Abstract
The leaves of Campylospermum elongatum have furnished the cyano-glycoside (lithospermoside), nine isomeric biflavonoid derivatives among which five are I3–II6 linked (robustaflavone; 4′-O-methyl robustaflavone; 4′,4″′-di-O-methyl robustaflavone; 7,4′,4″-tri-O-methyl robustaflavone; 4′,7″-di-O-methyl robustaflavone) and four I3–II8 linked (amentoflavone; 7-O-methyl amentoflavone; 7,7″-di-O-methyl amentoflavone; 7, 4′,7″-tri-O-methyl amentoflavone) and a flavone glycoside, 4″-O-methyl-7-O-β-d-galactosylapigenin. All structures were established from a complete spectroscopic analysis (MS, IR, 1D, and 2D NMR, including HSQC, HMBC, and NOESY) as well as by comparing the obtained spectroscopic data with literature. This is the first report on the characterization of 4′-O-methyl-7-O-β-d-galactosylapigenin from the genus campylospermum and thus has important chemotaxonomic implications.
1 Introduction
Campylospermum elongatum (Oliv.) Tiegh, a plant of the Ochnaceae family, is represented as shrubs growing up to 2.5 m high under dense secondary forests, well exposed to sunlight [1]. In Cameroun, this plant is found in the eastern and southern regions where natives of the Baka pigmy tribe use the leaves and alcohol to prepare medicinal portions destined to remedy many health problems such as palpitations, heart pain, and stomach disorders. According to our knowledge, no previous phytochemical work has been reported on this species.
2 Results and discussion
In the continuation of the phytochemical investigations of our local plants used in folk medicine, we have isolated and characterized from the leaves of C. elongatum 11 secondary metabolites (Figure 1). The structures of these compounds were established from a complete spectroscopic study (IR, UV, RMN, and MS), as well as by comparing the obtained data with literature. These include the biflavonoids: robustaflavone 1; 4′-O-methyl robustaflavone 2; 4′,4″′-di-O-methyl robustaflavone 3; 7,4′,4″-tri-O-methyl robustaflavone 4; 4′,7″-di-O-methyl robustaflavone 5; amentoflavone 6; 7-O-methyl amentoflavone 7; 7,7″-di-O-methyl amentoflavone 8; 7,4′,7″-tri-O-methyl amentoflavone 9; and a cyano-glycoside, lithospermoside 10 [2], [3], [4], [5], [6], [7], [8], [9]. All were earlier reported as secondary metabolites from other sources with important biological activities [10], [11], [12], [13], [14], [15].
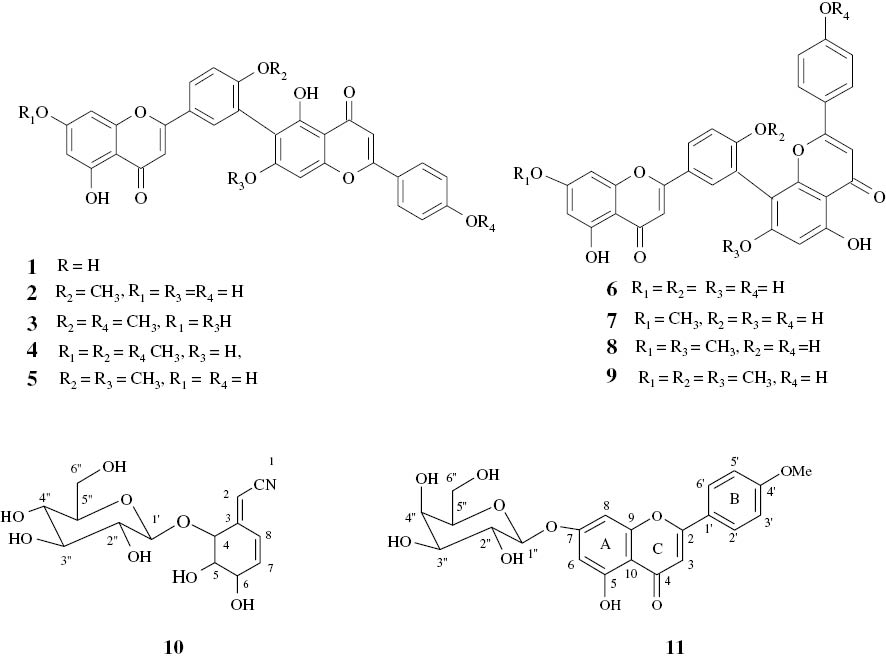
Structures of compounds 1–11.
Equally was obtained compound 11 as an amorphous pale yellow solid for which the molecular formula C22H22O10 was assigned in conformity with its high- resolution mass spectrum in which the [M+H]+ peak appeared at m/z 447.1282. This compound is a flavonoid glycoside since it gave a dark coloration with aqueous FeCl3 solution, a brick red coloration with Mg turnings in the presence of concentrated HCl [16] and a violet coloration with Molish reagent [17].
The IR spectrum was consistent with that of a flavonoid glycoside as it displayed important absorption bands for hydroxyl and phenol functions (3318 and 3120 cm−1), the chromanone carbonyl (1642 cm−1), the conjugated double bond (1622 cm−1), and aromatic rings (1601 and 1596 cm−1) [18].
The UV spectrum of compound 11 confirmed the implication of the flavone motif as it displayed two intense bands at 257 and 349 nm attributed to the benzoyl and the cinnamoyl chromophores, respectively [19]. Evidence of the absence of free OH groups at positions 4′ and 7 was obtained when the benzoyl band was stable with methanol as solvent and the cinnamoyl band varied very little with addition of NaOAc. On the other hand, the presence of a free hydroxyl group at the position 5 of the flavone moiety was confirmed by the bathochromic shift of 16 nm of the benzoyl band with the addition of HCl.
The 1H NMR (1D and 2D) spectra of compound 11 confirmed the presence of the flavone motif as the characteristic signals of protons corresponding to the four different proton systems on the flavone nucleus were identified (Table 1). These included a proton singlet at δH 6.76 (1H, s, H-3); an AB system of two meta coupled protons on ring A at δH 6.44 (1H, d, 2.1 Hz, H-6) and at δH 6.82 (1H, d, 2.1 Hz, H-8); an AA′BB′ system of four protons on a para disubstituted benzene ring B at δH 7.90 (2H, d, 9.0 Hz, H-2′/H-6′) and at δH 6.90 (2H, d, 9.0 Hz, H-3′/H-5′). In addition, two singlet signals observed at δH 3.86 ppm (3H, s, CH3-O-4′) and at δH 12.96 (1H, s, OH-5) were accounted for by an O-methyl substituent on ring B and a much deshielded phenol proton peri to the chromanone carbonyl function. The connections displayed in the NOESY spectrum between the protons of methoxy group at δH 3.86 and the two aromatic protons at δH 6.90 (H-3′/H-5′, ring B), suggested the implication of 4′-O-methylapigenin as aglycone in the structure of 11.
NMR (1H 500 MHz) and (13C 125 MHz) data for compounds 11 and 12 in acetone d6.
| 11 | 12 4′-O-methylapigenin [20] | |||||
|---|---|---|---|---|---|---|
| Position | δC ppm | DEPT | δH ppm (J, Hz) | δC HMBC correlations | δC ppm | δH ppm (J, Hz) |
| 2 | 163.2 | C | – | – | 164.4 | – |
| 3 | 102.6 | CH | 6.76 (1H, s) | 182.2, 163.2, 121.4, 103.1 | 106.6 | 6.58 (1H, s) |
| 4 | 182.2 | C | – | – | 180.5 | – |
| 5 | 161.3 | C | 12.96 (1H, s, OH) | 161.3, 103.1, 98.7 | 164.9 | 13.11 (1H, s, OH) |
| 6 | 98.7 | CH | 6.44 (1H, d, 2.1 Hz) | 161.8, 161.3, 103.1, 94.1 | 104.8 | 6.83 (1H, d, 2.1 Hz) |
| 7 | 163.8 | C | – | – | 160.2 | – |
| 8 | 94.1 | CH | 6.82 (1H, d, 2.1 Hz) | 163.8, 157.6, 98.7, 103.1 | 99.3 | 6.71 (1H, d, 2.1 Hz) |
| 9 | 157.6 | C | – | – | 160.7 | – |
| 10 | 103.1 | C | – | – | 109.4 | – |
| 1′ | 121.4 | C | – | – | 123.1 | – |
| 2′/6′ | 128.2 | CH | 7.90 (2H, d, 9.0 Hz) | 163.2, 161.5, 121.4, 116.1 | 129.3 | 7.83 (2H, d, 8.8 Hz) |
| 3′/5′ | 116.1 | CH | 6.90 (2H, d, 9.0 Hz) | 161.5, 128.2, 121.4 | 117.1 | 6.92 (2H, d, 8.8 Hz) |
| 4′ | 161.5 | C | – | – | 162.6 | – |
| CH3-O-4′ | 56.6 | CH3 | 3.86 (3H, s) | – | 56.4 | 3.86 (3H, s) |
| 1″ | 102.2 | CH | 5.34 (1H, d, 7.8 Hz) | 163.8, 77.6, 71.4 | ||
| 2″ | 71.4 | CH | 3.58 (1H, dd, 7.8 and 9.1 Hz) | 102.2, 77.6, 68.4 | ||
| 3″ | 77.6 | CH | 3.34 (1H, dd, 3.0 and 9.1 Hz) | 102.2, 71.4, 76.2, 68.4 | ||
| 4″ | 68.4 | CH | 3.64 (1H, dd, 3.0 and 3.3 Hz) | 77.6, 76.2, 71.4, 60.1 | ||
| 5″ | 76.2 | CH | 3.31 (1H, ddd, 3.3, 5.6, and 6.1 Hz) | 77.6, 68.4, 60.1, | ||
| 6″ | 60.1 | CH2 | 3.37 (1H, dd, 6.1 and 10.3 Hz) | 76.2, 68.4 | ||
| 3.45 (1H, dd, 5.6 and 10.3 Hz) | 76.2, 68.4 | |||||
In addition, the signals of a system of seven sugar protons observed at δH 5.34 (1H, d, 7.8 Hz, H-1″), 3.58 (1H, dd, 7.8 and 9.1 Hz, H-2″), 3.34 (1H, dd, 3.0 and 9.1 Hz, H-3″), 3.64 (1H, dd, 3.0 and 3.3 Hz, H-4″), 3.31 (1H, ddd, 3.3, 5.6 and 6.1 Hz, H-5″), 3.37 (1H, dd, 6.1 and 10.3 Hz, H-6″a) and 3.45 (1H, dd, 5.6 and 10.3 Hz, H-6″b) correspond to those reported for galactose [21].
These structural elements were confirmed when after the acid hydrolysis of 11, the spectral data of the aglycone was very similar to those described for 4′-O-methylapigenin [20] while those of the sugar were identical to the values obtained for a commercial sample of d-galactose.
The 13C NMR spectrum of 11 ( Table 1) displayed signals indicating the presence of all the 20 two carbon atoms given in the molecular formula among which were identified seven quaternary carbon atoms, 10 methines, a methyl, and a methylene groups. In its HSQC spectrum, the signal of the anomeric carbon C-1″ of the sugar appeared at δC 102.2, that of the methylene carbon at δC 60.1, while those of the other sugar carbons appear at δC 71.4, 77.6, 68.4, and δC 76.2. All these values correspond to those of the carbon atoms in galactose [20]. Signals of protonated carbon atoms on the aglycone include that of the methoxy carbon at δC 56.6, the carbons C-3, C-6, and C-8 of the chromanone moiety that appear, respectively, at δC 102.6, 98.7, and 94.1. HMBC correlations confirm that the signals at δC 182.2, 163.8 and at 161.5 are those of the carbonyl C-4, and the carbon atoms C-7 and C-4′, respectively.
The position of the glycosidic bond was deduced from the connections observed in the HMBC spectrum of 11 which included a correlation between the anomeric proton of the sugar H-1″ (δH 5.34) and the carbon C-7 of the aglycone at δC 163.8. This suggests that the glycosidic bond is between the anomeric carbon C-1′ (δC 102.2) and the oxygen on the aglycone carbon C-7 (δC 163.8). This was confirmed by the NOESY spectrum of 11 that displayed cross peaks between the anomeric proton (δH 5.34) and the aglycone protons H-6 (δH 6.44) and H-8 (δH 6.82), thus establishing its structure as 4′-O-methyl-7-O-β-d-galactosylapigenin. This glycoside had earlier been characterized as an active anti-HIV agent in Chrysanthemum morifolium [22], but the given spectroscopic information is incomplete with several overlapping peaks and missing coupling constants. Our report gives both the complete assignments of all the chemical shifts of the protons and carbon atoms in the molecule of 4′-O-methyl-7-O-β-d-galactosylapigenin and all the proton–proton coupling constants thus completing this earlier reported data.
2.1 Chemotaxonomic significance
Flavonoids, biflavonoids, and cyanoglycosides have been reported as regular constituents of many species of Campylospermum [23], [24], [25]. To our knowledge, this is the first report of the characterization of a flavone galactoside from this genus placing it as a potential source of this bioactive galactoside.
3 Materials and methods
3.1 General experimental procedures
Ethanolic solutions of compounds were used to record UV spectra on a Krontron-Uvikon 930 spectrometer (San Diego, CA, USA), whereas transparent KBr pellets were used on a Jasco FTIR-3000E spectrometer (Tokyo, Japan) to obtain IR spectra. Optical rotations were measured on a PERKIN Elma polarimeter (Überlingen, Germany). HR-CIMS was recorded on a Riber Nermag V3.0 spectrometer (Rueil-Malmaison, France) and using NH3 as ionizing gaz. Solutions of compounds in either CD3COCD3 or DMSO-d6 were used to record the 500 MHz 1H and the 125 MHz 13C spectra on a Bruker WM500 spectrometer (Rheinstettten, Germany). Sephadex LH20 (Pharmacia Fine Chemicals, Uppsala, Sweden) and kieselgel 60 (mesh 0.063, 0.200 mm, Merck, Darmstadt, Germany) were used for column chromatography. Precoated fluorescent silica gel 60 F254 aluminum sheets (Merck, Germany) used for thin layer chromatography (TLC) were developed in the eluent mixture CH2Cl2/MeOH (1:1, v/v). TLC chromatograms were visualized by spraying plates with 3% H2SO4 followed by heating in an oven at 60 °C for 10 min. Preparative TLC plates on glass support were prepared using fluorescent silica gel 60 F254 and developed in the same solvent system as above and visualized with a UV lamp of wavelength 254 nm. Bands resulting from the separation were isolated, scraped off, and recovered with methanol and evaporated to get pure compounds.
3.2 Plant material
The leaves of C. elongatum were harvested in Massok (Cameroon) in August 2009 and identified by Mr. Paul Mezili, botanist in the National Herbarium, Yaounde, Cameroon where a voucher specimen (#18360) was deposited.
3.3 Extraction and purification
Air-dried ground leaves of C. elongatum reduced into fine powder (1.5 kg) and exhaustively extracted with cold methanol in a percolator gave a gum (54 g) after removal of solvent. Warm EtOAc was used to wash the gum leading to an EtOAc soluble fraction further concentrated to give a crude extract (32 g), which was then fractionated by gel exclusion chromatography on a Sephadex LH-20 column, eluted with MeOH to give eight fractions: F1 20.5 g, F2 6.5 g, F3 2.8 g, F4 0.6 g, F5 0.8 g, F6 0.5 g, F7 0.2 g, and F8 0.1 g. The purification of F8 by preparative TLC on silica gel plates, eluted with the mixture CH2Cl2/MeOH (10:1, v/v) and using the technique of multiple migrations gave five major bands which were scraped off, recovered with MeOH, and finally concentrated to give five compounds: 1 (22 mg), 2 (8 mg), 3 (16 mg), 4 (12 mg), and 5 (10 mg). The purification of F7 following the same procedure as above led to the isolation of four other compounds 6 (8 mg), 7 (4 mg), 8 (10 mg), and 9 (9 mg).
Fraction F6 was subjected to repeated LH-20 separation to give three main fractions: F6a (214 mg), F6b (188 mg), and F6c (98 mg). The last fraction was purified by repeated TLC on silica gel plates developed with the eluent CH2Cl2-MeOH (5:1, v/v) to give compounds 10 (23 mg) and 11 (18 mg).
3.4 Hydrolysis of compound 11
Compound 11 (8 mg) was placed in a 10-ml flask and dilute HCl (10%, 3 ml) was added and the set was put under reflux for 1 h after which it was allowed to cool, and water (2 ml) was added. A yellow deposit formed was filtered and washed with water and finally placed in a dessicator under vacuum for 24 h to give the aglycone as yellow powder. Acetone (3 ml) was added to the combined aqueous filtrate and a white precipitate of the sugar deposited was filtered and dried under vacuum for 24 h to give a white amorphous powder.
4′-O-methyl-7-O-β-d-galactopyranosylapigenin 11: amorphous light yellow powder.
– [α]D – 148 (c 0.1 MeOH) – UV: λmax (nm) log ε: 257 (4,3); 349 (3,8); + NaOAc: 257 (4,3); 348 (3,8) + HCl: 273 (4,3); 352 (3,4) – IR (KBr disc) νmax cm−1: 3318, 3120, 3048, 1642, 1622, 1601, 1596. – ESI-HRMS: m/z (%): [M+H]+m/z 447.1282 (calculated for C22H23O10 447.1291). – NMR (1H 500 MHz and 13C 125 MHz), see Table 1.
Acknowledgments:
We thank Mr. Mezili for the identification and harvest of the plant material and the University of Yaounde 1 grants committee for financial assistance.
References
1. Zhang Z, Amaral ME. In: Ochnaceae. Flora of China, 2007;12:361–3.Suche in Google Scholar
2. Junxia Z, Naili W, Ming F, Haifeng C, Hongwei L, Xinsheng Y. A new biflavonoid from Selaginella uncinata. Asian J Trad Med 2007;2:92–7.Suche in Google Scholar
3. Freitas AM, Almeida MT, Andrighetti-Fröhner CR, Cardozo FT, Barardi CR, Farias MR, et al. Antiviral activity-guided fractionation from Araucaria angustifolia leaves extract. J Ethnopharmacol 2009;126:512–7.10.1016/j.jep.2009.09.005Suche in Google Scholar
4. Chen JJ, Duh CY, Chen JF. New cytotoxic biflavonoids from Selaginella delicatula. Planta Med 2005;71:659–65.10.1055/s-2005-871273Suche in Google Scholar
5. Lee NY, Min HY, Lee J, Nam J-W, Lee YJ, Han AR. Identification of a new cytotoxic biflavanone from Selaginella doederleinii. Chem Pharm Bull 2008;56:1360–1.10.1248/cpb.56.1360Suche in Google Scholar
6. Kan H, Timmermann BN, Aladesanmi AJ, Lu Z. A biflavonoid from Dysoxylum lenticellare Gillespie. Phytochemistry 1996;42:1199–201.10.1016/0031-9422(96)00010-6Suche in Google Scholar
7. Hu XY, Guo YQ, Gao WY, Chen HX, Zhang TJ. A new triterpenoid from Alisma orientalis. Chinese Chem Lett 2008;19:438–40.10.1016/j.cclet.2008.01.019Suche in Google Scholar
8. Baranowska MK, Mardarowicz M, Wiwart M. The chemical composition of Microbiota decussate. Z Naturforsch 2002;57c:998–1003.10.1515/znc-2002-11-1208Suche in Google Scholar
9. Erdemgil FZ, Baser KH, Akbay P, Sticher O, Çalis I. A new phenolic compound from Thalictrum orientale. Z Naturforsch 2003;58c:632–6.10.1515/znc-2003-9-1005Suche in Google Scholar
10. Havsteen B. A class of natural products of high pharmacological potency. Biochem Pharmacol 1983;32:1141–8.10.1016/0006-2952(83)90262-9Suche in Google Scholar
11. Satyawan AD. Natural products from genus Salagenilla. Nus Biosci 2011;3:44–58.Suche in Google Scholar
12. Lin YM, Zembower DE, Flavin MT, Schurer RM, Anderson HM, Korba BE, et al. Robusta flavone, a naturally occurring biflavanoid is a potent non-nucleoside in vitro inhibitor of hepatitis B virus replication. Bioorg Med Chem Lett 1997;7:2325–8.10.1016/S0960-894X(97)00422-8Suche in Google Scholar
13. Sloley BD, Urichuk LJ, Morley P, Durkin J, Shan JJ, Pang PK, et al. Identification of Kaempferol as a monoamine oxidase inhibitor and potential neuroprotectant in extracts of Ginkgo Biloba leaves. J Pharm Pharmacol 2000;52:451–9.10.1211/0022357001774075Suche in Google Scholar PubMed
14. Lin YM, Flavin MT, Cassidy CS, Mar A, Chen F-C. Biflavonoids as novel antituberculosis agents. Bioorg Med Chem Lett 2001;11:2101–4.10.1016/S0960-894X(01)00382-1Suche in Google Scholar
15. Lin YM, Flavin MT, Schure R, Chen FC, Sidwell R, Barnard DL,et al. Antiviral activities of biflavonoids. Planta Med 1999;65:120–5.10.1055/s-1999-13971Suche in Google Scholar
16. Danjuma NM, Zezi AU, Yaro AH, Musa AM, Ahmed A, Sanni HA, et al. Residual aqueous fraction of stem bark extract of Xeromphis nilotica and behavioral effects in mice. Int J App Res Nat Prod 2009;2:5–12.Suche in Google Scholar
17. Satheesh KB, Suchetha KN, Vadisha SB, Sharmila KP, Mahesh PB. Preliminary phytochemical screening of various extracts of punica granatum peel, whole fruit and seeds. NUJHS 2012;2:34–8.10.1055/s-0040-1703609Suche in Google Scholar
18. Heneczkowski M, Kopacz M, Nowak D, Kuzniar A. Infrared spectrum analysis of some flavonoids. Acta Pol Pharm 2001;58:415–20.Suche in Google Scholar
19. Patora J, Klimek B. Flavonoids from Lemon balm, Melissa oficinalis L. Lamiaceae. Acta Pol Pharm 2002;59:139–43.Suche in Google Scholar
20. Munoz O, Pena RC, Ureta E, Motenegro G, Cadwell C, Timmerman BN. Phenolic compounds of propolis from central chilean mattorial. Z Naturforsch 2001;56c:273–7.10.1515/znc-2001-3-417Suche in Google Scholar
21. Colson P, King RR. The 13C-N.M.R. Spectra of disaccharides of D-glucose, D-galactose, and L-rhamnose as models for immunological polysaccharides. Carbohydr Res 1979;47:1–13.10.1016/S0008-6215(00)83543-0Suche in Google Scholar
22. Hu CQ, Chen K, Shi Q, Kilkuskie RE, Cheng YC, Lee KH. Anti-AIDS agents, 10. Acacetin-7-O-beta-D-galactopyranoside, an anti-HIV principle from Chrysanthemum morifolium and a structure-activity correlation with some related flavonoids. J N Prod 1994;57:42–51.10.1021/np50103a006Suche in Google Scholar PubMed
23. Elo-Manga SS, Tih AE, Ghogomu RT, Blond A, Bodo B. Biflavonoid constituents of Campylospermum mannii. Biochem Syst Ecol 2009;37:402–4.10.1016/j.bse.2009.04.002Suche in Google Scholar
24. Ndongo JT, Shaaban M, Ngo-Mbing J, Ngono BD, Atchadé AT, Pegnyemb DE, et al. Phenolic dimers and an indole alkaloid from Campylospermum flavum (Ochnaceae). Phytochemistry 2010;71:1872–8.10.1016/j.phytochem.2010.08.006Suche in Google Scholar PubMed
25. Abouem AZ, Ngono BD, Atchadé AT, Ngo-Mbing J, Gangoue-Pieboji J, Ghogomu TR, et al. Nitrile glucosides and serotobenine from Campylospermum glaucum and Ouratea turnarea. Phytochemistry 2008;69:2209–13.10.1016/j.phytochem.2008.04.013Suche in Google Scholar PubMed
©2017 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Review Article
- Structural diversity in echinocandin biosynthesis: the impact of oxidation steps and approaches toward an evolutionary explanation
- Research Articles
- Cytotoxic constituents of Alocasia macrorrhiza
- Isolation, antimicrobial and antitumor activities of a new polyhydroxysteroid and a new diterpenoid from the soft coral Xenia umbellata
- Volatile oil profile of some lamiaceous plants growing in Saudi Arabia and their biological activities
- Membrane permeabilizing action of amphidinol 3 and theonellamide A in raft-forming lipid mixtures
- Discovery and preliminary structure-activity relationship of the marine natural product manzamines as herpes simplex virus type-1 inhibitors
- NMR reassignment of stictic acid isolated from a Sumatran lichen Stereocaulon montagneanum (Stereocaulaceae) with superoxide anion scavenging activities
- Simultaneous formulation of terbinafine and salvia monoterpenes into chitosan hydrogel with testing biological activity of corresponding dialysates against C. albicans yeast
- Rapid Communication
- Chemical constituents of the leaves of Campylospermum elongatum
Artikel in diesem Heft
- Frontmatter
- Review Article
- Structural diversity in echinocandin biosynthesis: the impact of oxidation steps and approaches toward an evolutionary explanation
- Research Articles
- Cytotoxic constituents of Alocasia macrorrhiza
- Isolation, antimicrobial and antitumor activities of a new polyhydroxysteroid and a new diterpenoid from the soft coral Xenia umbellata
- Volatile oil profile of some lamiaceous plants growing in Saudi Arabia and their biological activities
- Membrane permeabilizing action of amphidinol 3 and theonellamide A in raft-forming lipid mixtures
- Discovery and preliminary structure-activity relationship of the marine natural product manzamines as herpes simplex virus type-1 inhibitors
- NMR reassignment of stictic acid isolated from a Sumatran lichen Stereocaulon montagneanum (Stereocaulaceae) with superoxide anion scavenging activities
- Simultaneous formulation of terbinafine and salvia monoterpenes into chitosan hydrogel with testing biological activity of corresponding dialysates against C. albicans yeast
- Rapid Communication
- Chemical constituents of the leaves of Campylospermum elongatum

