A novel wide-band dielectric imaging system for electro-anatomic mapping and monitoring in radiofrequency ablation and cryoablation
-
Jian Li
and Yanli Chen
Abstract
Background and Objectives
A novel wide-band dielectric mapping system, named as KODEX-EPD (EPD Solutions, Philips, Best, the Netherlands), was effectively used in the EA mapping for atrial fibrillation (AF) ablation. To date, only a few studies have concentrated on the application of the KODEX-EPD system for ablating supraventricular tachycardia or ventricular premature beats (VPBs) in human models. This study aims to assess the applicability and efficiency of a novel three-dimensional electro-anatomic (EA) mapping system to improve the success rate of ablation.
Methods
This study included 11 consecutive patients who underwent ablation after EA mapping with the KODEX-EPD system.
Results
All surgeries were successfully performed using the KODEX-EPD system, including 6 cases who underwent ablation of paroxysmal supraventricular tachycardia (PSVT), 2 cases who received ablation of VPBs from right ventricular outflow tract (RVOT), and 3 cases who underwent cryoablation of AF. For ablation of PSVT or VPBs, the operation time was 31.4 (range, 24.0–38.0) min, in which a median operation time of 2.9 min was used to create anatomic images, and the median fluoroscopic dose was 7.4 mGy. For ablation of AF, the operation time was 56.0 (range, 49.0–62.0) min, in which a median of 4.3 (range, 3.4–5.2) min was used for constructing left atrium map, and the median fluoroscopic dose was 15.0 mGy. The operation time and the fluoroscopic dose were greatly shortened for all surgeries.
Conclusion
The KODEX-EPD system is an effective and safe tool to guide the EA mapping, leading to improvement in the success rate of ablation. It can promote the ablation process with the reduced fluoroscopic dose, and it is also a promising tool for complex surgeries.
Introduction
The novel wide-band dielectric mapping (KODEX-EPD) system (EPD Solutions, Philips, Best, the Netherlands) measures changes in electric field gradients induced by intracardiac electrodes to enable catheter localization and real-time three-dimensional (3D) cardiac mapping.[1,2] An electric field is formed with the patches on the body surface. Using a set of electric field signs at each location of the catheter, the 3D electro-anatomic (EA) map of the chamber can be created to locate the catheter in real time.[3,4] The KODEX-EPD system was innovatively reported in radiofrequency ablation (RFA) or cryoablation of atrial fibrillation (AF). Several studies have concentrated on the applicability of the KODEX-EPD system in cryoablation.[4, 5, 6, 7, 8] Novel applications of the KODEX-EPD in pacemaker implantation have also been recently reported.[9,10] However, there are only a few studies concerning the utility of the KODEX-EPD system for the ablation of ventricular premature beats (VPBs) and paroxysmal supraventricular tachycardia (PSVT) in human models.
In the present study, we aimed to assess the applicability and efficacy of the KODEX-EPD system for cardiac EA mapping (including atrium and ventricle) and ablation for VPBs and PSVT. In addition, we studied the reliability of the KODEX-EPD occlusion tool software to detect real-time pulmonary vein occlusion and to avoid contrast injection for cryoballoon ablation of AF.
Methods
Study Population
Data of a total of 11 consecutive patients undergoing RFA for symptomatic PSVT, VPB, and drug-refractory paroxysmal or persistent AF from October 2020 to December 2021 were retrospectively analyzed. The KODEX-EPD system was used to perform real time, in vivo 3D cardiac mapping. All patients signed the written informed consent form before enrollment. The study was approved by the Ethics Committee of the First Affiliated Hospital of China Medical University (AF-SOP-07-1.0-01), and it was conducted in accordance with the Declaration of Helsinki.
Preoperative Preparation
Echocardiography or cardiac computed tomography (CT) scan was performed preoperatively. Seven patches were placed on patient’s body surface, producing an electric field with the wideband (10–100 kHz) across his thorax. By comparing the potential difference between the intracardiac catheter electrode and the reference sensor from the right leg, the electric field line distribution in the heart was measured to locate the catheter and construct the anatomic map with or without physical contact between the catheter and cardiac wall. All procedures were conducted in accordance with the routine surgical guidelines of AF, VPB, and PSVT.[11, 12, 13, 14]
Cardiac Imaging
The KODEX-EPD system created high-resolution images of cardiac anatomy by exploiting the distinct dielectric properties of biological tissue. It provided fast, real-time, and accurate images without rectifications.[5,8] The KODEX-EPD allowed a flat 3D view of the entire chamber – the panoramic view (PANO view) – to present anatomic details and accurately facilitate ablation surgeries. The internal structure of the cardiac chamber was clearly shown with the PANO view to confirm the definitive ablation target.[15]
Endpoints
The primary endpoint of the study was the successful rate of surgeries, guided with the 3D images of the KODEX-EPD. The secondary endpoints included the following items: (1) procedural parameters, such as the operation time and fluoroscopic dose; (2) procedure-related complications, defined as death, transient ischemic attack, stroke, pericardial tamponade or pericardial effusion, bleeding requiring blood transfusion, etc.
Results
Procedural Parameters
All procedures were completed successfully, including 6 cases who underwent ablation of PSVT, 2 cases who received ablation of VPBs from right ventricular outflow tract (RVOT), and 3 cases who underwent cryoablation of AF. Patients’ data are shown in Table 1. For ablation of PSVT or VPB, median operation time was 31.4 (range, 24.0–38.0) min, and median fluoroscopic dose was 7.4 (range, 5.0–14.0) mGy, in which a median of 2.9 (range, 2.0–4.0) min was used for mapping. For cryoablation of AF, median operation time was 56.0 (range, 49.0–62.0) min, and median fluoroscopic dose was 15.0 (range, 13.0–17.0) mGy, in which a median of 4.3 (range, 3.4–5.2) min was used to map the left atrium (Table 1). Additionally, the average fluoroscopic dose for catheter ablation with CARTO 3 (Biosense Webster Inc., Irvine, CA, USA) or cryoablation with angiography of pulmonary veins (PVs) was 60.0 (range, 50.0–63.0) mGy and 256.0 (range, 226.0–298.0) mGy in our center (data were not shown), respectively. High-resolution anatomic images were successfully built and the fluoroscopic dose used for cryoablation with the KODEX-EPD system was greatly reduced, compared with other traditional mapping methods (P < 0.05). All the 11 surgeries were carried out without any complications.
Patients’ clinical characteristics
| Patient No. | Age, (years) | Gender | Weight (kg) | Height (cm) | Symptom time (month) | Diagnosis | Medical history | Total procedure time (min) | Total fluoroscopic dose (mGy) | Mapping time with KODEX-EPD (min) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 59 | Female | 65 | 170 | 24 | AF | Amiodarone | 49 | 13 | 14 |
| 2 | 41 | Female | 67 | 164 | 240 | AVRT | Diltiazem, metoprolol, verapamil | 24 | 6 | 2 |
| 3 | 50 | Male | 84 | 168 | 7 | AF | Propafenone | 57 | 15 | 17 |
| 4 | 41 | Female | 65 | 165 | 3 | VPB | Metoprolol | 36 | 5 | 2 |
| 5 | 49 | Male | 79 | 168 | 6 | AVNRT | Metoprolol | 36 | 14 | 3 |
| 6 | 48 | Male | 75 | 170 | 36 | AVRT | Metoprolol | 31 | 7 | 2 |
| 7 | 60 | Male | 80 | 180 | 84 | AF | Bisolol, propafenone, amiodarone | 62 | 17 | 17 |
| 8 | 17 | Male | 84 | 182 | 24 | VPB | Metoprolol, propafenone | 28 | 6 | 4 |
| 9 | 41 | Male | 72 | 170 | 72 | AVNRT | Metoprolol | 25 | 6 | 2 |
| 10 | 67 | Female | 70 | 158 | 60 | AVNRT | Metoprolol | 38 | 7 | 4 |
| 11 | 31 | Female | 65 | 172 | 1 | AVNRT | No | 33 | 8 | 4 |
AF: atrial fibrillation; AVNRT: atrioventricular nodal reentrant tachycardia; AVRT: atrioventricular reentrant tachycardia; VPB: ventricular premature beat; KODEX-EPD: novel wide-band dielectric mapping.
3D Imaging of RVOT
The 3D images of RVOT and endocardial or epicardial views are shown in Figure 1. Electrocardiogram (ECG) of 2 patients showed VPB from RVOT. The ablation catheter was delivered into RVOT and used for constructing 3D maps of passing chambers of the heart with KODEX-EPD. Under the guidance of multi-channel recorder and ECG in KODEX, ablation target was accurately located in the map. At last, all patients successfully underwent ablation. After 6 months of follow-up, no frequent VPB was found in 24 h Holter monitoring.
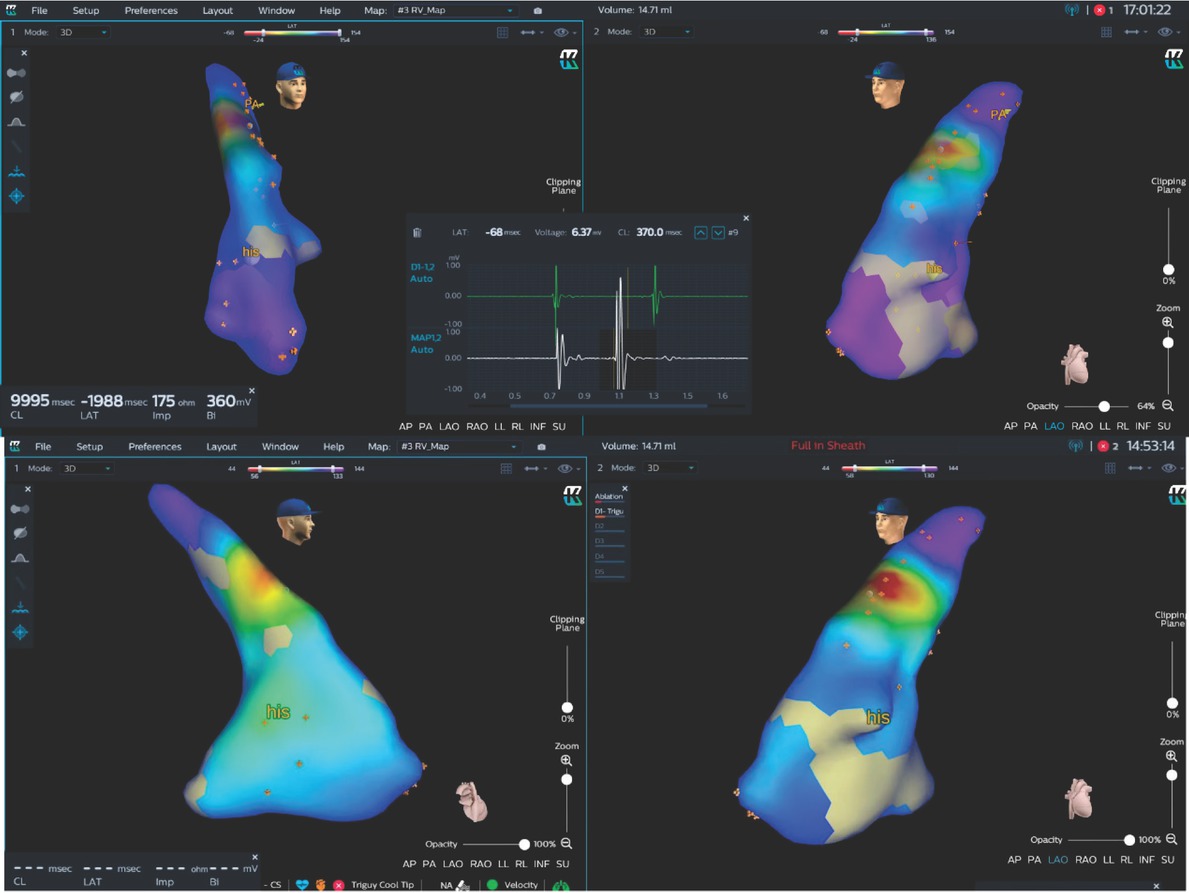
KODEX map of one patient with ventricular premature beats. The anatomic map of RVOT and right ventricle were plotted. The earliest target was clearly located at the septum of RVOT, with early fragmentation potential in the ECG lead window. After two seconds of ablation, ventricular premature beats disappeared. his: His bundle; RVOT: right ventricular outflow tract.
3D Imaging of the right atrium
An irrigated ablation catheter (Triguy, TPT Medical Co., Ltd., Shenzhen, China) was used to establish the anatomic model (Figure 2A) of coronary sinus, right atrium, and tricuspid annulus in 6 PSVT patients. PANO views (Figure 2B) of the right atrium were constructed subsequently. Then, the KODEX-EPD system rendered all major anatomic landmarks related to intracardiac catheter placement and navigation. Using 3D visualization, two diagnostic catheters (ten poles and four poles, respectively; Abbott Laboratories Inc., Chicago, IL, USA) were promptly placed into the coronary sinus and right ventricle without fluoroscopy. The EA map was used to reveal the activation and propagation sequence of cardiac electrical activity in the two PANO views.
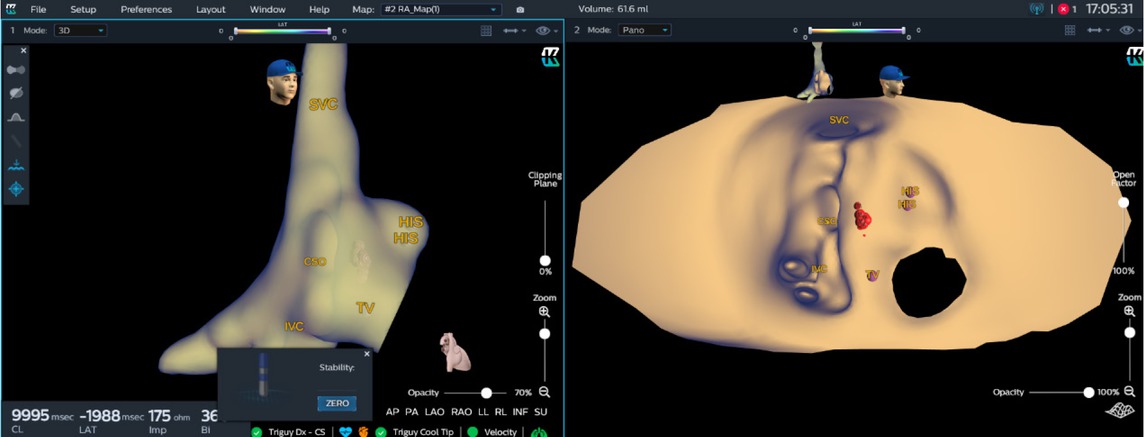
KODEX map of atrioventricular junctional region. Three-dimensional map (left) and PANO view (right) of right atrium and atrioventricular junctional region were presented. After isoprenaline infusion, a typical atrioventricular nodal re-entry tachycardia was induced. The position of His bundle, coronary sinus orifice and the ablation mark were clearly marked in PANO view to indicate the ablation tissue. After 1.5 minutes of ablation (35W, 55°C), the arrhythmia was successfully ablated. PANO view clearly showed the right atrial endocardial view of atrioventricular junctional region. CSO: coronary sinus orifice; HIS: His bundle; IVC: inferior vena cava; SVC: superior vena cava; TV: tricuspid valve.
3D Imaging of the left atrium
The KODEX-EPD system clearly constructed 3D images of the left atrium and PVs for cryoablation in 3 AF patients. The map of the left atrium and PVs for one patient is shown in Figure 3. Tiny malformations of PVs could be clearly observed in a 3D image plotted by the KODEX-EPD system. The accuracy of the model was similar to that of preoperative CT venography of PVs.[3,16] Meanwhile, it could indicate whether the balloon completely blocked PVs or not, with changes of dielectric coefficients discerned by the annular electrode at the head of the balloon. This greatly reduced or even avoided the fluoroscopic exposure and the usage of the contrast agent. High-quality voltage images of left atrium before and after operation are attached in the Supplementary Material.
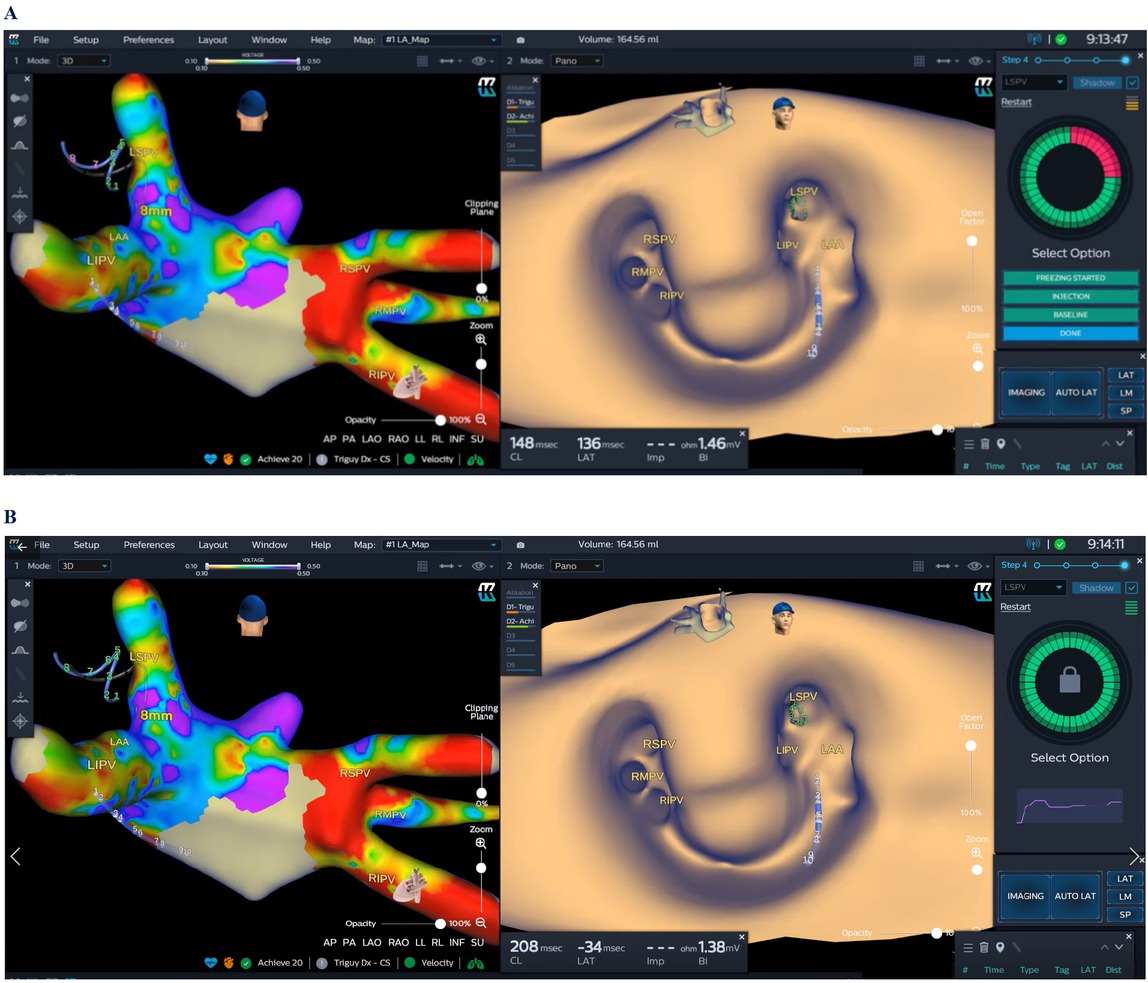
KODEX mapping for AF cryoablation. A 28 mm cryoballoon (Arctic Front Advance Pro; Medtronic), including the circular diagnostic catheter, was placed in the PV antrum. On the roof of the left atrium, there was a small diverticulum between the left and right superior PVs. Three branches were found for the right PV (left). When the cryoballoon occluded the pulmonary vein, “Select PV” displayed a circle with part of red color due to incomplete occlusion or leakage of contrast agent (A). If the PV was occluded completely, the “Select PV” displayed a circle of green color (B). AF: atrial fibrillation; PV: pulmonary vein; LAA: Left atrial appendage; LIPV: Left inferior pulmonary vein; LSPV: Left superior pulmonary vein; RIPV: Right inferior pulmonary vein; RMPV: Right middle pulmonary vein; RSPV: Right superior pulmonary vein.
Surgical Results and Complications
All the surgeries were successfully carried out. After 6–12 months of follow-up, all patients had no recurrent events of previously diagnosed arrhythmia, neither enlargement of cardiac chambers nor reduced ejection factors. The follow-up data of echocardiography are presented in Table 2.
Preoperational and post-operational electrocardiograms and echocardiograms
| Patient No. | Preoperational materials |
Post-operational materials |
||||||
|---|---|---|---|---|---|---|---|---|
| ECG | LA (mm) | LVDd (mm) | EF (%) | ECG | LA (mm) | LVDd (mm) | EF (%) | |
| 1 | AF | 42 | 50 | 58 | SR | 40 | 49 | 60 |
| 2 | SR | 39 | 53 | 63 | SR | 38 | 51 | 65 |
| 3 | AF | 40 | 46 | 57 | SR | 39 | 47 | 60 |
| 4 | SR and VPB | 29 | 41 | 67 | SR | 30 | 45 | 66 |
| 5 | SR | 35 | 56 | 57 | SR | 36 | 55 | 58 |
| 6 | SR | 34 | 43 | 67 | SR | 34 | 45 | 65 |
| 7 | AF | 42 | 47 | 67 | SR | 41 | 46 | 66 |
| 8 | VPB | 36 | 55 | 60 | SR | 35 | 54 | 61 |
| 9 | Pre-excitation syndrome | 36 | 48 | 61 | SR | 37 | 46 | 60 |
| 10 | SR | 37 | 42 | 63 | SR | 38 | 42 | 62 |
| 11 | SR | 33 | 47 | 67 | SR | 34 | 48 | 66 |
AF: atrial fibrillation; ECG: electrocardiogram; EF: ejection factor; LA: left atrium; LVDd: left ventricular end-diastolic dimension; SR: sinus rhythm; VPB: ventricular premature beat.
Discussion
The present study explored the safety, feasibility, and efficacy of using a novel wide-band dielectric mapping system (KODEX-EPD) to treat various arrhythmias, including PSVT, AF, and VPB (RVOT). The KODEX-EPD system can accurately guide the catheter location, and form real-time mapping of atrium, PV antrum, RVOT, and atrioventricular junctional region with valuable anatomic data.
The KODEX-EPD system constructs a high-resolution image of cardiac anatomy by measuring the distinct dielectric properties of heart with catheter moving within an electric field produced by body surface patches.[3,4] The heart, as a dielectric material, has dielectric coefficients and can be polarized by an applied electric field, storing and discharging energy.[17,18] Using this property, the KODEX-EPD system measures the dielectric properties and determines catheter location, as well as contact pressure.[2]
The KODEX-EPD system was first reported in the surgery of human atrial flutter and proved to be safe, feasible, and practical.[2] Several studies have subsequently reported the application of the KODEX-EPD system in the AF ablation.[4, 5, 6, 7, 8,19] AF is associated with significantly high morbidity and mortality.[20, 21, 22] The KODEX-EPD system was validated for accurate distance measuring and catheter positioning, capturing high-quality images.[23] It could construct 3D images of atrium with a quality non-inferior to that of CARTO 3 in human applications, and provide real-time images of mapping.[3,5] The real-time 3D and high-resolution imaging capability of the KODEX-EPD system revealed PV ostium structures that are critical in predicting and identifying leaks of PV occlusion.[1,24] Cryoablation is a safe and effective method for PVI.[25,26] Complete occlusion of the antrum of the PV with the inflated balloon is the key in the surgical steps for AF cryoablation.[22,27,28] The KODEX-EPD system could indicate and guide the occlusion degree by measuring the changes of dielectric properties over the spiral mapping catheter during positioning of the balloon, rather than traditional fluoroscopic guidance of the cryoballoon-based PVI with contrast.[4,6] The KODEX-EPD system provides PANO view to indicate the degree of PV occlusion (green for full occlusion, red for partial occlusion) after 3D imaging even without any radiation or contrast.[1,24,29] In the conventional strategy without using 3D mapping, radiation dose and left atrial procedure time for AF cryoablation were reported to be 8000 mGy and 50 min, respectively.[30] The use of contrast agents can lead to allergy or kidney injury.[31, 32, 33] The present study showed the reduced radiation dose and a short time required for mapping of the left atrium (Table 1) using the KODEX-EPD system. All cases of AF in the present study underwent cryoablation without any contrast. KODEX-EPD provided a good 3D image of the cardiac structure in real time.[29] High-quality images before and after surgery were advantageous to accurately evaluate the damage range and improve the success rate of freezing and assist physicians to make reliable clinical decisions.
Catheter ablation has been recommended as an effective modality for frequent VPBs, which are resistant to antiarrhythmic medications, leading to the reduced left ventricular ejection fraction.[14] The success rate of ablating VPB is highly variable due to its multiple origins, diversity of ventricular structure, and different underlying structural heart diseases. The KODEX-EPD system can produce the reconstructed 3D tomography of the heart without pre-acquired CT or other imaging methods. It can visualize any catheter and has the potential to characterize tissue. Images for the atrioventricular valves or ventricles in swine hearts captured with the KODEX-EPD system showed to be more similar to the CT images than CARTO 3’s map.[3] The surgeon can navigate the catheter in real time and observe the position and orientation of the catheter during mapping. Based on the accurate activation mapping, the arrhythmogenic target could be promptly located to ablate with multiple anatomic images. To our knowledge, there are only a few studies about the application of the KODEX-EPD system for human VPB ablation. The present study included two patients with RVOT VPBs who successfully underwent RVOT mapping and ablation with the KODEX-EPD system. Dielectric imaging is a new approach that may improve some limitations of other EA mapping systems and has the potential to change the way of mapping and ablation.[2,34] It will help to improve the success rate of ventricular substrate ablation for VPBs with exploiting specific dielectric properties of different tissues or even measuring myocardial thickness in the next version of KODEX-EPD.[35, 36, 37, 38] The KODEX-EPD system is also an open platform that is compatible with validated electrophysiological catheters, providing CT-like images of cardiac anatomy.
In conclusion, the KODEX-EPD dielectric imaging system has the following advantages:
Based on the dielectric imaging mechanism, the KODEX-EPD system has the characteristics of high precision, good stability, and anti-interference ability. The KODEX-EPD system has a high spatial resolution to distinguish two points with a distance of at least 0.3 mm, even without catheter–tissue contact.[3]
The KODEX-EPD system continuously detects voltage changes of target chamber and locates the earliest ectopic activation point in real time using the local activation time and the propagation map. A 3D map produced by the KODEX-EPD system can be promptly and safely operated with multiple angles to locate the arrhythmogenic target.[5]
Contact pressure of catheter can be obtained with the changes in dielectric coefficients by the KODEX-EPD system, even combined with ablation catheter without pressure-sensing function, leading to a reduction in the operation cost.
The KODEX-EPD system is highly compatible with other electrophysiological catheters.
Limitations
Several limitations of the present study should be pointed out. First, it was a single-center study with a small sample size. However, this study showed a significant reduction in the fluoroscopic time and dose, suggesting that this novel KODEX-EPD system could be a novel zero fluoroscopic guidance tool for the most of electrophysiological surgeries. Second, during AF cryoablation, the catheter and balloon cannot be displayed in the constructed 3D image. Therefore, completely zero fluoroscopic exposure cannot be achieved during RFA. Third, there is only one simulated lead for the KODEX-EPD system to assist the mapping, which may cause errors in the mapping and highly depends on the operator’s experience. Last but not least, tissue information obtained through dielectric measurement and its value for RFA should be developed and validated.
Conclusions
The novel wide-band dielectric KODEX-EPD mapping system can generate high-resolution 3D images in real time to guide RFA. It facilitates AF cryoablation with significantly reduced operation time and fluoroscopic dose. It has the potential to improve the ablation of VPB and PSVT. However, further studies with larger sample sizes are needed to confirm these findings.
Acknowledgements
We thank all technical support on KODEX-EPD mapping from Philips Medical System (Netherlands).
-
Supplementary Materials
Supplementary materials mentioned in this article are online available at the journal's official site only.
-
Authors’ Contributions
Sun Y, Chen Y, Zhang X, and Li J conceived and designed the research and drafted the manuscript. Li J and Chen Y collected the data. Li J, Chen Y, and Pang X completed the surgeries as the main surgeons. Jia H, Hua Y, Qiao L, Wang B, and Yu Y participated in surgeries and perioperative management. Yu B, Zhang X, and Chen Y assisted to draft the manuscript and made critical revision of the manuscript. All the authors read and approved the final manuscript.
-
Ethics Approval and Consent to Participate
The study was approved by the Ethics Committee of the First Affiliated Hospital of China Medical University (AF-SOP-07-1.0-01). All participants signed the written informed consent form to participate in this study.
-
Conflict of Interest
Yingxian Sun is an Associate Editor-in-Chief of the journal. The article was subject to the journal's standard procedures, and peer review was handled independently of this editor and his research groups.
References
1 Nicholls M. KODEX-EPD mapping for AF ablation. Eur Heart J 2019;40:3003–5.10.1093/eurheartj/ehz647Search in Google Scholar PubMed
2 Abeln BGS, van den Broek JLPM, van Dijk VF, Balt JC, Wijffels MCEF, Dekker LRC, et al. Dielectric imaging for electrophysiology procedures: The technology, current state, and future potential. J Cardiovasc Electrophysiol 2021;32:1140–6.10.1111/jce.14971Search in Google Scholar PubMed
3 Romanov A, Dichterman E, Schwartz Y, Ibragimov Z, Ben-David Y, Rodriguez H, et al. High-resolution, real-time, and nonfluoroscopic 3-dimensional cardiac imaging and catheter navigation in humans using a novel dielectric-based system. Heart Rhythm 2019;16:1883–9.10.1016/j.hrthm.2019.06.020Search in Google Scholar PubMed
4 Rottner L, Sinning C, Reissmann B, Schleberger R, Dinshaw L, Münkler P, et al. Wide-Band Dielectric Imaging and the Novel Cryoballoon-Occlusion Tool to Guide Cryoballoon-Based Pulmonary Vein Isolation. Circ Arrhythm Electrophysiol 2020;13:e008507.10.1161/CIRCEP.120.008507Search in Google Scholar PubMed
5 Maurer T, Mathew S, Schlüter M, Lemes C, Riedl J, Inaba O, et al. High-resolution imaging of LA anatomy using a novel wide-band dielectric mapping system: first clinical experience. JACC Clin Electrophysiol 2019;5:1344–54.10.1016/j.jacep.2019.06.020Search in Google Scholar PubMed
6 Rillig A, Rottner L, Nodorp M, Lin T, Weimann J, Münkler P, et al. Novel Wide-Band Dielectric Imaging System and Occlusion Tool to Guide Cryoballoon-Based Pulmonary Vein Isolation: Feasibility and First Insights. Circ Arrhythm Electrophysiol 2020;13:e009219.10.1161/CIRCEP.120.009219Search in Google Scholar PubMed
7 Schillaci V, Stabile G, Shopova G, Arestia A, Solimene F. Utility of a new imaging system for displaying complex anatomy during AF ablation with cryoenergy. Int J Arrhythm 2020;21:11.10.1186/s42444-020-00019-3Search in Google Scholar
8 Cauti FM, Rossi P, Polselli M, Iaia L, Giannitti CM, Bianchi S. Occlusion tool software for pulmonary vein occlusion verification in atrial fibrillation cryoballoon ablation to avoid the use of contrast injection. HeartRhythm Case Rep 2020;6:516–19.10.1016/j.hrcr.2020.05.011Search in Google Scholar PubMed PubMed Central
9 Hua W, Liu X, Gu M, Niu HX, Chen X, Tang M, et al. Novel Wide-Band Dielectric Imaging System Guided Lead Deployment for His Bundle Pacing: A Feasibility Study. Front Cardiovasc Med 2021;8:712051.10.3389/fcvm.2021.712051Search in Google Scholar PubMed PubMed Central
10 Scarà A, Sciarra L, Bressi E, De Ruvo E, Grieco D, Borrelli A, et al. Cardiac resynchronization therapy guided by the new KODEX-EPD imaging system. J Arrhythm 2021;37:1383–7.10.1002/joa3.12627Search in Google Scholar PubMed PubMed Central
11 January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. A report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 2014;64:e1–76.10.1161/CIR.0000000000000041Search in Google Scholar PubMed PubMed Central
12 Cronin EM, Bogun FM, Maury P, Peichl P, Chen M, Namboodiri N, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation. Heart Rhythm 2020;17:e2–154.10.1016/j.hrthm.2019.03.002Search in Google Scholar PubMed PubMed Central
13 Page RL, Joglar JA, Caldwell MA, Calkins H, Conti JB, Deal BJ, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2016;134:e506–74.10.1161/CIR.0000000000000311Search in Google Scholar PubMed
14 Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/ American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2018;72:e91–220.10.1161/CIR.0000000000000549Search in Google Scholar PubMed
15 Fink T, Imnadze G, Sciacca V, Braun M, Khalaph M, El Hamriti M, et al. Feasibility of wideband dielectric imaging to guide temperature-controlled atrial fibrillation ablation. Heart Rhythm 2022;S1547-5271(22)02052–5.10.1016/j.hrthm.2022.05.035Search in Google Scholar PubMed
16 Tovia Brodie O, Rav-Acha M, Wolak A, Ilan M, Orenstein DJ, Abuhatzera S, et al. Anatomical accuracy of the KODEX-EPD novel 3D mapping system of the left atrium during pulmonary vein isolation: A correlation with computer tomography imaging. J Cardiovasc Electrophysiol 2022;33:618–25.10.1111/jce.15391Search in Google Scholar PubMed PubMed Central
17 Salahuddin S. Acquisition of Dielectric Properties of Tissues in the Microwave Range. Galway: National University of Ireland Galway, 2019.Search in Google Scholar
18 Ištuk N, Porter E, O'Loughlin D, McDermott B, Santorelli A, Abedi S, et al. Dielectric Properties of Ovine Heart at Microwave Frequencies. Diagnostics (Basel) 2021;11:531.10.3390/diagnostics11030531Search in Google Scholar PubMed PubMed Central
19 Yoshida K. Potential advantages of the KODEX-EPD system as the fourth 3D mapping system for atrial fibrillation ablation. J Cardiovasc Electrophysiol 2022;33:626–28.10.1111/jce.15390Search in Google Scholar PubMed
20 Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: The Framingham Heart Study. Circulation 1998;98:946–52.10.1161/01.CIR.98.10.946Search in Google Scholar PubMed
21 Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129:837–47.10.1161/CIRCULATIONAHA.113.005119Search in Google Scholar PubMed PubMed Central
22 Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373–98.10.1093/eurheartj/ehaa612Search in Google Scholar PubMed
23 Romanov A, Shabanov V, Mikheenko I, Losik D, Stenin I, Elesin D, et al. Usefulness of real-time 3D panoramic view for execution of guided ablation procedures for cardiac arrhythmias. Eur Heart J 2018;39:P1917.10.1093/eurheartj/ehy565.P1917Search in Google Scholar
24 Pongratz J, Dorwarth U, Riess L, Schwartz Y, Wankerl M, Hoffmann E, et al. Catheter Ablation in Complex Atrial Arrhythmias: Pilot Study Evaluating a 3D Wide-Band Dielectric Imaging System. Front Cardiovasc Med 2022;8:817299.10.3389/fcvm.2021.817299Search in Google Scholar PubMed PubMed Central
25 Andrade JG, Champagne J, Dubuc M, Deyell MW, Verma A, Macle L, et al. Cryoballoon or Radiofrequency Ablation for Atrial Fibrillation Assessed by Continuous Monitoring: A Randomized Clinical Trial. Circulation 2019;140:1779–88.10.1161/CIRCULATIONAHA.119.042622Search in Google Scholar PubMed
26 Kuck K-H, Brugada J, Fürnkranz A, Metzner A, Ouyang F, Chun KRJ, et al. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. N Engl J Med 2016;374:2235–45.10.1056/NEJMoa1602014Search in Google Scholar PubMed
27 Kimura M, Kobori A, Nitta J, Hirao K, Shizuta S, Kurita T, et al. Cryoballoon ablation for paroxysmal atrial fibrillation in Japan: 2-year safety and efficacy results from the Cryo AF Global Registry. J Interv Card Electrophysiol. 2022. [Epub ahead of print]10.1007/s10840-022-01132-0Search in Google Scholar PubMed PubMed Central
28 Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719–47.Search in Google Scholar
29 Cauti FM, Solimene F, Stabile G, Polselli M, Schillaci V, Arestia A, et al. Occlusion tool software for pulmonary vein occlusion verification in atrial fibrillation cryoballoon ablation. Pacing Clin Electrophysiol 2021;44:63–70.10.1111/pace.14130Search in Google Scholar PubMed
30 Straube F, Pongratz J, Kosmalla A, Brueck B, Riess L, Hartl S, et al. Cryoballoon ablation strategy in persistent atrial fibrillation. Front Cardiovasc Med 2021;8:758408.10.3389/fcvm.2021.758408Search in Google Scholar PubMed PubMed Central
31 Schmidt M, Daccarett M, Marschang H, Intracardiac echocardiography improves procedural efficiency during cryoballoon ablation for atrial fibrillation: a pilot study. J Cardiovasc Electrophysiol 2010;21:1202–7.10.1111/j.1540-8167.2010.01796.xSearch in Google Scholar PubMed
32 Yanagisawa S, Inden Y, Kato H, Fujii A, Mizutani Y, Ito T, et al. Impaired renal function is associated with recurrence after cryoballoon catheter ablation for paroxysmal atrial fibrillation: A potential effect of non-pulmonary vein foci. J Cardiol 2017;69:3–10.10.1016/j.jjcc.2016.07.008Search in Google Scholar PubMed
33 Chao TF, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, et al. Associations between renal function, atrial substrate properties and outcome of catheter ablation in patients with paroxysmal atrial fibrillation. Circ J 2011;75:2326–32.10.1253/circj.CJ-11-0178Search in Google Scholar
34 Khan JM, Rogers T, Greenbaum AB, Babaliaros VC, Yildirim DK, Bruce CG, et al. Transcatheter Electrosurgery: JACC State-of-the-Art Review. J Am Coll Cardiol 2020;75:1455–70.10.1016/j.jacc.2020.01.035Search in Google Scholar PubMed PubMed Central
35 Schillaci V, Stabile G, Arestia A, Shopova G, Agresta A, Salito A, et al. Dielectric-based tissue thickness measured during radiofrequency catheter ablation. J Cardiovasc Electrophysiol 2022;33:1587–9.10.1111/jce.15530Search in Google Scholar PubMed
36 Tondo C. Dielectric concept: "A Magnification Lens in EP Lab?". J Cardiovasc Electrophysiol 2022;33:1590–1.10.1111/jce.15529Search in Google Scholar PubMed
37 Kotadia I, Williams ISM, Roney CH, Lemus JS, Razeghi O, Daniel C, et al. Dielectric Imaging accurately measures regional cardiac chamber wall thickness-an in vivo study. Heart Rhythm 2021;18:S227.10.1016/j.hrthm.2021.06.570Search in Google Scholar
38 Chinitz LA, Barbhaiya C, Fabbricatore D, Viville A, Groenendijk A, Haagen T, et al. Dielectric‐based tissue thickness measured with a radiofrequency ablation catheter: initial clinical results. Heart Rhythm 2021;18:S8.10.1016/j.hrthm.2021.07.041Search in Google Scholar
© 2022 Jian Li, Yu Hua, Lei Qiao, Bo Wang, Xuefeng Pang, He Jia, Yang Yu, Bo Yu, Yingxian Sun, Xingang Zhang, Yanli Chen, published by Sciendo
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Editorial
- Inhaled antibiotics and airway bacterial decolonization for patients with chronic obstructive pulmonary disease: The rationale and future
- Perspective
- Retinal examination modalities in the early detection of Alzheimer's disease: Seeing brain through the eye
- Commentary
- Hydrogel: A promising new technique for treating Alzheimer’s disease
- Metabolic adaptation in lactation: Insulin-dependent and -independent glycemic control
- Review Article
- Aberrant energy metabolism in Alzheimer’s disease
- Obesity and coronavirus disease 2019
- Crosstalk between adipose tissue and the heart: An update
- Delta variant: Partially sensitive to vaccination, but still worth global attention
- Benefits of physical activity on cardiometabolic diseases in obese children and adolescents
- Original Article
- TCDD-inducible poly (ADP-ribose) polymerase promotes adipogenesis of both brown and white preadipocytes
- Functional evaluation of intermediate coronary lesions with integrated computed tomography angiography and invasive angiography in patients with stable coronary artery disease
- A novel wide-band dielectric imaging system for electro-anatomic mapping and monitoring in radiofrequency ablation and cryoablation
- Brief Report
- Impact of COVID-19 pandemic control measures on infection of other respiratory pathogens: A real-world data research in Guangzhou, China
Articles in the same Issue
- Editorial
- Inhaled antibiotics and airway bacterial decolonization for patients with chronic obstructive pulmonary disease: The rationale and future
- Perspective
- Retinal examination modalities in the early detection of Alzheimer's disease: Seeing brain through the eye
- Commentary
- Hydrogel: A promising new technique for treating Alzheimer’s disease
- Metabolic adaptation in lactation: Insulin-dependent and -independent glycemic control
- Review Article
- Aberrant energy metabolism in Alzheimer’s disease
- Obesity and coronavirus disease 2019
- Crosstalk between adipose tissue and the heart: An update
- Delta variant: Partially sensitive to vaccination, but still worth global attention
- Benefits of physical activity on cardiometabolic diseases in obese children and adolescents
- Original Article
- TCDD-inducible poly (ADP-ribose) polymerase promotes adipogenesis of both brown and white preadipocytes
- Functional evaluation of intermediate coronary lesions with integrated computed tomography angiography and invasive angiography in patients with stable coronary artery disease
- A novel wide-band dielectric imaging system for electro-anatomic mapping and monitoring in radiofrequency ablation and cryoablation
- Brief Report
- Impact of COVID-19 pandemic control measures on infection of other respiratory pathogens: A real-world data research in Guangzhou, China

