Abstract
Polycrystalline samples of Fe3−yGeTe2 (0.08 ≤ y ≤ 0.29) and the solid solutions Fe3−x−yNixGeTe2 (0.24 ≤ x ≤ 1.32; 0.14 ≤ y ≤ 0.41) were synthesized at 898–973 K in a resistance furnace and characterized by X-ray and neutron powder diffraction with Rietveld analysis (Fe3GeTe2 type, P63/mmc, a = 402.665(3), c = 1632.820(14) pm for x = 0, y = 0.08). Fe3Ge layers with planar FeGe hexagons and additional iron atoms above and below the rings are separated by double layers of tellurium atoms. Fe3GeTe2 is ferromagnetic below TC = 230 K with magnetic moments aligned along the c axis. TC depends on the iron content and decreases with increasing iron vacancies continuously to 153 K in Fe2.71GeTe2. Further reduction of TC is possible by nickel substitution until magnetic ordering is nearly absent in Fe1.33Ni1.32GeTe2. The suppression of the magnetic ordering is caused by random dilution of the magnetic iron atoms either by vacancies or by non-magnetic nickel atoms.
1 Introduction
Metallic compounds with quasi two-dimensional crystal structures and itinerant magnetism attract considerable interest [1, 2]. In contrast to localized magnetism of unpaired electrons in 3d or 4f shells, itinerant magnetism is not an atomic phenomenon but caused by correlations between the conduction electrons. Magnetic fluctuations occurring in such itinerant magnetic systems are currently believed to be one key to unconventional superconductivity [3, 4]. Therefore the search for new materials with weak itinerant magnetism is well underway. A compound of recent awakening interest is the telluride Fe3GeTe2 which is metallic and ferromagnetic below TC = 230 K with a relatively low saturation moment of 1.2 μB per iron atom [5]. Recent more detailed studies [6, 7] revealed strongly anisotropic ferromagnetism with moments aligned along the c axis as expected from the pronounced two-dimensional character of the crystal structure. Interestingly, the analogous nickel compound Ni3GeTe2 shows no magnetic ordering but is a Pauli paramagnetic metal [5]. In contrast to Fe3GeTe2, the nickel compound shows significant electron density in the van der Waals gap between the tellurium layers in the crystal structure. The partial occupancy of the additional site is accompanied by a nickel deficiency in the NiGe hexagons. Due to the differences in the magnetic behavior of the iron and nickel compounds interesting properties of the solid solutions Fe3−xNixGeTe2 are conceivable. These could occur if magnetic fluctuations persist while the ordering is suppressed. Furthermore Fe3GeTe2 has a composition range according to Fe3−yGeTe2 which also affects the Curie temperature [8]. Here we study the crystal structures and magnetic properties of the iron-deficient compounds Fe3−yGeTe2 and the solid solutions Fe3−x−yNixGeTe2.
2 Experimental section
Polycrystalline samples of Fe3−yGeTe2 with nominal y = 0, 0.2, 0.4 and Fe3−x−yNixGeTe2 with nominal x = 0.25, 0.5, 0.75, 1.0, 1.5 and y = 0.1 were synthesized from Fe, Ni, Ge and Te powder in alumina crucibles sealed in silica ampoules under argon atmosphere. In a first step the stoichiometric mixture was heated up to 898 K at a rate of 100 K h−1 and kept at this temperature for 60 h. After cooling at a rate of 100 K h−1 or 200 K h−1 the samples were ground in a mortar and – except for Fe3−yGeTe2 with nominal y = 0.2 and 0.4 – sintered at 973 K for 60 h with a heating and cooling rate of 100 K h−1, respectively. For nominal Fe2.8GeTe2 the sintering step was carried out like the first one.
X-ray powder diffraction analysis of the samples was performed on a STOE STADI P diffractometer using MoKα1 radiation. Rietveld refinements were done using the Topas program package [9]. Energy-dispersive spectroscopy measurements were performed on a ZEISS EVO-MA 10 microscope. Data was collected using a BRUKER X-Flash 410-M detector and the software Quantax 200. Magnetic measurements of the powder samples were carried out with a Quantum Design SQUID-Magnetometer MPMS XL-5. Neutron powder diffraction analysis was realized at the Laboratory for Neutron Scattering and Imaging at the Paul Scherrer Institute (Villigen, Switzerland) with the High-Resolution Powder Diffractometer for Thermal Neutrons. Data was collected with a wavelength of 188.570 pm at 3–300 K. Data refinement was done with the Jana2006 program package [10].
3 Results and discussion
3.1 Crystal and magnetic structure
The crystallographic data obtained from Rietveld refinements of X-ray and neutron diffraction patterns are fully consistent with those given in refs. [5, 7]. Neutron patterns at 3 K and 300 K are shown in Fig. 1. Small impurities of Fe2.25Te2 (1.0 wt %) and Fe3O4 (0.8 wt %) are identified by Rietveld analysis. Fe3GeTe2 crystallizes in the hexagonal space group P63/mmc in its own structure type (Fig. 2), which contains Fe3Ge layers similar to those present in Fe2Ge [8, 9]. Fe2Ge can be derived from the AlB2-type structure with planar FeGe hexagons separated by additional iron atoms (Fe[FeGe]). Fe2Ge is not stoichiometric but iron-deficient according to Fe2−xGe (x ≈ 0.1–0.5) with Fe vacancies on the Fe2 site in the FeGe hexagons as it is the case in Fe3−yGeTe2. The Fe3Ge sheets are well separated by a double layers of tellurium atoms at an interlayer distance of 815 pm.
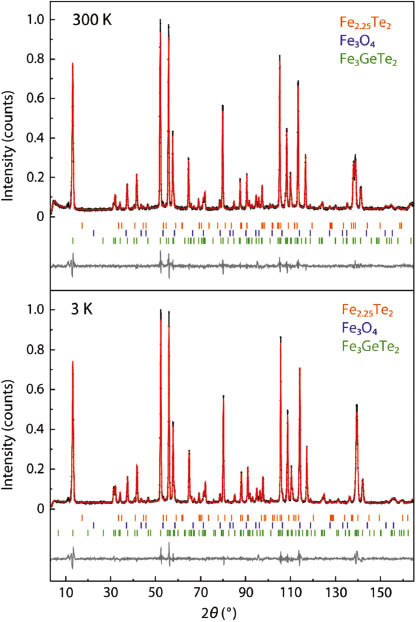
Neutron diffraction patterns (λ = 188.57 pm) of Fe3GeTe2 at room temperature and at 3 K with Rietveld fits.
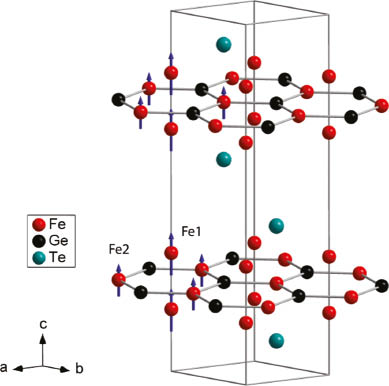
Crystal structure of Fe3GeTe2 with magnetic moments (μFe1 = 1.75(6) μB, μFe2 = 1.07(16) μB) aligned ferromagnetically along the crystallographic c axis.
The compositions of the samples Fe3−yGeTe2 and Fe3−x−yNixGeTe2 were determined by EDX measurements. Samples of Fe3−yGeTe2 are homogeneous up to Fe2.7GeTe2. The iron vacancies cause a shrinking of the unit cell volume through smaller lattice parameters a even though the c axis increases slightly (Table 1, Fig. 3). Further reduction of the iron content down to 1.33 is possible by substitution with nickel. The lattice parameters a, c and the cell volumes of the solid solution Fe3−x−yNixGeTe2 decrease continuously but with smaller slopes than in Fe3−yGeTe2 because iron vacancies are now filled with nickel atoms. We point out that no nickel atoms are found in the van-der-Waals gap in our Fe3−x−yNixGeTe2 samples up to x = 1.32, as it is the case for the pure nickel compound Ni3GeTe2 [5].
Lattice parameters a and c, unit cell volumes V, Curie temperatures TC and saturation magnetizations μsat of Fe3−yGeTe2 and Fe3−x−yNixGeTe2.
| y (± 0.05) | x (± 0.05) | a (pm) (± 0.07) | c (pm) (± 0.3) | V (nm3) (± 0.0005) | TC (K) (± 1) | μsat (μB) (± 0.05 μB) |
|---|---|---|---|---|---|---|
| 0.08 | 0 | 402.7 | 1632.8 | 0.2293 | 229 | 1.56 |
| 0.14 | 0 | 399.0 | 1636.0 | 0.2260 | 208 | 1.39 |
| 0.29 | 0 | 395.6 | 1639.5 | 0.2222 | 153 | 0.85 |
| 0.14 | 0.24 | 399.5 | 1630.9 | 0.2254 | 158 | 1.43 |
| 0.29 | 0.41 | 398.1 | 1629.6 | 0.2236 | 108 | 1.22 |
| 0.14 | 0.73 | 396.5 | 1627.4 | 0.2215 | 49 | 0.89 |
| 0.41 | 0.89 | 395.4 | 1625.2 | 0.2200 | 30 | 0.55 |
| 0.35 | 1.32 | 393.9 | 1620.2 | 0.2177 | 22 | 0.38 |
| 0.21 | 2.79 | 390.2 | 1602.2 | 0.2113 | – | – |
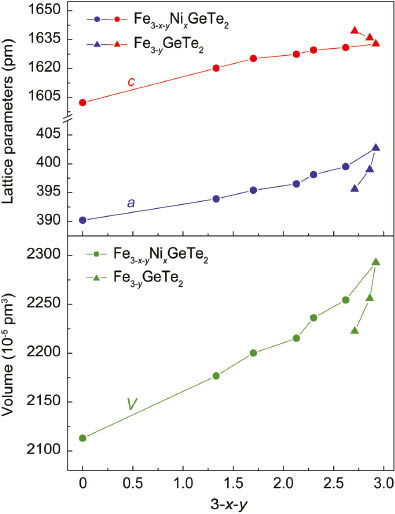
Lattice parameters (top) and unit cell volumes (bottom) of Fe3−yGeTe2 and Fe3−x−yNixGeTe2.
3.2 Magnetic properties
The top panel of Fig. 4 shows magnetic susceptibilities of Fe3−yGeTe2 and Fe3−x−yNixGeTe2 measured from 300 to 2 K at 20 kOe external fields (1 kOe = 7.96 × 104 A m−1). The zero points of the second derivatives
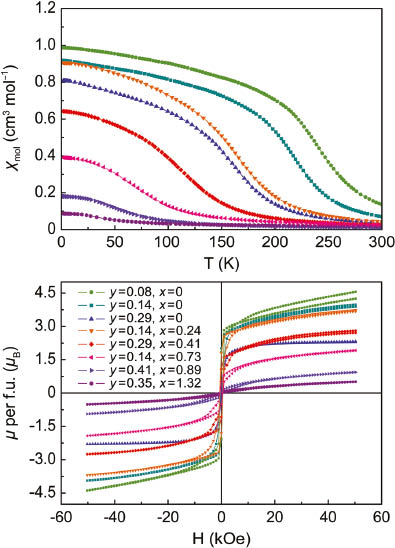
Magnetic susceptibilities measured at 20 kOe (top) and isothermal magnetizations at 1.8 K (bottom) of Fe3−yGeTe2 and Fe3−x−yNixGeTe2.
The ferromagnetism of these compounds in terms of TC and μsat is not proportional to the valence electron count but depends on the iron concentration, as depicted in Fig. 5. Nickel remains non-magnetic as it is known from other nickel tellurides with layered structures which are exclusively Pauli paramagnetic metals [5, 10, 11]. The suppression of magnetic ordering turns out to be more efficient by introducing iron vacancies. However, this is only possible up to Fe2.7GeTe2 where the ferromagnetism is still present.
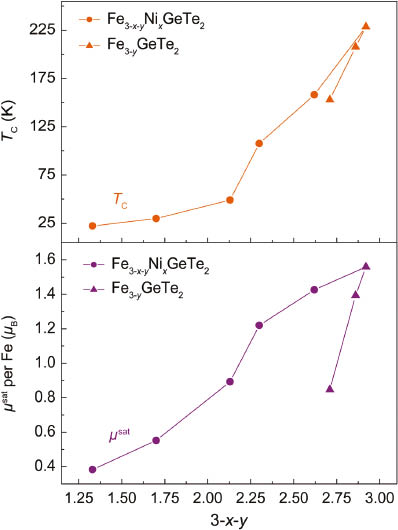
Curie temperatures TC (top) and saturation magnetizations μsat (bottom) of Fe3−yGeTe2 and Fe3−x−yNixGeTe2 against the iron concentration.
4 Conclusion
Ferromagnetism in the compounds Fe3−x−yNixGeTe2 is suppressed through random dilution of the magnetic iron atoms by either vacancies (most efficient) or by non-magnetic nickel atoms. The Curie temperatures as well as the saturation moments decrease continuously as the magnetic coupling between the iron atoms becomes weaker. Similar suppression of magnetic ordering has also been observed in other layered tellurides, for example Fe1.1−xNixTe (x = 0.02, 0.04, 0.08, 0.12) [12], as well as in the diluted antiferromagnet MnxZn1−xPS3 [13].
References
[1] C. Pfleiderer, S. R. Julian, G. G. Lonzarich, Nature2001, 414, 427.10.1038/35106527Search in Google Scholar PubMed
[2] Y. Ishikawa, Y. Noda, Y. J. Uemura, C. F. Majkrzak, G. Shirane, Phys. Rev. B.1985, 31, 5884.Search in Google Scholar
[3] D. J. Scalapino, Rev. Mod. Phys.2012, 84, 1383.Search in Google Scholar
[4] D. Johrendt, J. Mater. Chem.2011, 21, 13726.Search in Google Scholar
[5] H.-J. Deiseroth, K. Aleksandrov, C. Reiner, L. Kienle, R. K. Kremer, Eur. J. Inorg. Chem.2006, 2006, 1561.Search in Google Scholar
[6] B. Chen, J. Yang, H. Wang, M. Imai, H. Ohta, C. Michioka, K. Yoshimura, M. Fang, J. Phys. Soc. Jpn.2013, 82, 124711.Search in Google Scholar
[7] V. Y. Verchenko, A. A. Tsirlin, A. V. Sobolev, I. A. Presniakov, A. V. Shevelkov, Inorg. Chem.2015, 54, 8598.Search in Google Scholar
[8] F. Laves, H. J. Wallbaum, Z. Angew. Mineral.1942, 4, 17.Search in Google Scholar
[9] P. J. Schurer, N. J. G. Hall, A. H. Morrish, Phys. Rev. B.1978, 18, 4860.Search in Google Scholar
[10] T. K. Reynolds, R. F. Kelley, F. J. DiSalvo, J. Alloys Compd.2004, 366, 136.Search in Google Scholar
[11] A. A. Isaeva, O. N. Makarevich, A. N. Kuznetsov, T. Doert, A. M. Abakumov, G. Van Tendeloo, Eur. J. Inorg. Chem.2010, 9, 1395.Search in Google Scholar
[12] J. Janaki, T. Geetha Kumary, N. Thirumurugan, A. Mani, A. Das, G. V. Narasimha Rao, A. Bharathi, J. Supercond. Novel Magn.2012, 25, 209.Search in Google Scholar
[13] N. Chandrasekharan, S. Vasudevan, Phys. Rev. B.1996, 54, 14903.Search in Google Scholar
©2016 by De Gruyter
Articles in the same Issue
- Frontmatter
- In this Issue
- Ferromagnetism in Fe3−x−yNixGeTe2
- A novel potentiometric sensor based on urease/ bovine serum albumin-poly(3,4-ethylenedioxythiophene)/Pt for urea detection
- Two new glycosidal metabolites of endophytic fungus Penicillium sp. (NO.4) from Tapiscia sinensis
- New bioactive metabolites from Penicillium purpurogenum MM
- Long-chain alkyl-substituted gentisic acid and benzoquinone derivatives from the root of Micronychia tsiramiramy (Anacardiaceae)
- A tetranuclear copper (II) complex with pyrazole-3,5-dicarboxylate ligands: synthesis, characterization and electrochemical properties
- Synthesis and structure of a cobalt coordination polymer based on 2,8-di(pyridin-4-yl)dibenzothiophene and 4,4-dicarboxydiphenylsulfone
- nBu4NI-catalyzed direct amination of benzoxazoles with tertiary amines using TBHP as oxidant under microwave irradiation
- Synthesis, single-crystal structure determination and Raman spectra of the tricyanomelaminates NaA5[C6N9]2 · 4 H2O (A = Rb, Cs)
- Synthesis of structural analogues of GGT1-DU40, a potent GGTase-1 inhibitor
- Note
- Crystal structure of a dimeric 1-benzothiepin
Articles in the same Issue
- Frontmatter
- In this Issue
- Ferromagnetism in Fe3−x−yNixGeTe2
- A novel potentiometric sensor based on urease/ bovine serum albumin-poly(3,4-ethylenedioxythiophene)/Pt for urea detection
- Two new glycosidal metabolites of endophytic fungus Penicillium sp. (NO.4) from Tapiscia sinensis
- New bioactive metabolites from Penicillium purpurogenum MM
- Long-chain alkyl-substituted gentisic acid and benzoquinone derivatives from the root of Micronychia tsiramiramy (Anacardiaceae)
- A tetranuclear copper (II) complex with pyrazole-3,5-dicarboxylate ligands: synthesis, characterization and electrochemical properties
- Synthesis and structure of a cobalt coordination polymer based on 2,8-di(pyridin-4-yl)dibenzothiophene and 4,4-dicarboxydiphenylsulfone
- nBu4NI-catalyzed direct amination of benzoxazoles with tertiary amines using TBHP as oxidant under microwave irradiation
- Synthesis, single-crystal structure determination and Raman spectra of the tricyanomelaminates NaA5[C6N9]2 · 4 H2O (A = Rb, Cs)
- Synthesis of structural analogues of GGT1-DU40, a potent GGTase-1 inhibitor
- Note
- Crystal structure of a dimeric 1-benzothiepin

