Focal liver lesions other than hepatocellular carcinoma in cirrhosis: Diagnostic challenges
-
Kathleen Möller
Abstract
Liver cirrhosis is associated with regenerative nodules and an increased risk of developing hepatocellular carcinoma (HCC). However, other benign and malignant liver lesions may also occur. Differentiating the other lesions from HCC is important for further therapeutic decisions. This review discusses the characteristics of non-HCC liver lesions in cirrhosis and their consequent appearance on contrast-enhanced ultrasonography (CEUS) with consideration of other imaging. Knowledge of this data would be helpful in avoiding misdiagnoses.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver with over 500,000 new cases diagnosed annually worldwide.[1,2] It is the second leading cause of cancer-related mortality in the world.[3] The yearly incidence of HCC among patients with clinical liver cirrhosis ranges from 0.5% to 11.0%.[4, 5, 6]
Seventy-six percent of all solid lesions in 282 cases of liver cirrhosis corresponded to HCC in a German multicenter study.[7] In a contrast-enhanced ultrasonography (CEUS) study by Terzi et al.[8] 81% of 1006 solid lesions in surveillance in chronic liver disease corresponded to HCCs.[8] HCC has typical findings on CEUS, computed tomography (CT), and magnetic resonance imaging (MRI) due to the peculiarity of its vascularization, which make it possible to diagnose it noninvasively. According to the European Association for the Study of the liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) guidelines, contrast-enhanced CT including the arterial and portal venous phase and MRI with liver-specific contrast medium should be used to diagnose/rule out HCC in cirrhosis. Other international guidelines also give equivalent importance to CEUS,[9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19] and overall, the diagnosis of HCC and other non-HCC lesions is primarily made by contrast-enhanced imaging.
In this context, all other focal liver lesions can also occur in liver cirrhosis with a frequency of about 20%.[7,8] Table 1 summarizes an overview of the frequency of HCC and non-HCC in liver cirrhosis.
Frequency of HCC and non-HCC lesions in liver cirrhosis in autopsy and imaging studies
| Author | Method | HCC | Non- HCC | RN/DN | FNH; FNH- like lesion | Hemangioma | HCA | Metastases | CCC | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| Guinaldo et al. 1997[20] | Autopsy | 90.3% | 9.6% | NA | 0 | 1.2% | 1.5% | 4.2% | 2.3% | Hamartomas 0.8% |
| Dodd et al.1999[21] | Autopsy | 9% | NA | 11.2% | 0 | 1.7% | 0 | 0 | 0 | Peribiliary cysts 2.6% |
| Forner et al. 2008[22] | CT, MRI, biopsy | 67.4% | 31.5% | 27% | 1.1% | 3.4% | NA | NA | 1.1% | NA |
| D’Onofrio et al. 2008[23] | CEUS, CT, MRI, histology | 46.4% | 53.6% | 32.3% | 3.8% | 17.4% | 0 | 0 | 0 | NA |
| Seitz et al. 2011[7] | CEUS | 76.6% | 23.4% | 5.7% | 0 | 2.8% | 0.3% | 4.3% | 2.5% | Various 7.8% |
| Serste et al. 2012[24] | CT, MRI, biopsy | 63.5% | 57.4% | 25.2% | 0 | 0 | 0 | 0 | 1.4% | Epithelioid hemangioendothelioma 1.4% |
| Victor et al. 2011[25] | MRI | NA | NA | NA | 8.8% | NA | NA | NA | NA | Cysts 17.5% |
| Terzi et al.2018[8] | CEUS, CT, MRI, histology | 81% | 19% | 11% | 0 | 1% | NA | 0.2% | 4% | HCC/CCC 0.9% Other 1.9% |
HCC: hepatocellular carcinoma; RN: regenerative nodule; DN: dysplastic nodule; FNH: focal nodular hyperplasia; HCA: hepatocellular adenoma; CCC: cholangiocellular carcinoma; CEUS: contrast-enhanced ultrasonography; MRI: magnetic resonance imaging; CT: computed tomography; NA: not applicable.
Knowledge of the different types of benign and malignant lesions that can arise in the context of cirrhosis and about their ultrasound appearance on B-mode and CEUS is important to avoid misdiagnoses and, thus, the wrong therapeutic decision. This review focuses on this topic.
Not every lesion in liver cirrhosis is an HCC
Imaging diagnosis of HCC in liver cirrhosis is based on the identification of the typical characteristics, which differ according to imaging techniques or contrast agents and are as follows: arterial phase hyperenhancement (APHE) followed by washout in the portal venous or delayed phases on CT and MRI using extracellular contrast agents or gadobenate dimeglumine, APHE with washout in the portal venous phase on MRI using gadoxetic acid, and APHE with late-onset (> 60 s) washout of mild intensity on CEUS.[26]
Arterial hyperenhancement is also shown by other FLL: hemangiomas, focal nodular hyperplasia (FNH)/FNH-like lesions, hepatocellular adenomas (HCAs), many dysplastic and very few regenerative nodules, cholangiocellular carcinoma (CCC), metastases, and lymphomas, whereas in CEUS, very specific diagnostic vascularization patterns of the vascular architecture in the arterial phase are revealed in detail.[9, 10, 11, 17, 19, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41]
The probability that a new lesion smaller than 10 mm corresponds to HCC is low.[26,42,43] When a small hypervascular lesion is first detected on CT or MRI, it may be a true nodule, but it may also be a vascular phenomenon such as an arterioportal shunt. It has been published that 70%–90% of small (1–2 or < 2 cm) hypervascular foci that can only be seen on arterial phase (hyper arterial phase enhancement [HAPE]) imaging do not correspond to HCC.[44,45] Holland et al.[45] assessed small liver lesions < 20 mm on MRI, which exclusively showed hyperenhancement in the arterial phase on MRI. In the liver explants, 93% of these lesions were benign.
The prevalence of HAPE-only lesions in patients with severe cirrhosis before transplantation was 35%. The majority of HAPE-only lesions (93%) had no correlative pathologic findings, were benign, and may have represented regenerative nodules or arterial-portal venous shunts.[45] In the study by Jang et al.[46] of 59 patients at high risk of HCC and having 1–2 cm nodules with APHE on CEUS, only 26 (44%) corresponded to HCC and 33 (56%) were benign (20 regenerative nodules [RNs]/dysplastic nodules [DNs], 11 hemangiomas, two focal fat sparring). Khalili et al.[47] found in 93 indeterminate 1–2 cm nodules on dynamic CT, MRI, and CEUS that the prevalence of HCC was low. Eighty-five percent of the FLL showed characteristics of benignity and the only significant predictors of malignancy were arterial phase hypervascularity and synchronous HCC elsewhere in the liver. Among a total of 138 nodules, 4% were hemangiomas.[47] In the study by Kim et al.[48] 23% of all newly diagnosed solid lesions measuring 10–20 mm on MRI corresponded to hemangiomas during surveillance for HCC.[48]
CEUS might be effectively used for characterizing indeterminate lesions on CT and MRI or when these two are contraindicated.[49] If a small nodule does not show arterial hypervascularity on CEUS, it is unlikely to show typical features of hypervascular HCC on CT or MRI.[44]
Forner et al.[22] differentiated 89 nodules detected by ultrasound surveillance in liver cirrhosis. Among them, 67.4% were HCC, 1.1% were CCC, and 31.5% were benign lesions (RN/DN 27.0%, hemangioma 3.4%, FNH 1.1%).
In a retrospective study by Compagnon et al.[50] of explanted livers following liver transplantation, 16.7% were false positive for pretransplant HCC diagnosed on imaging without biopsy. These were nine DNs, five RNs, one CCC, one hemangioma, and four were not lesions. All lesions were smaller than 30 mm. Sensitivity, specificity, positive predictive value, and negative predictive value for the preoperative clinical and radiologic diagnosis of HCC were 89%, 94.3%, 77%, and 93.3%, respectively.[50]
In a study by Lee et al.[51] among 837 liver resection cases for presurgical imaging diagnosed as HCC without biopsy confirmation, 2.2% false positively diagnosed HCC cases were found. Among the false positives, 0.8% were benign FLL (hemangioma, inflammation, cortical adenoma, DN, angiomyolipoma, bile duct adenoma, and non-neoplastic liver parenchyma) and 1.3% were malignant FLL (cholangiocarcinoma, hepatoblastoma, lymphoepithelioma-like carcinoma, ovarian cystadenocarcinoma, and nasopharynx carcinoma metastasis). The false-positive rate in nodules ≤ 2 cm was 3.4%.
Table 2 presents the false-positive diagnoses on imaging without CEUS and biopsy for HCC.
False-positive diagnosis of HCC in liver cirrhosis in liver explants and resections
| Author | Methods and patients | Benign lesions with false-positive diagnosis of HCC | Malignant lesions with false-positive diagnosis of HCC |
|---|---|---|---|
| Compagnon et al.[50] | n = 120 patients; liver transplantation Imaging without biopsy (US, CT, MRI) False positive 16.7%; all < 30 mm | Dysplastic nodules 9 Regenerative nodules 5 Hemangioma 1 No lesions 4 | Cholangiocarcinoma 1 |
| Lee et al.[51] | n = 837 liver resection cases Presurgical imaging diagnosed HCC without biopsy (US, CT, MRI) False positive 2.2% (n = 18) | n = 7 (38.9%) Hemangioma 1 Inflammation 1 Cortical adenoma 1 Regenerative nodule 1 Angiomyolipoma 1 Bile duct adenoma 1 Non-neoplastic liver parenchyma 1 | n = 11 (61.1%) Cholangiocarcinoma 6 Hepatoblastoma 2 Lymphoepithelioma-like carcinoma 1 Ovarian cystadenocarcinoma 1 Metastasis from nasopharyngeal carcinoma 1 |
HCC: hepatocellular carcinoma; MRI: magnetic resonance imaging; US: ultrasound; CT: computed tomography.
Table 3 shows that lesions other than HCC are not uncommon when only APHE lesions are small or when the diagnosis is made solely on the basis of hypervascularization in the arterial phase.
Lesions in liver cirrhosis with arterial phase hyperenhancement only
| Author | Method, imaging | Number of patients Size of lesion | HCC | Non-HCC |
|---|---|---|---|---|
| Holland et al.[45] | MRI and liver transplantation, exclusively hyperenhancement in the arterial phase | 16 patients with APHE- only, 45 lesions < 20 mm | 7% | 93% benign (no correlative findings, regenerative nodules, arterioportal shunts) |
| Jang et al.[46] | CEUS and histology Arterial phase vascular intensity without hemangioma pattern | 59 patients 10–20 mm | 44% | 56% benign (20 regenerative and dysplastic nodules, 11 hemangiomas, two focal fat sparing) |
HCC: hepatocellular carcinoma; CEUS: contrast-enhanced ultrasonography; APHE: arterial phase hyperenhancement; MRI: magnetic resonance imaging.
Benign liver lesions
Cysts
In an autopsy study without liver cirrhosis, the prevalence of liver cysts was 15%.[52,53] An MRI study revealed no significant differences between the frequency of liver cysts in cirrhotic and noncirrhotic livers (17.5% and 13.8%, respectively) (Table 4).[54]
Cysts in liver cirrhosis: Similarities and differences with the noncirrhotic liver
| Common features with cysts in noncirrhotic liver | Special features in the cirrhotic liver |
|---|---|
| No significant differences between the frequencies[54] | Large cysts are rare[21] |
| Small liver cysts occur with similar rates[21] | Peribiliary cysts (retention cysts of the peribiliary glands; the accumulation of fibrosis encases the glands, leading to an increase in size)[21,55] |
Dodd et al.’s[21] autopsy study of 508 liver cirrhosis explants discovered that small liver cysts occur with similar rates in healthy and cirrhotic livers. Large liver cysts are a rare finding in cirrhosis, and 13 out of 508 were peribiliary cysts.[21] Peribiliary cysts are retention cysts of the peribiliary glands, located in the periductal connective tissue. They typically present as small cysts on both sides of the portal vein, often with a hilar distribution, and can be confounded with dilated bile ducts; they, however, have no connection with biliary ducts (biliary cysts are one side of the portal vein). This asymptomatic condition has been reported under the labels “multiple cysts in the hepatic hilum”, “hepatic cysts of periductal origin”, and “multiple hepatic peribiliary cysts”,[56] The first description was given by Nakanuma et al.[57] in 1984. Cirrhosis and portal hypertension are the most frequently associated conditions with peribiliary cysts.[55] The frequency of peribiliary cysts in liver explants was 1%.[55]
Liver cysts are easy to diagnose on B-mode abdominal sonography, and usually, CEUS examination and other radiologic imaging are not necessary.
Hemangioma
Regarding the frequency of liver hemangiomas in liver cirrhosis, there are reports of a lower frequency[7,20,21] comparable to patients without liver cirrhosis.[54] In an autopsy study by Dodd et al.[21] hemangiomas were found in only 1.7% of all cirrhotic livers.[21] This is significantly lower than in non-selected autopsy studies without liver cirrhosis, which had a 20% incidence of hemangioma.[52,53] It appears that the process of cirrhosis (i.e., necrosis and fibrosis) obliterates existing hemangiomas.[21] Autopsy studies reveal that previous imaging (CT, MRI) had missed hemangiomas in liver cirrhosis.[21,58]
Hemangiomas in liver cirrhosis usually become smaller and gradually develop fibrosis. This process already begins with liver fibrosis.[59] Nevertheless, some controversial data exists here. Brancatelli et al.[58] reported hemangioma sizes up to 10.0 cm in the cirrhotic liver. In patients with liver disease, the hemangiomas were more often solitary.[60] In a retrospective CT follow-up of hemangiomas in cirrhotic liver, Brancatelli et al.[58] demonstrated a decrease in size in 44% of cases. Retraction of the liver capsule was discovered in 31% of the patients. Vernuccio et al.[61] demonstrated in a case example that the process of sclerosis generally starts at the center and then extends to the entire lesion. Fibrotic degeneration may lead to retraction of the capsule or concavity of the entire lesion. These changes can reduce the size of the hemangiomas. This may explain why the hemangiomas in liver cirrhosis are smaller compared to a healthy liver.[21,58,59,61] The sclerosing process leads to a reduced contrast uptake to varying degrees.
On ultrasonography in the healthy liver with normal echogenicity, hemangiomas present as smoothly circumscribed, mostly homogeneous, hyperechogenic lesions and do not require contrast-enhanced sonography.[9,19,62] For this reason, typical hemangiomas are number-wise underrepresented in CEUS studies for the evaluation of liver lesions.[7] Depending on the liver echogenicity, hemangiomas may also appear isoechogenic or hypoechogenic. This means that other differential diagnoses come into consideration. Forty-three percent of the hemangiomas showed feeding vessels in duplex sonography and 7% showed homogeneous hyperenhancement.[62]
A typical feature of hemangiomas in CEUS is a smooth contrast-receiving ring in the arterial phase with a peripheral, discontinuous nodular (syn.: globular) enhancement with progressive centripetal contrast (Figure 1). The fill-in in the late phase can be complete or incomplete. (Partially) thrombosed hemangiomas do not fill up completely (Figure 2).[9,19]
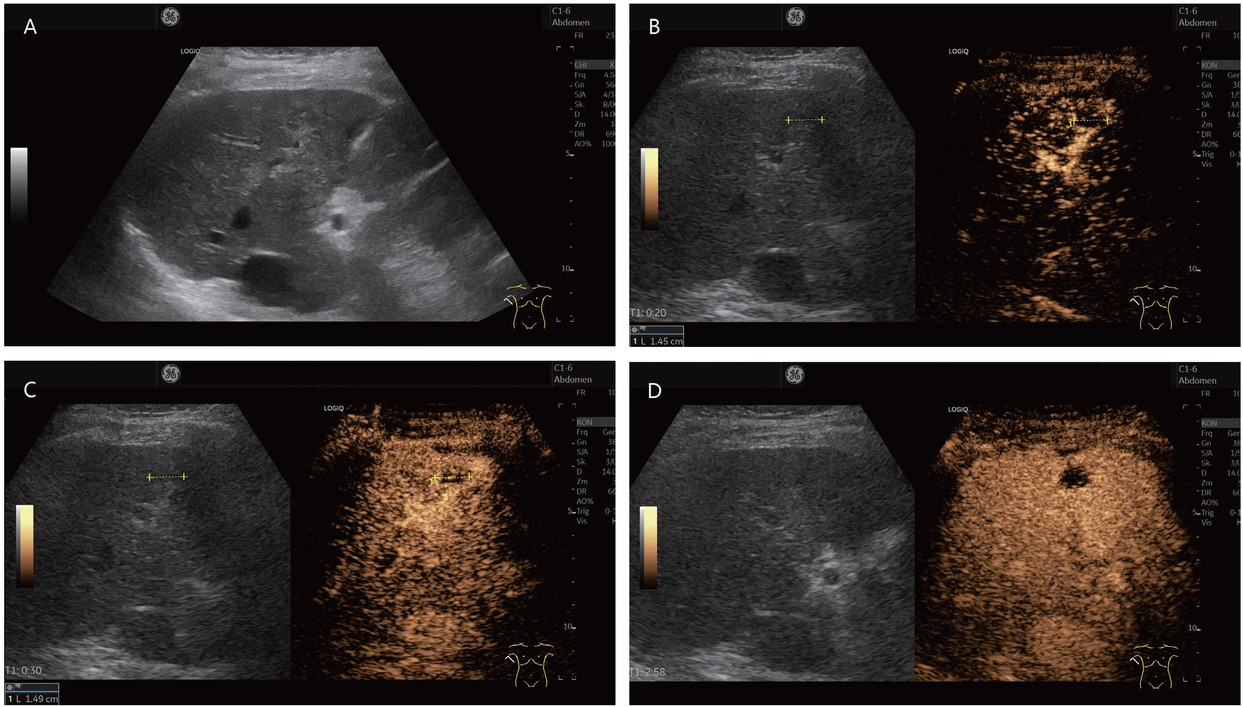
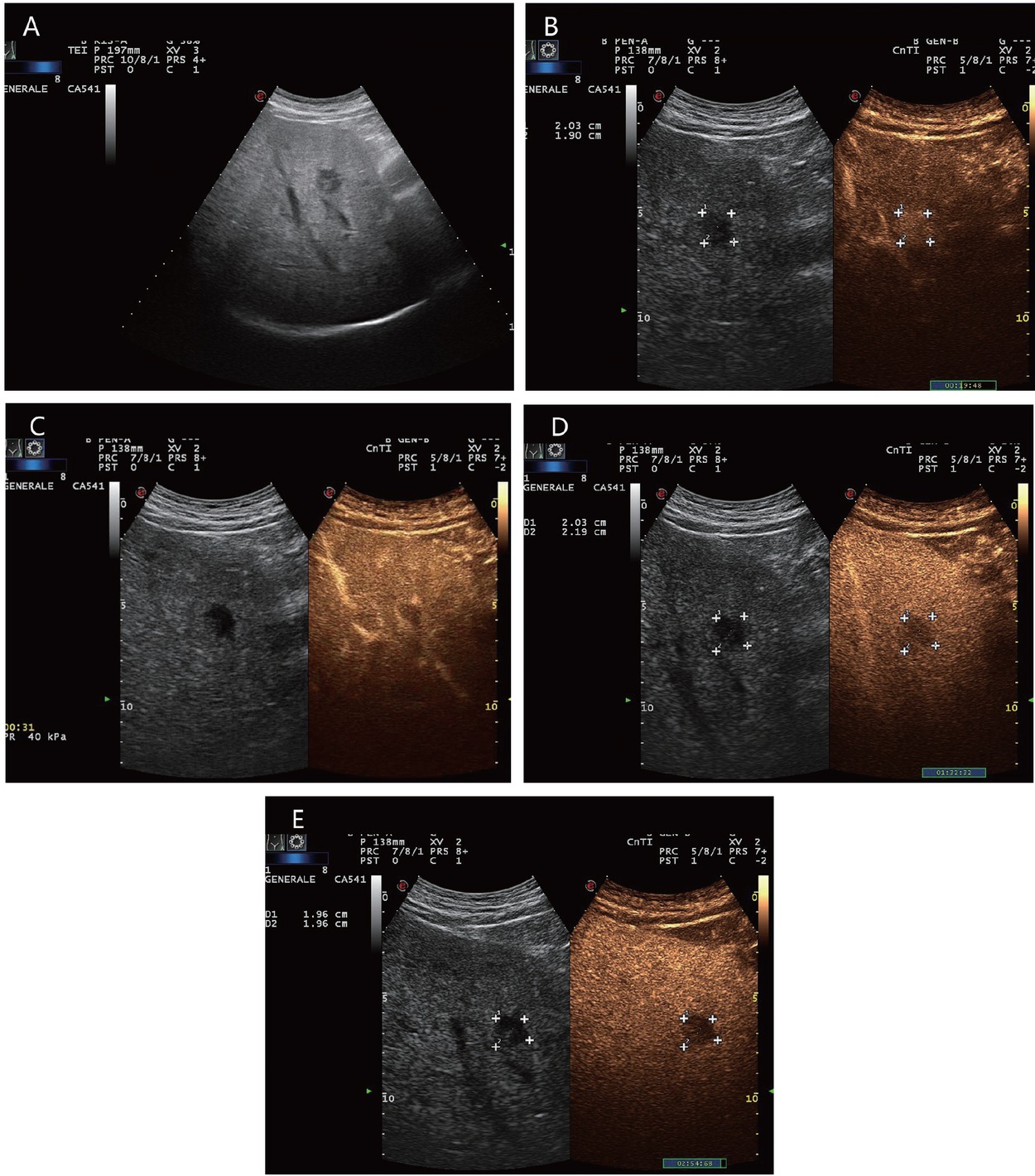
An 80-year-old female presented with thrombosed hemangioma (in between markers) and liver cirrhosis after hepatitis C infection. She had normal AFP and no previous known tumor disease. Hypoechoic inhomogeneous liver lesion with echogenic rim on B-mode ultrasonography (A). No enhancement in the arterial phase, only suggested rim (B). At the beginning of the portal venous phase, the lesion is not enhanced, except for a few vascular pixels. These are seen in the marginal area and cannot be clearly assigned for differential diagnosis (C). In the late CEUS phase, the lesion remains completely avascular. A retraction of the liver contour is seen above the lesion. On MRI, the lesion was assigned as a thrombosed hemangioma. Thrombosed hemangioma with capsular retraction is a finding that has been described on MRI for hemangiomas in liver cirrhosis. Capsular retraction can also be seen in tumors (D). CEUS: contrast-enhanced ultrasonography; MRI: magnetic resonance imaging; AFP: alpha-fetoprotein.
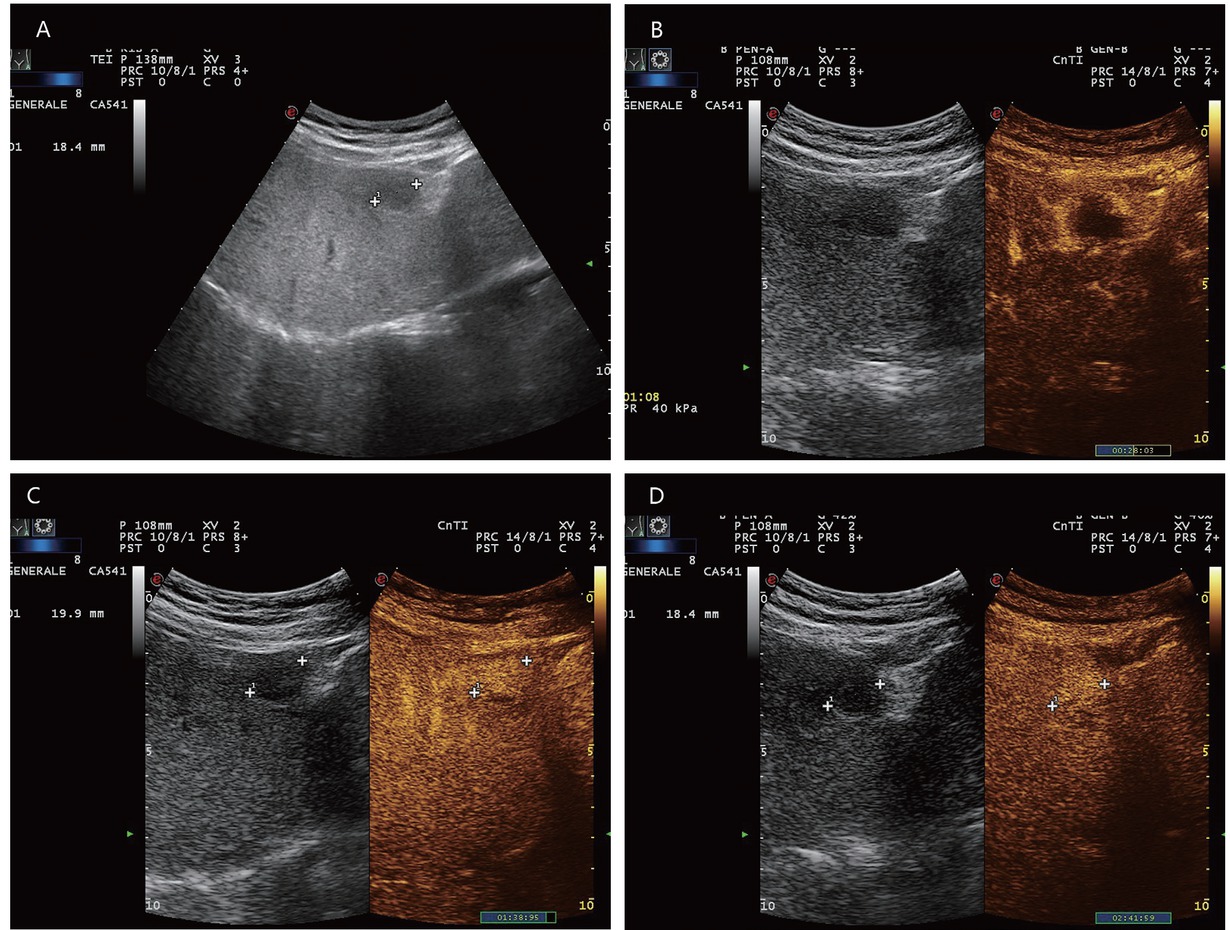
A 65-year-old male with diabetes and past history of obesity. Hemangioma (in between markers) on the background of steatosis hepatis with severe fibrosis at shear wave elastography (9.5 kPa). Hypoechoic liver lesion in B-mode ultrasonography (A). On CEUS, smooth annular enhancement in the arterial phase (B). Smooth contrast medium lake in the marginal area in the portal venous phase (C). Complete centripetal filling in the late phase (D). Here typical hemangioma. CEUS provides the exact differential diagnostic assignment of a hypoechoic liver lesion in liver cirrhosis and makes further contrast-enhanced radiologic diagnostics unnecessary. CEUS: contrast-enhanced ultrasonography.
Atypical CEUS features show small hemangiomas (< 15 mm) and larger hemangiomas. Hemangiomas below 10 mm, in particular, tend to lack the typical hemangioma contrast enhancement and are a challenge.[62] Small lesions, in particular, can fill up very fast and completely in the arterial phase. Hemangiomas with arteriovenous shunts (high-flow or shunt hemangiomas) show a very rapid homogeneous hyperenhancement in the arterial phase and sometimes do not reveal the annular centripetal flow pattern. Here, differential diagnosis with other completely hyperenhanced lesions in the arterial phase is difficult (FNH, adenomas, HCCs, some CCCs and some metastases).[9,19] A possible explanation for the rapid enhancement of small hemangiomas is a hyperdynamic status with large arterial inflow, rapid tumor enhancement, and consequently large and rapid outflow.[62]
But mostly these shunt hemangiomas are hyperenhanced in the portal venous and late phase, which is considered a benign criterion in the healthy liver.[29] Some hemangiomas are hypoenhanced in the late phase (especially when located superficially near the transducer head or after continuous insonation).[63] This makes it difficult to distinguish them from malignant liver lesions. Regressively altered hemangiomas may be completely sclerosed and are smoothly irradiated, but remain avascular and do not fill up at all. It is to be expected that the regressive changes that hepatic hemangiomas undergo in liver cirrhosis and the specific features of vascularization in liver cirrhosis are also reflected in the CEUS appearance of hemangiomas in liver cirrhosis. Since every arterially hypervascularized hepatic lesion in liver cirrhosis is suspicious for HCC and not every HCC shows hypoenhancement in the late phase, difficult differential diagnostic challenges can arise, especially in the case of shunt hemangiomas. The coincidence of HCC and hemangiomas in liver cirrhosis has been described.[62] Also, in patients with liver cirrhosis, 13% (2/15) hemangiomas corresponded to shunt hemangiomas.[62] The correct diagnosis of each individual focal lesion in cirrhosis is important for further therapeutic decisions. Misdiagnosis of a hemangioma as HCC can misinterpret a curative therapeutic option into a supposedly palliative situation. D’Onofrio et al.[23] studied 36 hemangiomas in liver cirrhosis, with a range of 0.6–3.5 cm. On B-mode ultrasound examination, 32 were hyperechoic, one was hyperechoic with a peripheral hypoechoic halo, two were hypoechoic, and one was hypoechoic with a peripheral hyperechoic halo. In Doppler ultrasound (US), all the hemangiomas displayed peripheral or intranodular venous vessels. Furthermore, in CEUS imaging, all the cases exhibited peripheral globular enhancement with centripetal fill-in in the arterial phase; in the late phase, two appeared isoechoic and 34 appeared homogeneously hyperechoic.[23]
In the study by Jang et al.[46] 59 patients were monitored for HCC. In this study, 23% of all nodules with arterial hyperenhancement corresponded to hemangiomas on CEUS. All of them showed peripheral nodular enhancement and progressive fill-in.[44] In these studies, hemangiomas in liver cirrhosis in the arterial phase showed typical contrast behaviors for diagnosis. Diagnostic difficulties were not evident here.
In the study by Terzi et al.[8] of 1006 nodules in cirrhosis, a few high-flow hemangiomas fell in the LR-4 category. The authors concluded that this finding is not worrisome not only for its minimal numeric impact (< 1% of all lesions), but also mostly for the fact that MRI is highly accurate in establishing the diagnosis of hemangioma.[8] However, there are also a few experiences to the contrary. Smaller, rapidly enhancing hemangiomas appear as uniformly enhancing lesions in the arterial phase, and therefore, it can be difficult to differentiate them from small HCCs simply based on the contrast patterns, especially in the MRI performed with hepatobiliary contrast agents such as gadoxetate disodium. On images obtained with this agent, hemangiomas can exhibit pseudo washout in the transitional phase: the lesion appears hypointense relative to the surrounding parenchyma owing to the rapid uptake of gadoxetate disodium by the background parenchyma. This pseudo washout phenomenon is more gradual than true washout in malignant tumors.[64,65] Therefore, for the differential diagnosis, the pattern in the T2 nonenhanced phases is highly relevant. Brancatelli et al.[58] reported in the follow-up of hemangiomas in liver cirrhosis the loss of some typical features, such as nodular peripheral enhancement and isoattenuation to blood vessels.[58] Vernuccio et al.[61] reported that the fibrotic degeneration may lead to peripheral capsular retraction or concavity over the lesion and loss of the typical imaging features of hemangiomas, including T2 hyperintensity, nodular peripheral enhancement with centripetal filling, and the enhancement parallel to blood vessels, and that central fibrotic degeneration might result in central hypointensity in T2-weighted images and a lack of T2 shine-through compared to lesions occurring in healthy or mildly fibrotic livers. Unfortunately, there are no comparative statements on the CEUS patterns in hemangiomas not showing a typical appearance on MRI. Sclerosed hemangioma may appear as a hypoenhancing lesion or display arterial phase rim hyperenhancement. The decreased enhancement in the dynamic study correlates with the histologic degree of sclerosis. Galia et al.[66] noted that fibrotic hemangiomas are usually irregular in shape. Duran et al.[59] observed a smaller T2 shine-through effect for hemangiomas in liver cirrhosis with MRI, and this effect is less common in flash-filling hemangiomas than in other types. All other MRI parameters were similar compared to noncirrhotic patients. Hemangiomas are rarely surrounded by fibrotic tissue distorting the liver, paradoxically appearing hypovascular. To differentiate them from other lesions, especially hypovascular HCC, one should remember that these hemangiomas commonly exhibit irregular margins and marked hypoattenuation compared to the surrounding liver in noncontrast and postcontrast images. Of course, marked signal hyperintensity in T2-weighted MRI remains helpful.[59,67] The changes in hemangiomas in the cirrhotic liver are shown in Table 5.
Features of hemangiomas in liver cirrhosis
| Pathogenetic processes | Process of fibrosis and cirrhosis obliterates existing hemangiomas Fibrosis and sclerosis of hemangiomas[21] |
|---|---|
| Morphological changes of | Lower frequency in liver cirrhosis[7,20,21] |
| hemangiomas | Decrease in size[21,58,59,61] |
| Retraction of liver capsule[58] | |
| Concavity[61,66] | |
| Imaging changes and problems | Missed hemangiomas on imaging[21,58] |
| Difficult demarcation in the inhomogeneous, nodular, altered cirrhosis on ultrasound | |
| Decreased enhancement due to sclerosis | |
| Loss of some typical features in the follow-up (nodular peripheral enhancement, centripetal filling)[58] | |
| Loss of T2 hyperintensity, central hypointensity in T2-weighted images, lack of T2 shine-through[61] | |
| Similarities to the noncirrhotic liver | Typical CEUS enhancement of hemangiomas in liver cirrhosis[23,44] |
| Special differential diagnostic problems | Shunt hemangiomas with isoenhancement in the portal venous and late phase on CEUS[8] |
CEUS: contrast-enhanced ultrasonography.
FNH and FNH-like nodules
The FNH in the normal liver and the FNH-like nodule which only develops in the cirrhotic liver are morphologically identical. Whether it is an FNH or an FNH-like lesion in liver cirrhosis cannot be distinguished on imaging. FNH-like nodules are focal lesions occurring in liver cirrhosis and are morphologically and immunohistochemically very similar to classical FNH in an otherwise normal liver.[68, 69, 70, 71] Histologic analyses revealed that the FNH-like nodules showed many unpaired arteries with thick-walled blood vessels, and this resulted in hypervascular enhancement that mimicked HCC on contrast-enhanced CT.[69]
However, it is striking that FNH is diagnosed much less frequently on imaging in liver cirrhosis than in noncirrhotic liver. From this, it can be assumed that FNH also undergoes a morphological change in the cirrhotic liver or is more difficult to identify. In two autopsy studies[20,21] and the German multicenter study,[7] no cases of FNH in liver cirrhosis were described. This entity is the exception in liver cirrhosis and in the mirror of so far published papers, practically irrelevant. But FNH-like lesions should not result in the misdiagnosis of HCC. The risk of a false-negative diagnosis is more relevant than that of a false-positive diagnosis of HCC. However, the concern of this review is to consider the changes that non-HCC lesions undergo in the process of liver cirrhosis. Therefore, FNH and FNH-like lesions are also discussed and examples shown.
FNH-like nodules were diagnosed in 15% of 130 explanted cirrhotic livers. Of them, 75% were smaller than 10 mm.[71] It was hypothesized that the FNH-like nodules arise as a local hyperplastic response to vascular alterations in cirrhosis. The presence of esophageal varices and pretransplant treatment with chemoembolization were independently and significantly associated with the presence of FNH-like nodules.[71] Multiple FNH-like nodules were seen in 37% of cases.[71] No associations were found between FNH-like nodules, on the one hand, and low-grade DNs, high-grade DNs, and HCCs, on the other hand.
In the DEGUM multicenter study with > 1300 patients, 6.4% of FNH showed hypoenhancement in the late phase. This study also included patients with liver cirrhosis. It did not further differentiate which patients with FNH and hypoenhancement in the late phase were involved.[72] When FNH in liver cirrhosis in the arterial phase does not show the wheel spoke pattern and centrifugal enhancement flow pattern and the lesion is iso- or even hypoenhanced in the late phase, differentiation from HCC can be very difficult.
In the report by D’Onofrio et al.[23] among 128 patients with liver cirrhosis and 207 focal lesions, 101 were benign lesions and eight of them were FNH (mean diameter 3.45 ± 1.11 cm; range 2.5–6.0 cm). On B-modeultrasonography, these appeared hypoechoic in six cases, slightly and heterogeneously hyperechoic in one case, and heterogeneous in the remaining one case. On Doppler US, six displayed peripheral and intranodular arterial vessels. In CEUS, seven lesions enhanced during the arterial phase. In the late phase, six lesions appeared isoechoic and two appeared hypoechoic. The two hypoechoic lesions were properly characterized via MRI with a liver-specific contrast agent.
FNH-like nodules are rare and small.[71] Some of them were detected by radiologic imaging and misclassified as HCC, as described in the studies by Quaglia[73] and Libbrecht.[71] In both studies, only larger FNH-like nodules were detected.[71,73] Lee et al.[69] described that FNH-like nodules showed hypervascular enhancement in the arterial phase of the contrast-enhanced CT images, and this was followed by a washout pattern in the delayed phase, which is a feature generally considered to be highly suggestive of HCC in liver cirrhosis. They appeared as high-signal-intensity masses on SPIO-enhanced MRI. In the study by Choi et al.[74] 33% (3/9) FNH-like lesions in liver cirrhosis or Budd Chiari syndrome were misinterpreted as HCC by radiologic imaging (CT, MRI). All of them showed nodules > 1 cm in diameter with arterial enhancement and portal/delayed washout on dynamic CT.[74] Among 62 patients with FNH-like lesion or FNH who underwent percutaneous needle biopsy, four patients (6.5%) were misdiagnosed as having HCC and two patients (3.2%) had inconclusive Results by a first needle biopsy.[74] Histologic differentiation of FNH or FNH-like lesion from well-differentiated HCC by needle biopsy may be difficult due to a modest increase in cell density with an irregular trabecular pattern and unpaired arteries and a diffuse capillarization of the sinusoids.[71,74] Loh et al.[75] described an FNH-like lesion in a cirrhotic liver with radiologic features on CT and MRI that mimicked an intrahepatic cholangiocarcinoma (ICC) (Figure 3).[75]
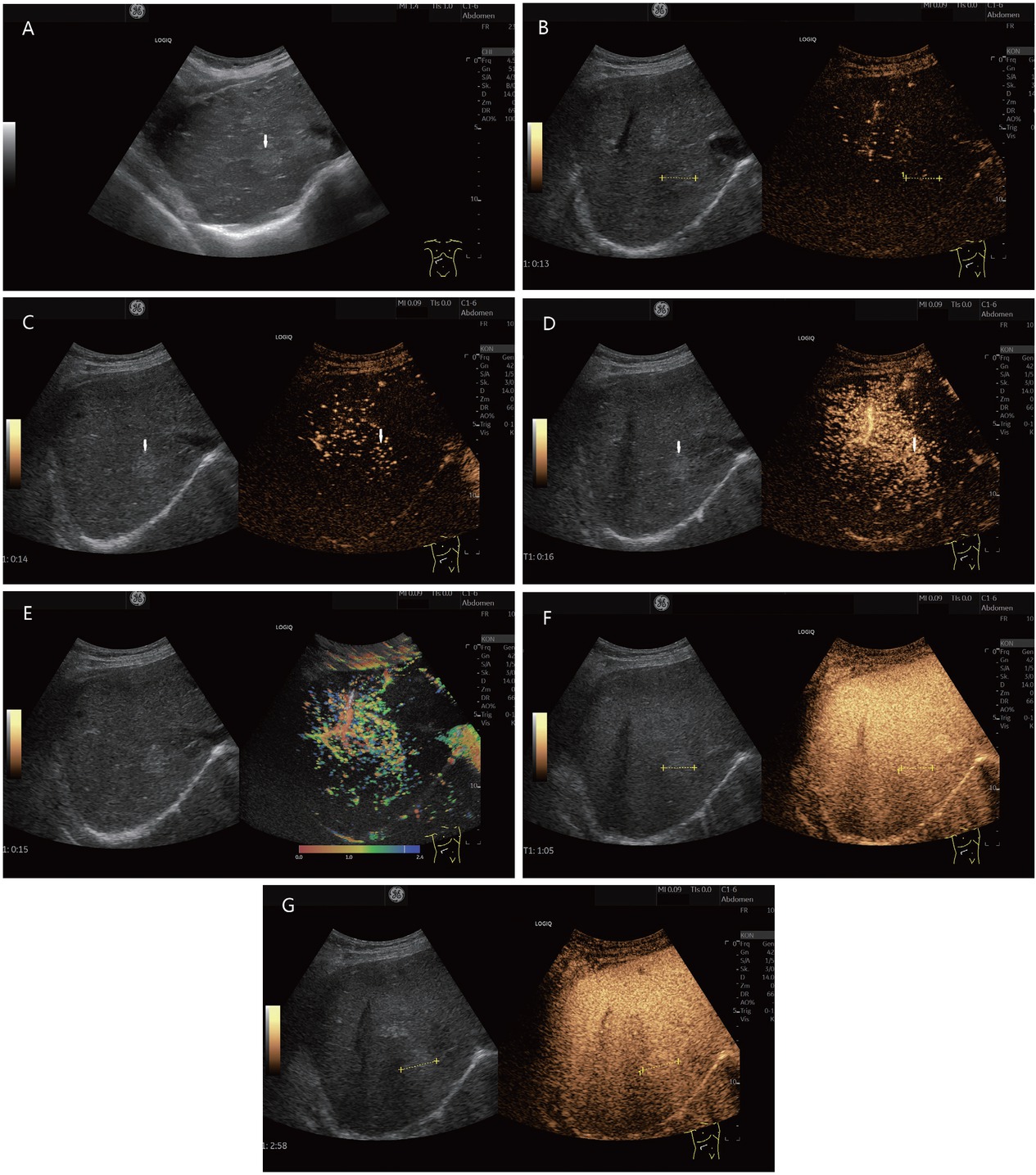
FNH or FNH-like lesion (histologically proven). In a 78-year-old female, ascites was diagnosed during gynecological examination. Further diagnosis revealed cirrhosis cardiac with a liver lesion. A 22-mm, slightly hyperechoic lesion was located in the right liver lobe adjacent to the inferior vena cava (in between markers and shown by arrows). This is shown on B-mode ultrasonography (A). A central vessel is visible on CEUS at the beginning of the arterial phase. The lesion is marked in the dual measurement (B). In the arterial phase, an evenly distributed contrast uptake is seen in the further course (C). The lesion is homogeneously enhanced. Increased contrast is conspicuous adjacent to the caudal part of the liver. Here, the contrast of a strong arterial vessel is visible (D). These phenomena are also seen in parametric imaging (E). In the portal venous phase (F) and late phase (G), the lesion remains hyperenhanced to the surrounding liver parenchyma. This was considered benign. On MRI, the lesion could not be clearly classified. Due to the hyperenhancement in the arterial phase and the diffusion disturbance in the MRI, the lesion remained suspicious. A sonographic guide biopsy was performed. The histology described an FNH. Follow-up over 6 months showed no change in findings. CEUS: contrast-enhanced ultrasonography; MRI: magnetic resonance imaging; FNH: Focal nodular hyperplasia.
It is possible that with increasingly sensitive high-resolution modern imaging, more of these small FNH-like lesions could be detected in liver cirrhosis, but then also, they would be difficult to be differentiated (Figure 4).[69,71,73] As some FNH lesions (2/8, 25%) in cirrhotic liver in the study of D’Onofrio et al.[23] also showed hypoenhancement in the late phase on CEUS, differential diagnosis with HCC is very difficult in these lesions.[23] Also, in radiologic imaging with CT and MRI, 33% of FNH/FNH-like lesions in liver cirrhosis were misinterpreted as HCC.[74] One should consider the possibility of an FNH-like nodule and, in case of doubt, perform histologic confirmation. However, as the data of Choi et al.[74] show, histology by needle aspiration also does not provide definitive confirmation in all cases.[74] Table 6 summarizes the specifics of FNH.
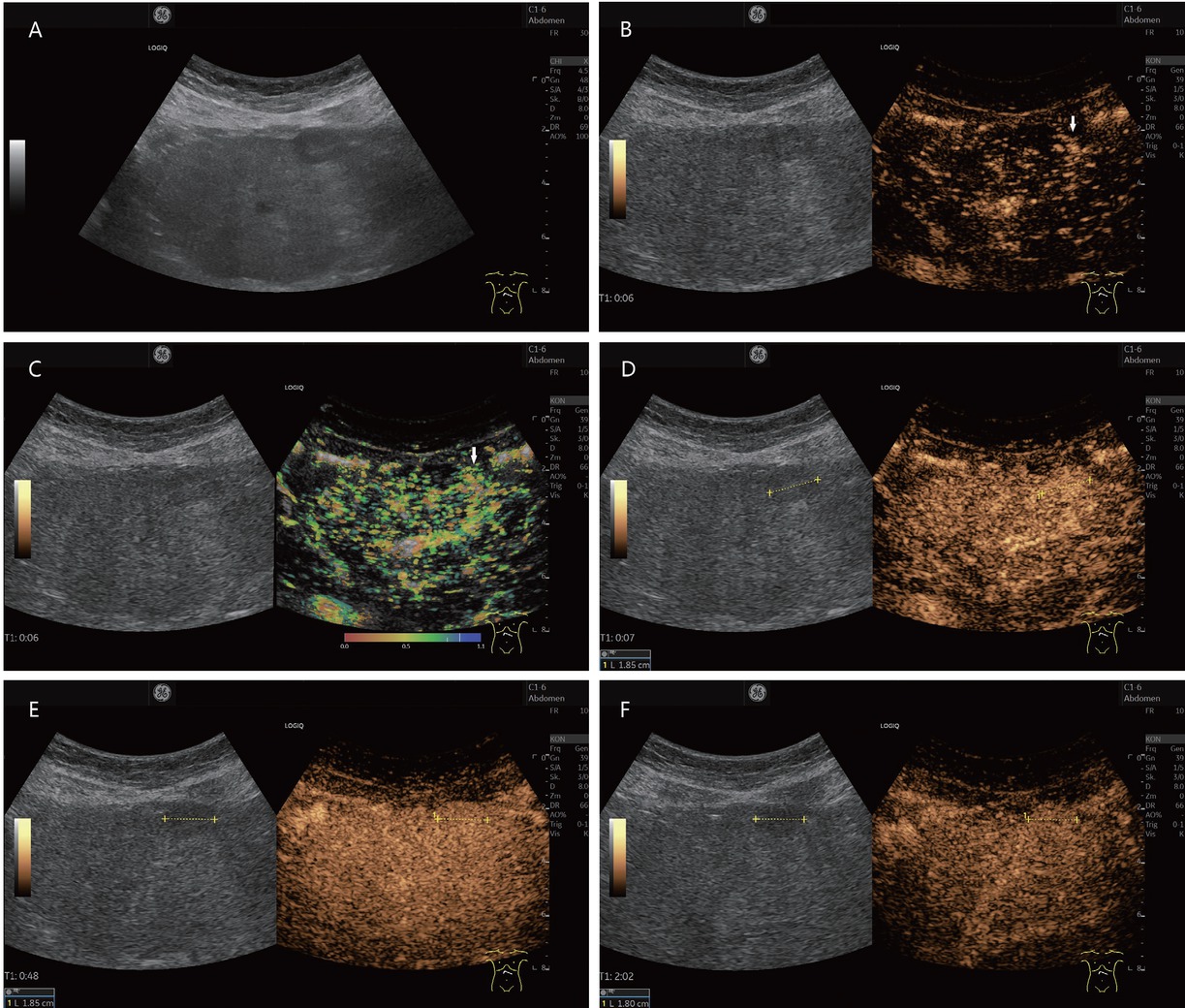
A 38-year-old female presented with suspected FNH-like nodule, alcohol toxic cirrhosis (in between markers and shown by arrows). She had a history of continued alcohol abuse and had untreated hepatitis B and hepatitis C. She had undergone repeated hospitalizations with ascitic decompensation. Newly diagnosed hepatic lesion not present 6 months previously on B-mode ultrasonography (A). In CEUS, wheel spoke enhancement early arterial (B), corresponding temporal mapping also in parametric imaging (C). The lesion was enhanced in the arterial phase (D), isoenhanced or slightly hyperenhanced in the portal venous phase (E), and slightly hyperenhanced in the late phase (F). The lesion also remained iso- to slightly hyperenhanced in the further course of examination until 3 min p.i. MRI also showed an APHE hepatic lesion. The wheel spoke enhancement and persistent hyperenhancement on CEUS were suggestive of FNH. However, a corresponding lesion was not pre-documented. Therefore, sonographically assisted biopsy with 4× percutaneous access was performed. This did not result in a definitive diagnosis – no HCC, but also no FNH. The CEUS findings were compatible with an FNH. Since the lesion was not known before, it could be in the overall context with an FNH-like nodule. The contrasting course in the CEUS argues against a regenerated node. On further hospitalization with ascitic decompensation, the lesion remained unchanged and the AFP was normal even after 6 months. FNH: focal nodular hyperplasia; CEUS: contrast-enhanced ultrasonography; APHE: arterial phase hyperenhancement; HCC: hepatocellular carcinoma; MRI: magnetic resonance imaging; AFP: alpha-fetoprotein.
Peculiarities of FNH and FNH-like nodules in liver cirrhosis
| Peculiarities | Descriptions |
|---|---|
| Peculiarities of FNH in liver cirrhosis compared to noncirrhotic liver | FNH is very rare in comparison to noncirrhotic liver;[7,8,20,21] FNH-like nodules arise as a response to vascular alterations in cirrhosis[71] |
| Differential diagnostic problems on imaging compared to HCC | Arterial hyperenhancement with iso- and/or hypoenhancement in the late phase (75% respectively 25%);[23] Hypervascular enhancement with washout in the delayed phase on CE-CT[69,74] |
FNH: focal nodular hyperplasia; HCC: hepatocellular carcinoma; MRI: magnetic resonance imaging; CEUS: contrast-enhanced ultrasonography.
Hepatocellular adenoma
HCA is a tumor that is rarely diagnosed in the noncirrhotic liver. Four subtypes are distinguished:[76] inflammatory HCA (30%–50% of all HCAs), hepatocyte nuclear factor 1 alpha (HNF1α)-inactivated HCA (approx. 40% of cases), β-catenin-mutated HCA (5%–10% of cases), and unclassified HCA (5%–10% of cases). The β-catenin-mutated HCA has an increased risk of malignant transformation, and the HCA is > 5 cm in males. In the German multicenter study, HCA was diagnosed in one of the 216 patients with liver cirrhosis (0.3%).[7] In an autopsy study with 596 cases of liver cirrhosis, 43.6% had a liver lesion, among which 1.5% were adenomas, surprisingly more than hemangiomas (1.2%).[20]
In CEUS, the typical behavior of HCA includes rapid centripetal filling in the arterial phase and persistent enhancement in the portal and delayed phases. None of the filling patterns in the arterial phase is specific to HCA, and they may also be observed in HCC or in hypervascularized metastasis. In the portal venous phase, they may show slight hypoenhancement, resulting in a difficult differential diagnosis from HCCs, whereas hypoenhancement in HCC
typically starts after 60 s.[9,19,27,28,77] However, depending on the subtype, the behavior of HCA in the portal venous and late phases may differ.[78] Inflammatory HCA showed arterial hyperenhancement with centripetal filling, peripheral rims with persistent enhancement, and central washout in the late venous phase (sensitivity 64%; specificity 100%). In HNF1α-inactivated HCA, isoenhancement or moderate hyperenhancement with mixed filling in the arterial phase and isoenhancement in the portal and late portal venous phases were detected. Homogeneous hyperechogenicity on B-mode ultrasonography was the most specific pattern (sensitivity 88%; specificity 91%) and correlated with diffuse fat distribution on MR images. In the unclassified and β-catenin-activated HCA, CEUS showed features of benign hepatocellular tumors without specific features.[78]
On MRI, HCAs are hyperintense or isointense on precontrast T2-weighted images and isointense or hypointense on T1-weighted images. HCA shows arterial enhancement and washout in the portal venous phase and hypointensity in the hepatobiliary phase.[79]
There are few case reports of HCA in the cirrhotic liver, including a patient with liver cirrhosis in hepatitis B[79] and another with glycogen storage disease IV.[80]
Seo et al.[79] reported an FLL in hepatitis B that appeared on MRI as a benign hepatocellular lesion such as FNH. Because of an increase in size in the follow-up, an ultrasound-guided biopsy was performed. Histologic findings revealed a benign hepatocellular nodule indicative of HCA. In addition, imaging showed liver cirrhosis. Alpha-fetoprotein (AFP) was normal. On follow-up, surgical resection was performed due to increase in size and a “nodule-in nodule” appearance on CT image. The histology revealed an HCC. This case demonstrates the difficulty of diagnosing HCA on imaging, especially in liver cirrhosis.[79]
Calderaro et al.[81] described two patients with liver cirrhosis caused by metabolic syndrome and alcohol use (patient 1: female, alcohol intake, obesity [body mass index {BMI} = 30], type 2 diabetes; patient 2: male, alcohol intake, obesity [BMI = 30], and inflammatory HCA) and one patient with metabolic syndrome without alcohol use (patient 3: male, obesity [BMI = 37], type 2 diabetes) with severe steatosis hepatis/histologically liver cirrhosis and multiple inflammatory HCAs. Microscopic examination initially diagnosed FNH in patient 1 and an RN in patient 2. Only complete immunohistochemical staining and molecular analysis confirmed the diagnosis of inflammatory HCA. The authors concluded that if HCA is known to arise in nonfibrotic, noncirrhotic liver, their observations showed that inflammatory adenoma and inflammatory liver adenomatosis might also rarely develop in the setting of chronic liver disease and cirrhosis. Interestingly, all three patients had chronic liver disease related to well-established risk factors for inflammatory adenoma development in patients without fibrotic livers, namely, metabolic syndrome and alcohol intake.[81]
HCAs are usually homogeneously hyperenhanced on CEUS in the arterial phase. They may show slight hypoenhancement in the portal venous phase.[9,19] Whether HCAs in liver cirrhosis show a different appearance in CEUS is unclear. This makes the differential diagnosis to HCC in liver cirrhosis difficult. Moreover, as the case report of Calderaro et al.[81] showed, needle biopsy is also not reliable in diagnosing HCA.
The challenge in differential diagnosis for liver cirrhosis is primarily with HCC. According to data from Seitz et al.[7] the ratio of HCA to HCC in liver cirrhosis is 1:216. The incidence of HCA in liver cirrhosis is many times lower than HCC.
Malignant Lesions
Metastases
There are a large number of autopsy studies[82, 83, 84, 85, 86] and meta-analyses of autopsy studies[86, 87, 88] showing that metastases of extrahepatic tumors are rarer in liver cirrhosis than in noncirrhotic livers. In a recent meta-analysis with biopsies, excisions, and autopsies from 1453 cirrhotic livers with liver masses, only 1.7% were metastases.[88] This applies to metastases of colorectal carcinomas as well as other tumors.
Several possible causes for the lower frequency of metastases in liver cirrhosis are discussed. A cirrhotic liver does not represent a breeding ground for the development of tumor cells because fibrosis and distortion of small hepatic capillaries, which occur in cirrhosis, known as sinusoid capillarization, represent a mechanical obstacle to the establishing of tumors. Portal venous flow is reduced by portal hypertension, and tumor seeding is impeded. Hepatofugal flow impedes the spread of metastasis to the liver. Other factors are Kupffer cell activation, increased concentrations of metalloproteinase inhibitors, and dysfunction of the lectins.[88, 89, 90]
In the noncirrhotic liver, metastases can have a variable and unpredictable appearance in native B-mode ultrasonography: not only hyperechoic and isoechoic, but also hypoechoic lesions, different types of boundaries, a hypoechoic rim (halo sign). The lesion may contain necrotic avascular anechoic or hypoechoic areas. How metastases are to be differentiated in the cirrhotic liver depends on the degree of alteration and the RNs present, but in general, B-mode gray-scale US is often insufficient in small lesions to achieve a diagnosis of metastasis or even of malignancy. A typical feature in CEUS is hypoenhancement from the early portal venous to the late phase. Most metastases show early hypoenhancement from the portal venous phase. In the arterial phase, rim enhancement with irregular vessels is a typical feature. The extent of arterial contrast depends, however, on the primary tumor. For example, metastases of neuroendocrine carcinomas may be arterially hyperenhanced and show hypoenhancement only late. This Results in diagnostic challenges with HCC. If hypoechoic lesions in a cirrhotic liver show a washout very early in the portal venous phase, metastases should be included in the differential diagnostic considerations,[9,19,72] following what has been described here just above.
Metastases are classified as LR-M (Liver Imaging Reporting and Data System [LIRADS] malignant) in the CEUS-LIRADS algorithm.[12,14,40,91–93] In contrast to HCC, metastases often have a rim sign and usually an early washout in the portal venous phase.[9,19,72] In the study by Terzi et al.[8] with 848 patients with liver cirrhosis and 1006 nodules, 87% were malignant, but only 0.25% were metastases. These are classified as LM-R according to CEUS-LIRADS. The two metastases showed arterial phase rim enhancement or APHE and an early washout. The CEUS pattern LR-M showed few HCC, CCC, HCC/ CC, and lymphoma. The authors concluded that the LR-M class requires histologic confirmation (Figure 5).[8]
![Figure 5 Liver metastases in a liver cirrhosis (histologically confirmed [in between markers and shown by arrows]). A 78-year-old male presented with hepatitis B and C and a history of continued alcohol abuse and operated sigmoid carcinoma. He currently had a peripheral lung lesion. AFP was normal. Computed tomography demonstrated hypodense liver lesions. Sonographically, irregularly circumscribed hyperechogenic liver lesions are seen subdiaphragmatically in the right liver lobe in B-mode (A). Adjacent to this, the right posterior branch of the ramus principalis is dilated and filled with thrombi (B). There is also a small thrombus in the pars umbilicalis, which is hyperenhanced in the arterial phase in CEUS, and thus corresponds to a tumor thrombus (C). On CEUS, the hyperechogenic lesions in the right hepatic lobe are isoechogenic, slightly inhomogeneously enhanced in the arterial (D) and portal venous (E) phases. The adjacent thrombosed portal venous branch is hypoenhanced (F). In the late phase, the liver lesions are hypoenhanced (G). CEUS was used to perform ultrasonography-guided puncture of the liver lesions and the thrombosed portal vein branch. Histologically, a metastasis of the sigmoid carcinoma was found. There was no evidence of hepatocellular carcinoma. CEUS: contrast-enhanced ultrasonography; AFP: alpha-fetoprotein.](/document/doi/10.2478/jtim-2022-0068/asset/graphic/j_jtim-2022-0068_fig_005.jpg)
Liver metastases in a liver cirrhosis (histologically confirmed [in between markers and shown by arrows]). A 78-year-old male presented with hepatitis B and C and a history of continued alcohol abuse and operated sigmoid carcinoma. He currently had a peripheral lung lesion. AFP was normal. Computed tomography demonstrated hypodense liver lesions. Sonographically, irregularly circumscribed hyperechogenic liver lesions are seen subdiaphragmatically in the right liver lobe in B-mode (A). Adjacent to this, the right posterior branch of the ramus principalis is dilated and filled with thrombi (B). There is also a small thrombus in the pars umbilicalis, which is hyperenhanced in the arterial phase in CEUS, and thus corresponds to a tumor thrombus (C). On CEUS, the hyperechogenic lesions in the right hepatic lobe are isoechogenic, slightly inhomogeneously enhanced in the arterial (D) and portal venous (E) phases. The adjacent thrombosed portal venous branch is hypoenhanced (F). In the late phase, the liver lesions are hypoenhanced (G). CEUS was used to perform ultrasonography-guided puncture of the liver lesions and the thrombosed portal vein branch. Histologically, a metastasis of the sigmoid carcinoma was found. There was no evidence of hepatocellular carcinoma. CEUS: contrast-enhanced ultrasonography; AFP: alpha-fetoprotein.
The following conclusion could be drawn from this: if a solid lesion is detected in a cirrhotic liver, it is very likely to be HCC. However, this does not mean that patients with liver cirrhosis can never develop liver metastases.
Intrahepatic cholangiocarcinoma
CCC may be a mimicker of HCC in the setting of noninvasive diagnosis of HCC as both can show hypervascularity and washout on CEUS. After HCC, ICC is the second most common primary tumor in liver cirrhosis. The combined HCC/ ICC is also possible and can only be differentiated histologically. The most important differential diagnoses of CCC are HCC and liver metastases or all liver tumors presenting as LR-M because of the imaging characteristics.[12,14,40,91, 92, 93]
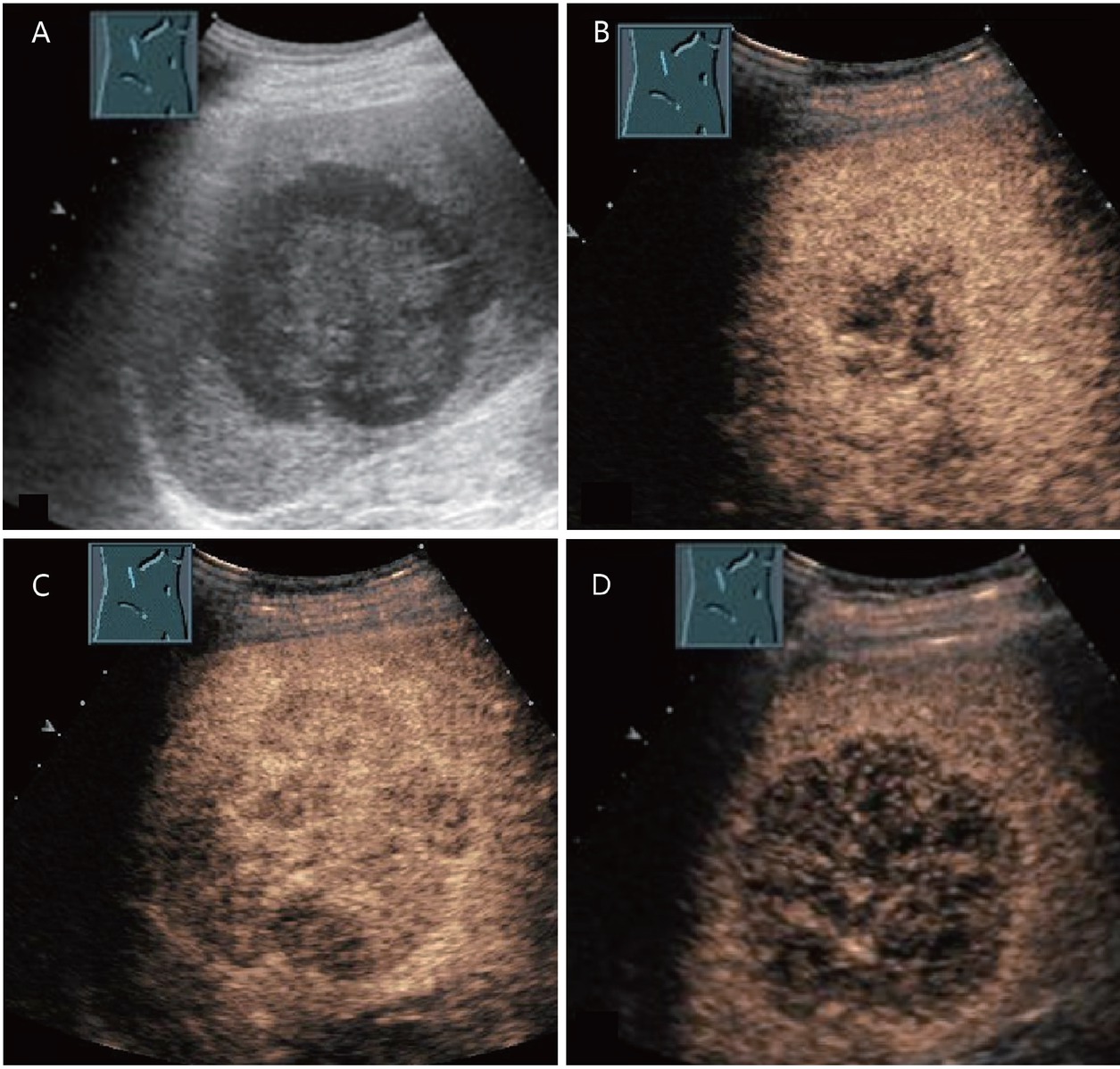
ICC can appear on CEUS with different enhancement patterns in the arterial phase: peripheral irregular rim-like enhancement, heterogeneous hyperenhancement, homogeneous hyperenhancement, and heterogeneous hypoenhancement.[9,19] ICC is characterized by washout in the early portal venous phase and marked hypoenhancement in the late phase (Figure 6).[9,19]
Histologically confirmed intrahepatic cholangiocellular carcinoma (in between markers) in a 60-year-old mildly obese male with metabolic liver cirrhosis and moderate alcohol consumption until detection of chronic liver disease. B-mode ultrasonography revealed a newly detected hypoechoic liver lesion (A). In CEUS, this showed complete arterial hyperenhancement in the arterial phase (19 s) and no rim sign (B). Already at the onset to the portal venous phase (31 s), the lesion had started to appear hypoenhanced (C). The progressive hypoenhancement continued in the portal venous and late phases (D and E). The early washout is atypical for HCC and suspicious for the presence of metastasis or intrahepatic cholangiocellular carcinoma. Therefore, histologic confirmation was performed, which diagnosed a CCC. CEUS: contrast-enhanced ultrasonography; CCC: cholangiocellular carcinoma; HCC: hepatocellular carcinoma.
On noncontrast CT, CCCs are usually seen as hypoattenuating focal liver masses with irregular margins. On MRI, CCCs are hypointense on T1-weighted images and moderately hyperintense on T2-weighted images, with the latter often associated with central hypointensity corresponding to areas of fibrosis. Typically, contrast-enhanced images show rim enhancement during the arterial phase, followed by progressive and concentric enhancement after extracellular contrast administration due to the presence of marked fibrous stroma.[67]
There has been a controversy on the use of CEUS because ICC can be misdiagnosed as HCC, and CEUS has been subsequently excluded from the diagnostic tests for HCC in the AASLD practice guidelines from 2011 onward. But CCC in liver cirrhosis is significantly rare compared to HCC. In the German multicenter study, 2.5% of patients with liver cirrhosis had CCC. However, the pattern of CEUS HCC diagnosis at that time (in the year 2011) did not adopt the refinements first introduced by the European Federation of Societies for Ultrasound in Medicine and Biology guidelines in 2013 and subsequently by the LIRADS system in 2017 even in the group without liver cirrhosis, CCC was rare (only 3.3%).[7] In other studies, only 1.1%[22] and 1.3%[24] of all newly detected nodules in liver cirrhosis were histologically confirmed as CCC. In the study by Terzi et al.[8] 4% of all nodules in liver cirrhosis corresponded to a CCC.[8] On CEUS, cholangiocarcinoma shows arterial hyperenhancement, often a rim sign, and a rapid washout in the portal venous phase. Unlike HCC, the onset of washout occurs early in CCC, usually before 1 min, and the degree of hypoenhancement in the venous phase is more marked in CCC than in HCC.[8,94–96] On CEUS, CCC shows the criteria of LR-M. Differential diagnoses are primarily metastases. In the study by Terzi et al.[8] the majority of CCC tended to segregate in the LR-M category, together with the mixed HCC-CCC tumors and metastases. Also, 12.5% (5/40) of the CCCs showed CEUS behavior of LR-3 and 10% (4/40 ) showed LR-4 behavior. The mean size was 1.6 cm in CEUS-LR-3 and 2.7 cm in LR-4; no case of misdiagnosis of LR-5 for CCC occurred.[8] Using the LR-M criteria to differentiate CCC from HCC, sensitivity, specificity, and accuracy were 97.3%, 87.7%, and 92.3%, respectively.[97] In the differentiation of ICC from LR-M HCC, the rim APHE is the most important feature. Rim APHE plus elevated CA19-9 and normal AFP are strong predictors of CCC rather than LR-M HCC. In differentiation of CCC and LR-M HCC, early washout and marked washout have limited value.[98]
For differential diagnostic reasons, histologic confirmation of any suspected malignant lesion without the typical features of HCC (corresponding to the LR5 pattern in cirrhosis) usually should be performed.
Lymphoma
Primary hepatic lymphoma (PHL) manifests in the liver and perihepatic lymph nodes without any involvement of other organs or leukemic changes in the peripheral blood for at least 6 months after diagnosis. PHL is rare, less than 1% of all non-Hodgkin’s lymphomas; most PHLs are B-cell lymphomas.[99] Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of PHL.[100] PHL accounts for 0.4% of extranodal NHL and 0.016% of all NHL.[100,101] PHL has also been described in liver cirrhosis.[100,101] Primary hepatic extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT) has been reported to be the second most common variant of lymphoma after DLBCL. Histologic liver assessment is required for the accurate diagnosis of primary hepatic extranodal marginal zone lymphoma of MALT.[102] Preexisting liver disease, which may be etiologically important for the development of nongastric hepatic MALT lymphoma, is, in turn, a risk factor for the development of HCC. In a secondary liver manifestation of lymphoma, other organ and lymph node manifestations are already present. More often, non-Hodgkin’s lymphoma affects the liver in advanced stages of a systemic disease. DLBCL is the most common lymphoma subtype in the western countries.[100]
Most often, solitary liver lesions are seen in PHL (60%), but multiple lesions are also possible.[100,101] In contrast, multiple liver lesions are seen in about 90% of cases in secondary lymphoma involvement of the liver.
On B-mode ultrasonography, lymphoma lesions may be hypoechoic or almost nonechoic like cysts. But a hyperechoic center with a hypoechoic rim is also possible.[99]
On CEUS, the lymphoma lesions show inhomogeneous hyperenhancement in the arterial phase and contrast agent washout in the portal and late phases.[103] Homogeneous hyperenhancement in the arterial phase followed by washout on CEUS is also described. Hypoenhancement on CEUS already occurs in the early portal venous phase.[102] Yamashita et al.[102] compared the appearance of hepatic extranodal marginal zone lymphoma of MALT from published case reports with HCC on CEUS, CT, MRI, and positron emission tomography (PET). The appearance is very similar, and a reliable differential diagnosis based on imaging is not possible.[102] However, the “vascular penetration sign” was described as a special characteristic. The hepatic lymphomas showed penetrating vessels in the arterial phase. This sign is sometimes also present in metastatic liver tumors and CCCs, but not in HCC.[102] In the study by Terzi et al.[8] 0.2% (2/1006) lesions in liver cirrhosis corresponded to lymphoma infiltrations (without specifying primary or secondary). These showed CEUS-LIRADS pattern of LR-M. Histologic assignment by ultrasound-guided needle biopsy is necessary, taking into account anamnestic data (Figure 7).[12]
Diffuse large cell non-Hodgkin’s lymphoma and alcoholic liver cirrhosis of Child–Pugh class B in a 74-year-old male. Large hypoechoic mass in segment 5/6 on B-mode ultrasonography (A). On contrast-enhanced ultrasound, the lesion showed an inhomogeneous, nearly isoechoic enhancement after 15 s (B), with hypoenhancement after 30 s (C) and 3 min (D). A biopsy of the lesion revealed a diagnosis of diffuse large cell non-Hodgkin’s lymphoma. After chemotherapy, a complete regression of the tumor was observed.
While the presentation of lymphomas on imaging is variable, they may be hyperenhanced on arterial phase images and hypointense on hepatobiliary MR phase due to lack of hepatocytes. They show signal restriction on high-b value diffusion-weighted imaging. Nevertheless, differentiation from HCC can be difficult.[67,99] Fu et al.[104] describe the misdiagnosis of HCC in primary hepatic MALT lymphoma and hepatitis B. Under antiviral therapy, newly diagnosed FLL of 11 and 5 mm were held as HCC on MRI and PET-CT. Laparoscopic resection of the left lateral lobe was performed. Postoperative histology diagnosed hepatic MALT lymphoma.[105]
Due to this lack of specific clinical, laboratory, and imaging features, definitive diagnosis of PHL requires liver biopsy compatible with lymphoma in the absence of extrahepatic disease.[100] The differential diagnosis of PHL includes HCC, especially in patients with preexisting hepatitis B or C infection and liver cirrhosis, which may be present in PHL. However, HCC is substantially more common than PHL. In the study by Terzi et al.[8] with 1006 FLL in liver cirrhosis, 82% HCC were compared to 0.2% lymphoma infiltration in the liver, although it is not known whether this was PHL or secondary lymphoma infiltration. The ratio of HCC:hepatic lymphoma is 410:1.[8] Both HCC and PHL may occur in patients who have cirrhosis with viral hepatitis.
The behavior of metastases, cholangiocarcinomas, and PHL in CEUS is summarized in Table 7.
CEUS features of metastases, cholangiocarcinoma, and primary hepatic lymphoma[8, 9, 10, 19, 102, 103]
| Phases on CEUS | Metastases | Cholangiocarcinoma | Primary hepatic lymphoma |
|---|---|---|---|
| Arterial | Rim enhancement, varying degrees of contrast intensity depending on the primary tumor and necrosis | Rim-like enhancement Heterogeneous hyperenhancement Homogeneous hyperenhancement Heterogeneous hypoenhancement | Mostly heterogeneous hyperenhancement “Vascular penetration sign” |
| Portal venous | Early hypoenhancement | Early washout in early portal venous phase | Early hypoenhancement |
| Late | Progredient hypoenhancement | Marked hypoenhancement | Hypoenhancement |
| CEUS-LIRADS | LR-M | LR-M | LR-M |
CEUS: contrast-enhanced ultrasonography; LIRADS: Liver Imaging Reporting and Data System. LR-M: LIRADS malignant.
Discussion
Newly discovered focal liver lesions in liver cirrhosis are primarily suspicious for HCC. Diagnosis is based on radiologic imaging with contrast-enhanced MRI and CT, but CEUS is also accepted by many, despite not all, scientific societies. Typical appearance of HCC is arterial hyperenhancement with hypoenhancement in the late phase. CEUS is used for unclear radiologic findings[26,42] as well as when CT and MRI are contraindicated or not available. The probability of HCC and of malignancy, in general, increases with the size of the nodule. The likelihood of HCC is low in FLL < 10 mm compared to nonmalignant lesions such as RN with or without dysplasia.[45] Although any newly diagnosed solid nodule in liver cirrhosis is primarily suspicious for HCC, small nodules or nodules that show only arterial hyperenhancement may still be nonmalignant. It is important to differentiate HCC from other arterially hypervascularized FLL such as hemangiomas, FNH or FNH-like lesion, HCA, metastases, CCC, and lymphoma. CEUS is excellent in the diagnosis of HCC, as well as in the differential diagnostic evaluation of all other liver lesions,[9,19] but usually histologic confirmation is required in all these instances to achieve definitive, apart from hemangiomas.
Data on the frequency of benign focal liver lesions in the noncirrhotic conditions vary widely. This, however, depends on the type of imaging and information from autopsy studies.[7,20,21,52,53,106]
Hemangiomas, in particular, undergo a morphological change in liver cirrhosis, where liver cirrhosis leads to obliteration of preexisting hemangiomas.[21] These changes start to occur early in the course of the chronic liver disease with liver fibrosis deposition, even before the development of liver cirrhosis,[59,107] and it is thought of as a dynamic process. Accordingly, hemangiomas in cirrhosis may show fibrotic changes, become smaller, and change shape.[58,59,61] In CEUS, the majority of hemangiomas showed a typical peripheral nodular enhancement.[23] Shunt hemangiomas, which are homogeneously enhanced in the arterial phase and show isoenhancement in the late phase, can be problematic in the differential diagnosis with HCC.[21] On CEUS, however, the majority of hemangiomas show a typical contrast course[23] comparable to hemangiomas in the noncirrhotic liver.[19,29]
FNH is diagnosed much less frequently in liver cirrhosis than in the normal population.[7,21] The development of FNH-like nodules, despite rare, is specific to liver cirrhosis; these are morphologically identical to FNH, but exist only in liver cirrhosis.[68, 69, 70, 71] They are often very small and only discovered on the liver explant.[71] The only significance of both types of liver lesions is in the differential diagnosis with HCC, especially if the typical wheel spoke-like vessel architecture cannot be differentiated in the early arterial phase and the lesions only appear arterially hyperenhanced. In patients with cirrhotic liver, the FNH tends to show isoenhancement in the late phase, but 25% show even hypoenhancement.[23] A biopsy is usually performed. False positives for HCC in the percutaneous biopsy were exceptionally reported, particularly in case of FNH-like lesions.[74]
HCA is a rare tumor in the healthy population.[7,52,53,106] Adenomas have also been exceptionally described in liver cirrhosis.[7,20,79–81] In the noncirrhotic liver, many HCAs show mild hypoenhancement in the portal venous and delayed phases.[17,19,29,77] Thus, if an adenoma in liver cirrhosis shows isoenhancement or a mild washout with CEUS in the portal venous and late phases, it cannot be reliably differentiated from an HCC. Further evaluation by means of MRI is required. These characteristics on CEUS have a threefold probability of an HCC rather than an adenoma on a background of a cirrhotic liver.[7] Thus, an HCC must be assumed until proven otherwise in this scenario.
Liver metastases in hepatic cirrhosis are rarer than in noncirrhotic livers in autopsy studies.[86, 87, 88, 108, 109] Nevertheless, patients with cirrhosis can also develop liver metastases. On CEUS, the metastases are classified as LR-M. Differentiation from HCC is usually not difficult,[12,14,40,91] but nonetheless, biopsy is usually required to achieve a specific diagnosis of the malignant cellular type.[110]
Intrahepatic CCC is similarly rare as in the noncirrhotic liver. It should be emphasized that CCC can be reliably differentiated from HCC by CEUS. The most important distinguishing feature from HCC is the rapid distinctive washout, occurring within 60 s from contrast injection in cholangiocarcinoma.[7,94,95,111]
Small lesions, in particular, can be a diagnostic challenge. In case of doubt, a biopsy is recommended by the EASL for all indeterminate lesions > 10 mm when various contrast-enhanced imaging modalities cannot assign the lesion.[26] In fact, HCC not reaching the typical imaging diagnostic criteria (i.e., CEUS-LIRADS LR5) does not have better prognosis than the typical ones.[112] Therefore, early diagnosis is not less important in these instances than in HCC with a typical enhancement pattern. However, it is also important to keep in mind that false-negative Results have been reported in up to 30%, especially in small lesions.[71,74] In these cases, a decision must be made in the overall context with the aid of all diagnostic possibilities for short-term follow-up or for therapeutic intervention, including repeat biopsy.
Conclusions
Liver cirrhosis comprises necrosis, fibrosis, and regeneration. RNs, FNH-like nodules, DNs, and HCC frequently develop, and sometimes existing benign liver lesions may change through the process of fibrosis, inflammation, and vascular obliteration. Hemangiomas are more easily missed on imaging, and true FNH is a rarity. Differentiation of HCCs from FNH and adenoma can be difficult in these conditions. Metastases are also infrequent. The most important aim of diagnosis is to differentiate these liver lesions in liver cirrhosis from HCC in order to make the right therapeutic decisions. Due to the characteristics of the lesions in liver cirrhosis, this can be challenging for both imaging.
-
Declaration
Institutional review board gave approval and subjects granted permission for using their data/ images.
-
Conflict of Interest
Christoph F. Dietrich is an Editorial Board Member of the journal. The article was subject to the journal’s standard procedures, with peer review handled independently of this editor and his research groups.
References
1 Ridola L, Faccioli J, Nardelli S, Gioia S, Riggio O. Hepatic Encephalopathy: Diagnosis and Management. J Transl Intern Med 2020;8:210–9.10.2478/jtim-2020-0034Search in Google Scholar PubMed PubMed Central
2 Tasneem AA, Luck NH. Autoimmune Hepatitis: Clinical Characteristics and Predictors of Biochemical Response to Treatment J Transl Intern Med 2020;8:106–11.10.2478/jtim-2020-0016Search in Google Scholar PubMed PubMed Central
3 Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol 2013;4:S2–6.10.1097/MCG.0b013e3182872f29Search in Google Scholar PubMed PubMed Central
4 Yu MW, Hsu FC, Sheen IS, Chu CM, Lin DY, Chen CJ, et al. Prospective study of hepatocellular carcinoma and liver cirrhosis in asymptomatic chronic hepatitis B virus carriers. Am J Epidemiol 1997;145:1039–47.10.1093/oxfordjournals.aje.a009060Search in Google Scholar PubMed
5 Pocha C, Xie C. Hepatocellular carcinoma in alcoholic and non-alcoholic fatty liver disease-one of a kind or two different enemies? Transl Gastroenterol Hepatol 2019;4:72.10.21037/tgh.2019.09.01Search in Google Scholar PubMed PubMed Central
6 Gallo A, Dedionigi C, Civitelli C, Panzeri A, Corradi C, Squizzato A. Optimal Management of Cirrhotic Ascites: A Review for Internal Medicine Physicians. J Transl Intern Med 2020;8:220–36.10.2478/jtim-2020-0035Search in Google Scholar PubMed PubMed Central
7 Seitz K, Greis C, Schuler A, Bernatik T, Blank W, Dietrich CF, et al. Frequency of tumor entities among liver tumors of unclear etiology initially detected by sonography in the noncirrhotic or cirrhotic livers of 1349 patients. Results of the DEGUM multicenter study. Ultraschall Med 2011;32:598–603.10.1055/s-0031-1281858Search in Google Scholar PubMed
8 Terzi E, Iavarone M, Pompili M, Veronese L, Cabibbo G, Fraquelli M, et al. Contrast ultrasound LI-RADS LR-5 identifies hepatocellular carcinoma in cirrhosis in a multicenter restropective study of 1,006 nodules. J Hepatol 2018;68:485–92.10.1016/j.jhep.2017.11.007Search in Google Scholar PubMed
9 Dietrich CF, Nolsoe CP, Barr RG, Berzigotti A, Burns PN, Cantisani V, et al. Guidelines and Good Clinical Practice Recommendations for Contrast Enhanced Ultrasound (CEUS) in the Liver - Update 2020 - WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultraschall Med 2020;41:562–85.10.1055/a-1177-0530Search in Google Scholar PubMed
10 Dietrich CF, Dong Y, Kono Y, Caraiani C, Sirlin CB, Cui XW, et al. LI-RADS ancillary features on contrast-enhanced ultrasonography. Ultrasonography 2020;39:221–8.10.14366/usg.19052Search in Google Scholar PubMed PubMed Central
11 Schellhaas B, Bernatik T, Bohle W, Borowitzka F, Chang J, Dietrich CF, et al. Contrast-Enhanced Ultrasound Algorithms (CEUS-LIRADS/ ESCULAP) for the Noninvasive Diagnosis of Hepatocellular Carcinoma - A Prospective Multicenter DEGUM Study. Ultraschall Med 2021;42:178–86.10.1055/a-1198-4874Search in Google Scholar PubMed
12 Wilson SR, Lyshchik A, Piscaglia F, Cosgrove D, Jang HJ, Sirlin C, et al. CEUS LI-RADS: algorithm, implementation, and key differences from CT/MRI. Abdom Radiol (NY) 2018;43:127–42.10.1007/s00261-017-1250-0Search in Google Scholar PubMed
13 Tang A, Bashir MR, Corwin MT, Cruite I, Dietrich CF, Do RKG, et al. Evidence Supporting LI-RADS Major Features for CT- and MR Imaging-based Diagnosis of Hepatocellular Carcinoma: A Systematic Review. Radiology 2018;286:29–48.10.1148/radiol.2017170554Search in Google Scholar PubMed PubMed Central
14 Piscaglia F, Wilson SR, Lyshchik A, Cosgrove D, Dietrich CF, Jang HJ, et al. American College of Radiology Contrast Enhanced Ultrasound Liver Imaging Reporting and Data System (CEUS LI-RADS) for the diagnosis of Hepatocellular Carcinoma: a pictorial essay. Ultraschall Med 2017;38:320–4.10.1055/s-0042-124661Search in Google Scholar PubMed
15 Kono Y, Lyshchik A, Cosgrove D, Dietrich CF, Jang HJ, Kim TK, et al. Contrast Enhanced Ultrasound (CEUS) Liver Imaging Reporting and Data System (LI-RADS(R)): the official version by the American College of Radiology (ACR). Ultraschall Med 2017;38:85–6.10.1055/s-0042-124369Search in Google Scholar PubMed
16 Kono Y, Sirlin CB, Fetzer DT, Kim TK, Rodgers SK, Piscaglia F, et al. Time to Clarify Common Misconceptions about the Liver Imaging Reporting and Data System for Contrast-enhanced US. Radiology 2020;295:245–7.10.1148/radiol.2020192557Search in Google Scholar PubMed
17 Dietrich CF, Averkiou M, Nielsen MB, Barr RG, Burns PN, Calliada F, et al. How to perform Contrast-Enhanced Ultrasound (CEUS). Ultrasound Int Open 2018;4:E2–E15.10.1055/s-0043-123931Search in Google Scholar PubMed PubMed Central
18 Bartolotta TV, Terranova MC, Gagliardo C, Taibbi A. CEUS LI-RADS: a pictorial review. Insights Imaging 2020;11:9.10.1186/s13244-019-0819-2Search in Google Scholar PubMed PubMed Central
19 Dietrich CF, Nolsoe CP, Barr RG, Berzigotti A, Burns PN, Cantisani V, et al. Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the Liver-Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med Biol 2020;46:2579–604.10.1016/j.ultrasmedbio.2020.04.030Search in Google Scholar PubMed
20 Ruiz Guinaldo A, Martin Herrera L, Roldan Cuadra R. Hepatic tumors in patients with cirrhosis: an autopsy study. Rev Esp Enferm Dig 1997;89:771–80.Search in Google Scholar
21 Dodd GD 3rd, Baron RL, Oliver JH 3rd, Federle MP. Spectrum of imaging findings of the liver in end-stage cirrhosis: Part II, focal abnormalities. AJR Am J Roentgenol 1999;173:1185–92.10.2214/ajr.173.5.10541086Search in Google Scholar PubMed
22 Forner A, Vilana R, Ayuso C, Bianchi L, Sole M, Ayuso JR, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 2008;47:97–104.10.1002/hep.21966Search in Google Scholar PubMed
23 D’Onofrio M, Faccioli N, Zamboni G, Malago R, Caffarri S, Fattovich G, et al. Focal liver lesions in cirrhosis: value of contrast-enhanced ultrasonography compared with Doppler ultrasound and alpha-fetoprotein levels. Radiol Med 2008;113:978–91.10.1007/s11547-008-0316-zSearch in Google Scholar PubMed
24 Serste T, Barrau V, Ozenne V, Vullierme MP, Bedossa P, Farges O, et al. Accuracy and disagreement of computed tomography and magnetic resonance imaging for the diagnosis of small hepatocellular carcinoma and dysplastic nodules: role of biopsy. Hepatology 2012;55:800–6.10.1002/hep.24746Search in Google Scholar PubMed
25 Victor B, Galvão T, rios torres L, Cardia P, Nunes T, Salvadori P, et al. Prevalence of simple liver cysts and hemangiomas in cirrhotic and non-cirrhotic patients submitted to magnetic resonance imaging. Radiol Bras 2013;46:203–8.10.1590/S0100-39842013000400005Search in Google Scholar
26 European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182–236.Search in Google Scholar
27 Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsoe CP, et al. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver - update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol 2013;39:187–210.10.1016/j.ultrasmedbio.2012.09.002Search in Google Scholar PubMed
28 Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsoe CP, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver--update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med 2013;34:11–29.10.1055/s-0032-1325499Search in Google Scholar PubMed
29 Dietrich CF. Contrast-Enhanced Ultrasound of Benign Focal Liver Lesions. Ultraschall Med 2019;40:12–29.10.1055/a-0668-5746Search in Google Scholar PubMed
30 Alhyari A, Gorg C, Alakhras R, Dietrich CF, Trenker C, Safai Zadeh E. HCC or Something Else? Frequency of Various Benign and Malignant Etiologies in Cirrhotic Patients with Newly Detected Focal Liver Lesions in Relation to Different Clinical and Sonographic Parameters. Diagnostics (Basel) 2022;12:2079.10.3390/diagnostics12092079Search in Google Scholar PubMed PubMed Central
31 Dong Y, Wang WP, Lee WJ, Meloni MF, Clevert DA, Chammas MC, et al. Contrast-Enhanced Ultrasound Features of Histopathologically Proven Hepatocellular Carcinoma in the Non-cirrhotic Liver: A Multicenter Study. Ultrasound Med Biol 2022;48:1797–805.10.1016/j.ultrasmedbio.2022.05.005Search in Google Scholar PubMed
32 Dong Y, Wang WP, Lee WJ, Meloni MF, Clevert DA, Chammas MC, et al. Hepatocellular carcinoma in the non-cirrhotic liver. Clin Hemorheol Microcirc 2022;80:423–36.10.3233/CH-211309Search in Google Scholar PubMed
33 Liu W, Liu X, Peng M, Chen GQ, Liu PH, Cui XW, et al. Artificial intelligence for hepatitis evaluation. World J Gastroenterol 2021;27:5715–26.10.3748/wjg.v27.i34.5715Search in Google Scholar PubMed PubMed Central
34 Zhang D, Wei Q, Wu GG, Zhang XY, Lu WW, Lv WZ, et al. Preoperative Prediction of Microvascular Invasion in Patients With Hepatocellular Carcinoma Based on Radiomics Nomogram Using Contrast-Enhanced Ultrasound. Front Oncol 2021;11:709339.10.3389/fonc.2021.709339Search in Google Scholar PubMed PubMed Central
35 Strobel D, Jung EM, Ziesch M, Praktiknjo M, Link A, Dietrich CF, et al. Real-life assessment of standardized contrast-enhanced ultrasound (CEUS) and CEUS algorithms (CEUS LI-RADS(R)/ESCULAP) in hepatic nodules in cirrhotic patients-a prospective multicenter study. Eur Radiol 2021;31:7614–25.10.1007/s00330-021-07872-3Search in Google Scholar PubMed PubMed Central
36 Dong Y, Teufel A, Wang WP, Dietrich CF. Current Opinion about Hepatocellular Carcinoma <10 mm. Digestion 2021;102:335–41.10.1159/000507923Search in Google Scholar PubMed
37 Tang A, Abukasm K, Moura Cunha G, Song B, Wang J, Wagner M, et al. Imaging of hepatocellular carcinoma: a pilot international survey. Abdom Radiol (NY) 2021;46:205–15.10.1007/s00261-020-02598-0Search in Google Scholar PubMed
38 Dong Y, Wang WP, Mao F, Zhang Q, Yang D, Tannapfel A, et al. Imaging Features of Fibrolamellar Hepatocellular Carcinoma with Contrast-Enhanced Ultrasound. Ultraschall Med 2021;42:306–13.10.1055/a-1110-7124Search in Google Scholar PubMed
39 Wang JY, Feng SY, Yi AJ, Zhu D, Xu JW, Li J, et al. Comparison of Contrast-Enhanced Ultrasound versus Contrast-Enhanced Magnetic Resonance Imaging for the Diagnosis of Focal Liver Lesions Using the Liver Imaging Reporting and Data System. Ultrasound Med Biol 2020;46:1216–23.10.1016/j.ultrasmedbio.2020.01.023Search in Google Scholar PubMed
40 Dietrich CF, Potthoff A, Helmberger T, Ignee A, Willmann JK, Group CL-RW.[Contrast-enhanced ultrasound: Liver Imaging Reporting and Data System (CEUS LI-RADS)]. Z Gastroenterol 2018;56:499–506.10.1016/j.ultrasmedbio.2017.08.1072Search in Google Scholar
41 Dong Y, Teufel A, Trojan J, Berzigotti A, Cui XW, Dietrich CF. Contrast enhanced ultrasound in mixed hepatocellular cholangiocarcinoma: Case series and review of the literature. Dig Liver Dis 2018;50:401–7.10.1016/j.dld.2017.11.003Search in Google Scholar PubMed
42 Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358–80.10.1002/hep.29086Search in Google Scholar PubMed
43 Yang JD. Detect or not to detect very early stage hepatocellular carcinoma? The western perspective. Clin Mol Hepatol 2019;25:335–43.10.3350/cmh.2019.0010Search in Google Scholar PubMed PubMed Central
44 Jang HJ, Kim TK, Burns PN, Wilson SR. CEUS: An essential component in a multimodality approach to small nodules in patients at high-risk for hepatocellular carcinoma. Eur J Radiol 2015;84:1623–35.10.1016/j.ejrad.2015.05.020Search in Google Scholar PubMed
45 Holland AE, Hecht EM, Hahn WY, Kim DC, Babb JS, Lee VS, et al. Importance of small (< or = 20-mm) enhancing lesions seen only during the hepatic arterial phase at MR imaging of the cirrhotic liver: evaluation and comparison with whole explanted liver. Radiology 2005;237:938–44.10.1148/radiol.2373041364Search in Google Scholar PubMed
46 Jang HJ, Kim TK, Wilson SR. Small nodules (1-2 cm) in liver cirrhosis: characterization with contrast-enhanced ultrasound. Eur J Radiol 2009;72:418–24.10.1016/j.ejrad.2008.08.011Search in Google Scholar PubMed
47 Khalili K, Kim TK, Jang HJ, Yazdi LK, Guindi M, Sherman M. Indeterminate 1-2-cm nodules found on hepatocellular carcinoma surveillance: biopsy for all, some, or none? Hepatology 2011;54:2048–54.10.1002/hep.24638Search in Google Scholar PubMed
48 Kim TK, Lee KH, Jang HJ, Haider MA, Jacks LM, Menezes RJ, et al. Analysis of gadobenate dimeglumine-enhanced MR findings for characterizing small (1-2-cm) hepatic nodules in patients at high risk for hepatocellular carcinoma. Radiology 2011;259:730–8.10.1148/radiol.11101549Search in Google Scholar PubMed
49 Duan Y, Xie X, Li Q, Mercaldo N, Samir AE, Kuang M, et al. Differentiation of regenerative nodule, dysplastic nodule, and small hepatocellular carcinoma in cirrhotic patients: a contrast-enhanced ultrasound-based multivariable model analysis. Eur Radiol 2020;30:4741–51.10.1007/s00330-020-06834-5Search in Google Scholar PubMed
50 Compagnon P, Grandadam S, Lorho R, Turlin B, Camus C, Jianrong Y, et al. Liver transplantation for hepatocellular carcinoma without preoperative tumor biopsy. Transplantation 2008;86:1068–76.10.1097/TP.0b013e318187754cSearch in Google Scholar PubMed
51 Lee H, Yoon JH, Kim H, Yi NJ, Hong SK, Yoon KC, et al. False Positive Diagnosis of Hepatocellular Carcinoma in Liver Resection Patients. J Korean Med Sci 2017;32:315–20.10.3346/jkms.2017.32.2.315Search in Google Scholar PubMed PubMed Central
52 Karhunen PJ. Benign hepatic tumours and tumour like conditions in men. J Clin Pathol 1986;39:183–8.10.1136/jcp.39.2.183Search in Google Scholar PubMed PubMed Central
53 Karhunen PJ, Penttila A, Liesto K, Mannikko A, Mottonen M. Benign bile duct tumours, non-parasitic liver cysts and liver damage in males. J Hepatol 1986;2:89–99.10.1016/S0168-8278(86)80012-5Search in Google Scholar
54 Galvao B, Torres LR, Cardia PP, Nunes TF, Salvadori PS, D’Ippolito G. Prevalence of simple liver cysts and hemangiomas in cirrhotic and non-cirrhotic patients submitted to magnetic resonance imaging. J Radiologia Brasileira 2013;46:203–8.10.1590/S0100-39842013000400005Search in Google Scholar
55 Bazerbachi F, Haffar S, Sugihara T, Mounajjed TM, Takahashi N, Murad MH, et al. Peribiliary cysts: a systematic review and proposal of a classification framework. BMJ Open Gastroenterol 2018;5:e000204.10.1136/bmjgast-2018-000204Search in Google Scholar PubMed PubMed Central
56 Seguchi T, Akiyama Y, Itoh H, Tanaka H, Naganuma S, Nagaike K, et al. Multiple hepatic peribiliary cysts with cirrhosis. J Gastroenterol 2004;39:384–90.10.1007/s00535-003-1307-4Search in Google Scholar PubMed
57 Nakanuma Y, Kurumaya H, Ohta G. Multiple cysts in the hepatic hilum and their pathogenesis. A suggestion of periductal gland origin. Virchows Arch A Pathol Anat Histopathol 1984;404:341–50.10.1007/BF00695218Search in Google Scholar PubMed
58 Brancatelli G, Federle MP, Blachar A, Grazioli L. Hemangioma in the cirrhotic liver: diagnosis and natural history. Radiology 2001;219:69–74.10.1148/radiology.219.1.r01ap3269Search in Google Scholar PubMed
59 Duran R, Ronot M, Di Renzo S, Gregoli B, Van Beers BE, Vilgrain V. Is magnetic resonance imaging of hepatic hemangioma any different in liver fibrosis and cirrhosis compared to normal liver? Eur J Radiol 2015;84:816–22.10.1016/j.ejrad.2015.01.016Search in Google Scholar PubMed
60 Mastropasqua M, Kanematsu M, Leonardou P, Braga L, Woosley JT, Semelka RC. Cavernous hemangiomas in patients with chronic liver disease: MR imaging findings. Magn Reson Imaging 2004;22:15–8.10.1016/j.mri.2003.02.001Search in Google Scholar PubMed
61 Vernuccio F, Cannella R, Porrello G, Calandra A, Midiri M, Furlan A, et al. Uncommon imaging evolutions of focal liver lesions in cirrhosis. Abdom Radiol (NY) 2019;44:3069–77.10.1007/s00261-019-02101-4Search in Google Scholar PubMed
62 Dietrich CF, Mertens JC, Braden B, Schuessler G, Ott M, Ignee A. Contrast-enhanced ultrasound of histologically proven liver hemangiomas. Hepatology 2007;45:1139–45.10.1002/hep.21615Search in Google Scholar PubMed
63 Bernatik T, Seitz K, Blank W, Schuler A, Dietrich CF, Strobel D. Unclear focal liver lesions in contrast-enhanced ultrasonography--lessons to be learned from the DEGUM multicenter study for the characterization of liver tumors. Ultraschall Med 2010;31:577–81.10.1055/s-0029-1245649Search in Google Scholar PubMed
64 Elsayes KM, Chernyak V, Morshid AI, Tang A, Kielar AZ, Bashir MR, et al. Spectrum of Pitfalls, Pseudolesions, and Potential Misdiagnoses in Cirrhosis. AJR Am J Roentgenol 2018;211:87–96.10.2214/AJR.18.19781Search in Google Scholar PubMed
65 Renzulli M, Brocchi S, Ierardi AM, Milandri M, Pettinari I, Lucidi V, et al. Imaging-based diagnosis of benign lesions and pseudolesions in the cirrhotic liver. Magn Reson Imaging 2021;75:9–20.10.1016/j.mri.2020.09.008Search in Google Scholar PubMed
66 Galia M, Taibbi A, Marin D, Furlan A, Dioguardi Burgio M, Agnello F, et al. Focal lesions in cirrhotic liver: what else beyond hepatocellular carcinoma? Diagn Interv Radiol 2014;20:222–8.10.5152/dir.2014.13184Search in Google Scholar PubMed PubMed Central
67 Ronot M, Dioguardi Burgio M, Purcell Y, Pommier R, Brancatelli G, Vilgrain V. Focal lesions in cirrhosis: Not always HCC. Eur J Radiol 2017;93:157–68.10.1016/j.ejrad.2017.05.040Search in Google Scholar PubMed
68 Kim MJ, Lee S, An C. Problematic lesions in cirrhotic liver mimicking hepatocellular carcinoma. Eur Radiol 2019;29:5101–10.10.1007/s00330-019-06030-0Search in Google Scholar PubMed
69 Lee YH, Kim SH, Cho MY, Shim KY, Kim MS. Focal nodular hyperplasia-like nodules in alcoholic liver cirrhosis: radiologic-pathologic correlation. AJR Am J Roentgenol 2007;188:W459–63.10.2214/AJR.05.1998Search in Google Scholar PubMed
70 Marin D, Galluzzo A, Plessier A, Brancatelli G, Valla D, Vilgrain V. Focal nodular hyperplasia-like lesions in patients with cavernous transformation of the portal vein: prevalence, MR findings and natural history. Eur Radiol 2011;21:2074–82.10.1007/s00330-011-2161-zSearch in Google Scholar PubMed
71 Libbrecht L, Cassiman D, Verslype C, Maleux G, Van Hees D, Pirenne J, et al. Clinicopathological features of focal nodular hyperplasia-like nodules in 130 cirrhotic explant livers. Am J Gastroenterol 2006;101:2341–6.10.1111/j.1572-0241.2006.00783.xSearch in Google Scholar PubMed
72 Strobel D, Seitz K, Blank W, Schuler A, Dietrich CF, von Herbay A, et al. Tumor-specific vascularization pattern of liver metastasis, hepatocellular carcinoma, hemangioma and focal nodular hyperplasia in the differential diagnosis of 1,349 liver lesions in contrast-enhanced ultrasound (CEUS). Ultraschall Med 2009;30:376–82.10.1055/s-0028-1109672Search in Google Scholar PubMed
73 Quaglia A, Tibballs J, Grasso A, Prasad N, Nozza P, Davies SE, et al. Focal nodular hyperplasia-like areas in cirrhosis. Histopathology 2003;42:14–21.10.1046/j.1365-2559.2003.01550.xSearch in Google Scholar PubMed
74 Choi JY, Lee HC, Yim JH, Shim JH, Lim YS, Shin YM, et al. Focal nodular hyperplasia or focal nodular hyperplasia-like lesions of the liver: a special emphasis on diagnosis. J Gastroenterol Hepatol 2011;26:1004–9.10.1111/j.1440-1746.2011.06659.xSearch in Google Scholar PubMed
75 Loh JT, Chea YW, Lim KH, Wan WK, Wong JS. Focal nodular hyperplasia-like lesion in a cirrhotic liver mimicking a cholangiocarcinoma. J Clin Pathol 2014;67:377–80.10.1136/jclinpath-2013-202048Search in Google Scholar PubMed
76 Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020;76:182–8.10.1111/his.13975Search in Google Scholar PubMed PubMed Central
77 Dietrich CF, Tannapfel A, Jang HJ, Kim TK, Burns PN, Dong Y. Ultrasound Imaging of Hepatocellular Adenoma Using the New Histology Classification. Ultrasound Med Biol 2019;45:1–10.10.1016/j.ultrasmedbio.2018.06.015Search in Google Scholar PubMed
78 Laumonier H, Cailliez H, Balabaud C, Possenti L, Zucman-Rossi J, Bioulac-Sage P, et al. Role of contrast-enhanced sonography in differentiation of subtypes of hepatocellular adenoma: correlation with MRI findings. AJR Am J Roentgenol 2012;199:341–48.10.2214/AJR.11.7046Search in Google Scholar PubMed
79 Seo JM, Lee SJ, Kim SH, Park CK, Ha SY. Hepatocellular carcinoma arising from hepatocellular adenoma in a hepatitis B virus-associated cirrhotic liver. Clin Radiol 2012;67:329–33.10.1016/j.crad.2011.09.003Search in Google Scholar PubMed
80 Alshak NS, Cocjin J, Podesta L, van de Velde R, Makowka L, Rosenthal P, et al. Hepatocellular adenoma in glycogen storage disease type IV. Arch Pathol Lab Med 1994;118:88–91.Search in Google Scholar
81 Calderaro J, Nault JC, Balabaud C, Couchy G, Saint-Paul MC, Azoulay D, et al. Inflammatory hepatocellular adenomas developed in the setting of chronic liver disease and cirrhosis. Mod Pathol 2016;29:43–50.10.1038/modpathol.2015.119Search in Google Scholar PubMed
82 Goldstein MJ. Metastases in the cirrhotic liver. N Engl J Med 1969;281:221.10.1056/NEJM196907242810421Search in Google Scholar PubMed
83 Hamaya K, Hashimoto H, Maeda Y. Metastatic carcinoma in cirrhotic liver--Statistical survey of autopsies in Japan. Acta Pathol Jpn 1975;25:153–9.10.1111/j.1440-1827.1975.tb00854.xSearch in Google Scholar PubMed
84 Pereira-Lima JE, Lichtenfels E, Barbosa FS, Zettler CG, Kulczynski JM. Prevalence study of metastases in cirrhotic livers. Hepatogastroenterology 2003;50:1490–5.Search in Google Scholar
85 Vanbockrijck M, Kloppel G. Incidence and morphology of liver metastasis from extrahepatic malignancies to cirrhotic livers. Zentralbl Pathol 1992;138:91–6.Search in Google Scholar
86 Melato M, Laurino L, Mucli E, Valente M, Okuda K. Relationship between cirrhosis, liver cancer, and hepatic metastases. An autopsy study. Cancer 1989;64:455–9.10.1002/1097-0142(19890715)64:2<455::AID-CNCR2820640219>3.0.CO;2-CSearch in Google Scholar
87 Seymour K, Charnley RM. Evidence that metastasis is less common in cirrhotic than normal liver: a systematic review of post-mortem case-control studies. Br J Surg 1999;86:1237–42.10.1046/j.1365-2168.1999.01228.xSearch in Google Scholar
88 Mahdi Z, Ettel MG, Gonzalez RS, Hart J, Alpert L, Fang J, et al. Metastases can occur in cirrhotic livers with patent portal veins. Diagn Pathol 2021;16:18.10.1186/s13000-021-01076-5Search in Google Scholar
89 Seitz G.[Why are metastases in cirrhotic livers so rare?]. Ultraschall Med 1989;10:123–6.10.1055/s-2007-1005976Search in Google Scholar
90 Ramia JM, Lopez-Andujar R, Torras J, Falgueras L, Gonzalez JA, Sanchez B, et al. Multicentre study of liver metastases from colorectal cancer in pathological livers. HPB (Oxford) 2011;13:320–3.10.1111/j.1477-2574.2010.00287.xSearch in Google Scholar
91 Lyshchik A, Kono Y, Dietrich CF, Jang HJ, Kim TK, Piscaglia F, et al. Contrast-enhanced ultrasound of the liver: technical and lexicon recommendations from the ACR CEUS LI-RADS working group. Abdom Radiol (NY) 2018;43:861–79.10.1007/s00261-017-1392-0Search in Google Scholar
92 Kim TK, Noh SY, Wilson SR, Kono Y, Piscaglia F, Jang HJ, et al. Contrast-enhanced ultrasound (CEUS) liver imaging reporting and data system (LI-RADS) 2017 - a review of important differences compared to the CT/MRI system. Clin Mol Hepatol 2017;23:280–89.10.3350/cmh.2017.0037Search in Google Scholar
93 Caraiani C, Boca B, Bura V, Sparchez Z, Dong Y, Dietrich C. CT/MRI LI-RADS v2018 vs. CEUS LI-RADS v2017-Can Things Be Put Together? Biology (Basel) 2021;10:412.10.3390/biology10050412Search in Google Scholar
94 Strobel D, Bernatik T, Blank W, Schuler A, Greis C, Dietrich CF, et al. Diagnostic accuracy of CEUS in the differential diagnosis of small (</= 20 mm) and subcentimetric (</= 10 mm) focal liver lesions in comparison with histology. Results of the DEGUM multicenter trial. Ultraschall Med 2011;32:593–7.10.1055/s-0031-1271114Search in Google Scholar
95 Wildner D, Bernatik T, Greis C, Seitz K, Neurath MF, Strobel D. CEUS in hepatocellular carcinoma and intrahepatic cholangiocellular carcinoma in 320 patients - early or late washout matters: a subanalysis of the DEGUM multicenter trial. Ultraschall Med 2015;36:132–9.10.1055/s-0034-1399147Search in Google Scholar
96 Cemeroglu AP, Sarialioglu F, Belen-Apak FB, Terzi YK. Persistent Hyperinsulinemic Hypoglycemia with Pancreatic Teratoma in Infancy: A Case Report. Am J Case Rep 2020;21:e925273.10.12659/AJCR.925273Search in Google Scholar
97 Li F, Li Q, Liu Y, Han J, Zheng W, Huang Y, et al. Distinguishing intrahepatic cholangiocarcinoma from hepatocellular carcinoma in patients with and without risks: the evaluation of the LR-M criteria of contrast-enhanced ultrasound liver imaging reporting and data system version 2017. Eur Radiol 2020;30:461–70.10.1007/s00330-019-06317-2Search in Google Scholar PubMed
98 Huang JY, Li JW, Ling WW, Li T, Luo Y, Liu JB, et al. Can contrast enhanced ultrasound differentiate intrahepatic cholangiocarcinoma from hepatocellular carcinoma? World J Gastroenterol 2020;26:3938–51.10.3748/wjg.v26.i27.3938Search in Google Scholar PubMed PubMed Central
99 Tomasian A, Sandrasegaran K, Elsayes KM, Shanbhogue A, Shaaban A, Menias CO. Hematologic malignancies of the liver: spectrum of disease. Radiographics 2015;35:71–86.10.1148/rg.351130008Search in Google Scholar PubMed
100 Dantas E, Santos J, Coelho M, Sequeira C, Santos I, Cardoso C, et al. Primary hepatic lymphoma in a patient with cirrhosis: a case report. J Med Case Rep 2020;14:168.10.1186/s13256-020-02471-0Search in Google Scholar PubMed PubMed Central
101 Imrani K, Znati K, Amouri W, Nassar I, Billah NM. Primary hepatic lymphoma in liver cirrhosis: A rare case report. Radiol Case Rep 2021;16:2179–83.10.1016/j.radcr.2021.05.028Search in Google Scholar PubMed PubMed Central
102 Yamashita Y, Joshita S, Kobayashi H, Wakabayashi SI, Sugiura A, Yamazaki T, et al. Primary Hepatic Extranodal Marginal Zone Lymphoma of Mucosa-Associated Lymphoid Tissue in a Patient with Chronic Hepatitis B Virus Infection: Case Report and Summary of the Literature. Medicina (Kaunas) 2021;57:28010.3390/medicina57030280Search in Google Scholar PubMed PubMed Central
103 Foschi FG, Dall’Aglio AC, Marano G, Lanzi A, Savini P, Piscaglia F, et al. Role of contrast-enhanced ultrasonography in primary hepatic lymphoma. J Ultrasound Med 2010;29:1353–6.10.7863/jum.2010.29.9.1353Search in Google Scholar PubMed
104 Fu Z, Wu L, Chen J, Zheng Q, Li P, Zhang L, et al. Primary hepatic mucosa-associated lymphoid tissue lymphoma: case report and literature review. Int J Clin Exp Pathol 2021;14:375–82.Search in Google Scholar
105 Burton A, Tataru D, Driver RJ, Bird TG, Huws D, Wallace D, et al. Primary liver cancer in the UK: Incidence, incidence-based mortality, and survival by subtype, sex, and nation. JHEP Rep 2021;3:100232.10.1016/j.jhepr.2021.100232Search in Google Scholar PubMed PubMed Central
106 Kaltenbach TE, Engler P, Kratzer W, Oeztuerk S, Seufferlein T, Haenle MM, et al. Prevalence of benign focal liver lesions: ultrasound investigation of 45,319 hospital patients. Abdom Radiol (NY) 2016;41:25–32.10.1007/s00261-015-0605-7Search in Google Scholar PubMed PubMed Central
107 Benz F, Mohr R, Tacke F, Roderburg C. Pulmonary Complications in Patients with Liver Cirrhosis. J Transl Intern Med 2020;8:150–8.10.2478/jtim-2020-0024Search in Google Scholar PubMed PubMed Central
108 Cai B, Liao K, Song XQ, Wei WY, Zhuang Y, Zhang S. Patients with chronically diseased livers have lower incidence of colorectal liver metastases: a meta-analysis. PLoS One 2014;9:e108618.10.1371/journal.pone.0108618Search in Google Scholar PubMed PubMed Central
109 Papamichalis PA, Zachou K, Papamichali RA, Ioannou M, Gatselis NK, Dalekos GN, et al. Promyelocytic Leukemia Antigen Expression: a Histological Marker for Primary Biliary Cholangitis Diagnosis? J Transl Intern Med 2021;9:43–51.10.2478/jtim-2021-0008Search in Google Scholar PubMed PubMed Central
110 Mohamed AA, Omar AAA, El-Awady RR, Hassan SMA, Eitah WMS, Ahmed R, et al. MiR-155 and MiR-665 Role as Potential Non-invasive Biomarkers for Hepatocellular Carcinoma in Egyptian Patients with Chronic Hepatitis C Virus Infection. J Transl Intern Med 2020;8:32–40.10.2478/jtim-2020-0006Search in Google Scholar PubMed PubMed Central
111 Chiou WY, Chang CM, Tseng KC, Hung SK, Lin HY, Chen YC, et al. Effect of liver cirrhosis on metastasis in colorectal cancer patients: a nationwide population-based cohort study. Jpn J Clin Oncol 2015;45:160–8.10.1093/jjco/hyu178Search in Google Scholar PubMed
112 Terzi E, Giamperoli A, Iavarone M, Leoni S, De Bonis L, Granito A, et al. Prognosis of Single Early-Stage Hepatocellular Carcinoma (HCC) with CEUS Inconclusive Imaging (LI-RADS LR-3 and LR-4) Is No Better than Typical HCC (LR-5). Cancers (Basel) 2022;14:336.10.3390/cancers14020336Search in Google Scholar PubMed PubMed Central
© 2022 Kathleen Möller, Ehsan Safai Zadeh, Christian Görg, Yi Dong, Xinwu Cui, Adrian Lim, Chiara de Molo, Carla Serra, Ana Martín Algíbez, Analisa Berzigotti, Fabio Piscaglia, Siegbert Faiss, Christoph F. Dietrich, published by Sciendo
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Editorial
- Interpretation of the key issues of expert consensus on immunomodulatory therapies for chronic obstructive pulmonary disease
- Perspective
- Emerging noninvasive neuromodulation methods for functional gastrointestinal diseases
- Gastric electrical stimulation: Overview and summary
- Antibiotic stewardship: Dead bugs do not mutate
- The long and the short of current nanomedicines for treating Alzheimer's disease
- Monkeypox: Clinical issues of concern
- Cortical synaptic mechanism for chronic pain and anxiety in Parkinson’s disease
- Commentary
- Chemical therapy for chronic pancreatitis: An assumption or an alternative?
- Review Article
- Focal liver lesions other than hepatocellular carcinoma in cirrhosis: Diagnostic challenges
- The emergence of travel-related infections in critical care units
- Research progress on N6-adenosylate methylation RNA modification in heart failure remodeling
- Original Article
- Characteristics of the severe acute respiratory syndrome coronavirus 2 omicron BA.2 subvariant in Jilin, China from March to May 2022
- A comprehensive weighted gene co-expression network analysis uncovers potential targets in diabetic kidney disease
- Letter to Editor
- Acute kidney injury associated with severe hypouricemia caused by a novel SLC2A9 mutation: Enlightenment from rare disease to common disease
- Noted tension headache, anxiety, and depression in a Chinese patient with spinocerebellar ataxia, autosomal recessive 10 caused by a novel anoctamin 10 mutation
Articles in the same Issue
- Editorial
- Interpretation of the key issues of expert consensus on immunomodulatory therapies for chronic obstructive pulmonary disease
- Perspective
- Emerging noninvasive neuromodulation methods for functional gastrointestinal diseases
- Gastric electrical stimulation: Overview and summary
- Antibiotic stewardship: Dead bugs do not mutate
- The long and the short of current nanomedicines for treating Alzheimer's disease
- Monkeypox: Clinical issues of concern
- Cortical synaptic mechanism for chronic pain and anxiety in Parkinson’s disease
- Commentary
- Chemical therapy for chronic pancreatitis: An assumption or an alternative?
- Review Article
- Focal liver lesions other than hepatocellular carcinoma in cirrhosis: Diagnostic challenges
- The emergence of travel-related infections in critical care units
- Research progress on N6-adenosylate methylation RNA modification in heart failure remodeling
- Original Article
- Characteristics of the severe acute respiratory syndrome coronavirus 2 omicron BA.2 subvariant in Jilin, China from March to May 2022
- A comprehensive weighted gene co-expression network analysis uncovers potential targets in diabetic kidney disease
- Letter to Editor
- Acute kidney injury associated with severe hypouricemia caused by a novel SLC2A9 mutation: Enlightenment from rare disease to common disease
- Noted tension headache, anxiety, and depression in a Chinese patient with spinocerebellar ataxia, autosomal recessive 10 caused by a novel anoctamin 10 mutation

