Synthesis and structure of a cobalt coordination polymer based on 2,8-di(pyridin-4-yl)dibenzothiophene and 4,4-dicarboxydiphenylsulfone
-
Yan Jiao
and Min-Dong Chen
Abstract
A new coordination polymer {[Co2(DPDBT)2(DCPS)2(H2O)](H2O)}n (1) [DPDBT = 2,8-di(pyridin-4-yl)dibenzothiophene, H2DCPS = 4,4′-dicarboxydiphenyl sulfone], was synthesized under solvothermal conditions. Complex 1 exhibits a 2D structure with two different kinds of binuclear cobalt cluster units. The UV/Vis spectra of 1 and of the ligands were investigated, indicating that complex 1 is a potential wide gap semiconductor material.
1 Introduction
Coordination polymers are currently under intense academic and industrial investigations, because of their potential applications in the fields of photochemistry [1], molecular magnetism [2], gas adsorption and separation [3], heterogeneous catalysis [4], nonlinear optics [5], as well as their artistic architectures [6–8]. In the self-assembly processes of coordination polymers, using mixed ligands is undoubtedly a good choice for the construction of new polymeric structures with greater tunability than that only present with a single type of ligand [9, 10]. Coordination polymers based on pyridyl ligands with excellent coordination ability have attracted more and more attention due to their novel architectures and special properties [11, 12].
Recently, we synthesized a new V-shaped pyridyl ligand, 2,8-di(pyridin-4-yl)dibenzo[b,d]thiophene (DPDBT) [10, 13], which may be regarded as a rigid ligand (Scheme 1). Meanwhile, we adopted 4,4′-dicarboxydiphenyl sulfone (H2DCPS) [14] as a co-ligand to react with the DPDBT ligand and various bivalent metal salts. A coordination polymer with an intriguing structure was obtained under solvothermal conditions, namely {[Co2(DPDBT)2(DCPS)2(H2O)](H2O)}n (1). Complex 1 was characterized by elemental analysis, IR spectra, and powder and single crystal X-ray crystallography. The UV/Vis spectra of 1 and the ligands were investigated.
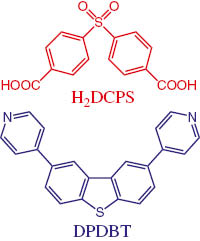
Structures of ligands DPDBT and H2DCPS.
2 Results and discussion
2.1 Crystal and molecular structure of {[Co2 (DPDBT)2(DCPS)2(H2O)](H2O)}n (1)
Complex 1 and the Cd(II) complex {[Cd2(DPDBT)2(DCPS)2 (H2O)](H2O)}n with the same ligand composition as reported in [13] have very similar molecular and crystal structures despite the utilization of different bivalent metal salts. As {[Cd2(DPDBT)2(DCPS)2(H2O)](H2O)}n [13], complex 1 crystallizes in the triclinic space group P1̅ with Z = 2 (Table 1). The differences in the cell constants do not point to isotypism. The coordination geometry around the Co2+ atoms is depicted in Fig. 1. The Co–O lengths are in the range of 2.039(1)–2.267(1) Å, and the Co–N lengths are 2.138(8)–2.149(2) Å. They are both close to that of the reported Co complex {[Co2(BPPA)(hfipbb)2(H2O)(DMF)2](H2O)4}n (Co–N 2.120(2) Å, Co–O 2.052–2.224 Å, BPPA = bi(4-pyridylphenyl)amine, H2DCPS = 4,4′-dicarboxydiphenyl sulfone) [8].
Crystallographic data of {[Co2(DPDBT)2(DCPS)2(H2O)](H2O)}n (1).
| Complex | 1 |
| Empirical formula | C72H46Co2N4O13S4 |
| Formula weight | 1421.23 |
| T, K | 296(2) |
| Crystal system | Triclinic |
| Space group | P1 |
| a, Å | 15.5070(10) |
| b, Å | 16.6545(10) |
| c, Å | 17.7571(19) |
| a, deg | 99.0360(10) |
| β, deg | 97.7370(10) |
| γ, deg | 117.4260(10) |
| V, Å−3 | 3906.4(5) |
| Z | 2 |
| Dcalcd, g m−3 | 1.21 |
| μ(MoKα), mm–1 | 0.6 |
| F(000), e | 1456 |
| θ range, deg | 1.19–25.00 |
| hkl range | ±18, ±19, −19→9 |
| Refl. collected/unique/Rint | 22 031/13 681/0.015 |
| Data/restraints/ref. param. | 13 681/3/849 |
| Goodness-of-fit on F2 | 1.020 |
| R1/wR2 [I > 2σ(I)] | 0.0401/0.1171 |
| R1/wR2 (all data) | 0.0470/0.1219 |
| Δρfin (max/min), e Å–3 | 0.95/−0.99 |
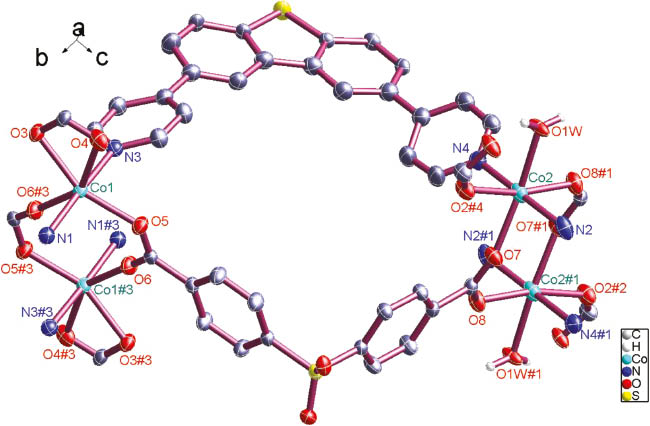
Coordination environments of the Co2+ atoms in 1. Most hydrogen atoms are omitted for clarity (30 % ellipsoid probability). Symmetry codes: #1 = −x, −y, 2 − z; #2 = −1 + x, −1 + y, 1 + z; #3 = −x, 1 − y, 1 − z; #4 = 1 − x, 1 − y, 1 − z.
There are two kinds of binuclear cobalt units [Co2(CO2)2] (Fig. 1), in which the Co1···Co1#3 and Co2···Co1#1 distances are 4.012(8) and 4.518(8) Å, respectively. There are three different types of bonding modes for the carboxylate groups in 1: monodentate (O1–C72–O2), chelate (O3–C59–O4), and bridging bidentate (O5–C58–O6 and O7–C45–O8). The dihedral angles between two phenyl rings of DCPS– anions are 75.3° and 77.3°. For DCPS2– anions connecting two cobalt cluster units, the [Co2(CO2)2]–S–[Co2(CO2)2] angles are 105.8° and 123.9°.
For the DPDBT molecules, the angle N3–S1–N4 is 75.19°, and N1–S2–N2 is 77.01°. The dihedral angles between pyridyl and adjacent phenyl rings of the BPPA molecules in 1 are 34.91°, 25.17°, 29.37°, and 17.92°. The crystal structure of 1 along the crystallographic a axis is shown in Fig. 2.
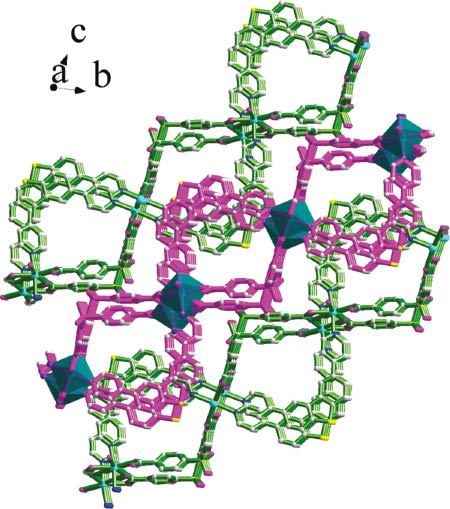
Crystal structure of 1 seen along the crystallographic a axis.
2.2 UV/Vis spectra
The UV/Vis absorption spectra of free H2DCPS and DPDBT ligands were obtained in the crystalline state at room temperature (Fig. 3). The H2DCPS and DPDBT ligands exhibit absorption bands in the ranges of 200–310 and 200–480 nm, respectively, which can be ascribed to the n → π* and π → π* transitions of the ligands. A lower energy band from 510 to 665 nm for complex 1 can be attributed to the spin-allowed d–d transition of the d7 (Co2+) cation [15, 16]. The diffuse reflectance data have been transformed into a Kubelka–Munk function to obtain the band gaps (Eg). As shown in Fig. 4, the Eg value assessed from the steep absorption edge is 2.40 eV.
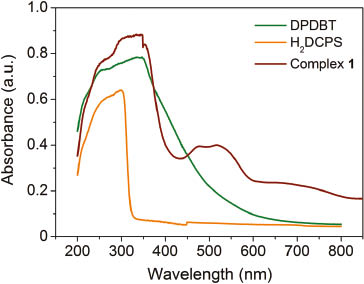
UV/Vis absorption spectra of 1 and the ligands at room temperature.
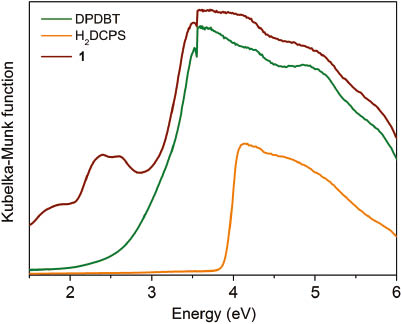
Kubelka–Munk plots as a function of the energy of 1 and the ligands at room temperature.
2.3 Powder X-ray diffraction measurements
To confirm whether the crystal structure is truly representative of the bulk material tested in the photochemical studies, powder X-ray diffraction (PXRD) was carried out for complex 1. The experimental and computer-simulated patterns are shown in Fig. 5, proving that the bulk synthesized material and the measured single crystal for 1 are the same.
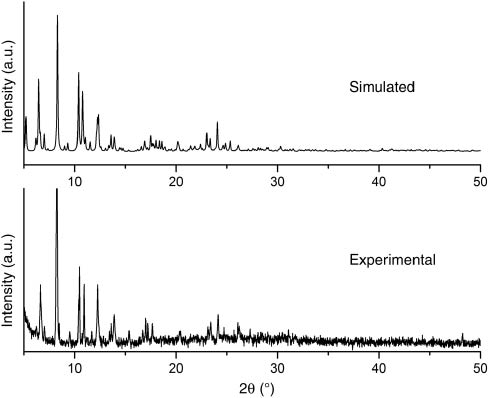
Powder X-ray diffraction pattern of complex 1.
3 Conclusion
In summary, a new coordination polymer has been successfully synthesized by self-assembly of DPDBT and carboxylate ligands DCPS2– and Co2+ ions under solvothermal conditions. Complex 1 shows a 2D structure containing two different binuclear cobalt units. The band gap (Eg) value assessed from the steep absorption edge is 2.40 eV.
4 Experimental section
4.1 Materials and physical measurements
The carboxylic acid H2DCPS, and other reagents and solvents employed were commercially available. The DPDBT ligand was synthesized by Suzuki cross-coupling reaction [10, 13]. The crystal sample was stored in mother solutions until the study of its properties. The IR absorption spectrum of complex 1 was recorded in the range of 400–4000 cm–1 on a Nicolet (Impact 410) spectrometer with KBr pellets. C, H, and N analysis was carried out with a Perkin Elmer 240C elemental analyzer. The UV/Vis absorption spectrum was recorded by a Shimadzu UV-3600 spectrometer for the solid phase.
4.2 Syntheses of {[Cd2(DPDBT)2(DCPS)2 (H2O)](H2O)}n (1)
A mixture of Co(NO3)2 · 6H2O (58.2 mg, 0.2 mmol), DPDBT (67.6 mg, 0.2 mmol), and 4,4′-dicarboxydiphenyl sulfone (60.12 mg, 0.20 mmol) was dissolved in 15 mL of DMF-H2O (v/v, 1:1). The final mixture was placed in a Parr Teflon-lined stainless steel vessel (25 mL) under autogenous pressure and heated at 90 °C for 3 days. Red crystals of 1 were collected in 77 % yield based on DPDBT ligand. – Co2C72H48N4O14S4: calcd. C 60.08, H 3.36, N 3.89; found: C 59.97, H 3.43, N 3.81. – IR (KBr, cm–1): 3429 (s), 1672 (s), 1612 (s), 1556 (s), 1470 (m), 1399 (s), 1324 (m), 1295 (s), 1226 (w), 1162 (s), 1136 (w), 1098 (s), 1070 (w), 1014 (w), 839 (w), 807 (s), 780 (w), 742 (s), 720 (w), 697 (w), 621 (s), 580 (s), 464 (w).
4.3 X-ray crystallography
Intensity measurements were made on a Bruker Smart-1000CCD diffractometer with graphite-monochromatized MoKα radiation (λ = 0.71073 Å) by using a φ–ω scan mode at 296(2) K. Lp corrections were applied. An absorption correction was done using Sadabs [17]. The structure was solved by Direct Methods [18] with Shelxtl (version 6.10) [18] and refined by full-matrix least squares on F2 with Shelxtl [19]. All non-hydrogen atoms were refined anisotropically and the hydrogen atoms were generated geometrically. It should be noted that the guest molecules in complex 1 are too disordered to be modeled properly in the refinement. Therefore, the respective electron density was removed with the routine SQUEEZE in Platon [20, 21]. The details of the crystal and refinement data are given in Table 1. Selected bond lengths and bond angles are listed in Table 2.
Selected bond lengths (Å) and bond angles (deg) for {[Co2(DPDBT)2(DCPS)2(H2O)](H2O)}n (1).a
| Distances | |||
| Co(1)–O(6)#1 | 2.0391(15) | Co(1)–O(5) | 2.0654(15) |
| Co(1)–N(3) | 2.1388(19) | Co(1)–N(1) | 2.1414(19) |
| Co(1)–O(3) | 2.1579(15) | Co(1)–O(4) | 2.2671(17) |
| Co(2)–O(8)#2 | 2.0594(15) | Co(2)–O(7) | 2.0772(16) |
| Co(2)–O(2)#3 | 2.1069(16) | Co(2)–N(4) | 2.147(2) |
| Co(2)–N(2) | 2.149(2) | Co(2)–O(1W) | 2.162(2) |
| Angles | |||
| O(6)#1–Co(1)–O(5) | 122.58(7) | O(6)#1–Co(1)–N(3) | 90.22(7) |
| O(5)–Co(1)–N(3) | 90.81(7) | O(6)#1–Co(1)–N(1) | 87.79(7) |
| O(5)–Co(1)–N(1) | 87.91(7) | N(3)–Co(1)–N(1) | 176.58(7) |
| O(6)#1–Co(1)–O(3) | 89.00(6) | O(5)–Co(1)–O(3) | 148.41(6) |
| N(3)–Co(1)–O(3) | 88.00(7) | N(1)–Co(1)–O(3) | 94.75(7) |
| O(6)#1–Co(1)–O(4) | 147.89(7) | O(5)–Co(1)–O(4) | 89.52(7) |
| N(3)–Co(1)–O(4) | 89.69(6) | N(1)–Co(1)–O(4) | 93.48(7) |
| O(3)–Co(1)–O(4) | 58.91(6) | O(8)##2–Co(2)–O(7) | 105.47(7) |
| O(8)#2–Co(2)–O(2)#3 | 168.87(7) | O(7)–Co(2)–O(2)#3 | 85.56(7) |
| O(8)#2–Co(2)–N(4) | 87.77(7) | O(7)–Co(2)–N(4) | 92.11(8) |
| O(2)#3–Co(2)–N(4) | 90.33(8) | O(8)#2–Co(2)–N(2) | 88.05(7) |
| O(7)–Co(2)–N(2) | 86.34(8) | O(2)#3–Co(2)–N(2) | 94.30(8) |
| N(4)–Co(2)–N(2) | 174.98(8) | O(8)#2–Co(2)–O(1W) | 84.46(8) |
| O(7)–Co(2)–O(1W) | 169.55(8) | O(2)#3–Co(2)–O(1W) | 84.63(8) |
| N(4)–Co(2)–O(1W) | 91.55(11) | N(2)–Co(2)–O(1W) | 90.81(11) |
aSymmetry transformations used to generate equivalent atoms: #1 = −x, 1 − y, 1 − z; #2 = −x, −y, 2 − z; #3 = 1 − x, 1 − y, 1 − z.
PXRD measurements were done on a Bruker D8 Advance X-ray diffractometer using MoKα radiation (λ = 0.71073 Å), the X-ray tube being operated at 40 kV and 40 mA.
CCDC 1439158 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre viawww.ccdc.cam.ac.uk/data_request/cif.
Acknowledgments
This work was supported by grants from the Natural Science Foundation of China (Nos. 21401107 and 21577065), the Natural Science Foundation of Jiangsu, China (Nos. BK20140986, BK20130986, and BK20131429), International ST Cooperation Program of China (2014DFA90780), and Natural Science Foundation of the Jiangsu Higher Education Institutions of China (No. 14KJB150013) for the grants. Meanwhile, this work was supported by a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), Jiangsu Joint Laboratory of Atmospheric Pollution Control, and Jiangsu Engineering Technology Research Center of Environmental Cleaning Materials.
References
[1] Y. Cui, Y. Yue, G. Qian, B. Chen, Chem. Rev.2012, 112, 1126.10.1021/cr200101dSearch in Google Scholar PubMed
[2] W. Li, C.-H Li, Y.-Q. Yang, H.-F. Li, Z. Naturforsch.2015, 70b, 215.10.1016/j.snb.2015.03.054Search in Google Scholar
[3] D. Wang, Q. L. Xu, S. Zhang, H. Y. Li, C. C. Wang, T. Y. Li, Y. M. Jing, W. Huang, Y. X. Zheng, G. Accorsi, Dalton Trans.2013, 42, 2716.10.1039/C2DT32154HSearch in Google Scholar
[4] Y. Liu, W. Xuan, Y. Cui, Adv. Mater.2010, 22, 4112.10.1002/adma.201000197Search in Google Scholar PubMed
[5] X. Q. Yao, Z. R. Pan, J. S. Hu, Y. Z. Li, Z. J. Guo, H. G. Zheng, Chem. Commun.2011, 47, 10049.10.1039/c1cc12691aSearch in Google Scholar PubMed
[6] B. Xiao, L.-J. Yang, H.-Y. Xiao, S.-M. Fang, J. Coord. Chem.2011, 64, 4408.10.1080/00958972.2011.639875Search in Google Scholar
[7] J. Xu, M.-D. Chen, Supramol. Chem.2013, 25, 204.10.1027/1864-1105/a000107Search in Google Scholar
[8] J.-S. Hu, X.-H. Huang, C.-L. Pan, L. Zhang, Cryst. Growth Des.2015, 15, 2272.10.1021/acs.cgd.5b00067Search in Google Scholar
[9] G.-F. Wang, Z. Naturforsch.2015, 70b, 165.Search in Google Scholar
[10] M.-D. Zhang, B.-H. Zheng, Y. Jiao, M.-D. Chen, Mendeleev Commun.2014, 24, 180.10.1016/j.mencom.2014.04.020Search in Google Scholar
[11] Y.-H. Zhou, J. Li, T. Wu, X.-P. Zhao, Q.-L. Xu, X.-L. Li, M.-B. Yu, L.-L. Wang, P. Sun, Y.-X. Zheng, Inorg. Chem. Commun.2013, 29, 18.10.1016/j.inoche.2012.11.027Search in Google Scholar
[12] J. Xu, M.-D. Chen, J. Coord. Chem.2013, 66, 509.10.1080/00958972.2012.762975Search in Google Scholar
[13] C.-Y. Huang, J. Wang, Z.-Y. Ding, K. Cui, J. Mol. Struct.2015, 1086, 118.10.1016/j.molstruc.2015.01.020Search in Google Scholar
[14] H. Furukawa, J. Kim, N. W. Ockwig, M. O’Keeffe, O. M. Yaghi, J. Am. Chem. Soc.2008, 130, 11650.10.1021/ja803783cSearch in Google Scholar PubMed
[15] B. K. Tripuramallu, P. Manna, S. N. Reddy, S. K. Das, Cryst. Growth Des.2012, 12, 777.Search in Google Scholar
[16] D. Sarma, K. V. Ramanujachary, S. E. Lofland, T. Magdaleno, S. Natarajan, Inorg. Chem.2009, 48, 11660.10.1021/ic901678cSearch in Google Scholar PubMed
[17] G. M. Sheldrick, Sadabs, Program for Empirical Absorption Correction of Area Detector Data, University of Göttingen, Göttingen (Germany) 2002.Search in Google Scholar
[18] G. M. Sheldrick, SHELXTL (version 5.1), Software Reference Manual, Bruker Analytical X-ray Instruments Inc., Madison, WI (USA) 1997.Search in Google Scholar
[19] G. M. Sheldric, Acta Crystallogr.2008, A64, 112.10.1107/S0108767307043930Search in Google Scholar PubMed
[20] A. L. Spek, J. Appl. Crystallogr.2003, 36, 7.10.1107/S0021889802022112Search in Google Scholar
[21] A. L. Spek, Acta Crystallogr.2009, D65, 148.10.1107/S090744490804362XSearch in Google Scholar PubMed PubMed Central
©2016 by De Gruyter
Articles in the same Issue
- Frontmatter
- In this Issue
- Ferromagnetism in Fe3−x−yNixGeTe2
- A novel potentiometric sensor based on urease/ bovine serum albumin-poly(3,4-ethylenedioxythiophene)/Pt for urea detection
- Two new glycosidal metabolites of endophytic fungus Penicillium sp. (NO.4) from Tapiscia sinensis
- New bioactive metabolites from Penicillium purpurogenum MM
- Long-chain alkyl-substituted gentisic acid and benzoquinone derivatives from the root of Micronychia tsiramiramy (Anacardiaceae)
- A tetranuclear copper (II) complex with pyrazole-3,5-dicarboxylate ligands: synthesis, characterization and electrochemical properties
- Synthesis and structure of a cobalt coordination polymer based on 2,8-di(pyridin-4-yl)dibenzothiophene and 4,4-dicarboxydiphenylsulfone
- nBu4NI-catalyzed direct amination of benzoxazoles with tertiary amines using TBHP as oxidant under microwave irradiation
- Synthesis, single-crystal structure determination and Raman spectra of the tricyanomelaminates NaA5[C6N9]2 · 4 H2O (A = Rb, Cs)
- Synthesis of structural analogues of GGT1-DU40, a potent GGTase-1 inhibitor
- Note
- Crystal structure of a dimeric 1-benzothiepin
Articles in the same Issue
- Frontmatter
- In this Issue
- Ferromagnetism in Fe3−x−yNixGeTe2
- A novel potentiometric sensor based on urease/ bovine serum albumin-poly(3,4-ethylenedioxythiophene)/Pt for urea detection
- Two new glycosidal metabolites of endophytic fungus Penicillium sp. (NO.4) from Tapiscia sinensis
- New bioactive metabolites from Penicillium purpurogenum MM
- Long-chain alkyl-substituted gentisic acid and benzoquinone derivatives from the root of Micronychia tsiramiramy (Anacardiaceae)
- A tetranuclear copper (II) complex with pyrazole-3,5-dicarboxylate ligands: synthesis, characterization and electrochemical properties
- Synthesis and structure of a cobalt coordination polymer based on 2,8-di(pyridin-4-yl)dibenzothiophene and 4,4-dicarboxydiphenylsulfone
- nBu4NI-catalyzed direct amination of benzoxazoles with tertiary amines using TBHP as oxidant under microwave irradiation
- Synthesis, single-crystal structure determination and Raman spectra of the tricyanomelaminates NaA5[C6N9]2 · 4 H2O (A = Rb, Cs)
- Synthesis of structural analogues of GGT1-DU40, a potent GGTase-1 inhibitor
- Note
- Crystal structure of a dimeric 1-benzothiepin

