Abstract
It is important to understand how different human organs coordinate and interact with each other. Since obesity and cardiac disease frequently coincide, the crosstalk between adipose tissues and heart has drawn attention. We appreciate that specific peptides/proteins, lipids, nucleic acids, and even organelles shuttle between the adipose tissues and heart. These bioactive components can profoundly affect the metabolism of cells in distal organs, including heart. Importantly, this process can be dysregulated under pathophysiological conditions. This also opens the door to efforts targeting these mediators as potential therapeutic strategies to treat patients who manifest diabetes and cardiovascular disease. Here, we summarize the recent progress toward a better understanding of how the adipose tissues and heart interact with each other.
Introduction
Adipose tissue (AT) in human adults accounts for 20%–50% of the body mass and is considered to be the second largest organ after skin.[1] Excessive expansion of AT defines the various forms of obesity, depending on which fat pads absorb the bulk of the calories. The prevalence of obesity has been continuously rising in recent decades.[2] Obesity is well established as an independent risk factor for cardiovascular disease, and has a pronounced association with coronary artery disease, heart failure, and atrial fibrillation.[3] Therefore, it is of paramount importance to better understand the mechanistic basis for the tight correlations between AT dysfunction and cardiovascular pathophysiology. Here, we pay particular attention to the crosstalk between AT and heart, in which significant progress has been made recently.
The breakthrough discoveries of adiponectin and leptin have completely changed the view of AT as a simple energy reservoir to a highly active and complex endocrine organ. The secretory fingerprints of the various ATs are now much better defined and stretch from simple metabolites to a variety of bioactive molecules, including adipokines, inflammatory cytokines, lipids, carbohydrates, miRNA, and extracellular vesicles (EVs).[4, 5, 6] We review the potential impact of these mediators on the physiological or pathological functions of heart. We also need to focus on the functions of epicardial AT, a unique fat depot anatomically located adjacent to the myocardium. There is, in fact, emerging evidence for a reverse crosstalk from the heart to AT, which also deserves some discussion (Figure 1).
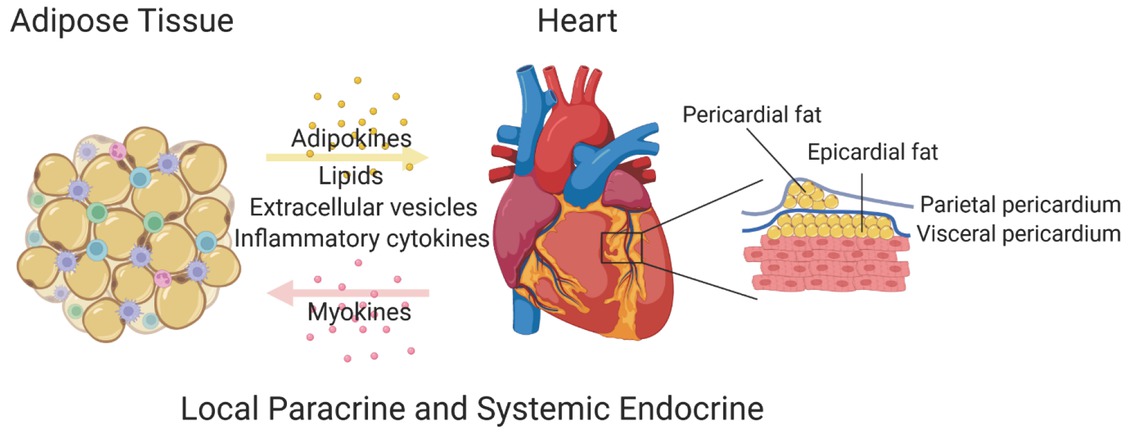
The crosstalk between adipose tissue and the heart.
Adipokines
Adipokines are factors mainly secreted by adipocytes. Since there is a significant amount of AT in the body, fairly large amounts of adipokines enter the blood circulation and coordinate the physiological state of the AT with the function of distal organs as well as with the local microenvironment of the adipocyte. Depending on how loose a definition we use for the term adipokine, a fair number of these proteins have been reported. Here, we would only summarize the ones that exert a clear influence on cardiac function. These include adiponectin, leptin, resistin, and fibroblast growth factor 21 (FGF21).
Adiponectin
Adiponectin, initially identified in 1995,[7] is a complex adipokine. Adiponectin exerts pleiotropic effects on multiple tissues, which includes the brain, heart, liver, kidneys, bone, blood vessels, pancreatic β-cells, and immune cells,[8,9] and is beneficial for healthspan and lifespan.[10] Low levels of adiponectin are tightly associated with increased incidence of cardiac diseases.[11] However, in the setting of high plasma adiponectin levels in the context of end-stage heart disease, it is associated with increased mortality. Further complicating the issue is the fact that adiponectin may have differential effects on patients with ischemic heart disease and heart failure as a function of ethnic background.[12, 13, 14, 15]
Atherosclerosis is the main cause for myocardial infarction in man. In mice, adiponectin inhibits the inflammatory responses in immune cells, and therefore is tightly associated with reduced atherosclerosis.[16,17] Moreover, adiponectin deficiency further worsens and adiponectin administration attenuates ischemia/ reperfusion (I/R)-induced cardiac injury.[18] In a murine model for cardiac hypertrophy, induced by pressure overload and angiotensin II infusion, adiponectin represses cardiac hypertrophy and remodeling.[19,20] Adiponectin exerts its effect on the heart mainly through binding with its receptors in the heart. There are three receptors for adiponectin, including AdipoR1, AdipoR2, and T-cadherin.[21,22] Multiple mouse studies show that T-cadherin plays a protective role in the progression of cardiac diseases. However, the detailed function of AdipoR1 and AdipoR2 in cardiomyocytes is still somewhat obscure. T-cadherin may be the main mediator for adiponectin’s effect on cardiomyocytes, even though this receptor lacks a cytoplasmic signaling domain.[23] Adiponectin administration into T-cadherin-knockout mice has no beneficial effects on pressure overload-induced cardiac hypertrophy, indicating that the other two receptors, AdipoR1 and AdipoR2, do not play a major role in this process.[24]
Leptin
Discovered in 1994,[25] leptin is predominantly secreted by AT.[26] Leptin is a pleiotrophic hormone that can regulate food intake, energy expenditure, reproduction, hemostasis, angiogenesis, blood pressure, and immune responses8. Clinical observations have shown that hyperleptinemia is positively correlated with adverse cardiovascular disease outcome.[27] Plasma leptin is acutely increased in patients suffering from a myocardial infarction.[28] In fact, high plasma levels of leptin is an independent risk factor to predict the occurrence of cardiac death in patients with coronary artery disease.[29] In addition, high levels of leptin, independent of body mass index (BMI) and blood pressure, is linked to increased myocardial wall thickness.[30]
In rodent models with cardiac disease, the contributions of leptin and its receptors to the disease are quite complex. In atherosclerosis, leptin deficiency represses disease progression in ApoE-knockout mice,[31] but promotes atherosclerosis in low-density lipoprotein receptor (LDLR)-knockout mice.[32] Both ob/ob mice (leptin deficiency) and db/db mice (leptin receptor [LEPR] deficiency) manifest age-dependent progression of cardiac hypertrophy.[33,34] Particularly, cardiac diastolic and systolic function is impaired in db/db mice.[35,36] Acute deletion of LEPR in adult cardiomyocytes leads to lethal heart failure[37] and worsens myocardial infarction-induced injury.[38] Interestingly, myocardial infarction in rats upon neutralizing leptin receptor with antibodies has no impact on the infarct size, but mitigates cardiac dysfunction and hypertrophy.[39] Either way, it seems that leptin–LEPR pathway is essential to maintain normal function of the heart. However, upon a pathological insult, overactivation of this pathway may compromise cardiac function.
How can both low and high leptin levels be associated with cardiac disease? Our recent observations on leptin action may provide some clues. Complete lack of leptin in mice and humans leads to morbid obesity, while hyperleptinemia is also associated with an obese phenotype.[40] These observations clearly imply that circulating leptin levels need to be maintained in a narrow range to sustain normal physiological functions: too much leptin is detrimental to cardiac function, while the complete lack of leptin is also harmful. We have to bear this in mind regarding various leptin-associated human diseases, including cardiac disease. In patients with very low or no circulating leptin levels, elevating its levels by supplementing exogenous leptin may be highly effective in preventing disease progression. However, in a conventional obese patient with high circulating leptin levels, reducing the leptin levels, by either genetic or pharmaceutical approaches, is beneficial toward achieving metabolic health.[41,42]
Resistin
Resistin was named because of its effect in inducing insulin resistance. It was identified independently by several laboratories.[43, 44, 45] In mice and humans, resistin exerts similar functions, even though it displays completely different expression patterns. In mice, resistin is mainly secreted by adipocytes, while in humans, it is largely derived from macrophages.[46] Resistin can bind to two receptors, the toll-like receptor 4 (TLR4) and adenylyl cyclase-associated protein 1 (CAP1), to promote inflammatory responses.[47,48] The function of resistin is diverse under different pathological conditions. Resistin protects the heart from I/R injury through activating the AKT pathway.[49] However, resistin also exacerbates pressure overload-induced heart failure due to activating a DNA damage response.[50]
Fibroblast growth factor 21
FGF21 is a unique member of the fibroblast growth factor (FGF) family. It affects the cell metabolism rather than proliferation, a common function that the other members of the family exert.[51] FGF21 is a hepatokine, adipokine, and myokine, and has profound beneficial effects on obesity and type 2 diabetes.[52] It is also emerging as a crucial link between AT and the heart. FGF21 can repress cardiac hypertrophy and protect the heart from I/R injury.[53,54] Under regular physiological conditions, FGF21 is mainly secreted by the liver. However, under some pathophysiological conditions, FGF21 can be secreted by other tissues, including brown AT and even the heart itself. Ruan et al. establish a clear link between brown AT and heart with respect to FGF21. They demonstrate that in rodents, brown AT can secrete FGF21 into the circulation under hypertensive conditions. This FGF21 directly targets the heart to attenuate cardiac remodeling.[55] Moreover, it is possible that cardiomyocyte-derived FGF21 could constitute a feedback signal to AT. Serum concentrations of FGF21 are significantly elevated after myocardial infarction both clinically as well as in murine models.[56] Under these conditions, FGF21 may impact systemic glucose and lipid metabolism. As such, FGF21 constitutes a two-way communication axis between AT and the heart.
Inflammatory cytokines
Obesity is generally accompanied by subclinical but chronic inflammation, and the severity of type 2 diabetic state correlates well with the degree of inflammation.[57] In the diabetic state, the immune cells together with adipocytes produce a considerable number of inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-6, IL-1, IL-17, and IL-22.[58] This systemic inflammatory state can further worsen the progression of ischemic and hypertensive heart disease.[59] Meantime, cardiomyocytes and infiltrated immune cells in the damaged heart secrete considerable amounts of inflammatory cytokines as well,[60] which may also affect the function of AT. Therefore, the inflammatory response is an integral part in the crosstalk between AT and the heart.
Lipids
ATs are actively secreting, fatty acid-derived bioactive lipids. While some of the lipids are retained in AT, others enter the bloodstream and possess a broad effect on distal organs, including liver, heart, muscle, and pancreas.[61,62] Here, we highlight two kinds of lipids that may have direct effects on cardiac diseases.
Ceramides
Ceramides are produced either by hydrolysis of membrane-located sphingomyelin or by de novo synthesis.[63] Ceramide levels in human plasma have emerged as prognostic indicators of major adverse cardiovascular events.[63,64] In atherosclerotic plaques, ceramides are enriched. They may initiate the formation of lipoprotein aggregation[65] and impair the plaque stability.[66] Importantly, animal studies show that inhibiting the synthesis of ceramide alleviates atherosclerosis.[67] Zhang et al. report that Hypoxia-inducible factor 2α (HIF2α) deficiency in adipocytes exacerbates western diet-induced atherosclerosis. Mechanistically, HIF2α initiates the transcription of alkaline ceramidase 2, which hydrolyzes ceramides. Therefore, HIF2α deficiency results in an elevation of ceramides and a worsening of atherosclerosis.[68] Moreover, ceramides play a detrimental role in heart failure. Heart biopsies reveal that high levels of ceramides accumulate in the failing myocardium as well.[69] This accumulation impairs cardiac function.[70] In contrast, decreasing the levels of ceramides improves myocardial infarction-induced heart failure.[69] In addition, our own studies have shown that adiponectin and its receptors enhance ceramide catabolism,[71] which is likely to be the basis for the cardioprotective effects of adiponectin.
Palmitoleate
Palmitoelate is a unique monosaturated fatty acid, in which the double bond is at position n-6. It was first identified as an adipose-derived bioactive lipid in Fabp4/5 double-knockout mice.[72] Intriguingly, palmitoleate is found in postprandial python plasma and can promote physiological heart growth.[73] Palmitoleate can also repress inflammatory responses, thereby attenuating the progression of atherosclerosis.[74]
Extracellular vesicles
EVs are cell-derived membranous structures which can be sorted into three subtypes, microvesicles, exosomes, and apoptotic bodies, according to their origins and the pathways that lead to their release.[75,76] Proteins, lipids, nucleic acid, and even submitochondrial particles can be packaged into EVs and then transported to other cells or organs.[77,78] Clinical studies have shown that some specific plasma EVs are correlated with a high incidence of myocardial infarction and mortality in obese patients with vascular diseases.[79, 80, 81] Here, we focus on the recent advances regarding the EV-mediated crosstalk between AT and heart.
In AT, adipocytes, immune cells, mesenchymal stem cells, and endothelial cells actively produce a large number of EVs, which affect the immune response, adipogenesis, thermogenesis, and adipokine release.[82] These EVs transport messages from ATs to distal organs, including heart, liver, skeleton muscle, pancreas, and brain. Importantly, several reports have directly demonstrated that AT-derived EVs play an important role in ischemic heart diseases. Lu et al. reveal that adipocytes from high fat diet (HFD) mice secrete miR-130b-3p-containing EVs, which exacerbate cardiac I/ R injury.[83] Our lab recently reported that mitochondria-containing EVs derived from dysfunctional adipocytes can trigger a burst of reactive oxygen species (ROS) and a compensatory antioxidant response in cardiomyocytes that protects the heart from the damage triggered by an I/R insult.[84]
EVs can also be secreted by cells in the heart, including cardiomyocytes, endothelial cells, and fibroblasts.[85] Similarly, these EVs not only play a role locally, but also transport cargos to other organs such as AT. Lu et al. recently showed that EVs derived from the mouse heart upon an I/R insult can induce endoplasmic reticulum (ER) stress in adipocytes and impair their endocrine function.[86] Interestingly, we found that in adipocytes, caveolin 1 can effectively be replenished by EVs secreted by endothelial cells. Combined, all the observations highlight the very active communication between cardiovascular system and ATs.[87]
Epicardial at and the heart
As a specific subtype of visceral fat, epicardial AT directly interacts with the myocardium and is located between epicardium and heart (Figure 1). Epicardial AT is completely absent in mice. However, in humans, it can account for 20% of the total ventricular weight,[88] covering 80% of the area of human heart,[89] even infiltrating into the myocardium.[90] Epicardial AT originates from the splanchnopleuric mesoderm as well as the heart itself.[91] The composition of epicardial AT is similar to that of the subcutaneous and other visceral fat tissues. Interestingly, the size of adipocytes in epicardial AT is comparatively smaller, probably due to a higher ratio of pre-adipocytes to mature adipocytes, which is not seen to the same extent in other fat pads.[92]
Clinical studies have shown that the volume of epicardial AT is highly associated with cardiac disease. The thickness of epicardial AT positively correlates with the degree of the metabolic syndrome[93] and has significant potential predicting a high risk to develop cardiovascular disease.[94] A higher volume of epicardial AT tends to impair the stability of plaques in atherosclerosis,[95,96] which leads to an increase in the events of ischemic heart disease. Besides, epicardial AT is a risk factor for ventricular hypertrophy, heart failure with preserved ejection fraction,[97] and atrial fibrillation.[98] All these clinical observations suggest that epicardial AT may be a driving force for cardiac diseases. However, the detailed mechanistic involvement of epicardial AT in the processes still needs to be worked out.
In contrast to its aforementioned role in cardiac disease, epicardial fat in humans may also exert some beneficial roles in preserving normal heart function based on a limited set of observations: (1) epicardial AT can protect the heart from mechanical stress; (2) epicardial AT can serve as a local energy sink for cardiac muscle to protect against high levels of free fatty acids in coronary circulation;[99] (3) epicardial AT functions as a brown fat to defend the myocardium and coronary vessels against hypothermia;[100] and (4) epicardial AT provides the anatomical site for the ganglia innervating the myocardium.[101] However, due to the complete absence of epicardial fat in current existing mouse models, it is very difficult to clarify the exact role(s) of epicardial AT in heart function under normal physiological and pathological conditions. Nonhuman primate models may have to provide the necessary insights to unravel a direct functional involvement of epicardial AT in cardiovascular disease.
Beyond that, similar to the other fat depots, epicardial AT can secrete adipokines, EVs, and lipids to affect heart, and we appreciate that the heart can regulate the status of epicardial AT. Coronary atherosclerosis correlates well with a proinflammatory phenotype in epicardial AT.[102] Interestingly, although there is no epicardial AT around heart in normal mice, it can be induced after the mice are subjected to myocardial infarction,[103] thereby opening up the possibility to study its impact on the mouse heart. In addition, cardiac hormones, such as ANP and BNP, and cytokines secreted from heart can affect epicardial AT directly.
Conclusions and perspectives
Ample evidence supports the notion that an active crosstalk exists between AT and the heart via adipokines, cytokines, lipids, and EVs. Here, we provide a very high-level assessment of some of the mechanisms in place to mediate this crosstalk. Considering the high prevalence of obesity and cardiovascular disease, this area will receive more attention in the future, as many gaps persist. Once we identify the key players, they may not only serve as important biomarkers, but also have the potential to be targets for intervention to resolve aspects of various pathophysiological cardiac disease states.
Funding statement: Authors were supported by US National Institutes of Health (NIH) grants R01-DK55758, R01-DK127274, R01-DK099110, R01-DK131537, and RC2-DK118620 (P.E.S.). Zhao S was supported by an AHA postdoctoral fellowship (No. 825982) and NIH Grant K99-AG068239.
Acknowledgments
We would like to thank Dr. Zhao V. Wang for helpful discussions. The figure was generated using Biorender.
-
Conflict of Interest
None declared.
References
1 Barbara Y, Geraldine OD, Phillip W. Wheater’s Functional Histology. 6th ed. Amsterdam: Elsevier, 2013.Search in Google Scholar
2 Jaacks LM, Vandevijvere S, Pan A, McGowan CJ, Wallace C, Imamura F, et al. The obesity transition: stages of the global epidemic. Lancet Diabetes Endocrinol 2019;7:231–40.10.1016/S2213-8587(19)30026-9Search in Google Scholar PubMed PubMed Central
3 Powell-Wiley TM, Poirier P, Burke LE, Després JP, Gordon-Larsen P, Lavie CJ, et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021;143:e984–e1010.10.1161/CIR.0000000000000973Search in Google Scholar PubMed PubMed Central
4 Richard AJ, White U, Elks CM, Stephens JM. Adipose Tissue: Physiology to Metabolic Dysfunction. 2020. Available at: https://www.ncbi.nlm.nih.gov/books/NBK555602/ Accessed August 25, 2022.Search in Google Scholar
5 Zhu Y, Li N, Huang M, Bartels M, Dogné S, Zhao S, et al. Adipose tissue hyaluronan production improves systemic glucose homeostasis and primes adipocytes for CL 316,243-stimulated lipolysis. Nat Commun 2021;12:4829.10.1038/s41467-021-25025-4Search in Google Scholar PubMed PubMed Central
6 Funcke JB, Scherer PE. Beyond adiponectin and leptin: adipose tissue-derived mediators of inter-organ communication. J Lipid Res 2019;60:1648–84.10.1194/jlr.R094060Search in Google Scholar PubMed PubMed Central
7 Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 1995;270:26746–9.10.1074/jbc.270.45.26746Search in Google Scholar PubMed
8 Zhao S, Kusminski CM, Scherer PE. Adiponectin, Leptin and Cardiovascular Disorders. Circ Res 2021;128:136-49.10.1161/CIRCRESAHA.120.314458Search in Google Scholar PubMed PubMed Central
9 Straub LG, Scherer PE. Metabolic Messengers: Adiponectin. Nat Metab 2019;1:334–9.10.1038/s42255-019-0041-zSearch in Google Scholar PubMed PubMed Central
10 Li N, Zhao S, Zhang Z, Zhu Y, Gliniak CM, Vishvanath L, et al. Adiponectin preserves metabolic fitness during aging. Elife 2021;10:e65108.10.7554/eLife.65108Search in Google Scholar PubMed PubMed Central
11 Shibata R, Ouchi N, Murohara T. Adiponectin and cardiovascular disease. Circ J 2009;73:608–14.10.1253/circj.CJ-09-0057Search in Google Scholar PubMed
12 Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. Jama 2004;291:1730–7.10.1001/jama.291.14.1730Search in Google Scholar PubMed
13 Cavusoglu E, Ruwende C, Chopra V, Yanamadala S, Eng C, Clark LT, et al. Adiponectin is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction in patients presenting with chest pain. Eur Heart J 2006;27:2300–9.10.1093/eurheartj/ehl153Search in Google Scholar PubMed
14 Kistorp C, Faber J, Galatius S, Gustafsson F, Frystyk J, Flyvbjerg A, et al. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation 2005;112:1756–62.10.1161/CIRCULATIONAHA.104.530972Search in Google Scholar PubMed
15 Tsutamoto T, Tanaka T, Sakai H, Ishikawa C, Fujii M, Yamamoto T, et al. Total and high molecular weight adiponectin, haemodynamics, and mortality in patients with chronic heart failure. Eur Heart J 2007;28:1723– 30.10.1093/eurheartj/ehm154Search in Google Scholar PubMed
16 Okamoto Y, Folco EJ, Minami M, Wara AK, Feinberg MW, Sukhova GK, et al. Adiponectin inhibits the production of CXC receptor 3 chemokine ligands in macrophages and reduces T-lymphocyte recruitment in atherogenesis. Circ Res 2008;102:218–25.10.1161/CIRCRESAHA.107.164988Search in Google Scholar PubMed
17 Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation 2002;106:2767–70.10.1161/01.CIR.0000042707.50032.19Search in Google Scholar PubMed
18 Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med 2005;11:1096–103.10.1038/nm1295Search in Google Scholar PubMed PubMed Central
19 Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med 2004;10:1384–9.10.1038/nm1137Search in Google Scholar PubMed PubMed Central
20 Fujita K, Maeda N, Sonoda M, Ohashi K, Hibuse T, Nishizawa H, et al. Adiponectin protects against angiotensin II-induced cardiac fibrosis through activation of PPAR-alpha. Arterioscler Thromb Vasc Biol 2008;28:863–70.10.1161/ATVBAHA.107.156687Search in Google Scholar PubMed
21 Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003;423:762–9.10.1038/nature01705Search in Google Scholar PubMed
22 Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/ adiponectin. Proc Natl Acad Sci U S A 2004;101:10308–13.10.1073/pnas.0403382101Search in Google Scholar PubMed PubMed Central
23 Yamauchi T, Iwabu M, Okada-Iwabu M, Kadowaki T. Adiponectin receptors: a review of their structure, function and how they work. Best Pract Res Clin Endocrinol Metab 2014;28:15–23.10.1016/j.beem.2013.09.003Search in Google Scholar PubMed
24 Denzel MS, Scimia MC, Zumstein PM, Walsh K, Ruiz-Lozano P, Ranscht B. T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J Clin Invest 2010;120:4342–52.10.1172/JCI43464Search in Google Scholar PubMed PubMed Central
25 Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 1994;372:425–32.10.1038/372425a0Search in Google Scholar PubMed
26 Caron A, Lee S, Elmquist JK, Gautron L. Leptin and brain–adipose crosstalks. Nat Rev Neurosci 2018;19:153–65.10.1038/nrn.2018.7Search in Google Scholar PubMed PubMed Central
27 Hall ME, Harmancey R, Stec DE. Lean heart: Role of leptin in cardiac hypertrophy and metabolism. World J Cardiol 2015;7:511–24.10.4330/wjc.v7.i9.511Search in Google Scholar PubMed PubMed Central
28 Khafaji HA, Bener AB, Rizk NM, Al Suwaidi J. Elevated serum leptin levels in patients with acute myocardial infarction; correlation with coronary angiographic and echocardiographic findings. BMC Res Notes 2012;5:262.10.1186/1756-0500-5-262Search in Google Scholar PubMed PubMed Central
29 Puurunen VP, Kiviniemi A, Lepojärvi S, Piira OP, Hedberg P, Junttila J, et al. Leptin predicts short-term major adverse cardiac events in patients with coronary artery disease. Annals of Medicine 2017;49:448–54.10.1080/07853890.2017.1301678Search in Google Scholar PubMed
30 Paolisso G, Tagliamonte MR, Galderisi M, Zito GA, D’Errico A, Marfella R, et al. Plasma leptin concentration, insulin sensitivity, and 24-hour ambulatory blood pressure and left ventricular geometry. Am J Hypertens 2001;14:114–20.10.1016/S0895-7061(00)01241-3Search in Google Scholar PubMed
31 Chiba T, Shinozaki S, Nakazawa T, Kawakami A, Ai M, Kaneko E, et al. Leptin deficiency suppresses progression of atherosclerosis in apoE-deficient mice. Atherosclerosis 2008;196:68–75.10.1016/j.atherosclerosis.2007.01.040Search in Google Scholar PubMed
32 Hasty AH, Shimano H, Osuga J, Namatame I, Takahashi A, Yahagi N, et al. Severe hypercholesterolemia, hypertriglyceridemia, and atherosclerosis in mice lacking both leptin and the low density lipoprotein receptor. J Biol Chem 2001;276:37402–8.10.1074/jbc.M010176200Search in Google Scholar PubMed
33 Barouch LA, Berkowitz DE, Harrison RW, O’Donnell CP, Hare JM. Disruption of leptin signaling contributes to cardiac hypertrophy independently of body weight in mice. Circulation 2003;108:754–9.10.1161/01.CIR.0000083716.82622.FDSearch in Google Scholar PubMed
34 Yue P, Arai T, Terashima M, Sheikh AY, Cao F, Charo D, et al. Magnetic resonance imaging of progressive cardiomyopathic changes in the db/ db mouse. Am J Physiol Heart Circ Physiol 2007;292:H2106–18.10.1152/ajpheart.00856.2006Search in Google Scholar PubMed
35 Semeniuk LM, Kryski AJ, Severson DL. Echocardiographic assessment of cardiac function in diabetic db/db and transgenic db/db-hGLUT4 mice. Am J Physiol Heart Circ Physiol 2002;283:H976–82.10.1152/ajpheart.00088.2002Search in Google Scholar PubMed
36 Van den Bergh A, Flameng W, Herijgers P. Type II diabetic mice exhibit contractile dysfunction but maintain cardiac output by favourable loading conditions. Eur J Heart Fail 2006;8:777–83.10.1016/j.ejheart.2006.03.001Search in Google Scholar PubMed
37 Hall ME, Smith G, Hall JE, Stec DE. Cardiomyocyte-specific deletion of leptin receptors causes lethal heart failure in Cre-recombinase-mediated cardiotoxicity. Am J Physiol Regul Integr Comp Physiol 2012;303:R1241– 50.10.1152/ajpregu.00292.2012Search in Google Scholar PubMed PubMed Central
38 Witham W, Yester K, O’Donnell CP, McGaffin KR. Restoration of glucose metabolism in leptin-resistant mouse hearts after acute myocardial infarction through the activation of survival kinase pathways. J Mol Cell Cardiol 2012;53:91–100.10.1016/j.yjmcc.2012.03.016Search in Google Scholar PubMed
39 Purdham DM, Rajapurohitam V, Zeidan A, Huang C, Gross GJ, Karmazyn M. A neutralizing leptin receptor antibody mitigates hypertrophy and hemodynamic dysfunction in the postinfarcted rat heart. Am J Physiol Heart Circ Physiol 2008;295:H441–6.10.1152/ajpheart.91537.2007Search in Google Scholar PubMed
40 Zhao S, Zhu Y, Schultz RD, Li N, He Z, Zhang Z, et al. Partial Leptin Reduction as an Insulin Sensitization and Weight Loss Strategy. Cell Metab 2019;30:706–19.e6.10.1016/j.cmet.2019.08.005Search in Google Scholar PubMed PubMed Central
41 Zhao S, Li N, Zhu Y, Straub L, Zhang Z, Wang MY, et al. Partial leptin deficiency confers resistance to diet-induced obesity in mice. Mol Metab 2020;37:100995.10.1016/j.molmet.2020.100995Search in Google Scholar PubMed PubMed Central
42 Zhao S, Kusminski CM, Elmquist JK, Scherer PE. Leptin: Less Is More. Diabetes 2020;69:823–9.10.2337/dbi19-0018Search in Google Scholar PubMed PubMed Central
43 Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature 2001;409:307–12.10.1038/35053000Search in Google Scholar PubMed
44 Kim KH, Lee K, Moon YS, Sul HS. A cysteine-rich adipose tissue-specific secretory factor inhibits adipocyte differentiation. J Biol Chem 2001;276:11252–6.10.1074/jbc.C100028200Search in Google Scholar PubMed
45 Rajala MW, Lin Y, Ranalletta M, Yang XM, Qian H, Gingerich R, et al. Cell type-specific expression and coregulation of murine resistin and resistin-like molecule-alpha in adipose tissue. Mol Endocrinol 2002;16:1920–30.10.1210/me.2002-0048Search in Google Scholar PubMed
46 Patel L, Buckels AC, Kinghorn IJ, Murdock PR, Holbrook JD, Plumpton C, et al. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun 2003;300:472–6.10.1016/S0006-291X(02)02841-3Search in Google Scholar PubMed
47 Tarkowski A, Bjersing J, Shestakov A, Bokarewa MI. Resistin competes with lipopolysaccharide for binding to toll-like receptor 4. J Cell Mol Med 2010;14:1419–31.10.1111/j.1582-4934.2009.00899.xSearch in Google Scholar PubMed PubMed Central
48 Lee S, Lee HC, Kwon YW, Lee SE, Cho Y, Kim J, et al. Adenylyl cyclase-associated protein 1 is a receptor for human resistin and mediates inflammatory actions of human monocytes. Cell Metab 2014;19:484–97.10.1016/j.cmet.2014.01.013Search in Google Scholar PubMed PubMed Central
49 Gao J, Chang Chua C, Chen Z, Wang H, Xu X, C Hamdy R, et al. Resistin, an adipocytokine, offers protection against acute myocardial infarction. J Mol Cell Cardiol 2007;43:601–9.10.1016/j.yjmcc.2007.08.009Search in Google Scholar PubMed PubMed Central
50 Zhao B, Bouchareb R, Lebeche D. Resistin deletion protects against heart failure injury by targeting DNA damage response. Cardiovasc Res 2021:cvab234.10.1093/cvr/cvab234Search in Google Scholar PubMed PubMed Central
51 Itoh N, Ornitz DM. Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. J Biochem 2011;149:121–30.10.1093/jb/mvq121Search in Google Scholar PubMed PubMed Central
52 Scarpellini E, Arts J, Karamanolis G, Laurenius A, Siquini W, Suzuki H, et al. International consensus on the diagnosis and management of dumping syndrome. Nat Rev Endocrinol 2020;16:448–66.10.1038/s41574-020-0357-5Search in Google Scholar PubMed PubMed Central
53 Planavila A, Redondo-Angulo I, Villarroya F. FGF21 and Cardiac Physiopathology. Front Endocrinol (Lausanne) 2015;6:133.10.3389/fendo.2015.00133Search in Google Scholar PubMed PubMed Central
54 Liu SQ, Roberts D, Kharitonenkov A, Zhang B, Hanson SM, Li YC, et al. Endocrine protection of ischemic myocardium by FGF21 from the liver and adipose tissue. Sci Rep 2013;3:2767.10.1038/srep02767Search in Google Scholar PubMed PubMed Central
55 Ruan CC, Kong LR, Chen XH, Ma Y, Pan XX, Zhang ZB, et al. A(2A) Receptor Activation Attenuates Hypertensive Cardiac Remodeling via Promoting Brown Adipose Tissue-Derived FGF21. Cell Metab 2018;28:476–89.e5.10.1016/j.cmet.2018.06.013Search in Google Scholar PubMed
56 Sunaga H, Koitabashi N, Iso T, Matsui H, Obokata M, Kawakami R, et al. Activation of cardiac AMPK-FGF21 feed-forward loop in acute myocardial infarction: Role of adrenergic overdrive and lipolysis byproducts. Sci Rep 2019;9:11841.10.1038/s41598-019-48356-1Search in Google Scholar PubMed PubMed Central
57 Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 2010;72:219–46.10.1146/annurev-physiol-021909-135846Search in Google Scholar PubMed
58 Caër C, Rouault C, Le Roy T, Poitou C, Aron-Wisnewsky J, Torcivia A, et al. Immune cell-derived cytokines contribute to obesity-related inflammation, fibrogenesis and metabolic deregulation in human adipose tissue. Sci Rep 2017;7:3000.10.1038/s41598-017-02660-wSearch in Google Scholar PubMed PubMed Central
59 Ridker PM. Targeting inflammatory pathways for the treatment of cardiovascular disease. Eur Heart J 2014;35:540–3.10.1093/eurheartj/eht398Search in Google Scholar PubMed PubMed Central
60 Ruparelia N, Chai JT, Fisher EA, Choudhury RP. Inflammatory processes in cardiovascular disease: a route to targeted therapies. Nat Rev Cardiol 2017;14:133–44.10.1038/nrcardio.2016.185Search in Google Scholar PubMed PubMed Central
61 Li VL, Kim JT, Long JZ. Adipose Tissue Lipokines: Recent Progress and Future Directions. Diabetes 2020;69:2541–8.10.2337/dbi20-0012Search in Google Scholar PubMed PubMed Central
62 Zhang Z, Funcke JB, Zi Z, Zhao S, Straub LG, Zhu Y, et al. Adipocyte iron levels impinge on a fat-gut crosstalk to regulate intestinal lipid absorption and mediate protection from obesity. Cell Metab 2021;33:1624–39.e9.10.1016/j.cmet.2021.06.001Search in Google Scholar PubMed PubMed Central
63 Choi RH, Tatum SM, Symons JD, Summers SA, Holland WL. Ceramides and other sphingolipids as drivers of cardiovascular disease. Nat Rev Cardiol 2021;18:701–11.10.1038/s41569-021-00536-1Search in Google Scholar PubMed PubMed Central
64 Field BC, Gordillo R, Scherer PE. The Role of Ceramides in Diabetes and Cardiovascular Disease Regulation of Ceramides by Adipokines. Front Endocrinol (Lausanne) 2020;11:569250.10.3389/fendo.2020.569250Search in Google Scholar PubMed PubMed Central
65 Schissel SL, Tweedie-Hardman J, Rapp JH, Graham G, Williams KJ, Tabas I. Rabbit aorta and human atherosclerotic lesions hydrolyze the sphingomyelin of retained low-density lipoprotein. Proposed role for arterial-wall sphingomyelinase in subendothelial retention and aggregation of atherogenic lipoproteins. J Clin Invest 1996;98:1455–64.10.1172/JCI118934Search in Google Scholar PubMed PubMed Central
66 Cheng JM, Suoniemi M, Kardys I, Vihervaara T, de Boer SP, Akkerhuis KM, et al. Plasma concentrations of molecular lipid species in relation to coronary plaque characteristics and cardiovascular outcome: Results of the ATHEROREMO-IVUS study. Atherosclerosis 2015;243:560–6.10.1016/j.atherosclerosis.2015.10.022Search in Google Scholar PubMed
67 Park TS, Panek RL, Mueller SB, Hanselman JC, Rosebury WS, Robertson AW, et al. Inhibition of sphingomyelin synthesis reduces atherogenesis in apolipoprotein E-knockout mice. Circulation 2004;110:3465–71.10.1161/01.CIR.0000148370.60535.22Search in Google Scholar PubMed
68 Zhang X, Zhang Y, Wang P, Zhang SY, Dong Y, Zeng G, et al. Adipocyte Hypoxia-Inducible Factor 2α Suppresses Atherosclerosis by Promoting Adipose Ceramide Catabolism. Cell Metab 2019;30:937–51.e5.10.1016/j.cmet.2019.09.016Search in Google Scholar PubMed
69 Ji R, Akashi H, Drosatos K, Liao X, Jiang H, Kennel PJ, et al. Increased de novo ceramide synthesis and accumulation in failing myocardium. JCI Insight 2017;2:e82922.10.1172/jci.insight.82922Search in Google Scholar PubMed PubMed Central
70 Park TS, Hu Y, Noh HL, Drosatos K, Okajima K, Buchanan J, et al. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J Lipid Res 2008;49:2101–12.10.1194/jlr.M800147-JLR200Search in Google Scholar PubMed PubMed Central
71 Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med 2011;17:55–63.10.1038/nm.2277Search in Google Scholar PubMed PubMed Central
72 Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a Lipokine, a Lipid Hormone Linking Adipose Tissue to Systemic Metabolism. Cell 2008;134:933–44.10.1016/j.cell.2008.07.048Search in Google Scholar PubMed PubMed Central
73 Riquelme CA, Magida JA, Harrison BC, Wall CE, Marr TG, Secor SM, et al. Fatty acids identified in the Burmese python promote beneficial cardiac growth. Science 2011;334:528–31.10.1126/science.1210558Search in Google Scholar PubMed PubMed Central
74 Çimen I, Kocatürk B, Koyuncu S, Tufanlı Ö, Onat UI, Yıldırım AD, et al. Prevention of atherosclerosis by bioactive palmitoleate through suppression of organelle stress and inflammasome activation. Sci Transl Med 2016;8:358ra126.10.1126/scitranslmed.aaf9087Search in Google Scholar PubMed
75 Zaborowski MP, Balaj L, Breakefield XO, Lai CP. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015;65:783–97.10.1093/biosci/biv084Search in Google Scholar PubMed PubMed Central
76 van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 2018;19:213–28.10.1038/nrm.2017.125Search in Google Scholar PubMed
77 Doyle LM, Wang MZ. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019;8:727.10.3390/cells8070727Search in Google Scholar PubMed PubMed Central
78 Amari L, Germain M. Mitochondrial Extracellular Vesicles - Origins and Roles. Front Mol Neurosci 2021;14:767219.10.3389/fnmol.2021.767219Search in Google Scholar PubMed PubMed Central
79 Eguchi A, Lazic M, Armando AM, Phillips SA, Katebian R, Maraka S, et al. Circulating adipocyte-derived extracellular vesicles are novel markers of metabolic stress. J Mol Med (Berl) 2016;94:1241–53.10.1007/s00109-016-1446-8Search in Google Scholar PubMed PubMed Central
80 Kanhai DA, Visseren FL, van der Graaf Y, Schoneveld AH, Catanzariti LM, Timmers L, et al. Microvesicle protein levels are associated with increased risk for future vascular events and mortality in patients with clinically manifest vascular disease. Int J Cardiol 2013;168:2358–63.10.1016/j.ijcard.2013.01.231Search in Google Scholar PubMed
81 Kranendonk ME, de Kleijn DP, Kalkhoven E, Kanhai DA, Uiterwaal CS, van der Graaf Y, et al. Extracellular vesicle markers in relation to obesity and metabolic complications in patients with manifest cardiovascular disease. Cardiovasc Diabetol 2014;13:37.10.1186/1475-2840-13-37Search in Google Scholar PubMed PubMed Central
82 Huang Z, Xu A. Adipose Extracellular Vesicles in Intercellular and Inter-Organ Crosstalk in Metabolic Health and Diseases. Front Immunol 2021;12:608680.10.3389/fimmu.2021.608680Search in Google Scholar PubMed PubMed Central
83 Gan L, Xie D, Liu J, Bond Lau W, Christopher TA, Lopez B, et al. Small Extracellular Microvesicles Mediated Pathological Communications Between Dysfunctional Adipocytes and Cardiomyocytes as a Novel Mechanism Exacerbating Ischemia/Reperfusion Injury in Diabetic Mice. Circulation 2020;141:968–83.10.1161/CIRCULATIONAHA.119.042640Search in Google Scholar PubMed PubMed Central
84 Crewe C, Funcke JB, Li S, Joffin N, Gliniak CM, Ghaben AL, et al. Extracellular vesicle-based interorgan transport of mitochondria from energetically stressed adipocytes. Cell Metab 2021;33:1853–68.e11.10.1016/j.cmet.2021.08.002Search in Google Scholar PubMed PubMed Central
85 Wagner KT, Radisic M. A New Role for Extracellular Vesicles in Cardiac Tissue Engineering and Regenerative Medicine. Adv Nanobiomed Res 2021;1:2100047.10.1002/anbr.202100047Search in Google Scholar PubMed PubMed Central
86 Gan L, Liu D, Xie D, Bond Lau W, Liu J, Christopher TA, et al. Ischemic Heart-Derived Small Extracellular Vesicles Impair Adipocyte Function. Circ Res 2022;130:48–66.10.1161/CIRCRESAHA.121.320157Search in Google Scholar PubMed
87 Crewe C, Joffin N, Rutkowski JM, Kim M, Zhang F, Towler DA, et al. An Endothelial-to-Adipocyte Extracellular Vesicle Axis Governed by Metabolic State. Cell 2018;175:695–708.e13.10.1016/j.cell.2018.09.005Search in Google Scholar PubMed PubMed Central
88 Corradi D, Maestri R, Callegari S, Pastori P, Goldoni M, Luong TV, et al. The ventricular epicardial fat is related to the myocardial mass in normal, ischemic and hypertrophic hearts. Cardiovasc Pathol 2004;13:313–6.10.1016/j.carpath.2004.08.005Search in Google Scholar PubMed
89 Iacobellis G, Pistilli D, Gucciardo M, Leonetti F, Miraldi F, Brancaccio G, et al. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine 2005;29:251–5.10.1016/j.cyto.2004.11.002Search in Google Scholar PubMed
90 Cherian S, Lopaschuk GD, Carvalho E. Cellular cross-talk between epicardial adipose tissue and myocardium in relation to the pathogenesis of cardiovascular disease. Am J Physiol Endocrinol Metab 2012;303:E937–49.10.1152/ajpendo.00061.2012Search in Google Scholar PubMed
91 Villasante Fricke AC, Iacobellis G. Epicardial Adipose Tissue: Clinical Biomarker of Cardio-Metabolic Risk. Int J Mol Sci 2019;20:5989.10.3390/ijms20235989Search in Google Scholar PubMed PubMed Central
92 Bambace C, Telesca M, Zoico E, Sepe A, Olioso D, Rossi A, et al. Adiponectin gene expression and adipocyte diameter: a comparison between epicardial and subcutaneous adipose tissue in men. Cardiovascular Pathology 2011;20:e153–6.10.1016/j.carpath.2010.07.005Search in Google Scholar PubMed
93 Pierdomenico SD, Pierdomenico AM, Cuccurullo F, Iacobellis G. Meta-analysis of the relation of echocardiographic epicardial adipose tissue thickness and the metabolic syndrome. Am J Cardiol 2013;111:73-8.10.1016/j.amjcard.2012.08.044Search in Google Scholar PubMed
94 Wilson PW, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005;112:3066–72.10.1161/CIRCULATIONAHA.105.539528Search in Google Scholar PubMed
95 Oka T, Yamamoto H, Ohashi N, Kitagawa T, Kunita E, Utsunomiya H, et al. Association between epicardial adipose tissue volume and characteristics of non-calcified plaques assessed by coronary computed tomographic angiography. Int J Cardiol 2012;161:45–9.10.1016/j.ijcard.2011.04.021Search in Google Scholar PubMed
96 Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol 2009;54:49–57.10.1016/j.jacc.2009.02.068Search in Google Scholar PubMed
97 Mahabadi AA, Berg MH, Lehmann N, Kälsch H, Bauer M, Kara K, et al. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall Study. J Am Coll Cardiol 2013;61:1388–95.10.1016/j.jacc.2012.11.062Search in Google Scholar PubMed
98 Zhu W, Zhang H, Guo L, Hong K. Relationship between epicardial adipose tissue volume and atrial fibrillation : A systematic review and meta-analysis. Herz 2016;41:421–7.10.1007/s00059-015-4387-zSearch in Google Scholar PubMed
99 Marchington JM, Pond CM. Site-specific properties of pericardial and epicardial adipose tissue: the effects of insulin and high-fat feeding on lipogenesis and the incorporation of fatty acids in vitro. Int J Obes 1990;14:1013–22.Search in Google Scholar
100 Sacks HS, Fain JN, Holman B, Cheema P, Chary A, Parks F, et al. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J Clin Endocrinol Metab 2009;94:3611–5.10.1210/jc.2009-0571Search in Google Scholar PubMed
101 Chiou CW, Eble JN, Zipes DP. Efferent vagal innervation of the canine atria and sinus and atrioventricular nodes. The third fat pad. Circulation 1997;95:2573–84.10.1161/01.CIR.95.11.2573Search in Google Scholar
102 Shimabukuro M, Hirata Y, Tabata M, Dagvasumberel M, Sato H, Kurobe H, et al. Epicardial adipose tissue volume and adipocytokine imbalance are strongly linked to human coronary atherosclerosis. Arterioscler Thromb Vasc Biol 2013;33:1077–84.10.1161/ATVBAHA.112.300829Search in Google Scholar PubMed
103 Zangi L, Oliveira MS, Ye LY, Ma Q, Sultana N, Hadas Y, et al. Insulin-Like Growth Factor 1 Receptor-Dependent Pathway Drives Epicardial Adipose Tissue Formation After Myocardial Injury. Circulation 2017;135:59–72.10.1161/CIRCULATIONAHA.116.022064Search in Google Scholar PubMed PubMed Central
© 2022 Chao Li, Xue-Nan Sun, Shangang Zhao, Philipp E. Scherer, published by Sciendo
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Editorial
- Inhaled antibiotics and airway bacterial decolonization for patients with chronic obstructive pulmonary disease: The rationale and future
- Perspective
- Retinal examination modalities in the early detection of Alzheimer's disease: Seeing brain through the eye
- Commentary
- Hydrogel: A promising new technique for treating Alzheimer’s disease
- Metabolic adaptation in lactation: Insulin-dependent and -independent glycemic control
- Review Article
- Aberrant energy metabolism in Alzheimer’s disease
- Obesity and coronavirus disease 2019
- Crosstalk between adipose tissue and the heart: An update
- Delta variant: Partially sensitive to vaccination, but still worth global attention
- Benefits of physical activity on cardiometabolic diseases in obese children and adolescents
- Original Article
- TCDD-inducible poly (ADP-ribose) polymerase promotes adipogenesis of both brown and white preadipocytes
- Functional evaluation of intermediate coronary lesions with integrated computed tomography angiography and invasive angiography in patients with stable coronary artery disease
- A novel wide-band dielectric imaging system for electro-anatomic mapping and monitoring in radiofrequency ablation and cryoablation
- Brief Report
- Impact of COVID-19 pandemic control measures on infection of other respiratory pathogens: A real-world data research in Guangzhou, China
Articles in the same Issue
- Editorial
- Inhaled antibiotics and airway bacterial decolonization for patients with chronic obstructive pulmonary disease: The rationale and future
- Perspective
- Retinal examination modalities in the early detection of Alzheimer's disease: Seeing brain through the eye
- Commentary
- Hydrogel: A promising new technique for treating Alzheimer’s disease
- Metabolic adaptation in lactation: Insulin-dependent and -independent glycemic control
- Review Article
- Aberrant energy metabolism in Alzheimer’s disease
- Obesity and coronavirus disease 2019
- Crosstalk between adipose tissue and the heart: An update
- Delta variant: Partially sensitive to vaccination, but still worth global attention
- Benefits of physical activity on cardiometabolic diseases in obese children and adolescents
- Original Article
- TCDD-inducible poly (ADP-ribose) polymerase promotes adipogenesis of both brown and white preadipocytes
- Functional evaluation of intermediate coronary lesions with integrated computed tomography angiography and invasive angiography in patients with stable coronary artery disease
- A novel wide-band dielectric imaging system for electro-anatomic mapping and monitoring in radiofrequency ablation and cryoablation
- Brief Report
- Impact of COVID-19 pandemic control measures on infection of other respiratory pathogens: A real-world data research in Guangzhou, China

