Abstract
Background
Ocimum basilicum, Thymus vulgaris, and Rosmarinus officinalis have been used for the treatment of different ailments for a historically long time but there have been no safety studies of these plants. Phytoconstituents of these plants were found to be good potential therapeutic agents which could be used in treatment regimens as a replacement of synthetic drugs if they are safe.
Experimental
Standard Folin ciocalteu reagent assay, aluminum chloride colorimetric assay and DPPH assay were employed to determine total phenolic, flavonoids and antioxidant activity, respectively. Broth microdilution method was used for investigation of synergistic effects of plant extracts with antibiotics. For toxicity assay, rats were treated with extracts of three plants at 1000 and 1500 mg/kg body weight.
Results
Ocimum basilicum extract showed highest total phenols, flavonoids, antioxidant and antibacterial activities. Thymus vulgaris extract caused hypertrophy of liver while Rosmarinus officinalis caused atrophy of spleen at both doses showing no significant histomorphological changes. Thymus vulgaris and O. basilicum extract significantly increased red blood cells, packed cell volume, hemoglobin and mean corpuscular volume at 1500 mg/kg body weight.
Conclusion
Ocimum basilicum, Thymus vulgaris and Rosmarinus officinalis have good phenolics and flavonoid content that shows antioxidant and antibacterial potential whilst having no evident toxic side effects on mammalian tissue and hematological parameters.
Özet
Amaç
O. basilicum, T. vulgaris ve R. officinalis, farklı hastalıkların tedavisinde uzun zamandır kullanılmaktadır ancak bu bitkilerin hiçbir güvenlik araştırması yapılmamıştır. Bu bitkilerin fito-konstitütiflerinin, tedavi rejimlerinde sentetik uyuşturucuların yerini alması halinde kullanılabilecek iyi potansiyel terapötik ajanlar olduğu tespit edildi.
Deneysel
Sırasıyla toplam fenolik, flavonoidleri ve antioksidan aktiviteyi belirlemek için Standart Folin ciocalteu reaktif testi, alüminyum klorür kolorimetrik assay ve DPPH testi kullanıldı. Bitki özlerinin antibiyotik ile sinerjik etkilerinin araştırılması için et suyu mikrodilüsyon yöntemi kullanılmıştır. Toksisite tahlili için sıçanlar üç bitki ekstraktları ile 1000 ve 1500 mg/kg vücut ağırlığı ile muamele edildi.
Bulgular
O. basilicum özütü, toplam fenol, flavonoid, antioksidan ve antibakteriyel aktivitelerde en yüksek oranda saptandı. T. vulgaris ekstraktı karaciğer hipertrofisine neden olurken, R. officinalis her iki dozda da dalak atrofisine neden olmuş, önemli bir histomorfolojik değişiklik göstermemiştir. T. vulgaris ve O. basilicum özü, 1500 mg/kg vücut ağırlığında kırmızı kan hücrelerini, paketlenmiş hücre hacmini, hemoglobin ve ortalama vücut hacmini önemli ölçüde arttırdı.
Sonuç
O. basilicum, T. vulgaris ve R. officinalis, antioksidan ve antibakteriyel potansiyel gösteren iyi fenolik ve flavonoid içeriğine sahipken, memeli dokusu ve hematolojik parametreler üzerinde belirgin toksik yan etkilere sahip olmamıştır.
Introduction
Increasing awareness about the use of medicinal herbs to be used as alternative and natural antioxidant and antimicrobial sources makes them potential therapeutic agents. Use of synthetic antioxidants in food industry is restricted to concentration and application [1, 2]. Growing evidence about the carcinogenic effects of food preservatives created a strong debate about the safety features of these chemicals [3]. Due to the side effects of these chemicals used as food additives, scientists progressed their research to explore some natural sources to be used as food preservatives having antioxidant and antimicrobial potential. In this respect, plants derived polyphenols obtained considerable attention [4].
Plants belonging to Lamiaceae family have good medicinal values and these plants have various applications in different industries, including cosmetics, pharmaceutical and perfume industry [5]. Ocimum basilicum (O. basilicum), Rosmarinus officinalis (R. officinalis) and Thymus vulgaris (T. vulgaris) which belong to Lamiaceae family are commonly used for potential flavors of foods [6, 7].
Ocimum basilicum, a major culinary herb of Lamiaceae has two important polyphenolic compounds, rosmarinic acid and caffeic acid. Extract of O. basilicum has been extensively studied for its various bioactivities, including antibacterial, antifungal, antioxidant, anticancer and antiviral activities [8]. Considering these pharmacological properties of O. basilicum it has been concluded that this plant has extensive therapeutic and pharmacological properties [9].
Thyme (T. vulgaris) is the most important commercially available medicinal plant belonging to genus Thymus. Thyme extract and essential oils are reported to be rich sources of antioxidant compounds [10]. The major compounds identified in thyme include, thymol, carvacrol and γ-terpinene. The antibacterial and antioxidant activities exhibited by thyme are due to the presence of these compounds [11]. Commonly, extract of T. vulgaris is used for the treatment of bronchial problems, gastritis, whooping cough, and diarrhea [12].
Phytochemical screening of R. officinalis explored the presence of several important constituents like phenolic diterpenes and diterpenoid quinine [13]. Among most important phytochemical constituents of this medicinal herb are carnosol, carnosic acid, rosmarinic acid and rosmaricine. Strong antioxidant activities exhibited by rosemary are reported to be due to the presence of carnosol, carnosic acid and rosmarinic acid whereas good antibacterial and antifungal potential of rosemary is due to the essential oil [14]. Medicinal plants are reported to have most potent therapeutic effects. Limited information is available about the safe dose of these selected medicinal herbs.
The aim of the present study was to evaluate the in vitro bioactivities and toxicity profile of the methanolic extracts of three selected medicinal herbs O. basilicum, T. vulgaris and R. officinalis.
Materials and methods
Plant material collection
Ocimum basilicum (O. basilicum), Thymus vulgaris (T. vulgaris) and Rosmarinus officinalis (R. officinalis) were collected from clonal repository in green house conditions at National Agriculture Research Centre (NARC), Islamabad, Pakistan (Latitude: 33.6982 and Longitude: 73.0393).
Preparation of plant material
Leaves of the three selected medicinal plants were collected and shadow dried under glass house conditions. Dried leaves were subjected to grinding to make coarse powder for extraction process. Seventy grams each of the dried powdered plant material was macerated in 200 mL methanol (Daejung Chemicals, Shiheung-city, Gyeonggi-do, South Korea) for 48 h in air tight bottles. Filtration of this mixture was carried out using Whatman filter paper 1. Methanol was evaporated from the filtrate of each plant material using Rotary evaporator (Bibby, RE200B, Yamato Scientific America Inc., Santa Clara, CA, USA). The dried plant material was collected, weighed, labeled and stored at 4˚C.
Total phenolic content measurement
Total phenolic content of the three medicinal plants was measured through Folin ciocalteu reagent (Sigma Aldrich, USA) assay following standard protocol. Gallic acid (Sigma Aldrich, USA) was used as a standard [14]. Nine milliliter of double distilled water (ddH2O) was added to each of the three extracts and gallic acid standard solution (20, 40, 60, 80 and 100 mg/L) in 25 mL volumetric flask. Blank used was ddH2O. One milliliter of Folin ciocalteu reagent was added to each of the flask and then was vigoreously shaken. After 5 min, 10 mL of 7% Na2CO3 was added to each of the flask. Solution volume was raised up to 25 mL using distilled water. Incubation of samples was carried out at room temperature for 90 min and absorbance of the samples was measured against the prepared reagent blank at 750 nm using UV-VIS Spectrophotometer Lambda 5 (Perkin Elmer, USA). Data was recorded as milligrams of gallic acid equivalents (GAE) per 100 g dry mass (mg GAE/100 g DW).
Total flavonoid assay
Total Flavonoid content of the three selected medicinal herbs was measured through Aluminum Chloride colorimetric assay. UV-VIS catechin (HYDRATE SC-204673A, Malaysia) was used as a standard [14]. Nine milliliter ddH2O was added to 1 mL of extracts of three medicinal herbs and standard solution of catechin. Three hundred microliter of 5% NaNO2 as added to the flask. Samples were incubated for 5 min at room temperature and 300 μL of 10% AlCl3 was added. At sixth minute of incubation 2 mL of 1 M NaOH was added to each of the flask and volume was raised up to 10 mL with ddH2O. Absorbance of the solution was measured against the prepared reagent blank at 510 nm through UV-VIS Spectrophotometer Lambda 5. The data of the total flavonoid contents of the dry herbs were expressed as milligrams catechin equivalents (CE) per 100 g dry mass (CE/100 g DW). All samples were analyzed in duplicates.
DPPH free radical scavenging activity
Antioxidant activity of ascorbic acid and methanolic extracts of three medicinal herbs was measured through standard DPPH assay [15]. Three different concentrations of ascorbic acid and plant extracts 25 μg/mL, 50 μg/mL and 100 μg/mL were used. Percent scavenging activity of samples was measured using the following formula:
where I=inhibition of DPPH radical, Ablank=absorbance of blank and Asample=absorbance of sample.
Determination of MICs
Minimum inhibitory concentration (MIC) was determined against three bacterial strains, Escherichia coli, Staphylococcus aureus and Streptococcus mutans through standard broth microdilution method according to the guidelines of clinical and laboratory standard institute [16]. Different serial dilutions of the three plant extracts were prepared using DMSO in micro tubes and 96 well micro plates. Final inocula of the bacteria were adjusted to 1.5×105 CFU/spot. The concentration of plant extracts at which there was no visible growth after 24 h incubation at 37°C was defined as MIC. The plates were examined visually for turbidity at the end of 24 h incubation, where cloudy appearance of the medium was considered growth of bacteria.
Determination of the in vitro effects of combinations of antimicrobial agents
Checkerboard test was used to assess the antimicrobial potential of different combinations of plant extracts and antibiotics. Two antibiotics amoxicillin and ciprofloxacin and methanolic extracts of three medicinal plants were used in combination. Serial dilutions of drugs and plant extracts were mixed in nutrient broth (Sigma Aldrich, USA). Inocula of the three bacterial strains were prepared from the colonies grown on NB overnight. MIC of the drug alone or in combination with plant extracts was determined after 24 h incubation at 37°C. Each experiment was performed in triplicate. The interaction of drug with plant extracts was determined through fractional inhibitory concentration (FIC). The FIC index (FICI) was calculated using the following formula:
where [A] is the concentration of drug A, MICA and FICA are the MIC and the FIC of drug A for the organism, respectively; whereas [B], MICB, and FICB are similarly defined for drug B. The FIC index obtained was interpreted as follows: <0.5 denoting synergy; 0.5–0.75 denoting partial synergy; 0.76–1 denoting an additive effect; 1–4 denoting indifference; and >4 denoting antagonism [17].
Experimental animals
Male albino rats of Sprague Dawley strain were randomly divided into seven groups for treatment with three different plant extracts at two different doses. They were kept in separate cages in a well ventilated environment and were allowed freely to access rodent pellets and water ad labitum.
Subacute toxicity assay
Rats were divided randomly into seven groups each of eight rats for subacute toxicity study of selected three medicinal plants. Each of the plant extract was administered orally to two groups at two different concentrations that are 1000 mg/kg body weight and 1500 mg/kg body weight daily for 1 month. Group A was administered only with 1 mL distilled water to serve as a control group. Rats in group B, C and D were administered with methanolic extracts of O. basilicum, T. vulgaris, and R. officinalis at a dose of 1000 mg/kg body weight while rats in group E, F and G were administered with methanolic extracts of O. basilicum, T. vulgaris and R. officinalis at a dose of 1500 mg/kg body weight. All the rats were weighed weekly. Overnight fasting of the rats was carried out on 28th and 29th day of the treatment. On 30th day of the treatment rats were weighed and sacrificed and blood samples were collected in EDTA tubes. Four organs including heart, liver, kidney, and spleen of each rat were taken and weighed. Organs were preserved in 10% buffered formalin solution for histopathological examination.
Histopathology analysis
All the four organs liver, heart, kidney and spleen were fixed in paraffin and sectioned 5 microns in thickness. Organ sections were stained using Hamatoxylin and Eosin (H&E). Tissue sections of organs were examined microscopically using light microscope. Histological profile was examined through representative images of organs of each group.
Hematological assays
The anticoagulant added blood was used for different hematological parameters measurement including total leukocytes count, red blood cells count, packed cell volume (PCV), hemoglobin mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC) and platelets using Sysmex KX21, a product of SYSMEX Corporation, Japan [18].
Statistical analysis
All the experiments were performed in triplicates. Standard curves were drawn for phenolics and flavonoids and data has been recorded as average±standard deviation. To compare antioxidant activity of plants extracts with acorbic acid as a standard and different parameters of rats treated with plant extracts and control rats, data were subjected to one way analysis of variance followed by LSD using software Statistix 8.1. Data were expressed as mean of eight replicates. Statistical significance was considered at p<0.05.
Results and discussion
Among the three medicinal herbs studied, highest total phenols 138.65 mg GE/100 g DW and flavonoids (102.45 mg CE/100 g DW) were recorded in methanolic extract of O. basilicum as indicated in Figure 1.
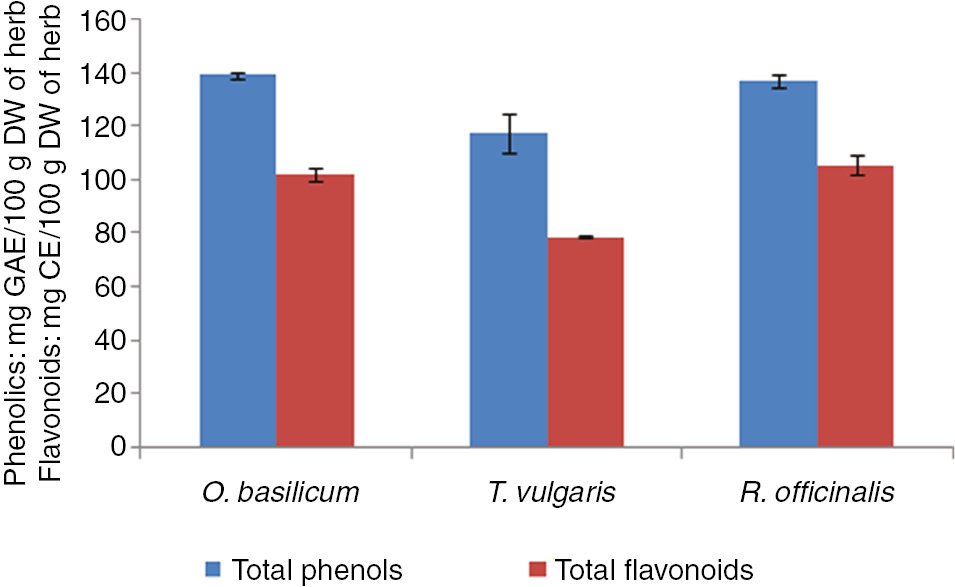
Total phenolics and flavonoids of O. basilicum, T. vulgaris and R. officinalis.
Percent antioxidant potential of the three medicinal herbs and ascorbic acid at three different concentrations (25 μg/mL, 50 μg/mL and 100 μg/mL) is shown in Figure 2. The percent free radical scavenging activity of medicinal herbs and ascorbic acid increased in a dose dependent manner. Antioxidant activity of O. basilicum and R. officinalis was found significantly higher than ascorbic acid at 25 mg/mL and 100 mg/mL. Ascorbic acid used as a standard showed highest antioxidant activity at all the three doses than plant extracts. Among plants, highest antioxidant activity was recorded for O. basilicum extract at 100 mg/mL concentration (86.99%), while lowest was observed for T. vulgaris extract at 25 mg/mL concentration (49.86%).

Percent antioxidant activity of O. basilicum, T. vulgaris and R. officinalis at three different concentrations (25 μg/mL, 50 μg/mL and 100 μg/mL). Bars represented with * have shown values significantly different from control.
The findings of this study demonstrate that the plant extracts used in this study are good sources of phenolics, flavonoids, useful antioxidant and antibacterial agents. It can also be concluded from this study that these extracts can be used as useful therapeutic agents. However, further study is needed to explore the medicinal uses of these plants.
The effects of three medicinal plant extracts were tested in combination with two antibiotics namely amoxicillin and ciprofloxacin on three bacterial strains, E. coli, S. aureus and S. mutans. MICs of the antibiotics were lowered markedly by plant extracts against the three bacterial strains. Good synergistic effects were recorded for plant extract of O. basilicum followed by R. officinalis extract, while lowest synergism was observed for T. vulgaris extract with two antibiotics as shown in Tables 1–3.
Results of the combination of O. basilicum plant extract and antibiotics against three bacterial strains.
| Bacterial strains | Agent | Alone | PE+antibiotics | FIC | FICI | Outcome |
|---|---|---|---|---|---|---|
| E. coli | PE | 200 | 100 | 0.50 | Partial synergy | |
| Ciprofloxacin | 150 | 25 | 0.17 | 0.67 | ||
| Amoxicillin | 250 | 50 | 0.20 | 0.70 | Partial synergy | |
| S. aureus | PE | 240 | 80 | 0.33 | ||
| Ciprofloxacin | 175 | 25 | 0.14 | 0.47 | Synergy | |
| Amoxicillin | 200 | 30 | 0.15 | 0.48 | Synergy | |
| S. mutans | PE | 300 | 125 | 0.42 | Partial synergy | |
| Ciprofloxacin | 175 | 30 | 0.17 | 0.59 | Partial synergy | |
| Amoxicillin | 300 | 75 | 0.25 | 0.67 |
Results of the combination of T. vulgar is plant extract and antibiotics against three bacterial strains.
| Bacterial strains | Agent | MIC μg/mL | FIC | FICI | Outcome | |
|---|---|---|---|---|---|---|
| Alone | PE+antibiotics | |||||
| E. coli | PE | 200 | 125 | 0.62 | ||
| Ciprofloxacin | 150 | 50 | 0.33 | 0.95 | Additive effect | |
| Amoxicillin | 250 | 100 | 0.4 | 1.02 | Indifference | |
| S. aureus | PE | 250 | 100 | 0.4 | ||
| Ciprofloxacin | 175 | 50 | 0.28 | 0.68 | Partial synergy | |
| Amoxicillin | 200 | 75 | 0.37 | 0.77 | Additive effect | |
| S. mutans | PE | 300 | 150 | 0.5 | ||
| Ciprofloxacin | 175 | 65 | 0.37 | 0.87 | Additive effect | |
| Amoxicillin | 300 | 150 | 0.5 | 1.00 | Additive effect | |
Results of the combination of R. officinalis plant extract and antibiotics against three bacterial strains.
| Bacterial strains | Agent | MIC μg/mL | FIC | FICI | Outcome | |
|---|---|---|---|---|---|---|
| Alone | PE+antibiotics | |||||
| E. coli | PE | 100 | 45 | 0.45 | ||
| Ciprofloxacin | 150 | 30 | 0.2 | 0.65 | Partial synergy | |
| Amoxicillin | 250 | 50 | 0.2 | 0.65 | Partial synergy | |
| S. aureus | PE | 200 | 75 | 0.37 | ||
| Ciprofloxacin | 175 | 25 | 0.14 | 0.51 | Partial synergy | |
| Amoxicillin | 200 | 40 | 0.2 | 0.57 | Partial synergy | |
| S. mutans | PE | 150 | 75 | 0.5 | ||
| Ciprofloxacin | 175 | 50 | 0.28 | 0.78 | Additive effect | |
| Amoxicillin | 300 | 75 | 0.25 | 0.75 | Partial synergy | |
The high antibacterial potential can be attributed to presence of phytoconstituents in basil extract. Medicinal plants are considered promising natural therapeutic agents in certain bacterial, fungal and viral diseases [19]. Several medicinal herbs possess aromatic and phenolic compounds that are best antimicrobial agents [20]. In another study carried out by Ilhan and coworkers it has been shown that E. coli and S. aureus cells treated with O. basilicum extract were completely damaged when observed through scanning electron microscope. It has also been reported that their cell walls were lysed by plant extract comparable to the antibiotics. In several previous studies, best antimicrobial potential has been reported for O. basilicum extract against various bacterial strains including candida albicans, Pseudomonas aeruginosa, E. coli, Salmonella paratyphi and Shigellady senterea [21]. The antibacterial activity exhibited by basil is considered to be due to its phytoconstituents that are linalool, methyl chavicol, camphor, thymol and eugenol [22]. Rosemary has also been reported to be a rich source of natural antioxidant and antimicrobial compounds. The major compounds related to its antioxidant activity are carnosic acid and carnosol whereas those involved in contributing antimicrobial potential are α-pinene, bornyl acetate, camphor and 1,8-cineole [23]. Thymol and carvacrol are the major constituents of thyme plant. These constituents have prominent antibacterial potential by disintegrating the outer membrane which in turn leads to increased ATP permeability through cytoplasmic membrane [24].
Effects of administration of methanolic extracts of O. basilicum, T. vulgaris and R. officinalis on body weight and four different organs including liver, kidneys, heart and spleen of rats are recorded in Table 4. No significant differences were recorded in body weights of rats and weight of organs in rats treated with methanolic extracts of the three selected herbs in comparison with control at two different doses, except for R. officinalis which causes a significant decrease in weight of spleen at both doses.
Effect of administration of methanolic extracts of O. basilicum, T. vulgaris and R. officinalis on body weight and some organs weight of Sprague Dawley rats.
| Parameters | Control | 1000 mg/kg BW | 1500 mg/kg BW | ||||
|---|---|---|---|---|---|---|---|
| O. basilicum | T. vulgaris | R. officinalis | O. basilicum | T. vulgaris | R. officinalis | ||
| Initial body weight | 199.37±18.32a | 185.25±15.43a | 183.88±9.98a | 197±38.28a | 208.25±15a | 201.75±19.5a | 188.37±12.37a |
| Final body weight | 218.12±13.8a | 207.38±14.21a | 201.62±13.5a | 218.25±31.95a | 225±17.15a | 220.75±20a | 210.87±10.72a |
| Weight of liver | 8.01±0.25a | 8.16±0.37a | 8.75±1.15a | 7.55±0.15a | 8.06±0.28a | 8.18±0.18a | 8±0.13a |
| Weight of kidneys | 2.07±0.24a | 2.05±0.25a | 2.08±0.22a | 2.07±0.1a | 2.00±0.09a | 2.09±0.28a | 2.12±0.09a |
| Weight of heart | 1±0.15a | 0.93±0.16a | 0.94±0.13a | 0.99±0.01a | 1.02±0.17a | 0.95±0.11a | 0.92±0.01a |
| Weight of spleen | 0.62±0.08a | 0.64±0.06a | 0.60±0.07a | 0.41±0.01c | 0.62±0.06a | 0.59±0.11a | 0.415±0.009c |
| Liver-body weight (%) | 3.68±0.22bb,c | 3.81±0.29b | 4.13±0.82a | 3.59±0.46c | 3.77±0.29b | 4.06±0.25a | 3.67±0.16c |
| Kidney body weight (%) | 0.94±0.143a | 0.99±0.11a | 1.03±0.17a | 0.94±0.12a | 0.88±0.05a,b | 0.95±0.13a | 1.00±0.07a |
| Heart body weight (%) | 0.46±0.06a | 0.43±0.09a,b | 0.47±0.07a | 0.45±0.04a | 0.45±0.08a | 0.43±0.05a | 0.44±0.02a |
| Spleen body weight (%) | 0.28±0.03a | 0.30±0.03a | 0.29±0.03a | 0.19±0.02c | 0.28±0.02a | 0.27±0.05a | 0.19±0.01c |
Mean±SD values carrying different superscripts from the control for each parameter are significantly different (p<0.05).
Organ-body weight ratio determines whether plant extracts are causing hypertrophy or atrophy [25]. No hypertrophic or atrophic results were recorded for plant extracts of the selected herbs but T. vulgaris extract caused hypertrophy of liver while R. officinalis extract caused atrophy of spleen. It can be argued that these were not significant changes as histomorphological studies did not show any differences from control. No previous data is available for the hypertrophic or atrophic effects of these plants.
Methanolic extract of T. vulgaris increased the liver-body weight ratio, while for other plant extracts no significant alterations were observed in the liver-body weight ratio. No significant differences were recorded in any other organ-body weight ratio except R. officinalis decreased spleen-body weight ratio significantly as depicted in Table 4.
Histomorphology of liver, kidney, heart and spleen tissues of control rat and plant extracts treated rats is shown in Figure 3. No significant histomorphological differences were observed in organ tissues of treated rats from the control.
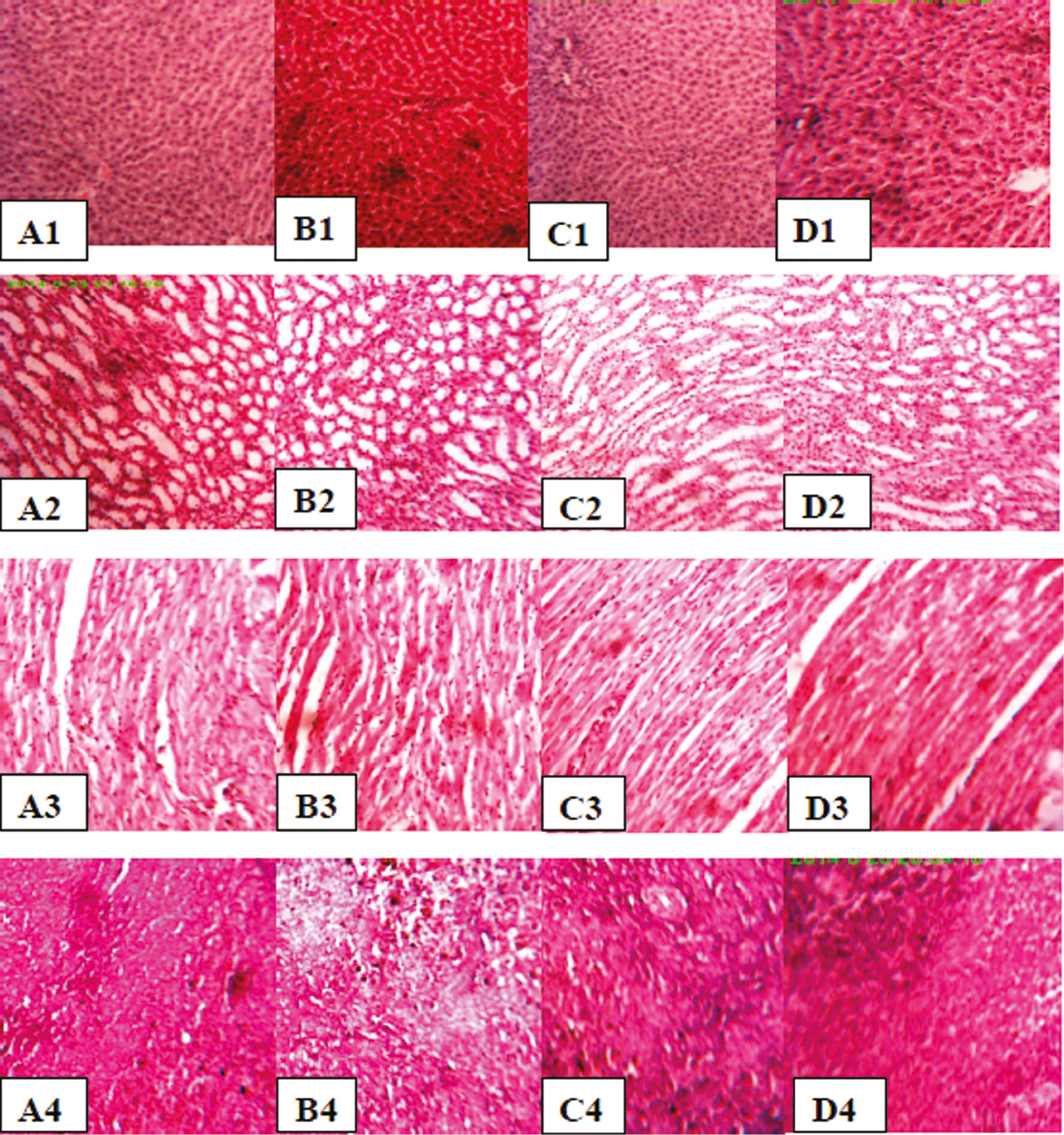
Histological sections of Liver, kidney, heart and spleen tissue stained with H&E×400 (A1, A2, A3, A4: Liver, kidney, heart and spleen tissue of control rat, B1, B2, B3, B4: Liver, kidney, heart and spleen tissue of rat treated with O. basilicum extract, C1, C2, C3, C4: Liver, kidney, heart and spleen tissue of rat treated with T. vulgaris extract, D1, D2, D3, D4: Liver, kidney, heart and spleen tissue of rat treated with R. officinalis extract).
Table 5 shows the results of administration of methanolic extracts of three selected medicinal herbs on heamatological parameters including white blood cells, platelets, red blood cells, hemoglobin, PCV, MCV, MCH and MCHC. No significant differences were recorded among control and plant extract treated rats for WBCs, MCH, MCHC and platelet count while RBCs, PCV, Hband MCV were significantly increased in rats treated with O. basilicum and T. vulgaris extracts at a dose of 1500 mg/kg body weight.
Effect of administration of methanolic extracts of O. basilicum, T. vulgaris and R. officinalis on some hematological parameters of Sprague Dawley rats (n=8).
| Parameters | Control | 1000 mg/kg BW | 1500 mg/kg BW | ||||
|---|---|---|---|---|---|---|---|
| O. basillicum | T. vulgaris | R. officinalis | O. basillicum | T. vulgaris | R. officinalis | ||
| WBCs (/μL) | 7975±768.57a | 7550±767.18a | 7952.5±512.46a | 7886.13±751.05a | 7525±1059.31a | 7512.5±816.68a | 7987.5±1126.86a |
| RBCs (×106/L) | 6.53±0.53b | 6.7325±0.5b | 6.86±0.77a,b | 6.9825±0.82a,b | 7.225±0.91a | 7.30±0.69a | 6.5575±0.49b |
| PCV (%) | 39.32±3.89b | 39.11±3.03b | 38.9±3.59b | 40.37±4.34b | 42.39±3.95a | 42.58±4.36a | 40.338029±5.12b |
| Hb (g/dL) | 12.09±1.43a,b | 12.51±1.59b | 12.62±0.98b | 12.68±1.16b | 13.99±1.19a | 14.20±1.19a | 12.87±0.89b |
| MCV (fL) | 51.85±3.29b | 52.59±2.78a,b | 51.12±2.17b | 52.69±2.21a,b | 53.23±3.87a | 53.72±1.92a | 52.05±2.62a,b |
| MCH (Pg) | 18.96±0.81a | 19.59±1.33a | 19.11±1.37a | 18.54±1.39a | 18.44±1.16a | 18.87±0.98a | 18.79±1.14a |
| MCHC (g/dL) | 36.78±1.96a | 35.26±1.64a | 35.83±2.15a | 33.21±1.95a | 35.82±2.62a | 36.05±2.46a | 36.32±1.52154a |
| Platlets (/μL) | 938,500±100,440 | 868,500±166,491 | 925,000±94 984.21a | 880,250±2,977,097a | 914,125±79 954.34a | 943,250±99 753.63a | 921,625±61976.81a |
Mean±SD values carrying different superscripts from the control for each parameter are significantly different (p<0.05).
Measurement of hematological parameters helps in understanding the effects of chemical compounds on blood constituents and indexing the toxicity and pathological status. Changes in hematological parameters are used to assess the toxicity of chemical compounds or plant extracts [26]. Significant increase in RBC count, PCV, Hb and MCV were recorded in rats treated with plant extracts of O. basilicum and T. vulgaris at 1500 mg/kg BW. This increase in T. vugaris treated rats can be attributed to high content of iron in T. vulgaris as reported previously that leads to stimulation of hemoglobin synthesis and also the presence of many important phenolics and flavonoids having good antioxidant potential which improves the heamatological picture [27]. The significant increase in above mentioned hematological parameters due to O. basilicum extract is in accordance with another study carried out on the aqueous extract of similar specie O. gratissimum, where increase in PCV, RBC and Hb was observed. Normally anoxia of tissues causes an increase in the production of erythropoietin that in turn leads to increased production of RBCs [28]. Probably, it might be due to the constituents of O. basilicum that act like erythropoietin and lead to increased production of erythrocytes. Contradictory results were recorded in another study carried out by Rasekh et al. where reduction in RBCs, Hb, PCV and platelets was observed. They attributed this reduction to suppression of bone marrow which might lead to reduced RBC and platelet counts [29].
Conclusions
The findings of this study demonstrate that the plant extracts of O. basilicum, R. officinalis and T. vulgaris are good sources of phenolics, flavonoids and are useful antioxidant and antibacterial agents. It can also be concluded from this study that these extracts can be used as useful therapeutic agents for the ailment of various diseases having no toxic effects up to a dose of 1500 mg/kg BW on body organs and hematological parameters of rats. Further study is needed to explore the medicinal uses of these plants.
Conflict of interest: The authors have no conflict of interest to disclose.
References
1. Tawaha K, Alali FQ, Gharaibeh M, Mohammad M, El-Elimat T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem 2007;104:1372–8.10.1016/j.foodchem.2007.01.064Search in Google Scholar
2. Peng Y, Yuan J, Liu F, Ye J. Determination of active components in rosemary by capillary electrophoresis with electrochemical detection. J Pharm Biomed Anal 2005;39:431–7.10.1016/j.jpba.2005.03.033Search in Google Scholar PubMed
3. Moreira M, Ponce A, Del Valle C, Roura S. Inhibitory parameters of essential oils to reduce a foodborne pathogen. LWT-Food Sci Technol 2005;38:565–70.10.1016/j.lwt.2004.07.012Search in Google Scholar
4. Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2009;2:270–8.10.4161/oxim.2.5.9498Search in Google Scholar PubMed PubMed Central
5. Sharma S, Bhadange D. Antimicrobial potential of Lamiaceae members. Int J Pharma Sci 2013;3:324–7.Search in Google Scholar
6. Orhan I, Aslan S, Kartal M, Şener B, Başer KH. Inhibitory effect of Turkish Rosmarinus officinalis L. on acetylcholinesterase and butyrylcholinesterase enzymes. Food Chem 2008;108:663–8.10.1016/j.foodchem.2007.11.023Search in Google Scholar PubMed
7. Soyal D, Jindal A, Singh I, Goyal P. Modulation of radiation-induced biochemical alterations in mice by rosemary (Rosemarinus officinalis) extract. Phytomedicine 2007;14:701–5.10.1016/j.phymed.2006.12.011Search in Google Scholar PubMed
8. Marwat SK, Khan MS, Ghulam S, Anwar N, Mustafa G, Usman K. Phytochemical constituents and pharmacological activities of sweet Basil-Ocimum basilicum L. (Lamiaceae). Asian J Chem 2011;23:3773–82.Search in Google Scholar
9. Shirazi MT, Gholami H, Kavoosi G, Rowshan V, Tafsiry A. Chemical composition, antioxidant, antimicrobial and cytotoxic activities of Tagetes minuta and Ocimum basilicum essential oils. Food Sci Nutr 2014;2:146–55.10.1002/fsn3.85Search in Google Scholar PubMed PubMed Central
10. Lee S-J, Umano K, Shibamoto T, Lee K-G. Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem 2005;91:131–7.10.1016/j.foodchem.2004.05.056Search in Google Scholar
11. Baranauskienė R, Venskutonis PR, Viškelis P, Dambrauskienė E. Influence of nitrogen fertilizers on the yield and composition of thyme (Thymus vulgaris). J Agric Food Chem 2003;51:7751–8.10.1021/jf0303316Search in Google Scholar PubMed
12. Behnia M, Haghighi A, Komeylizadeh H, Tabaei S-J, Abadi A. Inhibitory effects of Iranian Thymus vulgaris extracts on in vitro growth of Entamoeba histolytica. Korean J Parasitol 2008;46:153–6.10.3347/kjp.2008.46.3.153Search in Google Scholar PubMed PubMed Central
13. Mahmoud AA, Al-Shihry SS, Son BW. Diterpenoid quinones from Rosemary (Rosmarinus officinalis L.). Phytochemistry 2005;66:1685–90.10.1016/j.phytochem.2005.04.041Search in Google Scholar
14. Atanassova M, Georgieva S, Ivancheva K. Total phenolic and total flavonoid contents, antioxidant capacity and biological contaminants in medicinal herbs. J Univ Chem Technol Metallurgy 2011;46:81–8.Search in Google Scholar
15. Kulisic T, Radonic A, Katalinic V, Milos M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem 2004;85:633–40.10.1016/j.foodchem.2003.07.024Search in Google Scholar
16. Wikler MA, Cockerill FR, Craig WA, Dudley MN, Eliopoulos GM, Hecht DW, et al. editors. Clinical and laboratory standards institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard – seventh edition. Clinical and Laboratory Standards Institute document M7-A7 [ISBN 1-56238-587-9]. Wayne, PA, USA: Clinical and Laboratory Standards Institute, 2006:1–49.Search in Google Scholar
17. Mun S-H, Joung D-K, Kim Y-S, Kang O-H, Kim S-B, Seo Y-S, et al. Synergistic antibacterial effect of curcumin against methicillin-resistant Staphylococcus aureus. Phytomedicine 2013;20:714–8.10.1016/j.phymed.2013.02.006Search in Google Scholar
18. Dacie JV, Lewis SM, editors. Practical Haematology. 7th edition. UK: ELBS with Churchill Livingstone, Longman Group, 1991:5–82.Search in Google Scholar
19. Saad B, Azaizeh H, Abu-Hijleh G, Said O. Safety of traditional Arab herbal medicine. Evid Based Complement Alternat Med 2006;3:433–9.10.1093/ecam/nel058Search in Google Scholar
20. Holley RA, Patel D. Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microbiol 2005;22:273–92.10.1016/j.fm.2004.08.006Search in Google Scholar
21. Kaya I, Yigit N, Benli M. Antimicrobial activity of various extracts of Ocimum basilicum L. and observation of the inhibition effect on bacterial cells by use of scanning electron microscopy. Afr J Tradit Complement Altern Med 2008;5:363–9.10.4314/ajtcam.v5i4.31291Search in Google Scholar
22. Joshi RK. Chemical composition and antimicrobial activity of the essential oil of Ocimum basilicum L. (sweet basil) from Western Ghats of North West Karnataka, India. Anc Sci Life 2014;33:151–6.10.4103/0257-7941.144618Search in Google Scholar
23. Pintore G, Usai M, Bradesi P, Juliano C, Boatto G, Tomi F, et al. Chemical composition and antimicrobial activity of Rosmarinus officinalis L. oils from Sardinia and Corsica. Flavour Frag J 2002;17:15–9.10.1002/ffj.1022Search in Google Scholar
24. Nazzaro F, Fratianni F, De Martino L, Coppola R, De Feo V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013;6:1451–74.10.3390/ph6121451Search in Google Scholar
25. Ashafa AO, Orekoya LO, Yakubu MT. Toxicity profile of ethanolic extract of Azadirachta indica stem bark in male Wistar rats. Asian Pac J Trop Biomed 2012;2:811–7.10.1016/S2221-1691(12)60234-2Search in Google Scholar
26. Olson H, Betton G, Robinson D, Thomas K, Monro A, Kolaja G, et al. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul Toxicol Pharmacol 2000;32:56–67.10.1006/rtph.2000.1399Search in Google Scholar PubMed
27. Kaviarasan S, Vijayalakshmi K, Anuradha C. Polyphenol-rich extract of fenugreek seeds protect erythrocytes from oxidative damage. Plant Foods Hum Nutr 2004;59:143–7.10.1007/s11130-004-0025-2Search in Google Scholar PubMed
28. Ofem O, Ani E, Eno A. Effect of aqueous leaves extract of Ocimum gratissimum on hematological parameters in rats. Int J App Basic Med Res 2012;2:38–42.10.4103/2229-516X.96807Search in Google Scholar PubMed PubMed Central
29. Rasekh HR, Hosseinzadeh L, Mehri S, Kamli-Nejad M, Aslani M, Tanbakoosazan F. Safety assessment of Ocimum basilicum hydroalcoholic extract in wistar rats: acute and subchronic toxicity studies. Iran J Basic Med Sci 2012;15:645–53.Search in Google Scholar
Article note
Translated from English to Turkish by: Prof. Dr. Mustafa Soylak, Erciyes University, Faculty of Sciences, Department of Chemistry, 38039 Kayseri-Turkey.
©2018 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Research Articles
- Betaine treatment decreased serum glucose and lipid levels, hepatic and renal oxidative stress in streptozotocin-induced diabetic rats
- Oxidative stress and response of antioxidant system in Nostoc muscorum exposed to different forms of Zinc
- An investigation on Trakya region Oak (Quercus spp.) honeys of Turkey: their physico-chemical, antioxidant and phenolic compounds properties
- Effects of Shiranuhi flower extracts and fractions on lipopolysaccharide-induced inflammatory responses in murine RAW 264.7 cells
- Evaluation of resveratrol organogels prepared by micro-irradiation: fibroblast proliferation through in vitro wound healing
- Ecological and phytochemical attributes of endemic Ferula gummosa Boiss. at vegetative and generative stages
- Characterization of calmodulin in the clam Anodonta woodiana: differential expressions in response to environmental Ca2+ and Cd2+
- Foliar application effects of salicylic acid and jasmonic acid on the essential oil composition of Salvia officinalis
- Antioxidant and antimicrobial activities of four Astragalus species growing wild in Turkey
- Differences in structure, allergenic protein content and pectate lyase enzyme activity of some Cupressaceae pollen
- In vitro bioactivities and subacute toxicity study of O. basilicum, T. vulgaris and R. officinalis
- Wendlandia exserta: a pertinent source of antioxidant and antimicrobial agent
- Effects of epigallocatechin-3-gallate (EGCG) on a scleroderma model of fibrosis
Articles in the same Issue
- Frontmatter
- Research Articles
- Betaine treatment decreased serum glucose and lipid levels, hepatic and renal oxidative stress in streptozotocin-induced diabetic rats
- Oxidative stress and response of antioxidant system in Nostoc muscorum exposed to different forms of Zinc
- An investigation on Trakya region Oak (Quercus spp.) honeys of Turkey: their physico-chemical, antioxidant and phenolic compounds properties
- Effects of Shiranuhi flower extracts and fractions on lipopolysaccharide-induced inflammatory responses in murine RAW 264.7 cells
- Evaluation of resveratrol organogels prepared by micro-irradiation: fibroblast proliferation through in vitro wound healing
- Ecological and phytochemical attributes of endemic Ferula gummosa Boiss. at vegetative and generative stages
- Characterization of calmodulin in the clam Anodonta woodiana: differential expressions in response to environmental Ca2+ and Cd2+
- Foliar application effects of salicylic acid and jasmonic acid on the essential oil composition of Salvia officinalis
- Antioxidant and antimicrobial activities of four Astragalus species growing wild in Turkey
- Differences in structure, allergenic protein content and pectate lyase enzyme activity of some Cupressaceae pollen
- In vitro bioactivities and subacute toxicity study of O. basilicum, T. vulgaris and R. officinalis
- Wendlandia exserta: a pertinent source of antioxidant and antimicrobial agent
- Effects of epigallocatechin-3-gallate (EGCG) on a scleroderma model of fibrosis


