Abstract
C21H16N2O2, monoclinic, P21/n (no. 14), a = 13.4768(7) Å, b = 6.9225(3) Å, c = 18.2840(8) Å, β = 107.601(2)°, V = 1625.92(13) Å3, Z = 4, Rgt(F) = 0.0593, wRref(F2) = 0.1529, T = 100 K.
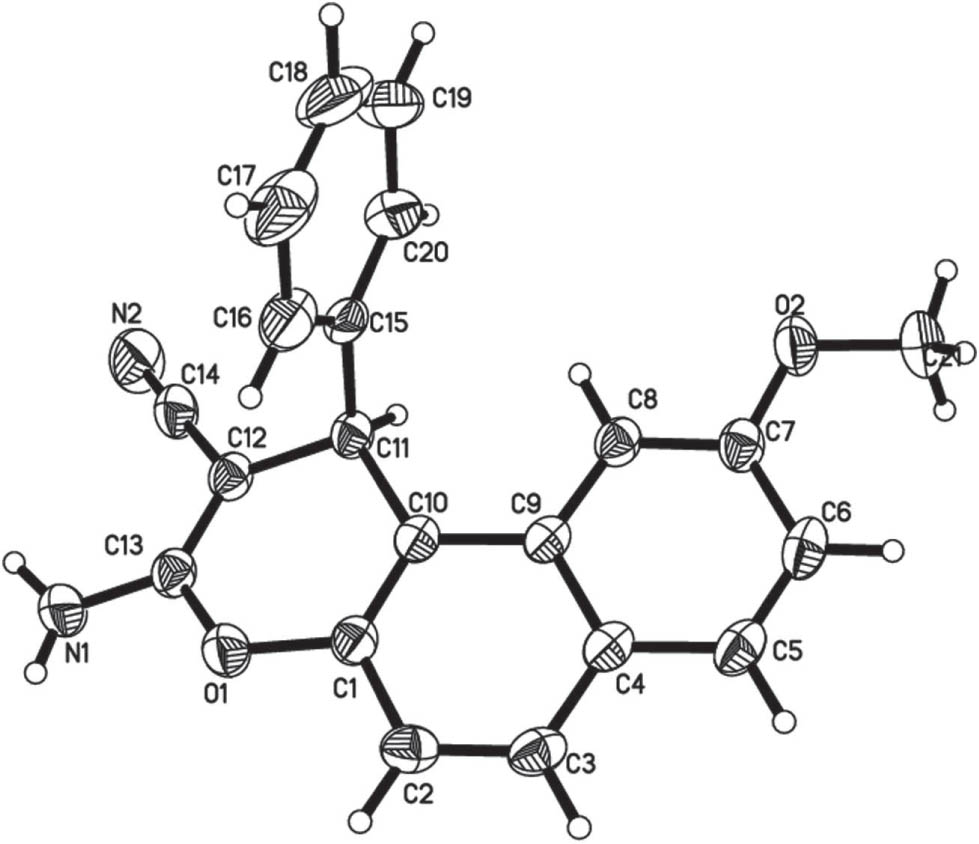
The asymmetric unit of the title crystal structure is shown in the figure. Tables 1 and 2 contain details of the measurement method and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless blocks Size 0.38 × 0.26 × 0.17 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.9 cm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| 2θmax, completeness: | 54°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 20809, 3545, 0.082 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2057 |
| N(param)refined: | 235 |
| Programs: | SHELX [20], Bruker programs [21] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1 | 0.59082(13) | 0.8745(2) | 0.82067(9) | 0.0455(4) |
| O2 | 0.57781(14) | 0.1833(2) | 0.52137(9) | 0.0534(5) |
| N1 | 0.57577(19) | 0.8190(4) | 0.93596(13) | 0.0532(6) |
| N2 | 0.5218(2) | 0.3050(4) | 0.94082(13) | 0.0724(8) |
| C1 | 0.61127(16) | 0.8104(3) | 0.75389(12) | 0.0359(5) |
| C2 | 0.66193(17) | 0.9495(3) | 0.72213(12) | 0.0420(6) |
| H2A | 0.6782 | 1.0732 | 0.7451 | 0.050* |
| C3 | 0.68721(18) | 0.9030(3) | 0.65776(13) | 0.0419(6) |
| H3A | 0.7221 | 0.9953 | 0.6359 | 0.050* |
| C4 | 0.66262(16) | 0.7201(3) | 0.62279(11) | 0.0357(5) |
| C5 | 0.68903(18) | 0.6689(4) | 0.55621(12) | 0.0439(6) |
| H5A | 0.7260 | 0.7591 | 0.5351 | 0.053* |
| C6 | 0.66320(19) | 0.4947(4) | 0.52143(13) | 0.0452(6) |
| H6A | 0.6812 | 0.4643 | 0.4764 | 0.054* |
| C7 | 0.60963(18) | 0.3606(3) | 0.55281(12) | 0.0393(6) |
| C8 | 0.58386(17) | 0.4031(3) | 0.61805(12) | 0.0375(5) |
| H8A | 0.5483 | 0.3093 | 0.6387 | 0.045* |
| C9 | 0.60900(16) | 0.5824(3) | 0.65500(11) | 0.0326(5) |
| C10 | 0.58411(16) | 0.6312(3) | 0.72383(11) | 0.0327(5) |
| C11 | 0.53168(17) | 0.4876(3) | 0.76307(11) | 0.0354(5) |
| H11A | 0.5679 | 0.3606 | 0.7655 | 0.043* |
| C12 | 0.54723(17) | 0.5550(3) | 0.84513(12) | 0.0365(5) |
| C13 | 0.57007(17) | 0.7403(3) | 0.86761(12) | 0.0379(5) |
| C14 | 0.5326(2) | 0.4192(4) | 0.89841(13) | 0.0475(6) |
| C15 | 0.41735(18) | 0.4575(4) | 0.71922(12) | 0.0421(6) |
| C16 | 0.3489(2) | 0.6117(5) | 0.70379(14) | 0.0568(7) |
| H16A | 0.3736 | 0.7369 | 0.7215 | 0.068* |
| C17 | 0.2453(2) | 0.5870(7) | 0.66315(17) | 0.0847(11) |
| H17A | 0.1995 | 0.6948 | 0.6528 | 0.102* |
| C18 | 0.2084(3) | 0.4068(9) | 0.6378(2) | 0.1089(18) |
| H18A | 0.1372 | 0.3896 | 0.6095 | 0.131* |
| C19 | 0.2745(3) | 0.2521(8) | 0.6532(2) | 0.1045(16) |
| H19A | 0.2487 | 0.1272 | 0.6358 | 0.125* |
| C20 | 0.3789(2) | 0.2758(5) | 0.69413(16) | 0.0676(9) |
| H20A | 0.4240 | 0.1671 | 0.7049 | 0.081* |
| C21 | 0.6061(2) | 0.1246(4) | 0.45597(13) | 0.0565(7) |
| H21A | 0.5792 | −0.0055 | 0.4406 | 0.085* |
| H21B | 0.6821 | 0.1239 | 0.4685 | 0.085* |
| H21C | 0.5766 | 0.2148 | 0.4137 | 0.085* |
| H2N1 | 0.550(2) | 0.756(4) | 0.9687(15) | 0.054(8)* |
| H1N1 | 0.590(3) | 0.935(5) | 0.9423(18) | 0.084(12)* |
Source of material
A mixture of 7-methoxy-2-naphthol (0.01 mol), malononitrile (0.01 mol), benzaldehyde (0.01 mol), ethanol (30 mL) and piperidine (0.5 mL) was heated under reflux for 1 h. After complete precipitation occurred the solid product was collected by filtration, washed by methanol and recrystallized from ethanol to give the title compound as colourless crystals; yield 89%; M.p.: 528–529 K.
Experimental details
Carbon-bound hydrogen atoms were placed in calculated positions and were included in the refinement in the riding model approximation. The H atoms of the methyl groups were allowed to rotate with a fixed angle around the C—C bond to best fit the experimental electron density.
Discussion
Chromene and benzochromene derivatives have attracted considerable interest owing to their biological and pharmaceutical activities such as antibacterial [1, 2, 3], antifungal [4, 5, 6], vascular-disrupting [7], antioxidant [8, 9], anticancer [10, 11, 12, 13], estrogenic anticoagulant and antispasmolytic [14], antileishmanial [15], antiproliferative and apoptosis inducing [16, 17, 18, 19].
In the title structure the asymmetric unit contains one independent molecule. All bond lengths and angles are in the expected ranges. The phenyl ring (C16—C20) is perpendicular to the plane of the rest of the molecule (84°). The molecules are connected in the crystal structure via two symmetry related strong classical intermolecular hydrogen bonds, N1—H2N1⋯N2i (H⋯A distance = 2.20(3) Å; N—H⋯N angle = 162(3)°; symmetry code: (i) −x + 1, −y + 1, −z + 2).
Acknowledgements:
The project was financially supported by King Saud University, Vice Deanship of Research Chairs.
References
1. Kidwai, M.; Poddar, R.; Bhardwaj, S.; Singh, S.; Mehta, L. P.: Aqua mediated synthesis of 2-amino-6-benzothiazol-2-ylsulfanyl-chromenes and its in vitro study, explanation of the structure–activity relationships (SARs) as antibacterial agent. Eur. J. Med. Chem. 45 (2010) 5031–5038.10.1016/j.ejmech.2010.08.010Suche in Google Scholar
2. Qian-Zhu, L.; Xiao-Yan, N.; Jie, L.: Novel coumarin and 4H-chromene derivatives containing 4,5-dihydropyrazole moiety: synthesis and antibacterial activity. Lett. Drug. Des. Discov. 8 (2010) 558–561.10.2174/157018011795906857Suche in Google Scholar
3. Xin-Hua, L.; Jin-Xin, L.; Lin-Shan, B.; Guo-Lin, L.; Chu-Xiou, P.: Novel dihydropyrazole derivatives linked with 4H-chromene: microwave-promoted synthesis and antibacterial activity. Lett. Drug. Des. Discov. 7 (2010) 487–490.10.2174/157017810791824847Suche in Google Scholar
4. Thomas, N.; Zachariah, S. M.: Pharmacological activities of chromene derivatives: an overview. Asian J. Pharm. Clin. Res. 6 (2013) 11–15.Suche in Google Scholar
5. Agarwal, S. K.; Verma, S.; Singh, S. S.; Tripathi, A. K.; Khan, Z. K.; Kumar, S.: Antifeedant and antifungal activity of chromene compounds isolated from Blepharispermum subsessile. J. Ethnopharm. 71 (2000) 231–234.10.1016/S0378-8741(00)00158-6Suche in Google Scholar
6. Abrunhosa, L.; Costa, M.; Areias, F.; Venâncio, A.; Proenca, F.: Antifungal activity of a novel chromene dimer. J. Ind. Microbiol. Biotechnol. 34 (2007) 787–792.10.1007/s10295-007-0255-zSuche in Google Scholar PubMed
7. Kasibhatla, S.; Gourdeau, H.; Meerovitch, K.; Drewe, J.; Reddy, S.; Qiu, L.; Zhang, H.; Bergeron, F.; Bouffard, D.; Yang, Q.; Herich, J.; Lamothe, S.; Cai, S. X.; Tseng, B.: Discovery and mechanism of action of a novel series of apoptosis inducers with potential vascular targeting activity. Mol. Cancer Ther. 3 (2004) 1365–1373.10.1158/1535-7163.1365.3.11Suche in Google Scholar
8. Singh, O. M.; Devi, N. S.; Thokchom, D. S.; Sharma, G. J.: Novel 3-alkanoyl/aroyl/heteroaroyl-2H-chromene-2-thiones: synthesis and evaluation of their antioxidant activities. Eur. J. Med. Chem. 45 (2010) 2250–2257.10.1016/j.ejmech.2010.01.070Suche in Google Scholar PubMed
9. Vukovic, N.; Sukdolak, S.; Solujic, S.; Niciforovic, N.: Substituted imino and amino derivatives of 4-hydroxycoumarins as novel antioxidant, antibacterial and antifungal agents: synthesis and in vitro assessments. Food Chem. 120 (2010) 1011–1018.10.1016/j.foodchem.2009.11.040Suche in Google Scholar
10. El-Agrody, A. M.; Fouda, A. M.; Khattab, E. S. A. E. H.: Synthesis, antitumor activity of 2-amino-4H-benzo[h]chromene derivatives, and structure–activity relationships of the 3- and 4-positions. Med. Chem. Res. 22 (2013) 6105–6120.10.1007/s00044-013-0602-8Suche in Google Scholar
11. El-Agrody, A. M.; Khattab, E. S. A. E. H.; Fouda, A. M.: Synthesis, structure-activity relationship (SAR) studies on some 4-Aryl-4Hchromenes and relationship between lipophilicity and antitumor activity. Lett. Drug. Des. Discov. 11 (2014) 1167–1176.10.2174/1570180811666140623204655Suche in Google Scholar
12. Sabry, N. M.; Mohamed, H. M.; Khattab, E. S. A. E. H.; Motlaq, S. S.; El-Agrody, A. M.: Synthesis of 4H-chromene, coumarin, 12H-chromeno[2,3-d]pyrimidine derivatives and some of their antimicrobial and cytotoxicity activities. Eur. J. Med. Chem. 46 (2011) 765–772.10.1016/j.ejmech.2010.12.015Suche in Google Scholar PubMed
13. Mahmoodi, M.; Aliabadi, A.; Emami, S.; Safavi, M.; Rajabalian, S.; Mohagheghi, M. A.; Khoshzaban, A.; Samzadeh-Kermani, A.; Lamei, N.; Shafiee, A.; Foroumadi, A.: Synthesis and in-vitro cytotoxicity of poly-functionalized 4-(2-Arylthiazol-4-yl)-4H-chromenes. Arch. Pharm. Chem. 343 (2010) 411–416.10.1002/ardp.200900198Suche in Google Scholar PubMed
14. Jain, N.; Xu, J.; Kanojia, R. M.; Du, F.; Guo, J.-Z.; Pacia, E.; Lai, M.-T.; Musto, A.; Allan, G.; Reuman, M.; Li, X.; Hahn, D.; Cousineau, M.; Sean Peng, S.; Ritchie, D.; Russell, R.; Lundeen, S.; Sui, Z.: Identification and structure-activity relationships of chromene-derived selective estrogen receptor modulators for treatment of postmenopausal symptoms. J. Med. Chem. 52 (2009) 7544–7569.10.1021/jm900146eSuche in Google Scholar PubMed
15. Tanaka, J. C. A.; Da Silva, C. C.; Ferreira, I. C. P.; Machado, G. M. C.; Leon, L. L.; De Oliveira, A. J. B.: Antileishmanial activity of indole alkaloids from Aspidosperma ramiflorum. Phytomedicine 14 (2007) 377–380.10.1016/j.phymed.2006.09.002Suche in Google Scholar PubMed
16. Zhang, D.; Ma, Y.; Liu, Y.; Liu, Z.-P.: Synthesis of sulfonylhydrazone- and acylhydrazone-substituted 8-ethoxy-3-nitro-2H-chromenes as potent antiproliferative and apoptosis inducing agents. Arch. Pharm. Chem. 347 (2014) 576–588.10.1002/ardp.201400082Suche in Google Scholar PubMed
17. Szulawska-Mroczek, A.; Szumilak, M.; Szczesio, M.; Olczak, A.; Nazarski, R. B.; Lewgowd, W.; Czyz, M.; Stanczak, A.: Synthesis and biological evaluation of new bischromone derivatives with antiproliferative activity. Arch. Pharm. Chem. 346 (2013) 34–43.10.1002/ardp.201200220Suche in Google Scholar PubMed
18. Xiao, G.-Q.; Liang, B.-X.; Chen, S.-H.; Ou, T.-M.; Bu, X.-Z.; Yan, M.: 3-Nitro-2H-chromenes as a new class of inhibitors against thioredoxin reductase and proliferation of cancer cells. Arch. Pharm. Chem. 345 (2012) 767–770.10.1002/ardp.201200121Suche in Google Scholar PubMed
19. Magedov, I. V.; Manpadi, M.; Evdokimov, N. M.; Elias, E. M.; Rozhkova, E.; Ogasawara, M. A.; Bettale, J. D.; Przheval'skii, N. M.; Rogelj, S.; Kornienko, A.: Antiproliferative and apoptosis inducing properties of pyrano[3,2-c]pyridones accessible by a one-step multicomponent synthesis. Bioorg. Med. Chem. Lett. 17 (2007) 3872–3876.10.1016/j.bmcl.2007.05.004Suche in Google Scholar PubMed PubMed Central
20. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Suche in Google Scholar PubMed
21. Bruker. APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, WI, USA, 2009.Suche in Google Scholar
©2016 Ahmed M. El-Agrody et al., published by De Gruyter.
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of the catena-poly-[bis(μ2-1-ethyl-6-fluoro-4-oxo-7-(piperazin-1-yl-κN)-1,4-dihydroquinoline-3-carboxylato-κ2O,O′)nickel(II)] 5.5 hydrate, C32H44N6NiO11F2
- Crystal structure of catena-poly-[(μ2-2-(2-((2,6-dimethylphenyl)amino)phenyl)acetato-κ2O:O′)(μ2-2-(2-((2,6-dimethylphenyl)amino)phenyl)acetate-κ3O,O′:O′)cadmium(II)], C28H20N2Cl4O4Cd
- Crystal structure of catena-poly[dichlorido-(μ2-4-(pyridin-4-yl)-isophthalate-κ2O, O′)cadmium(II)] monohydrate, C13H11NO5Cl2Cd
- Crystal structure of poly-{[μ2-(E)-1,4-di(1H-imidazol-1-yl)but-2-ene-κ2N:N′][μ2-cyclohexane-1,4-dicarboxylato-κ4O,O′:O′′,O′′′]nickel(II)}, C18H22N4NiO4
- Crystal structure of aqua (5,5′-dicarboxy-(1,1′-biphenyl)-2,3′-dicarboxylato-κO) bis(1,10-phenanthroline-κ2N,N′)cadmium monohydrate, C40H28CdN4O10
- Crystal structure of 5-methoxy-N′-[(3Z)-5-chloro-1-(4-fluorobenzyl)-2-oxo-1,2-dihydro-3H-indol-3-ylidene]-1H-indole-2-carbohydrazide-DMSO (1/1), C25H18ClFN4O3 · C2H6OS
- Crystal structure of 5-methoxy-N′-[(3Z)-1-benzyl-5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene]-1H-indole-2-carbohydrazide-DMSO (1/1), C27H25FN4O4S
- Crystal structure of poly-[bis{μ2-N-(4-(1H-imidazol-1-yl)phenyl)-4-(1H-imidazol-1-yl)-N-phenylaniline-κ2N:N′)}-(μ2-naphthalene-2,6-dicarboxylato)-(μ4-naphthalene-2,6-dicarboxylato)dicadmium(II)], C36H25N5O4Cd
- Crystal structure of 1-(adamantan-1-yl)-3-(4-bromophenyl)thiourea, C17H21BrN2S
- Crystal structure of N′-[(1E)-(2,6-dichlorophenyl)-methylidene]adamantane-1-carbohydrazide, C18H20Cl2N2O
- Crystal structure of dichlorido{bis(2-hydroxyethyl)-5′-([2,2′:6′,2′′-terpyridin]-4′-yl)-[1,1′:3′,1′′-terphenyl]-4,4′′-dicarboxylate-κ3N,N′,N′′}zinc(II), C39H31Cl2N3O6Zn
- Crystal structure of 4,4′-(1,3,5,7-tetraoxo-3a,4,4a,5,7,7a,8,8a-octahydro-4,8-ethenopyrrolo [3,4-f]isoindole-2,6(1H,3H)-diyl)dibenzoic acid, C26H18N2O8
- Crystal structure of (E)-1-(4-(((E)-3,5-dichloro-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C17H16Cl2N2O2
- The crystal structure of diaqua-(N-(2-hydroxy-5-nitrobenzyl)iminodiacetato-κ4-N,O,O′,O′′)chromium(III) based on synchrotron data, C11H13CrN2O9
- Crystal structure of ethyl 5-amino-3-(methylthio)-1-(1-phenyl-5-(thiophen-2-yl)-1H-pyrazole-3-carbonyl)-1H-pyrazole-4-carboxylate, C21H19N5O3S2
- Crystal structure of (E)-1-(4-(((E)-3,5-dibromo-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C17H16Br2N2O2
- Crystal structure of dibromido μ-oxalato-κ2O,O′:κ2O′′,O′′′−η6-p-cymenediosmium(II), C22H28Br2O4Os2
- Crystal structure of 2-(bromomethyl)-4-(4-chlorophenyl)-1-tosylpyrrolidine, C18H19BrClNO2S2
- Crystal structure of 5-(3-fluorobenzylidene)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione; C13H11FN2O3
- Crystal structure of diethylammonium 1,3-dimethyl-2,4,6-trioxohexahydropyrimidin-5-ide, C10H19N3O3
- Crystal structure of 1,1-dimethyl-3-(2-phenylethyl)urea, C11H16N2O
- Crystal structure of 2-(4-methoxyphenyl)-1,3-thiazolo[4,5-b]pyridine, C13H10N2OS
- Crystal structure of 3-tert-butyl-7-azadioxindole, C11H14N2O2
- Crystal structure of 1-ferrocenyl-6-bromopyrene, C26H17BrFe
- Crystal structure of 3-(4-bromophenyl)-5-(4-fluorophenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide, C16H13BrFN3S
- Crystal structure of 2-amino-4-(3,5-ditrifluoromethylphenyl)-3-cyano-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo[b]pyran, C20H16F6N2O2
- The crystal structure of 2-amino-4-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13N3O4
- Crystal structure of 2-amino-4-(2, 4-dichlorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H12Cl2N2O2
- Crystal structure of bis(μ2-chlorido)-bis(di-p-tolylhydroxyphosphine-κP)-bis(di-p-tolylphosphite-κP)dipalladium(II), C56H58Cl2O4P4Pd2
- Crystal structure of diaqua-bis(2-methyl-1H-imidazole-4,5-dicarboxylato-κ2-O,N)cadmium(II) tetrahydrate, C12H22CdN4O14
- Crystal structure of aqua-(5-nitrosalicylato-κ2O,O′)-(1,10-phenanthroline-κ2N,N′)copper(II), C19H13CuN3O6
- Crystal structure of bis(4-(2-phenylpropan-2-yl)phenyl)amine, C30H31N
- Crystal structure of 2-amino-4-(3,5-difluorophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C18H16F2N2O2
- Crystal structure of 2-amino-4-(3,4,5-trifluorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H11F3N2O2
- Crystal structure of an isomeric bis[(η5:η1-6,6-di-p-tolylpentafulvene)(η5-pentamethylcyclopentadienyl)titanium(III)]-μ2,η1:η1-dinitrogen complex, C60H66N2Ti2
- Crystal structure of 3,4-dinitropyrazole, C3H2N4O4
- Crystal structure of (4-vinylpyridine-κN)triphenyl tin(IV) chloride, C25H22ClNSn
- Crystal structure of tert-butyl 2-phenylethylcarbamate, C13H19NO2
- Crystal structure of (Z)-4-((E)-(4-chlorobenzyli-dene)hydrazono)-1-p-tolylpyrrolidine-3-carbonitrile, C19H17ClN4
- Crystal structure of bis(biphenyl-2,2′-dicarboxylato-κ2O:O′)-bis(1-cyclopropyl-6-fluoro-4-oxo-7-(1-piperazinyl)-1,4-dihydroquinoline-3-carboxylato-κ2O,O′)dizinc(II)2.5 hydrate, C62H57N6Zn2O16.5F2
- Crystal structure of dichloridobis{μ2-2,2′-((1E,1′E)-(ethane-1,2-diylbis(azanylylidene))bis(methanylylidene))bis(4-chlorophenolato)-κ5O,N,N′,O′:O′}diiron(III), C32H24Cl6Fe2N4O4
- Crystal structure of 4-((4,4-dimethyl-2, 6-dioxocyclohexylidine)methylamino)-N-(3,4-dimethylisoxazol-5-yl)benzenesulfonamide, C20H23N3O5S
- Crystal structure of poly-[aqua-μ2-aqua-μ2-(4,4′-oxybis(benzoato)-κ4O,O′:O′′,O′′′)cadmium(II)], C14H12O7Cd
- Crystal structure of aqua(μ2-biphenyl-2,2′-dicarboxylato-κ3O,O′:O′′)-(μ2-1-cyclopropyl-6-fluoro-4-oxo-7-(1-piperazinyl)-1,4-dihydroquinoline-3-carboxylato-κ2O,O′)cadmium(II) 1.5 hydrate, C62H60N6Cd2O19F2
- Crystal structure of dimethanolo-bis[μ-(2-(2-(5-(pyridin-2-yl)-1H-1,2,4-triazol-3-yl)phenoxy)benzoato)-κ5O,O′,N:N′,N′′]dicopper(II) — methanol (1/2), C46H48Cu2N8O12
- Crystal structure of poly-[tetraaqua-bis(μ4-2,5-dibenzoyl-1,4-benzenedicarboxylato-κ4O1:O2:O3:O4)-μ2-2,5-dibenzoyl-1,4-benzenedicarboxylato-k4O5,O6: O5′,O6′-didysprosium(III)] tetrahydrate C33H26O13Dy
- Crystal structure of hexaaqua-bis(3-(3-pyridin-4-yl-[1,2,4]oxadiazol-5-yl) propionato-κ3O,O′:O′)-bis(3-(3-pyridin-4-yl-[1,2,4]oxadiazol-5-yl)propionato-κO)-bis(3-(3-pyridin-4-yl-[1,2,4]oxadiazol-5-yl)propionato-κ2O,O′)dineodymium(III) octahydrate, C60H76N18O32Nd2
- Crystal structure of poly-[triaqua-(μ3-3,4,5,6-tetrafluoro-1,2-phthalato-κ4O:O′:O′′,O′′′) (2,3,4,5-tetrafluoro-benzoato-κ2O,O′) praseodymium(III)], C15H7F8O9Pr
- The crystal structure of dichlorido (1,3-dimesityl-1H-3λ4-imidazol-2-yl)(isoquinoline-κN)palladium(IV) – ethylacetate (1/1), C34H39Cl2N3O2Pd
- Crystal structure of dichlorido(1,3-bis(2,6-dimethyl-phenyl)-1H-3λ4-imidazol-2-yl)(isoquinolinyl)palladium(IV), C28H27Cl2N3Pd
- Crystal structure of 5-(4-(1H-tetrazol-5-yl)phenyl)-1H-imidazol-3-ium 7-carboxy-1,3-dioxo-1H,3H-benzo[de]isochromene-6-carboxylate monohydrate 4,5-anhydride, C24H16N6O8
- Crystal structure of poly-[diaqua-bis(μ2-2-((1H-1,2,4-triazol-5-yl)thio)acetato-κ2N:O) cadmium(II)], C8H8CdN6O6S2
- Crystal Structure of (E)-3-(4-methoxyphenyl)-1-(2,3,4-tris(benzyloxy)-6-hydroxyphenyl)prop-2-en-1-one, C37H32O6
- Structure and photochromism of 1-(1,2-dimethylindol-3-yl)-2-[2-methyl-5-(3-fluorophenyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C26H18F7NS
- Crystal structure of two-dimensional coordination polymer poly-[μ2-azido-aqua-(μ2-pyrazine-2-carboxylato-κ3O,N:N′)nickel(II)], C5H5N5O3Ni
- Crystal structure of 2-amino-5-oxo-4-(3,5-bis(trifluoromethyl)phenyl)-4H,5H-pyrano [3,2-c]chromene-3-carbonitrile, C21H10F6N2O3
- Crystal structure of 4-(5-((2-methylbenzyl)thio)-4-phenyl-4H-1,2,4-triazol-3-yl)pyridine, C21H18N4S
- Crystal structure of 5-(2-chloro-5-nitrophenyl)-3-(4-chlorophenyl)-N-ethyl-4,5-dihydro-1H-pyrazole-1-carbothioamide, C18H16Cl2N4O2S
- Crystal structure of 4-(benzofuran-2-yl)-2-(3-(4-fluorophenyl)-3,3a,4,5-tetrahydro-2H-benzo[g]indazol-2-yl)thiazole, C28H20FN3OS
- Crystal structure of bis(dicyanamido-κ1N)-tetrakis[1-benzyl-1H-1,2,4-triazole-κ1N]cobalt(II), CoC40H36N18
- Crystal structure of 1-benzyl-6-hydroxy-1,4,5,6-tetrahydropyridine-3-carbonitrile, C13H14N2O
- Crystal structure of 2-amino-7-methyl-4-(3,4-difluoro-phenyl)-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carbonitrile, C16H10F2N2O3
- The crystal structure of 4-[(benzo[1,3]dioxol-5-ylmethylene)-amino]-1,5-dimethyl-2-phenyl-1,2-dihydro-pyrazol-3-one, C19H17N3O3
- Crystal structure of 1,4-dihydro-1-phenylchromeno[4,3-c]pyrazole, C16H12N2O
- Crystal structure of N-(5-((3,5-dimethylisoxazol-4-yl)sulfonyl)quinolin-8-yl)benzamide, C21H17N3O4S
- Crystal structure of 3-amino-9-methoxy-1-phenyl-1H-benzo[f]chromene-2-carbonitrile, C21H16N2O2
- Crystal structure of 1,2-bis(4-methoxyphenyl)-2-((3-(trifluoromethyl)phenyl)amino)ethan-1-one, C23H20F3NO3
- Crystal structure of 2-amino-4-(2,4-dinitrophenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carbonitrile – ethanol (1:1), C21H16N4O8
- Crystal structure of catena-poly-[(1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-ium-1-yl)-1,4-dihydroquinoline-3-carboxylate-κ2O,O′)-(μ2-4,4′-sulfonyldibenzoato-κ4O,O′:O′′,O′′′)zinc(II)] hemihydrate, C31H27ZnFN3O9.5S
- Crystal structure of 2-(2-bromophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13BrO4
- Crystal structure of 2-(2-bromophenyl)-5-ethyl-1,3-dioxane-5-carboxylic acid, C13H15BrO4
- Crystal structure of 4-(4-((3-bromophenyl)amino)-6-(tert-butyl)-3-(2-hydroxypropan-2-yl)cinnolin-8-yl)-2-methylbut-3-yn-2-ol, C26H30BrN3O2
- Crystal structure poly-(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazolyl-κ2NN1:N2N)-(μ3-2,2′-(1,2-phenylene)diacetato-κ5-O1,O2:O2:O3,O4)cadmium(II), C22H19CdN5O4
- Crystal structure of bis(1-ethyl-6-fluoro-4-oxido-7-(piperazin-1-ium-1-yl)-1,8-naphthyridin-1-ium-3-carboxylate-κ2O,O′)copper(II) benzene-1, 4-dicarboxylate dihydrate, C38H42F2CuN8O12
- Redetermination of the crystal structure of potassium lithium molybdate monohydrate, KLiMoO4·H2O
- Crystal structure of [tris(2-benzimidazolylmethyl)amine-κ3N](isonicotinate-κO) cobalt(II) [tris(2-benzimidazolylmethyl)amine-κ3N](isonicotinic acid-κO) cobalt(II) triperchlorate, C60H51N16O16Cl3Co2
- The crystal structure of tris(μ2-1,3-bis(4,4,4-trifluoro-3-oxido-1-(oxo)but-2-en-1-yl)phenyl-κ4O,O′:O′′,O′′′)-bis(1,2-dimethoxyethane-κ2O,O′)dicerium(III), C50H38F18O16Ce2
- Crystal structure of 8-isopropyl-8-aza-bicyclo[3.2.1]octan-3-ol, C10H19NO
- Crystal structure of 2,4-dibenzoyl-N,N-dimethylbenzenamine, C22H19NO2
- The crystal structure of 2-(4-methoxyphenyl)-6,8-diphenyl-4-(phenylamino)quinazoline — acetonitrile (1/1), C35H28N4O
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of the catena-poly-[bis(μ2-1-ethyl-6-fluoro-4-oxo-7-(piperazin-1-yl-κN)-1,4-dihydroquinoline-3-carboxylato-κ2O,O′)nickel(II)] 5.5 hydrate, C32H44N6NiO11F2
- Crystal structure of catena-poly-[(μ2-2-(2-((2,6-dimethylphenyl)amino)phenyl)acetato-κ2O:O′)(μ2-2-(2-((2,6-dimethylphenyl)amino)phenyl)acetate-κ3O,O′:O′)cadmium(II)], C28H20N2Cl4O4Cd
- Crystal structure of catena-poly[dichlorido-(μ2-4-(pyridin-4-yl)-isophthalate-κ2O, O′)cadmium(II)] monohydrate, C13H11NO5Cl2Cd
- Crystal structure of poly-{[μ2-(E)-1,4-di(1H-imidazol-1-yl)but-2-ene-κ2N:N′][μ2-cyclohexane-1,4-dicarboxylato-κ4O,O′:O′′,O′′′]nickel(II)}, C18H22N4NiO4
- Crystal structure of aqua (5,5′-dicarboxy-(1,1′-biphenyl)-2,3′-dicarboxylato-κO) bis(1,10-phenanthroline-κ2N,N′)cadmium monohydrate, C40H28CdN4O10
- Crystal structure of 5-methoxy-N′-[(3Z)-5-chloro-1-(4-fluorobenzyl)-2-oxo-1,2-dihydro-3H-indol-3-ylidene]-1H-indole-2-carbohydrazide-DMSO (1/1), C25H18ClFN4O3 · C2H6OS
- Crystal structure of 5-methoxy-N′-[(3Z)-1-benzyl-5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene]-1H-indole-2-carbohydrazide-DMSO (1/1), C27H25FN4O4S
- Crystal structure of poly-[bis{μ2-N-(4-(1H-imidazol-1-yl)phenyl)-4-(1H-imidazol-1-yl)-N-phenylaniline-κ2N:N′)}-(μ2-naphthalene-2,6-dicarboxylato)-(μ4-naphthalene-2,6-dicarboxylato)dicadmium(II)], C36H25N5O4Cd
- Crystal structure of 1-(adamantan-1-yl)-3-(4-bromophenyl)thiourea, C17H21BrN2S
- Crystal structure of N′-[(1E)-(2,6-dichlorophenyl)-methylidene]adamantane-1-carbohydrazide, C18H20Cl2N2O
- Crystal structure of dichlorido{bis(2-hydroxyethyl)-5′-([2,2′:6′,2′′-terpyridin]-4′-yl)-[1,1′:3′,1′′-terphenyl]-4,4′′-dicarboxylate-κ3N,N′,N′′}zinc(II), C39H31Cl2N3O6Zn
- Crystal structure of 4,4′-(1,3,5,7-tetraoxo-3a,4,4a,5,7,7a,8,8a-octahydro-4,8-ethenopyrrolo [3,4-f]isoindole-2,6(1H,3H)-diyl)dibenzoic acid, C26H18N2O8
- Crystal structure of (E)-1-(4-(((E)-3,5-dichloro-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C17H16Cl2N2O2
- The crystal structure of diaqua-(N-(2-hydroxy-5-nitrobenzyl)iminodiacetato-κ4-N,O,O′,O′′)chromium(III) based on synchrotron data, C11H13CrN2O9
- Crystal structure of ethyl 5-amino-3-(methylthio)-1-(1-phenyl-5-(thiophen-2-yl)-1H-pyrazole-3-carbonyl)-1H-pyrazole-4-carboxylate, C21H19N5O3S2
- Crystal structure of (E)-1-(4-(((E)-3,5-dibromo-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C17H16Br2N2O2
- Crystal structure of dibromido μ-oxalato-κ2O,O′:κ2O′′,O′′′−η6-p-cymenediosmium(II), C22H28Br2O4Os2
- Crystal structure of 2-(bromomethyl)-4-(4-chlorophenyl)-1-tosylpyrrolidine, C18H19BrClNO2S2
- Crystal structure of 5-(3-fluorobenzylidene)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione; C13H11FN2O3
- Crystal structure of diethylammonium 1,3-dimethyl-2,4,6-trioxohexahydropyrimidin-5-ide, C10H19N3O3
- Crystal structure of 1,1-dimethyl-3-(2-phenylethyl)urea, C11H16N2O
- Crystal structure of 2-(4-methoxyphenyl)-1,3-thiazolo[4,5-b]pyridine, C13H10N2OS
- Crystal structure of 3-tert-butyl-7-azadioxindole, C11H14N2O2
- Crystal structure of 1-ferrocenyl-6-bromopyrene, C26H17BrFe
- Crystal structure of 3-(4-bromophenyl)-5-(4-fluorophenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide, C16H13BrFN3S
- Crystal structure of 2-amino-4-(3,5-ditrifluoromethylphenyl)-3-cyano-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo[b]pyran, C20H16F6N2O2
- The crystal structure of 2-amino-4-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13N3O4
- Crystal structure of 2-amino-4-(2, 4-dichlorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H12Cl2N2O2
- Crystal structure of bis(μ2-chlorido)-bis(di-p-tolylhydroxyphosphine-κP)-bis(di-p-tolylphosphite-κP)dipalladium(II), C56H58Cl2O4P4Pd2
- Crystal structure of diaqua-bis(2-methyl-1H-imidazole-4,5-dicarboxylato-κ2-O,N)cadmium(II) tetrahydrate, C12H22CdN4O14
- Crystal structure of aqua-(5-nitrosalicylato-κ2O,O′)-(1,10-phenanthroline-κ2N,N′)copper(II), C19H13CuN3O6
- Crystal structure of bis(4-(2-phenylpropan-2-yl)phenyl)amine, C30H31N
- Crystal structure of 2-amino-4-(3,5-difluorophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C18H16F2N2O2
- Crystal structure of 2-amino-4-(3,4,5-trifluorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H11F3N2O2
- Crystal structure of an isomeric bis[(η5:η1-6,6-di-p-tolylpentafulvene)(η5-pentamethylcyclopentadienyl)titanium(III)]-μ2,η1:η1-dinitrogen complex, C60H66N2Ti2
- Crystal structure of 3,4-dinitropyrazole, C3H2N4O4
- Crystal structure of (4-vinylpyridine-κN)triphenyl tin(IV) chloride, C25H22ClNSn
- Crystal structure of tert-butyl 2-phenylethylcarbamate, C13H19NO2
- Crystal structure of (Z)-4-((E)-(4-chlorobenzyli-dene)hydrazono)-1-p-tolylpyrrolidine-3-carbonitrile, C19H17ClN4
- Crystal structure of bis(biphenyl-2,2′-dicarboxylato-κ2O:O′)-bis(1-cyclopropyl-6-fluoro-4-oxo-7-(1-piperazinyl)-1,4-dihydroquinoline-3-carboxylato-κ2O,O′)dizinc(II)2.5 hydrate, C62H57N6Zn2O16.5F2
- Crystal structure of dichloridobis{μ2-2,2′-((1E,1′E)-(ethane-1,2-diylbis(azanylylidene))bis(methanylylidene))bis(4-chlorophenolato)-κ5O,N,N′,O′:O′}diiron(III), C32H24Cl6Fe2N4O4
- Crystal structure of 4-((4,4-dimethyl-2, 6-dioxocyclohexylidine)methylamino)-N-(3,4-dimethylisoxazol-5-yl)benzenesulfonamide, C20H23N3O5S
- Crystal structure of poly-[aqua-μ2-aqua-μ2-(4,4′-oxybis(benzoato)-κ4O,O′:O′′,O′′′)cadmium(II)], C14H12O7Cd
- Crystal structure of aqua(μ2-biphenyl-2,2′-dicarboxylato-κ3O,O′:O′′)-(μ2-1-cyclopropyl-6-fluoro-4-oxo-7-(1-piperazinyl)-1,4-dihydroquinoline-3-carboxylato-κ2O,O′)cadmium(II) 1.5 hydrate, C62H60N6Cd2O19F2
- Crystal structure of dimethanolo-bis[μ-(2-(2-(5-(pyridin-2-yl)-1H-1,2,4-triazol-3-yl)phenoxy)benzoato)-κ5O,O′,N:N′,N′′]dicopper(II) — methanol (1/2), C46H48Cu2N8O12
- Crystal structure of poly-[tetraaqua-bis(μ4-2,5-dibenzoyl-1,4-benzenedicarboxylato-κ4O1:O2:O3:O4)-μ2-2,5-dibenzoyl-1,4-benzenedicarboxylato-k4O5,O6: O5′,O6′-didysprosium(III)] tetrahydrate C33H26O13Dy
- Crystal structure of hexaaqua-bis(3-(3-pyridin-4-yl-[1,2,4]oxadiazol-5-yl) propionato-κ3O,O′:O′)-bis(3-(3-pyridin-4-yl-[1,2,4]oxadiazol-5-yl)propionato-κO)-bis(3-(3-pyridin-4-yl-[1,2,4]oxadiazol-5-yl)propionato-κ2O,O′)dineodymium(III) octahydrate, C60H76N18O32Nd2
- Crystal structure of poly-[triaqua-(μ3-3,4,5,6-tetrafluoro-1,2-phthalato-κ4O:O′:O′′,O′′′) (2,3,4,5-tetrafluoro-benzoato-κ2O,O′) praseodymium(III)], C15H7F8O9Pr
- The crystal structure of dichlorido (1,3-dimesityl-1H-3λ4-imidazol-2-yl)(isoquinoline-κN)palladium(IV) – ethylacetate (1/1), C34H39Cl2N3O2Pd
- Crystal structure of dichlorido(1,3-bis(2,6-dimethyl-phenyl)-1H-3λ4-imidazol-2-yl)(isoquinolinyl)palladium(IV), C28H27Cl2N3Pd
- Crystal structure of 5-(4-(1H-tetrazol-5-yl)phenyl)-1H-imidazol-3-ium 7-carboxy-1,3-dioxo-1H,3H-benzo[de]isochromene-6-carboxylate monohydrate 4,5-anhydride, C24H16N6O8
- Crystal structure of poly-[diaqua-bis(μ2-2-((1H-1,2,4-triazol-5-yl)thio)acetato-κ2N:O) cadmium(II)], C8H8CdN6O6S2
- Crystal Structure of (E)-3-(4-methoxyphenyl)-1-(2,3,4-tris(benzyloxy)-6-hydroxyphenyl)prop-2-en-1-one, C37H32O6
- Structure and photochromism of 1-(1,2-dimethylindol-3-yl)-2-[2-methyl-5-(3-fluorophenyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C26H18F7NS
- Crystal structure of two-dimensional coordination polymer poly-[μ2-azido-aqua-(μ2-pyrazine-2-carboxylato-κ3O,N:N′)nickel(II)], C5H5N5O3Ni
- Crystal structure of 2-amino-5-oxo-4-(3,5-bis(trifluoromethyl)phenyl)-4H,5H-pyrano [3,2-c]chromene-3-carbonitrile, C21H10F6N2O3
- Crystal structure of 4-(5-((2-methylbenzyl)thio)-4-phenyl-4H-1,2,4-triazol-3-yl)pyridine, C21H18N4S
- Crystal structure of 5-(2-chloro-5-nitrophenyl)-3-(4-chlorophenyl)-N-ethyl-4,5-dihydro-1H-pyrazole-1-carbothioamide, C18H16Cl2N4O2S
- Crystal structure of 4-(benzofuran-2-yl)-2-(3-(4-fluorophenyl)-3,3a,4,5-tetrahydro-2H-benzo[g]indazol-2-yl)thiazole, C28H20FN3OS
- Crystal structure of bis(dicyanamido-κ1N)-tetrakis[1-benzyl-1H-1,2,4-triazole-κ1N]cobalt(II), CoC40H36N18
- Crystal structure of 1-benzyl-6-hydroxy-1,4,5,6-tetrahydropyridine-3-carbonitrile, C13H14N2O
- Crystal structure of 2-amino-7-methyl-4-(3,4-difluoro-phenyl)-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carbonitrile, C16H10F2N2O3
- The crystal structure of 4-[(benzo[1,3]dioxol-5-ylmethylene)-amino]-1,5-dimethyl-2-phenyl-1,2-dihydro-pyrazol-3-one, C19H17N3O3
- Crystal structure of 1,4-dihydro-1-phenylchromeno[4,3-c]pyrazole, C16H12N2O
- Crystal structure of N-(5-((3,5-dimethylisoxazol-4-yl)sulfonyl)quinolin-8-yl)benzamide, C21H17N3O4S
- Crystal structure of 3-amino-9-methoxy-1-phenyl-1H-benzo[f]chromene-2-carbonitrile, C21H16N2O2
- Crystal structure of 1,2-bis(4-methoxyphenyl)-2-((3-(trifluoromethyl)phenyl)amino)ethan-1-one, C23H20F3NO3
- Crystal structure of 2-amino-4-(2,4-dinitrophenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carbonitrile – ethanol (1:1), C21H16N4O8
- Crystal structure of catena-poly-[(1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-ium-1-yl)-1,4-dihydroquinoline-3-carboxylate-κ2O,O′)-(μ2-4,4′-sulfonyldibenzoato-κ4O,O′:O′′,O′′′)zinc(II)] hemihydrate, C31H27ZnFN3O9.5S
- Crystal structure of 2-(2-bromophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13BrO4
- Crystal structure of 2-(2-bromophenyl)-5-ethyl-1,3-dioxane-5-carboxylic acid, C13H15BrO4
- Crystal structure of 4-(4-((3-bromophenyl)amino)-6-(tert-butyl)-3-(2-hydroxypropan-2-yl)cinnolin-8-yl)-2-methylbut-3-yn-2-ol, C26H30BrN3O2
- Crystal structure poly-(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazolyl-κ2NN1:N2N)-(μ3-2,2′-(1,2-phenylene)diacetato-κ5-O1,O2:O2:O3,O4)cadmium(II), C22H19CdN5O4
- Crystal structure of bis(1-ethyl-6-fluoro-4-oxido-7-(piperazin-1-ium-1-yl)-1,8-naphthyridin-1-ium-3-carboxylate-κ2O,O′)copper(II) benzene-1, 4-dicarboxylate dihydrate, C38H42F2CuN8O12
- Redetermination of the crystal structure of potassium lithium molybdate monohydrate, KLiMoO4·H2O
- Crystal structure of [tris(2-benzimidazolylmethyl)amine-κ3N](isonicotinate-κO) cobalt(II) [tris(2-benzimidazolylmethyl)amine-κ3N](isonicotinic acid-κO) cobalt(II) triperchlorate, C60H51N16O16Cl3Co2
- The crystal structure of tris(μ2-1,3-bis(4,4,4-trifluoro-3-oxido-1-(oxo)but-2-en-1-yl)phenyl-κ4O,O′:O′′,O′′′)-bis(1,2-dimethoxyethane-κ2O,O′)dicerium(III), C50H38F18O16Ce2
- Crystal structure of 8-isopropyl-8-aza-bicyclo[3.2.1]octan-3-ol, C10H19NO
- Crystal structure of 2,4-dibenzoyl-N,N-dimethylbenzenamine, C22H19NO2
- The crystal structure of 2-(4-methoxyphenyl)-6,8-diphenyl-4-(phenylamino)quinazoline — acetonitrile (1/1), C35H28N4O

