Abstract
C35H28N4O, triclinic, P1̅ (no. 2), a = 10.5972(11) Å, b = 11.0497(12) Å, c = 12.0241(12) Å, α = 89.499(4)°, β = 77.169(4)°, γ = 79.758(4)°, V = 1350.2(2) Å3, Z = 2, Rgt(F) = 0.0717, wRref(F2) = 0.1542, T = 173 K.
Data collection and handling.
| Crystal: | Yellow needle Size 0.36 × 0.10 × 0.08 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.8 cm−1 |
| Diffractometer, scan mode: | Bruker D8 Venture, ω (0.5°) |
| 2θmax, completeness: | 51°, >97% |
| N(hkl)measured, N(hkl)unique, Rint: | 18764, 4920, 0.040 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 4122 |
| N(param)refined: | 367 |
| Programs: | SHELX [2], Bruker programs [3], WinGX [4], Platon [5] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.2559(2) | 0.5795(2) | 0.41878(19) | 0.0200(5) |
| C2 | 0.3109(2) | 0.6347(2) | 0.3162(2) | 0.0211(5) |
| C3 | 0.4333(2) | 0.5798(2) | 0.2540(2) | 0.0239(5) |
| H3A | 0.4709 | 0.6181 | 0.1868 | 0.029* |
| C4 | 0.5051(2) | 0.4698(2) | 0.2851(2) | 0.0227(5) |
| C5 | 0.4509(2) | 0.4170(2) | 0.3844(2) | 0.0232(5) |
| H5 | 0.4976 | 0.3429 | 0.4071 | 0.028* |
| C6 | 0.3285(2) | 0.4701(2) | 0.4525(2) | 0.0204(5) |
| C7 | 0.2649(2) | 0.4201(2) | 0.5569(2) | 0.0201(5) |
| C8 | 0.0879(2) | 0.5768(2) | 0.5738(2) | 0.0209(5) |
| C9 | −0.0450(2) | 0.6344(2) | 0.6400(2) | 0.0228(5) |
| C10 | −0.1210(2) | 0.7309(2) | 0.5946(2) | 0.0251(6) |
| H10 | −0.0873 | 0.7584 | 0.5207 | 0.03* |
| C11 | −0.2437(3) | 0.7866(2) | 0.6549(2) | 0.0285(6) |
| H11 | −0.2937 | 0.8518 | 0.6225 | 0.034* |
| C12 | −0.2940(2) | 0.7474(2) | 0.7630(2) | 0.0276(6) |
| C13 | −0.2212(3) | 0.6521(3) | 0.8094(2) | 0.0303(6) |
| H13 | −0.2554 | 0.6251 | 0.8834 | 0.036* |
| C14 | −0.0974(3) | 0.5953(2) | 0.7478(2) | 0.0266(6) |
| H14 | −0.0484 | 0.5292 | 0.78 | 0.032* |
| C15 | 0.2921(2) | 0.2555(2) | 0.6982(2) | 0.0260(6) |
| C16 | 0.3383(3) | 0.1292(3) | 0.6972(3) | 0.0368(7) |
| H16 | 0.3875 | 0.0874 | 0.6282 | 0.044* |
| C17 | 0.3129(3) | 0.0647(3) | 0.7958(3) | 0.0503(9) |
| H17 | 0.3439 | −0.0215 | 0.7946 | 0.06* |
| C18 | 0.2429(3) | 0.1250(4) | 0.8955(3) | 0.0540(10) |
| H18 | 0.2255 | 0.0806 | 0.9635 | 0.065* |
| C19 | 0.1976(3) | 0.2507(4) | 0.8971(3) | 0.0487(9) |
| H19 | 0.1493 | 0.2919 | 0.9668 | 0.058* |
| C20 | 0.2214(3) | 0.3179(3) | 0.7985(2) | 0.0339(7) |
| H20 | 0.1901 | 0.404 | 0.7999 | 0.041* |
| C21 | 0.6313(2) | 0.4085(2) | 0.2102(2) | 0.0255(6) |
| C22 | 0.7232(3) | 0.3281(3) | 0.2551(3) | 0.0342(7) |
| H22 | 0.708 | 0.3173 | 0.3352 | 0.041* |
| C23 | 0.8365(3) | 0.2638(3) | 0.1843(3) | 0.0491(8) |
| H23 | 0.898 | 0.2089 | 0.2159 | 0.059* |
| C24 | 0.8600(3) | 0.2795(3) | 0.0676(3) | 0.0519(9) |
| H24 | 0.9356 | 0.2329 | 0.0186 | 0.062* |
| C25 | 0.7729(3) | 0.3635(3) | 0.0229(3) | 0.0474(8) |
| H25 | 0.7907 | 0.3772 | −0.0567 | 0.057* |
| C26 | 0.6599(3) | 0.4276(3) | 0.0933(2) | 0.0355(7) |
| H26 | 0.601 | 0.4853 | 0.0615 | 0.043* |
| C27 | 0.2408(2) | 0.7480(2) | 0.27376(19) | 0.0208(5) |
| C28 | 0.3101(3) | 0.8401(2) | 0.2293(2) | 0.0260(6) |
| H28 | 0.4008 | 0.8315 | 0.2297 | 0.031* |
| C29 | 0.2481(3) | 0.9438(3) | 0.1845(2) | 0.0354(7) |
| H29 | 0.2965 | 1.0059 | 0.1544 | 0.043* |
| C30 | 0.1166(3) | 0.9577(3) | 0.1834(2) | 0.0397(7) |
| H30 | 0.0744 | 1.0291 | 0.1526 | 0.048* |
| C31 | 0.0464(3) | 0.8675(3) | 0.2270(2) | 0.0337(7) |
| H31 | −0.0442 | 0.8769 | 0.2261 | 0.04* |
| C32 | 0.1074(2) | 0.7634(2) | 0.2720(2) | 0.0258(6) |
| H32 | 0.0582 | 0.7018 | 0.3021 | 0.031* |
| C33 | −0.4688(3) | 0.7846(3) | 0.9295(2) | 0.0446(8) |
| H33A | −0.474 | 0.697 | 0.9352 | 0.067* |
| H33B | −0.557 | 0.834 | 0.9551 | 0.067* |
| H33C | −0.4114 | 0.8049 | 0.9776 | 0.067* |
| N1 | 0.13625(19) | 0.63455(18) | 0.48237(16) | 0.0208(4) |
| N2 | 0.1464(2) | 0.47145(18) | 0.61433(16) | 0.0223(5) |
| N3 | 0.3293(2) | 0.3161(2) | 0.59496(18) | 0.0260(5) |
| O1 | −0.41672(18) | 0.81029(19) | 0.81495(17) | 0.0391(5) |
| H3 | 0.406(3) | 0.275(3) | 0.555(2) | 0.032(8)* |
| C1S | 0.7064(3) | 0.1106(2) | 0.4862(2) | 0.0327(6) |
| C2S | 0.8446(3) | 0.0641(3) | 0.4805(3) | 0.0376(7) |
| H2S1 | 0.8925 | 0.1329 | 0.4733 | 0.056* |
| H2S2 | 0.8805 | 0.0075 | 0.4142 | 0.056* |
| H2S3 | 0.8543 | 0.0203 | 0.5502 | 0.056* |
| N1S | 0.5979(3) | 0.1485(2) | 0.4909(2) | 0.0453(7) |
Source of material
A mixture of the previously prepared 2-(4-methoxyphenyl)-6,8-diphenylquinazolin-4(3H)-one[1] (0.40 g, 1.06 mmol) and POCl3 (15 mL) was heated at 120 °C for 3 h. The mixture was allowed to cool to room temperature and then quenched with a mixture of ice and ammonia. The resulting precipitate was filtered and washed with water followed by ice-cold ethanol. The product was dried in an oven to afford 4-chloro-2-(4-methoxyphenyl)-6,8-diphenylquinazoline. The 4-chloro-2-(4-methoxyphenyl)-6,8-diphenylquinazoline (0.40 g, 0.9 mmol) was in turn reacted with aniline (15 mL) under reflux at 120 °C for 4 h to afford the title compound (0.4 g, 95%), mp. 176–178 °C; νmax (ATR) 754, 804, 831, 1034, 1156, 1213, 1247, 1471, 1504, 1562, 1597, 3436 cm−1; 1H NMR (500 MHz, DMSO-d6) 3.81 (s, 3H), 7.03 (d, J 8.7Hz, 2H), 7.20 (t, J 7.5 Hz, 1H), 7.45–7.56 (m, 8H), 7.80 (d, J 8.7 Hz, 2H), 7.96–7.99 (m, 4H), 8.17 (d, J 1.8 Hz, 1H), 8.28 (d, J 7.5 Hz, 2H), 8.96 (d, J 1.8 Hz, 1H), 10.02 (s, 1H); 13C NMR (125 MHz, DMSO-d6) 55.7, 114.3, 115.0, 120.5, 122.9, 124.2, 127.7, 127.8, 128.2, 128.4, 129.0(2xC), 129.5, 130.0, 131.4, 131.5, 132.6, 137.3, 139.2, 139.6, 139.8, 139.9, 147.9, 158.8, 161.6; m/z 480 (MH+). HRMS (ESI): found: 480.2071. C33H26N3O requires 480.1998. Crystals were obtained by recrystalization from acetonitrile.
Experimental details
The crystal structure was solved using direct methods [3]. Hydrogen atoms were positioned geometrically and allowed to ride on their respective parent atoms with d(C—H) = 0.95 Å and Uiso(H) = 1.2Ueq(C).
Discussion
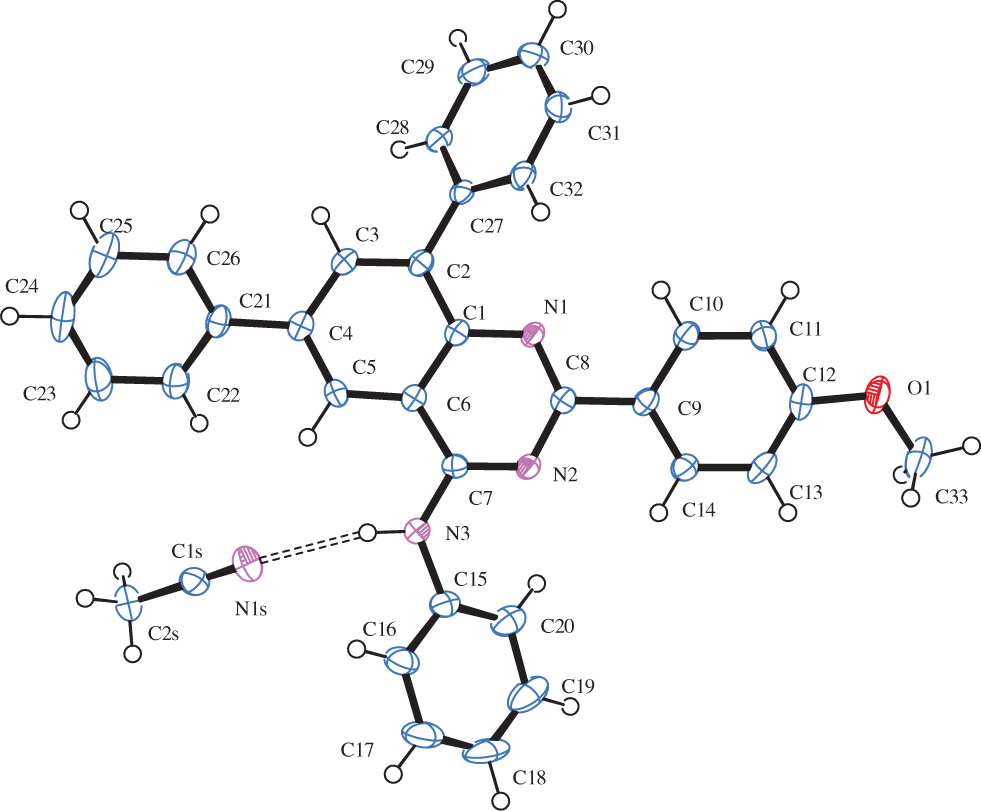
4-Amino–substituted quinazolines constitute an important class of compounds with a wide-ranging applications in the field of medicine and materials [6]. Continued interest in the synthesis of quinazoline derivatives substituted with a primary amino group at the 4-position stems from their importance as selective inhibitors of the epidermal growth factor receptor (EGFR) tyrosine kinase phosphorylation resulting from competitive binding at the ATP site [7]. In pharmaceutical industry, it is not only important to obtain the active ingredients in pure form, but also to have the knowledge of all possible forms of a drug in the solid state, because different forms can influence the physicochemical properties [8]. In this structure, the solvate (acetonitrile) is hydrogen bonded to the N—H group N(3)⋯N(1S) 3.113(3) Å; < N(3)H(3)N(1S) 167° (cf. the figure). In the crystal, the compound adopts the anti-orientation (anti-5) of the 4-phenylamino group with respect to the quinazoline 5-position to minimize steric interaction with Cquinaz-N and N—Ph torsion angles N(2)—C(7)—N(3)—C(15) = −6.2° and C(20)—C(15)—N(3)—C(7) = −31.9°, respectively. Moreover, the anti-arrangement of the N-phenyl ring enables hydrogen bonding. The 8-aryl ring, on the other hand, is strongly deformed out of plane of the quinazoline moiety (average torsion angle ca. −43°) to avoid steric interaction between its ortho hydrogen atoms and quinazoline H-7. The slight twist of the 6-aryl ring from co-planarity minimizes steric interaction with hydrogen atoms on C3 (H-7) and C5 (H-5). The same is true for the 2-aryl moiety.
Acknowledgements:
The authors are grateful to the University of South Africa and the National Research Foundation for financial assistance.
References
1. Mphahlele, M. J.; Maluleka, M. M.; Khoza, T. A.: 2-Aryl-6,8-dibromo-2,3-dihydroquinazolin-4(1H)-ones as substrates for the synthesis of 2,6,8-triarylquinazolin-4-ones. Bull. Chem. Soc. Ethiop. 28 (2014) 81–90.10.4314/bcse.v28i1.10Search in Google Scholar
2. Bruker: SAINT-Plus and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA, (2004).Search in Google Scholar
3. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
4. Farrugia, L. J.: WinGX suite for small-molecule single-crystal crystallography. J. Appl. Cryst. 32 (1999) 837–838.10.1107/S0021889899006020Search in Google Scholar
5. Spek, A. L.: Structure validation in chemical crystallography. Acta Crystallogr. D65 (2009) 148–155.10.1107/S090744490804362XSearch in Google Scholar PubMed PubMed Central
6. Wilson, J. N.; Liu, W.; Brown, A. S.; Landgraf, R.: Binding-induced, turn-on fluorescence of the EGFR/ERBB kinase inhibitor, lapatinib. Org. Biomol. Chem. 13 (2015) 5006–5011.10.1039/C5OB00239GSearch in Google Scholar
7. Abouzid, K.; Shouman, S.: Design, synthesis and in vitro antitumor activity of 4-aminoquinoline and 4-aminoquinazoline derivatives targeting EGFR tyrosine kinase. Bioorg. Med. Chem. 16 (2008) 7543–7551.10.1016/j.bmc.2008.07.038Search in Google Scholar PubMed
8. Schultheiss, N.; Newman, A.: Pharmaceutical co-crystals and their physicochemical properties. Cryst. Growth Des. 9 (2009) 2950–2967.10.1021/cg900129fSearch in Google Scholar PubMed PubMed Central
©2016 Marole M. Maluleka et al., published by De Gruyter.
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of the catena-poly-[bis(μ2-1-ethyl-6-fluoro-4-oxo-7-(piperazin-1-yl-κN)-1,4-dihydroquinoline-3-carboxylato-κ2O,O′)nickel(II)] 5.5 hydrate, C32H44N6NiO11F2
- Crystal structure of catena-poly-[(μ2-2-(2-((2,6-dimethylphenyl)amino)phenyl)acetato-κ2O:O′)(μ2-2-(2-((2,6-dimethylphenyl)amino)phenyl)acetate-κ3O,O′:O′)cadmium(II)], C28H20N2Cl4O4Cd
- Crystal structure of catena-poly[dichlorido-(μ2-4-(pyridin-4-yl)-isophthalate-κ2O, O′)cadmium(II)] monohydrate, C13H11NO5Cl2Cd
- Crystal structure of poly-{[μ2-(E)-1,4-di(1H-imidazol-1-yl)but-2-ene-κ2N:N′][μ2-cyclohexane-1,4-dicarboxylato-κ4O,O′:O′′,O′′′]nickel(II)}, C18H22N4NiO4
- Crystal structure of aqua (5,5′-dicarboxy-(1,1′-biphenyl)-2,3′-dicarboxylato-κO) bis(1,10-phenanthroline-κ2N,N′)cadmium monohydrate, C40H28CdN4O10
- Crystal structure of 5-methoxy-N′-[(3Z)-5-chloro-1-(4-fluorobenzyl)-2-oxo-1,2-dihydro-3H-indol-3-ylidene]-1H-indole-2-carbohydrazide-DMSO (1/1), C25H18ClFN4O3 · C2H6OS
- Crystal structure of 5-methoxy-N′-[(3Z)-1-benzyl-5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene]-1H-indole-2-carbohydrazide-DMSO (1/1), C27H25FN4O4S
- Crystal structure of poly-[bis{μ2-N-(4-(1H-imidazol-1-yl)phenyl)-4-(1H-imidazol-1-yl)-N-phenylaniline-κ2N:N′)}-(μ2-naphthalene-2,6-dicarboxylato)-(μ4-naphthalene-2,6-dicarboxylato)dicadmium(II)], C36H25N5O4Cd
- Crystal structure of 1-(adamantan-1-yl)-3-(4-bromophenyl)thiourea, C17H21BrN2S
- Crystal structure of N′-[(1E)-(2,6-dichlorophenyl)-methylidene]adamantane-1-carbohydrazide, C18H20Cl2N2O
- Crystal structure of dichlorido{bis(2-hydroxyethyl)-5′-([2,2′:6′,2′′-terpyridin]-4′-yl)-[1,1′:3′,1′′-terphenyl]-4,4′′-dicarboxylate-κ3N,N′,N′′}zinc(II), C39H31Cl2N3O6Zn
- Crystal structure of 4,4′-(1,3,5,7-tetraoxo-3a,4,4a,5,7,7a,8,8a-octahydro-4,8-ethenopyrrolo [3,4-f]isoindole-2,6(1H,3H)-diyl)dibenzoic acid, C26H18N2O8
- Crystal structure of (E)-1-(4-(((E)-3,5-dichloro-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C17H16Cl2N2O2
- The crystal structure of diaqua-(N-(2-hydroxy-5-nitrobenzyl)iminodiacetato-κ4-N,O,O′,O′′)chromium(III) based on synchrotron data, C11H13CrN2O9
- Crystal structure of ethyl 5-amino-3-(methylthio)-1-(1-phenyl-5-(thiophen-2-yl)-1H-pyrazole-3-carbonyl)-1H-pyrazole-4-carboxylate, C21H19N5O3S2
- Crystal structure of (E)-1-(4-(((E)-3,5-dibromo-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C17H16Br2N2O2
- Crystal structure of dibromido μ-oxalato-κ2O,O′:κ2O′′,O′′′−η6-p-cymenediosmium(II), C22H28Br2O4Os2
- Crystal structure of 2-(bromomethyl)-4-(4-chlorophenyl)-1-tosylpyrrolidine, C18H19BrClNO2S2
- Crystal structure of 5-(3-fluorobenzylidene)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione; C13H11FN2O3
- Crystal structure of diethylammonium 1,3-dimethyl-2,4,6-trioxohexahydropyrimidin-5-ide, C10H19N3O3
- Crystal structure of 1,1-dimethyl-3-(2-phenylethyl)urea, C11H16N2O
- Crystal structure of 2-(4-methoxyphenyl)-1,3-thiazolo[4,5-b]pyridine, C13H10N2OS
- Crystal structure of 3-tert-butyl-7-azadioxindole, C11H14N2O2
- Crystal structure of 1-ferrocenyl-6-bromopyrene, C26H17BrFe
- Crystal structure of 3-(4-bromophenyl)-5-(4-fluorophenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide, C16H13BrFN3S
- Crystal structure of 2-amino-4-(3,5-ditrifluoromethylphenyl)-3-cyano-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo[b]pyran, C20H16F6N2O2
- The crystal structure of 2-amino-4-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13N3O4
- Crystal structure of 2-amino-4-(2, 4-dichlorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H12Cl2N2O2
- Crystal structure of bis(μ2-chlorido)-bis(di-p-tolylhydroxyphosphine-κP)-bis(di-p-tolylphosphite-κP)dipalladium(II), C56H58Cl2O4P4Pd2
- Crystal structure of diaqua-bis(2-methyl-1H-imidazole-4,5-dicarboxylato-κ2-O,N)cadmium(II) tetrahydrate, C12H22CdN4O14
- Crystal structure of aqua-(5-nitrosalicylato-κ2O,O′)-(1,10-phenanthroline-κ2N,N′)copper(II), C19H13CuN3O6
- Crystal structure of bis(4-(2-phenylpropan-2-yl)phenyl)amine, C30H31N
- Crystal structure of 2-amino-4-(3,5-difluorophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C18H16F2N2O2
- Crystal structure of 2-amino-4-(3,4,5-trifluorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H11F3N2O2
- Crystal structure of an isomeric bis[(η5:η1-6,6-di-p-tolylpentafulvene)(η5-pentamethylcyclopentadienyl)titanium(III)]-μ2,η1:η1-dinitrogen complex, C60H66N2Ti2
- Crystal structure of 3,4-dinitropyrazole, C3H2N4O4
- Crystal structure of (4-vinylpyridine-κN)triphenyl tin(IV) chloride, C25H22ClNSn
- Crystal structure of tert-butyl 2-phenylethylcarbamate, C13H19NO2
- Crystal structure of (Z)-4-((E)-(4-chlorobenzyli-dene)hydrazono)-1-p-tolylpyrrolidine-3-carbonitrile, C19H17ClN4
- Crystal structure of bis(biphenyl-2,2′-dicarboxylato-κ2O:O′)-bis(1-cyclopropyl-6-fluoro-4-oxo-7-(1-piperazinyl)-1,4-dihydroquinoline-3-carboxylato-κ2O,O′)dizinc(II)2.5 hydrate, C62H57N6Zn2O16.5F2
- Crystal structure of dichloridobis{μ2-2,2′-((1E,1′E)-(ethane-1,2-diylbis(azanylylidene))bis(methanylylidene))bis(4-chlorophenolato)-κ5O,N,N′,O′:O′}diiron(III), C32H24Cl6Fe2N4O4
- Crystal structure of 4-((4,4-dimethyl-2, 6-dioxocyclohexylidine)methylamino)-N-(3,4-dimethylisoxazol-5-yl)benzenesulfonamide, C20H23N3O5S
- Crystal structure of poly-[aqua-μ2-aqua-μ2-(4,4′-oxybis(benzoato)-κ4O,O′:O′′,O′′′)cadmium(II)], C14H12O7Cd
- Crystal structure of aqua(μ2-biphenyl-2,2′-dicarboxylato-κ3O,O′:O′′)-(μ2-1-cyclopropyl-6-fluoro-4-oxo-7-(1-piperazinyl)-1,4-dihydroquinoline-3-carboxylato-κ2O,O′)cadmium(II) 1.5 hydrate, C62H60N6Cd2O19F2
- Crystal structure of dimethanolo-bis[μ-(2-(2-(5-(pyridin-2-yl)-1H-1,2,4-triazol-3-yl)phenoxy)benzoato)-κ5O,O′,N:N′,N′′]dicopper(II) — methanol (1/2), C46H48Cu2N8O12
- Crystal structure of poly-[tetraaqua-bis(μ4-2,5-dibenzoyl-1,4-benzenedicarboxylato-κ4O1:O2:O3:O4)-μ2-2,5-dibenzoyl-1,4-benzenedicarboxylato-k4O5,O6: O5′,O6′-didysprosium(III)] tetrahydrate C33H26O13Dy
- Crystal structure of hexaaqua-bis(3-(3-pyridin-4-yl-[1,2,4]oxadiazol-5-yl) propionato-κ3O,O′:O′)-bis(3-(3-pyridin-4-yl-[1,2,4]oxadiazol-5-yl)propionato-κO)-bis(3-(3-pyridin-4-yl-[1,2,4]oxadiazol-5-yl)propionato-κ2O,O′)dineodymium(III) octahydrate, C60H76N18O32Nd2
- Crystal structure of poly-[triaqua-(μ3-3,4,5,6-tetrafluoro-1,2-phthalato-κ4O:O′:O′′,O′′′) (2,3,4,5-tetrafluoro-benzoato-κ2O,O′) praseodymium(III)], C15H7F8O9Pr
- The crystal structure of dichlorido (1,3-dimesityl-1H-3λ4-imidazol-2-yl)(isoquinoline-κN)palladium(IV) – ethylacetate (1/1), C34H39Cl2N3O2Pd
- Crystal structure of dichlorido(1,3-bis(2,6-dimethyl-phenyl)-1H-3λ4-imidazol-2-yl)(isoquinolinyl)palladium(IV), C28H27Cl2N3Pd
- Crystal structure of 5-(4-(1H-tetrazol-5-yl)phenyl)-1H-imidazol-3-ium 7-carboxy-1,3-dioxo-1H,3H-benzo[de]isochromene-6-carboxylate monohydrate 4,5-anhydride, C24H16N6O8
- Crystal structure of poly-[diaqua-bis(μ2-2-((1H-1,2,4-triazol-5-yl)thio)acetato-κ2N:O) cadmium(II)], C8H8CdN6O6S2
- Crystal Structure of (E)-3-(4-methoxyphenyl)-1-(2,3,4-tris(benzyloxy)-6-hydroxyphenyl)prop-2-en-1-one, C37H32O6
- Structure and photochromism of 1-(1,2-dimethylindol-3-yl)-2-[2-methyl-5-(3-fluorophenyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C26H18F7NS
- Crystal structure of two-dimensional coordination polymer poly-[μ2-azido-aqua-(μ2-pyrazine-2-carboxylato-κ3O,N:N′)nickel(II)], C5H5N5O3Ni
- Crystal structure of 2-amino-5-oxo-4-(3,5-bis(trifluoromethyl)phenyl)-4H,5H-pyrano [3,2-c]chromene-3-carbonitrile, C21H10F6N2O3
- Crystal structure of 4-(5-((2-methylbenzyl)thio)-4-phenyl-4H-1,2,4-triazol-3-yl)pyridine, C21H18N4S
- Crystal structure of 5-(2-chloro-5-nitrophenyl)-3-(4-chlorophenyl)-N-ethyl-4,5-dihydro-1H-pyrazole-1-carbothioamide, C18H16Cl2N4O2S
- Crystal structure of 4-(benzofuran-2-yl)-2-(3-(4-fluorophenyl)-3,3a,4,5-tetrahydro-2H-benzo[g]indazol-2-yl)thiazole, C28H20FN3OS
- Crystal structure of bis(dicyanamido-κ1N)-tetrakis[1-benzyl-1H-1,2,4-triazole-κ1N]cobalt(II), CoC40H36N18
- Crystal structure of 1-benzyl-6-hydroxy-1,4,5,6-tetrahydropyridine-3-carbonitrile, C13H14N2O
- Crystal structure of 2-amino-7-methyl-4-(3,4-difluoro-phenyl)-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carbonitrile, C16H10F2N2O3
- The crystal structure of 4-[(benzo[1,3]dioxol-5-ylmethylene)-amino]-1,5-dimethyl-2-phenyl-1,2-dihydro-pyrazol-3-one, C19H17N3O3
- Crystal structure of 1,4-dihydro-1-phenylchromeno[4,3-c]pyrazole, C16H12N2O
- Crystal structure of N-(5-((3,5-dimethylisoxazol-4-yl)sulfonyl)quinolin-8-yl)benzamide, C21H17N3O4S
- Crystal structure of 3-amino-9-methoxy-1-phenyl-1H-benzo[f]chromene-2-carbonitrile, C21H16N2O2
- Crystal structure of 1,2-bis(4-methoxyphenyl)-2-((3-(trifluoromethyl)phenyl)amino)ethan-1-one, C23H20F3NO3
- Crystal structure of 2-amino-4-(2,4-dinitrophenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carbonitrile – ethanol (1:1), C21H16N4O8
- Crystal structure of catena-poly-[(1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-ium-1-yl)-1,4-dihydroquinoline-3-carboxylate-κ2O,O′)-(μ2-4,4′-sulfonyldibenzoato-κ4O,O′:O′′,O′′′)zinc(II)] hemihydrate, C31H27ZnFN3O9.5S
- Crystal structure of 2-(2-bromophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13BrO4
- Crystal structure of 2-(2-bromophenyl)-5-ethyl-1,3-dioxane-5-carboxylic acid, C13H15BrO4
- Crystal structure of 4-(4-((3-bromophenyl)amino)-6-(tert-butyl)-3-(2-hydroxypropan-2-yl)cinnolin-8-yl)-2-methylbut-3-yn-2-ol, C26H30BrN3O2
- Crystal structure poly-(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazolyl-κ2NN1:N2N)-(μ3-2,2′-(1,2-phenylene)diacetato-κ5-O1,O2:O2:O3,O4)cadmium(II), C22H19CdN5O4
- Crystal structure of bis(1-ethyl-6-fluoro-4-oxido-7-(piperazin-1-ium-1-yl)-1,8-naphthyridin-1-ium-3-carboxylate-κ2O,O′)copper(II) benzene-1, 4-dicarboxylate dihydrate, C38H42F2CuN8O12
- Redetermination of the crystal structure of potassium lithium molybdate monohydrate, KLiMoO4·H2O
- Crystal structure of [tris(2-benzimidazolylmethyl)amine-κ3N](isonicotinate-κO) cobalt(II) [tris(2-benzimidazolylmethyl)amine-κ3N](isonicotinic acid-κO) cobalt(II) triperchlorate, C60H51N16O16Cl3Co2
- The crystal structure of tris(μ2-1,3-bis(4,4,4-trifluoro-3-oxido-1-(oxo)but-2-en-1-yl)phenyl-κ4O,O′:O′′,O′′′)-bis(1,2-dimethoxyethane-κ2O,O′)dicerium(III), C50H38F18O16Ce2
- Crystal structure of 8-isopropyl-8-aza-bicyclo[3.2.1]octan-3-ol, C10H19NO
- Crystal structure of 2,4-dibenzoyl-N,N-dimethylbenzenamine, C22H19NO2
- The crystal structure of 2-(4-methoxyphenyl)-6,8-diphenyl-4-(phenylamino)quinazoline — acetonitrile (1/1), C35H28N4O
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of the catena-poly-[bis(μ2-1-ethyl-6-fluoro-4-oxo-7-(piperazin-1-yl-κN)-1,4-dihydroquinoline-3-carboxylato-κ2O,O′)nickel(II)] 5.5 hydrate, C32H44N6NiO11F2
- Crystal structure of catena-poly-[(μ2-2-(2-((2,6-dimethylphenyl)amino)phenyl)acetato-κ2O:O′)(μ2-2-(2-((2,6-dimethylphenyl)amino)phenyl)acetate-κ3O,O′:O′)cadmium(II)], C28H20N2Cl4O4Cd
- Crystal structure of catena-poly[dichlorido-(μ2-4-(pyridin-4-yl)-isophthalate-κ2O, O′)cadmium(II)] monohydrate, C13H11NO5Cl2Cd
- Crystal structure of poly-{[μ2-(E)-1,4-di(1H-imidazol-1-yl)but-2-ene-κ2N:N′][μ2-cyclohexane-1,4-dicarboxylato-κ4O,O′:O′′,O′′′]nickel(II)}, C18H22N4NiO4
- Crystal structure of aqua (5,5′-dicarboxy-(1,1′-biphenyl)-2,3′-dicarboxylato-κO) bis(1,10-phenanthroline-κ2N,N′)cadmium monohydrate, C40H28CdN4O10
- Crystal structure of 5-methoxy-N′-[(3Z)-5-chloro-1-(4-fluorobenzyl)-2-oxo-1,2-dihydro-3H-indol-3-ylidene]-1H-indole-2-carbohydrazide-DMSO (1/1), C25H18ClFN4O3 · C2H6OS
- Crystal structure of 5-methoxy-N′-[(3Z)-1-benzyl-5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene]-1H-indole-2-carbohydrazide-DMSO (1/1), C27H25FN4O4S
- Crystal structure of poly-[bis{μ2-N-(4-(1H-imidazol-1-yl)phenyl)-4-(1H-imidazol-1-yl)-N-phenylaniline-κ2N:N′)}-(μ2-naphthalene-2,6-dicarboxylato)-(μ4-naphthalene-2,6-dicarboxylato)dicadmium(II)], C36H25N5O4Cd
- Crystal structure of 1-(adamantan-1-yl)-3-(4-bromophenyl)thiourea, C17H21BrN2S
- Crystal structure of N′-[(1E)-(2,6-dichlorophenyl)-methylidene]adamantane-1-carbohydrazide, C18H20Cl2N2O
- Crystal structure of dichlorido{bis(2-hydroxyethyl)-5′-([2,2′:6′,2′′-terpyridin]-4′-yl)-[1,1′:3′,1′′-terphenyl]-4,4′′-dicarboxylate-κ3N,N′,N′′}zinc(II), C39H31Cl2N3O6Zn
- Crystal structure of 4,4′-(1,3,5,7-tetraoxo-3a,4,4a,5,7,7a,8,8a-octahydro-4,8-ethenopyrrolo [3,4-f]isoindole-2,6(1H,3H)-diyl)dibenzoic acid, C26H18N2O8
- Crystal structure of (E)-1-(4-(((E)-3,5-dichloro-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C17H16Cl2N2O2
- The crystal structure of diaqua-(N-(2-hydroxy-5-nitrobenzyl)iminodiacetato-κ4-N,O,O′,O′′)chromium(III) based on synchrotron data, C11H13CrN2O9
- Crystal structure of ethyl 5-amino-3-(methylthio)-1-(1-phenyl-5-(thiophen-2-yl)-1H-pyrazole-3-carbonyl)-1H-pyrazole-4-carboxylate, C21H19N5O3S2
- Crystal structure of (E)-1-(4-(((E)-3,5-dibromo-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C17H16Br2N2O2
- Crystal structure of dibromido μ-oxalato-κ2O,O′:κ2O′′,O′′′−η6-p-cymenediosmium(II), C22H28Br2O4Os2
- Crystal structure of 2-(bromomethyl)-4-(4-chlorophenyl)-1-tosylpyrrolidine, C18H19BrClNO2S2
- Crystal structure of 5-(3-fluorobenzylidene)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione; C13H11FN2O3
- Crystal structure of diethylammonium 1,3-dimethyl-2,4,6-trioxohexahydropyrimidin-5-ide, C10H19N3O3
- Crystal structure of 1,1-dimethyl-3-(2-phenylethyl)urea, C11H16N2O
- Crystal structure of 2-(4-methoxyphenyl)-1,3-thiazolo[4,5-b]pyridine, C13H10N2OS
- Crystal structure of 3-tert-butyl-7-azadioxindole, C11H14N2O2
- Crystal structure of 1-ferrocenyl-6-bromopyrene, C26H17BrFe
- Crystal structure of 3-(4-bromophenyl)-5-(4-fluorophenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide, C16H13BrFN3S
- Crystal structure of 2-amino-4-(3,5-ditrifluoromethylphenyl)-3-cyano-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo[b]pyran, C20H16F6N2O2
- The crystal structure of 2-amino-4-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13N3O4
- Crystal structure of 2-amino-4-(2, 4-dichlorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H12Cl2N2O2
- Crystal structure of bis(μ2-chlorido)-bis(di-p-tolylhydroxyphosphine-κP)-bis(di-p-tolylphosphite-κP)dipalladium(II), C56H58Cl2O4P4Pd2
- Crystal structure of diaqua-bis(2-methyl-1H-imidazole-4,5-dicarboxylato-κ2-O,N)cadmium(II) tetrahydrate, C12H22CdN4O14
- Crystal structure of aqua-(5-nitrosalicylato-κ2O,O′)-(1,10-phenanthroline-κ2N,N′)copper(II), C19H13CuN3O6
- Crystal structure of bis(4-(2-phenylpropan-2-yl)phenyl)amine, C30H31N
- Crystal structure of 2-amino-4-(3,5-difluorophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C18H16F2N2O2
- Crystal structure of 2-amino-4-(3,4,5-trifluorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H11F3N2O2
- Crystal structure of an isomeric bis[(η5:η1-6,6-di-p-tolylpentafulvene)(η5-pentamethylcyclopentadienyl)titanium(III)]-μ2,η1:η1-dinitrogen complex, C60H66N2Ti2
- Crystal structure of 3,4-dinitropyrazole, C3H2N4O4
- Crystal structure of (4-vinylpyridine-κN)triphenyl tin(IV) chloride, C25H22ClNSn
- Crystal structure of tert-butyl 2-phenylethylcarbamate, C13H19NO2
- Crystal structure of (Z)-4-((E)-(4-chlorobenzyli-dene)hydrazono)-1-p-tolylpyrrolidine-3-carbonitrile, C19H17ClN4
- Crystal structure of bis(biphenyl-2,2′-dicarboxylato-κ2O:O′)-bis(1-cyclopropyl-6-fluoro-4-oxo-7-(1-piperazinyl)-1,4-dihydroquinoline-3-carboxylato-κ2O,O′)dizinc(II)2.5 hydrate, C62H57N6Zn2O16.5F2
- Crystal structure of dichloridobis{μ2-2,2′-((1E,1′E)-(ethane-1,2-diylbis(azanylylidene))bis(methanylylidene))bis(4-chlorophenolato)-κ5O,N,N′,O′:O′}diiron(III), C32H24Cl6Fe2N4O4
- Crystal structure of 4-((4,4-dimethyl-2, 6-dioxocyclohexylidine)methylamino)-N-(3,4-dimethylisoxazol-5-yl)benzenesulfonamide, C20H23N3O5S
- Crystal structure of poly-[aqua-μ2-aqua-μ2-(4,4′-oxybis(benzoato)-κ4O,O′:O′′,O′′′)cadmium(II)], C14H12O7Cd
- Crystal structure of aqua(μ2-biphenyl-2,2′-dicarboxylato-κ3O,O′:O′′)-(μ2-1-cyclopropyl-6-fluoro-4-oxo-7-(1-piperazinyl)-1,4-dihydroquinoline-3-carboxylato-κ2O,O′)cadmium(II) 1.5 hydrate, C62H60N6Cd2O19F2
- Crystal structure of dimethanolo-bis[μ-(2-(2-(5-(pyridin-2-yl)-1H-1,2,4-triazol-3-yl)phenoxy)benzoato)-κ5O,O′,N:N′,N′′]dicopper(II) — methanol (1/2), C46H48Cu2N8O12
- Crystal structure of poly-[tetraaqua-bis(μ4-2,5-dibenzoyl-1,4-benzenedicarboxylato-κ4O1:O2:O3:O4)-μ2-2,5-dibenzoyl-1,4-benzenedicarboxylato-k4O5,O6: O5′,O6′-didysprosium(III)] tetrahydrate C33H26O13Dy
- Crystal structure of hexaaqua-bis(3-(3-pyridin-4-yl-[1,2,4]oxadiazol-5-yl) propionato-κ3O,O′:O′)-bis(3-(3-pyridin-4-yl-[1,2,4]oxadiazol-5-yl)propionato-κO)-bis(3-(3-pyridin-4-yl-[1,2,4]oxadiazol-5-yl)propionato-κ2O,O′)dineodymium(III) octahydrate, C60H76N18O32Nd2
- Crystal structure of poly-[triaqua-(μ3-3,4,5,6-tetrafluoro-1,2-phthalato-κ4O:O′:O′′,O′′′) (2,3,4,5-tetrafluoro-benzoato-κ2O,O′) praseodymium(III)], C15H7F8O9Pr
- The crystal structure of dichlorido (1,3-dimesityl-1H-3λ4-imidazol-2-yl)(isoquinoline-κN)palladium(IV) – ethylacetate (1/1), C34H39Cl2N3O2Pd
- Crystal structure of dichlorido(1,3-bis(2,6-dimethyl-phenyl)-1H-3λ4-imidazol-2-yl)(isoquinolinyl)palladium(IV), C28H27Cl2N3Pd
- Crystal structure of 5-(4-(1H-tetrazol-5-yl)phenyl)-1H-imidazol-3-ium 7-carboxy-1,3-dioxo-1H,3H-benzo[de]isochromene-6-carboxylate monohydrate 4,5-anhydride, C24H16N6O8
- Crystal structure of poly-[diaqua-bis(μ2-2-((1H-1,2,4-triazol-5-yl)thio)acetato-κ2N:O) cadmium(II)], C8H8CdN6O6S2
- Crystal Structure of (E)-3-(4-methoxyphenyl)-1-(2,3,4-tris(benzyloxy)-6-hydroxyphenyl)prop-2-en-1-one, C37H32O6
- Structure and photochromism of 1-(1,2-dimethylindol-3-yl)-2-[2-methyl-5-(3-fluorophenyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C26H18F7NS
- Crystal structure of two-dimensional coordination polymer poly-[μ2-azido-aqua-(μ2-pyrazine-2-carboxylato-κ3O,N:N′)nickel(II)], C5H5N5O3Ni
- Crystal structure of 2-amino-5-oxo-4-(3,5-bis(trifluoromethyl)phenyl)-4H,5H-pyrano [3,2-c]chromene-3-carbonitrile, C21H10F6N2O3
- Crystal structure of 4-(5-((2-methylbenzyl)thio)-4-phenyl-4H-1,2,4-triazol-3-yl)pyridine, C21H18N4S
- Crystal structure of 5-(2-chloro-5-nitrophenyl)-3-(4-chlorophenyl)-N-ethyl-4,5-dihydro-1H-pyrazole-1-carbothioamide, C18H16Cl2N4O2S
- Crystal structure of 4-(benzofuran-2-yl)-2-(3-(4-fluorophenyl)-3,3a,4,5-tetrahydro-2H-benzo[g]indazol-2-yl)thiazole, C28H20FN3OS
- Crystal structure of bis(dicyanamido-κ1N)-tetrakis[1-benzyl-1H-1,2,4-triazole-κ1N]cobalt(II), CoC40H36N18
- Crystal structure of 1-benzyl-6-hydroxy-1,4,5,6-tetrahydropyridine-3-carbonitrile, C13H14N2O
- Crystal structure of 2-amino-7-methyl-4-(3,4-difluoro-phenyl)-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carbonitrile, C16H10F2N2O3
- The crystal structure of 4-[(benzo[1,3]dioxol-5-ylmethylene)-amino]-1,5-dimethyl-2-phenyl-1,2-dihydro-pyrazol-3-one, C19H17N3O3
- Crystal structure of 1,4-dihydro-1-phenylchromeno[4,3-c]pyrazole, C16H12N2O
- Crystal structure of N-(5-((3,5-dimethylisoxazol-4-yl)sulfonyl)quinolin-8-yl)benzamide, C21H17N3O4S
- Crystal structure of 3-amino-9-methoxy-1-phenyl-1H-benzo[f]chromene-2-carbonitrile, C21H16N2O2
- Crystal structure of 1,2-bis(4-methoxyphenyl)-2-((3-(trifluoromethyl)phenyl)amino)ethan-1-one, C23H20F3NO3
- Crystal structure of 2-amino-4-(2,4-dinitrophenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carbonitrile – ethanol (1:1), C21H16N4O8
- Crystal structure of catena-poly-[(1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-ium-1-yl)-1,4-dihydroquinoline-3-carboxylate-κ2O,O′)-(μ2-4,4′-sulfonyldibenzoato-κ4O,O′:O′′,O′′′)zinc(II)] hemihydrate, C31H27ZnFN3O9.5S
- Crystal structure of 2-(2-bromophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13BrO4
- Crystal structure of 2-(2-bromophenyl)-5-ethyl-1,3-dioxane-5-carboxylic acid, C13H15BrO4
- Crystal structure of 4-(4-((3-bromophenyl)amino)-6-(tert-butyl)-3-(2-hydroxypropan-2-yl)cinnolin-8-yl)-2-methylbut-3-yn-2-ol, C26H30BrN3O2
- Crystal structure poly-(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazolyl-κ2NN1:N2N)-(μ3-2,2′-(1,2-phenylene)diacetato-κ5-O1,O2:O2:O3,O4)cadmium(II), C22H19CdN5O4
- Crystal structure of bis(1-ethyl-6-fluoro-4-oxido-7-(piperazin-1-ium-1-yl)-1,8-naphthyridin-1-ium-3-carboxylate-κ2O,O′)copper(II) benzene-1, 4-dicarboxylate dihydrate, C38H42F2CuN8O12
- Redetermination of the crystal structure of potassium lithium molybdate monohydrate, KLiMoO4·H2O
- Crystal structure of [tris(2-benzimidazolylmethyl)amine-κ3N](isonicotinate-κO) cobalt(II) [tris(2-benzimidazolylmethyl)amine-κ3N](isonicotinic acid-κO) cobalt(II) triperchlorate, C60H51N16O16Cl3Co2
- The crystal structure of tris(μ2-1,3-bis(4,4,4-trifluoro-3-oxido-1-(oxo)but-2-en-1-yl)phenyl-κ4O,O′:O′′,O′′′)-bis(1,2-dimethoxyethane-κ2O,O′)dicerium(III), C50H38F18O16Ce2
- Crystal structure of 8-isopropyl-8-aza-bicyclo[3.2.1]octan-3-ol, C10H19NO
- Crystal structure of 2,4-dibenzoyl-N,N-dimethylbenzenamine, C22H19NO2
- The crystal structure of 2-(4-methoxyphenyl)-6,8-diphenyl-4-(phenylamino)quinazoline — acetonitrile (1/1), C35H28N4O


