Abstract
Two 2,6-pyridinedicarboxamide derivatives containing arylboronic acid fragments were prepared and fully characterized including X-ray crystal diffraction analysis of a pinacol ester. These compounds are potential bifunctional receptors for sugars and anions. Acid dissociation and stability constants for complexation of both receptors with glucose and fructose were determined by potentiometric titrations in aqueous DMSO. Also, binding of alizarin red S indicator was studied spectrophotometrically and a highly sensitive detection of fructose by an indicator displacement assay was proposed. Complexation with anions was studied by 1H NMR titrations in DMSO-d6. Binding of acetate anion occurs only via hydrogen bonding to OH groups of boronic acid fragments and does not affect signals of NH protons but chloride anion induces large shift of the signals of NH protons and small shifts of the signals of OH groups. This behavior makes possible anion discrimination based on preference in the type of binding site rather than simply on anion basicity as is typical for majority of neutral hydrogen bonding anion receptors.
Introduction
There is a significant current interest in development of ‘multi-channel’ or ‘multifunctional’ sensors containing an array of recognition sites for detection of several analytes [1], [2], [3], [4]. By the moment most such sensors are designed for detection of multiple metal ions [2]. Important analytes of other types involve anions and carbohydrates which are frequent components of many biological, environmental and nutritional systems [5], [6], [7], [8], [9]. In this paper, we report synthesis and characterization of two dicarboxamidediboronic acids as first examples of receptors of this type exploiting dicarboxamide and boronic acid moieties as anion and sugar receptor sites.
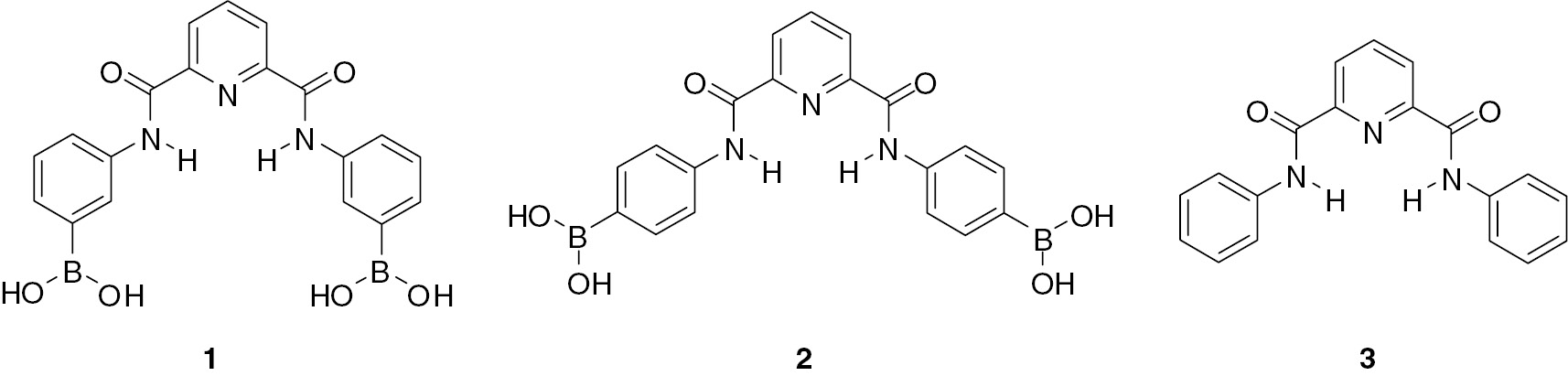
2,6-Pyridinedicarboxamide and structurally related isophthalamide derivatives initially suggested as simple yet efficient hydrogen bonding anion receptors [10] are currently among most popular building blocks and scaffolds employed in design of numerous cyclic and acyclic receptors for guests of distinct types including anions, cations and neutral molecules [11], [12], [13], [14], [15]. Pyridinedicarboxamides are used more often because they are better preorganized for anion binding [14], [15]. In a proposed here design of a bifunctional receptor a 2,6-pyridinedicarboxamide is used both as an anion recognition site and as a scaffold for incorporation of boronic acid groups for sugar recognition. However, the actual role of boronic acids may be more complex because they also may participate in anion recognition. Lewis acidity of boronic acids toward anions is well documented and widely employed in design of anion, mostly fluoride, receptors [16], [17], [18], [19]. It has been shown recently that the B(OH)2 group acts also as a strong proton donor toward anions [20], which possibly may compete with ‘classical’ hydrogen bonding anion receptors like dicarboxamides. It seems therefore interesting to see which site would be preferable for binding of anions of distinct types within a receptor possessing both a dicarboxamide and a boronic acid recognition sites. Following from above considerations we have prepared bifunctional receptors 1 and 2 as well as a simple monofunctional receptor 3 lacking boronic acid groups as a reference compound.
Numbered free-standing structures (without legends):
Results and discussion
Receptors 1–3 were prepared by reacting 2,6-pyridinedicarbonyl dichloride with respective isomers of aminophenylboronic acid or with aniline. Figure 1 shows the structure of pinacol ester of 2 determined by X-ray diffraction analysis of a crystal grown from methanol. Crystallographic data for pinacol ester of 2 are given in Table S1 (Supporting Information). The receptor is nearly planar with convergent NH bonds directed inside the cavity. The cavity size with distances between protons of NH groups, 3.063 Å, and between protons of ortho-CH groups, 4.101 Å, is optimal for inclusion of chloride anion via hydrogen bonding chelation with NH and CH donors [21]. Boronic acid groups are separated by a large distance of 12.7 Å, obviously too large to allow chelation of a guest.
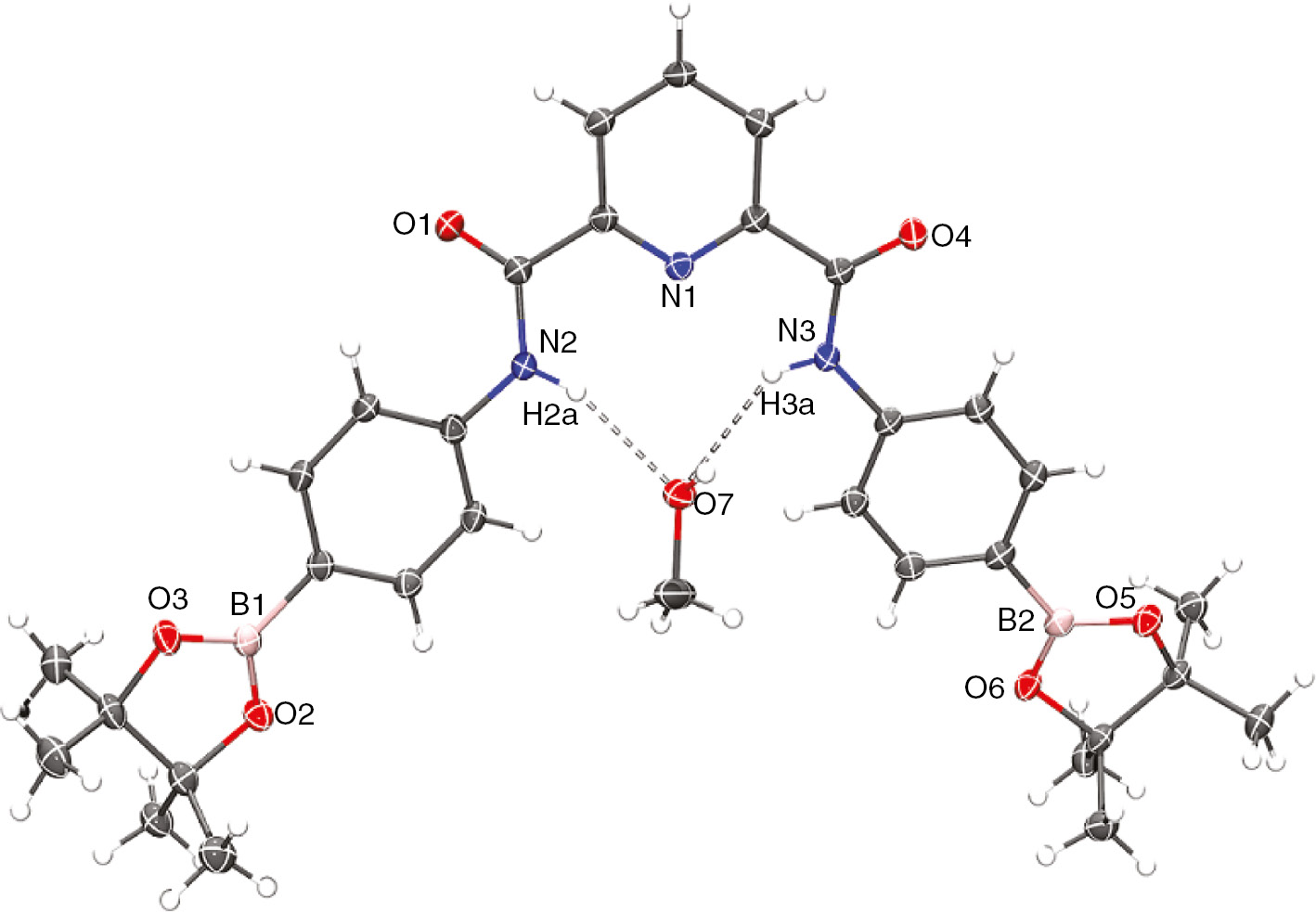
Molecular structure of pinacol ester of 2 crystallized with a methanol molecule.
Ellipsoids are drawn at the 50% probability level.
All compounds are practically insoluble in water. The acid-base and sugar binding properties of 1 and 2 were determined by potentiometric titrations in 50% vol. aqueous DMSO. Figure 2 shows the titration plots for 1 alone and in the presence of excess of glucose or fructose. Similar plots were obtained for 2. The plot for 1 alone has the shape of a single ‘wave’ with an upward break in pH at the point when two mole equivalents of NaOH are added. Such profile is indicative of deprotonation of two boronic acid groups with relatively close pKa values. Additions of sugars induce strong downward shifts of titration curves to lower pH values and a more pronounced break at two mole equivalents of added NaOH, confirming formation of anionic tetrahedral hydroxo-complexes of boronic acid esters [22], [23], [24] with both boronic acid groups.

Potentiometric titrations of 2.1 mM receptor 1 alone (solid squares) and in the presence of 0.2 M glucose (open squares) or fructose (solid triangles) in 50% vol. aqueous DMSO; a is the number of added equivalents of NaOH.
The titration results were analyzed in terms of general equation (1) where M is the neutral receptor molecule, L is the neutral form of the sugar, H is proton and stability constants βpqr are defined as in equation (2). By testing different combinations of p, q, r values for the fitting of the
titration results with Hyperquad program we found the best fit with a set of the equilibrium constants given in Table 1 which correspond to a set of deprotonation and association equilibria illustrated in Scheme 1. For comparison, corresponding literature data for phenylboronic acid in water are also included in Table 1.
Acid dissociation and stability constants of sugar complexes for receptors 1 and 2 (50% DMSO, 0.1 M NaCl).
| 1 | 2 | PhB(OH)2a | ||||
|---|---|---|---|---|---|---|
| pKa1 | 10.34±0.03 | 11.40±0.06 | 8.9 | |||
| pKa2 | 11.22±0.04 | 12.3±0.1 | ||||
| Glucose | Fructose | Glucose | Fructose | Glucose | Fructose | |
| logβ11-1 | −7.48±0.04 | −5.18±0.02 | −8.81±0.05 | −6.9±0.2 | −6.75 | −5.36 |
| logβ12-2 | −15.28±0.02 | −11.55±0.03 | −19.13±0.06 | NDb | ||
| logKtet1 | 2.86 | 5.16 | 2.59 | 4.5 | 2.15 | 3.54 |
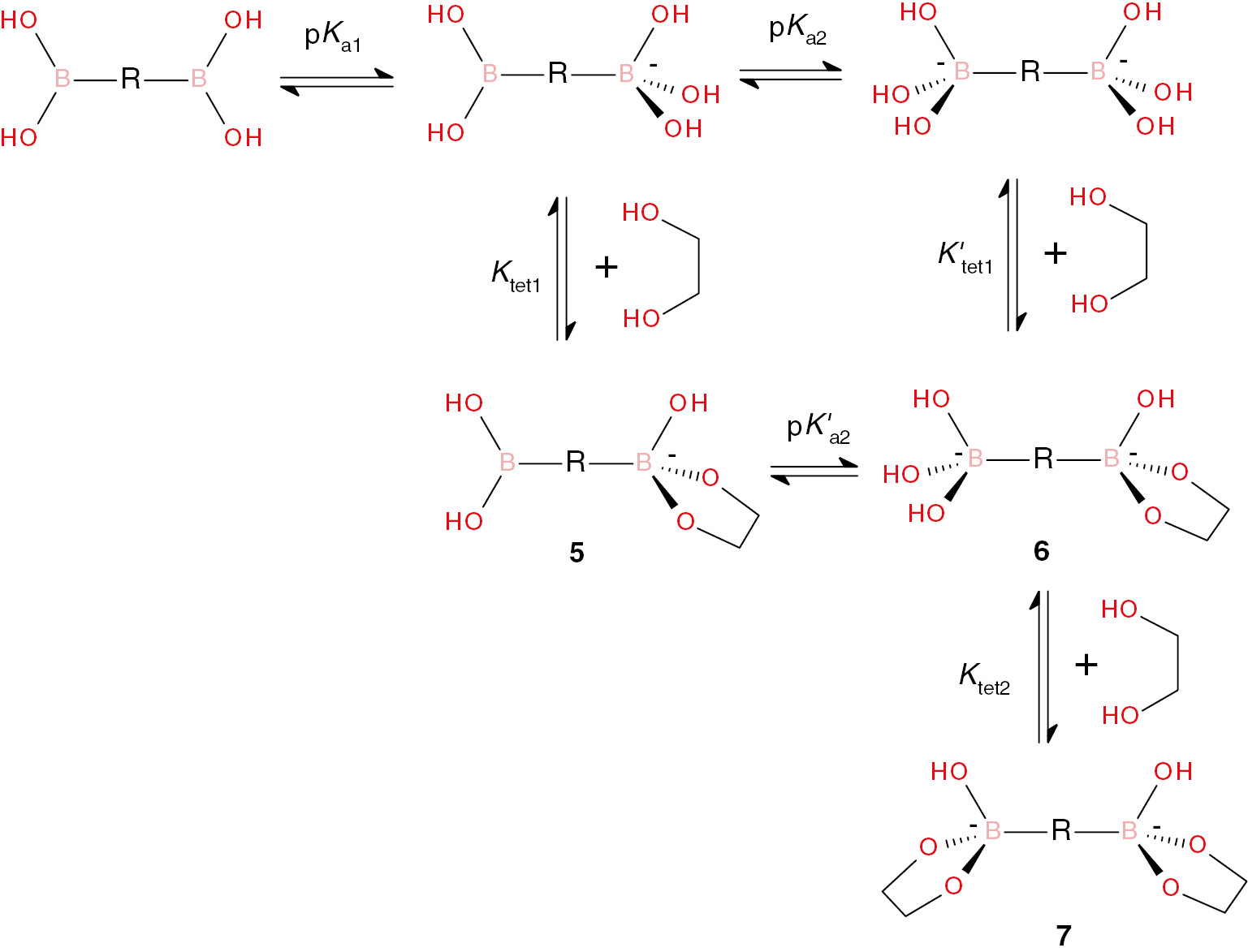
The first pKa values of receptors 1 and 2 are significantly higher than that of phenylboronic acid in water, most probably due to the solvent effect. A smaller pKa of 1 reflects a larger electron acceptor effect of the N-acyl group in meta position (more positive σm than σp) [25] of boronic acid. The second pKa values are larger than the first values due to both statistical and electronic effects. Increased pKa values of boronic acids 1 and 2 have the expected negative effect on ester formation with polyols, which is reflected in much smaller β11-1 values for 1 and 2 as compared to those for stronger phenylboronic acid. However, the stability of boronate esters expressed in terms of Ktet1 (Scheme 1) is higher for 1 and 2. The relationship between these constants is given by equation (3) [24] and the respective calculated values of logKtet1 are shown in Table 1. Evidently, Ktet1 for glucose is 5 and 3 times larger with 1 and 2, respectively, compared to phenylboronic acid. For fructose, the effect is even larger: 40 and 10 times, respectively.
The results of potentiometric titrations did not allow to determine β11-2 which would correspond to formation of the monoester with deprotonated second boronic acid group (6 in Scheme 1). This means low degree of formation of this species. Without this equilibrium constant we cannot, however, calculate the Ktet2 value corresponding to formation of the final dianionic diester 7 (Scheme 1), but the overall stability of this species should be very large as follows from the species distribution diagrams shown for 1 in Figure 3. This Figure illustrates the species distribution diagrams for interaction of 1 with glucose and fructose. It shows the degree of formation of distinct species as percentage of total concentration of the receptor at variable pH calculated for 0.1 mM receptor and excess of a sugar (10 mM for glucose and 1 mM for more tightly bound fructose) using the equilibrium constants from Table 1. For comparison, the dashed lines show calculated profiles for phenylboronic acid in water.

Species distribution diagrams for 0.1 mM receptor 1 in the presence of 10 mM glucose (A) or 1 mM fructose (B) in 50% v/v DMSO/water.
Red lines show the relative concentrations of boronate esters. Dashed lines show the distribution curves calculated for phenylboronic acid in water with the same concentrations of sugars.
Let us consider first the results for glucose (Figure 3A). The distribution curve for monoester 5 reaches the maximum of 40% at pH 9.7 and then goes down with concomitant increase in the fraction of the diester 7. The fraction of mono-deprotonated 1 reaches the maximum of 6% at the same pH and the doubly deprotonated form as well as the ester 6 do not contribute noticeably at any pH. Instead, a rapid growth of the fraction of diester 7 which exceeds 90% above pH 11 is observed. Such behavior may be observed if both Ktet2 and K′tet1 are larger than Ktet1. Currently, we cannot provide a satisfactory explanation for this phenomenon, but it is in line with our previous observation of co-operative formation of diester of 1,4-benzenediboronic acid with a catechol ligand [26]. Comparison of distribution curves for 1 with those for phenylboronic acid at first glance indicate worse performance of the former since the distribution curves for it are shifted to higher pH values. It must be considered, however, that the presence of DMSO as a co-solvent shifts the autoprotolysis constant of water (pKw) and therefore shifts the whole scale of pH [27]. In 50% (v/v) DMSO the value of pKw equals 15.9 instead of 13.8 in water (at ionic strength 0.1 M) which means that solutions of equal basicity have pH values by 2.1 units higher in 50% DMSO than in pure water. Thus, for a typical ‘physiological’ pH of 7.4 in water the equivalent value in 50% DMSO is pH 9.5. As can be seen from Figure 3A the degree of ester formation of phenylboronic acid with glucose at pH 7.4 is only about 4% while at pH 9.5 in 50% DMSO the total degree of ester formation (5+7, see Scheme 1) is about 70%.
In case of fructose with a much higher Ktet1 value an increased degree of ester formation is observed for 1 as compared to phenylboronic acid already at pH around 7 in both media (Figure 3B). At higher pH values the complexation of 1 becomes nearly quantitative with low fraction of free boronic acid anion. These results show that receptor 1 could serve as a sensitive sensor for fructose, but it lacks suitable spectral properties. The problem can be solved by using an indicator displacement assay. We chose as an indicator the most popular catechol-type dye alizarin red S (ARS) [23], [28]. Figure 4 shows the results of spectrophotometric titration of ARS by 1 at pH 8 which demonstrates characteristic blue shift of the absorption maximum. Fitting of the titration results to equation (4) allowed us to calculate the association constant K=(4.6±0.4)×104M−1. The fitting to equation (4), which implies a 1:1 complexation equilibrium was possible because in this experiment an excess of receptor to the guest was employed and therefore predominantly a 1:1 complex was formed. For isomeric receptor 2, K=(3.0±0.3)×104M−1 was obtained. The respective binding constant of ARS to phenylboronic acid in water at pH 7.4, K=1.5×103M−1 [23], is much lower. Additions of fructose to the mixture of 1 and ARS

Spectrophotometric titration of 0.1 mM ARS with 1 in 50% v/v DMSO/water at pH 8.0.
The inset shows the fitting of titration profile at 485 nm to equation (4).
were accompanied by restoration of the absorbance of free ARS and the calibration plot constructed at 555 nm at pH 8.0 showed a linear response to fructose concentration up to 8 mM with detection limit of 0.05 mM.
Anion recognition properties of 1–3 were studied by 1H NMR titrations in DMSO-d6. We chose two anions which represent two distinct types of anionic species: highly basic planar triangular AcO− and low basic spherical Cl−. Titration results for a monofunctional receptor 3 are shown in Figure 5. Both anions induce downfield shifts of the signals of NH protons and smaller downfield shifts of C-protons in ortho position of the phenyl ring (doublet at 7.92 ppm) together with small upfield shifts of the signals of meta and para protons of phenyl rings. Downfield shifts are indicative of hydrogen bonding, which therefore occurs as chelation of anions by NH and ortho-C-protons and upfield shifts reflect small electron donor inductive effect from the bound anion. Analysis of titration profiles by using a HypNMR program gives the association constants collected in Table 2 and the complexation induced shifts of the signals of NH protons at saturation 1.06 and 1.26 ppm for chloride and acetate anions, respectively. Titration results for bifunctional receptors 1 and 2 are shown in the respective Figures 6 and 7. Additions of acetate induce strong downfield shift in the signal of protons of B(OH)2 group characteristic of hydrogen bonding interaction and small upfield shifts in signals of aromatic protons of phenyl groups which can be attributed to an inductive effect of bound anion, but practically they do not affect the signals of NH protons. The fitting of titration results with HypNMR program allowed us to determine binding constants to both boronic acid groups given in Table 2. Formation constants for the first acetate anion are close to that reported for phenylboronic acid. Binding of the second anion has a smaller constant, apparently due to the electrostatic repulsion to the first bound anion. Binding of acetate to receptor 3 involves amide groups and occurs with much smaller binding constant (Table 2), however, in the range of acetate concentrations employed for titrations of 1 and 2 even with this binding constant the observed shift of the NH signal should be about 1 ppm. Thus, while affinity of boronic acid groups in 1 and 2 to acetate is approximately the same as that of phenylboronic acid, the dicarboxamide fragment loses the affinity to this anion.

1H NMR titrations of 5 mM3 by chloride (A) and acetate (B) anions in DMSO-d6.
Spectra were recorded at increased concentrations of added anion as a tetrabutylammonium salt from bottom to top of the Figure.
Complex formation constants for anions in DMSO.
| Anion | K (M−1) | |||
|---|---|---|---|---|
| 1 | 2 | 3 | PhB(OH)2a | |
| AcO | 630±50; 320±30b | 3200±300; 200±20b | 33±3 | 950 |
| Cl− | 9.8±0.8 | 3.6±0.5 12±1c | 5.1±0.9 | 60 |
aData from Ref. [20].
bFormation constants for the binding of first and second anion.
cFormation constant calculated from the chemical shift of the protons of PhB(OH)2 group.

1H NMR titrations of 5 mM1 by acetate (A) and chloride (B) anions in DMSO-d6.
Spectra were recorded at increased concentrations of added anion as a tetrabutylammonium salt from bottom to top of the Figure.

1H NMR titrations of 5 mM2 by acetate (A) and chloride (B) anions in DMSO-d6.
Spectra were recorded at increased concentrations of added anion as a tetrabutylammonium salt from bottom to top of the Figure.
Titration of 1 by chloride (Figure 6B) induces only small downfield shift of the signal of NH protons with the binding constant which is two times higher than that for 3. Although chloride forms hydrogen bonded complex with phenyl boronic acid and with larger constant (Table 2) no interaction with boronic acid group of 1 is observed. In the case of receptor 2 (Figure 7B) binding of chloride to dicarboxamide fragment occurs via hydrogen bonding to NH and ortho-CH (signal at 7.9 ppm experiencing significant downfield shift upon titration) in accordance with what is expected on basis of structure of the receptor (see above) and with the binding constant similar in limits of experimental errors to that for 3. A small downfield shift of the signal of protons of boronic acid groups indicates hydrogen bonding of chloride but with much smaller stability constant than in the case of phenylboronic acid as receptor (Table 2).
In general, acetate shows clearly selectivity toward boronic acid and chloride preferably binds to dicarboxamide NH groups. At the same time with both monofunctional receptors 3 and phenylboronic acid, more basic acetate always forms more stable complexes which induces larger shifts of the NMR signals of the respective protons. Such behavior is typical for neutral hydrogen bonding anion receptors which discriminate anions mostly by their basicity. Using of bifunctional receptors allows less trivial discrimination of anions determined by the type of preferable interaction site.
Conclusions
The first examples of bifunctional receptors designed for recognition of sugars/polyols and anions demonstrate good efficiency of detection of both types of analytes. Selectivity of polyol recognition follows usual trend catechol>fructose>glucose and receptors may be employed as sensors in indicator displacement mode. Recognition sites for anions are not independent. Boronic acid responds to acetate and the dicarboxamide fragment responds to chloride. This creates a different type of selectivity not based on anion basicity which usually is the principal factor in anion discrimination. Further progress in development of sugar/anion bifunctional sensors may be expected with more water soluble optically responsive receptors, which are currently in a process of design and screening in our laboratory.
Experimental
General procedure for the synthesis of compounds 1–3
A mixture of 2,6-pyridinedicarbonyl dichloride (50 mg, 0.25 mmol) and 3- or 4-aminophenylboronic acid (70 mg, 0.5 mmol) or aniline (47 mg, 0.5 mmol) in dry toluene was stirred under reflux in the atmosphere of nitrogen for 4 h. The resultant precipitate was collected by filtration and washed with 3% NaHCO3 aqueous solution and then with water to give 1–3 as a white powder in 92%–95% yield. Copies of 1H and 13C NMR spectra of compounds 1–3 are given in the online supplement.
[3-[[6-[(3-Boronophenyl)carbamoyl]pyridine-2-carbonyl]amino]phenyl]boronic acid (1) 1H NMR (300 MHz, 25°C, DMSO-d6): δ 11.06 (s, 2H), 8.42 (d, J=7 Hz, 2H), 8.30 (t, J=9 Hz, 1H), 8.16 (s, 2H), 8.11 (s, 4H), 7.93 (d, J=9 Hz, 2H), 7.63 (d, J=7 Hz, 2H), 7.45 (t, J=7 Hz, 2H); 13C NMR (75 MHz, 25°C, DMSO-d6): δ 161.7, 148.9, 139.8, 138.6, 137.1, 130.4, 127.7, 127.6, 125.2, 123.7; EI-MS: m/z 405 [M]+; ATR-IR: νmax 3382, 2977, 1355, 1398, 1141, 1087 cm−1. Anal. Calcd for C19H17B2N3O6: C, 56.35; H, 4.23; N, 10.38. Found: C, 56.37; H, 4.25; N, 10.75.
[4-[[6-[(4-Boronophenyl)carbamoyl]pyridine-2-carbonyl]amino]phenyl]boronic acid (2) 1H NMR (300 MHz, 25°C, DMSO-d6): δ 11.05 (s, 2H), 8.43 (d, J=7.1 Hz, 2H), 8.30 (t, J=8.7 Hz, 1H 1H), 8.01 (s, 4H), 7.89 (m, 8H); 13C NMR (75 MHz, 25°C, DMSO-d6): δ 161.9, 148.8, 140.9, 140.0, 135.2, 125.6, 124.1, 119.9; EI-MS: m/z 405 [M]+; ATR-IR: νmax 3299, 2976, 1675, 1586, 1356, 1139 cm−1. Anal. Calcd for C19H17B2N3O6: C, 56.35; H, 4.23; N, 10.38. Found: C, 56.38; H, 4.29; N, 10.29.
N2, N6-diphenylpyridine-2,6-dicarboxamide (3) 1H NMR (300 MHz, 25°C, DMSO-d6): δ 11.04 (s, 2H), 8.43 (d, J=8 Hz, 2H), 8.30 (t, J=9 Hz, 1H), 7.94 (d, J=9 Hz, 4H), 7.45 (t, J=8 Hz, 4H), 7.20 (t, J=8 Hz, 2H); 13C NMR (75 MHz, 25°C, DMSO-d6): δ 161.6, 148.9, 139.9, 138.0, 128.8, 125.3, 124.3, 121.4; EI-MS: m/z 317 [M]+; ATR-IR: νmax cm−1) 3270, 1672, 1659, 1533, 1533, 1079 cm−1. Anal. Calcd for C19H15N3O2: C, 71.91; H, 4.76; N, 13.24. Found: C, 71.98; H, 4.79; N, 13.19.
Crystallography
The relevant details of the crystals, data collection and structure refinement can be found in Table S1 (Supporting Information). Data for dipinacol ester of 2 crystalized from methanol were collected on a Bruker APEX II CCD diffractometer at 100 K, using Mo-Kα radiation (k=0.71073 Å) from an Incoatec ImuS source and Helios optic monochromator [29]. Suitable crystals were coated with hydrocarbon oil, picked up with a nylon loop, and mounted in the cold nitrogen stream of the diffractometer. The structures were solved by direct methods [30] and refined by full-matrix least-squares on F2 using the shelXle GUI [31], [32]. The hydrogen atoms of the C–H bonds were placed in idealized positions whereas the hydrogen atoms from the N–H, O–H moieties were localized from the difference electron density map, and their position was refined with Uiso=aUeq (where a is 1.5 for –CH3 and –OH moieties and 1.2 for others). X-ray crystallographic data in CIF format are available in Supporting Information.
Potentiometry
Potentiometric titrations were performed in a 25 mL cell kept under nitrogen at 25±0.1°C with 0.1 M NaCl as background electrolyte. Aqueous DMSO solutions (50% v/v) of receptors 1 or 2 (2 mM) alone and in the presence of sugar (glucose or fructose 0.2 M) were employed. The pKa values of all components were determined independently by potentiometric titrations under the same conditions and were used as fixed parameters in the fitting of results for the mixtures. Experimental details and procedure for the electrode calibration have been previously described [33]. The program Hyperquad 2008 [34], [35] was used to calculate all equilibrium constants.
1H NMR titrations
To a 5 mM solution of the receptor 1 or 2 in DMSO-d6 were added portions of concentrated solution of tetrabutylammonium salts of anions (AcO− or Cl−) in the same solvent and the mixture was incubated for 2 min after each addition before recording the spectrum. The observed equilibrium constants of the complex formation (K) were calculated from the profiles of the chemical shift δ versus salt concentration by fitting to the HypNMR program. The signals of B-OH or NH protons in the receptor were used for the fitting.
Acknowledgments
Mayte A. Martínez-Aguirre thanks CONACyT for the doctoral fellowship (271108). We thank M.Sc. María de la Nieves Zavala Segovia for technical assistance. The financial support of this research by CONACyT (281251, CB239648, PDCPN247495) and FQ-UNAM-PAIP is gratefully acknowledged. T. T.-B. and M. L. A.-H. are grateful to DGAPA-UNAM for scholarships.
References
[1] de Silva, A. P. Analytical chemistry: sense and versatility. Nature, 2007, 445, 718–719.10.1038/445718aSearch in Google Scholar PubMed
[2] Chhatwal, M.; Kumar, A.; Singh, V.; Gupta, R. D.; Awasthi, S. K. Addressing of multiple-metal ions on a single platform. Coord. Chem. Rev.2015, 292, 30–55.10.1016/j.ccr.2015.02.009Search in Google Scholar
[3] Gupta, V. K.; Mergu, N.; Kumawat, L. K. A new multifunctional rhodamine-derived probe for colorimetric sensing of Cu(II) and Al(III) and fluorometric sensing of Fe(III) in aqueous media. Sensors Actuators B, 2016, 223, 101–113.10.1016/j.snb.2015.09.060Search in Google Scholar
[4] Schmittel, M.; Lin, H.-W. Quadruple-channel sensing: a molecular sensor with a single type of receptor site for selective and quantitative multi-ion analysis. Angew. Chem. Int. Ed.2007, 46, 893–896.10.1002/anie.200603362Search in Google Scholar PubMed
[5] Gale, P. A.; Howe, E. N. W.; Wu, X. Anion receptor chemistry. Chem, 2016, 1, 351–422.10.1016/j.chempr.2016.08.004Search in Google Scholar
[6] Gale, P. A.; Busschaert, N.; Haynes, C. J. E.; Karagiannidis, L. E.; Kirby, I. L. Anion receptor chemistry: highlights from 2011 and 2012. Chem. Soc. Rev.2014, 43, 205–241.10.1039/C3CS60316DSearch in Google Scholar
[7] Busschaert, N.; Caltagirone, C.; Rossom, W. V.; Gale, P. A. Applications of supramolecular anion recognition. Chem. Rev.2015, 115, 8038–8155.10.1021/acs.chemrev.5b00099Search in Google Scholar PubMed
[8] Yan, J.; Fang, H.; Wang, B. Boronolectins and fluorescent boronolectins: an examination of the detailed chemistry issues important for the design. Med. Res. Rev.2005, 25, 490–520.10.1002/med.20038Search in Google Scholar PubMed
[9] James, T. D.; Phillips, M. D.; Shinkai, S. Boronic Acids in Saccharide Recognition; Royal Society of Chemistry: Cambridge, 2006.10.1039/9781847557612Search in Google Scholar
[10] Kavallieratos, K.; Bertao, C. M; Crabtree, R. H. Hydrogen bonding in anion recognition: a family of versatile, nonpreorganized neutral and acyclic receptors. J. Org. Chem.1999, 64, 1675–1683.10.1021/jo982382lSearch in Google Scholar PubMed
[11] Kumar, P.; Gupta, R. The wonderful world of pyridine-2,6-dicarboxamide based scaffolds. Dalton Trans.2016, 45, 18769–18783.10.1039/C6DT03578GSearch in Google Scholar PubMed
[12] Caltagirone, C.; Bazzicalupi, C.; Bencini, A.; Isaia, F.; Garau, A.; Lippolis, V. Anion recognition properties of pyridine-2,6-dicarboxamide and isophthalamide derivatives containing L-tryptophan moieties. Supramol. Chem. 2012, 24, 95–100.10.1080/10610278.2011.628391Search in Google Scholar
[13] Chmielewski, M. J.; Jurczak, J. Anion recognition by neutral macrocyclic amides. Chem. Eur. J.2005, 11, 6080–6094.10.1002/chem.200500232Search in Google Scholar PubMed
[14] Kang, S. O.; Begum, R. A.; Bowman-James, K. Amide-based ligands for anion coordination. Angew. Chem. Int. Ed.2006, 45, 7882–7894.10.1002/anie.200602006Search in Google Scholar PubMed
[15] Chmielewski, M. J.; Zielinski, T.; Jurczak, J. Synthesis, structure, and complexing properties of macrocyclic receptors for anions. Pure Appl. Chem.2007, 79, 1087–1096.10.1351/pac200779061087Search in Google Scholar
[16] Galbraith, E.; James, T. D. Boron based anion receptors as sensors. Chem. Soc. Rev.2010, 39, 3831–3842.10.1039/b926165fSearch in Google Scholar PubMed
[17] Guo, Z.; Shin, I.; Yoon, J. Recognition and sensing of various species using boronic acid derivatives. Chem. Commun.2012, 48, 5956–5967.10.1039/c2cc31985cSearch in Google Scholar PubMed
[18] Wade, C. R.; Broomsgrove, A. E. J.; Aldridge, S.; Gabbaï, F. P. Fluoride ion complexation and sensing using organoboron compounds. Chem. Rev.2010, 110, 3958–3984.10.1021/cr900401aSearch in Google Scholar PubMed
[19] Peters, J. A. Interactions between boric acid derivatives and saccharides in aqueous media: Structures and stabilities of resulting esters. Coord. Chem. Rev.2014, 268, 1–22.10.1016/j.ccr.2014.01.016Search in Google Scholar
[20] Martínez-Aguirre, M. A.; Yatsimirsky, A. K. Brønsted versus Lewis Acid Type Anion Recognition by Arylboronic Acids. J. Org. Chem.2015, 80, 4985–4993.10.1021/acs.joc.5b00377Search in Google Scholar PubMed
[21] Dorazco-Gonzalez, A.; Hopfl, H.; Medrano, F.; Yatsimirsky, A. K. Recognition of anions and neutral guests by dicationic pyridine-2,6-dicarboxamide receptors. J. Org. Chem.2010, 75, 2259–2273.10.1021/jo100037mSearch in Google Scholar PubMed
[22] Lorand, J. P.; Edwards, J. O. Polyol complexes and structure of the benzeneboronate ion. J. Org. Chem.1959, 24, 769–774.10.1021/jo01088a011Search in Google Scholar
[23] Springsteen, G.; Deeter, S.; Wang, B. A detailed examination of boronic acid–diol complexation. Tetrahedron2002, 58, 5291–5300.10.1016/S0040-4020(02)00489-1Search in Google Scholar
[24] Martínez-Aguirre, M. A.; Villamil-Ramos, R.; Guerrero-Alvarez, J. A.; Yatsimirsky, A. K. Substituent effects and pH profiles for stability constants of arylboronic acid diol esters. J. Org. Chem.2013, 78, 4674–4684.10.1021/jo400617jSearch in Google Scholar
[25] Hansch, C., Leo, A.; Taft, R. W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev.1991, 91, 165–195.10.1021/cr00002a004Search in Google Scholar
[26] Martínez-Aguirre, M. A.; Del Campo, J. M.; Escalante-Tovar, S.; Yatsimirsky, A. K. Self-assembly and recognition properties of a tetraanionic macrocyclic boronate ester in aqueous medium. RSC Adv.2015, 5, 30075–30083.10.1039/C5RA03291ASearch in Google Scholar
[27] Bosch, E.; Fonrodona, G.; Rafols, C.; Rosés, M. Autoprotolysis in aqueous organic solvent mixtures. Water/dipolar protophilic solvent binary systems. Anal. Chim. Acta1997, 349, 367–376.10.1016/S0003-2670(97)00191-8Search in Google Scholar
[28] Springsteen, G.; Wang, B. Alizarin Red S. as a general optical reporter for studying the binding of boronic acids with carbohydrates. Chem. Commun.2001, 1608–1609.10.1039/b104895nSearch in Google Scholar PubMed
[29] Bruker, APEX 2, (Ver. 1.0-22), Bruker AXS Inc.: Madison, Wisconsin, USA, 2004.Search in Google Scholar
[30] Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. Sect. A, 2008, 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
[31] Hübschle, C. B.; Sheldrick, G. M.; Dittrich, B. ShelXle: a Qt graphical user interface for SHELXL. J. Appl. Crystallogr.2011, 44, 1281–1284.10.1107/S0108767319098143Search in Google Scholar
[32] Sheldrick, G. M. SHELXL-97, Program for Crystal Structure Refinement; University of Göttingen: Germany, 1997.Search in Google Scholar
[33] Sánchez-Lombardo, I.; Yatsimirsky, A. K. Simplified speciation and improved phosphodiesterolytic activity of hydroxo complexes of trivalent lanthanides in aqueous DMSO. Inorg. Chem.2008, 47, 2514–2525.10.1021/ic701846eSearch in Google Scholar PubMed
[34] Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta1996, 43, 1739–1753.10.1016/0039-9140(96)01958-3Search in Google Scholar
[35] Alderlghi, L.; Gans, P.; Ienco, A.; Peters, D.; Sabatini, A.; Vacca, A. Hyperquad simulation and speciation (HySS): a utility program for the investigation of equilibria involving soluble and partially soluble species. Coord. Chem. Rev.1999, 184, 311–318.10.1016/S0010-8545(98)00260-4Search in Google Scholar
Supplemental Material:
The online version of this article (DOI: https://doi.org/10.1515/hc-2017-0054) offers supplementary material, available to authorized users.
©2017 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Editorial
- Carbohydrate chemistry/glycoscience
- Reviews
- Boron-based small molecules in disease detection and treatment (2013–2016)
- Impact of modified ribose sugars on nucleic acid conformation and function
- Preliminary Communication
- Crystallization-induced amide bond formation creates a boron-centered spirocyclic system
- Research Articles
- Anion and sugar recognition by 2,6-pyridinedicarboxamide bis-boronic acid derivatives
- Synthesis of biotinylated bivalent zanamivir analogs as probes for influenza viruses
- Synthesis of silodosin glucuronide and its deuterated counterpart: solving a problematic O-glycosylation of a nitrogen-containing molecule
- Synthesis and antimicrobial activity of 4-trifluoromethylpyridine nucleosides
- Synthesis of anti-inflammatory 2,3-unsaturated O-glycosides using conventional and microwave heating techniques
- Synthesis of various β-D-glucopyranosyl and β-D-xylopyranosyl hydroxybenzoates and evaluation of their antioxidant activities
- Synthesis of carbohydrate-substituted isoxazoles and evaluation of their antitubercular activity
- Synthesis and bioactivity of novel C2-glycosyl triazole derivatives as acetylcholinesterase inhibitors
- Modification of bovine serum albumin with aminophenylboronic acid as glycan sensor based on surface plasmon resonance and isothermal titration calorimetry
Articles in the same Issue
- Frontmatter
- Editorial
- Carbohydrate chemistry/glycoscience
- Reviews
- Boron-based small molecules in disease detection and treatment (2013–2016)
- Impact of modified ribose sugars on nucleic acid conformation and function
- Preliminary Communication
- Crystallization-induced amide bond formation creates a boron-centered spirocyclic system
- Research Articles
- Anion and sugar recognition by 2,6-pyridinedicarboxamide bis-boronic acid derivatives
- Synthesis of biotinylated bivalent zanamivir analogs as probes for influenza viruses
- Synthesis of silodosin glucuronide and its deuterated counterpart: solving a problematic O-glycosylation of a nitrogen-containing molecule
- Synthesis and antimicrobial activity of 4-trifluoromethylpyridine nucleosides
- Synthesis of anti-inflammatory 2,3-unsaturated O-glycosides using conventional and microwave heating techniques
- Synthesis of various β-D-glucopyranosyl and β-D-xylopyranosyl hydroxybenzoates and evaluation of their antioxidant activities
- Synthesis of carbohydrate-substituted isoxazoles and evaluation of their antitubercular activity
- Synthesis and bioactivity of novel C2-glycosyl triazole derivatives as acetylcholinesterase inhibitors
- Modification of bovine serum albumin with aminophenylboronic acid as glycan sensor based on surface plasmon resonance and isothermal titration calorimetry

