Abstract
The coronavirus disease 2019 (COVID-19) pandemic has brought severe challenges to global public health. Many studies have shown that obesity plays a vital role in the occurrence and development of COVID-19. Obesity exacerbates COVID-19, leading to increased intensive care unit hospitalization rate, high demand for invasive mechanical ventilation, and high mortality. The mechanisms of interaction between obesity and COVID-19 involve inflammation, immune response, changes in pulmonary dynamics, disruptions of receptor ligands, and dysfunction of endothelial cells. Therefore, for obese patients with COVID-19, the degree of obesity and related comorbidities should be evaluated. Treatment methods such as administration of anticoagulants and anti-inflammatory drugs like glucocorticoids and airway management should be actively initiated. We should also pay attention to long-term prognosis and vaccine immunity and actively address the physical and psychological problems caused by longterm staying-at-home during the pandemic. The present study summarized the research to investigate the role of obesity in the incidence and progression of COVID-19 and the psychosocial impact and treatment options for obese patients with COVID-19, to guide the understanding and management of the disease.
Introduction
At the end of 2019, there was an outbreak of a novel and highly infectious respiratory disease, the coronavirus disease 2019 (COVID-19).[1, 2] The causative organism is a virus named as severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which belongs to the family of encapsulated single-stranded RNA viruses.[1-4] COVID-19 spread rapidly worldwide and became a public health emergency of international concern.[1, 2, 5] On March 11, 2020, the World Health Organization (WHO) declared COVID-19 as a pandemic.[6] As of November 22, 2020, over 57.8 million cases of COVID-19 was reported globally, resulting in the deaths of more than 1.3 million people.[5]
The symptoms of COVID-19 are varied, including asymptomatic cases, upper respiratory tract pneumonia, and acute respiratory distress syndrome (ARDS). The spread of COVID-19 has led to a significant health issue in infected patients, especially for patients with several comorbidities such as obesity, diabetes, hypertension, cardiovascular disease, and other underlying diseases.[7, 8, 9]
Obesity is a common metabolic disorder worldwide that affects approximately 0.5 million people each year.[10] Obesity has been found to act as an independent risk factor for several diseases.[11, 12] The increasing prevalence of obesity indicates an urgency to assess this possible risk factor.
Several studies have reported that COVID-19 causes increased morbidity and mortality in obese people.[3, 13, 14, 15, 16] Obesity may contribute to long-term chronic inflammation, immune dysfunction, and the development of other comorbidities such as cardiovascular diseases and metabolic disorders.[9] Therefore, we summarized the research on obesity and COVID-19 to clarify the relationship between obesity and COVID-19, treatment strategies, social implications, and recommendations for COVID-19 patients with obesity.
Obesity and morbidity of COVID-19
In the last few decades, obesity has gradually shaped into a pandemic. As of 2016, there were about 1.9 billion people with a body mass index (BMI) of 25–30 kg/m2, and more than 650 million people were obese with a BMI of > 30 kg/m2.[6] The WHO estimated that 39% of adults aged 18 years and older were overweight and 13% of these adults were obese globally.[3, 17] If the morbidity of obesity continues, 57.8% of the world’s population will be overweight or obese by 2030.[18] According to research statistics, the prevalence rate of obesity and abdominal obesity in China is 13.2% and 44%, respectively, indicating that the number of patients should not be underestimated.[19]
Obesity is an independent risk factor for the onset of influenza. Generally, obese people with a BMI of 30–35 kg/m2 and more than 35 kg/m2 have 1.45 and 2.12 times, respectively, the risk of being hospitalizationed for influenza. Similarly, studies have shown that obese people have a higher incidence of COVID-19.[19, 20] In addition, the latest update from the WHO shows that countries with a high prevalence of obesity have a higher cumulative number of deaths per million people diagnosed with COVID-19; hence, the high prevalence of obesity worldwide makes people with COVID-19 more vulnerable to suffer from severe complications.[17, 19]
Obesity and severity of COVID-19
Obesity is correlated with a higher risk for hospitalization, intensive care unit (ICU) hospitalization, invasive mechanical ventilation (IMV),[3, 9, 12, 13, 14, 21, 22, 23, 24, 25, 26, 27, 28] and death.[3, 12, 13, 14, 21, 22, 24, 29] Studies have indicated an increased risk of adverse diseases in patients with COVID-19, especially among those who are overweight.[24] However, a few studies have identified obesity as a risk factor only for adverse diseases rather than for mortality.[15, 25, 28] In addition, some studies have reported that more visceral fat could lead to more severe disease.[17, 30]
Chang et al.[9] conducted a systematic review of the impact of BMI and obesity on COVID-19 and found that patients with severe COVID-19 had a higher BMI than those with mild or moderate disease. Moreover, increased BMI is associated with an increased need for IMV. Hoong et al.[24] showed that the prognosis was poorer in obese patients with COVID-19 than in nonobese patients, with an odds ratio (OR) of 1.51 (95% confidence interval [95% CI] 1.13–2.21; P = 0.006) for death and an OR of 2.26 (95% CI 1.47–3.48, P < 0.001) for severe disease. Pranata et al.[13] investigated the dose-response relationship between BMI and outcomes of patients with COVID-19. They suggested that an increase in BMI was positively associated with an increase in adverse outcomes in patients with COVID-19. Poly et al.[29] conducted a subgroup analysis to assess the risk of mortality in obese patients with COVID-19 with multiple comorbidities, including diabetes, hypertension, and stroke. The pooled relative risk (RR) among the patients with stroke and hypertension was 1.80 (95% CI 0.89–3.64, P < 0.001) and 1.07 (95% CI 0.92–1.25; P = 0.35), respectively.
Some studies have shown that patients with severe COVID-19 had a higher visceral fat area (VFA) value. A recent meta-analysis by Foldi et al.[30] included a comprehensive analysis of the relationship between computed tomography (CT)-based determination of quantitative fat mass distribution and COVID-19. The authors found that patients in ICU had higher VFA values than those in general wards. Moreover, patients requiring IMV had higher VFA values than those who did not require IMV. Battisti et al.[31] found that the severity of COVID-19 was related to the distribution of abdominal adipose tissue, and they highlighted the potential pathogenic role of visceral fat in severe disease.
Hypertension, diabetes, cardiovascular and respiratory diseases are the four most common comorbidities in COVID-19 patients, all of which are closely related to obesity and the severity of COVID-19.[32, 33] A study from Mexico showed that obesity combined with diabetes and hypertension significantly increased the risk of hospitalization and mortality in 10,544 COVID-19 cases.[32]
Several studies have reached the same conclusion that obesity is a risk factor for patients with COVID-19 regardless of the presence or absence of comorbidities. Therefore, we must investigate why obesity can aggravate the disease and lead to a worse prognosis in patients with COVID-19. Table 1 shows the meta-analysis of the impact of obesity on COVID-19 severity and mortality.
Meta-analysis to investigate the effects of obesity on the severity and mortality of COVID-19
| Author and publication year | Numbers of study | Country | Numbers of patients | Main results | Mortality |
|---|---|---|---|---|---|
| Du et al., 2020[14] | 16 | China, Mexico 3; USA 7; Italy 2; Kuwait 1 | 109,881 | 1. A linear relationship (Pnonlinearity= 0.242) between BMI and the risk of critical COVID-19 2. 1.09-fold increased risk (OR 1.09, 95% CI 1.04–1.14, P < 0.001) of critical COVID-19 for each 1 kg/m2 increase in BMI and a 1.19-fold increased risk (OR 1.19, 95% CI 1.08–1.30, P < 0.001) of critical COVID-19 for each 2 kg/m2 increase in BMI | 1. BMI ≥ 30 kg/m2 had a significantly higher risk of COVID-19 mortality (OR 2.68, 95% CI 1.65–4.37, P < 0.001) 2. The mortality of patients with COVID-19 increased by 6% (OR 1.06, 95% CI 1.02–1.10, P = 0.002) and 12% (OR 1.12, 95% CI 1.04–1.21, P = 0.002) for each 1 kg/m2 and 2 kg/m2 increase in BMI, respectively |
| Pranata et al., 2020[13] | 12 | China 2; USA 7; UK, France, Milian 1 | 34,930 | 1. Obesity was associated with composite poor outcome (OR 1.73 [1.40, 2.14], P < 0.001; I2 55.6%) and severity (OR 1.90 [1.45, 2.48], P < 0.001; I2 5.2%) in patients with COVID-19 2. 1.052-fold increased risk (OR 1.052 [1.028, 1.077], P < 0.001) of critical COVID-19 for every 5 kg/m2 increase in BMI (Pnonlinearity < 0.001) | 1.55-fold increased risk (OR 1.55 [1.16, 2.06], P = 0.003; I2 74.4%) of mortality for COVID-19 with obese |
| Chang et al., 2020[9] | 16 | China 10; USA 5; France 1 | 18,812 | 1. Higher BMI was found in patients with severe disease than in those with mild or moderate disease (MD 1.6, 95% CI 0.8–2.4, P = 0.0002) in China 2. Elevated BMI was associated with IMV use (MD 4.1, 95% CI 2.1–6.1, P <0.0001) in Western countries 3. There were increased odds ratios of IMV use (OR 2.0, 95% CI 1.4–2.9, P <0.0001) and hospitalization (OR 1.4, 95% CI 1.3–1.60, P < 0.0001) in patients with obesity | NA |
| Raeisi et al., 2020[22] | 54 | China 6; USA 22; UK 5; Mexico, Spain, Germany 2; Italy 8; Bolivia 1; France 5; Singapore 1 | 501,385 | Obesity was a significant risk factor for hospitalization (OR 1.75, 95% CI 1.47– 2.09; very low certainty), mechanical ventilation (OR 2.24, 95% CI 1.70–2.94; low certainty), ICU admission (OR 1.75, 95% CI 1.38–2.22; low certainty) in COVID-19 patients | Obesity was a significant risk factor for death (OR 1.23, 95% CI 1.06–1.41; low certainty) in COVID-19 patients |
| Poly et al., 2021[29] | 17 | USA 10; China, France, UK, Mexico, Multiple countries 1; Italy 2 | 543,399 | 1. Obesity was significantly associated with an increased risk of mortality among patients with COVID-19 (RRedjust 1.42; 95% CI 1.24–1.63, P < 0.001) 2. In subgroup analysis, the pooled risk ratio for the patients with stroke, COPD, CKD, and diabetes was 1.80 (95% CI 0.89–3.64, P = 0.10), 1.57 (95% CI 1.57–1.91, P < 0.001), 1.34 (95% CI 1.18–1.52, P < 0.001), and 1.19 (95% CI 1.07–1.32, P = 0.001), respectively | NA |
| Aghili et al., 2020[12] | 55 | China 18; USA 19; France 3; Italy 6; UK 4; Iran, German, Mexico, Singapore, Israel 1 | 260,693 | 1. Obesity (BMI ≥ 30) was associated with poor outcome (OR 1.297 [1.178–1.416], P < 0.001; I2 0.0%, heterogeneity = 13.69) 2. Obesity did not significantly increase the ICU admission (OR 1.189 [0.955–1.424], I2 0.0%, heterogeneity = 1.92) 3. Obesity (BMI ≥ 30) was associated with invasive mechanical ventilation (OR 2.049 [1.420–2.678], I2 75.3%, heterogeneity = 28.34) | Obesity (BMI ≥ 30) increased mortality (OR 1.35 [1.241–1.459], I2 76.6%, heterogeneity = 38.44) |
| Huang et al., 2020[23] | 33 | USA 18; Italy 6; Mexico 3; China, Spain, Greece, Kuwait, Switzerland, France 1 | 45,650 | 1. Univariate analyses showed significantly higher ORs of severe COVID-19 with higher BMI: 1.76 (95% CI 1.21, 2.56; P = 0.003) for hospitalization, 1.67 (95% CI 1.26, 2.21; P < 0.001) for ICU admission, 2.19 (95% CI 1.56, 3.07; P < 0.001) for IMV requirement, and giving an overall OR for severe COVID-19 of 1.67 (95% CI 1.43, 1.96; P < 0.001) 2. Multivariate analyses revealed increased ORs of severe COVID-19 associated with higher BMI: 2.36 (95% CI 1.37, 4.07; P = 0.002) for hospitalization, 2.32 (95% CI 1.38, 3.90; P = 0.001) for requiring ICU admission, 2.63 (95% CI 1.32, 5.25; P = 0.006) for IMV support, and 1.49 (95% CI 1.20, 1.85; P < 0.001) for mortality, giving an overall OR for severe COVID-19 of 2.09 (95% CI 1.67, 2.62; P < 0.001) | 1. Univariate analyses showed higher ORs of severe COVID-19 with higher BMI 1.37 (95% CI 1.06, 1.75; P = 0.014) for death 2. Multivariate analyses revealed increased ORs of severe COVID-19 associated with higher BMI 1.49 (95% CI 1.20, 1.85; P < 0.001) for mortality |
| Foldi et al., 2020[30] | 24 | NA | 33,987 | Obesity was a significant risk factor for ICU admission in a homogenous dataset (OR 1.21, 95% CI 1.002–1.46; I2 0.0%) as well as for IMV (OR 2.05, 95% CI 1.16–3.64; I2 34.86%) in COVID-19 | NA |
| Hoong et al., 2021[24] | 20 | China, Italy 3; USA 12; German, Mexico 1 | 28,355 | 1. A pooled OR of 2.02 (1.41–2.89, P < 0.001) for an unfavorable outcome in obese versus nonobese patients when adjusted for age, sex, and comorbidities 2. When unadjusted for confounders, the OR for unfavorable outcomes was 1.25 (95% CI 1.07–1.45; P = 0.005). An increased adjusted OR was also seen for severe illness (OR 2.26, 95% CI 1.47– 3.48; P < 0.001) | An increase adjusted OR was seen for death (OR 1.51, 95% CI 1.13–2.21; P = 0.006) |
| Cai et al., 2021[3] | 46 | China 7; USA 21; France 5; Mexico, UK 3; Bolivia 1; Spain 2; Italy 4 | 625,153 | Obese patients had a significantly increased risk of infection (OR 2.73, 95% CI 1.53–4.87; I2 96.8%), hospitalization (OR 1.72, 95% CI 1.55–1.92; I2 47.4%), clinically severe disease (OR 3.81, 95% CI 1.97–7.35; I2 57.4%), mechanical ventilation (OR 1.66, 95% CI 1.42–1.94; I2 41.3%), ICU admission (OR 2.25, 95% CI 1.55–3.27; I2 71.5%) | Obese patients had a higher mortality (OR 1.61, 95% CI 1.29–2.01; I2 83.1%) |
| Helvaci et al., 2021[25] | 19 | USA 8; China, Mexico, France, Italy 2; UK, Kuwait, Germany 1 | 47,872 | Obesity was associated with a higher risk for hospitalization [OR 1.3, 95% CI 1.00–1.69; I2 52%, P = 0.05], ICU admission (OR 1.51, 95% CI 1.16–1.97; I2 72%, P = 0.002), and IMV requirement (OR 1.77, 95% CI 1.34–2.35; I2 0%, P < 0.001) | The increase in risk of death did not reach statistical significance (OR 1.28, 95% CI 0.76–2.16, P = 0.35) |
| Zhang et al.[28] | 22 | China 5; USA 9; France, UK, Italy 2; Singapore, German 1 | 30,141 | Obesity is associated with an increased likelihood of presenting with more severe COVID-19 symptoms (OR 3.03, 95% CI 1.45–6.28, P = 0.003; 4 studies, n = 974), developing ARDS (OR 2.89, 95% CI 1.14–7.34, P = 0.025; 2 studies, n = 96), requiring hospitalization (OR 1.68, 95% CI 1.14–1.59, P < 0.001; 4 studies, n = 6611), being admitted to an ICU (OR 1.35, 95% CI 1.15–1.65, P = 0.001; 9 studies, n = 5298), and undergoing IMV (OR 1.76, 95% CI 1.29–2.40, P < 0.001; 7 studies, n = 1558) compared to nonobese patients | Obese patients had similar likelihoods of death from COVID-19 as nonobese patients (OR 0.96, 95% CI 0.74–1.25, P = 0.750; 9 studies, n = 20,597) |
COVID-19: coronavirus disease 2019; ARDS: acute respiratory distress syndrome; BMI: body mass index; COPD: chronic obstructive pulmonary disease; CKD: chronic kidney disease; ICU: intensive care unit; IMV: invasive mechanical ventilation; NA: not available.
Mechanism of interaction between obesity and SARS-CoV-2
Inflammatory changes in obese patients with COVID-19
Several studies have shown that obesity leads to a low-level inflammatory state.[10, 34, 35, 36] In obese people, because of excess adipose tissue (AT) and an increase in the total blood volume, the levels of many cytokines and chemokines are significantly increased, which might lead to insulin resistance, metabolic disorders, and cardiovascular diseases.[34, 35, 37, 38, 39, 40] This process increases innate and adaptive immune responses and tissue damage.[10] Several proinflammatory cytokines are secreted in this process, such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-β), interleukin-6 (IL-6), monocyte chemotactic protein-1 (MCP-1), and adipokines.
In obesity, AT is linked to the pathophysiology of obesity and metabolic dysfunction.[36]
In particular, AT secretes a variety of cell-signaling cytokines, known as adipokines, which participate in local and systemic inflammation.[32] In a normal body, macrophages are widely present in tissues and organs in a resting state, but they can polarize in infection. The polarized macrophages secrete MCP-1, which leads to an increase in inflammatory cytokines, including IL-6 and TNF-α. The number of macrophages in AT increases significantly, and they are surrounded by adipocytes with coronal structures (CLS).[18] The polarity of macrophages in AT changes from anti-inflammatory M2 to proinflammatory M1. This process further causes local and systemic chronic inflammation.[32, 38, 40]
The NLRP3 inflammasome is a multiprotein complex found in macrophages, dendritic cells, and other nonimmune cells. This complex is associated with metabolic and inflammatory conditions.[37] Several studies have confirmed that NLRP3 is activated in AT of obese people and is related to metabolic disorders.[41] Further activation of NLRP3 may cause systemic inflammatory response and cytokine storm, which subsequently produces a chain reaction throughout the body and leads to organ injury and multiple organ failure.[35] Cytokine storms are also caused by overproduction of interferons, TNF-a, interleukins, and various chemokines in patients with COVID-19. The cytokine storms also contribute to severe disease.[33]
Immune response changes in obese patients with COVID-19
The immune response also plays an important role in the mechanism of interaction between COVID-19 and obesity. Innate and adaptive immune cells in AT can maintain an anti-inflammatory environment; however, obesity disrupts this situation.[42]
Obesity is characterized by an excess of white adipose tissue (WAT). WAT is composed of different types of fat cells, immune cells, and endothelial cells. Immune cells are important for maintaining tissue homeostasis; however, apparent changes in their number and function are observed in obese patients.[43, 44] Immune cells in WAT secrete large amounts of IL-6 and TNF-a, which act as central cytokines to drive the inflammatory response associated with comorbidities.[10]
Higher BMI is related to the impaired immune function of T or B cells. Moreover, previous studies have shown that in some animal models, the influenza-specific CD8+ memory T cells were significantly reduced in obese mice.[9, 45] Lymphocytopenia and an increased neutrophil–lymphocyte ratio after SARS-CoV-2 infection are usually indicative of severe disease and poor prognosis.[46, 47] Obesity is also associated with the reduction in the production of type I interferon (IFN), which is a key factor in the antiviral immune response. The reduction in IFN production makes the body more susceptible to infection.[10]
Obese patients have poor physical fitness and immune system functions.[13] Moreover, because of excessive accumulation of AT, overweight and obesity alter innate and adaptive immune responses. This also causes the immune system to become more susceptible to infection.[10] AT regulates metabolism by secreting adiponectin, leptin, and other adipokines. Leptin has immune and neuroendocrine properties and plays a strong role in innate and adaptive immune responses; it can also exert effects on neutrophils, natural killer (NK) cells, monocytes, macrophages, and CD4 cells.[48] It is known that obese individuals have low adiponectin and high leptin levels. In one study, leptin was shown to promote fibrosis in a mouse model of ARDS,[49] suggesting that leptin might be related to severe pulmonary diseases.[34, 42] Another study showed that high levels of leptin could impair immune responses to viral infections, reduce vaccine efficacy, and result in chronic inflammatory responses.[48, 50, 51]
Multiple lines of evidence suggest that insulin might be a key regulator of T-cell metabolism and function. Insulin resistance impairs immune function.[52, 53, 54] Insulin signals actively control the growth and proliferation of T cells and stimulate their immune function, thereby strengthening the host’s defense against infection. However, obesity often leads to systemic insulin resistance and various metabolic abnormalities.
Pulmonary mechanics and physiological changes in obese patients with COVID-19
People with obesity show a variety of changes in pulmonary and chest wall function, which may further deteriorate following COVID-19 infection. Specifically, respiratory dysfunction in obese patients often increases the risk of hypoventilation-associated pneumonia, pulmonary hypertension, and cardiac stress. Besides, impairment of respiratory dynamics can lead to severe COVID-19. Obesity is associated with a reduction in an expiratory reserve capacity, functional capacity, and respiratory compliance. The pulmonary function of patients with abdominal obesity may be further impaired due to reduced diaphragmatic movement.[33, 45, 55, 56, 57] Moreover, obesity may lead to airway narrowing, uneven distribution of ventilation, and increased airway resistance.[19]
Mechanism of receptor–ligand interaction and its effect on increased infection and increased disease severity in obese patients with COVID-19
The first step for SARS-CoV-2 to enter a cell is the binding of its spike protein to angiotensin-converting enzyme 2 (ACE2) on the cell surface. ACE2 is expressed in many organs and tissues, including the respiratory epithelial cells, cells of the small intestine, and alveolar epithelial cells.[51, 58-61] In vitro tests have shown that the increased expression of ACE2 promotes the entry of SARS-CoV-2 into cells and its replication.[4, 62, 63] Thus, increased expression of ACE2 is considered as one of the mechanisms that facilitate the entry of SARS-CoV-2 into cells.[59]
The expression of ACE2 is higher in AT cells than in pulmonary cells, which suggests that AT may be more susceptible to COVID-19 infection.[59, 64] The presence of ACE2 may allow SARS-CoV-2 to enter AT cells, which suggests that AT may act as a viral reservoir. Moreover, the presence of ACE2 may prolong the shedding of SARS-COV-2 in obese individuals and exacerbate systemic inflammation and immune response.[32, 61, 65] Moreover, a few researchers have shown that some drugs used to treat obese patients with COVID-19 with complications may induce the overexpression of the ACE2 receptor in adipocytes, leading to more virus uptake and severe outcome.[51]
In addition, obese patients who exhibit overactivity of the renal–angiotensin–aldosterone system (RAAS) may show worse outcomes.[58] ACE catalyzes the conversion of angiotensin I to the octapeptide angiotensin II (Ang II), and ACE2 then converts Ang II to angiotensin 1-7. Angiotensin 1-7 stimulates vasodilation and inhibits cell growth.[61] It has been reported that coronavirus can cause the accumulation of Ang II by blocking ACE2 receptors during infection, which may further lead to pulmonary injury or tissue damage.[9, 60]
Changes in endothelial cells in obese patients with COVID-19
Endothelial cells play an important role in the regulation of vascular homeostasis. It can produce a series of bioactive mediators to regulate vascular tension, control permeability, regulate proliferation, and regulate platelet adhesion and aggregation. Obesity-related inflammation, however, can lead to an imbalance between the proinflammatory/ procoagulant and anti-inflammatory/anticoagulant status of endothelial cells. Obesity can induce pathological platelet hyperactivation, resulting in hemostasis.[66, 67]
Various studies have shown that chronic and progressive inflammatory processes in obese individuals lead to endothelial dysfunction.[32] Moreover, viral infection leads to an increase in endothelial cell death and promotes the release of proinflammatory mediators and inflammatory/ immune cell recruitment.[68] The cell environment of endothelial cells in obese patients promotes SARS-CoV-2 infection, resulting in coagulopathy and thrombosis.[69]
Obesity-related inflammation and metabolic molecular changes alter the overall structure of endothelial cells, along with multiple structural, functional, and molecular changes, including changes in the production of nitric oxide (NO) and reactive oxygen species (ROS). NO has many functions, including relaxation of vascular smooth muscle cells, increasing blood flow, inhibition of platelet aggregation, and inhibition of endothelial cell activation. The concentration and production of endothelial NO are, however, inhibited in obese patients.[32] A higher incidence of venous thromboembolism, arterial thrombosis, and thrombotic microangiopathy was found in patients with COVID-19.[67]
Vitamin D deficiency in obese patients with COVID-19
Vitamin D deficiency is prevalent and well documented in obese patients, and it disrupts immune function and increases the risk of infection.[56, 70] A higher prevalence of vitamin D deficiency was found in patients with COVID-19. Vitamin D deficiency is associated with atherosclerotic heart disease and impaired pancreatic islet function.[71] Moreover, vitamin D helps to control respiratory infections by regulating the RAAS and maintaining a balance between pro- and anti-inflammatory cytokines.[56] Consequently, vitamin D deficiency can block the activation of these defense pathways, trigger cytokine storms, and lead to immune dysfunction, which eventually exacerbates COVID-19.[56] Studies have also found that vitamin D supplementation can protect against acute respiratory tract infection.[72]
Comorbidities in obese patients with COVID-19
Obesity increases the risk of coronary heart disease due to epicardial AT and perivascular fat. Moreover, studies have indicated that obesity in children aged 11 to 12 years is positively correlated with the development of cardiovascular dysfunction.[73] Chronic renal diseases,[74] lipodystrophy, lipoatrophy, premature aging, and other disease states may also be associated with AT.[75] All these conditions may lead to an inadequate metabolic response to immune challenges during severe COVID-19 infection and result in a poor prognosis.[12] Figure 1 shows the mechanism by which obesity leads to increased severity of COVID-19.
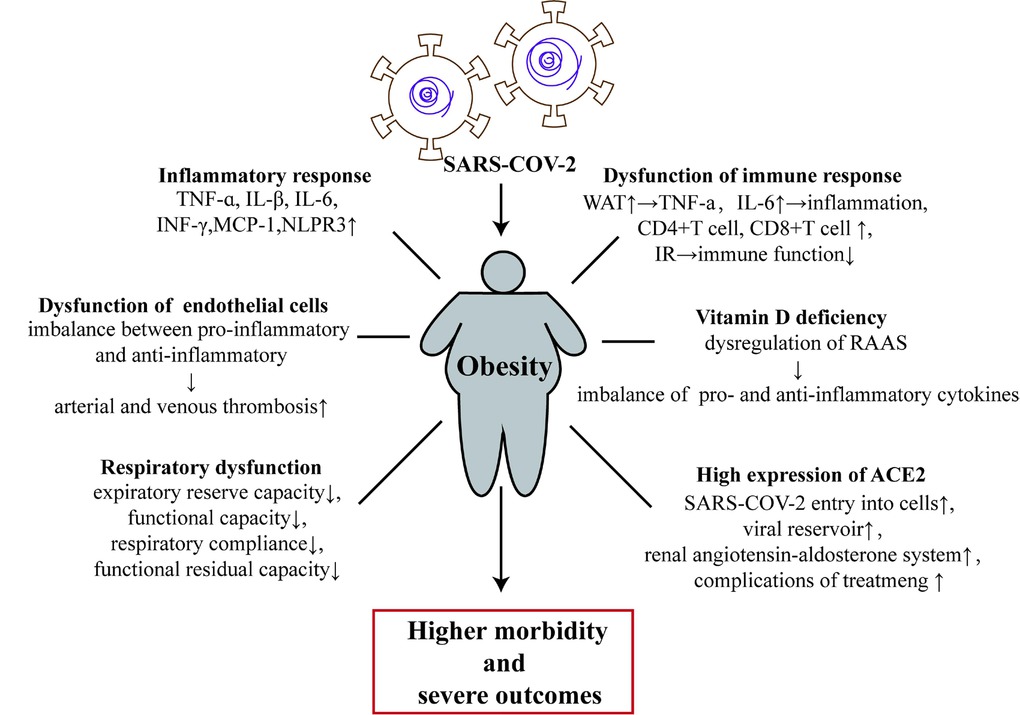
Obesity and related comorbidities are associated with the pathogenesis and physiological changes that lead to infection and pathogenicity of COVID-19. ACE2: angiotensin-converting enzyme 2; IR: insulin resistance; IL-1β: interleukin-1β; IL-6: interleukin-6; TNF-α: tumor necrosis factor-alpha; INF-γ: interferon-γ; MCP-1: monocyte chemoattractant protein-1; RAAS: renal–angiotensin–aldosterone system; SARS-CoV-2: severe acute respiratory syndrome coronavirus-2; TNFR: tumor necrosis factor receptor; WAT: white adipose tissue; COVID-19: coronavirus disease 2019.
Evaluation of obesity and related comorbidities
The WHO defines obesity as BMI ≥ 30 kg/m2, while the criterion for obesity in China is BMI ≥ 28 kg/m2. BMI should be measured in all patients with COVID-19, especially in younger and middle-aged people with fewer comorbidities, as a higher level of obesity may lead to a worse prognosis.[76] Compared to BMI, waist circumference and waist-to-hip ratio can better assess the inflammatory status of the body and the degree of abdominal obesity, and provide references for prognosis.[77, 78]
Obese patients often have underlying diseases, and therefore, the evaluation of comorbidities in these patients is essential.[79] Obese patients with COVID-19 are prone to have coagulation abnormalities and elevated D-dimer and fibrinogen levels. Coagulation indicators should be monitored and ultrasound screening should be used to signal the presence of deep vein thrombosis and pulmonary embolism.[80] Attention should be given to indicators such as ferritin, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and IL-6 to identify early critical patients.
Treatment strategies for obese patients with COVID-19
Anticoagulation therapy
All obese patients with COVID-19 should receive preventive anticoagulation therapy in the hospital, except for patients with contraindications for anticoagulation therapy. Low-molecular-weight heparin should be chosen first if the patient has no severe renal impairment. In patients with creatinine clearance (CrCl) > 30 mL/min, the routine preventive dose of enoxaparin is 40 mg or 4,000 U once daily; this dose can be administered every 12 h for patients with BMI ≥ 40 kg/m2 or high-risk patients with venous thromboembolism (VTE) after metabolic surgery.[81, 82] The dose of dalteparin can be increased by 30% based on standard preventive doses.[83] The INSPIRATION trial conducted an anticoagulant dose study on 600 critically ill COVID-19 ICU patients. The results showed that a moderate dose of enoxaparin did not improve thrombosis and reduce the rate of death and the use of extracorporeal membrane oxygenation (ECMO). Therefore, anticoagulation for patients with COVID-19 is recommended to be given as a preventive dose rather than a moderate or therapeutic dose.[84]
Airway management
Airway management is very important for obese patients with COVID-19. Hospital inpatients initially prefer high-flow oxygen through a nasal cannula (HFNC). They choose to tolerate the highest flow (usually 50 L/min) and meet the saturation of peripheral oxygen (SpO2) of 90%–96% required for minimum fraction inspired oxygen concentration (FiO2).[85] When the disease progresses rapidly within a few hours, with the oxygenation index PaO2/FiO2 at < 150 mmHg, HFNC at > 50 L/min, and FiO2 at > 0.6, and the condition remains deteriorated with unstable hemodynamics, early tracheal intubation is recommended.[86] Tracheal intubation is more difficult for obese patients with COVID-19, and deoxygenation is more likely to occur during operation,[87] and therefore, preoxygenation should be given during tracheal intubation.[88] It is recommended to use visual laryngoscope intubation to improve the success rate of intubation at the first attempt and reduce the risk of infection to healthcare workers.[89] It is also recommended to adopt a low tidal volume (4–8 mL/ kg ideal body weight) and low plateau pressure ventilation (< 30 cmH2O) while providing moderate-to-high positive end-expiratory pressure to prevent airway collapse.[90]
Glucocorticoids
Obese patients are more likely to have spontaneous breathing difficulties and diffuse pulmonary injury following COVID-19 infection. For patients with COVID-19 who need auxiliary oxygen supply, glucocorticoids can regulate inflammation-mediated lung injury and reduce the occurrence of respiratory failure and death.[91] A study of 6,425 hospitalized patients with COVID-19 showed that compared to conventional treatment, oral or intravenous dexamethasone (6 mg/ d, for up to 10 days or to receive usual care alone) can reduce the overall 28-day mortality of patients by 17%. However, the use of glucocorticoids is not recommended for the prevention or treatment of COVID-19 patients with mild-to-moderate illness and for those receiving no respiratory support.[92] Methylprednisolone is recommended as the first choice for critically ill patients. The recommended dose is 1–2 mg/(kg·d) or 40–80 mg/ d, and the course of treatment is 3–5 days.[93] For patients with body weight above 80 kg, the dose of methylprednisolone is 40 mg twice daily. The dose can be increased up to 60–80 mg twice daily if the body temperature is over 38℃.[94] Although the effective benefits of glucocorticoids such as hydrocortisone[95] and methylprednisolone[96] for critically ill patients with COVID-19 remain unclear, the key to the use of glucocorticoids for treating COVID-19 should not be ignored.
Inhibitors of the IL-6 pathway
Blocking the inflammatory pathway may prevent disease progression.[97] IL-6 pathway inhibitors include IL-6 receptor blockers and direct IL-6 inhibitors, which can be used for severe and critical patients with elevated IL-6 levels and extensive lesions detected on chest CT. Tocilizumab is the only approved IL-6 monoclonal inhibitor currently used in China. The first dose for adults is 4–8 mg/ kg. If the patient continues to have a high fever after use, one additional dose can be given; the administration interval is ≥ 12 h, and the total number of administration is ≤ 2 times. The Infectious Diseases Society of America (IDSA) and COVID-19 diagnosis and treatment guidelines recommend that for hospitalized adult patients with progressive severe or critical illness, rapid increase in oxygen demand, and elevated levels of systemic inflammatory markers (CRP level ≥ 75 mg/ L), tocilizumab can be used in addition to dexamethasone.[98] An open-label study of 4,116 patients in the United Kingdom showed that compared to conventional treatment, the addition of tocilizumab administered 1–2 times based on weight can reduce the 28-day mortality rate (31% vs. 35%). Eighty-two percent of the study participants were treated with dexamethasone at the same time, and the results suggested that dexamethasone combined with tocilizumab provided more benefits to the patients.[99] Another study of 803 ICU patients with severe COVID-19 showed that compared to the standard treatment group, tocilizumab and saliruzumab reduced in-hospital mortality (28% and 22%, respectively, vs. 36%).[100] We should also be aware of the risk of infection when using IL-6 pathway inhibitors.[101]
Long-term prognosis of obese patients with COVID-19
Prognosis study of obese patients with COVID-19
A one-year follow-up study of COVID-19 patients after discharge showed that obese patients had more severe metabolic disorders, pulmonary function, and residual lesions on chest CT scans than nonobese patients. There were no significant differences in age, gender, underlying diseases, and COVID-19 severity in this study.[102] The authors of a previous study believed that the addition of inspiratory muscle training (IMT) to the pulmonary rehabilitation (PR) program can improve abnormal breathing patterns,[103] balance the relationship between respiratory muscle demand and respiratory muscle energy supply,[104] and improve patients’ pulmonary function and exercise capacity.
Vaccine immunity study in obese patients with COVID-19
Serum IgG antibody level in obese patients with SARS-CoV-2 infection has a negative correlation with BMI. Irrespective of the individuals’ age, obesity damages the function of B cells, increases the secretion of pro-inflammatory factors, reduces the secretion of anti-inflammatory factors, and diminishes the vaccine’s ability to produce protective antibodies.[105] Vaccine studies on influenza, hepatitis B, and rabies showed that the immune response of obese people following vaccination was worse than that of nonobese people,[106] suggesting that the immune efficacy of the COVID-19 vaccine may be reduced by obesity.[107]
Physical and psychological problems caused by longterm staying-at-home for obese patients during covid-19 epidemic
Weight gain
To slow down the spread of the COVID-19 pandemic, many countries issued stay-at-home orders, which led to some physical and psychological problems. The most prominent concern was weight gain, especially among obese and overweight people. A survey of 1,516 stay-at-home people in the United States in 3 months showed that nearly 30% of the participants gained weight, and 26% of obese people gained weight more than 2 kg, which was higher than that of nonobese people (14.8%), and the average daily physical activity time was reduced by approximately 30 min compared to that before living at home during the COVID-19 pandemic.[108] Lin et al.[109] accurately evaluated the impact of home quarantine on weight gain among participants living at home during the COVID-19 pandemic; the weight was measured for each participant during 4 months and through the Bluetooth smart weight scale. The results showed that the weight increased by approximately 0.27 kg every 10 days. Previous studies also showed that some people may choose to decrease the intake of fresh vegetables and fruits and replace them with canned foods.[110] In addition, some people were accustomed to eating snacks after meals, not restricting their diets, and consuming more in response to anxiety. Therefore, the impact of diet on weight gain during the COVID-19 pandemic needs to be analyzed based on the changes in eating habits.
Increased anxiety and depression
The pressure of the COVID-19 pandemic also led to some psychological issues. A survey of 1,210 respondents from 194 cities showed that at the early stage of the COVID-19 pandemic in China, 53.8% of respondents described the psychological impact of the pandemic on them as moderate or severe, and 28.8% of these respondents had moderate-to-severe symptoms of anxiety, while 16.5% of the respondents had moderate-to-severe symptoms of depression.[111] A psychological survey of 4,397 young people showed that 42% of them had anxiety and depression, while 21.4% had insomnia. Isolation measures and social disconnection are more likely to cause anxiety and depression in the elderly.[112] In addition, for critically ill patients who underwent ICU treatment, one-third of them showed persistent anxiety within 1 year after discharge,[113] and psychological counseling and follow-up are required for these patients.
Conclusions
COVID-19 is an unprecedented pandemic of a highly contagious virus that is taking a heavy toll on people’s lives and health on a global scale. Obesity itself is an epidemic and can be an independent factor in exacerbating the illness of patients with COVID-19, leading to increased hospitalization, ICU admission, IMV use, and mortality. Obesity also increases the risk of COVID-19 complications through various mechanisms. It can also cause psychological issues in patients with COVID-19. After assessing the risk of complications, drug therapy and airway management can be used for the treatment of obese patients. Our goal is to investigate personalized interventions based on the understanding of the mechanisms of obesity that lead to more severe diseases in patients with COVID-19.
Funding statement: This study was funded by the National Key R&D Program of China (2020YF2009006).
-
Conflict of Interest
Qi Pan is an Editorial Board Member of the journal. The article was subject to the journal’s standard procedures, with peer review handled independently of this member and her research group.
-
Author Contributions
Pan Q and Guo L made substantial contributions to study concept and design and critically revised the manuscript for important intellectual content. Fei S and Feng X drafted the manuscript. Luo J helped to improve the English of the paper. All authors have read and approved the final version to be published.
References
1 Wolff D, Nee S, Hickey NS, Marschollek M. Risk factors for Covid-19 severity and fatality: a structured literature review. Infection 2021;49:15-28.10.1007/s15010-020-01509-1Search in Google Scholar PubMed PubMed Central
2 Zhou Y, Chi J, Lv W, Wang Y. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (Covid-19). Diabetes Metab Res Rev 2021;37:e3377.10.1002/dmrr.3377Search in Google Scholar PubMed PubMed Central
3 Cai Z, Yang Y, Zhang J. Obesity is associated with severe disease and mortality in patients with coronavirus disease 2019 (COVID-19): a meta-analysis. BMC Public Health 2021;21:1505.10.1186/s12889-021-11546-6Search in Google Scholar PubMed PubMed Central
4 Gasmi A, Peana M, Pivina L, Srinath S, Gasmi Benahmed A, Semenova Y, et al.Interrelations between COVID-19 and other disorders. Clin Immunol 2021;224:108651.10.1016/j.clim.2020.108651Search in Google Scholar PubMed PubMed Central
5 Higgins V, Sohaei D, Diamandis EP, Prassas I. COVID- 19: from an acute to chronic disease? Potential long-term health consequences. Crit Rev Clin Lab Sci 2021;58:297-310.10.1080/10408363.2020.1860895Search in Google Scholar PubMed
6 Zakka K, Chidambaram S, Mansour S, Mahawar K, Salminen P, Almino R, et al. SARS-CoV-2 and Obesity: “CoVesity”-a Pandemic Within a Pandemic. Obes Surg 2021;31:1745-54.10.1007/s11695-020-04919-0Search in Google Scholar PubMed PubMed Central
7 Yang J, Hu J, Zhu C. Obesity aggravates COVID- 19: A systematic review and meta-analysis. J Med Virol 2021;93:257-61.10.1002/jmv.26237Search in Google Scholar PubMed PubMed Central
8 Thakur B, Dubey P, Benitez J, Torres JP, Reddy S, Shokar N, et al. A systematic review and meta-analysis of geographic differences in comorbidities and associated severity and mortality among individuals with COVID-19. Sci Rep 2021;11:8562.10.1038/s41598-021-88130-wSearch in Google Scholar PubMed PubMed Central
9 Chang TH, Chou CC, Chang LY. Effect of obesity and body mass index on coronavirus disease 2019 severity: A systematic review and meta-analysis. Obes Rev 2020;21:e13089.10.1111/obr.13089Search in Google Scholar PubMed
10 Pasquarelli-do-Nascimento G, Braz-de-Melo HA, Faria SS, Santos IO, Kobinger GP, Magalhães KG. Hypercoagulopathy and Adipose Tissue Exacerbated Inflammation May Explain Higher Mortality in COVID- 19 Patients With Obesity. Front Endocrinol (Lausanne) 2020;11:530.10.3389/fendo.2020.00530Search in Google Scholar PubMed PubMed Central
11 Zhu X, Yang L, Huang K. COVID-19 and Obesity: Epidemiology, Pathogenesis and Treatment. Diabetes Metab Syndr Obes 2020;13:4953-9.10.2147/DMSO.S285197Search in Google Scholar PubMed PubMed Central
12 Aghili SMM, Ebrahimpur M, Arjmand B, Shadman Z, Pejman Sani M, Qorbani M, et al. Obesity in COVID- 19 era, implications for mechanisms, comorbidities, and prognosis: a review and meta-analysis. Int J Obes (Lond) 2021;45:998-1016.10.1038/s41366-021-00776-8Search in Google Scholar PubMed PubMed Central
13 Pranata R, Lim MA, Yonas E, Vania R, Lukito AA, Siswanto BB, et al. Body mass index and outcome in patients with COVID- 19: A dose-response meta-analysis. Diabetes Metab 2021;47:101178.10.1016/j.diabet.2020.07.005Search in Google Scholar PubMed PubMed Central
14 Du Y, Lv Y, Zha W, Zhou N, Hong X. Association of body mass index (BMI) with critical COVID- 19 and in-hospital mortality: A dose-response meta-analysis. Metabolism 2021;117:154373.10.1016/j.metabol.2020.154373Search in Google Scholar PubMed PubMed Central
15 Zhao X, Gang X, He G, Li Z, Lv Y, Han Q, et al. Obesity Increases the Severity and Mortality of Influenza and COVID- 19: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne) 2020;11:595109.10.3389/fendo.2020.595109Search in Google Scholar PubMed PubMed Central
16 Sharma A, Garg A, Rout A, Lavie CJ. Association of Obesity With More Critical Illness in COVID-19. Mayo Clin Proc 2020;95:2040-2.10.1016/j.mayocp.2020.06.046Search in Google Scholar PubMed PubMed Central
17 Stefan N, Birkenfeld AL, Schulze MB. Global pandemics interconnected - obesity, impaired metabolic health and COVID- 19. Nat Rev Endocrinol 2021;17:135-49.10.1038/s41574-020-00462-1Search in Google Scholar PubMed
18 Ahmed B, Sultana R, Greene MW. Adipose tissue and insulin resistance in obese. Biomed Pharmacother 2021;137:111315.10.1016/j.biopha.2021.111315Search in Google Scholar PubMed
19 Amin MT, Fatema K, Arefin S, Hussain F, Bhowmik DR, Hossain MS. Obesity, a major risk factor for immunity and severe outcomes of COVID-19. Biosci Rep 2021;41:BSR20210979.10.1042/BSR20210979Search in Google Scholar PubMed PubMed Central
20 Louie JK, Acosta M, Samuel MC, Schechter R, Vugia DJ, Harriman K, et al. A novel risk factor for a novel virus: obesity and 2009 pandemic influenza A (H1N1). Clin Infect Dis 2011;52:301-12.10.1093/cid/ciq152Search in Google Scholar PubMed
21 Huang Y, Lu Y, Huang YM, Wang M, Ling W, Sui Y, et al. Obesity in patients with COVID-19: a systematic review and meta-analysis. Metabolism 2020;113:154378.10.1016/j.metabol.2020.154378Search in Google Scholar PubMed PubMed Central
22 Raeisi T, Mozaffari H, Sepehri N, Darand M, Razi B, Garousi N, Alizadeh M, et al.The negative impact of obesity on the occurrence and prognosis of the 2019 novel coronavirus (COVID-19) disease: a systematic review and meta-analysis. Eat Weight Disord 2022;27:893-911.10.1007/s40519-021-01269-3Search in Google Scholar PubMed PubMed Central
23 Huang HK, Bukhari K, Peng CC, Hung DP, Shih MC, Chang RH, et al. The J-shaped relationship between body mass index and mortality in patients with COVID- 19: A dose-response meta-analysis. Diabetes Obes Metab 2021;23:1701-9.10.1111/dom.14382Search in Google Scholar PubMed PubMed Central
24 Hoong CWS, Hussain I, Aravamudan VM, Phyu EE, Lin JHX, Koh H. Obesity is Associated with Poor Covid-19 Outcomes: A Systematic Review and Meta-Analysis. Horm Metab Res 2021;53:85-93.10.1055/a-1326-2125Search in Google Scholar PubMed
25 Helvaci N, Eyupoglu ND, Karabulut E, Yildiz BO. Prevalence of Obesity and Its Impact on Outcome in Patients With COVID-19: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne) 2021;12:598249.10.3389/fendo.2021.598249Search in Google Scholar PubMed PubMed Central
26 Malik VS, Ravindra K, Attri SV, Bhadada SK, Singh M. Higher body mass index is an important risk factor in COVID-19 patients: a systematic review and meta-analysis. Environ Sci Pollut Res Int 2020;27:42115-23.10.1007/s11356-020-10132-4Search in Google Scholar PubMed PubMed Central
27 Földi M, Farkas N, Kiss S, Zádori N, Váncsa S, Szakó L, et al. Obesity is a risk factor for developing critical condition in COVID- 19 patients: A systematic review and meta-analysis. Obes Rev 2020;21:e13095.10.1111/obr.13095Search in Google Scholar PubMed PubMed Central
28 Zhang X, Lewis AM, Moley JR, Brestoff JR. A systematic review and meta-analysis of obesity and COVID- 19 outcomes. Sci Rep 2021;11:7193.10.1038/s41598-021-86694-1Search in Google Scholar PubMed PubMed Central
29 Poly TN, Islam MM, Yang HC, Lin MC, Jian WS, Hsu MH, et al. Obesity and Mortality Among Patients Diagnosed With COVID- 19: A Systematic Review and Meta-Analysis. Front Med (Lausanne) 2021;8:620044.10.3389/fmed.2021.620044Search in Google Scholar PubMed PubMed Central
30 Földi M, Farkas N, Kiss S, Dembrovszky F, Szakács Z, Balaskó M, et al. Visceral Adiposity Elevates the Risk of Critical Condition in COVID- 19: A Systematic Review and Meta-Analysis. Obesity (Silver Spring) 2021;29:521-8.10.1002/oby.23096Search in Google Scholar PubMed PubMed Central
31 Battisti S, Pedone C, Napoli N, Russo E, Agnoletti V, Nigra SG, et al. Computed Tomography Highlights Increased Visceral Adiposity Associated With Critical Illness in COVID-19. Diabetes Care 2020;43:e129-e130.10.2337/dc20-1333Search in Google Scholar PubMed
32 Ritter A, Kreis NN, Louwen F, Yuan J. Obesity and COVID- 19: Molecular Mechanisms Linking Both Pandemics. Int J Mol Sci 2020;21:5793.10.3390/ijms21165793Search in Google Scholar PubMed PubMed Central
33 Marazuela M, Giustina A, Puig-Domingo M. Endocrine and metabolic aspects of the COVID-19 pandemic. Rev Endocr Metab Disord 2020;21:495-507.10.1007/s11154-020-09569-2Search in Google Scholar PubMed PubMed Central
34 Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 2011;11:85-97.10.1038/nri2921Search in Google Scholar PubMed PubMed Central
35 Bertocchi I, Foglietta F, Collotta D, Eva C, Brancaleone V, Thiemermann C, et al. The hidden role of NLRP3 inflammasome in obesity-related COVID-19 exacerbations: Lessons for drug repurposing. Br J Pharmacol 2020;177:4921-30.10.1111/bph.15229Search in Google Scholar PubMed PubMed Central
36 López-Reyes A, Martinez-Armenta C, Espinosa-Velázquez R, Vázquez-Cárdenas P, Cruz-Ramos M, Palacios-Gonzalez B, et al. NLRP3 Inflammasome: The Stormy Link Between Obesity and COVID-19. Front Immunol 2020;11:570251.10.3389/fimmu.2020.570251Search in Google Scholar PubMed PubMed Central
37 Liu H, Xu R, Kong Q, Liu J, Yu Z, Zhao C. Downregulated NLRP3 and NLRP1 inflammasomes signaling pathways in the development and progression of type 1 diabetes mellitus. Biomed Pharmacother 2017;94:619-26.10.1016/j.biopha.2017.07.102Search in Google Scholar PubMed
38 Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796-808.10.1172/JCI200319246Search in Google Scholar
39 Esser N, L’Homme L, De Roover A, Kohnen L, Scheen AJ, Moutschen M, et al. Obesity phenotype is related to NLRP3 inflammasome activity and immunological profile of visceral adipose tissue. Diabetologia 2013;56:2487-97.10.1007/s00125-013-3023-9Search in Google Scholar PubMed
40 Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract 2005;69:29-35.10.1016/j.diabres.2004.11.007Search in Google Scholar PubMed
41 Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med 2011;17:179-88.10.1038/nm.2279Search in Google Scholar PubMed PubMed Central
42 Bhattacharya I, Ghayor C, Pérez Dominguez A, Weber FE. From Influenza Virus to Novel Corona Virus (SARS-CoV-2)-The Contribution of Obesity. Front Endocrinol (Lausanne) 2020;11:556962.10.3389/fendo.2020.556962Search in Google Scholar PubMed PubMed Central
43 Silverio R, Gonçalves DC, Andrade MF, Seelaender M. Coronavirus Disease 2019 (COVID-19) and Nutritional Status: The Missing Link? Adv Nutr 2021;12:682-92.10.1093/advances/nmaa125Search in Google Scholar PubMed PubMed Central
44 Kintscher U, Hartge M, Hess K, Foryst-Ludwig A, Clemenz M, Wabitsch M, et al. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol 2008;28:1304-10.10.1161/ATVBAHA.108.165100Search in Google Scholar PubMed
45 Popkin BM, Du S, Green WD, Beck MA, Algaith T, Herbst CH, et al. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes Rev 2020;21:e13128.10.1111/obr.13128Search in Google Scholar PubMed PubMed Central
46 Tan L, Wang Q, Zhang D, Ding J, Huang Q, Tang YQ, et al. Lymphopenia predicts disease severity of COVID- 19: a descriptive and predictive study. Signal Transduct Target Ther 2020;5:33.10.1038/s41392-020-0148-4Search in Google Scholar PubMed PubMed Central
47 Liu Y, Du X, Chen J, Jin Y, Peng L, Wang HHX, et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect 2020;81:e6-e12.10.1016/j.jinf.2020.04.002Search in Google Scholar PubMed PubMed Central
48 Kuperberg SJ, Navetta-Modrov B. The Role of Obesity in the Immunopathogenesis of COVID-19 Respiratory Disease and Critical Illness. Am J Respir Cell Mol Biol 2021;65:13-21.10.1165/rcmb.2020-0236TRSearch in Google Scholar PubMed PubMed Central
49 Jain M, Budinger GR, Lo A, Urich D, Rivera SE, Ghosh AK, et al. Leptin promotes fibroproliferative acute respiratory distress syndrome by inhibiting peroxisome proliferator-activated receptor-γ. Am J Respir Crit Care Med 2011;183:1490-8.10.1164/rccm.201009-1409OCSearch in Google Scholar PubMed PubMed Central
50 Ealey KN, Phillips J, Sung HK. COVID-19 and obesity: fighting two pandemics with intermittent fasting. Trends Endocrinol Metab 2021;32:706-20.10.1016/j.tem.2021.06.004Search in Google Scholar PubMed PubMed Central
51 Maurya R, Sebastian P, Namdeo M, Devender M, Gertler A. COVID- 19 Severity in Obesity: Leptin and Inflammatory Cytokine Interplay in the Link Between High Morbidity and Mortality. Front Immunol 2021;12:649359.10.3389/fimmu.2021.649359Search in Google Scholar PubMed PubMed Central
52 Tsai S, Clemente-Casares X, Zhou AC, Lei H, Ahn JJ, Chan YT, et al. Insulin Receptor-Mediated Stimulation Boosts T Cell Immunity during Inflammation and Infection. Cell Metab 2018;28:922-934.e924.10.1016/j.cmet.2018.08.003Search in Google Scholar PubMed
53 Fischer HJ, Sie C, Schumann E, Witte AK, Dressel R, van den Brandt J, et al. The Insulin Receptor Plays a Critical Role in T Cell Function and Adaptive Immunity. J Immunol 2017;198:1910-20.10.4049/jimmunol.1601011Search in Google Scholar PubMed
54 Mohammad S, Aziz R, Al Mahri S, Malik SS, Haji E, Khan AH, et al. Obesity and COVID-19: what makes obese host so vulnerable? Immun Ageing 2021;18:1.10.1186/s12979-020-00212-xSearch in Google Scholar PubMed PubMed Central
55 O’Rourke RW, Lumeng CN. Pathways to Severe COVID-19 for People with Obesity. Obesity (Silver Spring) 2021;29:645-53.10.1002/oby.23099Search in Google Scholar PubMed PubMed Central
56 Yan T, Xiao R, Wang N, Shang R, Lin G. Obesity and severe coronavirus disease 2019: molecular mechanisms, paths forward, and therapeutic opportunities. Theranostics 2021;11:8234-53.10.7150/thno.59293Search in Google Scholar PubMed PubMed Central
57 Gao YD, Ding M, Dong X, Zhang JJ, Kursat Azkur A, Azkur D, et al. Risk factors for severe and critically ill COVID- 19 patients: A review. Allergy 2021;76:428-55.10.1111/all.14657Search in Google Scholar PubMed
58 Bornstein SR, Dalan R, Hopkins D, Mingrone G, Boehm BO. Endocrine and metabolic link to coronavirus infection. Nat Rev Endocrinol 2020;16:297-8.10.1038/s41574-020-0353-9Search in Google Scholar PubMed PubMed Central
59 Engin AB, Engin ED, Engin A. Two important controversial risk factors in SARS-CoV-2 infection: Obesity and smoking. Environ Toxicol Pharmacol 2020;78:103411.10.1016/j.etap.2020.103411Search in Google Scholar PubMed PubMed Central
60 Hanff TC, Harhay MO, Brown TS, Cohen JB, Mohareb AM. Is There an Association Between COVID- 19 Mortality and the Renin-Angiotensin System? A Call for Epidemiologic Investigations. Clin Infect Dis 2020;71:870-4.10.1093/cid/ciaa329Search in Google Scholar PubMed PubMed Central
61 Bansal R, Gubbi S, Muniyappa R. Metabolic Syndrome and COVID 19: Endocrine-Immune-Vascular Interactions Shapes Clinical Course. Endocrinology 2020;161:bqaa112.10.1210/endocr/bqaa112Search in Google Scholar PubMed PubMed Central
62 Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID- 19 infection? Lancet Respir Med 2020;8:e21.10.1016/S2213-2600(20)30116-8Search in Google Scholar PubMed PubMed Central
63 Zamorano Cuervo N, Grandvaux N. ACE2: Evidence of role as entry receptor for SARS-CoV-2 and implications in comorbidities. Elife 2020;9:e61390.10.7554/eLife.61390Search in Google Scholar PubMed PubMed Central
64 Sanchis-Gomar F, Lavie CJ, Mehra MR, Henry BM, Lippi G. Obesity and Outcomes in COVID-19: When an Epidemic and Pandemic Collide. Mayo Clin Proc 2020;95:1445-53.10.1016/j.mayocp.2020.05.006Search in Google Scholar PubMed PubMed Central
65 Kruglikov IL, Scherer PE. The Role of Adipocytes and Adipocyte-Like Cells in the Severity of COVID-19 Infections. Obesity (Silver Spring) 2020;28:1187-90.10.1002/oby.22856Search in Google Scholar PubMed PubMed Central
66 Kwaifa IK, Bahari H, Yong YK, Noor SM. Endothelial Dysfunction in Obesity-Induced Inflammation: Molecular Mechanisms and Clinical Implications. Biomolecules 2020;10:291.10.3390/biom10020291Search in Google Scholar PubMed PubMed Central
67 Gu SX, Tyagi T, Jain K, Gu VW, Lee SH, Hwa JM, et al. Thrombocytopathy and endotheliopathy: crucial contributors to COVID- 19 thromboinflammation. Nat Rev Cardiol 2021;18:194-209.10.1038/s41569-020-00469-1Search in Google Scholar PubMed PubMed Central
68 Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004;203:631-7.10.1002/path.1570Search in Google Scholar PubMed PubMed Central
69 Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al.Endothelial cell infection and endotheliitis in COVID- 19. Lancet 2020;395:1417-8.10.1016/S0140-6736(20)30937-5Search in Google Scholar PubMed PubMed Central
70 Rhodes JM, Subramanian S, Laird E, Griffin G, Kenny RA. Perspective: Vitamin D deficiency and COVID-19 severity-plausibly linked by latitude, ethnicity, impacts on cytokines, ACE2 and thrombosis. J Intern Med 2021;289:97-115.10.1111/joim.13149Search in Google Scholar PubMed PubMed Central
71 Stokić E, Kupusinac A, Tomić-Naglić D, Zavišić BK, Mitrović M, Smiljenić D, et al. Obesity and vitamin D deficiency: trends to promote a more proatherogenic cardiometabolic risk profile. Angiology 2015;66:237-243.10.1177/0003319714528569Search in Google Scholar PubMed
72 Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. Bmj 2017;356:i6583.10.1136/bmj.i6583Search in Google Scholar PubMed PubMed Central
73 Zhao S, Kusminski CM, Scherer PE. Adiponectin, Leptin and Cardiovascular Disorders. Circ Res 2021;128:136-49.10.1161/CIRCRESAHA.120.314458Search in Google Scholar PubMed PubMed Central
74 Hammoud SH, AlZaim I, Al-Dhaheri Y, Eid AH, El-Yazbi AF. Perirenal Adipose Tissue Inflammation: Novel Insights Linking Metabolic Dysfunction to Renal Diseases. Front Endocrinol (Lausanne) 2021;12:707126.10.3389/fendo.2021.707126Search in Google Scholar PubMed PubMed Central
75 Kobayashi M, Nezu Y, Tagawa R, Higami Y. Mitochondrial Unfolded Protein Responses in White Adipose Tissue: Lipoatrophy, Whole-Body Metabolism and Lifespan. Int J Mol Sci 2021;22:2854.10.3390/ijms22062854Search in Google Scholar PubMed PubMed Central
76 Soeroto AY, Soetedjo NN, Purwiga A, Santoso P, Kulsum ID, Suryadinata H, et al. Effect of increased BMI and obesity on the outcome of COVID- 19 adult patients: A systematic review and meta-analysis. Diabetes Metab Syndr 2020;14:1897-904.10.1016/j.dsx.2020.09.029Search in Google Scholar PubMed PubMed Central
77 Ye Q, Wang B, Mao J. The pathogenesis and treatment of the 'Cytokine Storm' in COVID-19. J Infect 2020;80:607-13.10.1016/j.jinf.2020.03.037Search in Google Scholar PubMed PubMed Central
78 Watanabe M, Caruso D, Tuccinardi D, Risi R, Zerunian M, Polici M, et al. Visceral fat shows the strongest association with the need of intensive care in patients with COVID- 19. Metabolism 2020;111:154319.10.1016/j.metabol.2020.154319Search in Google Scholar PubMed PubMed Central
79 Ochoa JB, Cárdenas D, Goiburu ME, Bermúdez C, Carrasco F, Correia M. Lessons Learned in Nutrition Therapy in Patients With Severe COVID-19. JPEN J Parenter Enteral Nutr 2020;44:1369-75.10.1002/jpen.2005Search in Google Scholar PubMed PubMed Central
80 Al-Samkari H, Karp Leaf RS, Dzik WH, Carlson JCT, Fogerty AE, Waheed A, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood 2020;136:489-500.10.1182/blood.2020006520Search in Google Scholar PubMed PubMed Central
81 Martinelli I, Ciavarella A, Abbattista M, Aliberti S, De Zan V, Folli C, et al. Increasing dosages of low-molecular-weight heparin in hospitalized patients with Covid-19. Intern Emerg Med 2021;16:1223-9.10.1007/s11739-020-02585-9Search in Google Scholar PubMed PubMed Central
82 Scholten DJ, Hoedema RM, Scholten SE. A comparison of two different prophylactic dose regimens of low molecular weight heparin in bariatric surgery. Obes Surg 2002;12:19-24.10.1381/096089202321144522Search in Google Scholar PubMed
83 Nutescu EA, Spinler SA, Wittkowsky A, Dager WE. Low-molecular-weight heparins in renal impairment and obesity: available evidence and clinical practice recommendations across medical and surgical settings. Ann Pharmacother 2009;43:1064-83.10.1345/aph.1L194Search in Google Scholar PubMed
84 Sadeghipour P, Talasaz AH, Rashidi F, Sharif-Kashani B, Beigmohammadi MT, Farrokhpour M, et al. Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial. Jama 2021;325:1620-30.10.1001/jama.2021.4152Search in Google Scholar PubMed PubMed Central
85 Lemyze M, Courageux N, Maladobry T, Arumadura C, Pauquet P, Orfi A, et al. Implications of Obesity for the Management of Severe Coronavirus Disease 2019 Pneumonia. Crit Care Med 2020;48:e761-7.10.1097/CCM.0000000000004455Search in Google Scholar PubMed PubMed Central
86 Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, et al. Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19). Crit Care Med 2020;48:e440-9.10.1097/CCM.0000000000004363Search in Google Scholar PubMed PubMed Central
87 Natt BS, Malo J, Hypes CD, Sakles JC, Mosier JM. Strategies to improve first attempt success at intubation in critically ill patients. Br J Anaesth 2016;117 Suppl 1:i60-8.10.1093/bja/aew061Search in Google Scholar PubMed
88 Weingart SD, Trueger NS, Wong N, Scofi J, Singh N, Rudolph SS. Delayed sequence intubation: a prospective observational study. Ann Emerg Med 2015;65:349-55.10.1016/j.annemergmed.2014.09.025Search in Google Scholar PubMed
89 Hypes CD, Stolz U, Sakles JC, Joshi RR, Natt B, Malo J, et al. Video Laryngoscopy Improves Odds of First-Attempt Success at Intubation in the Intensive Care Unit. A Propensity-matched Analysis. Ann Am Thorac Soc 2016;13:382-90.10.1513/AnnalsATS.201508-505OCSearch in Google Scholar PubMed
90 Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/ Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2017;195:1253-63.10.1164/rccm.201703-0548STSearch in Google Scholar PubMed
91 Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46:846-8.10.1007/s00134-020-05991-xSearch in Google Scholar PubMed PubMed Central
92 Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med 2021;384:693-704.10.1056/NEJMoa2021436Search in Google Scholar PubMed PubMed Central
93 Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507-13.10.1016/S0140-6736(20)30211-7Search in Google Scholar PubMed PubMed Central
94 Zhao JP, Hu Y, Du RH, Chen ZS, Jin Y, Zhou M, et al. Expert consensus on the use of corticosteroid in patients with 2019-nCoV pneumonia. Zhonghua Jie He He Hu Xi Za Zhi 2020;43:E007.Search in Google Scholar
95 Dequin PF, Heming N, Meziani F, Plantefève G, Voiriot G, Badié J, et al. ll Patients With COVID-19: A Randomized Clinical Trial. Jama 2020;324:1298-306.10.1001/jama.2020.16761Search in Google Scholar PubMed PubMed Central
96 Jeronimo CMP, Farias MEL, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, et al. Methylprednisolone as Adjunctive Therapy for Patients Hospitalized With Coronavirus Disease 2019 (COVID-19; Metcovid): A Randomized, Double-blind, Phase IIb, Placebo-controlled Trial. Clin Infect Dis 2021;72:e373-81.10.1093/cid/ciaa1177Search in Google Scholar PubMed PubMed Central
97 Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID- 19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033-4.10.1016/S0140-6736(20)30628-0Search in Google Scholar PubMed PubMed Central
98 Bhimraj A, Morgan RL, Shumaker AH, Lavergne V, Baden L, Cheng VC, et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Clin Infect Dis 2020;ciaa478.10.1093/cid/ciaa478Search in Google Scholar PubMed PubMed Central
99 Horby PW, Campbell M, Staplin N. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2021;397:1637-45.10.1016/S0140-6736(21)00676-0Search in Google Scholar PubMed PubMed Central
100 Brown MJ, Alazawi W, Kanoni S. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N Engl J Med 2021;385:1147.10.1056/NEJMc2108482Search in Google Scholar PubMed
101 Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M Tocilizumab in patients admitted to hospital with COVID- 19 (RECOVERY): a randomised, controlled, open-label, platform trial. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol 2020;2:e474-84.10.1016/S2665-9913(20)30173-9Search in Google Scholar PubMed PubMed Central
102 Shang L, Wang L, Zhou F, Li J, Liu Y, Yang S. Long-term effects of obesity on COVID-19 patients discharged from hospital. Immun Inflamm Dis. 2021;9:1678-85.10.1002/iid3.522Search in Google Scholar PubMed PubMed Central
103 Maldaner V, Coutinho J, Santana A, Cipriano GFB, Oliveira MC, Carrijo MM, et al. Adjunctive inspiratory muscle training for patients with COVID-19 (COVIDIMT): protocol for randomised controlled double-blind trial. BMJ Open 2021;11:e049545.10.1136/bmjopen-2021-049545Search in Google Scholar PubMed PubMed Central
104 Hoffman M, Augusto VM, Eduardo DS, Silveira BMF, Lemos MD, Parreira VF. Inspiratory muscle training reduces dyspnea during activities of daily living and improves inspiratory muscle function and quality of life in patients with advanced lung disease. Physiother Theory Pract 2021;37:895-905.10.1080/09593985.2019.1656314Search in Google Scholar PubMed
105 Frasca D, Ferracci F, Diaz A, Romero M, Lechner S, Blomberg BB. Obesity decreases B cell responses in young and elderly individuals. Obesity (Silver Spring) 2016;24:615-25.10.1002/oby.21383Search in Google Scholar PubMed PubMed Central
106 Frasca D, Blomberg BB. The Impact of Obesity and Metabolic Syndrome on Vaccination Success. Interdiscip Top Gerontol Geriatr 2020, 43:86-97.10.1159/000504440Search in Google Scholar PubMed
107 Ledford H. How obesity could create problems for a COVID vaccine. Nature 2020;586:488-9.10.1038/d41586-020-02946-6Search in Google Scholar PubMed
108 Seal A, Schaffner A, Phelan S, Brunner-Gaydos H, Tseng M, Keadle S, et al. COVID-19 pandemic and stay-at-home mandates promote weight gain in US adults. Obesity (Silver Spring) 2022;30:240-8.10.1002/oby.23293Search in Google Scholar PubMed PubMed Central
109 Lin AL, Vittinghoff E, Olgin JE, Pletcher MJ, Marcus GM. Body Weight Changes During Pandemic-Related Shelter-in-Place in a Longitudinal Cohort Study. JAMA Netw Open 2021;4:e212536.10.1001/jamanetworkopen.2021.2536Search in Google Scholar PubMed PubMed Central
110 Litton MM, Beavers AW. The Relationship between Food Security Status and Fruit and Vegetable Intake during the COVID-19 Pandemic. Nutrients 2021;13:712.10.3390/nu13030712Search in Google Scholar PubMed PubMed Central
111 Wang C, Pan R, Wan X, Tan Y, Xu L, Ho CS, et al. Immediate Psychological Responses and Associated Factors during the Initial Stage of the 2019 Coronavirus Disease (COVID-19) Epidemic among the General Population in China. Int J Environ Res Public Health 2020;17:1729.10.3390/ijerph17051729Search in Google Scholar PubMed PubMed Central
112 Armitage R, Nellums LB. COVID-19 and the consequences of isolating the elderly. Lancet Public Health 2020;5:e256.10.1016/S2468-2667(20)30061-XSearch in Google Scholar PubMed PubMed Central
113 Nikayin S, Rabiee A, Hashem MD, Huang M, Bienvenu OJ, Turnbull AE, et al. Anxiety symptoms in survivors of critical illness: a systematic review and meta-analysis. Gen Hosp Psychiatry 2016;43:23-9.10.1016/j.genhosppsych.2016.08.005Search in Google Scholar PubMed PubMed Central
© 2022 Sijia Fei, Xinyuan Feng, Jingyi Luo, Lixin Guo, Qi Pan, published by Sciendo
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Editorial
- Inhaled antibiotics and airway bacterial decolonization for patients with chronic obstructive pulmonary disease: The rationale and future
- Perspective
- Retinal examination modalities in the early detection of Alzheimer's disease: Seeing brain through the eye
- Commentary
- Hydrogel: A promising new technique for treating Alzheimer’s disease
- Metabolic adaptation in lactation: Insulin-dependent and -independent glycemic control
- Review Article
- Aberrant energy metabolism in Alzheimer’s disease
- Obesity and coronavirus disease 2019
- Crosstalk between adipose tissue and the heart: An update
- Delta variant: Partially sensitive to vaccination, but still worth global attention
- Benefits of physical activity on cardiometabolic diseases in obese children and adolescents
- Original Article
- TCDD-inducible poly (ADP-ribose) polymerase promotes adipogenesis of both brown and white preadipocytes
- Functional evaluation of intermediate coronary lesions with integrated computed tomography angiography and invasive angiography in patients with stable coronary artery disease
- A novel wide-band dielectric imaging system for electro-anatomic mapping and monitoring in radiofrequency ablation and cryoablation
- Brief Report
- Impact of COVID-19 pandemic control measures on infection of other respiratory pathogens: A real-world data research in Guangzhou, China
Articles in the same Issue
- Editorial
- Inhaled antibiotics and airway bacterial decolonization for patients with chronic obstructive pulmonary disease: The rationale and future
- Perspective
- Retinal examination modalities in the early detection of Alzheimer's disease: Seeing brain through the eye
- Commentary
- Hydrogel: A promising new technique for treating Alzheimer’s disease
- Metabolic adaptation in lactation: Insulin-dependent and -independent glycemic control
- Review Article
- Aberrant energy metabolism in Alzheimer’s disease
- Obesity and coronavirus disease 2019
- Crosstalk between adipose tissue and the heart: An update
- Delta variant: Partially sensitive to vaccination, but still worth global attention
- Benefits of physical activity on cardiometabolic diseases in obese children and adolescents
- Original Article
- TCDD-inducible poly (ADP-ribose) polymerase promotes adipogenesis of both brown and white preadipocytes
- Functional evaluation of intermediate coronary lesions with integrated computed tomography angiography and invasive angiography in patients with stable coronary artery disease
- A novel wide-band dielectric imaging system for electro-anatomic mapping and monitoring in radiofrequency ablation and cryoablation
- Brief Report
- Impact of COVID-19 pandemic control measures on infection of other respiratory pathogens: A real-world data research in Guangzhou, China

