Abstract
The crystal structure of the 1-benzothiepin derivative meso-5 is presented. Compound 5 is the first dimeric 1-benzothiepin system.
1 Introduction
1-Benzothiepins (1) represent an important class of compounds for many applications in pharmacology and biology. Although more than 18,000 derivatives of 1-benzothiepin are registered in SciFinder, no simple dimer of 1-benzothiepin (1) is known. Exceptions exist only for some tetrahydrobi(dibenzothiepinyl)s 2 [1–3] and 3 [4–6], which are antihistaminic or neurotropic and psychotropic agents. We report here on an unexpected formation and the crystal structure of 4,4′,5,5′-tetrahydro-4,4′-bi(1-benzothiepinyl)-2,2′,4,4′-tetracarboxylic acid tetraethyl ester.
1.1 Results and discussion
2H-1-Benzothietes are versatile synthons for the preparation of various S-heterocycles [7–13]. Diester 4, our starting compound, was prepared by the reaction of diethyl (Z)-4-diazo-2-ethoxy-pent-2-enedioate, and 2H-1-benzothiete in the presence of rhodium (II) acetate as catalyst [14]. We tried to transform 4 to the corresponding thiepin by ether cleavage with BBr3 and dehydrobromination with NaOCH3 (Scheme 1). Instead of the expected 1-benzothiepin diester, we got a multicomponent mixture, which refrained from separation. However, after allowing an ethanol solution of the mixture to stand for several days at 0°C, we observed the formation of colorless crystals (13% yield). Their crystal structure analysis revealed the structure of the dimer meso-5. As its stereoselective formation is unlikely, we assume that the stereoisomers (R,R)-5 and (S,S)-5 were formed as well.
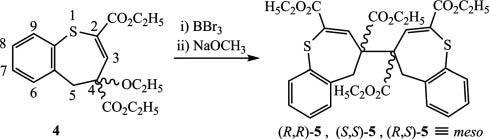
Formation of dimeric 1-benzothiepin derivatives.
Assuming that the dimerization proceeds via radical or ionic intermediates (Fig. 1), it may be expected that (R,R)-, (S,S)-, and (R,S)-2,2′-dimers as well as (2R,4′S)-, (2R,4′R)-, (2S,4′R)-, and (2S,4′S)-dimers could be formed in addition to (R,R)-5, (S,S)-5, and (R,S)-5. This might explain the complexity of the 1H NMR spectra of the crude product.
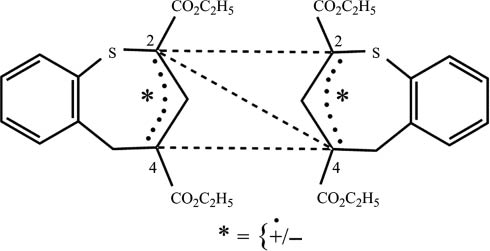
Possible dimerization reactions of radical or ionic intermediates.
Figure 2 shows the Schakal plot, and Fig. 3 shows the unit cell of the X-ray diffraction of meso-5. The most important bond lengths, bond angles, and torsion angles are listed in Table 1.
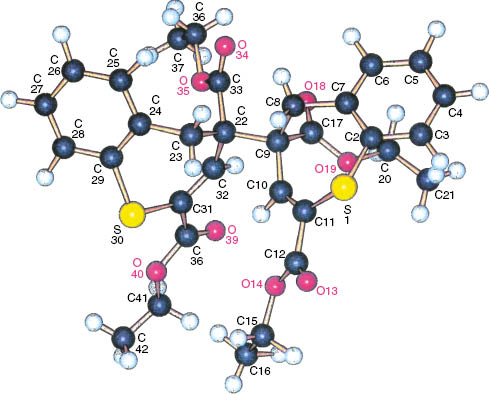
Molecular structure of meso-5 in the crystalline state (Schakal plot).
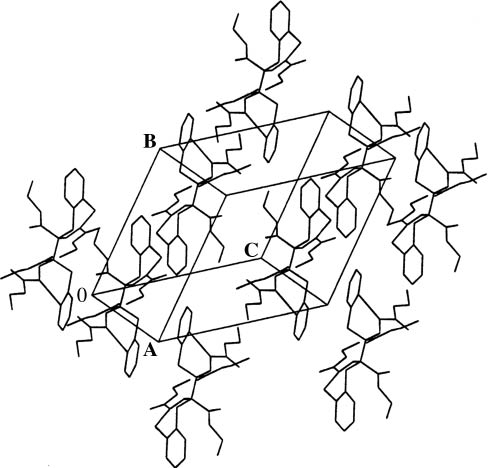
Projection of the crystal structure of meso-5.
Selected bond lengths (Å), bond angles (deg), and torsion angles (deg) of the molecular structure of meso-5 in the crystal.
| C(9)–C(22) | 1.599(2) |
| C(2)–S(1) | 1.757(2) |
| C(11)–(S(1) | 1.763(2) |
| C(29)–S(30) | 1.764(3) |
| C(31)–S(30) | 1.761(2) |
| C(2)–S(1)–C(11) | 106.34(9) |
| C(29)–S(30)–C(31) | 102.98(1) |
| C(2)–S(1)–C(11)–C(10) | –27.8(3) |
| C(7)–C(8)–C(9)–C(10) | 77.3(2) |
| C(24)–C(23)–C(22)–C(32) | –64.1(3) |
| C(29)–S(30)–C(31)–C(32) | 38.7(3) |
The length of the σ bond between the monomer units is extended to about 1.60 Å. The seven-membered rings are tilted against each other. Moreover, they are puckered, which is indicated by the torsion angles listed in Table 1.
2 Conclusion
Besides 2,2′-bithiepan [15] and the bi(dibenzothiepinyl)s 2 and 3, shown in Fig. 4, the compound meso-5 represents one of the very few known thiepin dimers and actually the first 1-benzothiepin dimer. The crystal structure analysis revealed tilted, puckered seven-membered rings, which are connected by a σ bond with an extended length of about 1.60 Å.
![Fig. 4: 1-Benzothiepin (1), 6,6′,11,11′-tetrahydro-11,11′-bi(dibenzo[b,e]thiepinyl) (2), and 10,10′,11,11′-tetrahydro-10,10′-bi(dibenzo[b,f]thiepinyl) (3).](/document/doi/10.1515/znb-2015-0201/asset/graphic/j_znb-2015-0201_fig_004.jpg)
1-Benzothiepin (1), 6,6′,11,11′-tetrahydro-11,11′-bi(dibenzo[b,e]thiepinyl) (2), and 10,10′,11,11′-tetrahydro-10,10′-bi(dibenzo[b,f]thiepinyl) (3).
3 Experimental section
3.1 Reaction of 4-ethoxy-4,5-dihydro- 1-benzothiepin-2,4-dicarboxylic acid diethyl ester (4)
To 4 (60 mg, 0.17 mmol), dissolved in 5 mL of dry CH2Cl2, a solution of 43 mg (0.17 mmol) BBr3 in 2 mL of dry CH2Cl2 was added slowly at –78°C. The mixture was stirred overnight, and the reaction temperature was slowly raised to room temperature. Water (10 mL) was added, and the aqueous layer was extracted with 30 mL of CH2Cl2. The unified organic layers were evaporated at 0.1 kPa, and the residue was treated at ambient temperature with a solution of Na (23 mg, 1.0 mmol) in 10 mL of anhydrous ethanol. A 1H NMR probe in CDCl3 revealed a complex mixture of products. However, after a few days at 0°C, colorless crystals of meso-5 (6.8 mg, 13%, m.p. 137°C) could be collected from the ethanol phase.
3.2 Crystal structure analysis
Details of the crystal structure analysis of meso-5 are summarized in Table 2. The measurement was performed with an Enraf-Nonius CAD-4 diffractometer applying the Enraf Nonius Software V5 [16]. Structure solution and refinement were done with the programs Sir-97 [17] and Shelxl-97 [18].
Crystal structure data of 5.
| Formula | C32H34O8S2 |
| Mr | 610.71 |
| Habit | Colorless block |
| Crystal size, mm3 | 0.500×0.625×0.750 |
| Crystal system | Triclinic |
| Space group | P1̅ |
| Cell parameters | |
| a, Å | 10.2936(9) |
| b, Å | 12.291(2) |
| c, Å | 13.648(1) |
| α, deg | 69.225(9) |
| β, deg | 89.351(9) |
| γ, deg | 76.659(9) |
| V, Å3 | 1566.1(3) |
| Z | 2 |
| D, g/cm3 | 1.295 |
| Radiation | MoKα |
| μ, mm−1 | 0.6 |
| F(000), e | 1160 |
| T, K | 298 K |
| θmax, deg | 29.97 |
| Measured refl | 9578 |
| Independent refl | 9106 |
| R (int) | 0.008 |
| Observed ref. [Fo/σ(Fo)>4.0] | 6328 |
| Refined parameters | 399 |
| R1 (F2>2 σ(F2)) | 0.0578 |
| wR2 | 0.1853 |
| S | 1.017 |
| Δρfin (max/min), e Å−3 | 0.71/–0.38 |
CCDC 150223 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre viawww.ccdc.cam.ac.uk/data_request/cif.
References
[1] Z. Polivka, V. Valenta, K. Sindelar, J. Holubek, M. Budesinsky, M. Ryska, I. Koruna, J. Kohoutova, J. Metys, J. Metysova, M. Valchar, M. Protiva, Coll. Czech. Chem. Commun. 1989, 54, 235.Search in Google Scholar
[2] Z. Polivka, J. Metys, M. Protiva, Coll. Czech. Chem. Commun. 1988, 53, 1806.Search in Google Scholar
[3] V. Valenta, F. Kvis, J. Nemec, M. Protiva, Coll. Czech. Chem. Commun. 1979, 44, 2689.Search in Google Scholar
[4] J. O. Jilek, I. Cervena, Z. Kopicova, K. Sindelar, E. Svatek, J. Metysova, A. Dlabac, J. Pomykacek, M. Protiva, Coll. Czech. Chem. Commun. 1976, 41, 443.Search in Google Scholar
[5] K. Sindelar, B. Kakac, E. Svatek, J. Holubek, M. Rajsner, J. Metysova, M. Protiva, Coll. Czech. Chem. Commun. 1974, 39, 333.Search in Google Scholar
[6] J. O. Jilek, V. Seidlova, E. Svatek, M. Protiva, J. Pomykacek, Z. Sedivy, Monatsh. Chem. 1965, 96, 182.Search in Google Scholar
[7] A. T. Balaban, A. Greer, J. F. Liebmann, Adv. Heterocycl. Chem. 2014, 113, 111.Search in Google Scholar
[8] H. Meier, Molecules2012, 17, 1548.10.3390/molecules17021548Search in Google Scholar PubMed PubMed Central
[9] H. Meier, M. Schmidt, A. Mayer, D. Schollmeyer, B. Beile, J. Heterocycl. Chem. 2012, 49, 516.Search in Google Scholar
[10] G. Rousseau, S. Robin, Mod. Heterocycl. Chem. 2011, 1, 163.Search in Google Scholar
[11] K. A. Kolmakov, A. J. Kresge, Can. J. Chem. 2008, 86, 119.Search in Google Scholar
[12] H. Meier, J. Prakt. Chem./Chem. Ztg. 1996, 338, 383.Search in Google Scholar
[13] H. Meier, A. Mayer, D. Gröschl, Sulfur Rep. 1994, 16, 23.Search in Google Scholar
[14] H. Meier, D. Gröschl, Tetrahedron Lett. 1995, 36, 6047.Search in Google Scholar
[15] A. Boege, J. Voss, Chem. Ber. 1990, 123, 1733.Search in Google Scholar
[16] B. V. Nonius, Enraf Nonius, Software V5, Delft (The Netherlands) 1989.Search in Google Scholar
[17] A. Altomare, M. C. Burla, M. C. M. Camalli, G. L. Cascarano, C. Giacovazzo, A. Guagliardi, A. G. G. Moliterni, G. Polidori, R. Spagna, J. Appl. Cryst. 1999,32, 115.Search in Google Scholar
[18] G. M. Sheldrick, Acta Cryst. 2008, A64, 112.Search in Google Scholar
©2016 by De Gruyter
Articles in the same Issue
- Frontmatter
- In this Issue
- Ferromagnetism in Fe3−x−yNixGeTe2
- A novel potentiometric sensor based on urease/ bovine serum albumin-poly(3,4-ethylenedioxythiophene)/Pt for urea detection
- Two new glycosidal metabolites of endophytic fungus Penicillium sp. (NO.4) from Tapiscia sinensis
- New bioactive metabolites from Penicillium purpurogenum MM
- Long-chain alkyl-substituted gentisic acid and benzoquinone derivatives from the root of Micronychia tsiramiramy (Anacardiaceae)
- A tetranuclear copper (II) complex with pyrazole-3,5-dicarboxylate ligands: synthesis, characterization and electrochemical properties
- Synthesis and structure of a cobalt coordination polymer based on 2,8-di(pyridin-4-yl)dibenzothiophene and 4,4-dicarboxydiphenylsulfone
- nBu4NI-catalyzed direct amination of benzoxazoles with tertiary amines using TBHP as oxidant under microwave irradiation
- Synthesis, single-crystal structure determination and Raman spectra of the tricyanomelaminates NaA5[C6N9]2 · 4 H2O (A = Rb, Cs)
- Synthesis of structural analogues of GGT1-DU40, a potent GGTase-1 inhibitor
- Note
- Crystal structure of a dimeric 1-benzothiepin
Articles in the same Issue
- Frontmatter
- In this Issue
- Ferromagnetism in Fe3−x−yNixGeTe2
- A novel potentiometric sensor based on urease/ bovine serum albumin-poly(3,4-ethylenedioxythiophene)/Pt for urea detection
- Two new glycosidal metabolites of endophytic fungus Penicillium sp. (NO.4) from Tapiscia sinensis
- New bioactive metabolites from Penicillium purpurogenum MM
- Long-chain alkyl-substituted gentisic acid and benzoquinone derivatives from the root of Micronychia tsiramiramy (Anacardiaceae)
- A tetranuclear copper (II) complex with pyrazole-3,5-dicarboxylate ligands: synthesis, characterization and electrochemical properties
- Synthesis and structure of a cobalt coordination polymer based on 2,8-di(pyridin-4-yl)dibenzothiophene and 4,4-dicarboxydiphenylsulfone
- nBu4NI-catalyzed direct amination of benzoxazoles with tertiary amines using TBHP as oxidant under microwave irradiation
- Synthesis, single-crystal structure determination and Raman spectra of the tricyanomelaminates NaA5[C6N9]2 · 4 H2O (A = Rb, Cs)
- Synthesis of structural analogues of GGT1-DU40, a potent GGTase-1 inhibitor
- Note
- Crystal structure of a dimeric 1-benzothiepin

