Abstract
Adipose-derived mesenchymal stem cells (ADSCs) and bone marrow-derived mesenchymal stem cells (BMSCs) have shown great potential in clinical applications. However, the similarities and differences between these two cell types have not been fully elucidated. Recent advances in transcriptomic and metabolomic research have provided valuable insight into the characteristics and functions of ADSCs and BMSCs. In this perspective article, we review the key findings from these studies, including cellular heterogeneity as well as differences in metabolic and secretory properties. We discuss how these insights can help guide the selection of the most suitable cell source for the clinic, and the optimization of preconditioning strategies prior to clinical deployment. Furthermore, we analyze the current landscape of products and clinical trials involving ADSCs and BMSCs, highlighting their therapeutic potential. We propose that the integration of multi-omics datasets will be crucial for establishing a comprehensive understanding of ADSC and BMSC identity and potency, and the provision of quality-assured stem cell-derived products for the clinic.
Biological insights from transcriptomic studies of ADSCs and BMSCs
Identifying transcriptomic heterogeneity of in vitro cultured ADSCs and BMSCs
Recent single-cell studies have shed light on the heterogeneity within in vitro cultured adipose-derived mesenchymal stem cells (ADSCs) and bone marrow-derived mesenchymal stem cells (BMSCs) (Table S1), whereas analyzing the heterogeneity of ADSCs and BMSCs simultaneously provides a more accurate reflection of the differences between these two cell types. Our previous research revealed that ADSCs had lower transcriptomic heterogeneity compared to BMSCs [1]. A cross-tissue investigation found that ADSCs and BMSCs were made up of three cell subpopulations: osteo-mesenchymal stem cells (MSCs), chondro-MSCs, and adipo/myo-MSCs [2]. Another cross-tissue study conducted by Zheng et al. identified five tissue source-conserved cell subpopulations, one BMSC-specific subpopulation, and two ADSC-specific subpopulations [3]. The conserved subpopulations highly expressed regulons associated with immunosuppression, proliferation and stem cell self-renewal, common characteristics of MSCs. The BMSC-specific subpopulation exhibited high levels of cytokine and chemokine gene expression, whereas the two ADSC-specific subpopulations were characterized by cell cycle regulation and extracellular matrix regulation, respectively.
Identifying transcriptomic heterogeneity of native ADSCs and BMSCs
In vitro cultured MSCs retain the main characteristics of their native counterparts. Therefore, the analysis of cell heterogeneity in the native state is of significance and provides a greater understanding of cell identity and potency following their expansion ex vivo. In general, two universal stem/progenitor subpopulations within adipose tissues have been identified: ICAM1-expressing committed pre-adipocytes and multipotential progenitor cells with the markers CD55, DPP4 and PI16 [4], 5] (Table S2). A comprehensive review of current single-cell transcriptome studies on native BMSCs revealed two main differentiation trajectories: osteogenesis and adipogenesis (Table S2). Tikhonova et al. identified two adipo- and two osteo-primed subpopulations in their study [6], while Wolock et al. and Zhong et al. reported higher-definition cellular architectures, including multipotent stromal cells, adipocyte progenitors, pre-adipocytes, osteoblast/chondrocyte progenitors, pre-osteoblast/chondrocytes, and pro-osteoblasts [7], 8]. In addition, a recent study identified a group of neural crest progenitor cells in BMSCs that have the ability to regenerate nerves [9]. These results indicate that native BMSCs contain multiple lineages, whereas the native ADSC subpopulations are mainly adipogenic. This may explain the lower transcriptomic heterogeneity we previously found in cultured ADSCs [1].
Biological insights from metabolomic studies of ADSCs and BMSCs
Identifying metabolic differences between ADSCs and BMSCs
Cell metabolites can reflect the biochemical phenotype of the cells. The pattern of endogenous metabolites can be used to distinguish MSCs from different tissues [10]. Li et al. conducted a more comprehensive investigation into the metabolic differences between ADSCs and BMSCs [11]. They found that ADSCs differ from BMSCs in the components of the linoleic acid pathway, including bovinic acid, 12,13-EpOME, 13-hydroxyoctadecadienoic acid, and 9,10-epoxyoctadecenoic acid. Metabolites in the linoleic acid pathway have been shown to protect against cardiovascular disease and inflammation by reducing oxidative stress [12]. Thus, the higher concentration of linoleic acid pathway metabolites in ADSCs suggests that they may have a greater therapeutic potential for treating cardiovascular disease compared to BMSCs.
Identifying secretory differences between ADSCs and BMSCs
Secretomics is an effective strategy for investigating the interactions between stem cells and their niche, providing valuable information on cellular properties. Although the conclusions of these studies are varied, several consistent patterns emerge (Table S3). The secretome of ADSCs exhibits neurotrophic, angiogenic, and detoxification properties. BMSCs, on the other hand, secrete factors that promote cellular differentiation and proliferation, chemotaxis, and both pro- and anti-inflammatory processes (Table S3). These distinct characteristics suggest that ADSCs and BMSCs are naturally suited to different therapeutic applications: ADSCs may be more beneficial for treating neurodegenerative diseases and ischemic conditions, whereas BMSCs may be more effective in modulating the immune response and promoting tissue repair through the recruitment and differentiation of endogenous cells.
Identifying bioenergetic differences between ADSCs and BMSCs
The bioenergetic profile is a crucial measure of the metabolic condition of stem cells. It is widely recognized that as stem cells differentiate, energy production shifts from anaerobic to aerobic metabolism. Recent research has highlighted the distinct metabolic patterns of stem cells as they differentiate into different lineages [13]. Osteogenically committed BMSCs exhibit increased oxidative phosphorylation (OXPHOS) activity but lower glycolysis, whereas adipogenically committed BMSCs display higher levels of glycolysis. Under inflammatory stimulation, BMSCs demonstrate impaired OXPHOS activity with reduced spare respiratory capacity [13]. In contrast, under similar inflammatory conditions, ADSCs exhibit more active OXPHOS and increased spare respiratory capacity [14], 15], which enhances osteoblastic differentiation but reduces adipogenic and chondrogenic specification [14]. Our previous research also revealed that, under identical in vitro culture conditions, ADSCs rely less on mitochondrial respiration compared to BMSCs, making them more resistant to hypoxic and serum-deprived environments [1]. These studies collectively suggest that ADSCs may thrive better in harsh in vivo environments, such as those encountered in tissue ischemia or inflammation.
Combining transcriptomic and metabolomic signatures to optimize the deployment of stem cell based therapies
Selecting the suitable source of MSCs for clinical applications
Studies showed that ADSCs possess superior pro-neurogenic and pro-angiogenic traits compared to BMSCs, making them preferable for treating nerve and vascular regeneration disorders (Table S3). Additionally, the metabolic response of ADSCs enables them to withstand harsh conditions, potentially increasing their efficacy in repairing tissues affected by hypoxia and ischemia [1], 14]. Conversely, BMSCs secrete a higher quantity of substances that promote proliferation, differentiation, and immunoregulation (Table S3), and demonstrate a rapid response to changes in the microenvironment [16], suggesting their suitability for regenerating parenchymal cells at injury sites. Besides, although both cell types have immunomodulatory functions, they perform differently in regulating specific immune responses. ADSCs are more effective at suppressing T-cell proliferation, whereas BMSCs excel at inhibiting NK cell growth [17]. Furthermore, ADSCs express high levels of CD55, a cell-surface complement regulator, making them more effective at avoiding complement system attacks [17]. The findings suggest that ADSCs may be more effective at regulating acquired immune responses, while BMSCs are better suited for regulating innate immune responses.
Medical conditions are commonly classified as either acute or chronic. Most acute lesions are accompanied by acute inflammatory responses and parenchymal cell damage. Treating acute damage necessitates a rapid response to the inflammatory microenvironment, immune regulation, and support for the organism’s regenerative functions. The rapid response to stimulation, immunoregulation, pro-proliferative and pro-differentiation properties of BMSCs may make them more advantageous in treating acute injuries. Chronic diseases, on the other hand, are characterized by progressive, persistent, and irreversible damage to multiple cells in the tissue, along with local vascular and nervous system lesions and a micro-environment marked by high inflammation, ischemia, and hypoxia. This often results in long-term non-healing ulceration or excessive fibrosis. ADSCs’ resistance to extreme environments, as well as their pro-neurogenic and pro-angiogenic properties, may give them an advantage in treating chronic diseases. Furthermore, since the properties of these two cells complement each other well, combining ADSCs and BMSCs may enhance the overall therapeutic outcome. For chronic disease, ADSCs can be administered first to promote nerve and blood vessel reconstruction in the injured area, followed by BMSCs to stimulate tissue regeneration. For acute injuries, BMSCs would be applied first to promote tissue healing, and ADSCs used later to prevent or mitigate the formation of chronic inflammation.
With the advancement of chemical reprogramming strategies in human cells [18], 19], the study and application of chemically induced pluripotent stem cells (CiPSCs) have received increased attention. This has naturally sparked discussions in the field about the functional capabilities and application prospects of CiPSCs compared to adult tissue-derived MSCs, such as ADSCs and BMSCs. iPSCs are characterized by their unlimited proliferation, rapid growth, and high differentiation potential. These characteristics endow iPSCs with inherent advantages in constructing in vitro disease models, drug screening, establishing off-the-shelf cell banks, and differentiating into specific cell types. Additionally, numerous studies have demonstrated that iPSC-derived MSCs possess immunomodulatory abilities comparable to those of adult tissue-derived MSCs. However, the application of CiPSCs is not without challenges [20]. Although the chemically induced approach avoids the potential for adverse effects associated with residual transcription factors, the high proliferation capacity and strong differentiation potential of CiPSCs present greater risks of genomic instability and tumorigenesis in vivo compared to ADSCs and BMSCs. In addition, immune rejection and heterogeneity remain issues that need to be addressed. Factors such as the tissue source of the parent cells, the physiological state of the donor, the combinations of small molecules, and subsequent lineage induction methods all influence the performance of the final cell products. Furthermore, a study found that the adipogenic and chondrogenic capacities of iPSC-derived MSCs were lower than those of BMSCs, and their gene expression profiles are significantly different [21]. Therefore, in practical clinical applications, CiPSCs cannot yet replace tissue-derived MSCs.
Evaluating the necessity of subpopulation purification
The presence of senescent and dysfunctional cell subpopulations significantly impacts the efficacy of stem cell-based therapies. Stem cells derived from older donors often contain a substantial number of P21-positive senescent cells, along with a depletion of the regenerative subpopulations [22], 23]. In contrast, stem cells derived from young donors exhibit a greater capacity for wound repair and functional restoration [22], 23]. Consequently, the removal of defective subpopulations has the potential to improve the safety and efficacy of stem cell therapies, particularly in the context of autologous transplantation. Furthermore, distinct subpopulations interact with the microenvironment in unique ways [24], 25]. Therefore, identifying and selecting cell subpopulations that favorably interact with the patient’s tissue holds great promise for enhancing the efficacy of stem cell-based therapies.
Understanding the impact of preconditioning on therapeutic outcome
Culture conditions significantly influence stem cell biology. ADSCs and BMSCs respond differently to the same preconditioning stimuli. For instance, Rapamycin treatment increases the secretion of prostaglandin E2 in BMSCs, thereby enhancing the efficacy of multiple sclerosis therapy, but it diminishes the therapeutic effects in ADSC [26]. BMSCs respond to stress by upregulating their metabolic activity, whereas ADSCs maintain stable metabolic rates even under hypoxic or inflammatory conditions [27]. Additionally, the sensitivity to preconditioning varies between the two cell types, with BMSCs displaying enhanced exosomal activity following short-term hypoxia, whereas ADSCs require prolonged exposure to achieve similar effects [16]. These observations support the hypothesis that ADSCs possess a unique energy metabolism that enables them to adapt more effectively to harsh environments. Conversely, BMSCs respond more actively to environmental stimuli, exhibiting unique secretory behaviors. These variations underscore the importance of appropriate preconditioning strategies to fine-tune stem cell populations prior to their therapeutic use.
Advancements in clinical research involving ADSCs and BMSCs
Our statistics reveal the existence of 4 ADSC products and 8 BMSC products worldwide, primarily approved for use in Japan and South Korea (Table S4). However, a comprehensive review of current and forthcoming clinical trials shows that the number of trials involving ADSCs now outnumbers those involving BMSCs (Figure 1A). A closer and more detailed examination of the specific indications targeted by ADSCs and BMSCs in these trials reveals a notable shift in trends. Notably, the volume of clinical research investigating the use of ADSCs for the treatment of osteoarthritis significantly exceeds that of BMSCs (Figure 1B). This trend deviates from the pattern observed in previous clinical trials [1]. Additionally, ADSCs are more commonly used in clinical trials to treat conditions like fistulas, ischemia or ulcers, neurodegenerative diseases, and scars (Figure 1B). This preference aligns with their unique capabilities in neurovascular regeneration, resistance to extreme environmental conditions, and advantages in treating chronic conditions. These findings emphasize the critical role of fundamental stem cell research in guiding clinical trials. On the other hand, clinical trials with MSCs for corona virus disease 2019 (COVID-19) have increased dramatically since the pandemic due to a lack of specific treatments. The data show that there are slightly more trials using BMSCs to treat COVID-19 or acute respiratory distress syndrome (ARDS) than ADSCs (Figure 1B). The characteristics of rapid response to stimulation, regulation of inflammation, and promotion of regeneration may indeed make BMSCs more effective in controlling COVID-19 and ARDS in the early stages. However, due to the heterogeneity and complexity of COVID-19, its therapeutic efficacy must be further investigated. As basic research progresses and provides more comprehensive insights into the unique characteristics of ADSCs and BMSCs, clinical applications are expected to become more focused and evidence-based in the future.
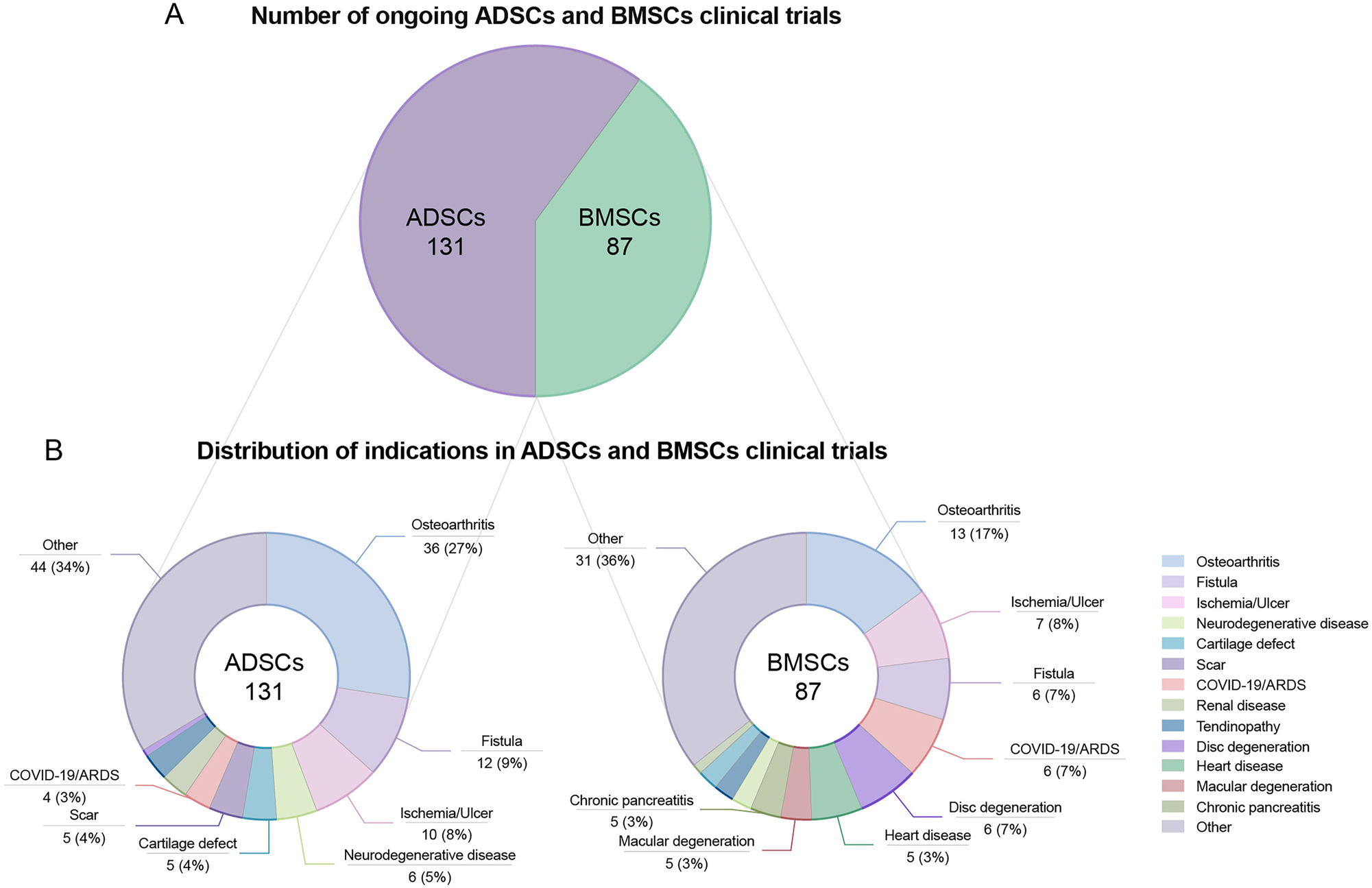
Ongoing ADSCs and BMSCs clinical trials. (A) Total number of ongoing ADSCs and BMSCs clinical trials. (B) Distribution of indications in ADSCs and BMSCs clinical trials. Data from ClinicalTrials.gov. We searched in the “Intervention/Treatment” section as follow: (Adipose-Derived Mesenchymal Stem Cells) OR (Adipose-Derived stem cells) OR (Adipose stromal cells) OR (Adipose Stromal Vascular Fraction) OR (Adipose stem cells) for ADSCs clinical trials, (Bone marrow mesenchymal stem cells) OR (Bone marrow stromal cells) OR (Bone marrow stem cells) for BMSCs clinical trials, records with the status “not yet recruiting”, “recruiting” and “active not recruiting” are kept for analysis, and records of non-compliance with MSC therapy from the retrieval results are moved. Other indications indicates that the percentage of the indications is 2 % or less in both ADSCs and BMSCs clinical trials. ADSC, adipose-derived mesenchymal stem cell; BMSC, bone marrow-derived mesenchymal stem cell.
Open questions and future directions
Despite advances in the application of transcriptomic and metabolomic technologies to the study of ADSCs and BMSCs, significant challenges persist. In the realm of transcriptomics, there is a lack of standardization in cell sorting strategies and bioinformatics algorithms across different studies. A key direction for future research is the creation of a comprehensive database that integrates all available single-cell transcriptomic data for ADSCs and BMSCs. Recently, some studies have presented a more comprehensive composition and interaction pattern of adipose tissue cell subpopulations by integrating published single-cell data [28], 29]. By integrating, normalizing, and reanalyzing this resource, researchers could better clarify subpopulation composition, discover novel subpopulations, and understand their therapeutic potential. Additionally, the identity of ADSCs and BMSCs following in vitro culture and subsequent in vivo transplantation remains underexplored. To address this knowledge gap, future research could combine cell tagging and single-cell technologies to track the fate of transplanted cells within the niche in vivo [30]. This approach would provide valuable insights into the stability and plasticity of stem cell subpopulations in response to different microenvironments.
Another promising avenue for future research is the application of single-cell metabolomics to the study of cellular heterogeneity. While this technology has been developed for other cell types [31], its application in ADSCs and BMSCs has been limited. Combining single-cell transcriptomics and metabolomics has the potential to reveal the complex interplay between gene expression and metabolic states at the individual cell level, thereby enabling a more comprehensive understanding of stem cell biology and function.
The integration and analysis of multi-omics data using artificial intelligence (AI) technologies, such as machine learning and deep learning algorithms, is an emerging and promising area of stem cell research. Some studies have employed machine learning to predict the differentiation of MSCs in the early stages of culture or to forecast the efficacy of MSC therapies for specific diseases [32], 33]. Future research can incorporate information on donor status, tissue source, cell morphology, and multi-omics data to enhance MSC quality control, and streamline quality control indicators. Additionally, analyzing the omics data of MSCs treated under different preconditioning conditions may contribute to the development of customized culture protocols for MSCs with specific functions, thereby further improving their efficacy.
Conclusions
In this article, we have provided an overview of recent achievements in the transcriptomic and metabolomic profiling of ADSCs and BMSCs. We provide a summary of new insights into the identity, properties, and function of these cells. Additionally, we have examined the current landscape of ADSC and BMSC products and clinical trials, highlighting the important relationship between fundamental research and clinical applications. We hope that our article will serve as a valuable resource for researchers, clinicians, and industry partners interested in the current state and future directions of ADSC and BMSC research and application.
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: T2121004, 31830029, 82302784
-
Research ethics: The local Institutional Review Board deemed the study exempt from review.
-
Informed consent: Not applicable.
-
Author contributions: Conception and design, financial support, Hongwei Ouyang; conception and design, manuscript writing, Wenyan Zhou; manuscript writing, Junxin Lin; review and editing, David C. Hay; review and editing, Xudong Yao. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: Authors state no conflict of interest.
-
Research funding: This work was supported by the National Natural Science Foundation of China (T2121004, 31830029, 82302784).
-
Data availability: Not applicable.
References
1. Zhou, W, Lin, J, Zhao, K, Jin, K, He, Q, Hu, Y, et al.. Single-cell profiles and clinically useful properties of human mesenchymal stem cells of adipose and bone marrow origin. Am J Sports Med 2019;47:1722–33. https://doi.org/10.1177/0363546519848678.Suche in Google Scholar PubMed
2. Hou, W, Duan, L, Huang, C, Li, X, Xu, X, Qin, P, et al.. Cross-tissue characterization of heterogeneities of mesenchymal stem cells and their differentiation potentials. Front Cell Dev Biol 2021;9:781021. https://doi.org/10.3389/fcell.2021.781021.Suche in Google Scholar PubMed PubMed Central
3. Wang, Z, Chai, C, Wang, R, Feng, Y, Huang, L, Zhang, Y, et al.. Single-cell transcriptome atlas of human mesenchymal stem cells exploring cellular heterogeneity. Clin Transl Med 2021;11:e650. https://doi.org/10.1002/ctm2.650.Suche in Google Scholar PubMed PubMed Central
4. Merrick, D, Sakers, A, Irgebay, Z, Okada, C, Calvert, C, Morley, MP, et al.. Identification of a mesenchymal progenitor cell hierarchy in adipose tissue. Science. 2019;364. https://doi.org/10.1126/science.aav2501.Suche in Google Scholar PubMed PubMed Central
5. Vijay, J, Gauthier, MF, Biswell, RL, Louiselle, DA, Johnston, JJ, Cheung, WA, et al.. Single-cell analysis of human adipose tissue identifies depot and disease specific cell types. Nat Metab 2020;2:97–109. https://doi.org/10.1038/s42255-019-0152-6.Suche in Google Scholar PubMed PubMed Central
6. Tikhonova, AN, Dolgalev, I, Hu, H, Sivaraj, KK, Hoxha, E, Cuesta-Dominguez, A, et al.. The bone marrow microenvironment at single-cell resolution. Nature 2019;569:222–8. https://doi.org/10.1038/s41586-019-1104-8.Suche in Google Scholar PubMed PubMed Central
7. Wolock, SL, Krishnan, I, Tenen, DE, Matkins, V, Camacho, V, Patel, S, et al.. Mapping distinct bone marrow niche populations and their differentiation paths. Cell Rep 2019;28:302–11 e5. https://doi.org/10.1016/j.celrep.2019.06.031.Suche in Google Scholar PubMed PubMed Central
8. Zhong, L, Yao, L, Tower, RJ, Wei, Y, Miao, Z, Park, J, et al.. Single cell transcriptomics identifies a unique adipose lineage cell population that regulates bone marrow environment. Elife 2020;9. https://doi.org/10.7554/elife.54695.Suche in Google Scholar PubMed PubMed Central
9. Changmeng, Z, Hongfei, W, Cheung, MC, Chan, YS, Shea, GK. Revealing the developmental origin and lineage predilection of neural progenitors within human bone marrow via single-cell analysis: implications for regenerative medicine. Genome Med 2023;15:66. https://doi.org/10.1186/s13073-023-01224-0.Suche in Google Scholar PubMed PubMed Central
10. Lee, SJ, Yi, T, Ahn, SH, Lim, DK, Kim, SN, Lee, HJ, et al.. Comparative study on metabolite level in tissue-specific human mesenchymal stem cells by an ultra-performance liquid chromatography quadrupole time of flight mass spectrometry. Anal Chim Acta 2018;1024:112–22. https://doi.org/10.1016/j.aca.2018.04.018.Suche in Google Scholar PubMed
11. Li, JZ, Qu, H, Wu, J, Zhang, F, Jia, ZB, Sun, JY, et al.. Metabolic profiles of adipose-derived and bone marrow-derived stromal cells from elderly coronary heart disease patients by capillary liquid chromatography quadrupole time-of-flight mass spectrometry. Int J Mol Med 2018;41:184–94. https://doi.org/10.3892/ijmm.2017.3198.Suche in Google Scholar PubMed PubMed Central
12. Hassan Eftekhari, M, Aliasghari, F, Babaei-Beigi, MA, Hasanzadeh, J. Effect of conjugated linoleic acid and omega-3 fatty acid supplementation on inflammatory and oxidative stress markers in atherosclerotic patients. ARYA Atheroscler 2013;9:311–8.Suche in Google Scholar
13. Li, B, Shi, Y, Liu, M, Wu, F, Hu, X, Yu, F, et al.. Attenuates of NAD(+) impair BMSC osteogenesis and fracture repair through OXPHOS. Stem Cell Res Ther 2022;13:77. https://doi.org/10.1186/s13287-022-02748-9.Suche in Google Scholar PubMed PubMed Central
14. Fang, J, Lu, R, Lin, Y, Wang, H, Wei, H, Wang, J, et al.. Effects of sepsis serum on the fate of adipose tissue-derived stem cells. Front Biosci 2023;28:72. https://doi.org/10.31083/j.fbl2804072.Suche in Google Scholar PubMed
15. Ye, G, Xie, Z, Zeng, H, Wang, P, Li, J, Zheng, G, et al.. Oxidative stress-mediated mitochondrial dysfunction facilitates mesenchymal stem cell senescence in ankylosing spondylitis. Cell Death Dis 2020;11:775. https://doi.org/10.1038/s41419-020-02993-x.Suche in Google Scholar PubMed PubMed Central
16. Gupta, S, Rawat, S, Krishnakumar, V, Rao, EP, Mohanty, S. Hypoxia preconditioning elicit differential response in tissue-specific MSCs via immunomodulation and exosomal secretion. Cell Tissue Res 2022;388:535–48. https://doi.org/10.1007/s00441-022-03615-y.Suche in Google Scholar PubMed
17. Menard, C, Dulong, J, Roulois, D, Hebraud, B, Verdiere, L, Pangault, C, et al.. Integrated transcriptomic, phenotypic, and functional study reveals tissue-specific immune properties of mesenchymal stromal cells. Stem Cell 2020;38:146–59. https://doi.org/10.1002/stem.3077.Suche in Google Scholar PubMed
18. Guan, J, Wang, G, Wang, J, Zhang, Z, Fu, Y, Cheng, L, et al.. Chemical reprogramming of human somatic cells to pluripotent stem cells. Nature 2022;605:325–31. https://doi.org/10.1038/s41586-022-04593-5.Suche in Google Scholar PubMed
19. Liuyang, S, Wang, G, Wang, Y, He, H, Lyu, Y, Cheng, L, et al.. Highly efficient and rapid generation of human pluripotent stem cells by chemical reprogramming. Cell Stem Cell 2023;30:450–9 e9. https://doi.org/10.1016/j.stem.2023.02.008.Suche in Google Scholar PubMed
20. Yamanaka, S. Pluripotent stem cell-based cell therapy-promise and challenges. Cell Stem Cell 2020;27:523–31. https://doi.org/10.1016/j.stem.2020.09.014.Suche in Google Scholar PubMed
21. Xu, M, Shaw, G, Murphy, M, Barry, F. Induced pluripotent stem cell-derived mesenchymal stromal cells are functionally and genetically different from bone marrow-derived mesenchymal stromal cells. Stem Cell 2019;37:754–65. https://doi.org/10.1002/stem.2993.Suche in Google Scholar PubMed PubMed Central
22. Khong, SML, Lee, M, Kosaric, N, Khong, DM, Dong, Y, Hopfner, U, et al.. Single-cell transcriptomics of human mesenchymal stem cells reveal age-related cellular subpopulation depletion and impaired regenerative function. Stem Cell 2019;37:240–6. https://doi.org/10.1002/stem.2934.Suche in Google Scholar PubMed PubMed Central
23. Wang, B, Liu, Z, Chen, VP, Wang, L, Inman, CL, Zhou, Y, et al.. Transplanting cells from old but not young donors causes physical dysfunction in older recipients. Aging Cell 2020;19:e13106. https://doi.org/10.1111/acel.13106.Suche in Google Scholar PubMed PubMed Central
24. Liang, ZX, Liu, HS, Xiong, L, Zeng, ZW, Zheng, XB, Kang, L, et al.. GAS6 from CD200(+) adipose-derived stem cells mitigates colonic inflammation in a macrophage-dependent manner. J Crohns Colitis 2023;17:289–301. https://doi.org/10.1093/ecco-jcc/jjac123.Suche in Google Scholar PubMed
25. Zhou, W, Lin, J, Xie, Y, Hu, X, Yao, X, Ou, Y, et al.. High-resolution aging niche of human adipose tissues. Signal Transduct Target Ther 2023;8:105. https://doi.org/10.1038/s41392-023-01315-9.Suche in Google Scholar PubMed PubMed Central
26. Wise, RM, Harrison, MAA, Sullivan, BN, Al-Ghadban, S, Aleman, SJ, Vinluan, AT, et al.. Short-term Rapamycin preconditioning diminishes therapeutic efficacy of human adipose-derived stem cells in a murine model of multiple sclerosis. Cells 2020;9. https://doi.org/10.3390/cells9102218.Suche in Google Scholar PubMed PubMed Central
27. Rodriguez, LA2nd, Mohammadipoor, A, Alvarado, L, Kamucheka, RM, Asher, AM, Cancio, LC, et al.. Preconditioning in an inflammatory milieu augments the immunotherapeutic function of mesenchymal stromal cells. Cells 2019;8. https://doi.org/10.3390/cells8050462.Suche in Google Scholar PubMed PubMed Central
28. Moller, AF, Madsen, JGS. JOINTLY: interpretable joint clustering of single-cell transcriptomes. Nat Commun 2023;14:8473. https://doi.org/10.1038/s41467-023-44279-8.Suche in Google Scholar PubMed PubMed Central
29. Massier, L, Jalkanen, J, Elmastas, M, Zhong, J, Wang, T, Nono Nankam, PA, et al.. An integrated single cell and spatial transcriptomic map of human white adipose tissue. Nat Commun 2023;14:1438. https://doi.org/10.1038/s41467-023-36983-2.Suche in Google Scholar PubMed PubMed Central
30. Weinreb, C, Rodriguez-Fraticelli, A, Camargo, FD, Klein, AM. Lineage tracing on transcriptional landscapes links state to fate during differentiation. Science 2020;367. https://doi.org/10.1126/science.aaw3381.Suche in Google Scholar PubMed PubMed Central
31. Nemes, P, Rubakhin, SS, Aerts, JT, Sweedler, JV. Qualitative and quantitative metabolomic investigation of single neurons by capillary electrophoresis electrospray ionization mass spectrometry. Nat Protoc 2013;8:783–99. https://doi.org/10.1038/nprot.2013.035.Suche in Google Scholar PubMed PubMed Central
32. Zhou, Y, Ping, X, Guo, Y, Heng, BC, Wang, Y, Meng, Y, et al.. Assessing biomaterial-induced stem cell lineage fate by machine learning-based artificial intelligence. Adv Mater 2023;35:e2210637. https://doi.org/10.1002/adma.202210637.Suche in Google Scholar PubMed
33. Liu, YYF, Lu, Y, Oh, S, Conduit, GJ. Machine learning to predict mesenchymal stem cell efficacy for cartilage repair. PLoS Comput Biol 2020;16:e1008275. https://doi.org/10.1371/journal.pcbi.1008275.Suche in Google Scholar PubMed PubMed Central
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/mr-2024-0056).
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Reviews
- Biophysical stimuli for promoting bone repair and regeneration
- A stepwise approach to deriving functional β-cells from human embryonic or induced pluripotent stem cells
- Eco-fertility: examining the climate change-total fertility rate nexus in the context of sustainable developmental goals in a systematic review approach
- Redefining chronic mountain sickness: insights from high-altitude research and clinical experience
- Review of organ damage from COVID and Long COVID: a disease with a spectrum of pathology
- Perspective
- Combining transcriptomic and metabolomic insights to guide the clinical application of adipose- and bone marrow-derived mesenchymal stem cells
- Research Highlight
- Precision phototherapy and imaging with aggregation-induced emission-based nanoparticles cloaked in macrophage membrane
Artikel in diesem Heft
- Frontmatter
- Reviews
- Biophysical stimuli for promoting bone repair and regeneration
- A stepwise approach to deriving functional β-cells from human embryonic or induced pluripotent stem cells
- Eco-fertility: examining the climate change-total fertility rate nexus in the context of sustainable developmental goals in a systematic review approach
- Redefining chronic mountain sickness: insights from high-altitude research and clinical experience
- Review of organ damage from COVID and Long COVID: a disease with a spectrum of pathology
- Perspective
- Combining transcriptomic and metabolomic insights to guide the clinical application of adipose- and bone marrow-derived mesenchymal stem cells
- Research Highlight
- Precision phototherapy and imaging with aggregation-induced emission-based nanoparticles cloaked in macrophage membrane


