Validation of the enhanced liver fibrosis (ELF)-test in heparinized and EDTA plasma for use in reflex testing algorithms for metabolic dysfunction-associated steatotic liver disease (MASLD)
-
Koen C. van Son
, Anne-Marieke van Dijk
To the Editor,
Metabolic dysfunction-associated steatotic liver disease (MASLD) is rapidly becoming the most common liver disease worldwide, currently effecting around a third of the adult population [1]. Diagnostic algorithms that use non-invasive tests (NITs) to stage MASLD and allow for the non-invasive and early detection of advanced stages of MASLD fibrosis are currently being developed and implemented [2], [3], [4]. An often-proposed strategy is the use of two or more sequential NITs to accurately and cost-effectively assess the risk of underlying fibrosis. Commonly, these combinations start with the FIB-4 score, a low-cost and easy-to-use NIT requiring age, AST, ALT and platelets, followed by a more expensive, more robust test such as the enhanced liver fibrosis (ELF)-test. Reflex testing with such combinations would enhance the diagnostic process for fibrotic MASLD. Yet, whereas transaminases are commonly measured in heparinized plasma and platelets are measured in EDTA plasma, the ELF-test has only been validated for use in serum. Therefore, we aimed to validate the ELF-test in both heparinized plasma and EDTA plasma.
To this end, we utilized the Nijmegen-Leiden-Amsterdam (NLA)2 study, consisting of patients at risk of MASLD from primary, secondary and tertiary care who were screened with NITs (i.e., FIB-4 score and vibration controlled transient elastography (VCTE)), as the derivation cohort. Blood samples consisting of serum, heparinized plasma and EDTA plasma were collected and stored in a designated biobank. The validation cohorts consist of a selection of the NAFLD In the healthy Life in an urban sEtting (NILE) cohort, representing a primary care population at risk of MASLD, and the Amsterdam NAFLD-NASH cohort (ANCHOR), representing a secondary and tertiary care population with histologically characterized MASLD [5, 6]. The three ELF proteins (hyaluronic acid (HA), PIIINP and TIMP-1) were analyzed separately using ELF-test kits provided by Siemens™ according to manufactures’ instructions using the Atellica IM analyser (Siemens Heathineers) [7]. All samples were analyzed simultaneously at the endocrinology laboratory at the Amsterdam UMC, location AMC.
The NLA2 derivation cohort consisted of 144 participants with a mean age of 57.9 (12.3) years (Supplementary Table 1). 63.9 % of participants were women and 55.6 % of participants had type 2 diabetes mellitus. Mean BMI was 31.5 (5.5) kg/m2, 90.2 % had BMI ≥25 kg/m2 and 57.6 % had BMI ≥30 kg/m2. 61.1 % of participants had controlled attenuation parameter (CAP) ≥290 dB/m and 22.9 % had a liver stiffness measurement (LSM) ≥8.2 kPa, suggesting the presence of ≥S3 steatosis and ≥F3 fibrosis [8]. Mean ELF-score in heparinized plasma was comparable to that in serum (9.06 (0.79) vs. 9.06 (0.76) (p=0.999)), while mean ELF-score in EDTA plasma was lower than ELF-score in serum (8.69 (0.78) vs. 9.06 (0.76) (p<0.001)) (Supplementary Table 2). Correlation coefficients between ELF-score in serum vs. heparinized plasma and EDTA plasma were 0.974 and 0.966 (p for both <0.001), respectively. Regarding the three individual components of ELF, median HA in heparinized plasma and EDTA plasma were not significantly different from median HA in serum (p=0.680 and p=0.613, respectively), but median PIIINP and TIMP-1 were significantly different (all p<0.001) (Supplementary Table 2). Given the differences in levels of the individual ELF proteins, correction factors were calculated using Passing–Bablok regression analyses and new formulas were designed using the original formula for the ELF-score as reference:
Heparinized plasma
EDTA plasma
Using the corrected formula for ELF-test in heparinized plasma, mean ELF-score was 9.07 (0.79) compared to 9.06 (0.76) in serum (p=0.983). Pearson’s correlation resulted in a correlation coefficient of 0.975 (p<0.001). Mean ELF-score using the corrected formula in EDTA plasma was also 9.07 (0.80) (p=0.974) and the correlation coefficient with ELF-score in serum was 0.969 (p<0.001).
In the NILE cohort, mean ELF-score in EDTA plasma using the unadjusted formula was significantly lower than mean ELF-score in serum (8.85 (0.82) vs. 9.26 (0.79) (p<0.001)). Application of the corrected formula resulted in a mean ELF-score in EDTA plasma comparable to that in serum (9.23 (0.82) vs. 9.26 (0.79) (p=0.823)) (Supplementary Table 2). Mean ELF-score in heparinized plasma was comparable to serum using the unadjusted and the corrected formulas. When comparing agreement between ELF-test conducted in heparinized plasma and serum, Cohen’s kappa increased from 0.84 to 0.87 when using the corrected formula compared to the unadjusted formula. When using ELF-test conducted in EDTA plasma, Cohen’s kappa increased from 0.68 to 0.90.
In the ANCHOR cohort, mean ELF-score in heparinized plasma and EDTA plasma using the unadjusted formulas was comparable to mean ELF-score in serum (9.17 (0.85) vs. 8.91 (0.86) (p=0.092), and 8.67 (0.86) vs. 8.91 (0.86) (p=0.133), respectively). When using the corrected formulas, the mean ELF-score remained comparable between serum and heparinized plasma and EDTA plasma (9.16 (0.84) vs. 8.91 (0.86) (p=0.100), and 9.03 (0.87) vs. 8.91 (0.86) (p=0.411), respectively) (Supplementary Table 2). Figure 1 shows Bland-Altman plots of serum and heparinized plasma and serum and EDTA plasma using the unadjusted and the corrected formulas in the validation cohorts. Sensitivity of the ELF-test for the detection of ≥F3 fibrosis was higher using the corrected formulas for heparinized plasma (0.53 (0.29, 0.77) vs. 0.47 (0.23, 0.71)) and EDTA plasma (0.53 (0.29, 0.77) vs. 0.29 (0.08, 0.51)) compared to the unadjusted formulas. The AUC of ELF-test with the predefined cut-off of 9.8 to detect ≥F3 fibrosis was higher for the corrected formulas for heparinized plasma (0.71 (0.58, 0.84) vs. 0.68 (0.55, 0.81)) and EDTA plasma (0.70 (0.56, 0.83) vs. 0.62 (0.51, 0.74)) compared to the unadjusted formulas (Supplementary Table 3).
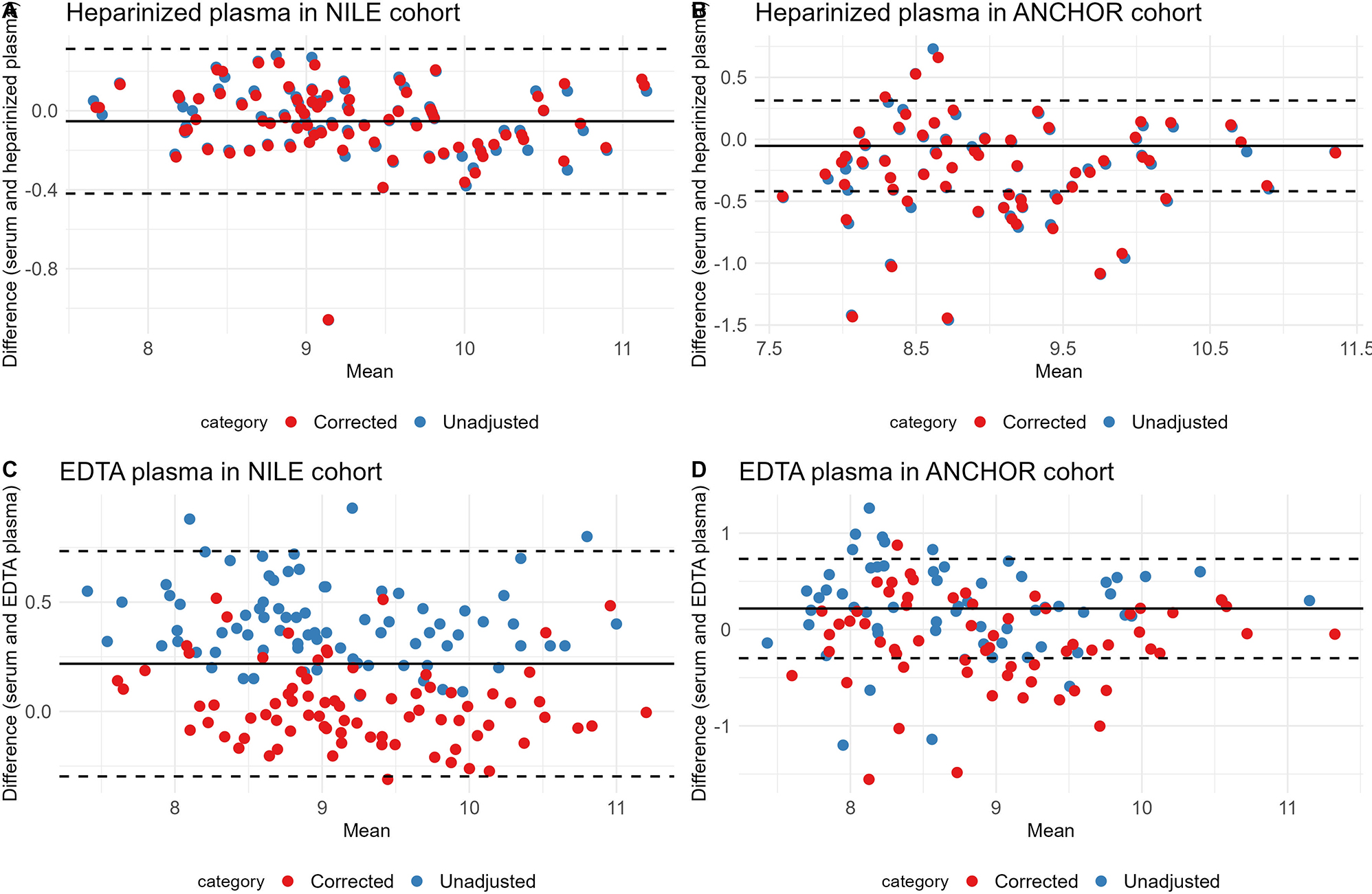
Bland–Altman plots of ELF-score in serum and heparinized plasma using the unadjusted and the corrected ELF-score formulas in the NILE (A) and ANCHOR validation cohorts (B) and ELF-score in serum and EDTA plasma using the unadjusted and the corrected ELF-score formulas in the NILE (C) and ANCHOR validation cohorts (D).
To explore the concept of reflex testing, the Camden & Islington algorithm [9] – in which an ELF-test follows an intermediate FIB-4 score (1.30–3.25) – was retrospectively applied in the ANCHOR cohort. 19 participants (31.7 %) had an intermediate FIB-4 score and would thus be provided an ELF-test for which the manufacturer’s cut-off of 9.8 was used (Supplementary Figure 1). One participants was omitted from analyses due to insufficient data to calculate the FIB-4 score. The sensitivity and specificity of this reflex testing algorithm using ELF-test performed in serum were 0.38 (0.14, 0.61) and 0.93 (0.86, 1.00), yielding an AUC of 0.70 (0.55, 0.84). Using heparinized plasma or EDTA plasma with the unadjusted formulas yielded AUCs of 0.69 (0.54, 0.83) and 0.67 (0.52, 0.82), and application of the corrected formulas yielded AUCs of 0.70 (0.56, 0.85) and 0.69 (0.55, 0.84), respectively (Supplementary Table 4).
Taken together, here we demonstrate that ELF-test performed in heparinized plasma and EDTA plasma has comparable performance to that of ELF-test performed in serum when using correction factors. The usefulness of the corrected ELF-test formulas is demonstrated in separate validation cohorts. This allows for the application of reflex testing algorithms with the ELF-test as a second-tiered test. Sensitivity and AUC for the detection of ≥F3 fibrosis increased when performing the ELF-test in heparinized plasma and EDTA plasma using the corrected compared to the unadjusted formulas. Interestingly, AUCs, including that of ELF-test performed in serum, are considerably lower than previously reported in a meta-analysis by Vali et al. who reported an AUC of 0.83 (0.71, 0.90) [10]. There are some study limitations including the relatively small sample size of the histologically characterized validation cohort and unequal distribution of MASLD-subtypes. Nevertheless, our study has strong clinical utility: the validation of ELF-test in heparinized plasma and EDTA plasma allows for direct applicability of the ELF-test in reflex testing algorithms for fibrotic MASLD.
Acknowledgments
We are most grateful to the participants of the NLA2, NILE and ANCHOR studies. We thank Siemens for supplying the ELF kits, especially Vincent Schaarman and Cees van Eegeraat. We thank Ulrika Boulund for helping with the analyses for the corrected ELF-test formulas.
-
Research ethics: All studies were approved by the Medical Ethical Committee of the Amsterdam UMC, Amsterdam, The Netherlands, and the study was conducted in accordance with the declaration of Helsinki (as revised in 2013).
-
Informed consent: Informed consent was obtained from all individuals included in this study, or their legal guardians or wards.
-
Author contributions: The authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: The authors state no competing interests.
-
Research funding: The HELIUS study is conducted by the Amsterdam University Medical Centers, location AMC and the Public Health Service of Amsterdam. Both organisations provided core support for HELIUS. The HELIUS study is also funded by the Dutch Heart Foundation, the Netherlands Organization for Health Research and Development (ZonMw), the European Union (FP-7), and the European Fund for the Integration of non-EU immigrants (EIF). This work has received funding from an ITN Marie Curie BestTreat – Building a Gut Microbiome Engineering Toolbox for In-Situ Therapeutic Treatments for Non-alcoholic Fatty Liver Disease No. 813781 ITN BestTreat (on which VH is appointed). MN is supported by a personal ZonMW-VICI grant 2020 (09150182010020). A.G.H. is supported by the Amsterdam UMC Fellowship grant, an Amsterdam UMC Innovation grant, two grants from the Dutch Gastroenterology & Hepatology Foundation MLDS and two TKI‐PPP grants from Health∼Holland.
-
Data availability: The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
1. Younossi, ZM, Golabi, P, Paik, JM, Henry, A, Van Dongen, C, Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology 2023;77:1335–47. https://doi.org/10.1097/hep.0000000000000004.Suche in Google Scholar PubMed PubMed Central
2. van Dijk, AM, Schattenberg, JM, Holleboom, AG, Tushuizen, ME. Referral care paths for non-alcoholic fatty liver disease-Gearing up for an ever more prevalent and severe liver disease. United Eur Gastroenterol J 2021;9:903–9. https://doi.org/10.1002/ueg2.12150.Suche in Google Scholar PubMed PubMed Central
3. Lazarus, JV, Anstee, QM, Hagström, H, Cusi, K, Cortez-Pinto, H, Mark, HE, et al.. Defining comprehensive models of care for NAFLD. Nat Rev Gastroenterol Hepatol 2021;18:717–29. https://doi.org/10.1038/s41575-021-00477-7.Suche in Google Scholar PubMed
4. Macpherson, I, Nobes, JH, Dow, E, Furrie, E, Miller, MH, Robinson, EM, et al.. Intelligent liver function testing: working smarter to improve patient outcomes in liver disease. J Appl Lab Med 2020;5:1090–100. https://doi.org/10.1093/jalm/jfaa109.Suche in Google Scholar PubMed
5. van Dijk, AM, Vali, Y, Mak, AL, Galenkamp, H, Nieuwdorp, M, van den Born, BJ, et al.. Noninvasive tests for nonalcoholic fatty liver disease in a multi-ethnic population: the HELIUS study. Hepatol Commun 2023;7:e2109. https://doi.org/10.1002/hep4.2109.Suche in Google Scholar PubMed PubMed Central
6. Troelstra, MA, Witjes, JJ, van Dijk, AM, Mak, AL, Gurney-Champion, O, Runge, JH, et al.. Assessment of imaging modalities against liver biopsy in nonalcoholic fatty liver disease: the Amsterdam NAFLD-NASH cohort. J Magn Reson Imag 2021;54:1937–49. https://doi.org/10.1002/jmri.27703.Suche in Google Scholar PubMed PubMed Central
7. I. U. enhanced liver fibrosis test (ELF test). Terrytown, NY: Siemens Healthcare Diagnostics; 2019:1–10 pp.Suche in Google Scholar
8. Eddowes, PJ, Sasso, M, Allison, M, Tsochatzis, E, Anstee, QM, Sheridan, D, et al.. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 2019;156:1717–30. https://doi.org/10.1053/j.gastro.2019.01.042.Suche in Google Scholar PubMed
9. Srivastava, A, Gailer, R, Tanwar, S, Trembling, P, Parkes, J, Rodger, A, et al.. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J Hepatol 2019;71:371–8. https://doi.org/10.1016/j.jhep.2019.03.033.Suche in Google Scholar PubMed
10. Vali, Y, Lee, J, Boursier, J, Spijker, R, Löffler, J, Verheij, J, et al.. Enhanced liver fibrosis test for the non-invasive diagnosis of fibrosis in patients with NAFLD: a systematic review and meta-analysis. J Hepatol 2020;73:252–62. https://doi.org/10.1016/j.jhep.2020.03.036.Suche in Google Scholar PubMed
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/cclm-2024-0470).
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Editorial
- Circulating tumor DNA measurement: a new pillar of medical oncology?
- Reviews
- Circulating tumor DNA: current implementation issues and future challenges for clinical utility
- Circulating tumor DNA methylation: a promising clinical tool for cancer diagnosis and management
- Opinion Papers
- The final part of the CRESS trilogy – how to evaluate the quality of stability studies
- The impact of physiological variations on personalized reference intervals and decision limits: an in-depth analysis
- Computational pathology: an evolving concept
- Perspectives
- Dynamic mirroring: unveiling the role of digital twins, artificial intelligence and synthetic data for personalized medicine in laboratory medicine
- General Clinical Chemistry and Laboratory Medicine
- Macroprolactin in mothers and their babies: what is its origin?
- The influence of undetected hemolysis on POCT potassium results in the emergency department
- Quality control in the Netherlands; todays practices and starting points for guidance and future research
- QC Constellation: a cutting-edge solution for risk and patient-based quality control in clinical laboratories
- OILVEQ: an Italian external quality control scheme for cannabinoids analysis in galenic preparations of cannabis oil
- Using Bland-Altman plot-based harmonization algorithm to optimize the harmonization for immunoassays
- Comparison of a two-step Tempus600 hub solution single-tube vs. container-based, one-step pneumatic transport system
- Evaluating the HYDRASHIFT 2/4 Daratumumab assay: a powerful approach to assess treatment response in multiple myeloma
- Insight into the status of plasma renin and aldosterone measurement: findings from 526 clinical laboratories in China
- Reference Values and Biological Variations
- Reference values for plasma and urine trace elements in a Swiss population-based cohort
- Stimulating thyrotropin receptor antibodies in early pregnancy
- Within- and between-subject biological variation estimates for the enumeration of lymphocyte deep immunophenotyping and monocyte subsets
- Diurnal and day-to-day biological variation of salivary cortisol and cortisone
- Web-accessible critical limits and critical values for urgent clinician notification
- Cancer Diagnostics
- Thyroglobulin measurement is the most powerful outcome predictor in differentiated thyroid cancer: a decision tree analysis in a European multicenter series
- Cardiovascular Diseases
- Interaction of heparin with human cardiac troponin complex and its influence on the immunodetection of troponins in human blood samples
- Diagnostic performance of a point of care high-sensitivity cardiac troponin I assay and single measurement evaluation to rule out and rule in acute coronary syndrome
- Corrigendum
- Reference intervals of 24 trace elements in blood, plasma and erythrocytes for the Slovenian adult population
- Letters to the Editor
- Disturbances of calcium, magnesium, and phosphate homeostasis: incidence, probable causes, and outcome
- Validation of the enhanced liver fibrosis (ELF)-test in heparinized and EDTA plasma for use in reflex testing algorithms for metabolic dysfunction-associated steatotic liver disease (MASLD)
- Detection of urinary foam cells diagnosing the XGP with thrombopenia preoperatively: a case report
- Methemoglobinemia after sodium nitrite poisoning: what blood gas analysis tells us (and what it might not)
- Novel thiopurine S-methyltransferase (TPMT) variant identified in Malay individuals
- Congress Abstracts
- 56th National Congress of the Italian Society of Clinical Biochemistry and Clinical Molecular Biology (SIBioC – Laboratory Medicine)
Artikel in diesem Heft
- Frontmatter
- Editorial
- Circulating tumor DNA measurement: a new pillar of medical oncology?
- Reviews
- Circulating tumor DNA: current implementation issues and future challenges for clinical utility
- Circulating tumor DNA methylation: a promising clinical tool for cancer diagnosis and management
- Opinion Papers
- The final part of the CRESS trilogy – how to evaluate the quality of stability studies
- The impact of physiological variations on personalized reference intervals and decision limits: an in-depth analysis
- Computational pathology: an evolving concept
- Perspectives
- Dynamic mirroring: unveiling the role of digital twins, artificial intelligence and synthetic data for personalized medicine in laboratory medicine
- General Clinical Chemistry and Laboratory Medicine
- Macroprolactin in mothers and their babies: what is its origin?
- The influence of undetected hemolysis on POCT potassium results in the emergency department
- Quality control in the Netherlands; todays practices and starting points for guidance and future research
- QC Constellation: a cutting-edge solution for risk and patient-based quality control in clinical laboratories
- OILVEQ: an Italian external quality control scheme for cannabinoids analysis in galenic preparations of cannabis oil
- Using Bland-Altman plot-based harmonization algorithm to optimize the harmonization for immunoassays
- Comparison of a two-step Tempus600 hub solution single-tube vs. container-based, one-step pneumatic transport system
- Evaluating the HYDRASHIFT 2/4 Daratumumab assay: a powerful approach to assess treatment response in multiple myeloma
- Insight into the status of plasma renin and aldosterone measurement: findings from 526 clinical laboratories in China
- Reference Values and Biological Variations
- Reference values for plasma and urine trace elements in a Swiss population-based cohort
- Stimulating thyrotropin receptor antibodies in early pregnancy
- Within- and between-subject biological variation estimates for the enumeration of lymphocyte deep immunophenotyping and monocyte subsets
- Diurnal and day-to-day biological variation of salivary cortisol and cortisone
- Web-accessible critical limits and critical values for urgent clinician notification
- Cancer Diagnostics
- Thyroglobulin measurement is the most powerful outcome predictor in differentiated thyroid cancer: a decision tree analysis in a European multicenter series
- Cardiovascular Diseases
- Interaction of heparin with human cardiac troponin complex and its influence on the immunodetection of troponins in human blood samples
- Diagnostic performance of a point of care high-sensitivity cardiac troponin I assay and single measurement evaluation to rule out and rule in acute coronary syndrome
- Corrigendum
- Reference intervals of 24 trace elements in blood, plasma and erythrocytes for the Slovenian adult population
- Letters to the Editor
- Disturbances of calcium, magnesium, and phosphate homeostasis: incidence, probable causes, and outcome
- Validation of the enhanced liver fibrosis (ELF)-test in heparinized and EDTA plasma for use in reflex testing algorithms for metabolic dysfunction-associated steatotic liver disease (MASLD)
- Detection of urinary foam cells diagnosing the XGP with thrombopenia preoperatively: a case report
- Methemoglobinemia after sodium nitrite poisoning: what blood gas analysis tells us (and what it might not)
- Novel thiopurine S-methyltransferase (TPMT) variant identified in Malay individuals
- Congress Abstracts
- 56th National Congress of the Italian Society of Clinical Biochemistry and Clinical Molecular Biology (SIBioC – Laboratory Medicine)

