Abstract
The 5-nitrosalicylate ester of 2-acetamidophenylboronic acid (C15H10BN2O6) is formed under crystallization conditions from the 5-nitrosalicylate ester of 2-aminophenylboronic acid. The boron at the center of this structure exists as a tetrahedral complex produced by a dative bond with the amide carbonyl. The perpendicular shape produces an unusual packing structure including a bifurcated hydrogen bond between the amide hydrogen and carbonyl groups on two neighboring molecules. We propose that this reaction occurs due to increased Lewis acidity of the nitrosalicylate ester of 2-aminophenylboronic acid.
Boron acids are useful catalysts for many transformations including amidation and esterification reactions [1], [2], [3]. In the first known example where 2-aminophenylboronic acid was used in amidation, it was shown by Groziak in 1994 that this compound could form a formamide by refluxing in formic acid [4]. It is unclear whether the boronic acid plays a role in catalyzing the amidation as the reaction can occur thermally at temperatures greater than 100°C [5]. Previously, we have demonstrated that boric acid is an effective catalyst for esterification of α-hydroxycarboxylic [6], [7], malonic [8] or salicylic acids [9]. By chelating the carboxylate and the alcohol (or second carboxylate), boron activates the carbonyl group toward esterification [10]. We reasoned that such chelation would allow for direct, facile amide bond formation between 2-aminophenylboronic acid and α-hydroxycarboxylic acids or salicylic acids (Scheme 1). Boron ester formation would place the activated carbonyl six atoms away from the amine.
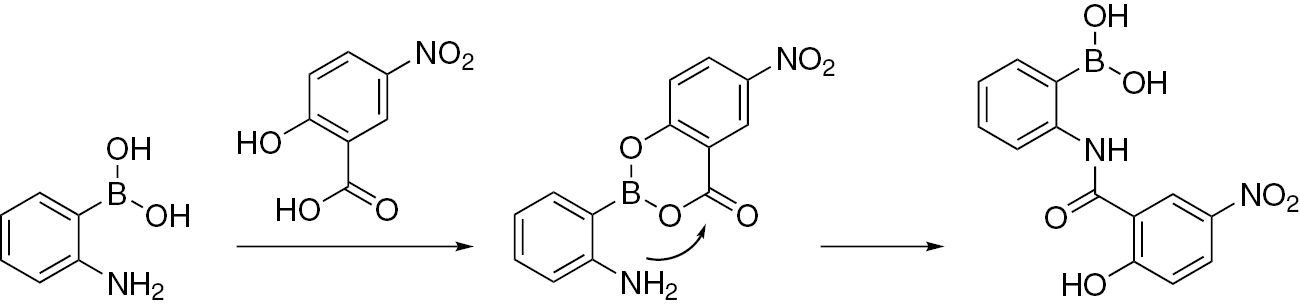
Proposed direct amide formation between 2-aminophenylboronic acid and 5-nitrosalicylic acid.
Heating a mixture of 2-aminophenylboronic acid and 5-nitrosalicylic acid in acetonitrile at 50°C resulted in formation of 1 as a light brown precipitate rather than the desired amide. Attempted crystallization of 1 from EtOAc/hexane produced the unexpected crystalline product 2 (Scheme 2). This amidation reaction with ethyl acetate does not occur when 2-aminophenylboronic acid alone is heated in this solvent system suggesting the nitrosalicylate ester plays a role in this reaction.
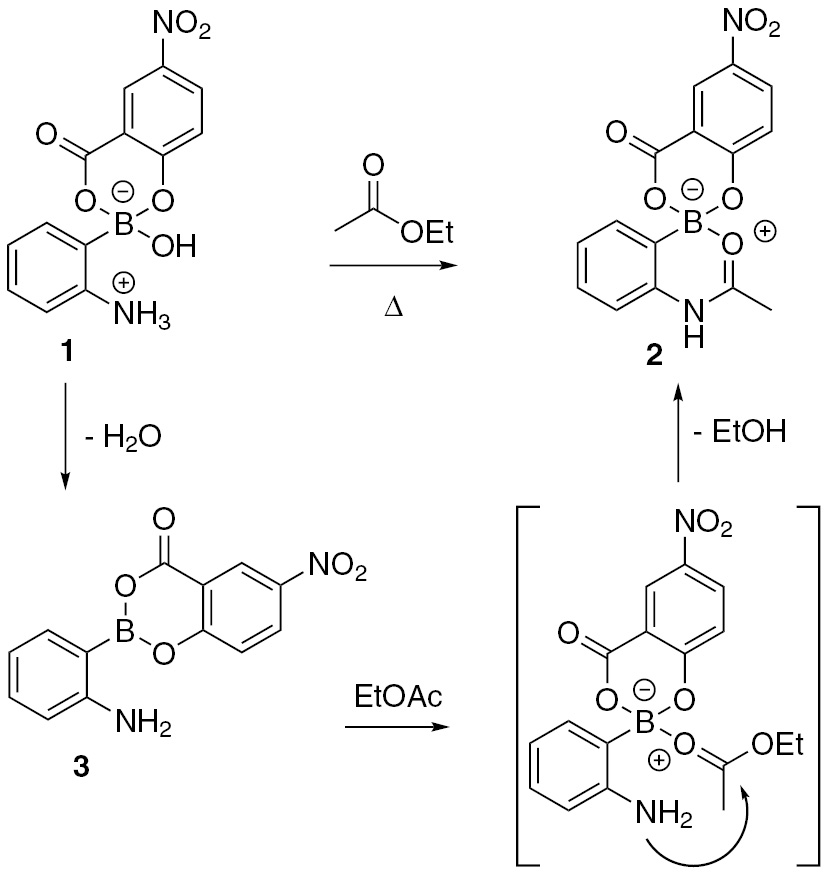
Postulated mechanism for amidation reaction of 1.
We propose that 1 likely undergoes dehydration to form a free amine and neutral boronate ester 3 that can subsequently activate the ester carbonyl as shown in the bracket. The nitrosalicylate ester of the boronate would make the boron more Lewis acidic than its parent boronic acid allowing it to better activate the carbonyl group of ethyl acetate [11], [12]. Compound 2 does not accumulate in solution and thus its formation appears to be a result of the crystallization process. The ORTEP diagram for the X-ray crystal structure of 2 is shown below in Figure 1.
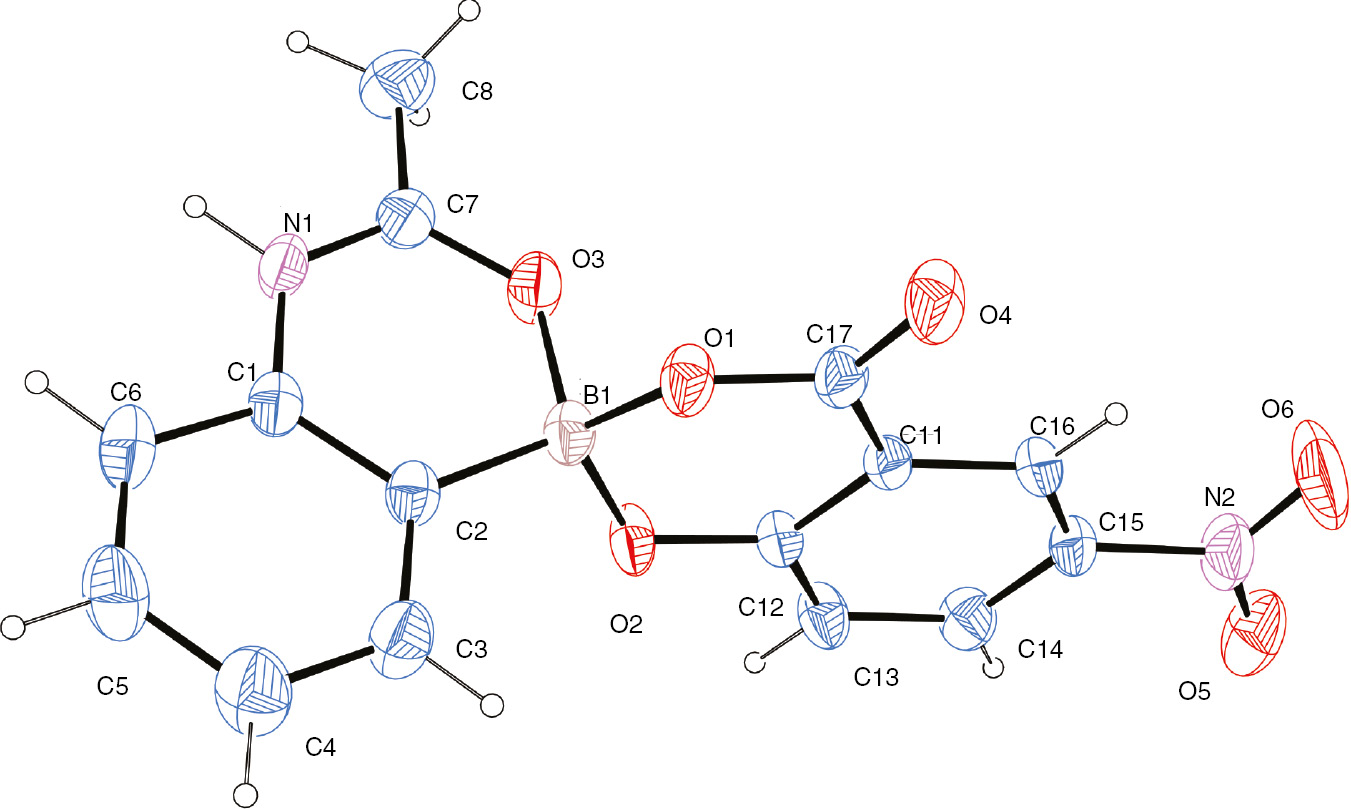
ORTEP diagram of compound 2.
Compound 2 demonstrates some interesting supramolecular features in its crystal lattice (Figure 2). The main hydrogen bond supporting the packing structure is a bifurcated hydrogen bond between the hydrogen on the nitrogen in the amide (N1) and two neighboring carbonyl oxygen (O4) from two other molecules. This hydrogen bond brings together two planar systems and two perpendicular systems together. The boron complex has put torsional strain on some bond lengths in the molecule, but the bond length of the B1–C1 of 1.58 Å is consistent with typical values in the literature [13]. Coordination of an amide oxygen to create a spirocyclic center at boron via a five-membered ring has also been observed [14]; however, the extended supramolecular topology of compound 2 appears unique.
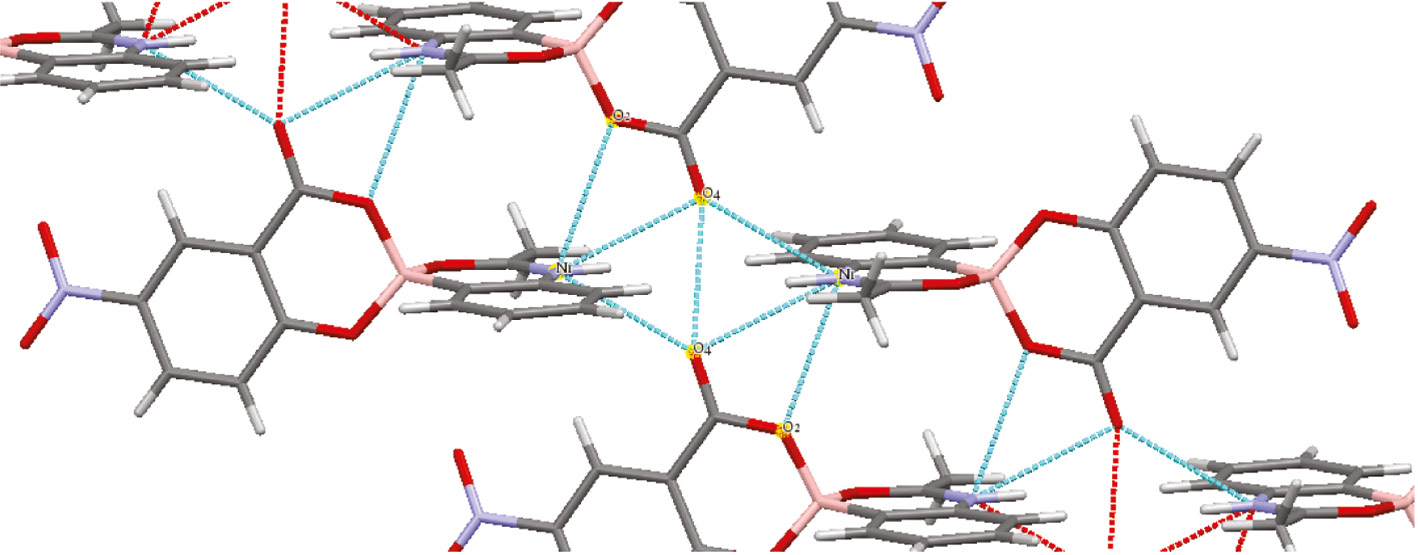
Hydrogen bonding network revealed in X-ray crystal structure of 2.
Formation of compound 2 occurs via amidation of 1 with ethyl acetate during the crystallization process. It is unclear at present whether amide bond formation is induced by the rich hydrogen bonding network within the crystal lattice, or whether compound 2 has a strong propensity to crystallize once formed. Nonetheless, we are exploring the proposed Lewis acid activation toward nucleophilic attack by the adjacent amine in other systems and will report on success in this arena in due course.
Experimental
4′-Hydroxy-3-methyl-6′-nitro-4′H-spiro[benzo[c][1,5,2]oxazaborinine-1,2′-benzo[d][1,3,2]dioxaborinin]-1-uide (2)
To a stirring solution of 2-aminophenylboronic acid (50.0 mg, 0.37 mmol) in acetonitrile (3.65 mL) was added 5-nitrosalicylic acid (66.9 mg, 0.37 mmol). The mixture was allowed to react at 323 K for 1 h and then the precipitated product 1 as a light brown powder was collected via vacuum filtration (109.2 mg, 99%). 11B NMR (DMSO-d6): δ 5.59 with BF3-OEt2/CDCl3 as standard. ESI-MS(-). Calcd for C13H10BN2O6−, (M-H)−: m/z 301.06. Found: m/z 301.1. Crystallization occurred via a slow evaporation process at room temperature from hot 1:1 EtOAc/hexane solvent system after purification with activated charcoal. The solution stood for 3 days producing crystals that were clear, small and rectangular in appearance. The crystals were identified as compound 2 by 1H NMR, MS and X-ray crystallography. 1H NMR (DMSO-d6): δ 2.35 (s, 3H, CH3), 7.10 (d, 1H, J=9.0 Hz, ArH), 7.16 (d, J=7.0 Hz, 1H, ArH), 7.27 (m, 1H, ArH), 7.42 (m, 2H, ArH), 8.32 (d, 1H, J=9.0 Hz, ArH), 8.61 (s, 1H, ArH). ESI-MS(-). Calcd for C15H10BN2O6−, (M-H)−: m/z 325.06. Found: m/z 324.9.
Crystal data for 2: C15H11BN2O6. M=326.1, monoclinic, space group P21/n, a=8.6642(4), b=11.2685(8), c=16.0267(10) Å, β=101.689(6)°, U=1532.3(2) Å3, Z=4, Dc=1.41 g cm−3, μ=0.110 mm−1, crystal size: 0.30 Å~ 0.20 Å~ 0.20 mm. Tmin/max=0.89, 1.00. 6797 reflections collected, 4092 unique (Rint=0.040), R=0.068 [2187 reflections with I>2s(I)], wRF2=0.130 (all data). Supplementary crystallographic data of 2 is deposited at the Cambridge Crystallography Data Centre (CCDC) as supplementary publication CCDC 1532315. Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK, (fax: +44-(0)1223-336033 or e-mail: deposit@ccdc.cam.ac.uk).
Acknowledgments
This paper is dedicated to Professor Binghe Wang of Georgia State University for his important contributions to the study of boronic acids across several fields from catalysis to chemical biology. We thank the Institute for Glycomics and Griffith University for funding.
References
[1] Latta, R.; Springsteen, G.; Wang, B. Development and synthesis of an arylboronic acid-based solid-phase amidation catalyst. Synthesis2001, 33, 1611–1613.10.1055/s-2001-16758Suche in Google Scholar
[2] Houston, T. A.; Levonis, S. M.; Kiefel, M. J. Tapping into boron/α-hydroxycarboxylic acid interactions in sensing and catalysis. Aust. J. Chem. 2007, 60, 811–815.10.1071/CH07222Suche in Google Scholar
[3] Gernigon, N.; Al-Zoubi, R. M.; Hall, D. G. Direct amidation of carboxylic acids catalyzed by ortho-iodo arylboronic acids: catalyst optimization, scope, and preliminary mechanistic study supporting a peculiar halogen acceleration effect. J. Org. Chem.2012, 77, 8386–8400.10.1021/jo3013258Suche in Google Scholar PubMed
[4] Groziak, M. P.; Ganguly, A. D.; Robinson, P. D. Boron heterocycles bearing a peripheral resemblance to naturally-occurring purines: design, syntheses, structures, and properties. J. Am. Chem. Soc.1994, 116, 7597–7599.10.1021/ja00096a017Suche in Google Scholar
[5] Arnold, K.; Davies, B.; Giles, R. L.; Grosjean, C.; Smith, G. E.; Whiting, A. To catalyze or not to catalyze? Insight into direct amide bond formation from amines and carboxylic acids under thermal and catalyzed conditions. Adv. Synth. Catal.2006, 348, 813–820.10.1002/adsc.200606018Suche in Google Scholar
[6] Houston, T. A.; Wilkinson, B. L.; Blanchfield, J. T. Boric acid catalyzed chemoselective esterification of α-hydroxycarboxylic acids. Org. Lett.2004, 6, 679–681.10.1021/ol036123gSuche in Google Scholar PubMed
[7] Levonis, S. M.; Pappin, B. B.; Sharp, A.; Kiefel, M. J.; Houston, T. A. Boric acid catalyzed methyl esterification of sugar acids. Aust. J. Chem.2014, 67, 528–530.10.1071/CH13459Suche in Google Scholar
[8] Levonis, S. M.; Bornaghi, L. F.; Houston, T. A. Selective monoesterification of malonic acid catalyzed by boric acid. Aust. J. Chem. 2007, 60, 821–823.10.1071/CH07231Suche in Google Scholar
[9] Levonis, S. M.; Kiefel, M. J.; Houston, T. A.; Healy, P. C. 2′-Propynyl 2-hydroxybenzoate. Acta Cryst.2010, E66, o226–o228.10.1107/S160053680905421XSuche in Google Scholar PubMed PubMed Central
[10] Maki, T.; Ishihara, K.; Yamamoto, H. N-Alkyl-4-boronopyridinium halides versus boric acid as catalysts for the esterification of α-hydroxycarboxylic acids. Org. Lett.2005, 7, 5047–5050.10.1021/ol052061dSuche in Google Scholar PubMed
[11] Springsteen, G.; Wang, B. A detailed examination of boronic acid–diol complexation. Tetrahedron2002, 58, 5291–5300.10.1016/S0040-4020(02)00489-1Suche in Google Scholar
[12] Aydin, R.; Ozer, U.; Turkel, N. Potentiometric and spectroscopic determination of acid dissociation constants of some phenols and salicylic acids. Turk. J. Chem. 1997, 21, 428–436.Suche in Google Scholar
[13] Allen, F. H.; Kennard, O.; Watson, D. G.; Brammer, L.; Orpen, A. G.; Taylor, R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc., Perkin Trans.1987, 2, S1–S19.10.1039/p298700000s1Suche in Google Scholar
[14] Inglis, S. R.; Esther C. Y. Woon, E. C. Y.; Amber L. Thompson, A. L.; Schofield, C. J. Observations on the deprotection of pinanediol and pinacol boronate esters via fluorinated intermediates. J. Org. Chem.2010, 75, 468–471.10.1021/jo901930vSuche in Google Scholar PubMed
©2017 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- Editorial
- Carbohydrate chemistry/glycoscience
- Reviews
- Boron-based small molecules in disease detection and treatment (2013–2016)
- Impact of modified ribose sugars on nucleic acid conformation and function
- Preliminary Communication
- Crystallization-induced amide bond formation creates a boron-centered spirocyclic system
- Research Articles
- Anion and sugar recognition by 2,6-pyridinedicarboxamide bis-boronic acid derivatives
- Synthesis of biotinylated bivalent zanamivir analogs as probes for influenza viruses
- Synthesis of silodosin glucuronide and its deuterated counterpart: solving a problematic O-glycosylation of a nitrogen-containing molecule
- Synthesis and antimicrobial activity of 4-trifluoromethylpyridine nucleosides
- Synthesis of anti-inflammatory 2,3-unsaturated O-glycosides using conventional and microwave heating techniques
- Synthesis of various β-D-glucopyranosyl and β-D-xylopyranosyl hydroxybenzoates and evaluation of their antioxidant activities
- Synthesis of carbohydrate-substituted isoxazoles and evaluation of their antitubercular activity
- Synthesis and bioactivity of novel C2-glycosyl triazole derivatives as acetylcholinesterase inhibitors
- Modification of bovine serum albumin with aminophenylboronic acid as glycan sensor based on surface plasmon resonance and isothermal titration calorimetry
Artikel in diesem Heft
- Frontmatter
- Editorial
- Carbohydrate chemistry/glycoscience
- Reviews
- Boron-based small molecules in disease detection and treatment (2013–2016)
- Impact of modified ribose sugars on nucleic acid conformation and function
- Preliminary Communication
- Crystallization-induced amide bond formation creates a boron-centered spirocyclic system
- Research Articles
- Anion and sugar recognition by 2,6-pyridinedicarboxamide bis-boronic acid derivatives
- Synthesis of biotinylated bivalent zanamivir analogs as probes for influenza viruses
- Synthesis of silodosin glucuronide and its deuterated counterpart: solving a problematic O-glycosylation of a nitrogen-containing molecule
- Synthesis and antimicrobial activity of 4-trifluoromethylpyridine nucleosides
- Synthesis of anti-inflammatory 2,3-unsaturated O-glycosides using conventional and microwave heating techniques
- Synthesis of various β-D-glucopyranosyl and β-D-xylopyranosyl hydroxybenzoates and evaluation of their antioxidant activities
- Synthesis of carbohydrate-substituted isoxazoles and evaluation of their antitubercular activity
- Synthesis and bioactivity of novel C2-glycosyl triazole derivatives as acetylcholinesterase inhibitors
- Modification of bovine serum albumin with aminophenylboronic acid as glycan sensor based on surface plasmon resonance and isothermal titration calorimetry

