Introduction
Monkeypox is a zoonotic disease caused by the monkeypox virus from the genus Orthopoxvirus. Monkeypox infections are often caused by the spillover from animals into human populations. The virus is infectious and can also spread among humans through close contact.[1] Since the first case of human infection with monkeypox virus was detected in the 1970s, multiple outbreaks have been reported in 11 African countries, with few global events detected. However, the recently identified global outbreak of monkeypox has no clear link to Africa. On May 29, 2022, the World Health Organization raised a moderate global public health concern because of the ongoing monkeypox outbreak. As of June 12, 2022, at least 1548 human monkeypox cases in 46 countries have been confirmed or suspected.[2]
Two main strains of the monkeypox virus exist—Congo clade and West African clade. The Congo clade strain typically causes more severe illness. Only the West African clade has been identified in the multi-country outbreak.[1] This article will briefly discuss monkeypox in the context of the coronavirus disease 2019 (COVID-19) pandemic, its transmission, and treatment.
The Covid-19 pandemic and the monkeypox endemic
The COVID-19 pandemic has significantly boosted the virus detection capacity of clinical laboratories worldwide. This enhanced capability has contributed to the timely detection of the monkeypox virus.[3] As a learning curve from the COVID-19 pandemic, the rapid spread of monkeypox has been prevented to a certain extent by traveler quarantine policies in various countries. However, because medical resources are directed to the management of the ongoing COVID-19 pandemic, the timely diagnosis and treatment of monkeypox is affected. Taking Nigeria as an example, the decline in reported monkeypox cases in 2020 may be due to the COVID-19 pandemic resulting in reduced monkeypox surveillance and reporting capacity.[4] To avoid this impact on the management of monkeypox, the COVID-19 pandemic needs to be well controlled.
Risk of monkeypox virus transmission
Animals-to-human transmission
Evidence suggests that the monkeypox virus can infect many animals under natural conditions, including rodents, especially squirrels, and non-human primates, but the definitive reservoir for human infection remains unknown (Figure 1).[1]
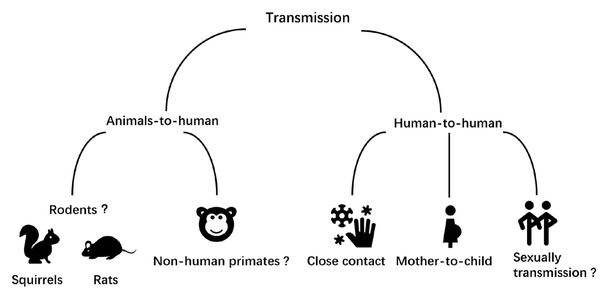
Possible transmission routes of the monkeypox virus.
Human-to-human transmission
Monkeypox virus is transmitted by close contact with the lesions and respiratory secretions of infected patients and their fomites (Figure 1).[1] Since June 8, 2022, 704 cases have been confirmed from 18 countries in the European Union/European Economic Area. Most patients are young homosexual men and present with lesions on the genitalia or peri-genital areas, indicating probable transmission through close physical contact during sexual activities.[5] Viral DNA was detected in the seminal fluid samples from three monkeypox patients in Italy, but the infectivity of seminal fluid remains unknown.[6] In the Republic of Congo, in 1971– 1976, human‑to‑human transmissions accounted for 28% cases; however, in 1996–1997, this rate increased to 78%.[7] The accelerated evolution of the monkeypox virus suggests that the virus has adapted to transfer via humans.[8] The sudden appearance and wide geographic reach of many sporadic cases suggest the start of widespread human-to-human transmission, and that the virus may have been circulating unrecognized for weeks or more.
The need for new vaccines
Replication-competent vaccinia virus, which is the smallpox vaccine, is at least 85% effective in preventing monkeypox.[9] Since the 1980s, when the World Health Organization declared that the smallpox virus has been eliminated, vaccination against smallpox also ceased. According to the Population Division of the United Nations Department of Economic and Social Affairs, people less than 40 years old constituting 60% of the world’s population are apparently not vaccinated against smallpox.[10] Similarly, most people older than 40 years, including immunosuppressive patients, have also not been vaccinated against smallpox. In addition, in countries such as Australia where smallpox was never endemic, residents have not been widely vaccinated.[11] Furthermore, the protective effect of the vaccine decreases with time. The protective efficacy of the vaccinia vaccine against smallpox was found to decrease by 1.41% per year.[12] Taken together, the population now has a low level of immunity to monkeypox.
Based on the experience of prevention and control of smallpox, “ring vaccination” can be used to prevent the spread of monkeypox.[13] Notably, 28 cases were reported in Portugal between May 1 and 23, 2022. Fourteen of these patients were coinfected with human immunodeficiency virus.[14] However, the currently used vaccinia vaccine is not recommended for immunosuppressed patients, especially acquired immunodeficiency syndrome (AIDS) patients.[15] The vaccinia vaccine has considerable side effects such as acute vaccinia syndrome at a high rate of 13%. Post-vaccinal encephalitis, progressive vaccinia, eczema vaccinatum, and generalized vaccinia are other relatively rare adverse reactions.[16] Therefore, a vaccine for immunosuppressed patients with less adverse reactions is urgently required. If cases increase further, the indications of several smallpox vaccines such as IMVAMUNE (Bavarian Nordic) and LC16M8 (Kaketsuken) could be extended for use against monkeypox.[17–20]
Management of severe monkeypox cases
“Four-Anti and Two-Balance” is a clinical strategy that includes antivirus, anti-shock, anti‑hypoxemia, and anti-secondary infection, and maintaining of water, electrolyte, and acid/base balance and microecological balance.[21,22] This clinical strategy has been found to improve the cure rate and reduce the mortality rate of patients with H7N9 avian influenza and COVID‑19. The same therapeutic strategy can be considered for the treatment of severe monkeypox.
Early antiviral therapy reduces disease severity and prevents disease progression. Brincidofovir and tecovirimat have been approved in the USA for the treatment of smallpox disease and have proven activity against monkeypox in animal studies.[23–25] Cytokine storm can lead to shock, hypoxemia, and secondary infection. Cytokine storm is associated with human monkeypox cases. More importantly, plasma granulocyte macrophage colony-stimulating factor, interleukin (IL)-10, and soluble IL-2 receptor concentrations are correlated with severe disease. Blood purification and anti-cytokine antibodies are promising treatment options for severe monkeypox. The study results are limited by a sample size of only 19 case sera, and more samples are required in the future to better identify key cytokines as therapeutic targets.[26]
Conclusion
Despite being a noteworthy disease, our knowledge about monkeypox is limited. Therefore, further study is needed in the areas of transmission route, vaccine research, and critical case treatment.
-
Conflict of Interest
The authors declare that they have no competing interests.
References
1 WHO. Monkeypox. 2022. Available at: https://www.who.int/news-room/fact-sheets/detail/monkeypox Accessed June 14, 2022.Suche in Google Scholar
2 Kraemer MUG, Tegally H, Pigott DM, Dasgupta A, Sheldon J, Wilkinson E, et al. Tracking the 2022 monkeypox outbreak with epidemiological data in real-time. Lancet Infect Dis 2022;22:941–2.10.1016/S1473-3099(22)00359-0Suche in Google Scholar PubMed PubMed Central
3 Hasell J, Mathieu E, Beltekian D, Macdonald B, Giattino C, Ortiz-Ospina E, et al. A cross-country database of COVID- 19 testing. Sci Data 2020;7:345.10.1038/s41597-020-00688-8Suche in Google Scholar PubMed PubMed Central
4 Amao LK, Olatunji DI, Igbodo G, Okoli SC, Amaechi I, Goni MI, et al. Trend and enhanced surveillance of Monkeypox during COVID-19 pandemic in Nigeria. J Public Health Afr 2022;13:2184.10.4081/jphia.2022.2184Suche in Google Scholar PubMed PubMed Central
5 European Centre for Disease Prevention and Control. Monkeypox multi-country outbreak. 2022. Available at: https://www.ecdc.europa.eu/en/monkeypox-outbreak Accessed June 14, 2022.Suche in Google Scholar
6 Antinori A, Mazzotta V, Vita S, Carletti F, Tacconi D, Lapini LE, et al. Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Euro Surveill 2022;27:2200421.10.2807/1560-7917.ES.2022.27.22.2200421Suche in Google Scholar PubMed PubMed Central
7 Heymann DL, Szczeniowski M, Esteves K. Re-emergence of monkeypox in Africa: a review of the past six years. Br Med Bull 1998;54:693–702.10.1093/oxfordjournals.bmb.a011720Suche in Google Scholar PubMed
8 Initial observations about putative APOBEC3 deaminase editing driving short-term evolution of MPXV since 2017 - Monkeypox / Evolution - Virological. 2022. Available at: https://virological.org/t/initial-observations-about-putative-apobec3-deaminase-editing-driving-short-term-evolution-of-mpxv-since-2017/830 Accessed June 14, 2022.Suche in Google Scholar
9 Fine PE, Jezek Z, Grab B, Dixon H. The transmission potential of monkeypox virus in human populations. Int J Epidemiol 1988;17:643–50.10.1093/ije/17.3.643Suche in Google Scholar PubMed
10 United Nations. World Population Prospects. 2019. Available at: https://population.un.org/wpp/dataquery/ Accessed June 14, 2022.Suche in Google Scholar
11 Kennedy RB, Lane JM, Henderson DA, Poland GA. Smallpox and vaccinia. In: Vaccines: Sixth Edition. Amsterdam: Elsevier Inc, 2012:718–45.10.1016/B978-1-4557-0090-5.00010-0Suche in Google Scholar
12 Eichner M. Analysis of historical data suggests long-lasting protective effects of smallpox vaccination. Am J Epidemiol 2003;158:717–23.10.1093/aje/kwg225Suche in Google Scholar PubMed
13 Kretzschmar M, van den Hof S, Wallinga J, van Wijngaarden J. Ring vaccination and smallpox control. Emerg Infect Dis 2004;10:832–41.10.3201/eid1005.030419Suche in Google Scholar PubMed PubMed Central
14 Perez Duque M, Ribeiro S, Martins JV, Casaca P, Leite PP, Tavares M, et al. Ongoing monkeypox virus outbreak, Portugal, 29 April to 23 May 2022. Euro Surveill 2022;27:2200424.10.2807/1560-7917.ES.2022.27.22.2200424Suche in Google Scholar PubMed PubMed Central
15 Redfield RR, Wright DC, James WD, Jones TS, Brown C, Burke DS. Disseminated vaccinia in a military recruit with human immunodeficiency virus (HIV) disease. N Engl J Med 1987;316:673–6.10.1056/NEJM198703123161106Suche in Google Scholar PubMed
16 Frey SE, Couch RB, Tacket CO, Treanor JJ, Wolff M, Newman FK, et al. Clinical responses to undiluted and diluted smallpox vaccine. N Engl J Med 2002;346:1265–74.10.1056/NEJMoa020534Suche in Google Scholar PubMed
17 Walsh SR, Wilck MB, Dominguez DJ, Zablowsky E, Bajimaya S, Gagne LS, et al. Safety and immunogenicity of modified vaccinia Ankara in hematopoietic stem cell transplant recipients: a randomized, controlled trial. J Infect Dis 2013;207:1888–97.10.1093/infdis/jit105Suche in Google Scholar PubMed PubMed Central
18 Kennedy JS, Greenberg RN. IMVAMUNE: modified vaccinia Ankara strain as an attenuated smallpox vaccine. Expert Rev Vaccines 2009;8:13–24.10.1586/14760584.8.1.13Suche in Google Scholar PubMed PubMed Central
19 Yokote H, Shinmura Y, Kanehara T, Maruno S, Kuranaga M, Matsui H, et al. Safety of attenuated smallpox vaccine LC16m8 in immunodeficient mice. Clin Vaccine Immunol 2014;21:1261–6.10.1128/CVI.00199-14Suche in Google Scholar PubMed PubMed Central
20 Kenner J, Cameron F, Empig C, Jobes DV, Gurwith M. LC16m8: an attenuated smallpox vaccine. Vaccine 2006;24:7009–22.10.1016/j.vaccine.2006.03.087Suche in Google Scholar PubMed PubMed Central
21 Gao HN, Lu HZ, Cao B, Du B, Shang H, Gan JH, et al. Clinical findings in 111 cases of influenza A (H7N9) virus infection. N Engl J Med 2013;368:2277–85.10.1056/NEJMoa1305584Suche in Google Scholar PubMed
22 Xu K, Cai H, Shen Y, Ni Q, Chen Y, Hu S, et al. [Management of corona virus disease-19 (COVID-19): the Zhejiang experience]. J Zhejiang Univ Med Sci 2020;49:147–57.Suche in Google Scholar
23 FDA. FDA approves drug to treat smallpox. 2021. Available at: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-drug-treat-smallpox Accessed June 14, 2022.Suche in Google Scholar
24 Chittick G, Morrison M, Brundage T, Nichols WG. Short-term clinical safety profile of brincidofovir: A favorable benefit-risk proposition in the treatment of smallpox. Antiviral Res 2017;143:269–77.10.1016/j.antiviral.2017.01.009Suche in Google Scholar PubMed
25 Grosenbach DW, Honeychurch K, Rose EA, et al. Oral Tecovirimat for the Treatment of Smallpox. N Engl J Med 2018;379:44–53.10.1056/NEJMoa1705688Suche in Google Scholar PubMed PubMed Central
26 Johnston SC, Johnson JC, Stonier SW, Lin KL, Kisalu NK, Hensley LE, et al. Cytokine modulation correlates with severity of monkeypox disease in humans. J Clin Virol 2015;63:42–5.10.1016/j.jcv.2014.12.001Suche in Google Scholar PubMed PubMed Central
© 2022 Xiantian Lin, Xiaoxin Wu, published by Sciendo
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Artikel in diesem Heft
- Editorial
- Interpretation of the key issues of expert consensus on immunomodulatory therapies for chronic obstructive pulmonary disease
- Perspective
- Emerging noninvasive neuromodulation methods for functional gastrointestinal diseases
- Gastric electrical stimulation: Overview and summary
- Antibiotic stewardship: Dead bugs do not mutate
- The long and the short of current nanomedicines for treating Alzheimer's disease
- Monkeypox: Clinical issues of concern
- Cortical synaptic mechanism for chronic pain and anxiety in Parkinson’s disease
- Commentary
- Chemical therapy for chronic pancreatitis: An assumption or an alternative?
- Review Article
- Focal liver lesions other than hepatocellular carcinoma in cirrhosis: Diagnostic challenges
- The emergence of travel-related infections in critical care units
- Research progress on N6-adenosylate methylation RNA modification in heart failure remodeling
- Original Article
- Characteristics of the severe acute respiratory syndrome coronavirus 2 omicron BA.2 subvariant in Jilin, China from March to May 2022
- A comprehensive weighted gene co-expression network analysis uncovers potential targets in diabetic kidney disease
- Letter to Editor
- Acute kidney injury associated with severe hypouricemia caused by a novel SLC2A9 mutation: Enlightenment from rare disease to common disease
- Noted tension headache, anxiety, and depression in a Chinese patient with spinocerebellar ataxia, autosomal recessive 10 caused by a novel anoctamin 10 mutation
Artikel in diesem Heft
- Editorial
- Interpretation of the key issues of expert consensus on immunomodulatory therapies for chronic obstructive pulmonary disease
- Perspective
- Emerging noninvasive neuromodulation methods for functional gastrointestinal diseases
- Gastric electrical stimulation: Overview and summary
- Antibiotic stewardship: Dead bugs do not mutate
- The long and the short of current nanomedicines for treating Alzheimer's disease
- Monkeypox: Clinical issues of concern
- Cortical synaptic mechanism for chronic pain and anxiety in Parkinson’s disease
- Commentary
- Chemical therapy for chronic pancreatitis: An assumption or an alternative?
- Review Article
- Focal liver lesions other than hepatocellular carcinoma in cirrhosis: Diagnostic challenges
- The emergence of travel-related infections in critical care units
- Research progress on N6-adenosylate methylation RNA modification in heart failure remodeling
- Original Article
- Characteristics of the severe acute respiratory syndrome coronavirus 2 omicron BA.2 subvariant in Jilin, China from March to May 2022
- A comprehensive weighted gene co-expression network analysis uncovers potential targets in diabetic kidney disease
- Letter to Editor
- Acute kidney injury associated with severe hypouricemia caused by a novel SLC2A9 mutation: Enlightenment from rare disease to common disease
- Noted tension headache, anxiety, and depression in a Chinese patient with spinocerebellar ataxia, autosomal recessive 10 caused by a novel anoctamin 10 mutation

