Abstract
3-Carboxy-1-sulfopyridin-1-ium chloride ([CPySO3H]+Cl−) was synthesized and evaluated as a recoverable catalyst for one-pot synthesis of hexahydroquinoline-3-carboxamide derivatives by a four-component reaction of an arylaldehyde, dimedone, acetoacetanilide and ammonium acetate. The [CPySO3H]+Cl− catalyst was characterized by infrared (IR), 1H nuclear magnetic resonance (NMR), 13C NMR, elemental analysis and thermal gravimetric analysis (TGA).
Introduction
Quinolines containing a 1,4-dihydropyridine (1,4-DHP) moiety show a variety of pharmacological properties [1], [2], [3], [4], [5], [6], [7], [8]. Acetoacetanilide is an important building block in the synthesis of heterocyclic compounds with antimicrobial [9], [10] and analgesic activities [11]. The synthesis of hexahydroquinoline-3-carboxamides via a four-component reaction of acetoacetanilide, aromatic aldehyde, dimedone and ammonium acetate in the presence of p-toluenesulfonic acid [12] and without catalyst under harsh conditions has been reported [13]. Herein, we report that this reaction can be conducted under much milder conditions in the presence of another catalyst.
Results and discussion
In this study, 3-carboxy-1-sulfopyridin-1-ium chloride ([CPySO3H]+Cl−) was successfully used as a catalyst in the reaction of arylaldehydes, dimedone, acetoacetanilide and ammonium acetate in ethanol at 70°C to furnish a series of hexahydroquinoline-3-carboxamides 5a–l in excellent yields (Scheme 1). The catalyst was obtained by treatment of nicotinic acid with chlorosulfonic acid (Scheme 2). The structure of [CPySO3H]+Cl− was supported by infrared (IR), 1H nuclear magnetic resonance (NMR), 13C NMR, thermal gravimetric analysis (TGA) and elemental analysis. In the IR spectrum, the stretching vibrations for O-H near 3151 cm−1, for C=O at 1728 cm−1, for C=N at 1633 cm−1, for C=C at 1600 cm−1 and 1537 cm−1 and the SO2 asymmetric and symmetric stretching at 1272 cm−1 and 1172 cm−1, respectively, were observed. These results provide evidence that sulfonic acid moiety is part of the molecular structure of the catalyst.
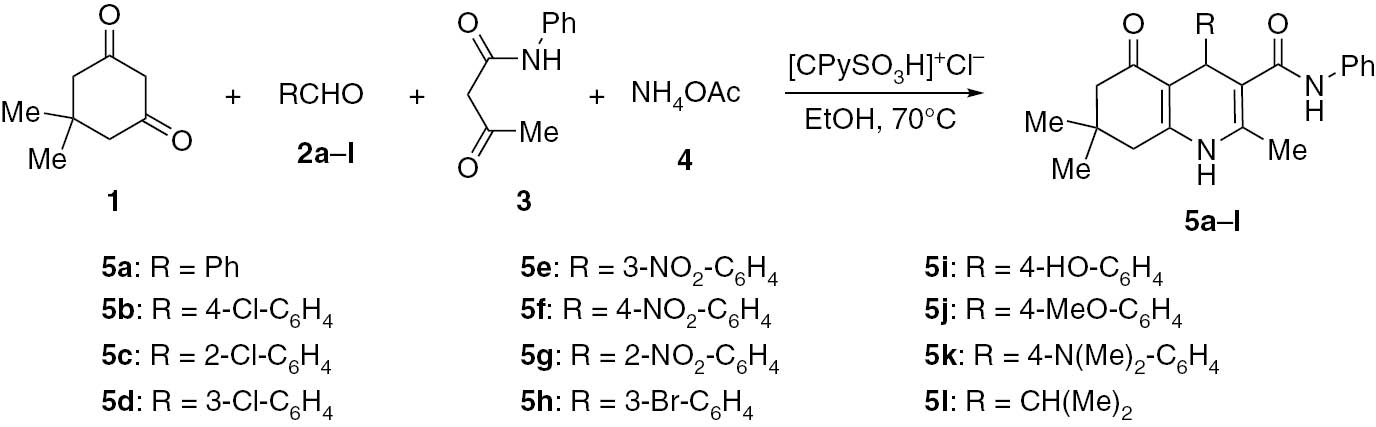
Synthesis of hexahydroquinoline-3-carboxamides 5a–l.
![Scheme 2 Preparation of [CPySO3H]+Cl−.](/document/doi/10.1515/hc-2017-0211/asset/graphic/j_hc-2017-0211_scheme_002.jpg)
Preparation of [CPySO3H]+Cl−.
The thermal behavior of [CPySO3H]+Cl− was studied by TGA. The thermal analysis indicated that the catalyst was stable up to 250°C. A slow decomposition could be observed starting around 300°C.
To optimize the reaction conditions, the four-component reaction of benzaldehyde, dimedone, acetoacetanilide and ammonium acetate was examined as a model reaction. The highest yield of 5a was obtained for the reaction conducted in ethanol at 70°C in the presence of 20 mol% of [CPySO3H]+Cl−. Under these optimized conditions, the reaction furnished products 5b–l in the range of yields from 88% to 96%. The optimum reaction time was analyzed using thin-layer chromatography (TLC) and was found to vary from 25 min to 45 min. The structure of all products was fully supported by IR, 1H NMR, 13C NMR and elemental analysis.
To study the recyclability of the catalyst, the synthesis of compound 5a was conducted 5 times. Upon completion of the reaction, the catalyst was filtered, washed with dichloromethane (2×10 mL) and then reused in the subsequent preparation of 5a. It was found that the initial yield of 5a of 95% decreased only to 90% in the fifth preparation. Also, the catalyst retained its activity after several months of storage.
Conclusions
A simple and efficient one-pot procedure for the synthesis of hexahydroquinolines using [CPySO3H]+Cl− as an ionic organocatalyst in ethanol at 70°C was decribed. Simplicity of the preparation, easy work-up, high yields and recyclability of the catalyst are the advantages of this method.
Experimental
Melting points were measured on an Electro-thermal 9100 apparatus and are uncorrected. Unless stated otherwise, 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were recorded in CDCl3 on a Bruker Avance DRX-400 spectrometer. Fourier-transform infrared (FT-IR) spectra were obtained for potassium bromide pellets on a Shimadzu SP-1100 instrument. The TGA was conducted using a Mettler TGA instrument. The thermograms were recorded at a heating rate of 10°C/min in the range of temperature from 25°C to 600°C in an inert atmosphere. Elemental analyses were done on a Carlo-Erba EA1110 analyzer.
Catalyst preparation
The catalyst was synthesized according to the reported method for the preparation of a similar catalyst [14], [15]. A solution of chlorosulfonic acid (10 mmol) in CH2Cl2 (15 mL) was added dropwise to a solution of nicotinic acid (10 mmol) in CH2Cl2 (25 mL), and the mixture was stirred for 2 h at room temperature. The resulting precipitate was filtered, washed with CH2Cl2 (2×10 mL) and dried in a desiccator under reduced pressure to give [CPySO3H]+Cl− as a white stable powder: Yield 94%; IR: 3157 (O-H stretch), 1731 (C=O stretch), 1631 (C=N stretch), 1621, 1533 (aromatic C=C stretch), 1176, 1107 cm−1 (SO2 asymmetric and symmetric stretch); 1H NMR (DMSO-d6): δ 11.74 (br s, 2H), 9.27 (d, J=1.6 Hz, 1H), 9.05 (dd, J=6.0, 1.6 Hz, 1H), 8.82 (m, 1H), 8.06 (m, 1H); 13C NMR (DMSO-d6): δ 164.0, 147.5, 145.2, 144.4, 129.7 127.2. Anal. Calcd C6H6NSO5Cl: C, 30.07; H, 2.52, N, 5.85. Found: C, 30.13; H, 2.64, N, 5.89.
General procedure for synthesis of hexahydroquinoline-3-carboxamides 5a–l
A mixture of aromatic aldehyde (1 mmol), dimedone (1 mmol, 0.14 g), acetoacetanilide (1 mmol, 0.18 g) and ammonium acetate (1.2 mmol, 0.09 g) in the presence of [CPySO3H]+Cl− (0.05 g, 0.2 mmol) was stirred in ethanol (5 mL) at 70°C for 15–45 min. Completion of the reaction was indicated by TLC monitoring. Then, the mixture was cooled to ambient temperature, and the resultant precipitate of 5a–l was crystallized from ethanol. All products were characterized by IR, 1H NMR and 13C NMR, and the data for known compounds were compared with those of the authentic samples reported in the literature. Yields for known compounds: 5a: 95%, reported: 78% [15], 89% [12]; 5b: 96%, reported: 92% [12]; 5d: 93%, reported 74% [15]; 5e: 95%, reported: 83% [12]; 5f: 94%, reported: 83% [12]; 5g: 92%, reported: 84% [15]; 5i: 87%, reported: 76% [15]; 5j: 88%, reported: 94% [12].
4-(2-Chlorophenyl)-2,7,7-trimethyl-5-oxo-N-phenyl-1,4,5,6,7,8-hexahydroquinoline-3-carboxamide (5c)
Reaction time 20 min; yield 90%; white solid; mp 225–227°C; IR: 3265 (N-H stretch), 2956 (aliphatic C-H stretch), 1677 (C=O), 1645 (C=O), 1498 (C=C stretch), 752 cm−1 (aromatic C-H out of plane bending); 1H NMR: δ 0.96 (s, 3H, CH3), 1.06 (s, 3H, CH3), 1.95 (s, 3H, CH3), 1.98 (d, J=13 Hz, 1H), 2.13 (d, J=16 Hz, 1H), 2.32 (d, J=16 Hz, 1H), 2.41 (d, J=16 Hz, 1H), 5.35 (s, 1H, CH), 6.97–7.29 (m, 7H, Ar), 7.53 (d, J=7.6 Hz, 2H, Ar), 8.74 (s, 1H, NH), 9.72 (s, 1H, NHCO); 13C NMR: δ 19.5, 27.1, 29.3, 32.6, 35.3, 41.2, 50.5, 108.6, 110.9, 120.3, 124.0, 127.7, 128.3, 128.9, 129.7, 131.0, 131.6, 138.1, 140.4, 143.5, 148.6, 165.8, 194.9 (C=O). Anal. Calcd for C25H25ClN2O2: C, 71.33, H, 5.99, N, 6.66. Found: C, 71.13, H, 6.04, N, 6.59.
4-(3-Bromophenyl)-2,7,7-trimethyl-5-oxo-N-phenyl-1,4,5,6,7,8-hexahydroquinoline-3-carbox amide (5h)
Reaction time 15 min; yield 96%; yellow solid; mp 211–213°C; IR: 3276 (N-H stretch), 3062 (aromatic C-H), 2956 (C-H stretch), 1674 (C=O stretch), 1643 (C=O stretch), 752 cm−1 (aromatic C-H out of plane bending); 1H NMR: δ 0.96 (s, 3H, CH3), 1.10 (s, 3H, CH3), 2.13 (s, 1H), 2.18–2.20 (m, 1H), 2.22–2.28 (m, 2H), 2.36 (s, 3H, CH3), 5.40 (s, 1H, CH), 6.09 (s,1H, NH), 7.08 (m, 1H, Ar), 7.11–7.15 (m, 1H, Ar), 7.21–7.25 (m, 1H, Ar), 7.28–7.27 (m, 2H, Ar), 7.31–7.35 (m, 2H, Ar), 7.45–7.48 (m, 2H, Ar), 7.61 (s, 1H, NH); 13C NMR: δ 19.0, 27.3, 29.5, 32.5, 36.6, 50.8, 108.2, 111.6, 119.9, 120.0, 123.2, 127.5, 127.7, 128.8, 129.1, 131.2, 131.8, 133.9, 139.8, 139.9, 145.3, 151.4, 151.5, 167.3, 193.7 (C=O). Anal. Calcd for C25H25BrN2O2: C, 64.25, H, 5.41, N, 6.02. Found: C, 63.83, H, 5.64, N, 6.09.
4-(4-(Dimethylamino)phenyl)-2,7,7-trimethyl-5-oxo-N-phenyl-1,4,5,6,7,8-hexahydroquinoline-3-carboxamide (5k)
Reaction time 30 min; yield 92%; orange solid; mp 248–250°C: IR: 3269 (N-H stretch), 3066 (aromatic C-H), 2952 (C-H stretch), 1674 (C=O stretch), 1637 (C=O stretch), 754 cm−1 (aromatic C-H out of plane bending); 1H NMR: δ 0.87 (s, 3H, CH3), 1.03 (s, 3H, CH3), 2.11–2.26 (m, 4H), 2.37 (s, 3H, CH3), 2.39 (s, 6H, 2CH3), 4.82 (s, 1H, CH), 6.72 (d, J=8 Hz, 2H), 6.94 (s, 1H, Ar), 7.04 (m, 1H, Ar), 7.23–7.34 (m, 4H, Ar), 7.36 (d, J=4 Hz, 2H, Ar), 7.52 (s, 1H, NH), 9.72 (s, 1H, NHCO): 13C NMR: δ 18.0, 27.2, 29.2, 32.5, 37.0, 40.1, 40.5, 50.7, 76.8, 77.1, 77.4, 108.3, 111.0, 111.05, 112.9, 119.8, 123.8, 128.7, 128.8, 133.6, 138.3, 141.3, 149.1, 167.0, 195.0 (C=O). Anal. Calcd for C27H31N3O2: C, 75.50, H, 7.27, N, 9.78. Found: C, 75.33, H, 7.35, N, 9.69.
4-Isopropyl-2,7,7-trimethyl-5-oxo-N-phenyl-1,4,5,6,7,8-hexahydroquinoline-3-carboxamide (5l)
Reaction time 45 min; yield 90%; yellow solid; mp 234–236°C, IR: 3296 (N-H stretch), 2956 (aliphatic C-H stretch), 1664 (C=O), 1637 (C=O), 1490 (C=C stretch), 752 cm−1 (aromatic C-H out of plane bending); 1H NMR: δ 0.74 (d, J=4.4 Hz, 3H), 0.758 (d, J=4.4 Hz, 3H, CH3), 1.05 (s, 6H, 2CH3), 1.64 (m, 1H), 2.01 (s, 3H, CH3), 2.07 (d, J=16 Hz, 1H), 2.16 (d, J=16 Hz, 1H), 2.22 (d, J=2 Hz, 1H), 2.33 (d, J=4.4 Hz, 1H), 3.81 (d, J=2 Hz, CH), 7.00 (m, 1H, Ar), 7.26 (br s, 2H, Ar), 7.62 (m, 2H, Ar), 8.48 (s, 1H, NH), 9.62 (s, 1H, NHCO); 13C NMR: δ 17.3, 18.2, 19.7, 27.01, 30.1, 32.3, 35.3, 38.2, 51.2, 107.1, 109.0, 120.1, 123.3, 128.9, 136.2, 140.1, 152.1, 169.7, 194.6 (C=O). Anal. Calcd for C22H27N2O2: C, 75.18, H, 7.74, N, 7.97. Found: C, 74.96, H, 7.85, N, 8.05.
Acknowledgments
Financial support provided by Rasht Branch, Islamic Azad University, under the Grant No. 4.5830 is gratefully acknowledged.
References
[1] Bossert, F.; Meyer, H.; Wehinger, E. 4-Aryldihydropyridines, a new class of highly active calcium antagonists. Angew. Chem. Int. Ed. 1981, 20, 762–769.10.1002/anie.198107621Search in Google Scholar
[2] Boer, R.; Gekeler, V. Chemosensitizers in tumor therapy: new compounds promise better efficacy. Drugs Future1995, 20, 499–509.Search in Google Scholar
[3] Wachter, G. A.; Davis, M. C. Antimycobacterial activity of substituted isosteres of pyridine- and pyrazinecarboxylic acids. J. Med. Chem.1998, 41, 2436–2438.10.1021/jm9708745Search in Google Scholar
[4] Gullapalli, S.; Ramarao, P. L-type Ca2+ channel modulation by dihydropyridines potentiates κ-opioid receptor agonist induced acute analgesia and inhibits development of tolerance in rats. Neuropharmacol.2002, 42, 467–475.10.1016/S0028-3908(01)00200-3Search in Google Scholar
[5] Sunkel, C. E.; de Casa-Juana, M. F.; Santos, L. 4-Alkyl-1,4-dihydropyridine derivatives as specific PAF-acether antagonists. J. Med. Chem. 1990, 33, 3205–3210.10.1021/jm00174a017Search in Google Scholar PubMed
[6] Gündüz, M. G.; Ragno, G.; Şimşek, R.; Deluca, M.; Şafak, C.; Grande, F.; El-Khouly, A.; İşli, F.; Yildirim, Ş.; Fincan, G. S. Ö.; et al. Synthesis and photodegradation studies of analogues of muscle relaxant 1,4-dihydropyridine compounds. Acta Pharm.2017, 67, 341–355.10.1515/acph-2017-0026Search in Google Scholar PubMed
[7] Klusa, V. Cerebrocrast. Neuroprotectant, cognition enhancer. Drugs Future1995, 20, 135–138.10.1358/dof.1995.020.02.284117Search in Google Scholar
[8] Retzel, R. G.; Bollen, C. C.; Maeser, E.; Federlin, K. F. Trombodipine platelet aggregation inhibitor antithrombotic. Drugs Future1992, 17, 465–468.10.1358/dof.1992.017.06.175816Search in Google Scholar
[9] Gein, V. L.; Kholkin, I. V.; Zamaraeva, T. M.; Voronina, E. V.; Vakhrin, M. I. Synthesis and antimicrobial activity of N,6-diaryl-4-methyl-2-thioxo-1,2,3,6-tetrahydropyrimidine-5-carboxamides. Pharm. Chem. J. 2012, 46, 49–51.10.1007/s11094-012-0743-ySearch in Google Scholar
[10] Gein, V. L.; Fedotov, A. Y.; Zamaraeva, T. M.; Bobyleva, A. A.; Kasimova, N. N. ; Odegova, T. F. Synthesis and antimicrobial activity of N,6-diaryl-4-methyl-2-oxo-1,2,3,6-tetrahydropyrimidine-5-carboxamides. Pharm.Chem. J.2013, 46, 24–26.10.1007/s11094-013-0877-6Search in Google Scholar
[11] Gein, V. L.; Zamaraeva, T. M.; Buzmakova, N. A.; Syropyatov, B. Ya.; Alikina, N. V. Synthesis and analgesic activity of N,6-diaryl-4-methyl-2-thioxo-1,2,3,6-tetrahydropyrimidine-5-carboxamides. Pharm. Chem. J.2016, 50, 19–21.10.1007/s11094-016-1427-9Search in Google Scholar
[12] Ahmed, K.; Jain, A. K.; Dubey, B.; Shrivastava, B.; Sharma, P.; Nadeem, S. p-TSA catalyzed synthesis of 4-aryl-2,7,7-trimethyl-5-oxo-N-phenyl-1,4,5,6,7,8-hexahydroquinoline-3-carboxamides derivatives as CNS active agents. Der Pharma Chemica2015, 7, 52–65.Search in Google Scholar
[13] Gein, V. L.; Kazantseva, M. I.; Kurbatova, A. A.; Vahrin, M. I. Synthesis of 4-aryl-2,7,7-trimethyl-5-oxo-n-phenyl-1,4,5,6,7,8-hexahydroquinoline-3-carboxamides. Chem. Heterocycl. Compd.2010, 46, 629–630.10.1007/s10593-010-0560-8Search in Google Scholar
[14] Moosavi-Zare, A. R.; Zolfigol, M. A.; Zarei, M.; Zare, A.; Khakyzadeh, V.; Hasaninejad, A. Design, characterization and application of new ionic liquid 1-sulfopyridinium chloride as an efficient catalyst for tandem Knoevenagel-Michael reaction of 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one with aldehydes. Appl. Catal. A. 2013, 467, 61–68.10.1016/j.apcata.2013.07.004Search in Google Scholar
[15] Moosavi-Zare, A. R.; Zolfigol, M. A.; Zarei, M.; Zare, A.; Khakyzadeh, V. Preparation, characterization and application of ionic liquid sulfonic acid functionalized pyridinium chloride as an efficient catalyst for the solvent-free synthesis of 12-aryl-8,9,10,12-tetrahydrobenzo[a]-xanthen-11-ones. J. Mol. Liq.2013, 186, 63–69.10.1016/j.molliq.2013.05.009Search in Google Scholar
©2018 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Review
- Research progress in quinazoline derivatives as multi-target tyrosine kinase inhibitors
- Research Articles
- A bio-inspired approach to proline-derived 2,4-disubstituted oxazoles
- Solvent-free preparation of 3-aryl-2-[(aryl)(arylamino)]methyl-4H-furo[3,2-c]chromen-4-one derivatives using ZnO-ZnAl2O4 nanocomposite as a heterogeneous catalyst
- Synthesis of 4-arylethyl-6-arylpyrimidine-2-thiols through aza-Michael addition/nucleophilic addition/aromatization tandem reactions
- 3-Carboxy-1-sulfopyridin-1-ium chloride ([CPySO3H]+Cl−): an efficient catalyst for one-pot synthesis of hexahydroquinoline-3-carboxamides
- Synthesis of new 3H-chromeno[2,3-d]pyrimidine-4,6(5H,7H)-diones via the tandem intramolecular Pinner/Dimroth rearrangement
- A new synthesis of pyrrolo[3,2-d]pyrimidine derivatives by a one-pot, three-component reaction in the presence of L-proline as an organocatalyst
- Diversity-oriented synthesis of amide derivatives of tricyclic thieno[2,3-d]pyrimidin-4(3H)-ones and evaluation of their influence on melanin synthesis in murine B16 cells
- Synthesis, crystal structure, molecular docking studies and bio-evaluation of some N4-benzyl-substituted isatin- 3-thiosemicarbazones as urease and glycation inhibitors
- Ultrasound-assisted synthesis and antimicrobial activity of tetrazole-based pyrazole and pyrimidine derivatives
Articles in the same Issue
- Frontmatter
- Review
- Research progress in quinazoline derivatives as multi-target tyrosine kinase inhibitors
- Research Articles
- A bio-inspired approach to proline-derived 2,4-disubstituted oxazoles
- Solvent-free preparation of 3-aryl-2-[(aryl)(arylamino)]methyl-4H-furo[3,2-c]chromen-4-one derivatives using ZnO-ZnAl2O4 nanocomposite as a heterogeneous catalyst
- Synthesis of 4-arylethyl-6-arylpyrimidine-2-thiols through aza-Michael addition/nucleophilic addition/aromatization tandem reactions
- 3-Carboxy-1-sulfopyridin-1-ium chloride ([CPySO3H]+Cl−): an efficient catalyst for one-pot synthesis of hexahydroquinoline-3-carboxamides
- Synthesis of new 3H-chromeno[2,3-d]pyrimidine-4,6(5H,7H)-diones via the tandem intramolecular Pinner/Dimroth rearrangement
- A new synthesis of pyrrolo[3,2-d]pyrimidine derivatives by a one-pot, three-component reaction in the presence of L-proline as an organocatalyst
- Diversity-oriented synthesis of amide derivatives of tricyclic thieno[2,3-d]pyrimidin-4(3H)-ones and evaluation of their influence on melanin synthesis in murine B16 cells
- Synthesis, crystal structure, molecular docking studies and bio-evaluation of some N4-benzyl-substituted isatin- 3-thiosemicarbazones as urease and glycation inhibitors
- Ultrasound-assisted synthesis and antimicrobial activity of tetrazole-based pyrazole and pyrimidine derivatives

