Synthesis, crystal structure, molecular docking studies and bio-evaluation of some N4-benzyl-substituted isatin- 3-thiosemicarbazones as urease and glycation inhibitors
-
Humayun Pervez
, Nazia Khan
Abstract
Fifteen N4-benzyl-substituted isatin-3-thiosemicarbazones 5a–o were synthesized and evaluated for their urease and glycation inhibitory potential. Lemna aequinocitalis growth and Artemia salina assays were also done to determine their phytotoxic and toxic effects. All compounds are potent inhibitors of the urease enzyme, displaying inhibition [half maximal inhibitory concentration (IC50)=1.08±0.12–11.23±0.19 μm] superior to that of the reference inhibitor thiourea (IC50=22.3±1.12 μm). Compounds 5c, 5d, 5h, 5j,k are potent antiglycating agents, showing glycation inhibitory activity better than that of the reference inhibitor rutin (IC50 values 209.87±0.37–231.70±6.71 vs. 294.5±1.5 μm). In the phytotoxicity assay, 11 thiosemicarbazones 5a–d, 5g, 5h, 5j–l, 5n,o are active, demonstrating 5–100% growth inhibition of L. aequinocitalis at the highest tested concentrations (1000 or 500 μg/mL). In the brine shrimp (A. salina) lethality bioassay, three derivatives 5b, 5j and 5o are active with median lethal dose (LD50) values of 3.63×10−5, 2.90×10−5 and 2.31×10−4m, respectively.
Introduction
Isatin and its derivatives, including thiosemicarbazones exhibit a variety of biological properties [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25] such as antiulcer [1], anticancer [1], [4], [7], [12], [21], [22], antimicrobial [1], [2], [3], [5], [7], [22], antitubercular [23], [24], [25], antiviral [1], [2], [5], [7], [12], [25] and enzymatic inhibition [1], [4], [11], [17] activities. We have recently reported the synthesis of a number of N4-aryl-substituted isatin-3-thiosemicarbazones (thiourea derivatives) as antimicrobial [26], [27], cytotoxic [27], [28], [29], phytotoxic [28], [29] and antiurease [27], [28] agents. The structure-activity relationship studies have revealed that in some cases, the kind, number and position of substituents on the phenyl ring attached to the N4 atom of the thiosemicarbazone moiety play a key role in the biological properties, including urease inhibition. Several thiourea derivatives demonstrate promising antiglycation activity [30], [31], [32], [33], [34] and certain isatin-imines (Schiff bases) are potent inhibitors of glycation [35], [36]. Stimulated by the above observations and in continuation of our efforts [37], [38], [39], [40], [41], [42], [43], [44] in search of novel isatin-based bioactive organic molecules with diverse pharmacological properties, we have synthesized 15 new title thiosemicarbazones and evaluated them for the first time for urease and glycation inhibitory effects. Lemna aequinocitalis growth and Artemia salina assays were also done to determine their phytotoxic and toxic influences, respectively.
Results and discussion
Chemistry of compounds 5a–o
Appropriate N-substituted thiosemicarbazides 3 were allowed to react with isatin 4 in aqueous ethanol to give the corresponding isatin-3-thiosemicarbazones 5a–o (Scheme 1) in good to excellent yields (73–95%). Compounds 5a and 5k have been reported previously [45], [46]. The structures of the synthesized thiosemicarbazones were confirmed by analytical and spectral data. In addition, X-ray crystallographic analysis for compound 5h was conducted. The spectral data are in line with the relevant literature [22], [24], [47], [48].
Reagents and conditions: (i) Et3N, MeOH, CS2, 30oC, 75 min, then r.t., 1 h; (ii) CH3I, MeOH, −10°C, 20 min, then r.t., 2 h; (iii) NH2NH2·H2O, EtOH, reflux, 2 h; (iv) 50% EtOH, reflux, 2 h.
X-ray crystallographic analysis of compound 5h
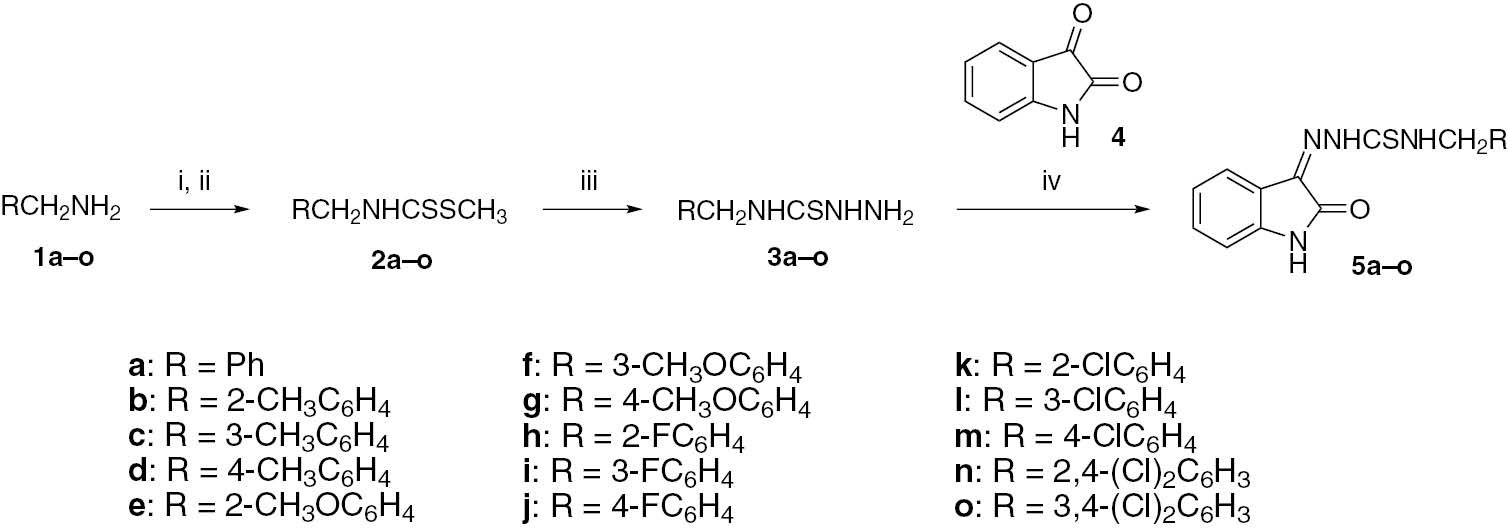
Quality crystals of 5h (Table S1) were cultivated by slow evaporation of the solution in CHCl3. The X-ray analysis shows that compound 5h crystallizes in monoclinic lattice with the P21/c space group. The molecular structure [Oak Ridge Thermal-Ellipsoid Plot (ORTEP) diagram] of 5h along with crystallographic numbering scheme is presented in Figure 1. The central thiosemicarbazone (N-iminothiourea) moiety is nearly planar adopting the S-cis/S-trans conformation, most probably due to the formation of intramolecular hydrogen bond [N(4)-H(4A)···N(2) 2.269 Å] [46], [49], [50]. The dihedral angles between S(1)-C(9)-N(3)-H(3A), S(1)-C(9)-N(4)-H(4A) and C(9)-N(3)-N(2)-C(8) are −6.45°, −174.20° and 178.73°, respectively. This planarity around the central thiosemicarbazone moiety may be attributed to substantial delocalization of N lone electron pairs onto the thiocarbonyl (C=S) group; this is clearly manifested by short N-C bond lengths [N(3)-C(9) 1.366(3) Å and N(4)-C(9) 1.333(3) Å], signifying the partial double bond character of the N-C bonds. The slightly longer N-C bond is directly connected to the imino (-C=N-) function of the thiosemicarbazone moiety.
The ORTEP diagram of 5h.
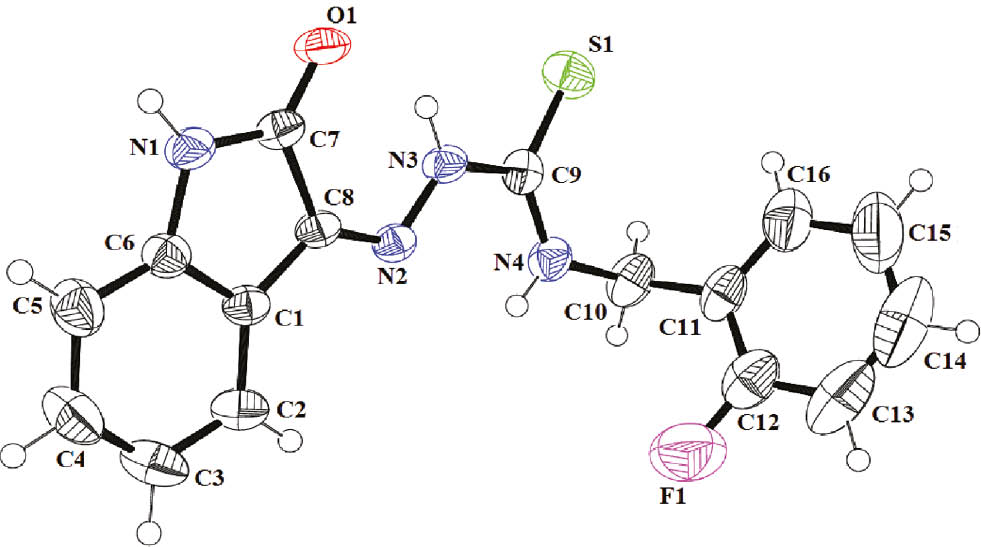
In crystal, compound 5h forms a centrosymmetric amide dimer as the major supramolecular interaction, dominating over a thioamide dimer. The three-dimensional (3D) network structure of 5h is composed of various one-dimensional (1D) tapes (Figure 2A), driven by supramolecular centrosymmetric amide [N(1)-H(1)···O(1) 2.05 Å] and centrosymmetric CH···F [C(4)-H(4)···F(1) 2.61 Å] interactions. These tapes are stacked on each other by means of CH···O [C(2)-H(2)···O(1) 2.71 Å] and π···π [C(6)···C(8) 3.38 Å, C(1)···C(5) 3.34 Å] interactions [51] to provide an overall 3D-network (Figure 2B and C).
The crystal packing of compound 5h. (A) 1D-tapes viewed along the c-axis, (B) 3D-network viewed along the c-axis, (C) 3D-network viewed along the b-axis.
In vitro antiurease activity
The N4-benzyl-substituted isatin-3-thiosemicarbazones 5a–o were evaluated for their antiurease activity against jack bean urease. Thiourea served as a reference inhibitor in this assay. All compounds are potent inhibitors of the enzyme, exhibiting inhibitory activity [half maximal inhibitory concentration (IC50)=1.08±0.12–11.23±0.19 μm], which is better than the activity of the reference inhibitor thiourea (IC50=22.3±1.12 μm) (Table 1). In comparison to compound 5a having no substituent in the phenyl ring of the benzyl group attached to N4 of the thiosemicarbazone moiety, all other compounds demonstrate enhanced enzymatic activity (IC50 values 1.08±0.12–7.97±0.14 vs. 11.23±0.19 μm). Compound 5f bearing a methoxy substituent at position 3 of the phenyl ring is the most potent urease inhibitor of the series, displaying 10- and 20-fold more activity than compound 5a and the reference inhibitor thiourea (IC50=1.08±0.12 vs. 11.23±0.19 and 22.3±1.12 μm, respectively). The other methoxy-substituted compounds 5e and 5g having the substituent at positions 2 and 4 of the phenyl ring are less active with IC50 values of 1.51±0.26 and 3.11±0.13 μm, respectively. The next most potent antiurease compound is 5b possessing the methyl substituent at position 2 of the phenyl ring. This compound exhibits slightly lower inhibitory activity than 5f but is more active than the reference inhibitor thiourea (IC50 value 1.22±0.17 vs. 1.08±0.12 and 22.3±1.12 μm, respectively). Comparison of the antiurease activity of compound 5b with that of closely related methyl-substituted compounds 5c and 5d having the substituent at positions 3 and 4 of the phenyl ring, respectively, reveals that methyl substitution at position 2 of the phenyl is more favorable than at positions 3 and 4. Compound 5c is less active than 5b but considerably more active than 5d (IC50 value 1.62±0.13 vs. 1.22±0.17 and 2.17±0.11 μm, respectively). The N4-benzyl-substituted isatin-thiosemicarbazones tested in the present assay, 5a–i and 5k–m, are more active in comparison to the corresponding N4-phenyl-substituted isatin-thiosemicarbazones tested in our earlier assays [27], [28].
Inhibition of urease by compounds 5a–o.
| Compound | IC50±SEM (μm) |
|---|---|
| 5a | 11.23±0.19 |
| 5b | 1.22±0.17 |
| 5c | 1.62±0.13 |
| 5d | 2.17±0.11 |
| 5e | 1.51±0.26 |
| 5f | 1.08±0.12 |
| 5g | 3.11±0.13 |
| 5h | 7.97±0.14 |
| 5i | 2.62±0.15 |
| 5j | 1.38±0.15 |
| 5k | 2.53±0.06 |
| 5l | 2.32±0.21 |
| 5m | 3.46±0.18 |
| 5n | 3.34±0.14 |
| 5o | 2.69±0.12 |
| Thioureaa | 22.3±1.06 |
SEM, Standard error of the mean. aReference inhibitor of urease enzyme.
In vitro antiglycation activity
The thiosemicarbazones 5a–o were further evaluated for their glycation inhibitory potential using rutin as a reference inhibitor. Seven compounds 5b–d, 5f, 5h, 5j,k, proved to be potent inhibitors of glycation. Compounds 5c, 5d, 5h, 5j,k show antiglycation activity even better than the reference inhibitor rutin (Table 2). Compound 5d bearing a methyl substituent at position 4 of the phenyl ring attached to N4 of the thiosemicarbazone moiety is the most potent antiglycating agent of the series, displaying inhibition of glycation with IC50 value of 209.87±0.37 μm. The isomer 5c having the substituent at position 3 of the phenyl ring has the IC50 value of 231.70±6.7 μm. This compound is significantly more active than the corresponding methoxy-substituted derivative 5f. The next most potent antiglycating agent is compound 5j possessing a fluoro substituent at position 4 of the phenyl ring. This compound exhibits slightly less inhibition than the most active derivative 5d but a great deal more than the reference inhibitor rutin. Overall, compounds with the CH3, F and Cl substitutions cause effective inhibition of glycation.
Antiglycation activity of compounds 5a–o.
| Compound | Concentration (μm) | Inhibition (%) | IC50±SEM (μm) |
|---|---|---|---|
| 5a | 500 | 40.98 | |
| 5b | 1000 | 62.89 | 605.26±2.24 |
| 5c | 500 | 57.97 | 231.70±6.71 |
| 5d | 500 | 70.12 | 209.87±0.37 |
| 5e | 1000 | 18.26 | |
| 5f | 1000 | 64.72 | 522.68±9.10 |
| 5g | 125 | 28.45 | |
| 5h | 1000 | 60.26 | 224.34±0.47 |
| 5i | 1000 | 25.54 | |
| 5j | 250 | 53.67 | 217.94±1.98 |
| 5k | 1000 | 59.78 | 228.55±2.30 |
| 5l | 125 | 13.71 | |
| 5m | 500 | 10.32 | |
| 5n | 500 | 42.04 | |
| 5o | 1000 | 39.57 | |
| Rutina | 1000 | 86.00 | 294.5±1.50 |
aStandard inhibitor of glycation.
In vitro phytotoxicity
Phytotoxic potential of compounds 5a–o was determined by L. aequinocitalis growth bioassay. Eleven compounds, 5a–d, 5g, 5h, 5j–l, 5n,o, are active in this assay, displaying weak or non-significant (5–100%) growth inhibition of L. aequinocitalis at the highest tested concentrations (1000 or 500 μg/mL) in comparison to the standard drug, paraquat, which shows 100% plant growth inhibition at 0.015 μg/mL concentration.
In vitro toxicity
Toxicity potential of compounds 5a–o was determined by a brine shrimp (A. salina) lethality bioassay, using etoposide (a standard anticancer drug) as a reference. Compounds 3b, 3j and 3o are active in this assay, demonstrating moderate to weak toxicity [median lethal dose (LD50)=3.63×10−5, 2.90×10−5 and 2.31×10−4m, respectively]. The remaining compounds gave LD50 values >2.64–3.22×10−4m and, therefore, can be considered to be almost inactive.
Molecular docking studies of compounds 5a–o
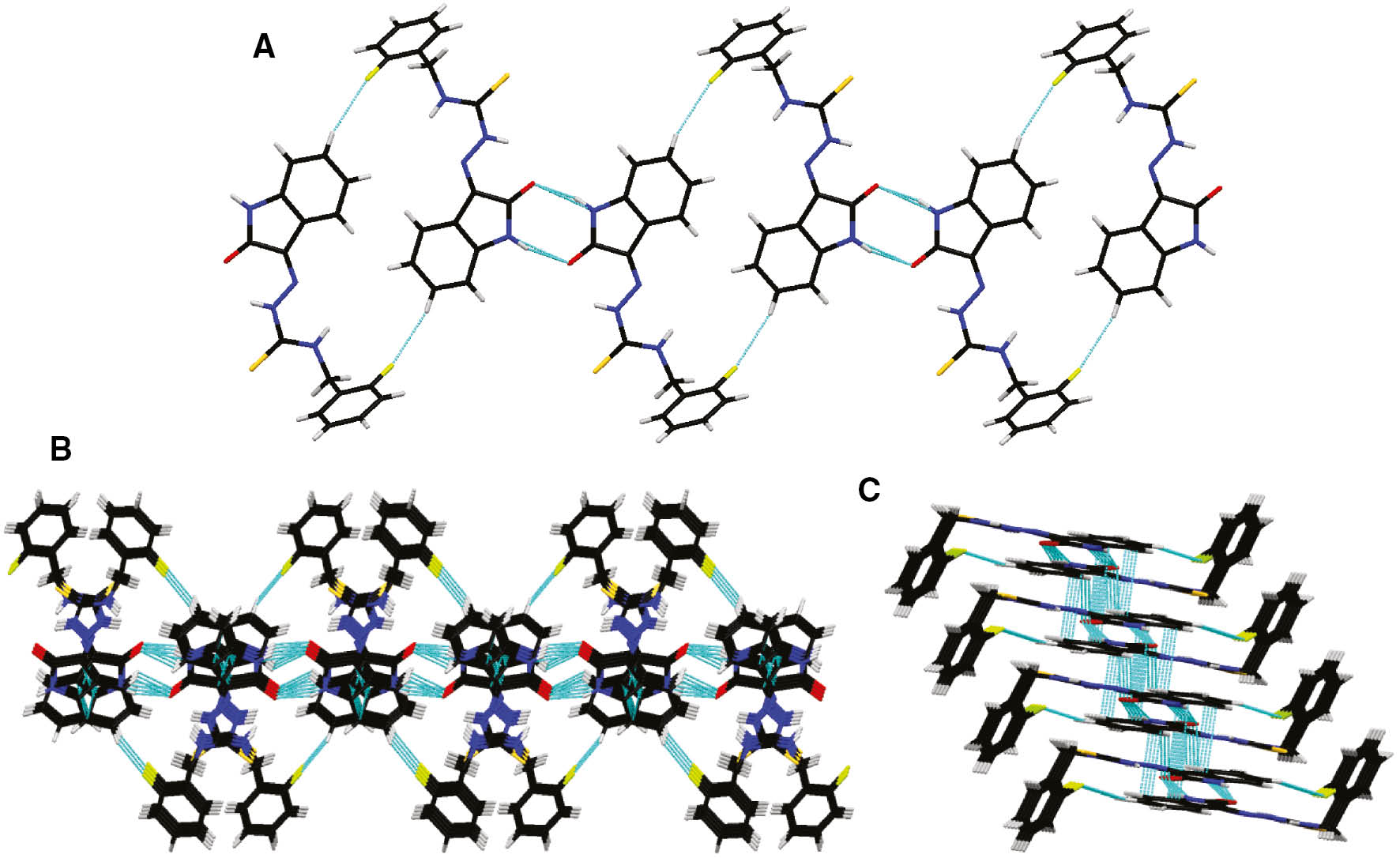
Molecular docking studies were performed as previously reported [52], [53], [54], [55], [56], [57], [58]. Docking studies of compound 5f against urease was carried out to gain further structural insights of the binding mode and possible interactions with the active site of the enzyme. Binding mode analysis of compound 5f with urease, selected after Hyde assessment, is given in Figure 3. Docking studies support the experimental results that inhibitors bind with the catalytic site of the enzyme. The interactions within the active pocket of the receptor for its ligand are very important in the design of inhibitors. The π-stacked interactions are suggested for 3-methoxyphenyl ring of the inhibitor in the active site with residues His519, His593 and Arg609. 2-Oxoindolin-3-ylidene moiety is oriented in the hydrophobic pocket of the active site, where amino acid residues Ala636 and Met637 are present. Moreover, polar interactions are observed by the central thiosemicarbazone moiety and the amino acid residues Arg439, Ala436 and Ala440.
A possible binding mode of compound 5f to urease. Carbon atoms of 5f are colored pink, while that of protein are light green; oxygen, sulfur and nitrogen atoms are colored red, yellow and blue, respectively. The two nickel ions in the active site are represented as small dark-green spheres.
Conclusions
All compounds 5a–o are extremely potent inhibitors of the urease enzyme. In addition, compounds 5c, 5d, 5h, 5j,k inhibit glycation quite convincingly. The glycation inhibitory activity of N4-benzyl-substituted isatin-3-thiosemicarbazones is described here for the first time.
Experimental
Melting points (uncorrected) were determined on a Fisher-Johns melting point apparatus. Elemental analyses were performed on a Leco CHNS-9320 (USA) elemental analyzer. Fourier transform-infrared (FT-IR) spectra (KBr discs) were recorded on a Shimadzu Prestige-21 FT-IR spectrophotometer. Proton nuclear magnetic resonance (1H NMR) spectra were measured in dimethyl sulfoxide-d6 (DMSO-d6) on Bruker Spectrospin 300 and Bruker AVANCE 400 spectrometers operating at 300 MHz and 400 MHz, respectively. Carbon-13 nuclear magnetic resonance (13C NMR) spectra were recorded in DMSO-d6 on Bruker AVANCE 300 operating at 75 MHz. Electron impact mass spectra were obtained at 70 eV on MAT312 and JEOL JMS-600 mass spectrometers. X-ray crystallographic data were taken on a Bruker Kappa APEXII charge coupled device (CCD) instrument. Compounds 5a and 5k have been reported previously [45], [46].
Synthesis of isatin-thiosemicarbazones 5a–o
N-substituted thiosemicarbazides 3a–o were synthesized in accordance with the literature route [59].
A solution of thiosemicarbazide 3 (5 mmol) in ethanol (10 mL) was added to a hot solution of isatin 4 (5 mmol) in 50% aqueous ethanol (15 mL), and the mixture was heated under reflux for 2 h. The resultant crystalline precipitate was filtered and washed with hot aqueous ethanol to afford the desired compounds 5a–o.
N-(2-Methylbenzyl)-2-[2-oxo-2,3-dihydro-1H-indol-3-ylidene]-1-hydrazinecarbothioamide (5b)
Yellow crystals; yield 73%; mp 240°C; IR: 3304, 3190 (NH), 1687 (C=O), 1616 (C=N), 1168 cm−1 (C=S); 1H NMR: δ 2.50 (s, 3H, CH3), 4.74 (d, J=6.0 Hz, 2H, benzyl CH2), 6.92 (d, J=8.0 Hz, 1H, isatin C7-H), 7.02–7.26 (m, 5H, benzyl C3-H, C4-H,C5-H,C6-H, isatin C5-H), 7.28–7.40 (m, 1H, isatin C6-H), 7.72 (d, J=7.6 Hz, 1H, isatin C4-H), 9.77 (t, J=6.0 Hz, 1H, CSNH), 11.30 (s, 1H, isatin NH), 12.76 (s, 1H, NNH); 13C NMR: δ 19.2 (CH3), 45.6 (CH2), 111.5, 120.4, 121.4, 122.8, 126.1, 127.1, 127.1, 127.6, 130.3, 131.7, 132.5, 137.8, 142.8, 163.1, 178.2; MS: m/z 324 (M+, 4), 179 (37), 161 (100), 136 (20), 121 (46), 118 (10), 91 (69), 77 (22), 65 (13%). Anal. Calcd for C17H16N4OS: C, 62.96; H, 4.94; N, 17.28. Found: C, 62.78; H, 4.83; N, 16.87.
N-(3-Methylbenzyl)-2-[2-oxo-2,3-dihydro-1H-indol-3-ylidene]-1- hydrazinecarbothioamide (5c)
Yellow crystals; yield 79%; mp 209°C; IR: 3358, 3248 (NH), 1693 (C=O), 1599 (C=N), 1149 cm−1 (C=S); 1H NMR: δ 2.50 (CH3), 3.30 (benzyl CH2), 6.96 (d, J=8.0 Hz, 1H, isatin C7-H), 7.15 (t, J=7.6 Hz, 1H, isatin C5-H), 7.19–7.57 (m, 5H, benzyl C2-H, C4-H, C5-H, C6-H, isatin C6-H), 7.78 (d, J=7.6 Hz, 1H, isatin C4-H), 10.75 (s, 1H, CSNH), 11.36 (s, 1H, isatin NH), 12.68 (s, 1H, NNH); 13C NMR: δ 21.5 (CH3), 47.7 (CH2), 111.5, 120.4, 121.4, 122.8, 125.0, 128.1, 128.4, 128.7, 131.7, 132.5, 137.8, 138.8, 142.8, 163.1, 178.1; MS: m/z 324 (M+, 6), 178 (28), 147 (79), 132 (10), 120 (77), 118 (74), 105 (100), 91 (65), 77 (75), 65 (36%). Anal. Calcd for C17H16N4OS: C, 62.96; H, 4.94; N, 17.28. Found: C, 62.86; H, 4.89; N, 17.22.
N-(4-Methylbenzyl)-2-(2-oxo-2,3-dihydro-1H-indol-3-ylidene)-1-hydrazinecarbothioamide (5d)
Yellow fluffy crystals; yield 78%; mp 200°C; IR: 3300, 3196 (NH), 1683 (C=O), 1600 (C=N), 1153 cm−1 (C=S); 1H NMR: δ 2.26 (s, 3H, CH3), 4.80 (d, J=6.0 Hz, 2H, benzyl CH2), 6.89 (d, J=8.8 Hz, 1H, isatin C7-H), 7.07 (t, J=7.6 Hz, 1H, isatin C5-H), 7.13 (d, J=7.6 Hz, 2H, benzyl C2-H, C6-H), 7.24 (d, J=8.0 Hz, 2H, benzyl C3-H, C5-H), 7.34 (t, J=7.6 Hz, 1H, isatin C6-H), 7.64 (d, J=7.6 Hz, 1H, isatin C4-H), 9.73 (t, J=6.0 Hz, 1H, CSNH), 11.18 (s, 1H, isatin NH), 12.62 (s, 1H, NNH); 13C NMR: 21.2 (CH3), 55.5, 111.5, 114.1, 120.4, 121.3, 122.7, 127.8, 129.3, 131.7, 132.4, 136.6, 142.8, 163.1, 178.0; MS: m/z 324 (M+, 7), 194 (13), 178 (38), 161(20), 147 (100), 132 (13), 120 (67), 118 (62), 105 (91), 91 (38), 77 (39), 65 (14%). Anal. Calcd for C17H16N4OS: C, 62.96; H, 4.94; N, 17.28. Found: C, 62.87; H, 4.89; N, 17.20.
N-(2-Methoxybenzyl)-2-[2-oxo-2,3-dihydro-1H-indol-3-ylidene]-1-hydrazinecarbothioamide (5e)
Yellow crystals; yield 76%; mp 205°C; IR: 3360, 3140 (NH), 1685 (C=O), 1598 (C=N), 1184 cm−1 (C=S); 1H NMR: δ 3.83 (s, 3H, CH3), 4.37 (d, J=5.0 Hz, 2H, benzyl CH2), 6.87 (d, J=8.0 Hz, 1H, isatin C7-H), 6.91 (t, J=8.4 Hz, 1H, benzyl C5-H), 7.01 (t, J=8.0 Hz, 2H, benzyl C4-H, isatin C5-H), 7.21–7.27 (m, 2H, benzyl C3-H, C6-H), 7.33 (t, J=8.0 Hz, 1H, isatin C6-H), 7.53 (t, J=6 Hz, 1H, CSNH), 8.05 (d, J=7.6 Hz, 1H, isatin C4-H), 10.30 (s, 1H, isatin NH), 10.67 (s, 1H, NNH); 13C NMR: δ 55.8 (CH2), 70.2 (CH3), 110.8, 111.0, 116.1, 120.6, 122.0, 125.7, 127.3, 128.3, 128.7, 132.1, 134.0, 143.4, 155.4, 157.2, 165.2; MS: m/z 340 (M+, 2), 235 (100), 221 (30), 178 (56), 163 (16), 145 (27), 120 (100), 118 (32), 105 (100), 93 (41), 77 (43), 58 (89%). Anal. Calcd for C17H16N4O2S: C, 60.00; H, 4.71; N, 16.47. Found: C, 59.89; H, 4.67; N, 16.39.
N-(3-Methoxybenzyl)-2-[2-oxo-2,3-dihydro-1H-indol-3-ylidene]-1-hydrazinecarbothioamide (5f)
Yellow fluffy crystals; yield 74%; mp 170°C; IR: 3350, 3194 (NH), 1686 (C=O), 1600 (C=N), 1151 cm−1 (C=S); 1H NMR: δ 3.74 (s, 3H, CH3), 4.85 (d, J=4.5 Hz, 2H, benzyl CH2), 6.83 (d, J=9.0 Hz, 1H, isatin C7-H), 6.85–6.95 (m, 3H, benzyl C2-H, C4-H, C6-H), 7.09 (t, J=9.0 Hz, 1H, benzyl C5-H), 7.26 (t, J=9.0 Hz, 1H, isatin C5-H), 7.36 (t, J=9.0 Hz, 1H, isatin C6-H), 7.65 (d, J=8.0 Hz, 1H, isatin C4-H), 9.79 (t, J=6.0 Hz, 1H, CSNH), 11.22 (s, 1H, isatin NH), 12.66 (s, 1H, NNH); 13C NMR: δ 47.1 (CH2), 55.0 (CH3), 111.0, 112.2, 113.2, 119.5, 119.9, 120.9, 122.3, 129.3, 131.2, 132.0, 139.9, 142.3, 159.2, 162.6, 177.7; MS: m/z 340 (M+, 7), 194 (24), 161 (18), 147 (93), 136 (92), 121 (95), 91 (100), 77 (95), 65 (55%). Anal. Calcd for C17H16N4O2S: C, 60.00; H, 4.71; N, 16.47. Found: C, 59.82; H, 4.67; N, 16.38.
N-(4-Methoxybenzyl)-2-[2-oxo-2,3-dihydro-1H-indol-3-ylidene]-1-hydrazinecarbothioamide (5g)
Yellow fluffy crystals; yield 82%; mp 230°C; IR: 3277, 3178 (NH), 1684 (C=O), 1605 (C=N), 1157 cm−1 (C=S); 1H NMR: δ 3.72 (s, 3H, CH3), 4.79 (d, J=6.0 Hz, 2H, benzyl CH2), 6.89 (d, J=8.8 Hz, 2H, benzyl C3-H, C5-H), 6.91 (d, J=8.0 Hz, 1H, isatin C7-H), 7.07 (t, J=7.6 Hz, 1H, isatin C5-H), 7.30 (d, J=8.8 Hz, 2H, benzyl C2-H, C6-H), 7.34 (td, J=8.0, 0.8 Hz, 1H, isatin C6-H), 7.64 (d, J=7.6 Hz, 1H, isatin C4-H), 9.70 (t, J=6.0 Hz, 1H, CSNH), 11.18 (s, 1H, isatin NH), 12.61 (s, 1H, NNH); 13C NMR: 47.2 (CH2), 55.5 (CH3), 111.5, 114.1, 120.4, 121.3, 122.7, 129.3, 130.8, 131.7, 132.4, 142.8, 158.9, 163.1, 177.8; MS: m/z 340 (M+, 15), 194 (55), 161 (47), 147 (52), 136 (52), 121 (100), 104 (13), 91 (17%). Anal. Calcd for C17H16N4O2S: C, 60.00; H, 4.71; N, 16.47. Found: C, 59.65; H, 4.66; N, 16.42.
N-(2-Fluorobenzyl)-2-[2-oxo-2,3-dihydro-1H-indol-3-ylidene]-1-hydrazinecarbothioamide (5h)
Yellow fluffy crystals; yield 79%; mp 210°C; IR: 3352, 3188 (NH), 1685 (C=O), 1610 (C=N), 1159 cm−1 (C=S); 1H NMR: δ 4.92 (d, J=6.0 Hz, 2H, benzyl CH2), 6.93 (d, J=8.0 Hz, 1H, isatin C7-H), 7.10 (t, J=8.0 Hz, 1H, isatin C5-H), 7.17–7.21 (m, 2H, benzyl C3-H, C6-H), 7.24–7.39 (m, 3H, benzyl C4-H, C5-H, isatin C6-H), 7.66 (d, J=6.0 Hz, 1H, isatin C4-H), 9.77 (t, J=6.0 Hz, 1H, CSNH), 11.24 (s, 1H, isatin NH), 12.71 (s, 1H, NNH); 13C NMR: δ 41.0 (CH2), 111.1, 114.9, 115.2, 119.9, 120.9, 122.3, 124.2, 124.3, 124.9, 125.1, 128.9, 128.9, 131.3, 132.2, 142.4, 161.4, 162.6, 178.03; MS: m/z 328 (M+, 10), 182 (13), 147 (51), 124 (52), 118 (31), 109 (100), 104 (24), 90 (16), 83 (47), 77 (32%). Anal. Calcd for C16H13FN4OS: C, 58.54; H, 3.96; N, 17.07. Found: C, 58.38; H, 3.87; N, 16.98.
N-(3-Fluorobenzyl)-2-[2-oxo-2,3-dihydro-1H-indol-3-ylidene]-1-hydrazinecarbothioamide (5i)
Yellow fluffy crystals; yield 80%; mp 215°C; IR: 3300, 3184 (NH), 1682 (C=O), 1600 (C=N), 1192 cm−1 (C=S); 1H-NMR: δ 4.87 (d, J=6.0 Hz, 2H, benzyl CH2), 6.92 (d, J=8.0 Hz, 1H, isatin C7-H), 7.08 (t, J=8.0 Hz, 2H, isatin C5-H, benzyl C5-H), 7.15–7.20 (m, 2H, benzyl C2-H, C6-H), 7.33–7.41 (m, 2H, benzyl C4-H, isatin C6-H), 7.64 (d, J=7.2 Hz, 1H, isatin C4-H), 9.81 (t, J=6.0 Hz, 1H, CSNH), 11.20 (s, 1H, isatin NH), 12.67 (s, 1H, NNH); 13C NMR: δ 47.18 (CH2), 111.56, 114.09, 114.33, 114.62, 120.39, 121.37, 122.77, 123.79, 123.83, 130.65, 130.76, 131.75, 132.71, 141.82, 141.91, 142.87, 161.01, 163.12, 164.23, 178.36; MS: m/z 328 (M+, 77), 300 (45), 252 (34), 203 (13), 182 (52), 161 (13), 147 (100), 132 (15), 124 (95), 118 (39), 109 (93), 104 (19), 83 (14), 77 (12%). Anal. Calcd for C16H13FN4OS: C, 58.54; H, 3.96; N, 17.07. Found: C, 58.43; H, 3.87; N, 17.02.
N-(4-Fluorobenzyl)-2-[2-oxo-2,3-dihydro-1H-indol-3-ylidene]-1-hydrazinecarbothioamide (5j)
Yellow fluffy crystals; yield 74%; mp 230°C; IR: 3374, 3248 (NH), 1699 (C=O), 1608 (C=N), 1163 cm−1 (C=S); 1H NMR: δ 3.30 (DMSO, benzyl CH2), 6.95 (d, J=8.0 Hz, 1H, isatin C7-H), 7.17 (tt, J=8.0, 2.4 Hz, 2H, benzyl C3-H, C5-H), 7.42 (dd, J=8.0, 2.4 Hz, 2H, benzyl C2-H, C6-H), 7.54–7.61 (m, 2H, isatin C5-H,C6-H), 7.85 (d, J=7.2 Hz, 1H, isatin C4-H), 10.93 (s, 1H, CSNH), 11.38 (s, 1H, isatin NH), 12.74 (s, 1H, NNH); 13C-NMR: δ 107.9, 108.3, 112.7, 121.1, 121.5, 126.6, 130.9, 131.8, 141.3, 160.3, 162.4, 163.5, 175.9; MS: m/z 328 (M+, 7), 182 (41), 161 (15), 147 (72), 132 (15), 124 (65), 118 (56), 109 (100), 104 (44), 90 (27), 83 (60), 77 (49%). Anal. Calcd for C16H13FN4OS: C, 58.54; H, 3.96; N, 17.07. Found: C, 58.45; H, 3.87; N, 17.01.
N-(3-Chlorobenzyl)-2-[2-oxo-2,3-dihydro-1H-indol-3-ylidene]-1-hydrazinecarbothioamide (5l)
Yellow crystals; yield 86%; mp 220°C; IR: 3350, 3142 (NH), 1695 (C=O), 1605 (C=N), 1148 cm−1 (C=S); 1H NMR: δ 3.30 (DMSO, benzyl CH2), 6.91 (d, J=7.6 Hz, 1H, isatin C7-H), 7.24 (t, J=7.6 Hz, 1H, isatin C5-H), 7.53 (d, J=7.8 Hz, 1H, benzyl C2-H) 7.64–7.72 (m, 3H, benzyl C4-H, C5-H, C6-H), 7.99–8.01 (m, 2H, isatin C4-H, C6-H), 9.76 (t, J=6.0 Hz, 1H, CSNH), 11.21 (s, 1H, isatin NH), 12.73 (s, 1H, NNH); 13C NMR: δ 93.6, 113.0, 114.2, 122.1, 123.7, 124.9, 130.3, 131.2, 133.6, 134.7, 139.6, 141.6, 162.3, 176.2; MS: m/z 346/344 (M+, 2/5), 200/198 (7/16), 147 (100), 142/140 (25/73), 132 (20), 127/125 (44/97), 118 (83), 104 (69), 89 (78), 77 (93%). Anal. Calcd for C16H13ClN4OS: C, 55.73; H, 3.77; N, 16.26. Found: C, 55.69; H, 3.69; N, 16.20.
N-(4-Chlorobenzyl)-2-[2-oxo-2,3-dihydro-1H-indol-3-ylidene]-1-hydrazinecarbothioamide (5m)
Yellow fluffy crystals; yield 85%; mp 250°C; IR: 3300, 3188 (NH), 1680 (C=O), 1604 (C=N), 1155 cm−1 (C=S); 1H NMR: δ 4.86 (d, J=6.0 Hz, 2H, benzyl CH2), 6.94 (d, J=8.0 Hz, 1H, isatin C7-H), 7.09 (t, J=7.6 Hz, 1H, isatin C5-H), 7.52–7.66 (m, 5H, benzyl C2-H. C3-H, C5-H, C6-H, isatin C6-H), 7.65 (d, J=6.0 Hz, 1H, isatin C4-H), 9.83 (t, J=6.0 Hz, 1H, CSNH), 11.23 (s, 1H, isatin NH), 12.68 (s, 1H, NNH); 13C NMR: δ 46.5 (CH2), 111.1, 119.9, 120.9, 122.3, 128.2, 129.2, 1313, 131.5, 132.2, 137.4, 142.4, 162.6, 177.8; MS: m/z 346/344 (M+, 3/1), 198(16), 147 (92), 140 (70), 125 (100), 118 (72), 104 (50), 90 (50), 77 (92%). Anal. Calcd for C16H13ClN4OS: C, 55.73; H, 3.77; N, 16.26. Found: C, 55.69; H, 3.77; N, 16.26.
N-(2, 4-Dichlorobenzyl)-2-[2-oxo-2,3-dihydro-1H-indol-3-ylidene]-1-hydrazinecarbothioamide (5n)
Yellow crystals; yield 82%; mp 265°C; IR: 3360, 3172 (NH), 1701 (C=O), 1599 (C=N), 1184 cm−1 (C=S); 1H NMR: δ 4.45 (d, J=5.6 Hz, 2H, benzyl CH2), 6.88 (d, J=7.6 Hz, 1H, isatin C7-H), 7.02 (t, J=8.0 Hz, 1H, isatin C5-H), 7.32–7.45 (m, 3H, benzyl C5-H, C6-H, isatin C6-H), 7.61 (d, J=2.0 Hz, 1H, benzyl C3-H), 7.85 (t, J=6 Hz, 1H, CSNH), 8.06 (d, J=7.6.Hz, 1H, isatin C4-H), 10.38 (s, 1H, isatin NH), 10.69 (s, 1H, NNH)); 13C NMR: δ 41.0 (CH2), 110.9, 116.1, 122.0, 125.8, 127.8, 129.0, 130.3, 132.3, 132.6, 133.2, 134.6, 136.4, 143.5, 155.7, 165.2; MS: m/z 364/362 (3/5), 329/327 (4/11), 186 (10), 166 (15), 161 (100), 140 (26), 132 (18), 104 (17%). Anal. Calcd for C16H12Cl2N4OS: C, 50.66; H, 3.17; N, 14.78. Found: C, 50.48; H, 3.14; N, 14.77.
N-(3, 4-Dichlorobenzyl)-2-[2-oxo-2,3-dihydro-1H-indol-3-ylidene]-1-hydrazinecarbothioamide (5o)
Yellow crystals; yield 95%; mp 230°C; IR: 3348, 3217 (NH), 1707 (C=O), 1610 (C=N), 1190 cm−1 (C=S); 1H NMR: δ 4.84 (d, J=6.0 Hz, 2H, benzyl CH2), 6.92 (d, J=8 Hz, 1H, isatin C7-H), 7.08 (t, J=7.6 Hz, 1H, isatin C5-H), 7.34–7.36 (m, 2H, isatin C6-H, benzyl C6-H), 7.59–7.64 (m, 3H, benzyl C2-H, C5-H, isatin C4-H), 9.81 (t, J=6.0 Hz, 1H, CSNH), 11.20 (s, 1H, isatin NH), 12.68 (s, 1H, NNH)); 13C NMR: δ 46.6 (CH2), 111.6, 120.3, 121.3, 122.8, 128.2, 129.8, 130.0, 130.9, 131.3, 131.8, 132.8, 140.1, 142.9, 163.1, 178.4; MS: m/z 380/378 (M+, 16/22), 352/350 (11/15), 234/232 (16/15), 203 (42), 176/174 (38/62), 161/159 (58/86), 147 (100), 144/142 (29/25), 140 (69), 118 (65), 104/102 (31/17), 91/89 (19/25), 77/75 (29/25), 63/61 (24/13%). Anal. Calcd for C16H12Cl2N4OS: C, 50.66; H, 3.17; N, 14.78. Found: C, 50.49; H 3.15; N, 14.76.
Crystallographic data collection and structural refinement
A crystal of 5h was mounted on a thin glass fiber at room temperature and the reflection data were collected on a Bruker Kappa APE XII CCD diffractometer equipped with graphite monochromated MoKα radiation (λ=0.71073 Å). The data were corrected for Lorentz and polarization effects. The structure was solved using SHELXS-97 [51]. A final refinement on F2 was carried out by full-matrix least-squares techniques using SHELXL-97 [51].
Biological assays
In vitro antiurease, antiglycation, phytotoxic and toxic activities of thiosemicarbazones 5a–o were performed by using the reported methods [52], [36], [38], [41].
Molecular docking studies
Docking studies of compounds 5a–o were carried out in accordance with the previously described protocols [52], [53], [54], [55], [56], [57], [58].
Acknowledgments
The authors thank the Higher Education Commission (HEC) of Pakistan for funding under the National Research Support Program for Universities (Project No. 20-873/R&D/07/452).
References
[1] da Silva, J. F. M.; Garden, S. J.; Pinto, A. C. The chemistry of isatins: a review from 1975 to 1999. J. Braz. Chem. Soc. 2001, 12, 273–324.10.1590/S0103-50532001000300002Suche in Google Scholar
[2] Pandeya, S. N.; Smitha, S.; Jyoti, M.; Sridhar, S. K. Biological activities of isatin and its derivatives. Acta Pharm. 2005, 55, 27–46.Suche in Google Scholar
[3] Cerchiaro, G.; Ferreira, A. M. C. Oxindoles and copper complexes with oxindole-derivatives as potential pharmacological agents. J. Braz. Chem. Soc. 2006, 17, 1473–1485.10.1590/S0103-50532006000800003Suche in Google Scholar
[4] Vine, K. L.; Matesic, L.; Locke, J. M.; Ranson, M.; Skropeta, D. Cytotoxic and anticancer activities of isatin and its derivatives: a comprehensive review from 2000–2008. Anti-Cancer Agents Med. Chem. 2009, 9, 397–414.10.2174/1871520610909040397Suche in Google Scholar
[5] Bhrigu, B.; Pathak, D.; Siddiqui, N.; Alam, M.S.; Ahsan, W. Search for biological active isatins: a short review. Int. J. Pharm. Drug Res. 2010, 2, 229–235.Suche in Google Scholar
[6] Aboul-Fadl, T.; Bin-Jubair, F. A. S. Anti-tubercular activity of isatin derivatives. Int. J. Res. Pharm. Sci. 2010, 1, 113–126.Suche in Google Scholar
[7] Pal, M.; Sharma, N. K.; Priyanka, J. K. K.; Jha, K. K. Synthetic and biological multiplicity of isatin: a review. J. Adv. Sci. Res. 2011, 2, 35–44.10.1002/chin.201144253Suche in Google Scholar
[8] Raj, V. Review on CNS activity of isatin derivatives. Int. J. Curr. Pharm. Res. 2012, 4, 1–9.Suche in Google Scholar
[9] Pakravan, P.; Kashanian, S.; Khodaei, M. M.; Harding, F. Biochemical and pharmacological characterization of isatin and its derivatives: from structure to activity. J. Pharmacol. Rep. 2013, 65, 313–335.10.1016/S1734-1140(13)71007-7Suche in Google Scholar
[10] Khan, K. M.; Khan, M.; Ali, M.; Qadir, M. I.; Perveen, S.; Karim, A.; Choudhary, M. I. Superoxide respiratory burst inhibitory activity of bis-Schiff bases of isatin. J. Chem. Soc. Pak. 2013, 35, 987–993.Suche in Google Scholar
[11] Vine, K. L.; Matesic, L.; Locke, J. M.; Scropeta, D. Recent highlights in the development of isatin based anticancer agents. In Advances in Anticancer Agents in Medicinal Chemistry. Prudhomme, M., Ed.; Bentham Science Publishers: Sharjah, UAE, 2013, pp 254–312.10.2174/9781608054961113020008Suche in Google Scholar
[12] Grewal, A. S. Isatin derivatives with several biological activities. Int. J. Pharm. Res. 2014, 6, 1–7.Suche in Google Scholar
[13] Mathur, G.; Nain, S. Recent advancement in synthesis of isatin as anticonvulsant agents: a review. Med. Chem. 2014, 4, 417–427.10.4172/2161-0444.1000173Suche in Google Scholar
[14] Adhikari, S.; Bari, S. B.; Samanta, A. Synthesis and screening of some new isatin containing thiazole derivatives for antimicrobial activity. J. Appl. Chem. Res. 2014, 8, 31–40.Suche in Google Scholar
[15] Phogat, P.; Singh, P. A mini review on central nervous system potential of isatin derivatives. Cent. Nerv. Syst. Agents Med. Chem. 2015, 15, 28–31.10.2174/1871524915666150213122246Suche in Google Scholar
[16] Hussain, A. Z.; Meeran, M. N. Synthesis, characterization and antimicrobial activity of some isatin based Schiff base compounds. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1598–1601.Suche in Google Scholar
[17] Rahim, F.; Malik, F.; Ullah, H.; Wadood, A.; Khan, F.; Javid, M. T.; Taha, M.; Rehman, W.; Rehman, A. U.; Khan, K. M. Isatin based Schiff bases as inhibitors of α-glucosidase: synthesis, characterization, in vitro evaluation and molecular docking studies. Bioorg. Chem. 2015, 60, 42–48.10.1016/j.bioorg.2015.03.005Suche in Google Scholar
[18] Khan, M.; Khan, K. M.; Rahim, F.; Samreen; Perveen, S.; Karim, A.; Imtiazuddin; Choudhary, M. I. Synthesis leishmanicidal activities of bis-Schiff bases of isatins. J. Chem. Soc. Pak. 2015, 37, 520–526.Suche in Google Scholar
[19] Corona, A.; Meleddu, R.; Esposito, F.; Distinto, S.; Bianco, G.; Masaoka, T.; Maccioni, E.; Menéndez-Arias, L.; Alcaro, S.; Le Grice, S. F. J.; et al. Ribonuclease H/DNA polymerase HIV-1 reverse transcriptase dual inhibitor: mechanistic studies on the allosteric mode of action of isatin-based compound RMNC6. PLoS One2016, 11, 1–18.10.1371/journal.pone.0147225Suche in Google Scholar
[20] Ziarani, G. M.; Moradi, R.; Lashgari, N. Synthesis of spiro-fused heterocyclic scaffolds through multicomponent reactions involving isatin. ARKIVOC2016, (i), 1–81.10.3998/ark.5550190.p009.385Suche in Google Scholar
[21] Karali, N.; Terzioğlu, N.; Gürsoy, A. Synthesis and primary cytotoxicity evaluation of new 5-bromo-3-substituted-hydrazono-1H-2-indolinones. Arch. Pharm. (Weinheim)2002, 335, 374–380.10.1002/1521-4184(200211)335:8<374::AID-ARDP374>3.0.CO;2-KSuche in Google Scholar
[22] Ermut, G.; Karalı, N.; Cetin, I.; Topcul, M.; Birteksoz, S. Synthesis and chemotherapeutic activities of 5-chloro-1H-indole-2,3-dione 3-thiosemicarbazones. Marmara Pharm. J. 2013, 17, 147–154.10.12991/201317383Suche in Google Scholar
[23] Karali, N.; Gürsoy, A.; Kandemirli, F.; Shvets, N.; Kaynak, F. B.; Özbey, S.; Kovalishyn, V.; Dimoglo, A. Synthesis and structure–antituberculosis activity relationship of 1H-indole-2,3-dione derivatives. Bioorg. Med. Chem. 2007, 15, 5888–5904.10.1016/j.bmc.2007.05.063Suche in Google Scholar PubMed
[24] Guzel, O.; Karali, N.; Salman, A. Synthesis and antituberculosis activity of 5-methyl/trifluoromethoxy-1H-indole-2,3-dione 3-thiosemicarbazone derivatives. Bioorg. Med. Chem. 2008, 16, 8976–8987.10.1016/j.bmc.2008.08.050Suche in Google Scholar PubMed
[25] Banerjee, D.; Yogeeswari, P.; Bhat, P.; Thomas, A.; Srividya, M.; Sriram, D. Novel isatinyl thiosemicarbazones derivatives as potential molecule to combat HIV-TB co-infection. Eur. J. Med. Chem. 2011, 46, 106–121.10.1016/j.ejmech.2010.10.020Suche in Google Scholar PubMed
[26] Pervez, H.; Iqbal, M. S.; Tahir, M. Y.; Choudhary, M. I.; Khan, K. M. Synthesis of some N4-substituted isatin-3-thiosemicarbazones. Nat. Prod. Res. 2007, 21, 1178–1186.10.1080/14786410601129770Suche in Google Scholar PubMed
[27] Pervez, H.; Iqbal, M. S.; Tahir, M. Y.; Nasim, F. H.; Choudhary, M. I.; Khan, K. M. In vitro cytotoxic, antibacterial, antifungal and urease inhibitory activities of some N4-substituted isatin-3-thiosemicarbazones. J. Enzyme Inhib. Med. Chem. 2008, 23, 848–854.10.1080/14756360701746179Suche in Google Scholar PubMed
[28] Pervez, H.; Chohan, Z. H.; Ramzan, M.; Nasim, F. H.; Khan, K. M. Synthesis and biological evaluation of some new N4-substituted isatin-3-thiosemicarbazones. J. Enzyme Inhib. Med. Chem. 2009, 24, 437–446.10.1080/14756360802188420Suche in Google Scholar PubMed
[29] Pervez, H.; Ramzan, M.; Yaqub, M.; Khan, K. M. Synthesis, cytotoxic and phytotoxic effects of some new N4-aryl substituted isatin-3-thiosemicarbazones. Lett. Drug. Des. Discov. 2011, 8, 452–458.10.2174/157018011795514159Suche in Google Scholar
[30] Faidullah, H. M.; Khan, K. A.; Asiri, A. M. Synthesis and biological evaluation of new 3-trifluoromethylpyrazolesulfonyl-urea and thiourea derivatives as antidiabetic and antimicrobial agents. J. Fluorine Chem. 2011, 132, 131–137.10.1016/j.jfluchem.2010.12.009Suche in Google Scholar
[31] Shantharam, C. S.; Suyoga Vardhan, D. M.; Suhas, R.; Sridhara, M. B.; Gowda, D. C. Inhibition of protein glycation by urea and thiourea derivatives of glycine/proline conjugated benzisoxazole analogue – synthesis and structure-activity studies. Eur. J. Med. Chem. 2013, 60, 325–332.10.1016/j.ejmech.2012.12.029Suche in Google Scholar PubMed
[32] Shantharam, C. S.; Suyoga Vardhan, D. M.; Suhas, R.; Channe Gowda, D. C. Design and synthesis of amino acids-conjugated heterocycle derived ureas/thioureas as potent inhibitors of protein glycation. Bioorg. Khim. 2014, 40, 443–454.10.1134/S1068162014040128Suche in Google Scholar
[33] Shantharam, C. S. N.; Raghu, M. S. Design and synthesis of novel N-capped urea/thiourea and C-capped [3-(4-piperidyl)-6-fluoro-1,2-benzisoxazole] linked amino acids as antiglycating agents. Eur. J. Pharmaceut. Medical Res. 2015, 2, 220–228.Suche in Google Scholar
[34] Suyoga Vardhan, D. M.; Shantharam, C. S.; Suhas, R.; Gowda, D. C. Synthesis and evaluation of novel ureido/thioureido derivatives of amino acid conjugated 2,3-dichlorophenyl piperazine as highly potent antiglycating agents. J. Saudi Chem. Soc. 2017, 21, S248–S257.10.1016/j.jscs.2014.02.006Suche in Google Scholar
[35] Khan, K. M.; Mughal, U. R.; Ambreen, N.; Khan, A.; Perveen, S.; Choudhary, M. I. Schiff bases of isatin: antiglycation activity. Lett. Drug Des. Discov. 2009, 6, 358–362.10.2174/1570180810906050358Suche in Google Scholar
[36] Khan, K. M.; Khan, M.; Ali, M.; Taha, M.; Rasheed, S.; Perveen, S.; Choudhary, M. I. Synthesis of bis-Schiff bases of isatins and their antiglycation activity. Bioorg. Med. Chem. 2009, 17, 7795–7801.10.1016/j.bmc.2009.09.028Suche in Google Scholar PubMed
[37] Pervez, H.; Manzoor, N.; Yaqub, M.; Khan, A.; Khan, K. M.; Nasim, F. H.; Choudhary, M. I. Synthesis and urease inhibitory properties of some new N4-substituted 5-nitroisatin-3-thiosemicarbazones. Lett. Drug Des. Discov. 2010, 7, 102–108.10.2174/157018010790225840Suche in Google Scholar
[38] Pervez, H.; Saira, N.; Iqbal, M. S.; Yaqub, M.; Khan, K. M. Synthesis and toxicity evaluation of some N4-aryl substituted 5-trifluoromethoxyisatin-3-thiosemicarbazones. Molecules2011, 16, 6408–6421.10.3390/molecules16086408Suche in Google Scholar PubMed PubMed Central
[39] Pervez, H.; Manzoor, N.; Yaqub, M.; Nasim, F. H.; Khan, K. M. Synthesis and biological evaluation of some N4-substituted 5-nitroisatin-3-thiosemicarbazones. Med. Chem. Res. 2012, 21, 2251–2262.10.1007/s00044-011-9745-7Suche in Google Scholar
[40] Pervez, H.; Ramzan, M.; Yaqub, M.; Nasim, F. H.; Khan, K. M. Synthesis and biological evaluation of some new N4-aryl substituted 5-chloroisatin-3-thiosemicarbazones. Med. Chem. 2012, 8, 505–514.10.2174/1573406411208030505Suche in Google Scholar PubMed
[41] Pervez, H.; Saira, N.; Iqbal, M. S.; Yaqub, M.; Khan, K. M. Synthesis and biological evaluation of some N4-aryl-substituted 5-fluoroisatin-3-thiosemicarbazones. Med. Chem. Res. 2013, 22, 5878–5889.10.1007/s00044-013-0575-7Suche in Google Scholar
[42] Pervez, H.; Manzoor, N.; Yaqub, M.; Khan, K. M. 5-Nitroisatin-derived thiosemicarbazones: potential antileishmanial agents. J. Enzyme Inhib. Med. Chem. 2014, 29, 628–632.10.3109/14756366.2013.836641Suche in Google Scholar PubMed
[43] Pervez, H.; Ahmad, M.; Zaib, S.; Yaqub, M.; Naseer, M. M.; Iqbal, J. Synthesis, cytotoxic and urease inhibitory activities of some novel isatin-derived bis-Schiff bases and their copper(II) complexes. Med. Chem. Commun. 2016, 7, 914–923.10.1039/C5MD00529ASuche in Google Scholar
[44] Ahmad, M.; Pervez, H.; Zaib, S.; Yaqub, M.; Naseer, M. M.; Khan, S. U.; Iqbal, J. Synthesis, biological evaluation and docking studies of some novel isatin-3-hydrazonothiazolines. RSC Adv. 2016, 6, 60826–60844.10.1039/C6RA10043KSuche in Google Scholar
[45] Hall, M. D.; Brimacombe, K. R.; Varonka, M. S.; Pluchino, K. M.; Monda, J. K.; Li, J.; Walsh, M. J.; Boxer, M. B.; Warren, T. H.; Fales, H. M.; et al. Synthesis and structure-activity evaluation of isatin-β-thiosemicarbazones with improved selective activity toward multidrug-resistant cells expressing P-glycoprotein. J. Med. Chem. 2011, 54, 5878–5889.10.1021/jm2006047Suche in Google Scholar
[46] Pervez, H.; Khan, N.; Iqbal, M. S.; Yaqub, M.; Tahir, M. N. (2Z)-N-(2-Chlorobenzyl)-2-(2-oxo-2,3-dihydro-1H-indol-3-ylidene)hydrazinecarbothioamide. Acta Crystallogr. 2012, E68, o2731.10.1107/S1600536812035076Suche in Google Scholar
[47] Omar, A.-M. M. E.; Eshba, N. H.; Salama, H. M. Syntheses of some substituted isatin-beta-thiosemicarbazones and isatin-beta-hydrazonothiazoline derivatives as potential antiviral and antimicrobial agents. Arch. Pharm. 1984, 317, 701–709.10.1002/ardp.19843170810Suche in Google Scholar
[48] Karali, N. Synthesis and primary cytotoxicity evaluation of new 5-nitroindole-2,3-dione derivatives. Eur. J. Med. Chem. 2002, 37, 909–918.10.1016/S0223-5234(02)01416-2Suche in Google Scholar
[49] Jawaria, R.; Hussain, M.; Shafiq, Z.; Ahmad, H. B.; Tahir, M. N.; Shad, H. A.; Naseer, M. M. Robustness of thioamide dimer synthon, carbon bonding and thioamide–thioamide stacking in ferrocene-based thiosemicarbazones. CrystEngComm. 2015, 17, 2553–2561.10.1039/C4CE02566KSuche in Google Scholar
[50] Hameed, A.; Shafiq, Z.; Yaqub, M.; Hussain, M.; Ahmad, H. B.; Tahir, M. N.; Naseer, M. M. Robustness of a thioamide synthon: synthesis and the effect of substituents on the formation of layered to cage-like supramolecular networks in coumarin–thiosemicarbazone hybrids. New J. Chem. 2015, 39, 6052–6061.10.1039/C5NJ00734HSuche in Google Scholar
[51] Sheldrick, G. M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122.10.1107/S0108767307043930Suche in Google Scholar PubMed
[52] Rauf, M. K.; Talib, A.; Badshah, A.; Zaib, S.; Shoaib, K.; Shahid, M.; Flörke, U.; Din, I. U.; Iqbal, J. Solution-phase microwave assisted parallel synthesis of N,N’-disubstituted thioureas derived from benzoic acid: biological evaluation and molecular docking studies. Eur. J. Med. Chem. 2013, 70, 487–496.10.1016/j.ejmech.2013.10.012Suche in Google Scholar PubMed
[53] Balasubramanian, A.; Ponnuraj, K. Crystal structure of the first plant urease from jack bean: 83 years of journey from its first crystal to molecular structure. J. Mol. Biol. 2010, 400, 274–283.10.1016/j.jmb.2010.05.009Suche in Google Scholar PubMed
[54] Labute, P. 3D: Assignment of macromolecular protonation state and geometry, Chemical Computing Group, 2007. http://www.chemcomp.com/journal/proton.htm.Suche in Google Scholar
[55] MOE, Version 2014.0901, Chemical Computing Group (CCG), Montreal, Canada; http://www.chemcomp.com/MOE-Molecular_Operating_Environment.htm. Accessed January 2017.Suche in Google Scholar
[56] LeadIT, 2014, at http://www.biosolveit.de/LeadIT/ index.html?ct=1. Accessed January 2017.Suche in Google Scholar
[57] Accelrys Software Inc. Discovery Studio Modeling Environment, Release 4.0; Accelrys Software: San Diego, CA, 2013.Suche in Google Scholar
[58] Schneider, N.; Lange, G.; Hindle, S.; Klein, R.; Rarey, M. A consistent description of HYdrogen bond and DEhydration energies in protein-ligand complexes: methods behind the HYDE scoring function. J. Comput. Aided Mol. Des.2013, 27, 15–29.10.1007/s10822-012-9626-2Suche in Google Scholar PubMed
[59] Ashton, W. T.; Cantone, C. L.; Chang, L. L.; Hutchins,S. M.; Strelitz, R. A.; MacCoss, M.; Chang, R. S. L.; Lotti, V. J.; Faust, K. A.; Chen, T-.B.; et al. Nonpeptide angiotensin II antagonists derived from 4H-1,2,4-triazoles and 3H-imidazo[1,2-b][1,2,4]triazoles. J. Med. Chem.1993, 36, 591–609.10.1021/jm00057a009Suche in Google Scholar PubMed
Supplementary Material:
The online version of this article offers supplementary material (https://doi.org/10.1515/hc-2017-0148).
©2018 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- Review
- Research progress in quinazoline derivatives as multi-target tyrosine kinase inhibitors
- Research Articles
- A bio-inspired approach to proline-derived 2,4-disubstituted oxazoles
- Solvent-free preparation of 3-aryl-2-[(aryl)(arylamino)]methyl-4H-furo[3,2-c]chromen-4-one derivatives using ZnO-ZnAl2O4 nanocomposite as a heterogeneous catalyst
- Synthesis of 4-arylethyl-6-arylpyrimidine-2-thiols through aza-Michael addition/nucleophilic addition/aromatization tandem reactions
- 3-Carboxy-1-sulfopyridin-1-ium chloride ([CPySO3H]+Cl−): an efficient catalyst for one-pot synthesis of hexahydroquinoline-3-carboxamides
- Synthesis of new 3H-chromeno[2,3-d]pyrimidine-4,6(5H,7H)-diones via the tandem intramolecular Pinner/Dimroth rearrangement
- A new synthesis of pyrrolo[3,2-d]pyrimidine derivatives by a one-pot, three-component reaction in the presence of L-proline as an organocatalyst
- Diversity-oriented synthesis of amide derivatives of tricyclic thieno[2,3-d]pyrimidin-4(3H)-ones and evaluation of their influence on melanin synthesis in murine B16 cells
- Synthesis, crystal structure, molecular docking studies and bio-evaluation of some N4-benzyl-substituted isatin- 3-thiosemicarbazones as urease and glycation inhibitors
- Ultrasound-assisted synthesis and antimicrobial activity of tetrazole-based pyrazole and pyrimidine derivatives
Artikel in diesem Heft
- Frontmatter
- Review
- Research progress in quinazoline derivatives as multi-target tyrosine kinase inhibitors
- Research Articles
- A bio-inspired approach to proline-derived 2,4-disubstituted oxazoles
- Solvent-free preparation of 3-aryl-2-[(aryl)(arylamino)]methyl-4H-furo[3,2-c]chromen-4-one derivatives using ZnO-ZnAl2O4 nanocomposite as a heterogeneous catalyst
- Synthesis of 4-arylethyl-6-arylpyrimidine-2-thiols through aza-Michael addition/nucleophilic addition/aromatization tandem reactions
- 3-Carboxy-1-sulfopyridin-1-ium chloride ([CPySO3H]+Cl−): an efficient catalyst for one-pot synthesis of hexahydroquinoline-3-carboxamides
- Synthesis of new 3H-chromeno[2,3-d]pyrimidine-4,6(5H,7H)-diones via the tandem intramolecular Pinner/Dimroth rearrangement
- A new synthesis of pyrrolo[3,2-d]pyrimidine derivatives by a one-pot, three-component reaction in the presence of L-proline as an organocatalyst
- Diversity-oriented synthesis of amide derivatives of tricyclic thieno[2,3-d]pyrimidin-4(3H)-ones and evaluation of their influence on melanin synthesis in murine B16 cells
- Synthesis, crystal structure, molecular docking studies and bio-evaluation of some N4-benzyl-substituted isatin- 3-thiosemicarbazones as urease and glycation inhibitors
- Ultrasound-assisted synthesis and antimicrobial activity of tetrazole-based pyrazole and pyrimidine derivatives


