Abstract
New tetrazole-based pyrazole and pyrimidine derivatives were synthesized by an ultrasound irradiation method. All compounds were characterized by infrared spectroscopy (IR), 1H nuclear magnetic resonance (NMR), 13C NMR, mass spectrometry (MS) and elemental analysis and assessed in vitro for their efficacy as antimicrobial agents against four bacteria (Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa) and two fungi (Candida albicans, Aspergillus niger). Compounds 8a, 8e, 9a, 9b and 9e show potent activity against the tested strains compared to the reference drugs chloramphenicol and clotrimazole.
Introduction
Ultrasound-accelerated chemical reactions proceed via the formation and adiabatic collapse of transient cavitation bubbles. Ultrasound irradiation has become an alternative energy source for organic reactions ordinarily accomplished by heating. Many reactions can be conducted smoothly by sonication to provide improved yields and increased selectivity [1].
Life-threatening invasive infections caused by microorganisms have increased to alarming levels all over the world [2]. In the past decades, antibiotics have been used as antimicrobial drugs. However, many human pathogens no longer respond to these antibiotics. Therefore, it is necessary to find new and efficacious drugs to treat these problems. The substitution of fluorine in potential drug molecules can improve their therapeutic efficacy through hydrogen bonding interactions at the active sites of the enzyme [3]. Thus, fluorine substitution remains an important aspect in the development of more active and selective drug candidates.
The synthetic versatility of tetrazole is due to its widespread applications in the field of medicinal chemistry. Tetrazole is considered a carboxylic acid analog because of their similar pKa values and planar delocalized systems. An advantage of tetrazole derivatives over carboxylic acids is that they are resistant to various biological degradation processes contributing to longer bioavailability of drugs [4, 5]. These factors, prime in the potential applications of tetrazole derivatives, are responsible for anticancer [6], antifungal [7], antitubercular [8], anti-HIV [9], antioxidant [10] and hormonal properties of the bioactive agents [11]. Pyrazole derivatives have showed significant biological activities, such as antimicrobial [12], analgesic [13], anti-inflammatory [14] and anticancer activities [15]. Pyrimidines also occupy a distinct and unique place in medicinal chemistry [16]. Pyrimidine derivatives are of interest due to their pharmacological properties such as antitumor [17], antimalarial [18], antiviral [19], antifungal [20], antibacterial [21], anti-inflammatory [22], analgesic [23] and antiprotozoal [24] activities. In continuation of our earlier work on design and synthesis of pharmacologically significant heterocycles [25], herein we report an efficient green synthesis and antimicrobial activity of new tetrazole containing pyrazole and pyrimidine derivatives.
Results and discussion
Chemistry
New tetrazole-based pyrazole derivatives 8a–f and tetrazole-based pyrimidine derivatives 9a–f were synthesized using conventional heating and ultrasound irradiation methods (Scheme 1). The precursors 4a–f were prepared in good yield according to the literature procedure [26, 27]. Compounds 4a–f were converted into corresponding substituted 3-hydroxychromones 5a–f by oxidative cyclization using hydrogen peroxide in ethanol. The hydroxychromones 5a–f were alkylated with 2-chloroacetonitrile in the presence of K2CO3 in N,N′-dimethylformamide (DMF) at room temperature to afford the substituted 2-(2-(4-fluorophenyl)-4-oxo-4H-chromen-3-yloxy)acetonitriles 6a–f. The reaction of 6a–f with sodium azide and zinc bromide in water at 100°C furnished 3-[(1H-tetrazol-5-yl)methoxy)-2-(4-fluorophenyl]-4H-chromen-4-one derivatives 7a–f. Treatment of 7a–f with hydrazine hydrate in ethanol under ultrasound irradiation yielded the desired pyrazoles 8a–f. In addition, treatment of compounds 7a–f with thiourea in ethanolic KOH under ultrasound irradiation furnished the desired pyrimidines 9a–f. All synthesized compounds were characterized by 1H nuclear magnetic resonance (NMR), 13C NMR, mass spectrometry (MS) and elemental analysis.
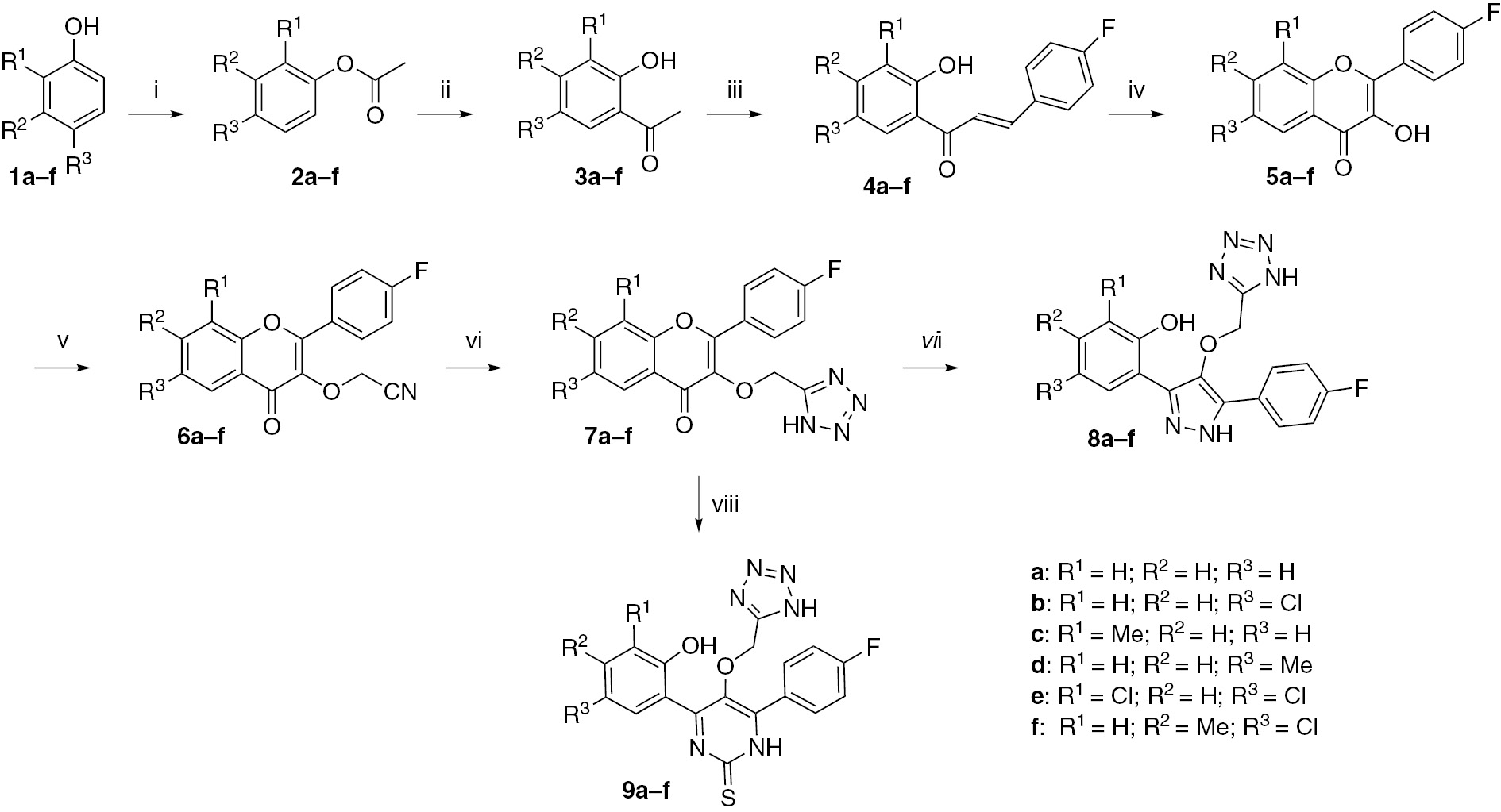
Reagents and conditions: (i) acetic anhydride, pyridine, 100°C, 3–4 h; (ii) AlCl3, 150°C, 3–4 h; (iii) 4-fluorobenzaldehyde, KOH, EtOH, room temperature (rt), 2–3 h; (iv) H2O2, NaOH, 0°C to rt, 2–3 h; (v) 2-chloroacetonitrile, K2CO3, DMF, rt, 3–4 h; (vi) sodium azide, zinc bromide, H2O, reflux, 100°C, 4–5 h; (vii) hydrazine hydrate, ethanol, )))), 65°C, 45–55 min; (viii) thiourea, KOH, ethanol, )))), 65°C, 15–25 min.
Antimicrobial activity
All compounds were evaluated for antibacterial activity against Gram-positive bacteria Staphylococcus aureus (NCIM 2079) and Bacillus subtilis (NCIM 2920) and Gram-negative bacteria Escherichia coli (NCIM 2065) and Pseudomonas aeruginosa (NCIM 2200) and for antifungal activity against Candida albicans (NCIM 3471) and Aspergillus niger (NCIM 596). The minimum inhibitory concentration (MIC) values were determined by the micro-broth dilution method. The antibacterial drug chloramphenicol and antifungal drug clotrimazole were used as reference antibiotics. The results are shown in Table 1. Of interest are high activities of compounds 9a and 9e against A. niger. Compound 9e is also active against C. albicans. The activities of 8e, 9a and 9b against S. aureus exceed those of the reference drug chloramphenicol.
Antimicrobial screening results of bioactive compounds 8 and 9 using the micro-broth dilution method.
| No. | Minimum inhibitory concentration (μg/mL) | |||||
|---|---|---|---|---|---|---|
| Bacteria | Fungi | |||||
| Sa | Bs | Ec | Pa | Ca | An | |
| 8e | 25 | 200 | 50 | 200 | 75 | 50 |
| 9a | 25 | 200 | 175 | 150 | 75 | 25 |
| 9b | 25 | 75 | 125 | 100 | 100 | 50 |
| 9e | 50 | 50 | 75 | 75 | 50 | 12.5 |
| Chloramphenicol | 50 | 25 | 50 | 50 | – | – |
| Clotrimazole | – | – | – | – | 50 | 25 |
Sa, Staphylococcus aureus; Bs, Bacillus subtilis; Ec, Escherichia coli; Pa, Pseudomonas aeruginosa; Ca, Candida albicans; An, Aspergillus niger.
Conclusion
Newly synthesized compounds 8e, 9a, 9b and 9e are highly potent antimicrobial agents.
Experimental
The progress of each reaction was monitored by thin-layer chromatography (TLC) using Merck silica gel 60 F254 plates and visualized with ultraviolet (UV) light and iodine. Melting points were determined in open capillaries and are uncorrected. Infrared spectra were recorded on a Carry 600 Series Fourier-transform infrared spectroscopy (FT-IR) spectrophotometer using KBr pellets. 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were recorded on a Bruker AVANCE II 400 NMR spectrometer in CDCl3 or DMSO-d6. Mass spectra were recorded on a Waters quadrupole time-of-flight (Q-TOF) micro instrument equipped with an electrospray ionization (ESI) source. Elemental analysis was performed on a Perkin-Elmer EAL-240 elemental analyzer.
General procedure for synthesis of compounds 5a–f
A mixture of (E)-3-(4-fluorophenyl)-1-(2-hydroxyphenyl)prop-2-en-1-one (4a–f, 1 mmol), ethanol (15 mL), NaOH (10%, 5 mL) and hydrogen peroxide (30%, 1.5 mL) was stirred vigorously for 30 min and then kept for 2–3 h in ice. The progress of the reaction was monitored by TLC using ethyl acetate/hexane as an eluent. After completion of the reaction, the mixture was poured into ice water and acidified with 1 m hydrochloric acid (HCl). The precipitate was collected by filtration, washed with water and crystalized from chloroform/ethanol to afford a pure product.
2-(4-Fluorophenyl)-3-hydroxy-4H-chromen-4-one (5a)
This compound was synthesized from (E)-3-(4-fluorophenyl)-1-(2-hydroxyphenyl)prop-2-en-1-one (4a); a yellow solid; yield 74%; mp 152–154°C; IR: 3297 (OH), 2925 (ArH), 1618 (C=O, pyrone), 1572 cm−1 (C=C); 1H NMR (CDCl3: δ 7.11 (br s, 1H, ArH), 7.22 (t, 2H, J=8.5 Hz, ArH), 7.42 (m 1H, ArH), 7.58 (d, 1H, J=8.5 Hz, ArH), 7.71 (m, 1H, ArH), 8.24 (d, 1H, J=1.8 Hz, ArH), 8.26 (s, 1H, OH), 8.27–8.30 (m, 1H, ArH); 13C NMR (CDCl3): δ 115.7, 115.9, 118.2, 120.7, 124.6, 125.5, 127.3, 129.9, 133.7, 138.2, 144.1, 155.3, 162.3, 164.8, 173.4. ESI-MS. Calcd for C15H9FO3 (M+H)+: m/z 257.0. Found: m/z 257.0.
6-Chloro-2-(4-fluorophenyl)-3-hydroxy-4H-chromen-4-one (5b)
This compound was synthesized from (E)-1-(5-chloro-2-hydroxyphenyl)-3-(4-fluorophenyl)prop-2-en-1-one (4b); a yellow solid; yield 77%; mp 213–215°C; 1H NMR (DMSO-d6): δ 7.08–7.25 (m, 2H, ArH), 7.68–7.82 (m, 2H, ArH), 8.12–8.23 (m, 2H, ArH), 8.51 (s, 1H, ArH), 9.08 (br s, 1H, OH); 13C NMR (DMSO-d6): δ 114.1, 117.5, 120.6, 125.4, 127.4, 128.4, 129.7, 130.2, 133.6, 148.5, 155.4, 157.6, 160.8, 162.5, 172.9. ESI-MS. Calcd for C15H8ClFO3 (M+H)+: m/z 291.0224. Found: m/z 291.2223.
2-(4-Fluorophenyl)-3-hydroxy-8-methyl-4H-chromen-4-one (5c)
This compound was synthesized from (E)-3-(4-fluorophenyl)-1-(2-hydroxy-3-methylphenyl)prop-2-en-1-one (4c); a yellow solid, yield 69%; mp 160–162°C; 1H NMR (DMSO-d6): δ 2.54 (s, 3H, CH3), 7.24–7.29 (m, 3H, ArH), 7.53 (d, 1H, J=7 Hz, ArH), 7.93 (d, 1H, J=7.7 Hz, ArH), 8.26 (m, 2H, ArH), 9.38 (s, 1H, OH); 13C NMR (DMSO-d6): δ 15.3, 115.1, 115.3, 121, 122.3, 123.6, 127.1, 127.9, 129.6, 133.7, 138.7, 143.5, 152.8, 161.3, 163.7, 173. ESI-MS. Calcd for C16H11FO3 (M+H)+: m/z 271.077. Found: m/z 271.2154.
2-(4-Fluorophenyl)-3-hydroxy-6-methyl-4H-chromen-4-one (5d)
This compound was synthesized from (E)-3-(4-fluorophenyl)-1-(2-hydroxy-5-methylphenyl)prop-2-en-1-one (4d); a yellow solid, yield 73%; mp 171–173°C; 1H NMR (DMSO-d6): δ 2.46 (s, 3H, CH3), 7.27 (t, 2H, J=8.6 Hz, ArH), 7.54 (s, 2H, ArH), 7.91 (s, 1H, ArH), 8.27–8.30 (m, 2H, ArH), 9.31 (s, 1H, OH); 13C NMR (DMSO-d6): δ 20.5, 115, 115.2, 117.7, 120.9, 123.8, 127.7, 129.8, 130.6, 134.5, 138.7, 143.9, 152.8, 161.3, 163.8, 172.8. ESI-MS. Calcd for C16H11FO3 (M+H)+: m/z 271.0770. Found: m/z 271.0234.
6,8-Dichloro-2-(4-fluorophenyl)-3-hydroxy-4H-chromen-4-one (5e)
This compound was synthesized from (E)-1-(3,5-dichloro-2-hydroxyphenyl)-3-(4-fluorophenyl)prop-2-en-1-one (4e); a yellow solid; yield 75%; mp 203–205°C; 1H NMR (DMSO-d6): δ 7.25 (t, 2H, J=8.8 Hz, ArH), 7.82 (d, 1H, J=2.6 Hz, ArH), 7.96 (d, 1H, J=2.2 Hz, ArH), 8.29 (m, 2H, ArH), 9.81 (s, 1H, OH); 13C NMR (DMSO-d6): δ 115.2, 115.4, 122.8, 123, 123.7, 127.1, 128.8, 129.8, 132.6, 139.2, 144.5, 148.4, 161.5, 164, 171.3. ESI-MS. Calcd for C15H7Cl2FO3 (M+H)+: m/z 324.9835. Found: m/z 325.0267.
6-Chloro-2-(4-fluorophenyl)-3-hydroxy-7-methyl-4H-chromen-4-one (5f)
This compound was synthesized from (E)-1-(5-chloro-2-hydroxy-4-methylphenyl)-3-(4-fluorophenyl)prop-2-en-1-one (4f); a yellow solid, yield 79%; mp 222–224°C; 1H NMR (DMSO-d6): δ 2.5 (s, 3H, CH3), 7.28 (m, 2H, ArH), 7.64 (br s, 1H, ArH), 8.03 (s, 1H, ArH), 8.27 (br s, 2H, ArH), 9.54 (s, 1H, OH); 13C NMR (DMSO-d6): δ 20.3, 115.1, 115.3, 120.1, 120.5, 124, 127.5, 129.9, 130.1, 138.8, 141.8, 144.3, 152.8, 161.4, 163.9, 171.8. ESI-MS. Calcd for C16H10ClFO3 (M+H)+: m/z 305.0381. Found: m/z 305.0251.
General procedure for synthesis of compounds 6a–f
To a stirred solution of 5a–f (1 mmol) in DMF was added potassium carbonate (2 mmol) and portion-wise 2-chloroacetonitrile (1 mmol) at room temperature for about 3–4 h. The mixture was quenched with crushed ice and the resultant precipitate was filtered and crystallized from ethanol.
2-(2-(4-Fluorophenyl)-4-oxo-4H-chromen-3-yloxy)acetonitrile (6a)
This compound was prepared from 5a; a white solid; yield 78%; mp 140–142°C; IR: υ 3012 (Ar-H), 2965 (C-H), 2346 (C≡N), 1634 (C=O), 1604 (C=C), 1238 (C-F) cm−1; 1H NMR (CDCl3): δ 5.11 (s, 2H, OCH2), 7.20–2.27 (m, 2H,ArH), 7.45 (m, 1H, ArH), 7.57 (dd, 1H, J=1.1, 8.6 Hz, ArH), 7.74 (ddt, 1H, J=1.5, 7.8, 8.0 Hz, ArH), 8.08 (m, 2H, ArH), 8.25 (dd, 1H, J=1.5, 7.8 Hz, ArH); 13C NMR (CDCl3): δ 56.1, 115.1, 115.9, 116.1, 118.1, 123.8, 125.4, 125.8, 126.1, 131.3, 134.2, 137.5, 155.3, 156.4, 163.1, 165.6, 174.1. Anal. Calcd for C17H10FNO3: C, 69.15; H, 3.41; N, 4.74. Found: C, 69.07; H, 3.35; N, 4.68.
2-(6-Chloro-2-(4-fluorophenyl)-4-oxo-4H-chromen-3-yloxy)acetonitrile (6b)
This compound was prepared from 5b; a white solid, yield 86%; mp 168–170°C; IR: υ 3069 (Ar-H), 2952 (C-H), 2360 (C≡N), 1628 (C=O), 1600 (C=C), 1144 (C-F) 755 (C-Cl) cm−1; 1H NMR (DMSO-d6): δ 5.14 (s, 2H, OCH2), 7.34 (t, 2H, J=8.0 Hz, ArH), 7.73 (d, 1H, J=8.0 Hz, ArH), 7.79 (dd, 1H, J=2.6, 9.2 Hz, ArH), 8.07 (d, 1H, J=2.6 Hz, ArH), 8.13 (m, 2H, ArH); 13C NMR (DMSO-d6): δ 56.4, 115.5, 115.7, 120.4, 124, 124.3, 125.7, 130.3, 131.1, 131.2, 134.1, 137.6, 153.1, 155.4, 162.4, 165, 172.1. Anal. Calcd for C17H9ClFNO3: C, 61.93; H, 2.75; N, 4.25. Found: C, 61.98; H, 2.82; N, 4.33.
2-(2-(4-Fluorophenyl)-8-methyl-4-oxo-4H-chromen-3-yloxy)acetonitrile (6c)
This compound was prepared from 5c; a white solid; yield 71%; mp 176–178°C; IR: υ 3032 (Ar-H), 2915 (C-H), 2360 (C≡N), 1633 (C=O), 1604 (C=C), 1193 (C-F) cm−1; 1H NMR (CDCl3): δ 2.58 (s, 3H, CH3), 5.12 (s, 2H, OCH2), 7.23–7.27 (m, 2H, ArH), 7.34 (t, 1H, J=7.0 Hz, ArH), 7.57 (d, 1H, J=7.0 Hz, ArH), 8.07–8.13 (m, 3H, ArH); 13C NMR (CDCl3): δ 15.8, 56, 115.1, 115.9, 116.2, 123.4, 123.8, 125.1, 126.5, 127.6, 131.3, 135, 137.4, 153.8, 155.8, 163.1, 165.6, 174.4. Anal. Calcd for C18H12FNO3: C, 69.90; H, 3.91; N, 4.53. Found: C, 69.96; H, 3.94; N, 4.56.
2-(2-(4-Fluorophenyl)-6-methyl-4-oxo-4H-chromen-3-yloxy)acetonitrile (6d)
This compound was prepared from 5d; a white solid; yield 74%; mp 162–164°C; IR: υ 3087 (Ar-H), 2974 (C-H), 2369 (C≡N), 1632 (C=O), 1603 (C=C), 1187 (C-F) cm−1; 1H NMR (DMSO-d6): δ 2.53 (s, 3H, CH3), 5.15 (s, 2H, OCH2), 7.36 (s, 2H, ArH), 7.61 (s, 2H, ArH), 7.90 (s, 1H, ArH), 8.12 (s, 2H, ArH); 13C NMR (DMSO-d6); δ 20.5, 56.4, 115.5, 115.7, 118, 122.9, 124.1, 126.2, 131.1, 131.1, 134.8, 135.4, 136.3, 137.5, 153, 154.9, 162.3, 173.1. Anal. Calcd for C18H12FNO3: C, 69.90; H, 3.91; N, 4.53. Found: C, 69.82; H, 3.95; N, 4.66.
2-(6,8-Dichloro-2-(4-fluorophenyl)-4-oxo-4H-chromen-3-yloxy)acetonitrile (6e)
This compound was prepared from 5e; a white solid; yield 77%; mp 177–179°C; IR: υ 3064 (Ar-H), 2916 (C-H), 2361 (C≡N), 1625 (C=O), 1601 (C=C), 1180 (C-F) 717 (C-Cl) cm−1; 1H NMR (CDCl3): δ 5.12 (s, 2H, OCH2), 7.26 (t, 2H, J=8.6 Hz, ArH), 7.77 (d, 1H, J=2.2 Hz, ArH), 8.10 (d, 1H, J=2.2 Hz, ArH), 8.18 (m, 2H, ArH); 13C NMR (CDCl3): δ 56.1, 114.8, 116.2, 116.4, 123.8, 124.6, 125.5, 131.2, 131.5, 131.6, 134.2, 137.6, 149.4, 156.3, 163.5, 166, 172.5. Anal. Calcd for C17H8Cl2FNO3: C, 56.07; H, 2.21; N, 3.85. Found: C, 56.18; H, 2.24; N, 3.79.
2-(6-Chloro-2-(4-fluorophenyl)-7-methyl-4-oxo-4H-chromen-3-yloxy)acetonitrile (6f)
This compound was prepared from 5f; a white solid, yield 82%; mp 170–172°C; IR: υ 3013 (Ar-H), 2915 (C-H), 2360 (C≡N), 1599 (C=O), 1552 (C=C), 1203 (C-F) 750 (C-Cl) cm−1; 1H NMR (DMSO-d6): 2.54 (s, 3H, CH3), 5.14 (s, 2H, OCH2), 7.35 (s, 2H, ArH), 7.70 (s, 1H, ArH), 8.04 (s, 1H, ArH), 8.12 (s, 2H, ArH); 13C NMR (DMSO-d6): δ 20.3, 56.4, 115.5, 115.6, 115.7, 120.3, 122.4, 124.2, 125.9, 125.9, 131.1, 131.1, 137.5, 142.9, 153.1, 155.1, 167.4, 172.1. Anal. Calcd for C18H11ClFNO3: C, 62.89; H, 3.23; N, 4.07. Found: C, 62.93; H, 3.28; N, 4.11.
General procedure for synthesis of compounds 7a–f
To a mixture of sodium azide (1.5 mmol) and zinc bromide (1.5 mmol) in water (20 mL) was added compound 6a–f (1 mmol). The mixture was then heated at reflux for 4–5 h with vigorous stirring. After completion of the reaction, as monitored by TLC using chloroform/ methanol as an eluent, the mixture was quenched with crushed ice, and the resultant solid was filtered and crystallized from ethanol.
3-[(1H-Tetrazol-5-yl)methoxy]-2-(4-fluorophenyl)-4H-chromen-4-one (7a)
This compound was prepared from 6a; a white solid, yield: 81%; mp 200–202°C; IR: υ 3444 (N-H), 3030 (Ar-H), 2917 (C-H), 1606 (C=O), 1554 (C=C), 1238 (C-F) cm−1; 1H NMR (DMSO-d6): δ 5.47 (s, 2H, OCH2), 7.26 (t, 2H, J=9.0 Hz, ArH), 7.48–7.52 (m, 1H, ArH), 7.70 (d, 1H, J=8.0 Hz, ArH), 7.82 (ddd, 1H, J=8.5, 7.1, 1.7 Hz, ArH), 8.01–8.05 (m, 2H, ArH), 8.16 (dd, 1H, J=8.0, 1.7 Hz, ArH); 13C NMR (DMSO-d6): δ 61.8 (O-CH2), 115.3, 115.6, 118.3, 123.4, 124.9, 125.1, 126.2, 130.9, 131, 134.1, 138.2, 154.7, 154.9, 162 (tetrazole C), 164.5 (C-F), 173.5 (C=O). Anal. Calcd for C17H11FN4O3: C, 60.36; H, 3.28; N, 16.56. Found: C, 60.29; H, 3.25; N, 16.51.
3-[(1H-Tetrazol-5-yl)methoxy]-6-chloro-2-(4-fluorophenyl)-4H-chromen-4-one (7b)
This compound was prepared from 6b; a white solid; yield 85%; mp 203–205°C; IR: υ 3435 (N-H), 3035 (Ar-H), 2992 (C-H), 1622 (C=O), 1605 (C=C), 1197 (C-F), 718 (C-Cl) cm−1; 1H NMR (DMSO-d6): δ 5.42 (s, 2H, OCH2), 7.17 (t, 2H, J=9.0 Hz, ArH), 7.64 (d, 1H, J=9.0 Hz, ArH), 7.72 (dd, 1H, J=9.0, 2.6 Hz, ArH), 7.98 (m, 2H, ArH), 8.04 (d, 1H, J=2.6 Hz, ArH); 13C NMR (DMSO-d6): δ 61.7 (O-CH2), 115.4, 115.6, 120.7, 123.8, 124.5, 125.9, 125.9, 129.9, 131, 134, 138.2, 153.2, 155.2, 162.1 (tetrazole C), 164.6 (C-F), 172.5 (C=O). Anal. Calcd for C17H10ClFN4O3: C, 54.78; H, 2.70; N, 15.03. Found: C, 54.84; H, 2.72; N, 15.23.
3-[(1H-Tetrazol-5-yl)methoxy]-2-(4-fluorophenyl)-8-methyl-4H-chromen-4-one (7c)
This compound was prepared from 6c; a white solid; yield 73%; mp 198–200°C; IR: υ 3435 (N-H), 3035 (Ar-H), 2992 (C-H), 1622 (C=O), 1605 (C=C), 1197 cm−1 (C-F); 1H NMR (DMSO-d6): δ 2.33 (s, 3H, CH3), 5.61 (s, 2H, OCH2), 6.97 (s, 1H, ArH), 7.10–7.22 (m, 2H, ArH), 7.53 (s, 1H, ArH), 7.69–7.80 (m, 3H, ArH), 8.41 (s, 1H, NH); 13C NMR (DMSO-d6): δ 15.1 (CH3), 63.5 (O-CH2), 114.9, 115.2, 122.3, 123, 124.3, 126.6, 127.1, 128.2, 130.4, 134.2, 139, 149.5, 152.6, 161.5 (tetrazole C), 164 (C-F), 173.8 (C=O). Anal. Calcd for C18H13FN4O3: C, 61.36; H, 3.72; N, 15.90. Found: C, 61.45; H, 3.69; N, 15.98.
3-[(1H-Tetrazol-5-yl)methoxy]-2-(4-fluorophenyl)-6-methyl-4H-chromen-4-one (7d)
This compound was prepared from 6d; a white solid; yield 75%; mp 202–204°C; IR: υ 3423 (N-H), 3021 (Ar-H), 2918 (C-H), 1602 (C=O), 1554 (C=C), 1174 cm−1 (C-F); 1H NMR (DMSO-d6): δ 2.46 (s, 3H, CH3), 5.47 (s, 2H, OCH2), 7.27 (t, 2H, J=9.0 Hz, ArH), 7.57–7.63 (m, 2H, ArH), 7.90 (s, 1H, ArH), 8.00 (dd, 2H, J=9.0, 5.3 Hz, ArH); 13C NMR (DMSO-d6): δ 20.4 (CH3), 61.8 (O-CH2), 115.3, 115.5, 118, 123.1, 124.1, 126.3, 126.3, 130.9, 134.7, 135.2, 138.2, 153, 154.7, 162 (tetrazole C), 164.5 (C-F), 173.4 (C=O). Anal. Calcd for C18H13FN4O3: C, 61.36; H, 3.72; N, 15.90. Found: C, 61.34; H, 3.65; N, 15.81.
3-[(1H-Tetrazol-5-yl)methoxy]-6,8-dichloro-2-(4-fluorophenyl)-4H-chromen-4-one (7e)
This compound was prepared from (6e); a white solid; yield 80%; mp 194–196°C; IR: υ 3423 (N-H), 3040 (Ar-H), 2917 (C-H), 1660 (C=O), 1603 (C=C), 1158 (C-F), 760 cm−1 (C-Cl); 1H NMR (DMSO-d6): δ 5.50 (s, 2H, OCH2), 7.24 (t, 2H, J=8.4 Hz, ArH), 7.33–7.37 (m, 1H, ArH), 8.00 (d, 2H, J=5.5 Hz, ArH), 8.08 (dd, 1H, J=8.4, 5.5 Hz, ArH); 13C NMR (DMSO-d6): δ 62 (O-CH2), 115.6, 115.8, 123, 123.8, 125.2, 129.6, 131, 133.5, 138.6, 139.4, 148.9, 154.6, 162.2 (tetrazole C), 164.7 (C-F), 172 (C=O). Anal. Calcd for C17H9Cl2FN4O3: C, 50.15; H, 2.23; N, 13.76. Found: C, 50.33; H, 2.32; N, 13.87.
3-[(1H-Tetrazol-5-yl)methoxy]-6-chloro-2-(4-fluorophenyl)-7-methyl-4H-chromen-4-one (7f)
This compound was prepared from 6f; a white solid; yield 78%; mp 178–180°C; IR: υ 3495 (N-H), 3077 (Ar-H), 2924 (C-H), 1638 (C=O), 1618 (C=C), 1166 (C-F) 771 cm−1 (C-Cl); 1H NMR (DMSO-d6): δ 2.44 (s, 3H, CH3), 5.45 (s, 2H, OCH2), 7.30 (t, 2H, J=9.0 Hz, ArH), 7.73–7.76 (m, 1H, ArH), 7.92–7.96 (m, 3H, ArH); 13C NMR (DMSO-d6): δ 20.1 (CH3), 61.8 (O-CH2), 115.4, 115.7, 120.6, 122.5, 123.9, 126.1, 130.6, 130.9, 131, 138.1, 142.7, 153, 154.9, 162 (tetrazole C), 164.5 (C-F), 172.4 (C=O). Anal. Calcd for C18H12ClFN4O3: C, 55.90; H, 3.13; N, 14.49. Found: C, 55.97; H, 3.21; N, 14.58.
General procedure for synthesis of compounds 8a–f
Conventional method
Hydrazine hydrate (1 mmol) was added to a solution of 7a–f (1 mmol) in ethanol (10 mL) and the mixture was heated for 5–6 h. After completion of the reaction, as monitored by TLC, 10 mL of water was added, and the resultant precipitate was filtered, dried under reduced pressure and crystallized from ethanol.
Ultrasound-assisted method
Hydrazine hydrate (1 mmol) was added to a solution of 7a–f (1 mmol) in ethanol (10 mL) and the mixture was ultrasonicated for 45–55 min at 65°C. After completion of the reaction, as monitored by TLC, 10 mL of water was added, and the resultant precipitate was filtered, dried and crystallized from ethanol.
2-[4-((1H-Tetrazol-5-yl)methoxy)-5-(4-fluorophenyl)-1H-pyrazol-3-yl]phenol (8a)
This compound was prepared from 7a; a yellow solid; conventional method time 340 min, yield 70%; ultrasound-assisted method time 51 min, yield 88%; mp 269–271°C; IR: υ 3943 (OH), 3352 (NH), 1884 (C=N), 1246 (C-F) cm−1; 1H NMR (DMSO-d6): δ 5.08 (s, 2H, OCH2), 6.89 (t, 1H, J=7.3 Hz, ArH), 6.96 (d, 1H, J=8.1 Hz, ArH), 7.19–7.23 (m, 3H, ArH), 7.63–7.88 (m, 3H, ArH), 10.80 (br s, 1H, OH), 12.69 (br s, 1H, NH), 13.54 (br s, 1H, NH); 13C NMR (DMSO-d6): δ 64.0, 99.5, 115.4, 115.6, 116.1, 119.2, 119.3, 127.7, 127.8, 129.0, 129.1, 136.5, 153.0, 153.1, 154.9, 160.5, 162.9. Anal. Calcd for C17H13FN6O2: C, 57.95; H, 3.72; N, 23.85. Found: C, 57.92; H, 3.69; N, 23.76.
2-[4-((1H-Tetrazol-5-yl)methoxy)-5-(4-fluorophenyl)-1H-pyrazol-3-yl]-4-chlorophenol (8b)
This compound was prepared from 7b; a yellow solid; conventional method time 315 min, yield 75%; ultrasound-assisted method time 45 min, yield 93%; mp 228–230°C; IR: υ 3905 (OH), 3387 (NH), 1852 (C=N), 1216 (C-F) cm−1; 1H NMR (DMSO-d6): δ 5.08 (s, 2H, OCH2), 6.97 (d, 1H, J=8.4 Hz, ArH), 7.24–7.30 (m, 3H, ArH), 7.58–7.89 (m, 3H, ArH), 10.72 (br s, 1H, OH), 12.86 (s, 1H, NH), 13.68 (s, 1H, NH); 13C NMR (DMSO-d6): δ 64.3, 99.5, 115.7, 117.3, 117.6, 123.0, 126.5, 126.6, 127.7, 127.8, 128.5, 136.7, 153.4, 153.7, 153.8, 160.6, 163.0. Anal. Calcd for C17H12ClFN6O2: C, 52.79; H, 3.13; N, 21.73. Found: C, 52.63; H, 3.10; N, 21.68.
2-[4-((1H-Tetrazol-5-yl)methoxy)-5-(4-fluorophenyl)-1H-pyrazol-3-yl]-6-methylphenol (8c)
This compound was prepared from 7c; a yellow solid; conventional method time 350 min, yield 77%; ultrasound-assisted method time 54 min, yield 84%; mp 250–252°C; IR: υ 3918 (OH), 3346 (NH), 1815 (C=N), 1245 (C-F) cm−1; 1H NMR (CDCl3): δ 2.57 (s, 3H, CH3), 5.14 (s, 2H, OCH2), 6.91–6.98 (m, 2H, ArH), 7.36 (t, 1H, J=7.7 Hz, ArH), 7.59 (d, 1H, J=7.7 Hz, ArH), 7.65–7.69 (m, 3H, ArH), 10.79 (br s, 1H, OH), 12.80 (s, 1H, NH), 13.71 (s, 1H, NH); 13C NMR (CDCl3): δ 15.8, 63.9, 99.8, 115.1, 115.9, 116.2, 119.4, 119.5, 127.6, 127.7, 129.5, 129.7, 137.0, 153.4, 153.6, 155.3, 160.7, 163.1. Anal. Calcd for C18H15FN6O2: C, 59.01; H, 4.13; N, 22.94. Found: C, 59.13; H, 3.98; N, 22.81.
2-[4-((1H-Tetrazol-5-yl)methoxy]-5-(4-fluorophenyl)-1H-pyrazol-3-yl]-4-methylphenol (8d)
This compound was prepared from 7d; a yellow solid; conventional method time 320 min, yield 79%; ultrasound-assisted method time 48 min, yield 91%; mp 240–242°C; IR: υ 3955 (OH), 3346 (NH), 1865 (C=N), 1258 (C-F) cm−1; 1H NMR (DMSO-d6): δ 2.52 (s, 3H, CH3), 5.19 (s, 2H, OCH2), 6.35–6.42 (m, 2H, ArH), 6.92–6.94 (m, 2H, ArH), 7.64–7.89 (m, 3H, ArH), 10.84 (br s, 1H, OH), 12.78 (br s, 1H, NH), 13.73 (br s, 1H, NH); 13C NMR (DMSO-d): δ 16.0, 64.2, 99.6, 115.5, 115.7, 116.5, 119.8, 119.9, 127.6, 127.7, 129.7, 129.8, 136.6, 154.2, 154.9, 155.8, 161.1, 163.6. Anal. Calcd for C18H15FN6O2: C, 59.01; H, 4.13; N, 22.94. Found: C, 58.96; H, 4.27; N, 22.98.
2-[4-((1H-Tetrazol-5-yl)methoxy]-5-(4-fluorophenyl)-1H-pyrazol-3-yl]-4,6-dichlorophenol (8e)
This compound was prepared from 7e; a yellow solid; conventional method time 330 min, yield 71%; ultrasound-assisted method time 45 min, yield 95%; mp 225–227°C; IR: υ 3936 (OH), 3386 (NH), 1855 (C=N), 1282 (C-F) cm−1; 1H NMR (CDCl3): δ 5.15 (s, 2H, OCH2), 6.42 (t, 2H, J=8.8 Hz, ArH), 6.91 (d, 1H, J=2.6 Hz, ArH), 7.69 (d, 1H, J=2.6 Hz, ArH), 7.90 (dd, 2H, J=8.8, 5.2 Hz, ArH), 10.81 (br s, 1H, OH), 12.73 (br s, 1H, NH), 13.82 (br s, 1H, NH); 13C NMR (CDCl3): δ 64.6, 99.9, 115.3, 115.4, 116.8, 120.1, 120.2, 127.9, 128.0, 129.4, 129.6, 137.3, 154.7, 155.0, 156.2, 161.3, 163.8. Anal. Calcd for C17H11Cl2FN6O2: C, 48.47; H, 2.63; N, 19.95. Found: C, 48.55; H, 2.61; N, 19.98.
2-[4-((1H-Tetrazol-5-yl)methoxy]-5-(4-fluorophenyl)-1H-pyrazol-3-yl]-4-chloro-5-methylphenol (8f)
This compound was prepared from 7f; a yellow solid; conventional method time 330 min, yield 72%; ultrasound-assisted method time 45 min, yield 92%; mp 278–280°C; IR: υ 3945 (OH), 3335 (NH), 1852 (C=N), 1273 (C-F) cm−1; 1H NMR (DMSO-d6): δ 2.52 (s, 3H, CH3), 5.19 (s, 2H, OCH2), 6.52 (s, 1H, ArH), 6.89 (s, 2H, ArH), 7.72–7.75 (m, 2H, ArH), 7.93 (s, 1H, ArH), 10.86 (br s, 1H, OH), 12.71 (br s, 1H, NH), 13.88 (br s, 1H, NH); 13C NMR (DMSO-d6): δ 16.4, 64.8, 99.4, 115.9, 116.0, 116.8, 119.6, 119.7, 127.9, 128.0, 129.5, 129.7, 136.8, 154.4, 155.1, 155.9, 160.8, 163.5. Anal. Calcd for C18H14ClFN6O2: C, 53.94; H, 3.52; N, 20.97. Found: C, 53.96; H, 3.50; N, 21.01.
General procedure for synthesis of compounds 9a–f
Conventional method
To a mixture of compound 7a–f (1 mmol) and KOH (1.5 mmol) in ethanol (10 mL), thiourea (1.5 mmol) was added and the mixture was heated for 3–4 h. After completion of the reaction, as monitored by TLC, the reaction mass was cooled to room temperature, poured on crushed ice and acidified with concentrated HCl. The resultant solid was filtered and crystallized from ethanol.
Ultrasound-assisted method
To a mixture of compound 7a–f (1 mmol) and KOH (1.5 mmol) in ethanol (10 mL), thiourea (1.5 mmol) was added and the reaction mass was ultrasonicated for 15–25 min at 65°C. After completion of the reaction, as monitored by TLC, the mixture was cooled to room temperature, poured on crushed ice and acidified with concentrated HCl. The resultant precipitate was filtered and crystallized from ethanol.
5-[(1H-Tetrazol-5-yl)methoxy]-6-(4-fluorophenyl)-4-(2-hydroxyphenyl)pyrimidine-2(1H)-thione (9a)
This compound was prepared from 7a; a yellow solid; conventional method time 230 min, yield 73%; ultrasound-assisted method time 21 min, yield 87%; mp 163–165°C; IR υ: 3919 (OH), 3423 (NH), 3313 (NH), 3040 (CH) cm−1; 1H NMR (DMSO-d6): δ 4.79 (s, 2H, OCH2), 7.19 (t, 2H, J=8.8 Hz, ArH), 7.26–7.30 (m, 2H, ArH), 7.43–7.46 (m, 1H, ArH), 7.76 (m, 1H, ArH), 7.97 (dd, 2H, J=5.5, 8.8 Hz, ArH), 10.10 (br s, 1H, OH), 10.81 (br s, 1H, NH), 11.19 (br s, 1H, NH); 13C NMR (DMSO-d6): δ 74.5, 115.2, 115.4, 116.5, 117.4, 119.1, 120.0, 120.9, 129.5, 130.1, 130.5, 131.7, 135.5, 155.8, 157.9, 159.6, 161.4, 198.7. Anal. Calcd for C18H13FN6O2S: C, 54.54; H, 3.31; N, 21.20; S, 8.09. Found: C, 54.47; H, 3.45; N, 21.06; S, 8.22.
5-[(1H-Tetrazol-5-yl)methoxy]-4-(5-chloro-2-hydroxyphenyl)-6-(4-fluorophenyl)pyrimidine-2(1H)-thione (9b)
This compound was prepared from 7b; a yellow solid; conventional method time 220 min, yield 80%; ultrasound-assisted method time 15 min, yield 95%; mp 148–150°C; IR: υ 3975 (OH), 3424 (NH), 3383 (NH), 3067 (CH) cm−1; 1H NMR (DMSO-d6): δ 4.81 (s, 2H, OCH2), 6.90 (d, 1H, J=8.8 Hz, ArH), 7.19 (t, 2H, J=8.8 Hz, ArH), 7.25 (dd, 1H, J=8.8, 2.6 Hz, ArH), 7.32 (d, 1H, J=2.6 Hz, ArH), 8.00 (m, 2H, ArH), 10.10 (br s, 1H, OH), 10.81 (br s, 1H, NH), 11.19 (br s, 1H, NH); 13C NMR (DMSO-d6): δ 63.5, 115.4, 115.6, 117.9, 122.5, 122.8, 129.4, 130.4, 130.9, 131.6, 131.7, 146.4, 157.9, 159.6, 161.7, 162.2, 164.7, 190.6. Anal. Calcd for C18H12ClFN6O2S: C, 50.18; H, 2.81; N, 19.51; S, 7.44. Found: C, 50.25; H, 2.87; N, 19.49; S, 7.48.
5-[(1H-Tetrazol-5-yl)methoxy]-6-(4-fluorophenyl)-4-(2-hydroxy-3-methylphenyl)pyrimidine-2(1H)-thione (9c)
This compound was prepared from 7c; a yellow solid; conventional method time 220 min, yield 74%; ultrasound-assisted method time 24 min, yield 82%; mp 215–217°C; IR: υ 3935 (OH), 3475 (NH), 3364 (NH), 3057 (CH) cm−1; 1H NMR (DMSO-d6): δ 2.49 (s, 3H, CH3), 4.85 (s, 2H, OCH2), 6.95 (s, 1H, ArH), 7.18–7.20 (m, 2H, ArH), 7.69 (s, 1H, ArH), 7.92–7.96 (m, 3H, ArH), 10.16 (br s, 1H, OH), 10.87 (br s, 1H, NH), 11.21 (br s, 1H, NH); 13C NMR (DMSO-d6): δ 15.8, 68.7, 115.7, 115.8, 118.1, 122.8, 122.9, 129.0, 130.2, 131.3, 131.8, 132.2, 146.9, 158.2, 159.3, 161.5, 161.9, 165.0, 195.2. Anal. Calcd for C19H15FN6O2S: C, 55.60; H, 3.68; N, 20.48; S, 7.81. Found: C, 55.57; H, 3.72; N, 20.39; S, 7.76.
5-[(1H-Tetrazol-5-yl)methoxy]-6-(4-fluorophenyl)-4-(2-hydroxy-5-methylphenyl)pyrimidine-2(1H)-thione (9d)
This compound was prepared from 7d; a yellow solid; conventional method time 235 min, yield 75%; ultrasound-assisted method time 24 min, yield 94%; mp 314–316°C; IR: υ 3954 (OH), 3424 (NH), 3364 (NH), 3095 (CH) cm−1; 1H NMR (DMSO-d6): δ 2.44 (s, 3H, CH3), 4.87 (s, 2H, OCH2), 6.97 (t, 2H, J=8.8 Hz, ArH), 7.17–7.23 (m, 2H, ArH), 7.74 (s, 1H, ArH), 7.90 (dd, 2H, J=8.8, 5.5 Hz, ArH), 10.11 (br s, 1H, OH), 10.82 (br s, 1H, NH), 11.19 (br s, 1H, NH); 13C NMR (DMSO-d6): δ 16.3, 68.4, 115.9, 116.0, 118.5, 123.1, 123.4, 128.6, 129.8, 131.2, 131.5, 132.6, 147.7, 158.4, 160.2, 161.6, 161.8, 165.3, 194.9. Anal. Calcd for C19H15FN6O2S: C, 55.60; H, 3.68; N, 20.48; S, 7.81. Found: C, 55.65; H, 3.61; N, 20.55; S, 7.74.
5-[(1H-Tetrazol-5-yl)methoxy]-4-(3,5-dichloro-2-hydroxyphenyl)-6-(4-fluorophenyl)pyrimidine-2(1H)-thione (9e)
This compound was prepared from 7e; a yellow solid; conventional method time 195 min, yield 78%; ultrasound-assisted method time 18 min, yield 91%; mp 183–185°C; IR: υ 3935 (OH), 3453 (NH), 3390 (NH), 3054 (CH) cm−1; 1H NMR (DMSO-d6): δ 4.83 (s, 2H, OCH2), 6.24 (t, 2H, J=8.5 Hz, ArH), 7.37–7.39 (m, 1H, ArH), 7.78 (d, 2H, J=5.5 Hz, ArH), 7.92 (dd, 1H, J=8.5, 5.5 Hz, ArH), 10.09 (br s, 1H, OH), 10.87 (br s, 1H, NH), 11.24 (br s, 1H, NH); 13C NMR (DMSO-d6): δ 67.1, 115.6, 115.9, 116.8, 117.7, 119.5, 120.2, 121.0, 129.8, 130.2, 130.7, 131.2, 136.1, 156.4, 157.5, 159.9, 162.0, 193.6. Anal. Calcd for C18H11Cl2FN6O2S: C, 46.46; H, 2.38; N, 18.06; S, 6.89. Found: C, 46.40; H, 2.48; N, 18.01; S, 6.97.
5-[(1H-Tetrazol-5-yl)methoxy]-4-(5-chloro-2-hydroxy-4-methylphenyl)-6-(4-fluorophenyl)pyrimidine-2(1H)-thione (9f)
This compound was prepared from 7f; a yellow solid; conventional method time 210 min, yield 76%; ultrasound-assisted method time 21 min, yield 92%; mp 277–279°C; IR: υ 3954 (OH), 3473 (NH), 3323 (NH), 3036 (CH) cm−1; 1H NMR (DMSO-d6): δ 2.45 (s, 3H, CH3), 4.86 (s, 2H, OCH2), 6.18 (t, 2H, J=8.8 Hz, ArH), 7.67–7.70 (m, 2H, ArH), 7.98–8.01 (m, 2H, ArH), 10.15 (br s, 1H, OH), 10.91 (br s, 1H, NH), 11.32 (br s, 1H, NH); 13C NMR (DMSO-d6): δ 17.3, 67.5, 115.9, 116.0, 116.9, 117.3, 119.6, 120.6, 121.4, 130.3, 130.7, 131.5, 131.9, 137.4, 155.6, 157.2, 160.4, 162.6, 194.2. Anal. Calcd for C19H14ClFN6O2S: C, 51.30; H, 3.17; N, 18.89; S, 7.21. Found: C, 51.38; H, 3.22; N, 18.91; S, 7.13.
Antimicrobial activity
Antibacterial activity of the synthesized compounds was tested in vitro against Gram-positive bacteria S. aureus (NCIM 2079) and B. subtilis (NCIM 2920) and Gram-negative bacteria E. coli (NCIM 2065) and P. aeruginosa (NCIM 2200). The compounds were also screened for antifungal activity against C. albicans (NCIM 3471) and A. niger (NCIM 596). Compounds were diluted in dimethyl sulfoxide (DMSO) with 1 μg/mL concentrations for bioassay. Micro-broth dilution method [28] was used to determine the MICs of compounds in 96-well microtiter plates. Test compounds were serially diluted in growth media. Plates were incubated at 30°C for fungi and 37°C for bacteria for 24 h. All experiments were carried out in triplicates.
Acknowledgments
V.S.D. is grateful to the Council of Scientific and Industrial Research (CSIR), New Delhi, India, for providing Senior Research Fellowship and Sophisticated Analytical Instrumentation Facility (SAIF). Panjab University provided spectral data. We are thankful to the Head, Department of Chemistry, Dr. Babasaheb Ambedkar Marathwada University, for providing laboratory facility. We also thank the Department of Microbiology, Maulana Azad College of Arts, Science and Commerce, Aurangabad, for providing biological assay results.
References
[1] Jin, H.; Zhao, H.; Zhao, F.; Li, S.; Liu, W.; Zhou, G.; Tao, K.; Hou, T. Efficient epoxidation of chalcones with urea-hydrogen peroxide under ultrasound irradiation. Ultrason. Sonochem.2009, 16, 304–307.10.1016/j.ultsonch.2008.10.013Search in Google Scholar PubMed
[2] Grare, M.; Mourer, M.; Fontanay, S.; Regnouf-de-Vains, J. B.; Finance, C.; Duval, R. E. In vitro activity of para-guanidinoethylcalix[4]arene against susceptible and antibiotic-resistant Gram-negative and Gram-positive bacteria. J. Antimicrob. Chemother.2007, 60, 575–581.10.1093/jac/dkm244Search in Google Scholar PubMed
[3] Anzahaee, M. Y.; Watts, J. K.; Alla, N. R.; Nicholson, A. W.; Damha, M. J. Energetically important C-H···F-C pseudohydrogen bonding in water: evidence and application to rational design of oligonucleotides with high binding affinity. J. Am. Chem. Soc.2011, 133, 728–731.10.1021/ja109817pSearch in Google Scholar PubMed
[4] Young, A. M.; Audus, K. L.; Proudfoot, J.; Yazdanian, M. Tetrazole compounds: the effect of structure and pH on Caco-2 cell permeability. J. Pharm. Sci.2006, 95, 717–725.10.1002/jps.20526Search in Google Scholar PubMed
[5] Myznikov, L. V.; Hrabalek, A.; Koldobskii, G. I. Drugs in the tetrazole series. Chem. Heterocycl. Compd.2007, 43, 1–9.10.1002/chin.200742264Search in Google Scholar
[6] Alam, M.; Nami, S. A.; Husain, A.; Lee, D.; Park, S. Synthesis, characterization, X-ray diffraction, antimicrobial and in vitro cytotoxicity studies of 7a-Aza-B-homostigmast-5-eno [7a,7-d]tetrazole. C. R. Chim.2013, 16, 201–206.10.1016/j.crci.2012.12.018Search in Google Scholar
[7] Bondaryk, M.; Lukowska-Chojnacka, E.; Staniszewska, M. Tetrazole activity against Candida albicans. The role of KEX2 mutations in the sensitivity to (±)-1-[5-(2-chlorophenyl)-2H-tetrazol-2-yl]propan-2-yl acetate. Bioorg. Med. Chem. Lett.2015, 25, 2657–2663.10.1016/j.bmcl.2015.04.078Search in Google Scholar PubMed
[8] Karabanovich, G.; Roh, J.; Smutny, T.; Nemecek, J.; Vicherek, P.; Stolaríkova, J.; Vejsova, M.; Dufkova, I.; Vavrova, K.; Pavek, P.; et al. 1-Substituted-5-[(3,5-dinitrobenzyl)sulfanyl]-1H-tetrazoles and their isostericanalogs: a new class of selective antitubercular agents active against drug-susceptible and multidrug-resistant mycobacteria. Eur. J. Med. Chem.2014, 82, 324–340.10.1016/j.ejmech.2014.05.069Search in Google Scholar PubMed
[9] Muraglia, E.; Kinzel, O. D.; Laufer, R.; Miller, M. D.; Moyer, G.; Munshi, V.; Orvieto, F.; Palumbi, M. C.; Pescatore, G.; Rowley, M.; et al. Tetrazolethioacetanilides: potent non-nucleoside inhibitors of WT HIV reverse transcriptase and its K103Nmutant. Bioorg. Med. Chem. Lett.2006, 16, 2748–2752.10.1016/j.bmcl.2006.02.024Search in Google Scholar PubMed
[10] Pegklidou, K.; Koukoulitsa, C.; Nicolaou, I.; Demopoulos, V. J. Design and synthesis of novel series of pyrrole based chemotypes and their evaluation as selective aldose reductase inhibitors. A case of bioisosterism between a carboxylic acid moiety and that of a tetrazole. Bioorg. Med. Chem.2010, 18, 2107–2114.10.1016/j.bmc.2010.02.010Search in Google Scholar PubMed
[11] Li, J.; Chen, S. Y.; Tao, S.; Wang, H.; Li, J. J.; Swartz, S.; Musial, C.; Hernandez, A. A.; Flynn, N.; Murphy, B. J.; et al. Design and synthesis of tetrazole-based growth hormone secretagogue: the SAR studies of the O-benzyl serine side chain. Bioorg. Med. Chem. Lett.2008, 18, 1825–1829.10.1016/j.bmcl.2008.02.021Search in Google Scholar PubMed
[12] Isloor, A. M.; Kalluraya, B.; Shetty, P. Regioselective reaction: synthesis, characterization and pharmacological studies of some new Mannich bases derived from 1,2,4-triazoles. Eur. J. Med. Chem.2009, 44, 3784–3787.10.1016/j.ejmech.2009.04.038Search in Google Scholar PubMed
[13] Isloor, A. M.; Kalluraya, B.; Rao, M. Sydnone derivatives: part IV; synthesis of 3-aryl-4-(substituted pyrazolidene hydrazine-4-thiazolyl) sydnones as possible analgesic and anticonvulsant agents. J. Saudi Chem. Soc.2000, 4, 265–270.Search in Google Scholar
[14] Bekhita, A. A.; Abdel-Aziem, T. Design, synthesis and biological evaluation of some pyrazole derivatives as anti-inflammatory-antimicrobial agents. Bioorg. Med. Chem.2004, 12, 1935–1945.10.1016/j.bmc.2004.01.037Search in Google Scholar PubMed
[15] Bertuzzi, G.; Locatelli, E.; Colecchia, D.; Calandro, P.; Bonini, B. F.; Chandanshive, J. Z.; Mazzanti, A.; Zani, P.; Chiariello, M.; Franchini, M. C. Straightforward synthesis of a novel ring-fused pyrazole-lactam and in vitro cytotoxic activity on cancer cell lines. Eur. J. Med. Chem.2016, 117, 1–7.10.1016/j.ejmech.2016.04.006Search in Google Scholar PubMed
[16] Mohamed, M. S.; Awad, S. M.; Sayed, A. I. Synthesis of certain pyrimidine derivatives as antimicrobial agents and anti-inflammatory agents. Molecules2010, 15, 1882–1890.10.3390/molecules15031882Search in Google Scholar PubMed PubMed Central
[17] Zhang, N.; Ayral-Kaloustian, S.; Nguyen, T.; Hernandezb, R.; Beyerb, C. 2-Cyanoaminopyrimidines as a class of antitumor agents that promote tubulin polymerization. Bioorg. Med. Chem. Lett.2007, 17, 3003–3005.10.1016/j.bmcl.2007.03.070Search in Google Scholar PubMed
[18] Katiyar, S. B.; Bansal, I.; Saxena, J. K.; Chauhan, P. M. S. Syntheses of 2,4,6-trisubstituted pyrimidine derivatives as a new class of antifilarial topoisomerase II inhibitors. Bioorg. Med. Chem. Lett.2005, 15, 47–50.10.1016/j.bmcl.2004.10.046Search in Google Scholar PubMed
[19] Lu, X.; Chen, Y.; Guo, Y.; Liu, Z.; Shi, Y.; Xu, Y.; Wang, X.; Zhang, Z.; Liu, J. The design and synthesis of N-1-alkylated-5-aminoaryalkyl-substituted-6-methluracils as potential non-nucleoside HIV-1 RT inhibitors. Bioorg. Med. Chem.2007, 15, 7399–7407.10.1016/j.bmc.2007.07.058Search in Google Scholar PubMed
[20] Morgan, A.; Cofer, C.; Stevens, D. L. Iclaprim: a novel dihydrofolate reductase inhibitor for skin and soft tissue infections. Future Microbiol.2009, 4, 131–144.10.2217/17460913.4.2.131Search in Google Scholar PubMed
[21] Prakash, O.; Kumar, R.; Kumar, R.; Tyagi, P.; Kuhad, R. C. Organoiodine(III) mediated synthesis of 3,9-diaryl- and 3,9-difuryl-bis-1,2,4-triazolo[4,3-a][4,3-c]pyrimidines as antibacterial agents. Eur. J. Med. Chem.2007, 42, 868–872.10.1016/j.ejmech.2006.11.019Search in Google Scholar PubMed
[22] Sondhi, S. M.; Jain, S.; Dinodia, M.; Shuklab, R.; Raghubir, R. One pot synthesis of pyrimidine and bispyrimidine derivatives and their evaluation for anti-inflammatory and analgesic activities. Bioorg. Med. Chem.2007, 15, 3334–3344.10.1016/j.bmc.2007.03.028Search in Google Scholar
[23] Bruno, O.; Brullo, C.; Schenone, S.; Ranise, A.; Bondavalli, F.; Barocelli, E.; Tognolini, M.; Magnanini, F.; Ballabeni, V. Progress in 5H[1]benzopyrano[4,3-d]pyrimidin-5-amine series: 2-Methoxy derivatives effective as antiplatelet agents with analgesic activity. Farmaco. 2002, 57, 753–758.10.1016/S0014-827X(02)01269-7Search in Google Scholar
[24] McCarthy, O.; Musso-Buendia, A.; Kaiser, M.; Brun, R.; Ruiz-Perez, L. M.; Johansson, N. G.; Pacanowska, D. G.; Gilbert, I. H. Design, synthesis and evaluation of novel uracil acetamide derivatives as potential inhibitors of plasmodium falciparum dUTP nucleotide hydrolase. Eur. J. Med. Chem.2009, 44, 678–688.10.1016/j.ejmech.2008.05.018Search in Google Scholar PubMed
[25] Diwakar, S. D.; Bhagwat, S. S.; Shingare, M. S.; Gill, C. H. Substituted 3-((Z)-2-(4-nitrophenyl)-2-(1H-tetrazol-5-yl) vinyl)-4H-chromen-4-ones as novel anti-MRSA agents: synthesis, SAR, and in-vitro assessment. Bioorg. Med. Chem. Lett.2008, 18, 4678–4681.10.1016/j.bmcl.2008.07.007Search in Google Scholar PubMed
[26] Gharpure, M.; Choudhary, R.; Ingle, V.; Juneja, H. Synthesis of new series of 3-hydroxy/acetoxy-2-phenyl-4H-chromen-4-ones and their biological importance. J. Chem. Sci.2013, 125, 575–582.10.1007/s12039-013-0420-zSearch in Google Scholar
[27] Dofe, V. S.; Sarkate, A. P.; Lokwani, D. K.; Kathwate, S. H.; Gill, C. H. Synthesis, antimicrobial evaluation, and molecular docking studies of novel chromone based 1,2,3-triazoles. Res. Chem. Int.2017, 43, 15–28.10.1007/s11164-016-2602-zSearch in Google Scholar
[28] Wiegand, I.; Hilpert, K.; Hancock, R. E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175.10.1038/nprot.2007.521Search in Google Scholar PubMed
©2018 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Review
- Research progress in quinazoline derivatives as multi-target tyrosine kinase inhibitors
- Research Articles
- A bio-inspired approach to proline-derived 2,4-disubstituted oxazoles
- Solvent-free preparation of 3-aryl-2-[(aryl)(arylamino)]methyl-4H-furo[3,2-c]chromen-4-one derivatives using ZnO-ZnAl2O4 nanocomposite as a heterogeneous catalyst
- Synthesis of 4-arylethyl-6-arylpyrimidine-2-thiols through aza-Michael addition/nucleophilic addition/aromatization tandem reactions
- 3-Carboxy-1-sulfopyridin-1-ium chloride ([CPySO3H]+Cl−): an efficient catalyst for one-pot synthesis of hexahydroquinoline-3-carboxamides
- Synthesis of new 3H-chromeno[2,3-d]pyrimidine-4,6(5H,7H)-diones via the tandem intramolecular Pinner/Dimroth rearrangement
- A new synthesis of pyrrolo[3,2-d]pyrimidine derivatives by a one-pot, three-component reaction in the presence of L-proline as an organocatalyst
- Diversity-oriented synthesis of amide derivatives of tricyclic thieno[2,3-d]pyrimidin-4(3H)-ones and evaluation of their influence on melanin synthesis in murine B16 cells
- Synthesis, crystal structure, molecular docking studies and bio-evaluation of some N4-benzyl-substituted isatin- 3-thiosemicarbazones as urease and glycation inhibitors
- Ultrasound-assisted synthesis and antimicrobial activity of tetrazole-based pyrazole and pyrimidine derivatives
Articles in the same Issue
- Frontmatter
- Review
- Research progress in quinazoline derivatives as multi-target tyrosine kinase inhibitors
- Research Articles
- A bio-inspired approach to proline-derived 2,4-disubstituted oxazoles
- Solvent-free preparation of 3-aryl-2-[(aryl)(arylamino)]methyl-4H-furo[3,2-c]chromen-4-one derivatives using ZnO-ZnAl2O4 nanocomposite as a heterogeneous catalyst
- Synthesis of 4-arylethyl-6-arylpyrimidine-2-thiols through aza-Michael addition/nucleophilic addition/aromatization tandem reactions
- 3-Carboxy-1-sulfopyridin-1-ium chloride ([CPySO3H]+Cl−): an efficient catalyst for one-pot synthesis of hexahydroquinoline-3-carboxamides
- Synthesis of new 3H-chromeno[2,3-d]pyrimidine-4,6(5H,7H)-diones via the tandem intramolecular Pinner/Dimroth rearrangement
- A new synthesis of pyrrolo[3,2-d]pyrimidine derivatives by a one-pot, three-component reaction in the presence of L-proline as an organocatalyst
- Diversity-oriented synthesis of amide derivatives of tricyclic thieno[2,3-d]pyrimidin-4(3H)-ones and evaluation of their influence on melanin synthesis in murine B16 cells
- Synthesis, crystal structure, molecular docking studies and bio-evaluation of some N4-benzyl-substituted isatin- 3-thiosemicarbazones as urease and glycation inhibitors
- Ultrasound-assisted synthesis and antimicrobial activity of tetrazole-based pyrazole and pyrimidine derivatives

