84. Lv, P., Dong, Z., Dai, X., Wang, H., Qiu, X. Synthesis and properties of ultralow dielectric porous polyimide films containing adamantane. J. Polym. Sci. Polym. Chem. 2018, 56, 549–559. https://doi.org/10.1002/pola.28928.Suche in Google Scholar
85. Wang, G., Zhao, G., Dong, G., Song, L., Park, C. B. Lightweight, thermally insulating, and low dielectric microcellular high-impact polystyrene (HIPS) foams fabricated by high-pressure foam injection molding with mold opening. J. Mater. Chem. C 2018, 6, 12294–12305. https://doi.org/10.1039/c8tc04248a.Suche in Google Scholar
86. Krause, B., Koops, G., Vegt, N., Wessling, M., Wübbenhorst, M., Turnhout, J. Ultralow-k dielectrics made by supercritical foaming of thin polymer films. Adv. Mater. 2002, 14, 1041–1046. https://doi.org/10.1002/1521-4095(20020805)14:15<1041::aid-adma1041>3.0.co;2-a.10.1002/1521-4095(20020805)14:15<1041::AID-ADMA1041>3.0.CO;2-ASuche in Google Scholar
87. Zhao, B., Zhao, C., Wang, C., Park, C. Poly(vinylidene fluoride) foams: a promising low-k dielectric and heat-insulating material. J. Mater. Chem. C 2018, 6, 3065–3073. https://doi.org/10.1039/c8tc00547h.Suche in Google Scholar
88. Chen, Y., Wang, W., Yu, W., Kang, E., Neoh, K., Vora, R., Ong, C., Chen, L. Ultra-low-k materials based on nanoporous fluorinated polyimide with well-defined pores via the RAFT-moderated graft polymerization process. J. Mater. Chem. 2004, 14, 1406–1412. https://doi.org/10.1039/b315129h.Suche in Google Scholar
89. Zhang, G., Li, D., Yan, G., Wang, H., Zhang, Y., Wu, Z., Liu, S., Wang, X., Yang, J. Design and fabrication of a low dielectric constant poly(aryleneether sulfone) film-containing cyclohexane group. Ind. Eng. Chem. Res. 2020, 59, 9541–9549. https://doi.org/10.1021/acs.iecr.0c01631.Suche in Google Scholar
90. Liu, Y., Liu, C., Cho, C., Hwu, M. Polyhedral oligomeric silsequioxane monolayer as a nanoporous interlayer for preparation of low-k dielectric films. Nanotechnology 2007, 18, 225701. https://doi.org/10.1088/0957-4484/18/22/225701.Suche in Google Scholar
91. Li, X., Feng, J., Zhang, S., Tang, Y., Hu, X., Liu, X., Liu, X. Epoxy/benzoxazinyl POSS nanocomposite resin with low dielectric constant and excellent thermal stability. J. Appl. Polym. Sci. 2020, 138, 49887. https://doi.org/10.1002/app.49887.Suche in Google Scholar
© 2022 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Material properties
- Research progress of low dielectric constant polymer materials
- Natural rubber reinforced with super-hydrophobic multiwalled carbon nanotubes: obvious improved abrasive resistance and enhanced thermal conductivity
- Epoxy resin/graphene nanoplatelets composites applied to galvanized steel with outstanding microwave absorber performance
- Enhancement of thermal conductivity in polymer composites by maximizing surface-contact area of polymer-filler interface
- Dynamic characterization of the magnetomechanical properties of off axis anisotropic magnetorheological elastomer
- Investigation of optical and biocompatible properties of polyethylene glycol-aspirin loaded commercial pure titanium for cardiovascular device applications
- Polylactic acid effectively reinforced with reduced graphitic oxide
- Preparation and assembly
- Assembled hybrid films based on sepiolite, phytic acid, polyaspartic acid and Fe3+ for flame-retardant cotton fabric
- Fabrication, characterization, and performance of poly (aryl ether nitrile) flat sheet ultrafiltration membranes with polyvinyl pyrrolidone as additives
- Synthesis of composite membranes from polyacrylonitrile/carbon resorcinol/formaldehyde xerogels: gamma effect study, characterization and ultrafiltration of salted oily wastewater
- Chitosan nanoparticles encapsulated into PLA/gelatin fibers for bFGF delivery
- Engineering and Processing
- Stable photoluminescent electrospun CdSe/CdS quantum dots-doped polyacrylonitrile composite nanofibers
Artikel in diesem Heft
- Frontmatter
- Material properties
- Research progress of low dielectric constant polymer materials
- Natural rubber reinforced with super-hydrophobic multiwalled carbon nanotubes: obvious improved abrasive resistance and enhanced thermal conductivity
- Epoxy resin/graphene nanoplatelets composites applied to galvanized steel with outstanding microwave absorber performance
- Enhancement of thermal conductivity in polymer composites by maximizing surface-contact area of polymer-filler interface
- Dynamic characterization of the magnetomechanical properties of off axis anisotropic magnetorheological elastomer
- Investigation of optical and biocompatible properties of polyethylene glycol-aspirin loaded commercial pure titanium for cardiovascular device applications
- Polylactic acid effectively reinforced with reduced graphitic oxide
- Preparation and assembly
- Assembled hybrid films based on sepiolite, phytic acid, polyaspartic acid and Fe3+ for flame-retardant cotton fabric
- Fabrication, characterization, and performance of poly (aryl ether nitrile) flat sheet ultrafiltration membranes with polyvinyl pyrrolidone as additives
- Synthesis of composite membranes from polyacrylonitrile/carbon resorcinol/formaldehyde xerogels: gamma effect study, characterization and ultrafiltration of salted oily wastewater
- Chitosan nanoparticles encapsulated into PLA/gelatin fibers for bFGF delivery
- Engineering and Processing
- Stable photoluminescent electrospun CdSe/CdS quantum dots-doped polyacrylonitrile composite nanofibers


