Abstract
Various β-D-glucopyranosyl and β-D-xylopyranosyl hydroxybenzoates were efficiently prepared from 2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide (TAGB) or 2,3,4-tri-O-acetyl-α-D-xylopyranosyl bromide (TAXB), respectively, by amine-promoted glycosylation. Regioselective deacetylation of the resulting acetylated β-D-gluco- and β-D-xylopyranosyl hydroxybenzoates was investigated using Novozym 435 as a lipase catalyst. In the case of β-D-glucopyranosyl hydroxybenzoates, Novozym 435-catalyzed deacetylation is regioselective at C-4 and C-6 positions. On the other hand, β-D-xylopyranosyl hydroxybenzoates are deacetylated only at the C-4 position. Antioxidant activities of free hydroxybenzoic acids and the respective β-D-gluco- and β-D-xylopyranosyl hydroxybenzoates were evaluated by DPPH˙ radical scavenging as well as their inhibitory effect on autoxidation of bulk methyl linoleate. The β-D-xylopyranosyl protocatechoate, as well as quercetin and α-tochopherol, show high antioxidant activity for the radical scavenging activity by 1,1-diphenyl-2-picrylhydrazyl (DPPH˙). In bulk methyl linoleate, the antioxidant activities of β-D-gluco- and β-D-xylopyranosyl protocatechoates are higher than that of α-tocopherol.
Introduction
Hydroxybenzoic acid derivatives such as protocatechuic, vanillic, and syringic acids are widely distributed in plants and foods derived from natural products [1], [2], [3], [4], [5], [6]. In recent years, there have been many reports on the biological activities of hydroxybenzoic acid derivatives. It has been reported that vanillic acid shows anticoagulant [7], anti-inflammatory [8], and nephroprotective effects [9], as well as estrogen-like [10] and antinociceptive activities [11]. Syringic acid has an antihyperglycemic effect [12] and antiangiogenic and antimicrobial activities [13], [14]. In many cases, these hydroxybenzoic acids exist predominantly as ester and glycoside forms with only lesser amounts of them being present in free carboxylic acid forms [15]. Many plants containing these hydroxybenzoic acid derivatives have been used as traditional medicines. Conyza sumatrensis leaves have been used for traditional treatment of malaria in Cameroon [16]. The stems of Berchemia racemosa are used in Japan for treatment of gall stones, liver diseases, neuralgia and stomach cramp [17]. Conyza sumatrensis contains various flavonoids as phenolic components [18]. The occurrence of β-D-glucopyranosyl syringoate in Berchemia racemosa has been confirmed by Inoshiri and coworkers [19]. Additionally, it is known that various Spanish Cistus species include β-D-glucopyranosyl vanilloate [20]. In traditional folk medicine, Cistus is used as anti-inflammatory, antiulcerogenic, wound healing, antimicrobial, cytotoxic and vasodilator remedies [21]. These activities of the plants containing hydroxybenzoic acid derivatives are related to their ability to act as antioxidants [22], metal ion chelators [23], radical scavengers [24] and inhibitors of prooxidant enzymes [25]. Many researchers have studied the antioxidant effects of hydroxybenzoic acid derivatives [22], [26], [27], [28]. We have previously reported preparation of β-D-gluco- and β-D-xylopyranosyl hydroxycinnamoates using amine-promoted glycosylation and Novozym 435-catalyzed regioselective deacetylation, and evaluation of their antioxidant activities [29], [30]. β-D-Glucopyranosyl feruloyl and synapinoates and β-D-xylopyranosyl cafferoate show similar or higher antioxidant activities compared with α-tocopherol. In this study, we investigated the synthesis of various β-D-gluco- and β-D-xylopyranosyl hydroxybenzoates and described the antioxidant activities of hydroxybenzoic acids and their gluco- and xylopyranosates as well as some vanillic acid-related compounds such as p-hydroxybenzoic, protocatechuic and syringic acids and their glycosates. Their antioxidant activities were evaluated based on their inhibitory effects on the autoxidation of methyl linoleate in a bulk system and the radical scavenging activity against 1,1-diphenyl-2-picrylhydrazyl (DPPH˙).
Results and discussion
Amine-promoted glycosylation of hydroxybenzoic acids and Novozym 435-catalyzed regioselective deacetylation
We have previously reported synthesis of β-D-glucopyranosyl hydroxycinnamoates by amine-promoted glycosylation and Novozym 435-catalyzed regioselective deacetylation [29]. β-D-Glucopyranosyl feruloyl and sinapinoates show high antioxidant activities compared with α-tocopherol and quercetin. It is known that glycosyl benzoate analogues have various biological activities such as antioxidant [31], cytotoxic [32], [33] and enzyme inhibitory properties [34]. Therefore, we synthesized β-D-glucopyranosyl hydroxybenzoates by previous methods using various hydroxybenzoic acids such as vanillic acid and protocatechuic acid as an aglycone (Scheme 1).
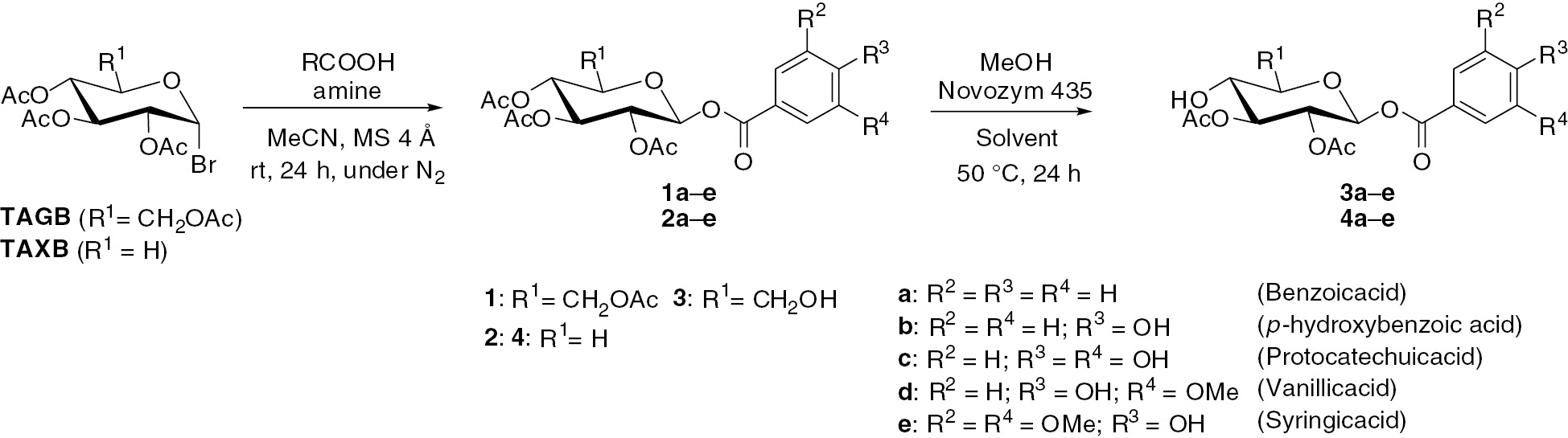
Glycosylation of hydroxybenzoic acid derivatives and deacetylation using Novozym 435.
β-D-Xylopyranosyl hydroxybenzoates were also synthesized because β-D-xylopyranosyl hydroxycinnamoates show higher antioxidant activities compared to the corresponding β-D-glycopyranosyl hydroxycinnamoates. Glycosylation of each hydroxybenzoic acid was performed under the previously described conditions [29], [30]. As in the case of glycosylation of hydroxycinnamic acids, the yields of β-D-glucopyranosyl p-hydroxybenzoyl and protocatechoates, which have phenolic hydroxyl groups, were lower than that of β-D-glucopyranosyl benzoate. Jover and co-workers have reported the relationship between chemical structures and acidity for benzoic acid derivatives [35]. The acidity of benzoic acid derivatives used in this work decreases in the order benzoic acid>syringic acid>protocatechuic acid>vanillic acid>p-hydroxybenzoic acid. On the other hand, the yields of β-D-glucopyranosyl hydroxybenzoates decrease in the order benzoic acid>vanillic acid>syringic acid>p-hydroxybenzoic acid>protocatechuic acid. It was expected that the yield of β-D-glucopyranosyl benzoate 1a would be highest because the stability of benzoate anion is highest among these benzoic acid derivatives. Galland and co-workers performed the glycosylation of hydroxycinnamic acids such as ferulic and caffeic acids after methateification at carboxylic group [36]. It seemed that the decrease in yield is not proportional to the acidity of benzoic acids themselves because glycosylation of competing phenoxy anion and carboxylate anion takes place. Additionally, it can be assumed that the rate of glycosylation of the phenolic hydroxyl groups for protocatechuic acid is higher than that of p-hydroxybenzoic acid because protocatechuic acid has two phenolic hydroxyl groups.
β-D-Xylopyranosyl hydroxybenzoates were synthesized using tri-O-acetyl-α-D-xylopyranosyl bromide (TAXB) as a saccharide donor (Scheme 1). Glycosylation was performed under previously described conditions that were used for the synthesis of β-D-xylopyranosyl hydroxycinnamoates [30]. As in the case of the synthesis of β-D-glucopyranosates 1b and 1c, yields of products 2b and 2c are lower than that of β-D-xylopyranosyl benzoate 2a. By contrast, reactions of vanillic and syringic acids give almost the same yields as β-D-xylopyranosyl benzoate 2a. The decrease of the yields occurrs for the same reason as in the case of synthesis of β-D-glucopyranosyl benzoate.
Subsequently, deacetylation of glucopyranosates 1 was investigated (Scheme 1). We have reported Novozym 435-catalyzed regioselective deacetylation of 2,3,4,6-tetraacetyl-β-D-glucopyranosyl hydroxycinnamoates [29]. Novozym 435 catalyzed deacetylation at C-4 and C-6 positions takes place regioselectively without methanolysis of a hydroxycinnamoyl group at the anomeric position. Since antioxidant activities of deacetylated β-D-glucopyranosyl cinnamoates were improved, deacetylation of β-D-glucopyranosyl hydroxybenzoates 1a–e was also investigated using Novozym 435 as a catalyst (Scheme 1). Methanolysis of 1 was performed in methyl tert-butyl ether (MTBE) for 24 h at 50°C. A mixture of MTBE/benzene, 3:1, was used as a solvent because β-D-glucopyranosyl p-hydroxybenzoyl, protocatechoyl and vanilloates 1b, 1c and 1d are sparingly soluble in MTBE. In the case of all substrates, methanolysis progressed to yields in a range of 84–90%. As with β-D-glucopyranosyl hydroxycinnamoates, methanolysis of the hydroxybenzoate moiety at the anomeric position was not observed. By using 2D NMR analysis, it was confirmed that acetyl groups at C-4 and C-6 positions were deacetylated regioselectively regardless of the difference in the benzoyl part. As with β-D-glucopyranosyl hydroxycinnamoates, Novozym 435-catalyzed methanolysis of β-D-glucopyranosates 1 exhibits high regio- and functional group selectivity. Additionally, Novozym 435 shows high affinity toward all substrates because all deacetylated β-D-glucopyranosates 2 were obtained in high yield after 24 h.
Methanolysis of β-D-xylopyranosyl hydroxybenzoates 2a–e was also performed using Novozym 435. The solubility of compounds 2b,c in MTBE is low, and a mixture of MTBE/benzene, 3/1, was used as a solvent. Novozym 435 catalyzed deacetylation of β-D-xylopyranosyl hydroxycinnamoates proceeds regioselectively at the C-4 position [30]. Deacetylation of β-D-xylopyranosyl hydroxybenzoates 2 was also investigated using Novozym 435 in the expectation that it would show high regio- and functional group selectivity. Methanolysis progressed to about 80% yields for all reactions. The hydroxybenzoate moiety did not react, and deacetylation at the C-4 position was only observed. Novozym 435 also shows high regio- and functional group selectivity toward β-D-xylopyranosyl hydroxybenzoates 2 and β-D-glucopyranosates 1.
Novozym 435 catalyzes methanolysis of acetyl groups at C-4 and C-6 positions for β-D-glucopyranosyl hydroxybenzoates 1 in MTBE. We have previously reported that deacetylation of β-D-gluco- and xylopyranosyl hydroxycinnamoates increases antioxidant properties [29], [30]. For deacetylation at C-2 and C-3 positions, regioselective hydrolysis of β-D-glucopyranosyl benzoate 1a was investigated using various lipases in pH 6 citrate buffer with MTBE as a co-solvent. To confirm that 1a is not hydrolyzed under mildly acidic conditions of pH 6, a citrate buffer solution of 1a without lipase was stirred at 40°C for 24 h; the hydrolysis was not observed. In addition to Novozym 435, lipase AS Amano (from Aspergillus niger), lipase AK Amano (from Pseudomonas fluorescens), lipase AYS (from Candia rugosa), lipase PS (from Burkholderia cepacia), and PPL (from porcine pancreas) were used. All these lipases show no reactivity for deacetylation of 1a. Deacetylation of β-D-xylopyranosyl benzoate 2a at C-2 and C-3 positions was also investigated using the same conditions described above. All lipases except lipase AYS Amano showed little reactivity. Hydrolysis of a benzoate group at the C-1 position was confirmed by analysis of NMR spectra. By contrast, deacetylated compound 4a was obtained by regioselective hydrolysis at the C-4 position for Novozym 435, lipase AK Amano and lipase PS Amano. Lipase can hydrolyze non-water soluble lipids and esterase catalyzes hydrolysis of water-soluble lipids [37]. We have previously reported PPL-catalyzed hydrolysis of N-methyl-5-acetoxyalkanamides [38]. Although the reaction mixture is a suspension because the water solubility of N-methyl-5-acetoxualkanamides is poor, lipase-catalyzed hydrolysis progresses with high enantioselectivity. The reaction mixtures of β-D-glucopyranosyl and β-D-xylopyranosyl benzoates 1a and 2a are also heterogeneous but the water solubility of xylose is higher than that of glucose [39]. It can be assumed that β-D-xylopyranosyl benzoate 2a shows slightly higher affinity for lipase compared with β-D-glucopyranosate 1a.
DPPH˙ radical scavenging activities
The antioxidant properties of hydroxybenzoyl acids and β-D-glucopyranosates 1b–e were evaluated by the demonstration of DPPH˙ radical scavenging activity (Figure 1). The activity decreases in the order of protocatechuic acid>syringic acid≈glucopyranosyl protocatechoates 1c and 3c>vanillic acid>glucopyranosyl syringoate 3e>deacetylated glucopyranosyl vanilloate 3d>deacetylated glucopyranosyl p-hydroxybenzoate 3b>glucopyranosyl syringoate 1e>glucopyranosyl vanilloate 1d≈p-hydroxybenzoic acid>glucopyranosyl p-hydroxybenzoate 1b.
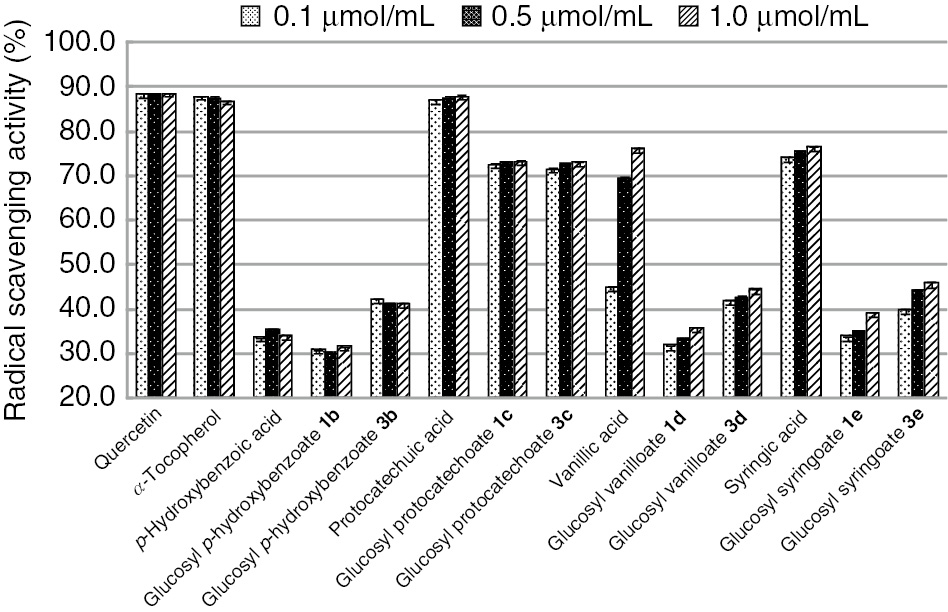
DPPH˙ radical scavenging activities of free hydroxylbenzoic acids and their β-D-glucopyranosates.
There are many reports of DPPH˙ radical scavenging activities of hydroxybenzoic acids. Karamać and co-workers have reported that the activities decrease in the order syringic acid>protocatechuic acid>>vanillic acid>>p-hydroxybenzoic acid [40]. Fujimoto and co-workers have reported different results: vanillic acid>>protocatechuic acid>syringic acid [22]. Furthermore, Noipa and co-workers have reported the order of syringic acid>protocatechuic acid>vanillic acid [28]. Still, different results were obtained in this work. It can be suggested that the differences between our and other results are due to the measurement conditions. On the other hand, our results are in good agreement with Gadow’s results [41]. Free protocatechuic acid shows almost the same high DPPH˙ radical scavenging activity as quercetin and α-tocopherol. Antioxidants that have many phenolic hydroxyl groups show high antioxidant activities [42], [43]. Free syringic and vanillic acids show high radical scavenging activities compared with free p-hydroxybenzoic acid. Ortho-methoxy groups as electron-donating groups at the phenolic hydroxyl group improve antioxidant activity and stabilize phenoxy radicals [42], [43]. The radical scavenging activity of free vanillic acid is higher than that of free p-hydroxybenzoic acid. Syringic acid has two methoxy groups at the ortho positions of phenolic hydroxyl groups. The phenoxy radical of syringic acid is more stable than that of vanillic acid. It can be assumed that syringic acid shows almost the same activity as vanillic acid because the steric hindrance of syringic phenoxy radicals is larger than that of vanillic acid. Only vanillic acid among free hydroxybenzoic acids shows concentration dependency. These results are in good agreement with those of Shyamala [44] and Sang [45]. DPPH˙ radical scavenging activities of glucopyranosyl protocatechoates 1c and 3c are highest in glucopyranosyl hydroxybenzoates 1 and 3. In the case of glucosyl p-hydroxybenzoyl, vanilloyl and syringoates, the corresponding deacetylated glucopyranosates 3b, 3d and 3e show about 10% higher activities than acetylated glucopyranosates 1b, 1d and 1e. Regioselective deacetylation at C-4 and C-6 positions increases water solubility and the increase of affinity to DPPH˙ free radicals increases the activity. All glucopyranosyl hydroxybenzoates except glucopyranosyl p-hydroxybenzoate 3b exhibit lower activities than the corresponding free hydroxybenzoic acids. Fukumoto and co-workers have suggested that the size of the steric hindrance of the sugar moiety reduces the antioxidant properties, and our results are in good agreement with their suggestion [22].
On the other hand, the DPPH˙ radical scavenging activities of xylopyranosates 2 and 4 decrease in the order of xylopyranosyl protocatechoate 2c>deacetylated xylopyranosyl protocatechoate 4c≈protocatechuic acid>syringic acid>vanillic acid>xylopyranosyl syringoates 2e and 4e≈xyropyranosyl vanilloates 2d and 4d>xylopyranosyl p-hydroxybenzoates 2b and 4b≈p-hydroxybenzoic acid (Figure 2). Xylopyranosyl protocatechuate 2c shows slightly higher activity compared with free protocatechuic acid. Deacetylated xylopyranosyl protocatechoate 4c also shows comparatively high activity with free protocatechuic acid.
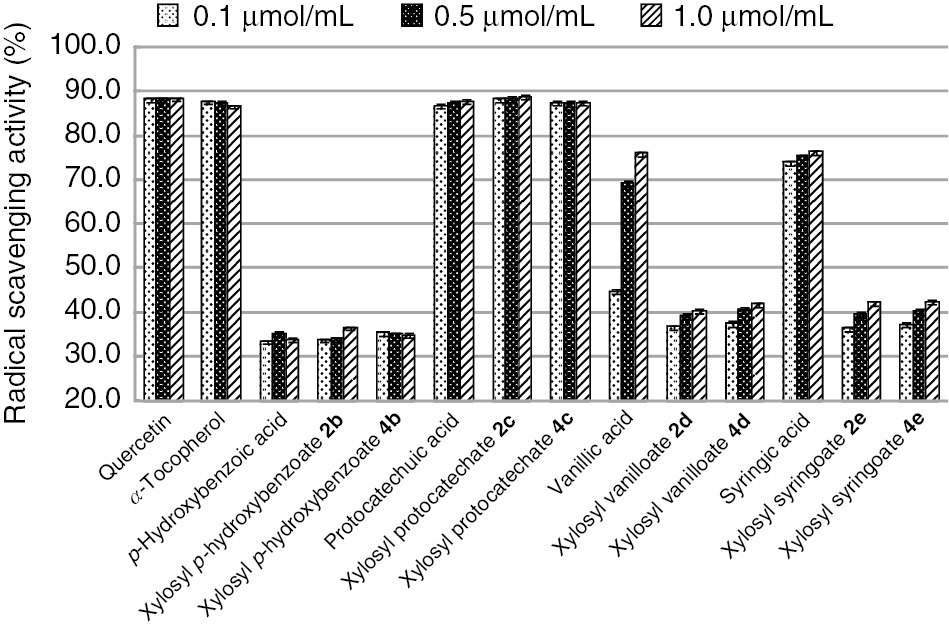
DPPH˙ radical scavenging activities of free hydroxybenzoic acids and their β-D-xylopyranosates.
These activities are almost of the same strength as those of quercetin and α-tocopherol. In the case of glucopyranosyl protocatechoates 1c and 3c, the activities decrease compared with that of free protocatechuic acid. The water solubility of xylose is higher than that of glucose [39]. It can be assumed that the activities of xylopyranosyl protocatechoates 2c and 4c increase compared with glucopyranosyl protocatechoates 1c and 3c because the affinities of xylopyranosates 2c and 4c with DPPH˙ radical are higher than those of glucopyranosates 1c and 3c. Whereas xylopyranosyl p-hydroxybenzoyl, vanilloyl and syringoates 2b, 2d and 2e show about 30% activities, deacetylation increases the activities about 10%. In contrast, there are no significant differences between activities of xylopyranosyl vanilloyl and syringoates 2d and 2e and deacetylated xylopyranosates 4d and 4e. The activities of xylopyranosyl p-hydoxybenzoates 2b and 4b and free p-hydroxybenzoic acid are similar. p-Hydroxybenzoic acid and its xylopyranosates show low activities compared with other hydroxybenzoic acid derivatives, and the sugar moiety does not affect activities. Xylopyranosyl protocatechoates 2c and 4c have the highest activities in this investigation.
Inhibitory effect on autoxidation of bulk methyl linoleate
Formation of hydroperoxides in bulk methyl linoleate at 40°C was estimated based on the absorbance at 234 nm generated during the initial oxidation stage (Figure 3). Modifications were made to the original method [46].
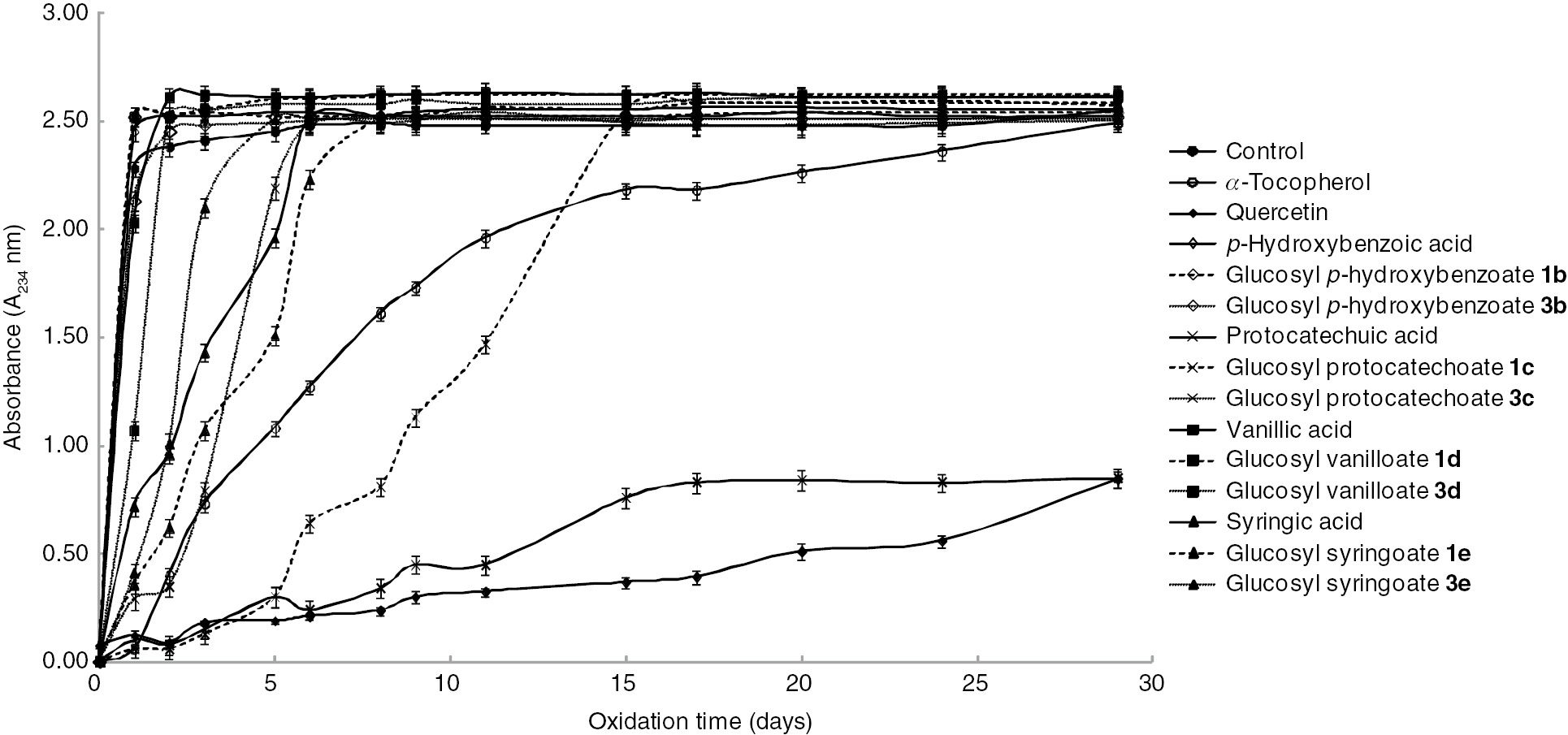
Inhibition of formation of hydroperoxide in bulk methyl linoleate by free hydroxybenzoic acids and their β-D-glucopyranosates.
The antioxidant activity decreases in the order of protocatechuic acid>glucopyranosyl protocatechoate 1c>α-tocopherol>glucopyranosyl syringoate 1e>deacetylated glucopyranosyl protocatechoate 3c≈syringic acid>deacetylated glucopyranosyl syringoate 3e>deacetylated glucopyranosyl vanilloate 3d>vanillic acid≈glucopyranosyl vanilloate 1d≈p-hydroxybenzoic acid≈glucopyranosyl p-hycroxybenzoates 1b and 3b. Free protocatechuic acid and glucopyranosyl protocatechoate 1c) show higher antioxidant activities than α-tocopherol. The antioxidant activity of protocatechuic acid is higher than that of other hydroxybenzoic acids. Glycopyranosyl protocatechoate 1c has high antioxidant activity among the glucosyl hydroxybenzoate synthesized in this investigation. Surprisingly, deacetylated glucopyranosyl protocatechoate 3c shows only the activity like free syringic acid. Comparing free hydroxybenzoic acids for DPPH˙ radical scavenging activity, syringic and vanillic acids have relatively high activities. In contrast, these hydroxybenzoic acids have very low activities on the inhibition of autoxidation of methyl linoleate. Porter has advanced the so-called ‘polar paradox’ by the observation that polar antioxidants are more effective in nonpolar lipids, whereas nonpolar antioxidants are more effective in polar lipid emulsions [47]. The water solubility of hydroxybenzoic acid derivatives used in this investigation decreases in the order of protocatechuic acid>p-hydroxybenzoic acid>syringic acid>vanillic acid [48], [49]. The antioxidant activity of p-hydroxybenzoic acid is one of the lowest because there is only one phenolic hydroxyl group and no methoxy group to stabilize the phenoxy radical. Glycosylation causes the decrease in antioxidant activity compared with free hydroxybenzoic acids in almost all cases. A possible explanation for the decrease of antioxidant activities might be a decrease of polarity by glycosylation compared with free hydroxybenzoic acids.
As with glucopyranosyl hydroxybenzoates 1 and 3, antioxidant activities of xylopyranosyl hydroxybenzoates 2 and 4 were also investigated using methyl linoleate (Figure 4). The antioxidant activity decreases in the order of protocatechuic acid>xylopyranosyl protocatechoate 2c>deacetylated xylopyranosyl protocatechoate 4c>α-tocopherol>xylopyranosyl syringoates 2e and 4e>syringic acid>p-hydroxybenzoic acid≈vanillic acid>xyropyranosyl vanilloates 2d and 4d≈xylopyranosyl p-hydroxybenzoates 2b and 4b. In addition to free protocatechuic acid, xylopyranosyl protocatechoates 2c and 4c have higher antioxidant activities than α-tocopherol.
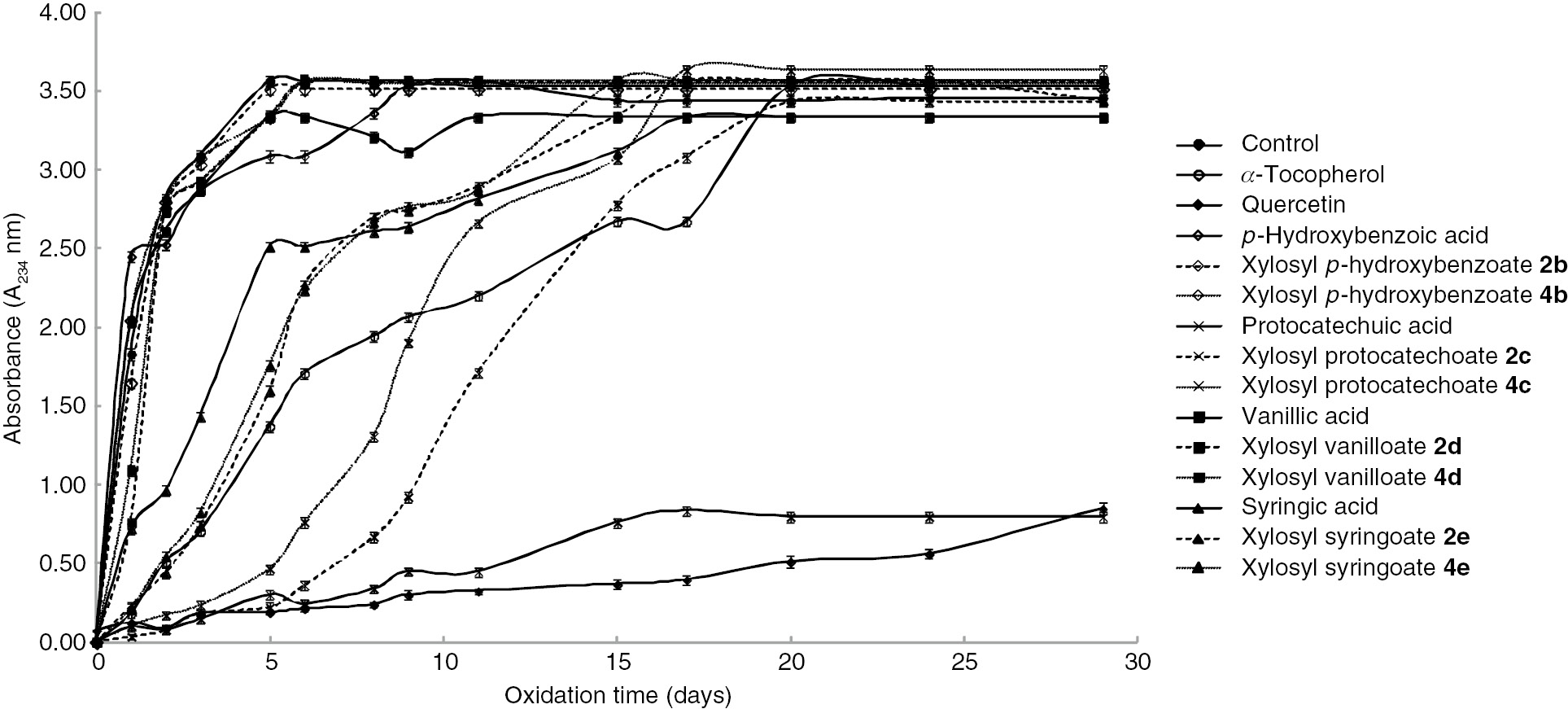
Inhibition of formation of hydroperoxide in bulk methyl linoleate by free hydroxybenzoic acids and their β-D-xylopyranosates.
Xylopyranosyl syringoates 2e and 4e show activities that are similar to that of α-tocopherol for as long as 5 days. Although glycosylation decreases the antioxidant activity of protocatechuic acid, deacetylated xylopyranosyl protocatechoate 4c is more active than the corresponding glucopyranosate 3c. Moreover, antioxidant activities of xylopyranosyl syringoates 2e and 4e are better compared with free syringic acid. Xylose exhibits 1.5 times higher water solubility than glucose [39]. It can be suggested that the water solubility of xylopyranosates is higher than that of glucopyranosates. For this reason, some xylopyranosyl hydroxybenzoates show higher antioxidant activities than the corresponding free hydroxyl benzoic acids and glucopyranosates. We previously reported a similar investigation of hydroxycinnamic acids derivatives [29], [30]. In almost all cases, hydroxybenzoic acid derivatives show lower antioxidant activities than hydroxycinnamic acid derivatives. The phenoxy radical of cinnamic acids is stabilized because these acids have an α,β-unsaturated C-C double bond in the phenylpropane structure. These results are in good agreement with related literature reports [46], [50].
Conclusions
Various β-D-gluco- and β-D-xylopyranosyl hydroxybenzoates 1 and 2 were efficiently synthesized by amine-promoted glycosylation. Deacetylation of products 1 and 2 was investigated using Novozym 435 as a lipase catalyst until the reaction progressed with over 80% yields in all cases. β-D-Glucopyranosyl hydroxybenzoates 1 are regioselectively deacetylated at C-4 and C-6 positions in the presence of Novozym 435. Regioselective deacetylation at the C-4 position exclusively was confirmed for β-D-xylopyranosyl hydroxybenzoates 2. Novozym 435 is most effective for regioselective deacetylation among various lipase catalysts used in this investigation. In the case of DPPH˙ radical scavenging activities, the activities of β-D-glucopyranosyl hydroxybenzoates 1 and 3 decrease compared with the corresponding free hydroxybenzoic acids. In contrast, β-D-xylopyranosyl protocatechoates 2c and 4c show almost the same high antioxidant activities as quercetin and α-tocopherol. Moreover, the antioxidant activities of β-D-glucopyranosyl and β-D-xylopyranosyl protocatechoates 1c, 2c and 4c in inhibition of autoxidation of bulk methyl linoleate are higher than that of α-tocopherol.
Experimental
1H NMR (500 MHz) and 13C NMR (126 MHz) spectra were recorded on a JNM-ECA-500 spectrometer with tetramethylsilane as the internal standard using CDCl3 and DMSO-d6 as solvents. Structural determination of all compounds was performed using COSY, HMQC and HMBC NMR techniques. ESI-MS spectra were taken on an AccuTof GCv 4G (JEOL, Tokyo, Japan) mass spectrometer. IR spectra were obtained in KBr pellets on an FT-IR JASCO 460 plus spectrometer. Melting points were determined on an MP-500D instrument and are uncorrected. Optical rotations were determined with a JASCO P-1010 polarimeter. Lipase AS Amano, lipase AK amano, lipase AYS amano and lipase PS Amano were purchased from Wako Pure Chemical Industries, Ltd. Japan. Porcine pancreas lipase (PPL) was obtained from Nacalai Tesque Inc., Japan.
General procedure for glycosylation of hydroxybenzoic acids
A mixture of 2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide (TAGB, 411 mg, 1.0 mmol) or 2,3,4-tri-O-acetyl-α-D-xylopyranosyl bromide (TAXB, 339 mg, 1.0 mmol), a hydroxybenzoic acid (2.0 mmol for 1, 3.0 mmol for 2) and i-Pr2NEt (388 mg, 3.0 mmol for 1, 129 mg, 1.0 mmol for 2) and 4 Å molecular sieves (1 g) in CH3CN (5 mL) was stirred for 24 h at room temperature under argon. Progress of the reaction was monitored by thin layer chromatography. Upon the completion of the reaction, the mixture was concentrated under reduced pressure, the residue was neutralized with aqueous NaHCO3 and extracted with AcOEt. The extract was washed with brine, dried over anhydrous MgSO4, and concentrated under reduced pressure. The crude product was purified by silica gel chromatography eluting with hexane/AcOEt.
2,3,4,6-Tetra-O-acetyl-β-D-glucopyranosyl benzoate (1a)
Yield 84%; colorless solid; mp 140–141°C; Rf 0.55 (hexane-AcOEt, 1:1);
2,3,4,6-Tetra-O-acetyl-β-D-glucopyranosyl p-hydroxybenzoate (1b)
Yield 49%; colorless solid; mp 131–132°C; Rf 0.45 (hexane-AcOEt, 1:1);
2,3,4,6-Tetra-O-acetyl-β-D-glucopyranosyl protocatechoate (1c)
Yield 29%; colorless solid; mp 65–66°C; Rf 0.33 (hexane-AcOEt, 1:1);
2,3,4,6-Tetra-O-acetyl-β-D-glucopyranosyl vaniloate (1d)
Yield 73%; colorless solid; mp 111–112°C; Rf 0.33 (hexane-AcOEt, 1:1);
2,3,4,6-Tetra-O-acetyl-β-D-glucopyranosyl syringoate (1e)
Yield 71%; colorless solid; mp 67–68°C; Rf 0.28 (hexane-AcOEt, 1:1);
2,3,4-Tri-O-acetyl-β-D-xylopyranosyl benzoate (2a)
Yield 70%; colorless solid; mp 119–120°C; Rf 0.63 (hexane-AcOEt, 1:1);
2,3,4-Tri-O-acetyl-β-D-xylopyranosyl p-hydroxybenzoate (2b)
Yield 51%; colorless solid; mp 205–206°C; Rf 0.43 (hexane-AcOEt, 1:1);
2,3,4-Tri-O-acetyl-β-D-xylopyranosyl protocatechuate (2c)
Yield 44%; colorless solid; mp 124–125°C; Rf 0.3 (hexane-AcOEt, 1:1);
2,3,4-Tri-O-acetyl-β-D-xylopyranosyl vaniloate (2d)
Yield 73%; colorless solid; mp 91–92°C; Rf=0.48 (hexane-AcOEt, 1:1);
2,3,4-Tri-O-acetyl-β-D-xylopyranosyl syringoate (2e)
Yield 71%; colorless solid; mp 103–104°C; Rf=0.33 (hexane-AcOEt, 1:1);
General procedure for Novozym 435-catalyzed regioselective deacetylation
A solution of 2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyl hydroxybenzoate 1a-e (1.0 mmol) or 2,3,4-tri-O-acetyl-β-D-xylopyranosyl hydroxybenzoates 2a-e (1.0 mmol), MeOH (384 mg, 12.0 mmol), and Novozym 435 (0.8 g) in tert-butyl methyl ether (MTBE, 10 mL) was stirred for 24 h at 50°C. The progress of the reaction was monitored with thin layer chromatography. Upon completion of the reaction, Novozym 435 was filtered off the mixture and MTBE was then removed under reduced pressure. The residue was subjected to flash chromatography (CHCl3/MeOH, 9:1) to give the partially deacetylated product 3a–e or 4a–e.
2,3-Di-O-acetyl-β-D-glucopyranosyl benzoate (3a)
Yield 85%; colorless solid; mp 136–137°C; Rf 0.48 (CHCl3/MeOH, 10:1);
2,3-Di-O-acetyl-β-D-glucopyranosyl p-hydroxybenzoate (3b)
Yield 88%; colorless solid; mp 131–132°C; Rf 0.33 (CHCl3/MeOH, 10:1);
2,3-Di-O-acetyl-β-D-glucopyranosyl protocatechuate (3c)
Yield 84%; colorless solid; mp 95–96°C; Rf 0.10 (CHCl3/MeOH, 20:1);
2,3-Di-O-acetyl-β-D-glucopyranosyl vaniloate (3d)
Yield 89%; colorless solid; mp 127–128°C; Rf 0.30 (CHCl3/MeOH, 10:1);
2,3-Di-O-acetyl-β-D-glucopyranosyl syringoate (3e)
Yield 90%; colorless solid; mp 159–160°C; Rf 0.73 (CHCl3/MeOH, 10:1);
2,3-Di-O-acetyl-β-D-xylopyranosyl benzoate (4a)
Yield 83%; colorless solid; mp 149–150°C; Rf 0.43 (CHCl3/MeOH, 20:1);
2,3-Di-O-acetyl-β-D-xylopyranosyl p-hydroxybenzoate (4b)
Yield 85%; colorless solid; mp 202–203°C; Rf 0.23 (CHCl3/MeOH, 20:1);
2,3-Di-O-acetyl-β-D-xylopyranosyl protocatechoate (4c)
Yield 80%; colorless solid; mp 78–79°C; Rf 0.13 (CHCl3-MeOH, 20:1);
2,3-Di-O-acetyl-β-D-xylopyranosyl vaniloate (4d)
Yield 88%; colorless solid; mp 40–41°C; Rf 0.33 (CHCl3/MeOH, 20:1);
2,3-Di-O-acetyl-β-D-xylopyranosyl syringoate (4e)
Yield 82%; colorless solid; mp 144–145°C; Rf=0.30 (CHCl3/MeOH, 20:1);
Lipase screening for deacetylation of 1a and 2a
Lipase (0.1 g) (AS from Aspergillus niger, AK from Pseudomonas fluorescens, AYS from Candida rugosa, PS from Burkholderia cepacia and PPL from porcine pancreas) was added to a mixture of 1a (45 mg, 0.1 mmol) or 2a (38 mg, 0.1 mmol) in pH 7 citrate buffer (10 mL) and MTBE (2.5 mL), and the mixture was stirred at 40°C for 24 h. The progress of the reaction was monitored with thin layer chromatography. After stirring, lipase was filtered off and the organic phase was extracted with AcOEt. The combined extracts were washed with brine, dried over anhydrous MgSO4 and concentrated under reduced pressure. The crude product was purified by flash chromatography (CHCl3/MeOH, 9:1).
DPPH˙ radical scavenging activity
The antioxidant activity of β-D-glycopyranosyl hydroxybenzoates was estimated by measuring their free radical scavenging activity using 2,2-diphenyl-1-picrylhydrazyl (DPPH˙) as free radical according to the literature report [51]. The reaction mixture (10 mL) comprised of freshly made 0.15 mm DPPH˙ in ethanol (7000 μL), different concentrations of each β-D-glycopyranosyl hydroxybenzoates (1, 5 and 10 μmol) in 300 μL DMSO and tris-HCl buffer (pH 7.4, 100 mm). The reaction mixture was kept for 30 min in a water bath at 25°C under dark and optical density was measured at 517 nm. DPPH˙ has an unpaired electron, which gave purple color, and when this electron is balanced, the color is lost. The compounds which can give an electron to the DPPH˙ bleaching the reagent, which is monitored as the decrease in absorbance of the DPPH˙ solution. All analyses were carried out in triplicate.
Inhibitory effect on autoxidation of bulk methyl linoleate
To 1 g of methyl linoleate, 25 μL of the acetone solution of the test compound was mixed in a 50-mL vial, and the mixture was agitated under ultrasonic wave for 30 s. A 25 μL aliquot of acetone without sample was added for control. After purging the acetone with nitrogen, the mixture was placed in an oven at 40°C in the dark. Final concentration of each sample was 0.05 μmol/g. An aliquot was dissolved in ethanol, and the conjugated diene absorbance was measured at 234 nm with an UVmini-2400 UV-vis spectrophotometer every 24 h at 20°C. All tests were run in triplicate.
References
[1] Šamec, D.; Valek-Žulj, L.; Martinez, S.; Grúz, J.; Piljac, A.; Piljac-Žegarac, J. Phenolic acids significantly contribute to antioxidant potency of Gynostemma pentaphyllum aqueous and methanol extracts. Ind. Crop. Prod.2016, 84, 104–107.10.1016/j.indcrop.2016.01.035Search in Google Scholar
[2] Tang, Y.; Zhang, B.; Li, X.; Chen, P. X.; Zhang, H.; Liu, R.; Tsao, R. Bound phenolics of quinoa seeds released by acid, alkaline, and enzymatic treatments and their antioxidant and α-glucosidase and pancreatic lipase inhibitory effects. J. Agric. Food Chem.2016, 64, 1712–1719.10.1021/acs.jafc.5b05761Search in Google Scholar PubMed
[3] Oniszczuk, A.; Olech, M. Optimization of ultrasound-assisted extraction and LC-ESI-MS/MS analysis of phenolic acids from Brassica oleracea L. var. sabellica. Ind. Crop. Prod.2016, 83, 359–363.10.1016/j.indcrop.2016.01.015Search in Google Scholar
[4] Rodríguez, J. C.; Gómez, D.; Pacetti, D.; Núñez, O.; Gagliardi, R.; Frega, N. G.; Ojeda, M. L.; Loizzo, M. R.; Tundis, R; Lucci, P. Effects of the fruit ripening stage on antioxidant capacity, total phenolics, and polyphenolic composition of crude palm oil from interspecific hybrid Elaeis olefera×Elaeis guineensis. J. Agric. Food Chem.2016, 64, 852–859.10.1021/acs.jafc.5b04990Search in Google Scholar PubMed
[5] Yu, L.; Li, G.; Li, M.; Xu, F.; Beta, T.; Bao, J. Genotypic variation in phenolic acids, vitamin E and fatty acids in whole grain rice. Food Chem.2016, 197, 776–782.10.1016/j.foodchem.2015.11.027Search in Google Scholar PubMed
[6] Peña-Neira, A.; Hernández, T.; García-Vallejo, C.; Estrella I.; Suarez, J. A. A survey of phenolic compounds in Spanish wines of different geographical origin. Eur. Food Res. Technol.2000, 210, 445–448.10.1007/s002170050579Search in Google Scholar
[7] Dhananjaya, B. L.; Nataraju, A.; Rajesh, R.; Gowda, C. D. R.; Sharath, B. K.; Vishwanath, B. S.; D’Souza, C. J. M. Anticoagulant effect of Naja naja venom 5’nucleotidase: demonstration using a novel specific inhibitor, vanillic acid. Toxicon2006, 48, 411–421.10.1016/j.toxicon.2006.06.017Search in Google Scholar PubMed
[8] Calixto-Campos, C.; Carvalho, T. T.; Hohmann, M. S. N.; Pinho-Ribeiro, F. A.; Fattori, V.; Manchope, M. F.; Zarpelon, A. C.; Baracat, M. M.; Georgetti, S. R.; Casagrande, R.; et al. Vanillic acid inhibits inflammatory pain by inhibiting neutrophil recruitment, oxidative stress, cytokine production and NFКB activation in mice. J. Nat. Prod.2015, 78, 1799–1808.10.1021/acs.jnatprod.5b00246Search in Google Scholar PubMed
[9] Sindhu, G.; Nishanthi, E.; Sharmila, R. Nephroprotective effect of vanillic acid against cisplatin induced nephrotoxicity in wistar rats: a biochemical and molecular study. Environ. Toxicol. Phar.2015, 39, 392–404.10.1016/j.etap.2014.12.008Search in Google Scholar PubMed
[10] Xiao, H.-H.; Gao, Q.-G.; Zhang, Y.; Wong, K.-C.; Dai, Y.; Yao, X.-S.; Wong, M.-S. Vanillic acid exerts oestrogen-like activities in osteoblast-like UMR 106 cells through MAP kinase (MEK/ERK)-mediated ER signaling pathway. J. Steroid Biochem. Mol. Biol.2014, 144, 382–391.10.1016/j.jsbmb.2014.08.002Search in Google Scholar PubMed
[11] Yrbas, M. A.; Morucci, F.; Alonso, R.; Gorzalczany, S. Pharmacological mechanism underlying the antinociceptive activity of vanillic acid. Pharmacol. Biochem. Behav.2015, 132, 88–95.10.1016/j.pbb.2015.02.016Search in Google Scholar
[12] Srinicasan, S.; Muthukumaran, J.; Muruganathan, U.; Venkatesan, R. S.; Jalaludeen, A. M. Antihyperglycemic effect of syringic acid on attenuating the key enzymes of carbohydrate metabolism in experimental diabetic rats. Biomed. Prevent. Nut.2014, 4, 595–602.10.1016/j.bionut.2014.07.010Search in Google Scholar
[13] Karthik, G.; Angappan, M.; VijayaKumar, A.; Natarajapillai, S. Syringic acid exerts antiangiogenic activity by downregulation of VEGF in zebrafish embryos. Biomed. Prevent. Nut.2014, 4, 203–208.10.1016/j.bionut.2014.01.007Search in Google Scholar
[14] Shi, C.; Sun, Y.; Zheng, Z.; Zhang, X.; Song, K.; Jia, Z.; Chen, Y.; Yang, M.; Liu, X.; Dong, R.; et al. Antimicrobial activity of syringic acid against Cronobacter sakazakii and its effect on cell membrane. Food Chem.2016, 197, 100–106.10.1016/j.foodchem.2015.10.100Search in Google Scholar
[15] Hermann, H. Contents of principle plant phenols in fruits. Fluess Obst.1992, 59, 66–70.Search in Google Scholar
[16] Boniface, P. K.; Verma, S.; Shukla, A.; Cheema, H. S.; Srivastava, S. K.; Khan, F.; Darokar, M. P.; Pal, A. Bioactivity-guided isolation of antiplasmodial constituents from Conyza sumatrensis. Parasitol. Int.2015, 64, 118–123.10.1016/j.parint.2014.10.010Search in Google Scholar
[17] Jiangsu Institute of New Medicine, Zhong Yao Da Ci Dian (Dictionary of Chinese Materia Medica), Shanghai Scientific and Technical Publications: Shanghai, 1977, p. 2068.Search in Google Scholar
[18] Chai, X.; Su. Y.-F.; Guo, L.-P.; Wu, D.; Zhang, J.-F.; Si, C.-L.; Kim, J.-K.; Bae, Y.-S. Phenolic constituents from Conyza sumatrensis. Biochem. System. Eco.2008, 36, 216–218.10.1016/j.bse.2007.07.002Search in Google Scholar
[19] Inoshiri, S.; Sasaki, M.; Kohda, H.; Otsuka, H.; Yamasaki, K. Aromatic glycosides from Berchemia racemose. Phytochem.1987, 26, 2811–2814.10.1016/S0031-9422(00)83595-5Search in Google Scholar
[20] Tomás-Menor, L.; Morales-Soto, A.; Barrajón-Catalán, E.; Roldán-Segura, C.; Segura-Carretero, A.; Micol, V. Correlation between the antibacterial activity and the composition of extracts derived from various Spanish Cistus species. Food Chem. Toxicol.2013, 55, 313–322.10.1016/j.fct.2013.01.006Search in Google Scholar PubMed
[21] Barrajón-Catalán, E.; Fernández-Arroyo, S.; Roldán, C.; Guillén, E.; Saura, D.; Segura-Carretero, A.; Micol, V. A systematic study of the polyphenolic composition of aqueous extracts deriving from several Cistus genus species: evolutionary relationship. Phytochem. Anal.2011, 22, 303–312.10.1002/pca.1281Search in Google Scholar PubMed
[22] Fukumoto, L. R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem.2000, 48, 3597–3604.10.1021/jf000220wSearch in Google Scholar PubMed
[23] Natella, F.; Nardini, M.; Felice, M. D.; Scaccini, C. Benzoic and cinnamic acid derivatives as antioxidants: structure-activity relation. J. Agric. Food Chem.1999, 47, 1453–1459.10.1021/jf980737wSearch in Google Scholar PubMed
[24] Payet, B.; Sing, A. S. C.; Smadja, J. Assessment of antioxidant activity of cane brown sugars by ABTS and DPPH radical scavenging assays: determination of their polyphenolic and volatile constituents. J. Agric. Food Chem.2005, 53, 10074–10079.10.1021/jf0517703Search in Google Scholar PubMed
[25] Russell, W. R.; Scobbie, L.; Duthie, G. G.; Chesson, A. Inhibition of 15-lipoxygenase-catalyzed oxygenation of arachidonic acid by substituted benzoic acids. Bioorg. Med. Chem.2008, 16, 4589–4593.10.1016/j.bmc.2008.02.041Search in Google Scholar PubMed
[26] Yeh, C.-T.; Yen, G.-C. Effects of phenolic acids on human phenolsulfotransferases in relation to their antioxidant activity. J. Agric. Food Chem.2003, 51, 1474–1479.10.1021/jf0208132Search in Google Scholar PubMed
[27] Ordoudi, S. A.; Tsimidou, M. Z.; Vafiadis, A. P.; Bakalbassis, E. G. Structure-DPPH˙ scavenging activity relationships: parallel study of catechol and guaiacol acid derivatives. J. Agric. Food Chem.2006, 54, 5763–5768.10.1021/jf060132xSearch in Google Scholar PubMed
[28] Noipa, T.; Srijaranai, S.; Tuntulani, T.; Ngeontae, W. New approach for evaluation of the antioxidant capacity based scavenging DPPH free radical in micelle systems. Food Res. Int.2011, 44, 789–806.10.1016/j.foodres.2011.01.034Search in Google Scholar
[29] Shimotori, Y.; Tsutano, K.; Soga, K.; Osawa, Y.; Aoyama, M.; Miyakoshi, T. Synthesis of glycosyl ferulate derivatives by amine-promoted glycosylation with regioselective hydrolysis using Novozym 435 and evaluation of their antioxidant properties. Carbohydr. Res.2012, 359, 11–17.10.1016/j.carres.2012.06.019Search in Google Scholar PubMed
[30] Shimotori, Y.; Hoshi, M.; Soga, K.; Osawa, Y.; Miyakoshi, T. Synthesis of hydroxycinnamoyl β-D-xylopyranosides and evaluation of their antioxidant properties. Carbohydr. Res.2014, 388, 138–146.10.1016/j.carres.2013.12.014Search in Google Scholar PubMed
[31] Wu, Q.; Fu, D.; Hou, A.; Lei, G.; Liu, Z.; Chen, J.; Zhou, T. Antioxidative phenols and phenolic glycosides from Curculigo orchioides. Chem. Pharm. Bull.2005, 53, 1065–1067.10.1248/cpb.53.1065Search in Google Scholar PubMed
[32] Wu, X.; Ruan, J.; Yang, V. C.; Wu, Z.; Lou, J.; Duan, H.; Zhang, J.; Zhang, Y.; Guo, D. Three new acetylated benzyl-beta-resorcylate glycosides from Cassia obtusifolia. Fitoterapia2012, 83, 166–169.10.1016/j.fitote.2011.10.009Search in Google Scholar PubMed
[33] Jiang, Z.; Li, S.; Li, W.; Guo, J.; Tian, K.; Hu, Q.; Huang, X. Phenolic glycosides from Ficus tikoua and their cytotoxic activities. Carbohydr. Res.2013, 382, 19–24.10.1016/j.carres.2013.09.008Search in Google Scholar
[34] Bokesch, H. R.; Wamiru, A.; Grice, S. F. J. L.; Beutler, J. A.; McKee, T. C.; McMahon, J. B. HIV-1 ribonuclease H inhibitory phenolic glycosides from Eugenia hyemalis. J. Nat. Prod.2008, 71, 1634–1636.10.1021/np8002518Search in Google Scholar
[35] Jover, J.; Bosque, R.; Sales, J. QSPR Prediction of pKa for benzoic acids in different solvents. QSAR Comb. Sci.2008, 25, 563–581.10.1002/qsar.200710095Search in Google Scholar
[36] Galland, S.; Mora, N.; Abert-Vian, M.; Rokatomanomana, N.; Dangles, O. Chemical synthesis of hydroxycinnamic acid glucosides and evaluation of their ability to stabilize natural colors via anthocyanin copigmentation. J. Agric. Food Chem.2007, 55, 7573–7579.10.1021/jf071205vSearch in Google Scholar
[37] Petersen, M. T. N.; Fojan, P.; Petersen, S. B. How do lipases and esterases work: the electrostatic contribution. J. Biotechnol.2001, 85, 115–147.10.1016/S0168-1656(00)00360-6Search in Google Scholar
[38] Shimotori, Y.; Sekine, K.; Miyakoshi, T. Asymmetric synthesis of δ-lactones with lipase catalyst. Flavour Fragr. J.2007, 22, 531–539.10.1002/ffj.1836Search in Google Scholar
[39] Gray, M. C.; Converse, A. O.; Wyman, C. E. Sugar monomer and oligomer solubility, Data and predictions for application to biomass hydrolysis. Appl. Biochem. Biotech.2003, 105–108, 179–193.10.1385/ABAB:105:1-3:179Search in Google Scholar
[40] Karamać, M.; Kosińska, A.; Pegg, R. B. Comparison of radical-scavenging activities for selected phenolic acids. Pol. J. Food Nutr. Sci.2005, 14, 165–170.Search in Google Scholar
[41] Gadow, A.; Joubert, E.; Hansmann, C. F. Comparison of the antioxidant activity of aspalathin with that of other plant phenols of rooibos tea (Aspalathus linearis), α-tocopherol, BHT and BHA. J. Agric. Food Chem.1997, 45, 632–638.10.1021/jf960281nSearch in Google Scholar
[42] Chen, J. H.; Ho, C.-T. Antioxidant activities of caffeic acid and its related hydroxycinnamic acid compounds. J. Agric. Food Chem.1997, 45, 2374–2378.10.1021/jf970055tSearch in Google Scholar
[43] Kylli, P.; Nousiainen, P.; Biely, P.; Sipilä, J.; Tenkanen, M.; Heinonen, M. Antioxidant potential of hydroxycinnamic acid glycoside esters. J. Agric. Food Chem.2008, 56, 4797–4805.10.1021/jf800317vSearch in Google Scholar
[44] Shyamala, B. N.; Naidu, M. M.; Sulochanamma. G.; Srinivas, P. Studies on the antioxidant activities of natural vanilla extract and its constituent compounds through in vitro models. J. Agric. Food Chem.2007, 55, 7738–7743.10.1021/jf071349+Search in Google Scholar PubMed
[45] Sang, S.; Lapsley, K.; Jeong, W.-S.; Lachance, P. A.; Ho, C.-T.; Rosen, R. T. Antioxidative phenolic compounds isolated from almond skins (Prunus amygdalus batsch). J. Agric. Food Chem.2002, 50, 2459–2463.10.1021/jf011533+Search in Google Scholar PubMed
[46] Pekkarinen, S. S.; Stöckmann, H.; Schwarz, K.; Heinonen, M.; Hopia, A. I. Antioxidant activity and partitioning of phenolic acids in bulk and emulsified methyl linoleate. J. Agric. Food Chem.1999, 47, 3036–3043.10.1021/jf9813236Search in Google Scholar PubMed
[47] Porter, W. L.; Black, E. D.; Drolet, A. M. Use of polyamide oxidative fluorescence test on lipid emulsions: contrast in relative effectiveness of antioxidants in bulk versus dispersed systems. J. Agric. Food Chem.1989, 37, 615–624.10.1021/jf00087a009Search in Google Scholar
[48] Gracin, S.; Rasmuson, Å. C. Solubility of phenylacetic acid, p-hydroxyphenylacetic acid, p-aminophenylacetic acid, p-hydroxybenzoic acid and ibuprofen in pure solvent. J. Chem. Eng. Data2002, 47, 1379–1383.10.1021/je0255170Search in Google Scholar
[49] Noubigh, A.; Abderrabba, M.; Provost, E. Temperature and salt addition effects on the solubility behavior of some phenolic compounds in water. J. Chem. Thermodyn.2007, 39, 297–303.10.1016/j.jct.2006.06.014Search in Google Scholar
[50] Szwajgier, D.; Pielecki, J.; Targoński, Z. Antioxidant activities of cinnamic and benzoic acid derivatives. Acta. Sci. Pol. Technol. Aliment.2005, 4, 129–142.Search in Google Scholar
[51] Silva, F. A. M.; Borges, F.; Guimaraes, C.; Lima, J.; Matos, C.; Reis, S. Phenolic acids and derivatives: studies on the relationship among structure, radical scavenging activity and physicochemical parameters. J. Agric. Food Chem.2000, 48, 2122–2126.10.1021/jf9913110Search in Google Scholar PubMed
©2017 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Editorial
- Carbohydrate chemistry/glycoscience
- Reviews
- Boron-based small molecules in disease detection and treatment (2013–2016)
- Impact of modified ribose sugars on nucleic acid conformation and function
- Preliminary Communication
- Crystallization-induced amide bond formation creates a boron-centered spirocyclic system
- Research Articles
- Anion and sugar recognition by 2,6-pyridinedicarboxamide bis-boronic acid derivatives
- Synthesis of biotinylated bivalent zanamivir analogs as probes for influenza viruses
- Synthesis of silodosin glucuronide and its deuterated counterpart: solving a problematic O-glycosylation of a nitrogen-containing molecule
- Synthesis and antimicrobial activity of 4-trifluoromethylpyridine nucleosides
- Synthesis of anti-inflammatory 2,3-unsaturated O-glycosides using conventional and microwave heating techniques
- Synthesis of various β-D-glucopyranosyl and β-D-xylopyranosyl hydroxybenzoates and evaluation of their antioxidant activities
- Synthesis of carbohydrate-substituted isoxazoles and evaluation of their antitubercular activity
- Synthesis and bioactivity of novel C2-glycosyl triazole derivatives as acetylcholinesterase inhibitors
- Modification of bovine serum albumin with aminophenylboronic acid as glycan sensor based on surface plasmon resonance and isothermal titration calorimetry
Articles in the same Issue
- Frontmatter
- Editorial
- Carbohydrate chemistry/glycoscience
- Reviews
- Boron-based small molecules in disease detection and treatment (2013–2016)
- Impact of modified ribose sugars on nucleic acid conformation and function
- Preliminary Communication
- Crystallization-induced amide bond formation creates a boron-centered spirocyclic system
- Research Articles
- Anion and sugar recognition by 2,6-pyridinedicarboxamide bis-boronic acid derivatives
- Synthesis of biotinylated bivalent zanamivir analogs as probes for influenza viruses
- Synthesis of silodosin glucuronide and its deuterated counterpart: solving a problematic O-glycosylation of a nitrogen-containing molecule
- Synthesis and antimicrobial activity of 4-trifluoromethylpyridine nucleosides
- Synthesis of anti-inflammatory 2,3-unsaturated O-glycosides using conventional and microwave heating techniques
- Synthesis of various β-D-glucopyranosyl and β-D-xylopyranosyl hydroxybenzoates and evaluation of their antioxidant activities
- Synthesis of carbohydrate-substituted isoxazoles and evaluation of their antitubercular activity
- Synthesis and bioactivity of novel C2-glycosyl triazole derivatives as acetylcholinesterase inhibitors
- Modification of bovine serum albumin with aminophenylboronic acid as glycan sensor based on surface plasmon resonance and isothermal titration calorimetry

