NMR reassignment of stictic acid isolated from a Sumatran lichen Stereocaulon montagneanum (Stereocaulaceae) with superoxide anion scavenging activities
-
Friardi Ismed
, Isabelle Rouaud
Abstract
The phytochemical study of Stereocaulon montagneanum harvested in Sumatra (Indonesia) led to the isolation of 11 known compounds including two metabolites not previously described in the genus Stereocaulon, peristictic acid (8) and menegazziaic acid (10). The complete 1H and 13C NMR spectral assignments of stictic acid derivatives are reported with some revisions. Five depsidones belonging to the stictic acid chemosyndrome were superoxide anion scavengers as potent as ascorbic acid and with no toxicity on two human cell lines.
1 Introduction
Stereocaulon represents a cosmopolitan lichen genus of over 130 species occurring from temperate to tropical regions. The main Stereocaulon metabolites have been initially studied by Duvigneaud [1] using microchemical studies, and they were also compiled by Lamb in the conspectus about the lichen genus Stereocaulon [2]. The depside atranorin was a constant marker for the genus, while lobaric, stictic and norstictic acids are the most common depsidones. In a previous study, we investigated the phytochemical content of Stereocaulon halei, harvested in Indonesia. Indeed, we isolated lobaric acid and a related metabolite, the diphenyl ether lobariol carboxylic acid, as a new natural product [3]. To implement the chemical knowledge of this tricky genus and find new photoprotective compounds, we focused our phytochemical investigation on another Indonesian species, Stereocaulon montagneanum, collected on rocks exposed to the sun. Like plants, lichens use various strategies to protect themselves against UV radiations and to inactivate the radical oxygen species generated. Mineral crystals of calcium oxalate and pigments such as melanins, phenols and carotenoids [4] are present in lichens and are supposed to block UV radiations. An increase in secondary metabolite content such as dibenzofurans, depsides and depsidones was also reported for lichens collected in sun-exposed conditions and at high altitude [5]. So, the current work aimed at investigating the chemical content of this Indonesian lichen and at exploring the photoprotective properties (antioxidant, sunscreen) and cytotoxicity of its main metabolites, highlighting their role in the lichen defense against UV.
2 Methods
2.1 General experimental
Melting points were measured on a hot-stage Kofler apparatus. Optical rotation was determined with a Perkin-Elmer model 341 polarimeter. UV spectra were performed on an Uvikon 931 spectrophotometer. FT-IR spectra were run on a Perkin-Elmer 16 PC IR spectrometer. 1H and 13C NMR spectra were recorded at 500 and 125 MHz, respectively, on a Bruker DMX 500 WB NMR spectrometer or at 300 and 67.5 MHz, respectively, on a Bruker 300 NMR spectrometer, using CDCl3, DMSO-d6, acetone-d6 and MeOH-d4 as solvents. High resolution mass spectrometry measurements for exact mass determination were performed on a Varian MAT 311 mass spectrometer for electron spray and a Micromass ZabspecTOF mass spectrometer for chemical ionization at the Centre Régional de Mesures Physiques de l′Ouest. Chromatographic separation was performed using vacuum liquid chromatography on silica gel (Merck 35–70 μm) and C18 Chromabond (45 mL/10 g). Medium pressure chromatography was conducted on a SPOT Flash Liquid Chromatography (Armen Instrument) using silica or C18 pre-packed columns (Super Vario Flash D26 cartridge SI60 40–63 µm, 30 g Merck, normal phase; SVF D26-RP18 25–40 µm, 31 g, Merck, reversed phase) or manually packed silica columns (40–63 µm, Kieselgel 60, Merck, 7667). Semi-preparative high-performance liquid chromatography (HPLC) was performed on Smartline pump 1000 Knauer equipped with a diode array detector (HPLC 540 DAD, Kontron Instruments), using Kromasil® C18 (5 µm, 250×10 mm, CIL Cluzeau, detector set at 254 and 310 nm) at a flow rate of 3.0 mL/min. Thin-layer chromatography (TLC) analytic and preparative plates (Merck silica gel 60F254) were eluted using five standard solvent systems: n-hexane/diethylether/formic acid (130:80:20 v/v/v) (B); toluene/acetic acid (85:15 v/v) (C); toluene/EtOAc/formic acid (139:83:8 v/v/v) (G); n-hexane/EtOAc (95:5 v/v) (K); CHCl3/MeOH/H2O (6:4:1 v/v/v) (L). Visualization of plates was carried out under UV light (254 and 365 nm) then by using anisaldehyde-H2SO4 reagent and heating.
2.2 Lichen material
S. montagneanum Lamb was collected on rocks exposed to the sun of Plateau Simanau (1500 m), Solok, West Sumatra, Indonesia. After identification by Harrie Sipman (Berlin Museum) and Martin Grube (Karl-Franzens University, Graz, Austria), the voucher specimens were deposited at the herbarium of Pharmacognosy and Mycology, Rennes and Biota Sumatran Laboratory, Andalas University, West Sumatra (Indonesia) with the reference codes JB/09/119 and SmV 3, respectively.
2.3 Extraction and isolation
The air-dried whole thalli of S. montagneanum (1.3 kg) were macerated with n-hexane, acetone and methanol successively. Each extract was concentrated in vacuo and precipitates formed after evaporation at room temperature in the n-hexane and acetone extracts afforded compound 1 (0.2 g) and compound 7 (11 g), respectively. The n-hexane filtrate (2 g) was chromatographed on vacuum liquid chromatography silica gel (150 g, 4×30 cm) with a solvent gradient consisting of n-hexane/EtOAc v/v (100:0→0:100) as the mobile phase. Six sub-fractions (SH1–SH6) were obtained. Sub-fraction SH3 (594 mg) was selected for further chromatography using a silica gel column (15 g, 3.5×60 cm) and was eluted employing n-hexane/CHCl3 v/v (60:40). From this, four fractions were obtained and compound 2 (13 mg) was purified by recrystallization in n-hexane. Sub-fraction SH2 was further purified by flash chromatography over a silica gel 60 with petroleum ether/EtOAc v/v (90:10) as the mobile phase and then subjected to preparative TLC with eluent n-hexane/ethyl acetate v/v (95:5). Compounds 3 (2 mg), 4 (10 mg) and 5 (2 mg) were obtained as crystalline residues. Flash chromatography on the C18 column of the acetone filtrate (4 g) with an increasing gradient solvent system of H2O/ACN v/v (100:0→0:100) yielded eight sub-fractions (A1–A8). Compound 8 (20 mg) was purified from sub-fraction A3 by recrystallization in diethyl ether. Sub-fraction A4 (905 mg) yielded compound 9 (38 mg) and compound 10 (4 mg) after separation on C18 flash chromatography (solvent system of H2O/ACN v/v, 60:40), and further purification by semi-preparative HPLC with the Kromasil® C18 column using H2O/MeOH v/v (60% for 20 min) and H2O/methanol-TFA v/v 0.2% with a non-linear gradient (50% for 20 min). Compound 11 (8 mg) was also obtained by flash chromatography on the C18 column with eluent H2O/ACN v/v (40:60) and recrystallization in ethyl acetate. The methanol extract (4 g) was purified using flash chromatography on the RP C18 column with eluent H2O/CAN v/v (100:0 → 0:100) to afford nine sub-fractions (M1–M9). The sub-fraction M1 gave compound 6 (20 mg) by liquid-liquid purification with isopropanol/water v/v (1:1).
2.4 Antioxidant assays
Two antioxidant assays were performed on compounds 1, 2, 7–9 and 11, one using the 1,1′-diphenyl-2-picrylhydrazyl free radical (DPPH) and the other based on the measurement of superoxide anion scavenging activity described previously [6]. Ascorbic acid was used as positive control. The percentage inhibition at a steady state for each dilution was used to calculate the IC50 values. This gave the amount of antioxidant required (measured as the concentration of the stock solution added to the reaction mixture) to scavenge 50% of O2−•, with lower values indicating more effective scavenging of O2−•. All tests were performed in triplicate and the results averaged.
2.5 Cytotoxic assays
Cytotoxic activities of compounds 1, 2, 7–9 and 11 were evaluated against B16-F10 (melanoma; ATCC CRL-6475) and HaCaT cells (Human Epidermal Keratinocytes, ATCC). In brief, the cells were maintained in RPMI medium culture with 5% foetal bovine serum at 37°C in an atmosphere of 10% CO2. Test compounds (100 mM) were prepared in dimethyl sulfoxide and added to each well 1 day after seeding. The amount of DMSO was adjusted to give a final concentration lower than 0.1%. Cytotoxic activity was determined on B16 and HaCaT cells seeded at 20,000 cells/mL at day 0. Compounds were serially diluted in RMPI 1640 at day 1 in a 96-well plate, with a concentration ranging from 1 to 100 µm. After a new incubation period, cell growth and viability were measured at day 5, using an MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay, as previously described [7, 8]. Doxorubicin was used as positive control. All tests were performed in triplicate and the results were subsequently averaged.
2.6 Photoprotective index calculation
The most used absolute indexes are UV-PF and UVA-PF. Relative indexes – UVA-PF/UV-PF ratio, critical wavelength λc – are indicators that reflect a ratio of the UV absorbing efficacy of the sunscreen in given UV regions. So, UV-PF, UVA-PF, critical wavelength (λc) calculation was assessed by an in vitro screening method [9]. Briefly, indexes were calculated from absorbances of the tested compounds in ethanolic solution at 10% through Diffey’s formulae taking into account the spectral irradiation of terrestrial sunlight and the erythemal action spectrum. Tinosorb M was used as positive control.
3 Results and discussion
The lichen was sequentially extracted with solvents of increasing polarity (n-hexane, acetone and methanol) to afford 11 compounds including known compounds, atranorin (1), methyl orcinol carboxylate (2), methyl hematommate (3), ethyl hematommate (4), atranol (5), mannitol (6), stictic acid (7) (Figures S1–S4) and norstictic acid (11) (Figures S13 and S14). All of them were determined by comparing their spectroscopic data with the reported literature values [10], leading to some necessary re-evaluation of their stictic acid datasets (Figure 1).
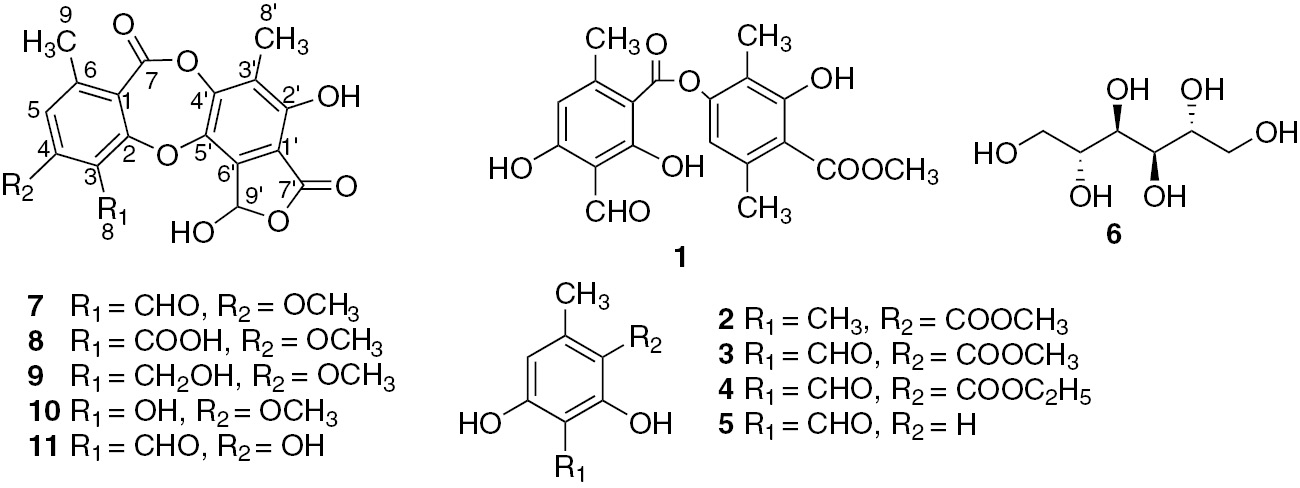
Compounds isolated from Stereocaulon montagneanum lichen thalli.
The complete assignments of 13C NMR signals for stictic acid (7) are given in Table 1. Directly bonded CH carbons were assigned by the heteronuclear single-quantum coherence (HSQC) experiment and the long-range couplings were established by a heteronuclear multiple-bond correlation (HMBC) spectrum (Figures S1–S4). A noticeable delta (δ 4.4 ppm) between δ 13C of C5 for compound 7 and other stictic derivatives is a characteristic downfield shift indicating the ortho phenol substitution at C4 [11, 12] as observed for subnorstictic and substictic acids [13]. Four of the 13C NMR signals exhibited close signals in the 160–167 ppm range, and the HMBC spectrum was useful to clarify the carbon attributions. The assignment of C-4 (δ 162.6) was suggested by the high correlation with OCH3-4 (δ 3.91) (Figure S4b), and C-7′ was assigned to δ 166.5 ppm because no HMBC correlation was observed with H-5 (δ 7.08) or H-9 (δ 2.50) in opposition to δ 160.8 and δ 163.0, which were both correlated with H-9 (δ 2.50). The strong correlation (4J) between H-5 (δ 7.08) and δ 160.8 suggested a W-coupling and was assigned to C-7. C-2 correlated only with H-9 (δ 2.50) corresponded to the signal at δ 163.0. The assignments of C-2 and C-7′ differed from the first attributions [11] and were in agreement with the revised assignments reported for salazinic acid [14].
1H and 13C NMR (500/125 MHz) data for stictic derivatives (δ in ppm, J in Hz).
| Position | Stictic acid (7) (DMSO-d6) | Peristictic acid (8) (acetone-d6) | Cryptostictic acid (9) (acetone-d6) | Menegazziaic acid (10) (DMSO-d6) | Norstictic acid (11) (DMSO-d6) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| δC | δH | HMBC | δC | δH | HMBC | δC | δH | HMBC | δC | δH | HMBC | δC | δH | HMBC | |
| 1 | 113.3 | – | 114.2 | – | – | 114.1 | – | – | 113.1 | – | – | 111.6 | – | – | |
| 2 | 163.0 | – | 161.3 | – | – | 162.1 | – | – | 161.4 | – | – | 160.3 | – | – | |
| 3 | 114.6 | – | 116.3 | – | – | 119.4 | – | – | 151.3 | – | – | 110.6 | – | – | |
| 4 | 162.6 | – | 160.3 | – | – | 162.8 | – | – | 162.4 | – | – | 163.9 | – | – | |
| 5 | 112.9 | 7.08 (1H, s) | 1, 3, 4, 6, 7, 8, 9 | 112.7 | 7.05 (1H, s) | 1, 3, 4, 7, 8, 9 | 112.1 | 6.94 (1H, s) | 1, 3, 9 | 111.6 | 6.80 (1H, s) | 1, 3 | 117.3 | 6.88 (1H, s) | 1, 3, 4, 7, 9 |
| 6 | 150.8 | – | 147.4 | – | – | 145.6 | – | – | 148.2 | – | – | 152.2 | – | – | |
| 7 | 160.8 | – | 158.4 | – | – | 160.0 | – | – | 160.7 | – | – | 163.5 | – | – | |
| 8 | 186.7 | 10.47 (1H, s) | 1, 3, 4, 5 | 165.5 | – | – | 53.1 | 4.96 (2H, s) | – | – | 10.14 (1H, s) | – | 192.6 | 10.50 (1H, s) | 4 |
| 9 | 21.5 | 2.50 (3H, s) | 1, 2, 5, 6, 7, 8 | 21.4 | 2.54 (3H, s) | 1, 5, 6 | 21.2 | 2.49 (3H, s) | 1, 5, 6 | 19.7 | 2.36 (3H, s) | 1, 5 | 21.3 | 2.48 (3H, s) | 1, 5, 6 |
| 1′ | 109.3 | – | 109.1 | – | – | 109.1 | – | – | 108.0 | – | – | 109.1 | – | – | |
| 2′ | 152.0 | – | 152.5 | – | – | 152.5 | – | – | 152.3 | – | – | 151.9 | – | – | |
| 3′ | 120.8 | – | 121.0 | – | – | 120.7 | – | – | 120.3 | – | – | 120.9 | – | – | |
| 4′ | 148.1 | – | 149.6 | – | – | 150.1 | – | – | 148.9 | – | – | 147.8 | – | – | |
| 5′ | 137.6 | – | 139.4 | – | – | 139.6 | – | – | 139.1 | – | – | 137.3 | – | – | |
| 6′ | 135.9 | – | 135.6 | – | – | 135.9 | – | – | 134.9 | – | – | 135.8 | – | – | |
| 7′ | 166.5 | – | 169.3 | – | – | 169.3 | – | – | 163.0 | – | – | 166.6 | – | – | |
| 8′ | 9.6 | 2.19 (3H, s) | 1′, 2′, 3′, 4′, 5′, 6′ | 9.2 | 2.25 (3H, s) | 2′, 3′, 4′ | 9.1 | 2.22 (3H, s) | 2′,3′, 4′, 6′ | 9.4 | 2.15 (3H, s) | 2′, 3′, 4′ | 9.8 | 2.20 (3H, s) | 2′, 3′, 4′, 5′, 6′ |
| 9′ | 95.4 | 6.62 (1H, s) | 98.5 | 6.98 (1H, s) | 7′ | 98.0 | 7.28 (1H, s) | 7′ | 95.4 | 6.65 (1H, s) | – | 94.6 | 6.79 (1H, s) | – | |
| OCH3-4 | 56.8 | 3.91 (3H, s) | 1, 4 | 56.9 | 3.98 (3H, s) | 4 | 56.6 | 3.94 (3H, s) | 4 | 56.7 | 3.85 (3H, s) | 4 | – | – | – |
| OH-4 | – | – | – | – | – | – | – | – | – | – | – | – | 12.10 (1H, s) | 4 | |
| OH | – | 8.19 (1H, s) | – | – | – | – | 7.72 (1H, s) | – | – | – | – | – | – | – | |
| OH | – | 10.11 (1H, s) | – | 8.69 (1H, s) | – | – | 8.76 (1H, s) | – | – | – | – | – | – | – | |
Compound 8 was obtained as a white amorphous powder. Its molecular formula was assigned as C19H13O10 on the basis of the ESIMS m/z 401.05087 [M-H]− (calcd. for C19H13O10=401.0511). The UV spectrum and the number of unsaturations were the same as for stictic acid. The IR bands were close to stictic acid (3419, 2951, 1735, 1731 and 1609 cm−1) except the signal at 1609 cm−1. No aldehyde signal was detected on the 13C NMR spectrum, but the presence of an additional carbonyl signal was confirmed around 160 ppm (Spectra S5–S6). The assignment of C-4 was confirmed by the HMBC correlation with OCH3-4 (δ 3.98); the deshielded signal (δ 169.3) was attributed to C-7′, which was correlated with H-9′ (δ 6.98) (Figure S7), suggesting a stabilizing hydrogen bond. C-7 (δ 158.4) and C-8 (δ 165.5) were both correlated with H-5 (δ 7.05), but an upfield shift is usually observed for an ester group. Compound 8, which differed from stictic acid by having a carboxylic group in C-3, was identified for peristictic acid (8) [15]. Compounds 9 and 10 were allocated to cryptostictic acid and menegazziaic acid, respectively, as suggested by their molecular formula with negative high-resolution electrospray ionisation mass spectrometry of C19H15O9 (m/z 387.0716 [M-H]−) and C18H13O9 (m/z 373.0566 [M-H]−). The UV spectra had the same profile as compounds 7 and 8, but one peak was missing on the IR absorption spectra (around 1690–1600 cm−1) and the number of unsaturations was calculated to be 12, suggesting a lack of a carbonyl group. The 1H NMR, 13C NMR along with HSQC experiment and HMBC spectra (Figures S1–S12) and DEPT spectra (Spectra S8–S12) confirmed this and showed as a difference a methylic alcohol at C-8 with a broad signal (δH 4.68) (Figure S10a) coupled with the methylenic carbon δC 53.1 ppm for compound 9 and the presence of a broad phenolic proton (δH 10.14) in replacement of the formoyl proton for compound 10. The spectra were acquired first in acetone-d6 for compound 9 to report the major assignments, but one experiment was carried out in DMSO-d6 and confirmed a correlation between C-7′ (δ 169.3) and H-9′ (δ 7.28) (Figure S10b) and attribution of the assignments for C-2 and C-4 as for stictic acid. This complete attribution for 13C NMR signals was first assigned for peristictic, cryptostictic and menegazziaic acids and then for norstictic acid (Table 1). Therefore, five β-orcinol depsidones related to the stictic acid chemosyndrome [15] were isolated. Structures of stictic acid (7), peristictic acid (8), cryptostictic acid (9), menegazziaic acid (10) and norstictic acid (11) showed some variation in the degree of oxidation of the C-8 side group R1 (i.e. CHO, COOH, CH2OH or OH) and the methylation of phenolic groups (i.e. R2=OCH3, OH) (Figure 1). Stictic acid derivatives have been found in many lichen families (Figure 2), in particular in the Parmeliaceae [16], Pertusariaceae and Usneaceae families. Concerning the Stereocaulaceae, peristictic acid and menegazziaic acid were isolated for the first time in the Stereocaulon genus, while cryptostictic acid was isolated in Stereocaulon azoreum [17]. S. montagneanum belongs to the Stereocaulon massartianum complex [18], which also has been found in Indonesia (Java, Sumatra, Borneo and Celebes), Malaya and New Guinea even if it is mainly distinguished from S. massartianum senso stricto by the larger size of its pseudopodetia. Four chemosyndromes are reported for S. massartianum correlated with phenotypic morphological differences, different distribution patterns and different ecological preferences [2, 19]. The typical strain I contains the depside atranorin and the depsidones stictic acid, norstictic acid and possibly constictic acid; the strain II contains atranorin, norstictic and connorstictic acids; the strain III contains atranorin and lobaric acid; and the strain IV contains atranorin and lobaric acid with additional stictic and norstictic acids. Interestingly, the strain I of S. massartianum from which S. montagneanum is chemically close was encountered in the same geographical places. A phylogenetic study has to be conducted to clarify the relative position of these two Stereocaulon species.
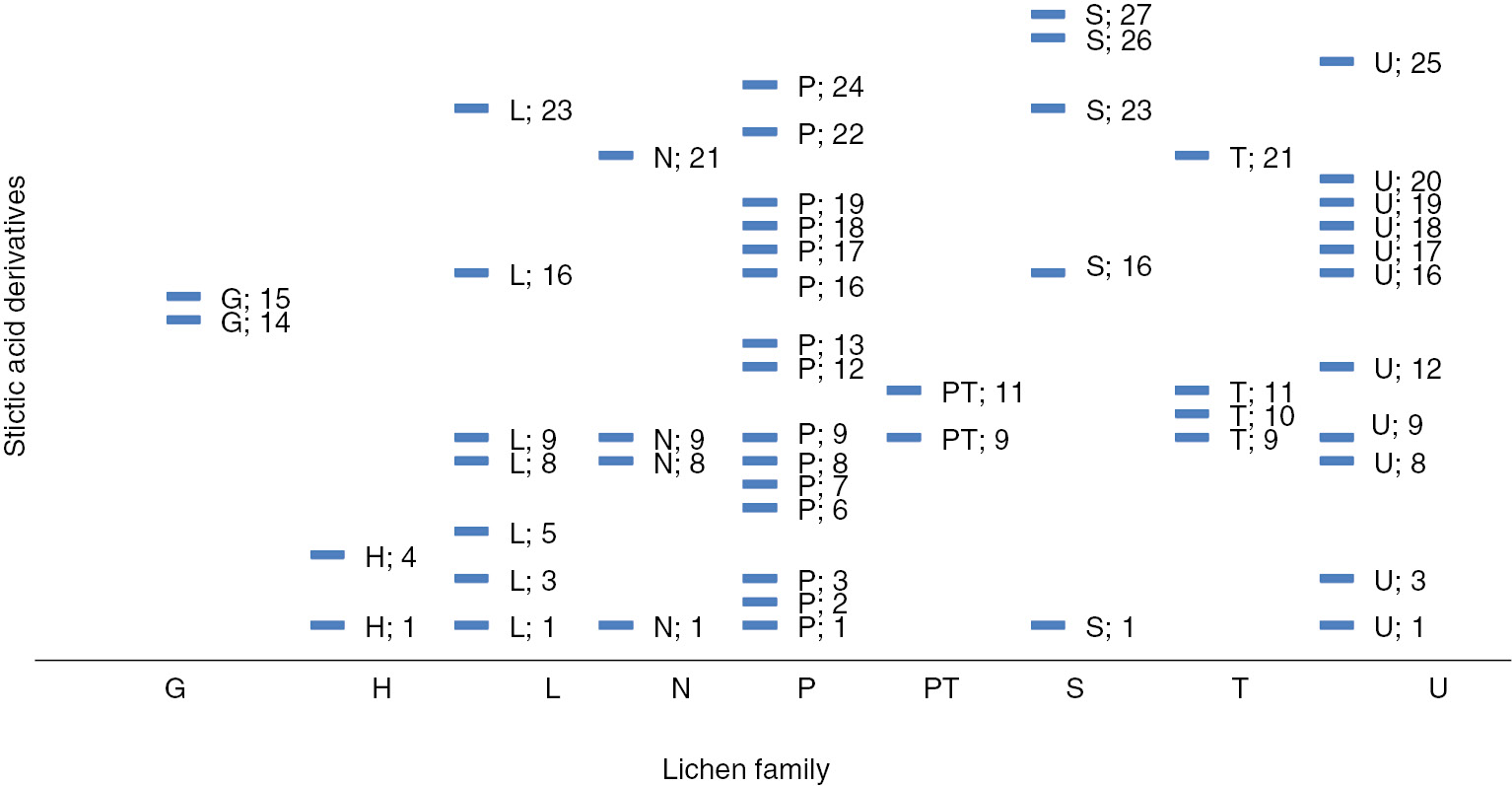
Correlation profile of the stictic acid derivatives and the lichen families (based on the literature, 1970–2013).
G, Graphidaceae; H, Hymeneliaceae; L, Lobariaceae; N, Nephromataceae; P, Parmeliaceae; PT, Pertusariaceae; S, Stereocaulaceae; T, Thelotremataceae; U, Usneaceae; 1, stictic acid; 2, deoxystictic acid; 3, 8′-methyl stictic acid; 4, substictic acid; 5, α-acetylconstictic acid; 6, 8′-ethyl stictic acid (vesuvianic acid); 7, 8′-methyl constictic acid; 8, constictic acid; 9, norstictic acid; 10, subnorstictic acid; 11, connorstictic acid; 12, peristictic acid; 13, lusitanic acid; 14, neotricone; 15, norperistictic acid; 16, cryptostictic acid; 17, cryptostictinolide; 18, menegazziaic acid; 19, 8′-methyl menegazziaic acid; 20, hypoconstictic acid; 21, hypostictic acid; 22, α-acetyl hypoconstictic acid; 23, isidiophorin; 24, verrucigeric acid; 25, 2′-O-methyl hypostictic acid; 26, 2′-O-isidiophorin; 27, 2′-O-methylcryptostictic acid. (Complete references in Table S2).
Compounds 1, 2, 7–9 and 11 were tested for radical-scavenging activity against 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals and superoxide anion (O2−•). The radical-scavenging effect of antioxidants on DPPH is a simple and reliable method to quantify the hydrogen-donating potency of chemicals. Lichen compounds did not seem to have labile hydrogen atoms even at 3000 µM for which the most active were compounds 7 and 8 with an activity of about 10% (Figure 3). This was in accordance with the literature; most of lichen compounds showed a weak reducing activity in electron transfer assays such as the DPPH test [20]. Interestingly, atranorin and all stictic acid derivatives exhibited an activity against superoxide anion equivalent to that of ascorbic acid (7, 9) as for the depside atranorin (1) or twofold better (8, 11) (Figure 3). Atranorin was already reported as a superoxide anion scavenger, but this activity was revealed for the first time for the stictic acid derivatives. As a preliminary assay to evaluate their safety in a possible cosmetic use, the cytotoxic activities of 1, 2, 7–9 and 11 were tested on HaCaT human keratinocyte cell lines (Figure 4). Additionally, cytotoxic activities on B16 murine melanoma were recorded (Figure S15). The dose-response curves showed that compounds 8, 9, 11 were safe for both cell lines with IC50 higher than 100 µM. So, compounds 8, 9 and 11 are valuable compounds with superoxide anion scavenging without toxicity on both cell lines. As sunscreen properties were previously reported for atranorin and methyl orcinol carboxylate [6, 21, 22, 23], their absorbances in the UVB and UVA regions and calculation of their UV-PF and UVA-PF indexes were compared with the broad spectrum UV-filter Tinosorb M (Table S1). None of the tested lichen compounds was found to pass the preliminary threshold to go on for a sunscreen development.
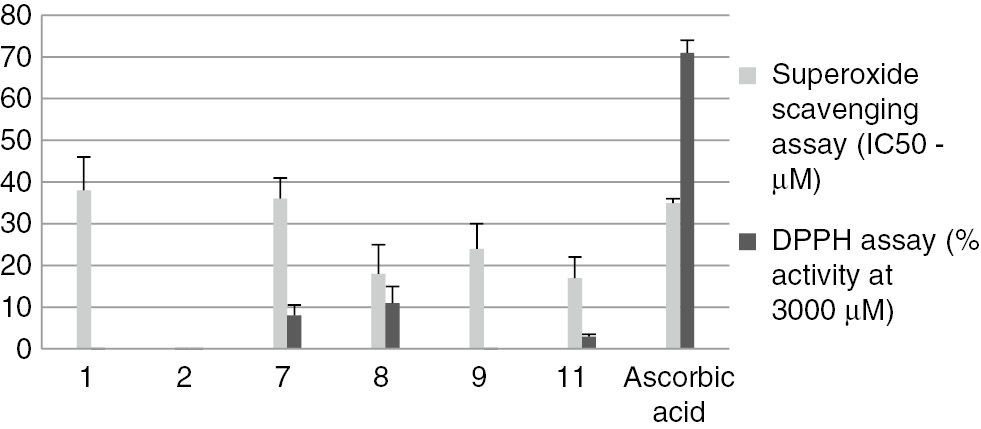
Antioxidant assays of compounds isolated from Stereocaulon montagneanum extract: scavenging ability on DPPH radicals and superoxide anion radicals.
Compounds 3–5, 10 and 6 are not reported on the figure because they were either in insufficient amounts or has no activity (compound 6). Data are means ± SD of triplicate experiment.
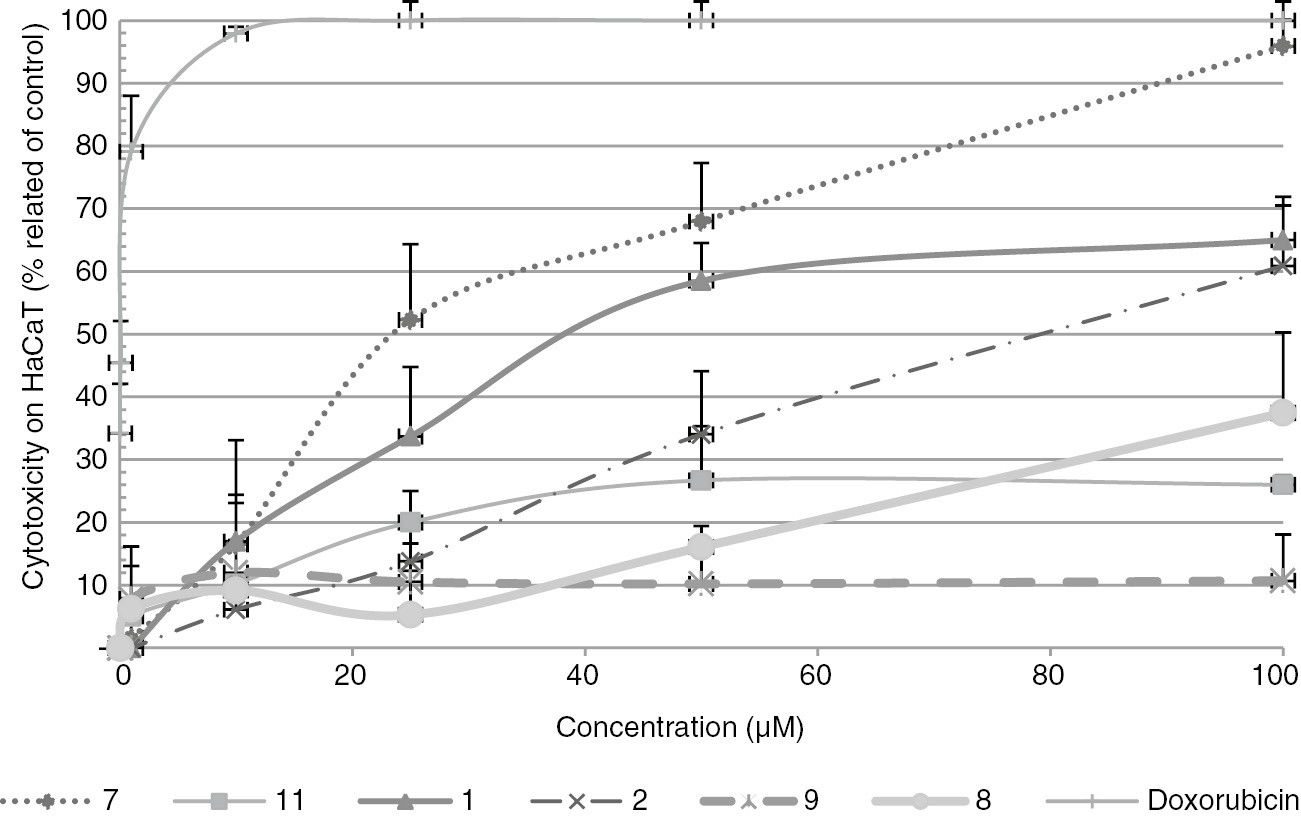
Dose-response curves determined with the MTT assays on HaCaT keratinocyte cells.
Compounds 3–5, 10 and 6 are not reported on the figure because they were either in insufficient amounts or has no activity (compound 6). Data are means ± SD of triplicate experiment.
4 Conclusions
Phytochemical studies of S. montagneanum harvested in Sumatra Island (Indonesia) at high altitude and exposed to the sun not only led to the isolation of the common atranorin and its derivatives but also to five stictic acid derivatives. The structural NMR data for stictic acid were re-examined and partially modified from originally published datasets. Stictic acid derivatives could be more involved in the protection of the lichen against the deleterious effects of UV via reactive oxygen species (ROS) formation than by filtering the UV. Lichens could be a valuable source of new active molecules by limiting ROS damages without a marked cytotoxicity on human keratinocytes.
5 Supplementary material
Experimental details relating to this article are available online alongside the structure NMR spectra of compounds 7–11 (Spectra S1–S14), tables where NMR shifts and attributions of stictic acid derivatives (Table S1) are reported, photoprotective activities and references used to build Figure 2.
Acknowledgements
We gratefully acknowledge the French Ministry of Research and Education [Bio-Asie Program, DREIC [International Relations and Cooperation Department)] and the French Embassy in Indonesia for a PhD grant to Friardi Ismed. We also thank Thi Hang Dao for contributing to the phytochemical work, Nova Syafni and the Sumatran Biota Laboratory team for their help to collect the lichens. We are grateful to P. Jéhan, F. Lambert and N. Le Yondre, CRMPO, Rennes, France, for the mass spectrometer measurements, B. Gargadennec for her help in the sun indexes calculation and P. Uriac. We also thank Arnaud Bondon (PRISM platform) for accurately recording stictic acid spectra as well as Stephane La Barre for critical comments on the text and proofreading service.
References
1. Duvigneaud P. Contribution à l′étude systématique du genre Stereocaulon. Biol Jaarb Dodonaea 1942;9:80–98.Search in Google Scholar
2. Lamb M. A conspectus of the lichen genus Stereocaulon (SCHREB.) HOFFM. J Hattori Bot Lab 1977;43:191–355.Search in Google Scholar
3. Ismed F, Lohézic-Le Dévéhat F, Delalande O, Sinbandhit S, Bakhtiar A, Boustie J. Lobarin from the Sumatran lichen, Stereocaulon halei. Fitoterapia 2012;83:1693–8.10.1016/j.fitote.2012.09.025Search in Google Scholar
4. Solhaug KA, Gauslaa Y, Nybakken L, Bilger W. UV-induction of sun-screening pigments in lichens. New Phytol 2003;158: 91–100.10.1046/j.1469-8137.2003.00708.xSearch in Google Scholar
5. Nguyen K-H, Chollet-Krugler M, Gouault N, Tomasi S. UV-protectant metabolites from lichens and their symbiotic partners. Nat Prod Rep 2013;30:1490–508.10.1039/c3np70064jSearch in Google Scholar
6. Lohézic-Le Dévéhat F, Legouin B, Couteau C, Boustie J, Coiffard L. Lichenic extracts and metabolites as UV filters. J Photochem Photobiol B 2013;120:17–28.10.1016/j.jphotobiol.2013.01.009Search in Google Scholar
7. Bézivin C, Tomasi S, Dévéhat FL, Boustie J. Cytotoxic activity of some lichen extracts on murine and human cancer cell lines. Phytomedicine 2003;10:499–503.10.1078/094471103322331458Search in Google Scholar
8. Millot M, Tomasi S, Articus K, Rouaud I, Bernard A, Boustie J. Metabolites from the lichen Ochrolechia parella growing under two different heliotropic conditions. J Nat Prod 2007;70:316–8.10.1021/np060561pSearch in Google Scholar
9. Lohézic-Le Dévéhat F, Legouin B, Malargé A, Couteau C, Coiffard L. Evaluation of a new UV-filter screening method. In 4èmes journées de l’AFERP “Biodiversité, chimie des substances naturelles et médicaments” et 3ème Symposium franco-pakistanais. France: Besançon, 2010.Search in Google Scholar
10. Huneck S, Yoshimura I. Identification of lichen substances. Berlin, Heidelberg, New York: Springer, 1996.10.1007/978-3-642-85243-5Search in Google Scholar
11. Ewing DF. 13C substituent effects in monosubstituted benzenes. Org Magn Reson 1979;12:499–524.10.1002/mrc.1270120902Search in Google Scholar
12. Stothers JB. Carbon-13 NMR Spectroscopy. Organic chemistry – A series of monographs, Vol. 24. New York and London: Academic Press, 1972.Search in Google Scholar
13. Elix JA, Adler MT, Wardlaw JH. A further three new lichen depsidones. Aust J Chem 1996;49:1175–8.10.1071/CH9961175Search in Google Scholar
14. Eifler-Lima VL, Sperry A, Sinbandhit S, Boustie J, Tomasi S, Schenkel E. NMR spectral data of salazinic acid isolated from some species of Parmotrema. Magn Reson Chem 2000;38:472–4.10.1002/1097-458X(200006)38:6<472::AID-MRC658>3.0.CO;2-PSearch in Google Scholar
15. Elix JA, Wardlaw JH. Lusitanic acid, peristictic acid and verrucigeric acid. Three new β-orcinol depsidones from the lichens Relicina sydneyensis and Xanthoparmelia verrucigera. Aust J Chem 2000;53:815–8.10.1071/CH00121Search in Google Scholar
16. Kosanić M, Ranković B, Stanojković T, Vasiljević P, Manojlović N. Biological activities and chemical composition of lichens from Serbia. EXCLI J 2014;13:1226–38.Search in Google Scholar
17. González AG, Pérez EM, Padrón CE, Barrera JB. Chemical constituents of the lichen Stereocaulon azoreum. Z Naturforsch C 1992;47:503–7.10.1515/znc-1992-7-802Search in Google Scholar
18. Lamb M. The Stereocaulon massartianum assemblage in East Asia. J Jap Bot 1965;40:270–5.Search in Google Scholar
19. Huang M-R. Noteworthy species of Stereocaulon from China. Mycosystema 2008;27:85–90.Search in Google Scholar
20. Lohézic-Le Dévéhat F, Delmail D, Thüs H, Boustie J. Oxidative stress regulation in lichens and its relevance for survival in coastal habitats. Adv Bot Res 2014;71:467–504.10.1016/B978-0-12-408062-1.00016-0Search in Google Scholar
21. Fernandez E, Reyes A, Hidalgo ME, Quilhot W. Photoprotector capacity of lichen metabolites assessed through the inhibition of the 8-methoxypsoralen photobinding to protein. J Photochem Photobiol B 1998;42:195–201.10.1016/S1011-1344(98)00070-0Search in Google Scholar
22. Fernandez E, Quilhot W, Gonzalez I, Hidalgo ME, Molina X, Meneses I. Lichen metabolites as UVB filters. Cosmet Toiletries 1996;111:69–74.Search in Google Scholar
23. Hidalgo ME, Bascunan L, Quilhot W, Fernandez E, Rubio C. Spectroscopic and photochemical properties of the lichen compound lobaric acid. Photochem Photobiol 2005;81:1447–9.10.1562/2005-05-17-RA-530Search in Google Scholar PubMed
Supplemental Material:
The online version of this article offers supplementary material (DOI: https://doi.org/10.1515/znc-2016-0148).
©2017 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Review Article
- Structural diversity in echinocandin biosynthesis: the impact of oxidation steps and approaches toward an evolutionary explanation
- Research Articles
- Cytotoxic constituents of Alocasia macrorrhiza
- Isolation, antimicrobial and antitumor activities of a new polyhydroxysteroid and a new diterpenoid from the soft coral Xenia umbellata
- Volatile oil profile of some lamiaceous plants growing in Saudi Arabia and their biological activities
- Membrane permeabilizing action of amphidinol 3 and theonellamide A in raft-forming lipid mixtures
- Discovery and preliminary structure-activity relationship of the marine natural product manzamines as herpes simplex virus type-1 inhibitors
- NMR reassignment of stictic acid isolated from a Sumatran lichen Stereocaulon montagneanum (Stereocaulaceae) with superoxide anion scavenging activities
- Simultaneous formulation of terbinafine and salvia monoterpenes into chitosan hydrogel with testing biological activity of corresponding dialysates against C. albicans yeast
- Rapid Communication
- Chemical constituents of the leaves of Campylospermum elongatum
Articles in the same Issue
- Frontmatter
- Review Article
- Structural diversity in echinocandin biosynthesis: the impact of oxidation steps and approaches toward an evolutionary explanation
- Research Articles
- Cytotoxic constituents of Alocasia macrorrhiza
- Isolation, antimicrobial and antitumor activities of a new polyhydroxysteroid and a new diterpenoid from the soft coral Xenia umbellata
- Volatile oil profile of some lamiaceous plants growing in Saudi Arabia and their biological activities
- Membrane permeabilizing action of amphidinol 3 and theonellamide A in raft-forming lipid mixtures
- Discovery and preliminary structure-activity relationship of the marine natural product manzamines as herpes simplex virus type-1 inhibitors
- NMR reassignment of stictic acid isolated from a Sumatran lichen Stereocaulon montagneanum (Stereocaulaceae) with superoxide anion scavenging activities
- Simultaneous formulation of terbinafine and salvia monoterpenes into chitosan hydrogel with testing biological activity of corresponding dialysates against C. albicans yeast
- Rapid Communication
- Chemical constituents of the leaves of Campylospermum elongatum

