Effects of Shiranuhi flower extracts and fractions on lipopolysaccharide-induced inflammatory responses in murine RAW 264.7 cells
-
Chang-Gu Hyun
, Min-Jin Kim
Abstract
Objective
In this study, we evaluated the anti-inflammatory effect of Shiranuhi flower in RAW 264.7 cells.
Methods
The effects of the extracts and solvent fractions on cell viability and LPS-induced inflammatory responses were investigated in RAW 264.7 cells.
Results
The results showed that the ethyl acetate fraction (HEF) significantly decreased NO production in RAW 264.7 cells; however, cell viability was not affected. In addition, ELISA assay revealed that HEF significantly inhibited the productions of PGE2, TNF-α, and IL-6. As well, using Western blot analysis, it was observed that HEF significantly reduced the expression levels of iNOS and COX-2 in a dose dependent manner. Furthermore, we detected a reduced phosphorylation of mitogen-activated protein kinases such as p38, JNK, and ERK1/2. This indicates that HEF regulates LPS-induced inflammatory responses, at least in part, via suppressing the MAPK signaling pathway. Correlation analysis also showed that anti-inflammatory activities were highly correlated to antioxidant activities in this study. Characterization of the Shiranuhi flowers for flavonoid contents using HPLC showed varied quantity of narirutin and hesperidin.
Conclusion
Overall, the results demonstrate that HEF may be a potential anti-inflammatory agent. In addition, our findings contribute to understanding the molecular mechanism underlying the anti-inflammatory effect of Shiranuhi flower.
Özet
Amaç
Bu çalışmada, RAW 264.7 hücrelerinde Shiranuhi çiçeğinin anti-inflamatuar etkisini değerlendirdik.
Yöntem
Ekstraktların ve çözücü fraksiyonlarının hücre sağlığı ve LPS ile uyarılan inflamatuvar tepkileri üzerindeki etkileri, RAW 264.7 hücrelerinde araştırılmıştır.
Bulgular
Sonuçlar etil asetat fraksiyonunun (HEF) RAW 264.7 hücrelerinde NO üretimini önemli ölçüde azalttığını gösterdi; ancak hücre canlılığı etkilenmemiştir. Buna ek olarak, ELISA tahlili, HEF’in PGE2, TNF-α ve IL-6 üretimlerini önemli ölçüde inhibe ettiğini ortaya koymuştur. Sonuçlar etil asetat fraksiyonunun (HEF) RAW 264.7 hücrelerinde NO üretimini önemli ölçüde azalttığını gösterdi; Ancak hücre canlılığı etkilenmemiştir. Buna ek olarak, ELISA tahlili, HEF’in PGE2, TNF-α ve IL-6 üretimlerini önemli ölçüde inhibe ettiğini ortaya koymuştur. Bunun yanı sıra, Western blot analizi kullanılarak, Hef’in doza bağımlı olarak iNOS ve COX-2 ekspresyonu anlamlı bir şekilde azalttığı gözlenmiştir. Dahası, p38, JNK ve ERK1/2 gibi mitojenle aktive olan protein kinazların fosforilasyonunun azaldığını tespit edilmiştir. Bu bulgular, HEF’in, en azından kısmen MAPK sinyal yolunu baskılayarak, LPS ile uyarılan inflamatuvar tepkileri regüle ettiğini gösterir. Ayrıca bu çalışmada yapılan korelasyon analizi, anti-inflamatuvar aktivitelerin antioksidan aktivitelerle yüksek oranda korele olduğunu göstermiştir. Shiranuhi çiçeğinin flavonoid içeriği HPLC kullanarak karakterize edildiğinde çeşitli miktarlarda narirütin ve hesperidin bulunmuştur.
Sonuç
Genel olarak, sonuçlar HEF’in potansiyel bir anti-inflamatuar ajan olabileceğini göstermektedir. Buna ek olarak, bulgularımız Shiranuhi çiçeğinin anti-inflamatuar etkisinin altında yatan moleküler mekanizmanın anlaşılmasına katkıda bulunmaktadır.
Introduction
Inflammation is a highly regulated defensive process that results from tissue injury or external stimuli. It leads to the release of numerous inflammatory mediators and ultimately triggers the restoration of tissue structure and function [1], [2], [3]. However, uncontrolled and excessive inflammatory responses can cause severe tissue damage and secondary inflammatory injuries, such as sepsis, asthma, rheumatoid arthritis, vascular diseases, and cancer [4], [5]. Macrophages play a crucial role in providing an immediate defense by directly counteracting the aforementioned stimuli by releasing cellular signaling molecules such as inflammatory mediators and cytokines [6], [7]. In a normal state, these inflammatory mediators and cytokines released from macrophages are essential for host survival and tissue repair [8]. Lipopolysaccharide (LPS) is a molecule obtained from Gram-negative bacteria. It induces the release of many pro-inflammatory mediators and cytokines such as nitric oxide (NO), prostaglandin E2 (PGE2), tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1β from macrophages [9], [10]. Therefore, a decrease in LPS-induced expression of pro-inflammatory mediators and cytokines in macrophages may be considered a significant therapeutic property during the development of anti-inflammatory agents [11], [12], [13]. Accumulating evidence indicates that mitogen-activated protein kinases (MAPKs) also induce the production of inflammatory molecules and immune-related cytotoxic factors via a sequence of phosphorylation events. The MAPK family is composed of subfamilies, which include members such as extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38. Furthermore, many studies have demonstrated that MAPKs are important in modulating the expressions of cyclooxygenase (COX)-2 and inducible NO synthase (iNOS) and the productions of NO, PGE2, and pro-inflammatory cytokines, such as IL-1β, TNF-α, and IL-6 [14], [15], [16]. Thus, regulating the activation of MAPK pathways may be a useful strategy in modulating inflammatory responses [17], [18], [19].
Shiranuhi [(Citrus unshiu Marcov×C. sinensis Osbeck)×C. reticulata Blanco] is a seedless and sweet variety of mandarin orange that has been cultivated on Jeju Island (Korea) since 1988, after its development in Japan in 1972. Its trade name is ‘Hallabong’, named after the Hallasan Mountain located on Jeju Island. Shiranuhi is also the name of a genus of flowering plants in the Rutaceae family. The Shiranuhi fruit is primarily used for its juice; however, its peel has diverse pharmacological properties including anti-tumor, anti-metastatic [20], anti-inflammatory [21], antioxidant [22], anti-obesity [23], and anti-angiogenic effects [24]. In addition, the peel has been noted to inhibit breast cancer cell migration. However, potential biological activities of Shiranuhi flower have not been explored. Therefore, in this study, the Shiranuhi flower was investigated for its potential anti-inflammatory activity. To the best of our knowledge, this is the first study to demonstrate that Shiranuhi flower shows an anti-inflammatory activity.
Materials and methods
Preparation of Shiranuhi flower extracts
Shiranuhi flowers were collected from Citrus Research Institute (RDA, Jeju Island) and were identified by Dr. Young Hun Choi. A voucher specimen was prepared and deposited at the Cosmetic Science Center at Jeju National University (Korea).
The dried flowers (1.39 kg) were soaked in 5 L of 80% ethanol at room temperature for 24 h, after which the solvent was evaporated under vacuum. The residue obtained was freeze-dried and stored in a desiccator at 4°C before use. The weight of the dried sample was 52.48 g; therefore, the yield of the extraction was 3.78%. The dried extract (20 g) was then sequentially suspended in water (1 L) and extracted with ethyl acetate (1 L). The recoveries of the extract from the ethyl acetate and water fractions were 12.5% (2.49 g) and 70.5% (14.1 g), respectively.
For the water extract, the dried flowers (700 g) were ground into powder and extracted with 3 L of water in a water bath at 80°C for 5 h. Next, the mixture was filtered and the filtrate was concentrated to about 250 mL. The water extract of the Shiranuhi flower was thereafter obtained by lyophilizing the concentrate. The yield was 25.4 g (3.6%). In this study, each fraction and extracts were dissolved in DMSO and filtered through syringe filters (0.45 μm pore size).
Reagents and cell culture
Murine RAW 264.7 macrophages were purchased from American Type Culture Collection (Manassas, VA, USA). Dulbecco’s Modified Eagle’s Medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco (Grand Island, NY, USA). LPS, antibiotics (penicillin and streptomycin), and bovine serum albumin (BSA) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Lactate dehydrogenase (LDH) cytotoxicity detection kit and enzyme-linked immunosorbent assay (ELISA) antibody sets were purchased from Promega (Madison, WI, USA) and R&D Systems Inc. (Minneapolis, MN, USA), respectively. Primary antibodies for iNOS, COX-2, phospho-ERK, ERK, phosphor-p38, p38, phospho-JNK, JNK, and horseradish peroxidase-conjugated secondary antibodies were purchased from Cell Signaling Technology Inc. (Danvers, MA, USA). β-actin antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Nitrocellulose membrane was obtained from Invitrogen Inc. (Seoul, Korea). The RAW 264.7 cells were maintained at subconfluence at 37°C in a humidified atmosphere of 95% air and 5% CO2. The medium used for routine subculture was DMEM containing 10% FBS, penicillin (100 units/mL), and streptomycin (100 μg/mL).
Cell viability assay
Cell viability was analyzed by using the LDH cytotoxicity detection kit according to the manufacturer’s instructions. The RAW 264.7 macrophages (1.8×105 cells/mL) were plated in 24-well plates and incubated for 18 h, after which they were treated with the extract samples for 2 h. The macrophages were then challenged with LPS (1 μg/mL) for an additional 24 h. The release of LDH from the macrophages was used to assess the cytotoxicity of each sample. This method determines LDH activity from the production of NADH during the conversion of lactate to pyruvate. Absorbance was measured at 450 nm using an ELISA multiplate reader (Sunrise, Tecan, Switzerland). Percentage cytotoxicity was determined relative to the viability of the control cells. All experiments were performed in triplicate.
Measurement of NO levels
The RAW 264.7 cells were plated at 1.8×105 cells/mL in 24-well plates and incubated for 18 h, and pretreated with the various samples for 2 h, after which they were stimulated with LPS (1 μg/mL) for 24 h. The nitrite levels in the culture media were determined using Griess reagent (1% sulfanilamide in 5% phosphoric acid and 0.1% N-(1-naphthyl)ethylenediamine dihydrochloride in distilled water) and incubated at room temperature for 5 min. Absorbance was then measured at 540 nm using the ELISA multiplate reader. The quantity of nitrite in the samples was calculated using sodium nitrite as the standard.
Determination of cytokine levels
The concentrations of PGE2, TNF-α, and IL-6 in the supernatant were assayed using a commercial mouse ELISA kit according to the manufacturer’s instructions. The RAW 264.7 cells were plated at 1.8×105 cells/mL in 24-well plates and incubated for 18 h, and pretreated in absence or presence the ethyl acetate fraction (HEF; 25, 50, or 100 μg/mL) for 2 h, after which they were stimulated with LPS (1 μg/mL) for 24 h. Supernatant samples were obtained 24 h later and frozen until analysis by ELISA. Cytokine concentrations were measured from standard curves that were constructed using known concentrations of recombinant TNF-α, IL-6, and IL-1β.
Western blot analysis
Western blot analysis was performed as previously described with some modifications [25]. First, for iNOS and COX-2 protein expression analysis, RAW 264.7 macrophages were pre incubated for 18 h in a 6-well culture plate at a density of 1.0×106 cells/well. And then, the cells were pretreated stimulated with LPS (1 μg/mL) in the absence or presence of HEF (25, 50, or 100 μg/mL) for 24 h, after which they were stimulated with LPS (1 μg/mL) for 24 h. Next, for MAPKs protein expression analysis, RAW 264.7 macrophages were pre incubated for 18 h in a 6-well culture plate at a density of 1.0×106 cells/well. And then, the cells were pretreated in the absence or presence of HEF (25, 50, or 100 μg/mL) for 2 h, after which they were stimulated with LPS (1 μg/mL) for 10 min. After incubation, the cells were collected and washed twice with cold phosphate-buffered saline. The macrophages were lysed in a lysis buffer (radioimmunoprecipitation assay buffer, 1% Nonidet P-40, and 1% protease inhibitor cocktail) for 1 h, collected into microtubes, and then centrifuged at 15,000 rpm for 15 min at 4°C. The protein contents of the cell lysates were then determined by the Bradford Assay (Bio-Rad, Richmond, CA, USA). BSA was used as the standard for the analysis. Equal amounts of the lysates (30–50 μg of protein) were separated on a 4–12% Bis-Tris mini gel. After electrophoresis, the proteins were transferred onto a nitrocellulose membrane (Invitrogen Inc.). The membrane was washed in Tris-buffered saline (TBS) containing 0.1% Tween 20 (TTBS) and blocked in TTBS containing 5% BSA solution for 2 h. The membrane was then incubated overnight at 4°C with primary antibodies (iNOS, COX-2, p-JNK, JNK, p-ERK, ERK, p-p38, and p38) diluted in TTBS (1:1000) and washed 4 times in TTBS. Each membrane was incubated for 1 h with secondary peroxidase-conjugated goat immunoglobulin G (IgG, 1:5000) and washed again with TTBS. The target proteins were detected using enhanced chemiluminescence solution. The immunoreactive bands were detected and then exposed to X-ray film. Protein levels were quantified by scanning the immunoblots.
2,2-diphenyl-1-picrylhydrazyl (DPPH assay)
The modified method of Kim et al. [26] was used. Twenty microliter of sample solution was mixed with 180 μL of 0.2 mM DPPH solution in a microtiter plate. After incubation for 15 min in the dark, the absorbance was measured at 517 nm. The % DPPH scavenging activity of samples are calculated as:
2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS assay)
The modified method of Yang et al. [27] was used. To make ABTS+solution, 7 mM ABTS and 2.45 mM potassium persulfate were mixed and left in the dark for 16 h. one hundred and eighty microliter of ABTS+ solution was added to 20 μL of sample solution in a microtiter plate. After incubating for 15 min in the dark, the absorbance was measured at 700 nm. The % ABTS scavenging activity of samples are calculated as:
HPLC fingerprint
Chromatographic analysis of the Shiranuhi flower performed using a HPLC system (Waters, Milford, MA, USA) with a YMC Pro-Triart C18 RS column (250×4.6 mm, S-5 μm, 8 nm; YMC Co., Kyoto, Japan) and a Waters 2489 UV visible detector. The mobile phases for the HPLC system were acetonitrile (A) and water containing 20 mM phosphoric acid (2:8) (B) at a flow rate of 2 mL/min. The samples were filtered through 0.22 μm polyvinylidene difluoride membrane filter. The following flavonoid compounds were analyzed in the citrus sample: rutin, naringin, hesperidin, neohesperidin, and narirutin. UV detection was performed at 280 nm.
Statistical analysis
The results have been expressed as mean±SD. All the quantitative data are representative of at least three independent experiments. Student’s t-test was used to determine statistically significant differences between each treated group and the negative control (LPS group). Statistical significance was considered at p<0.05 (*) or p<0.01 (**).
Results and discussion
Effect of Shiranuhi flower on NO synthesis in activated macrophages
In murine RAW 264.7 macrophages, bacterial LPS alone can induce iNOS transcription, protein synthesis, and subsequent NO production. Therefore, the RAW 264.7 cell line is a common model for evaluating anti-inflammatory agents by inhibiting pathways that trigger iNOS and NO productions [1, 2, 10]. To investigate the effect of Shiranuhi flower extract on NO production, we measured the levels of nitrite, a stable oxidized product of NO, in culture media containing RAW 264.7 cells that had been stimulated with LPS in the presence or absence of the Shiranuhi flower extract.
It was observed that the nitrite level in the medium containing the LPS-stimulated cells increased significantly and was higher than that in the medium containing the control cells. To evaluate whether Shiranuhi flower can modulate NO production by activated macrophages, we examined the effects of the hot water and ethanolic extracts, as well as those of the ethyl acetate and water fractions on NO production in the murine RAW 264.7 macrophages. As well, the effects of 2-amino-4-methylpyridine (10 μM) and dexamethasone (20 μM) on the cells were investigated. As shown in Figure 1A, among the four Shiranuhi flower preparations, HEF (100 μg/mL) markedly inhibited LPS-induced NO production in the RAW 264.7 cells by 85%. Furthermore, 2-amino-4-methylpyridine, which was used as an NO inhibitor and the positive control, also inhibited LPS-induced NO production by 91%. As shown in Figure 1B, the numbers of viable activated macrophages were not altered by HEF, 2-amino-4-methylpyridine, or dexamethasone, as were observed from the LDH assays. This was an indication that the inhibition of NO production by HEF was not simply due to cytotoxic effects.
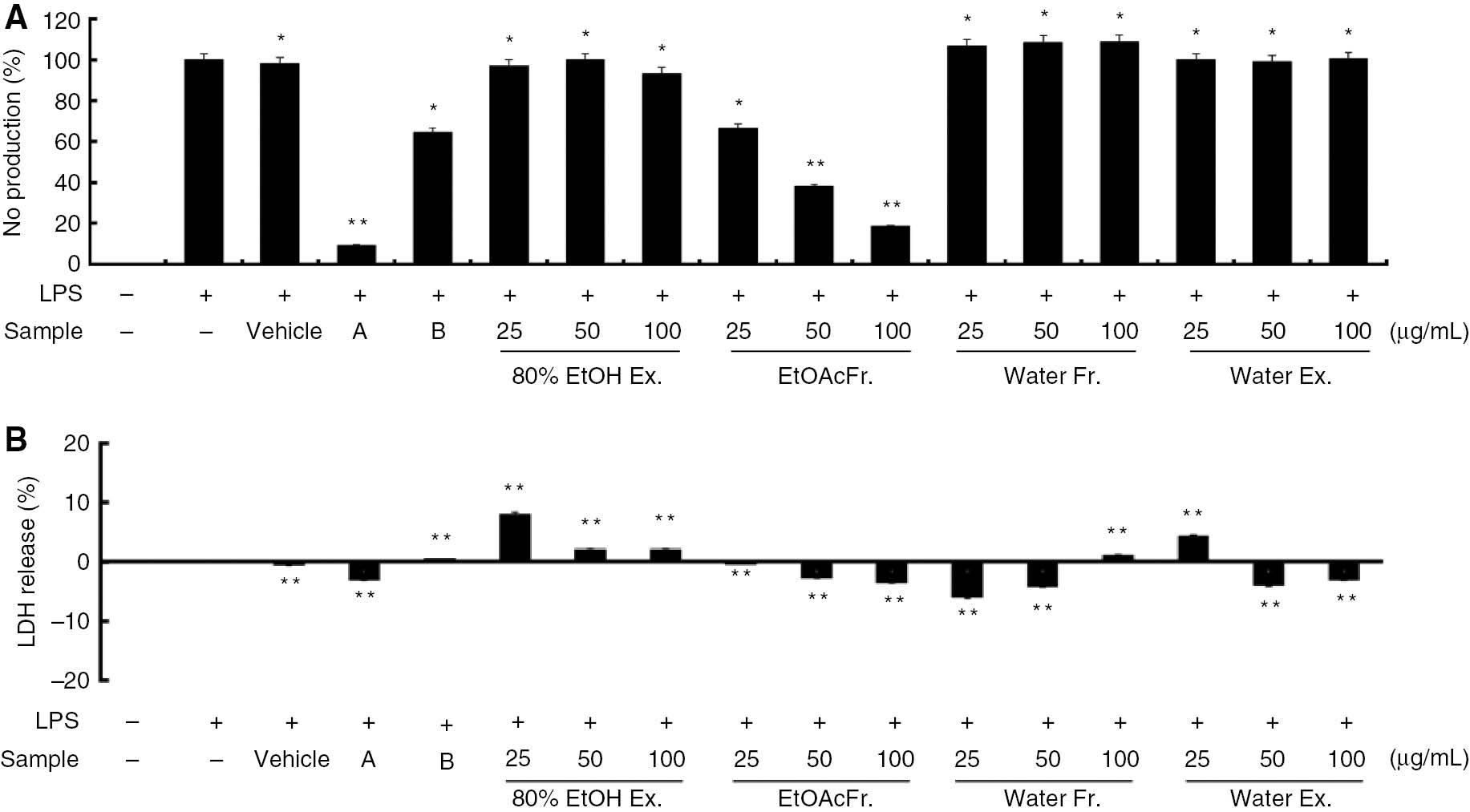
Inhibitory effects of Shiranuhi flower extracts and fractions on cytotoxicity and nitric oxide (NO) production in murine RAW 264.7 cells.
RAW 264.7 cells were pretreated with the Shiranuhi flower extracts and fractions (each at concentrations of 25, 50, and 100 μg/mL), 2-amino-4-methylpyridine (A, 10 μM), dexamethasone (B, 20 μM), and vehicle (DMSO, the solvent in which the dried extracts and fractions are dissolved) for 2 h, and then treated with lipopolysaccharide (1 μg/mL) for 24 h. (A) The nitrite levels in the culture media were determined using Griess reagent. (B) Cell viability was analyzed by using the LDH cytotoxicity detection kit according to the manufacturer’s instructions. The data are expressed as mean±SD (n=3). *indicates p<0.05 and **indicates p<0.01.
Effect of HEF on PGE2 production in LPS-induced RAW 264.7 cells
Prostaglandins are small pro-inflammatory molecules derived from arachidonic acid that play roles in a multitude of biological processes including inflammation. COX-2 expression, which is induced by cytokines and endotoxins such as LPS, results in the release of large amounts of PGE2 at sites of inflammation [1, 2, 10]. Therefore, we examined the effects of HEF on PGE2 production in the LPS-stimulated RAW 264.7 macrophages. As shown in Figure 2, LPS increased PGE2 production by approximately 12-fold, whereas pretreatment with HEF (25, 50, or 100 μg/mL) markedly reduced LPS-induced PGE2 production in the cells in a dose-dependent manner. Furthermore, dexamethasone, which is a COX-2 selective inhibitor, had a significant inhibitory effect on PGE2 production.
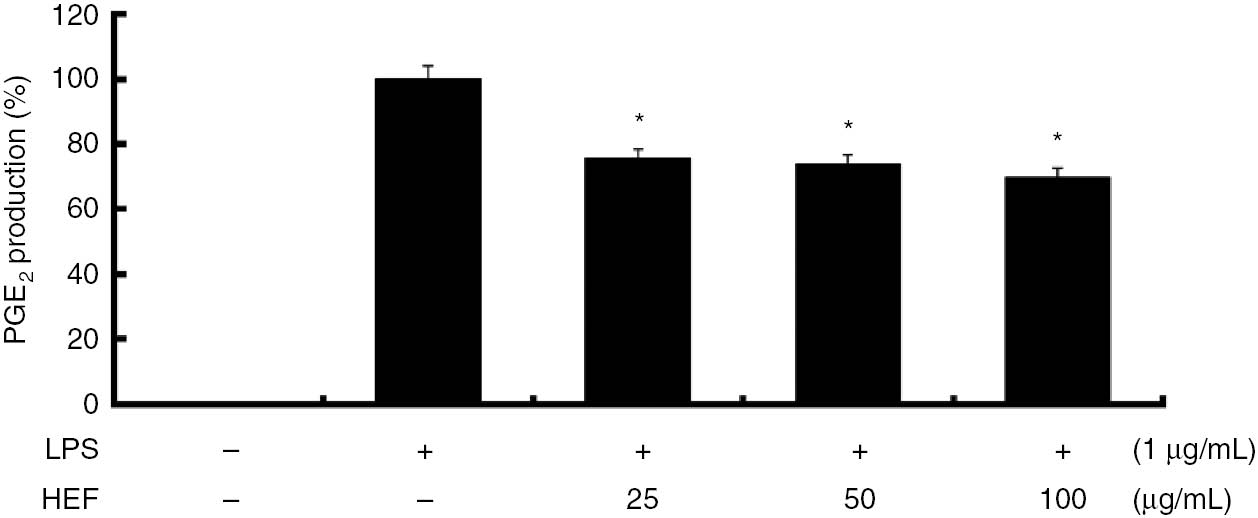
Inhibitory effect of the ethyl acetate fraction of Shiranuhi flower extract (HEF) on prostaglandin (PG)E2 production in murine RAW 264.7 cells.
HEF was prepared by suspending the residue obtained after drying the ethanolic extract of Shiranuhi flower in water, followed by extraction with ethyl acetate. The cells were stimulated with of LPS alone or with a combination of LPS and various concentrations (25, 50, or 100 μg/mL) of HEF for 24 h. PGE2 produced and released into the culture media were determined using ELISA Kit according to the manufacturer’s instructions. The data are expressed as mean±SD (n=3). *indicates p<0.05 and ** indicates p<0.01.
Effects of HEF on LPS-induced expression of iNOS and COX-2 proteins
Western blot analyses were performed to determine whether the inhibitory effects of HEF on NO and PGE2 productions were related to modulations of iNOS and COX-2 expressions, respectively. As shown in Figure 3, we did not detect iNOS and COX-2 protein expressions in the control cells. However, iNOS and COX-2 levels were markedly upregulated by the LPS treatment. On the other hand, HEF did not affect the expression of β-actin, which is a housekeeping gene. In general, these results suggest that the inhibitory effects of HEF on LPS-induced NO and PGE2 productions are the results of the suppression of iNOS and COX-2 expression levels, respectively.
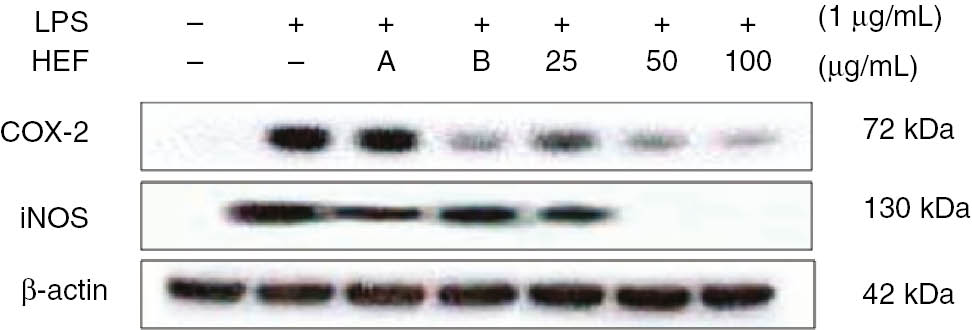
Inhibitory effects of the ethyl acetate fraction of Shiranuhi flower extract (HEF) on the expression levels of cyclooxygenase (COX)-2 and inducible nitric oxide synthase (iNOS) in murine RAW 264.7 cells.
The RAW 264.7 cells were plated at 1.0×106 cells/mL in 24-well plates and incubated for 18 h, and pretreated with HEF (25, 50, or 100 μg/mL), 2-amino-4-methylpyridine (A, 10 μM), or dexamethasone (B, 20 μM) for 2 h, after which they were stimulated with LPS (1 μg/mL) for 24 h. COX-2 and iNOS protein level was determined by immunoblotting.
Effect of HEF on the production of pro-inflammatory cytokines
Because TNF-α and IL-6 are markers of early-phase inflammation, elevated levels of these molecules can be detected during several acute and chronic inflammatory diseases. Therefore, we evaluated the effects of HEF on the levels of these cytokines in the LPS-treated cells by enzyme immunoassay. As expected, the LPS-treated RAW 264.7 cells displayed notable increases in the levels of TNF-α and IL-6. Moreover, the induction of pro-inflammatory cytokines was significantly decreased by HEF in a dose-dependent manner (p<0.05). As shown in Figure 4, the productions of TNF-α and IL-6 were decreased at the IC50 values of HEF (41 and 35 μg/mL, respectively). These indicate that HEF efficiently suppressed LPS-induced TNF-α and IL-6 productions, which suggests that HEF inhibited the initial phase of the LPS-induced inflammatory response.
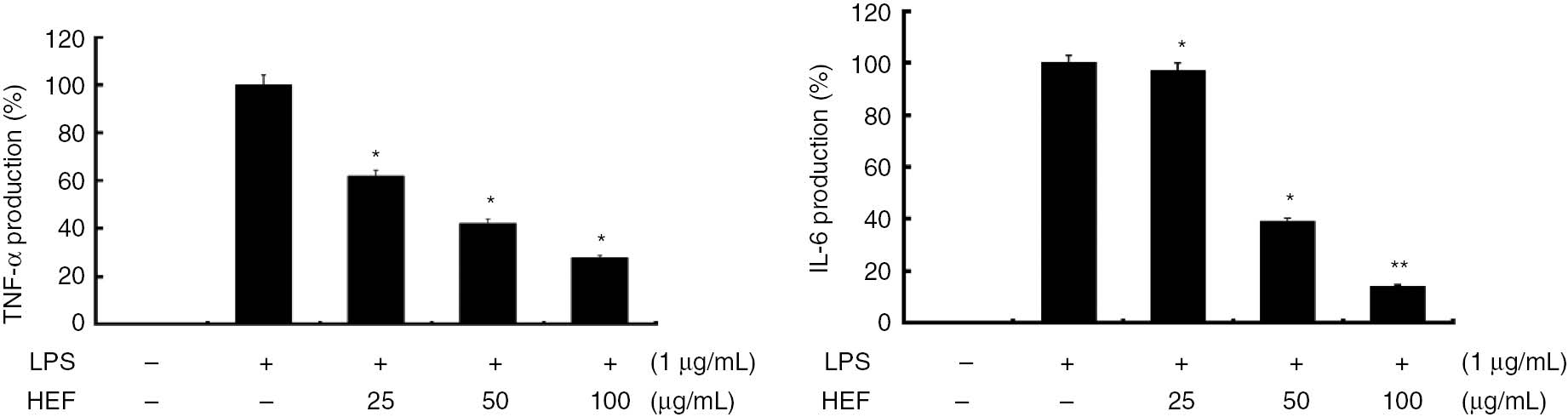
Inhibitory effects of the ethyl acetate fraction of Shiranuhi flower extract (HEF) on the productions of tumor necrosis factor (TNF)-α and interleukin (IL)-6 in murine RAW 264.7 cells.
The cells were stimulated with of LPS alone or with a combination of LPS and various concentrations (25, 50, or 100 μg/mL) of HEF for 24 h. TNF-α and IL-6 produced and released into the culture media were determined using ELISA Kit according to the manufacturer’s instructions. The data are expressed as mean±SD (n=3). *indicates p<0.05 and **indicates p<0.01.
Effect of HEF on the phosphorylation of MAPKs in LPS-stimulated RAW 264.7 cells
MAPKs play critical roles in the regulation of cell growth and differentiation, as well as in the control of cellular responses to cytokines and stressors. One of the most extensively investigated transduction pathways involved in inflammation is the MAPK pathway. Previous studies have shown that MAPKs play significant roles in regulating the levels of COX-2 and iNOS and the production of pro-inflammatory cytokines by modulating nuclear factor κB (NF-κB) and activator protein 1 (AP-1) signals in LPS-stimulated macrophages [3, 8, 13]. Therefore, Western blot analyses were performed to determine whether the inhibitory effects of HEF on the phosphorylations of ERK1/2, p38, and JNK MAPKs were related to the modulations of NF-κB and AP-1 signals. As shown in Figure 5, the LPS treatment significantly promoted the phosphorylations of ERK1/2, JNK, and p38 MAPKs in the RAW 264.7 cells. At all the concentrations studied (25, 50, and 100 μg/mL), HEF dramatically reduced the phosphorylations of ERK1/2, p38, and JNK MAPKs, each in a dose-dependent manner. However, the expression levels of unphosphorylated ERK1/2, p38, and JNK MAPKs were unaffected in cells that were treated with either LPS alone or both LPS and HEF. These results suggest that the phosphorylation of MAPKs may be involved in the mechanisms underlying the inhibitory effects of HEF on LPS-induced inflammation in murine RAW 264.7 cells.
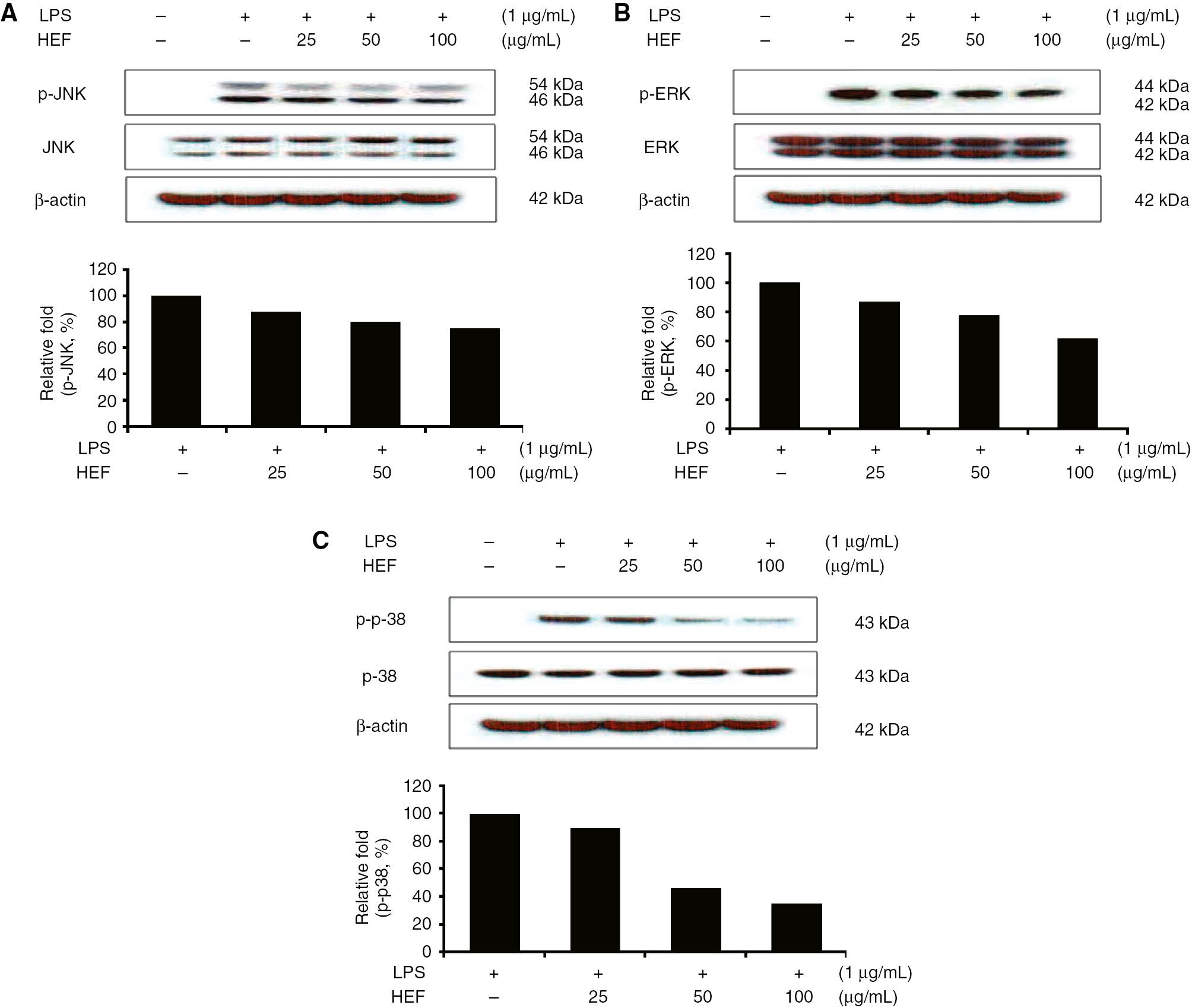
Effect of the ethyl acetate fraction of Shiranuhi flower extract (HEF) on lipopolysaccharide (LPS)-induced protein expression of p-JNK (phosphorylated-JNK)/JNK, p-ERK (phosphorylated-ERK)/ERK, and p-p-38 (phosphorylated-p-38)/p-38 in murine RAW 264.7 macrophages.
RAW 264.7 cells (1.0×106 cells/mL) were pre-incubated for 18 h, incubated for 2 h with HEF, and then stimulated for 10 min with LPS (1 μg/mL). Immunoblotting was used to determine the levels of p-JNK (phosphorylated-JNK)/JNK, p-ERK (phosphorylated-ERK)/ERK, and p-p-38 (phosphorylated-p-38)/p-38.
Investigation of the relationship between the anti-inflammatory and antioxidant activities
It has been shown that reactive oxygen species (ROS) trigger the phosphorylation of MAPKs and the production of pro-inflammatory cytokines [28], [29]. Therefore, 70% ethanol extracts and HEF of Shiranuhi flower was used to investigate the relationship between the data sets of anti-inflammatory and the anti-oxidant activities. In this study, 70% ethanol extracts and HEF were screened free radical-scavenging effects such as DPPH and ABTS. Two extracts, HEF was showed good anti-oxidative effects with IC50 of 149.44 μg/mL for DPPH and 54.57 μg/mL for ABTS (Figure 6). The anti-oxidative evaluation demonstrates that the same fraction was the best to inhibit the pro-inflammatory cytokines and MAPK mechanism.
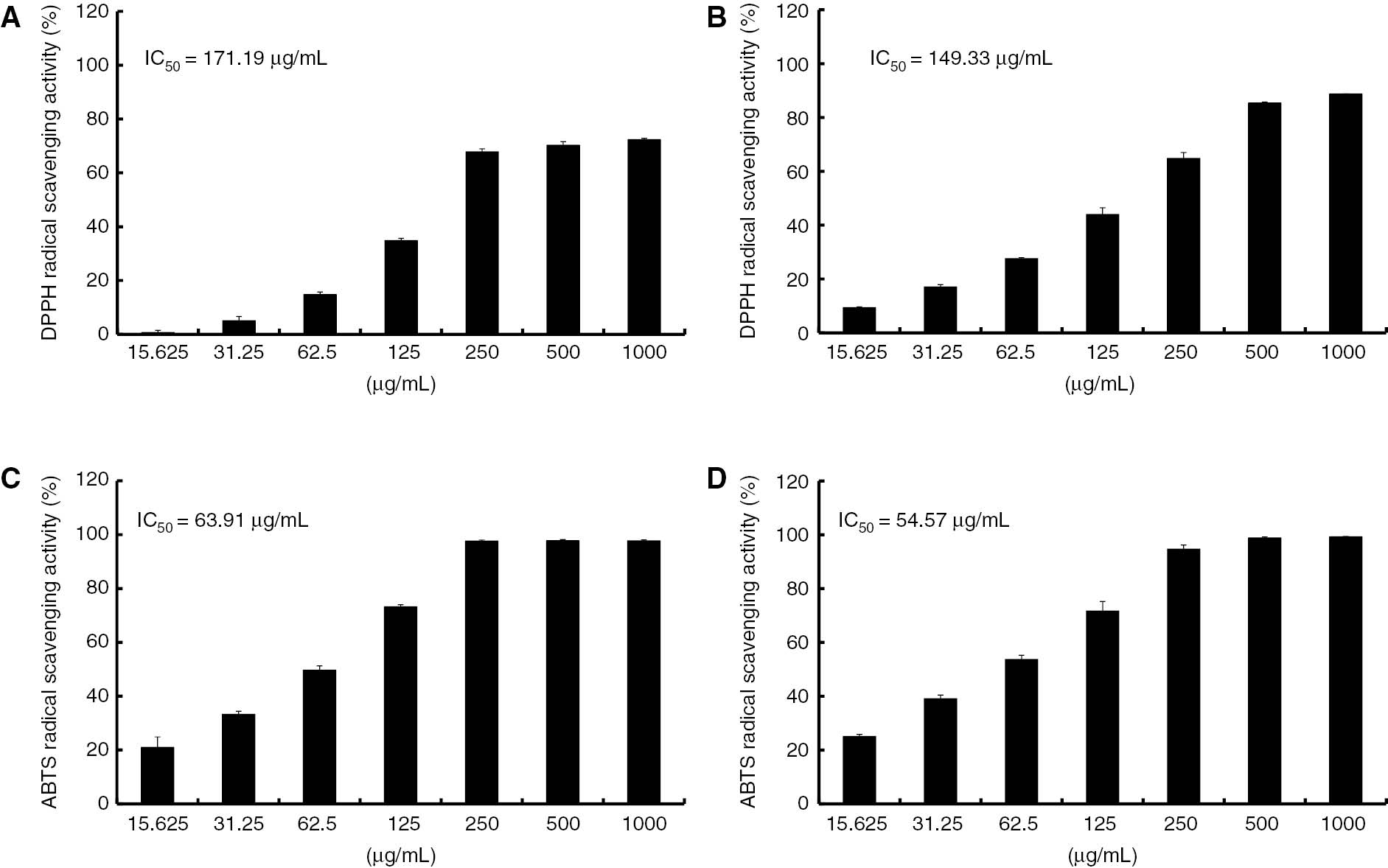
Antioxidant activities of 70% ethanol extracts and HEF.
DPPH and ABTS scavenging activities of 70% ethanol extracts (A and C) and HEF (B and D) at different concentrations. Each value represents mean±SEM of three replicates.
HPLC fingerprint of 70% ethanol extracts and HEF
Finally, in other to apply 70% ethanol extracts and HEF for anti-inflammatory agents in cosmetic industry, standard and/or functional materials have to be investigated. Therefore, the flavonoid constituents of the 70% ethanol extracts and HEF were also investigated using HPLC analysis in the present study. As shown in Figure 7, a total of 2 citrus flavonoids were identified on the basis of the compared to those in our HPLC library. The HEF is a predominantly composed of narirutin (3.13±0.43 g) and hesperidin (6.03±0.59 g) in 100 g of HEF. On the other hands, the 70% ethanol extract is composed of narirutin (0.69±0.07 g) and hesperidin (1.75±0.98 g).
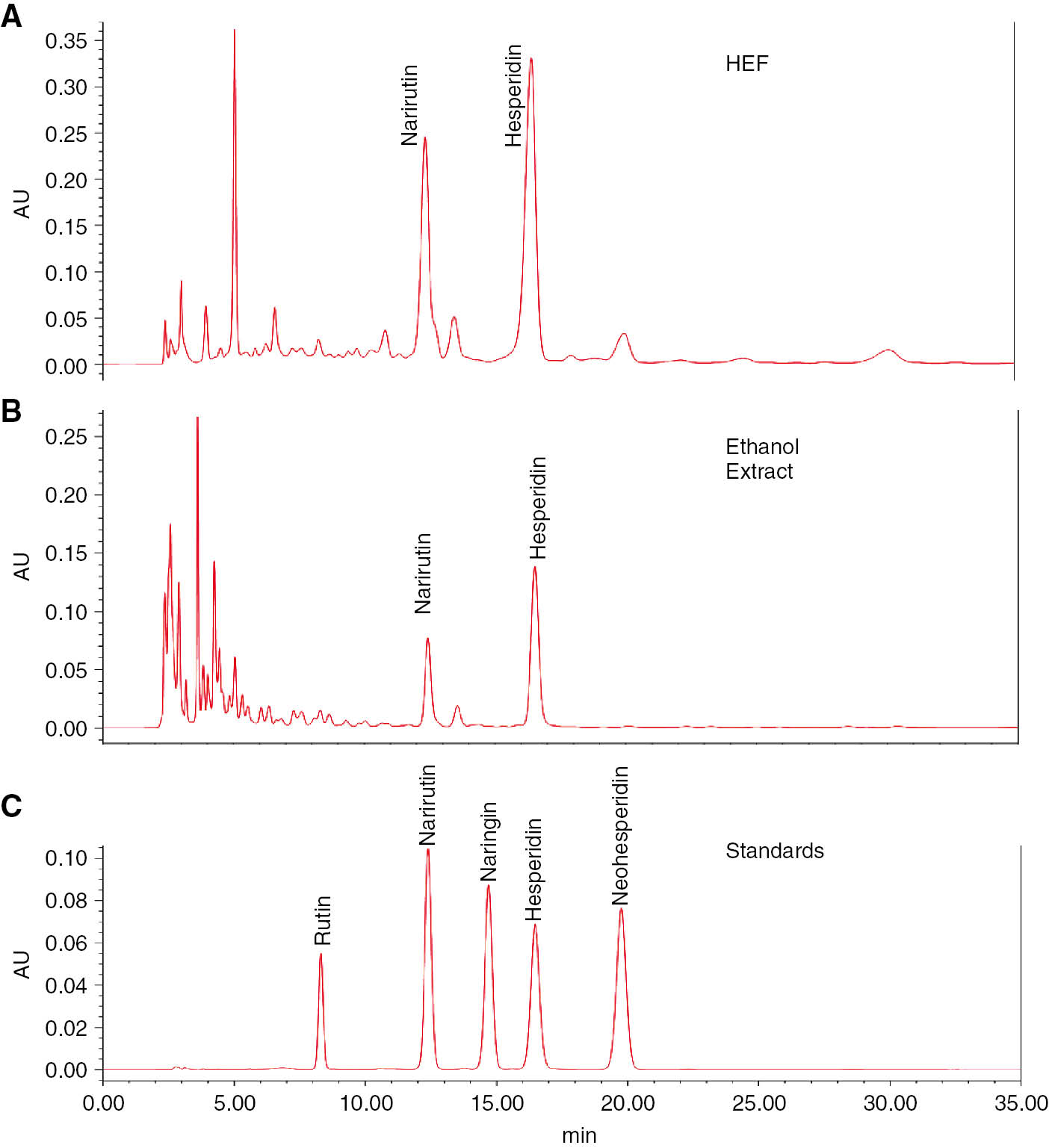
HPLC fingerprinting analysis of the HEF(A) and 70% ethanol (B) extracts.
The lower side (C) represents standard flavonoids. The wavelength of flavonoids is 280 nm.
Conclusions
The present study was undertaken to identify the anti-inflammatory effects of the hot water extract, ethanolic extract, and ethyl acetate and water fractions of the ethanolic extract of Shiranuhi flowers in murine RAW 264.7 macrophages. The results indicate that, in LPS-stimulated murine RAW 264.7 cells, HEF inhibits the productions of pro-inflammatory molecules such as NO, iNOS, PGE2, and COX-2, and cytokines such as TNF-α and IL-6. Furthermore, the phosphorylation of MAPKs such as p38, ERK, and JNK was suppressed by HEF in a concentration-dependent manner. Furthermore, HEF containing rutin, narirutin, hesperidin, naringenin, and hesperetin could be used as anti-inflammatory agents given their ability to suppress inflammatory cytokines and mediators. Based on these results, we suggest HEF as a potential anti-inflammatory candidate for pharmaceutical and cosmetic applications.
Funding source: Rural Development Administration
Award Identifier / Grant number: PJ010934082015
Funding statement: This work was supported by the Cooperative Research Program for Agriculture Science & Technology Development (Project no. PJ010934082015) through the Rural Development Administration (Republic of Korea).
Conflict of interest: The authors have no conflict of interest.
References
1. Jeong YH, Oh YC, Cho WK, Yim NH, Ma JY. Anti-inflammatory effect of Rhapontici radix ethanol extract via inhibition of NF-κB and MAPK andi of HO-1 in macrophages. Mediators Inflamm 2016;2016:7216912.10.1155/2016/7216912Search in Google Scholar PubMed PubMed Central
2. Jang KJ, Choi SH, Yu GJ, Hong SH, Chung YH, Kim CH, et al. Anti-inflammatory potential of total saponins derived from the roots of Panax ginseng in lipopolysaccharide-activated RAW 264.7 macrophages. Exp Ther Med 2016;11:1109–15.10.3892/etm.2015.2965Search in Google Scholar PubMed PubMed Central
3. Jeong DH, Kim KB, Kim MJ, Kang BK, Ahn DH. Skipjack tuna (Katsuwonus pelamis) eyeball oil exerts an anti-inflammatory effect by inhibiting NF-κB and MAPK activation in LPS-induced RAW 264.7 cells and croton oil-treated mice. Int Immunopharmacol 2016;40:50–6.10.1016/j.intimp.2016.07.005Search in Google Scholar PubMed
4. Lee WS, Shin JS, Jang DS, Lee KT. Cnidilide, an alkylphthalide isolated from the roots of Cnidium officinale, suppresses LPS-induced NO, PGE2, IL-1β, IL-6 and TNF-α production by AP-1 and NF-κB inactivation in RAW 264.7 macrophages. Int Immunopharmacol 2016;40:146–55.10.1016/j.intimp.2016.08.021Search in Google Scholar PubMed
5. Chun J, Tosun A, Kim YS. Anti-inflammatory effect of corymbocoumarin from Seseli gummiferum subsp. corymbosum through suppression of NF-κB signaling pathway and induction of HO-1 expression in LPS-stimulated RAW 264.7 cells. Int Immunopharmacol 2016;31:207–15.10.1016/j.intimp.2015.12.029Search in Google Scholar PubMed
6. Chen L, Teng H, Fang T, Xiao J. Agrimonolide from Agrimonia pilosa suppresses inflammatory responses through down-regulation of COX-2/iNOS and inactivation of NF-κB in lipopolysaccharide-stimulated macrophages. Phytomedicine 2016;23:846–55.10.1016/j.phymed.2016.03.016Search in Google Scholar PubMed
7. Intayoung P, Limtrakul P, Yodkeeree S. Anti-inflammatory activities of crebanine by inhibition of NF-κB and AP-1 activation through suppressing MAPKs and Akt signaling in LPS-induced RAW 264.7 macrophages. Biol Pharm Bull 2016;39:54–61.10.1248/bpb.b15-00479Search in Google Scholar PubMed
8. Cha SM, Cha JD, Jang EJ, Kim GU, Lee KY. Sophoraflavanone G prevents Streptococcus mutans surface antigen I/II-induced production of NO and PGE2 by inhibiting MAPK-mediated pathways in RAW 264.7 macrophages. Arch Oral Biol 2016;68:97–104.10.1016/j.archoralbio.2016.04.001Search in Google Scholar PubMed
9. Wu J, Zhang H, Hu B, Yang L, Wang P, Wang F, et al. Coptisine from Coptis chinensis inhibits production of inflammatory mediators in lipopolysaccharide-stimulated RAW 264.7 murine macrophage cells. Eur J Pharmacol 2016;780:106–14.10.1016/j.ejphar.2016.03.037Search in Google Scholar PubMed
10. Park JY, Moon JY, Park SD, Park WH, Kim H, Kim JE. Fruits extracts of Hovenia dulcis Thunb. Suppresses lipopolysaccharide-stimulated inflammatory responses through nuclear factor-kappaB pathway in Raw 264.7 cells. Asian Pac J Trop Med 2016;9:357–65.10.1016/j.apjtm.2016.03.017Search in Google Scholar PubMed
11. Ham YM, Yoon WJ, Lee WJ, Kim SC, Baik JS, Kim JH, et al. Anti-inflammatory effects of isoketocharbroic acid from brown alga, Sargassum micracanthum. EXCLI J 2015;14:1116–21.Search in Google Scholar
12. Kim MJ, Yang KW, Kim SS, Park SM, Park KJ, Kim KS, et al. Chemical composition and anti-inflammation activity of essential oils from Citrus unshiu flower. Nat Prod Commun 2014;9:727–30.10.1177/1934578X1400900538Search in Google Scholar
13. Kim MJ, Yang KW, Yang EJ, Kim SS, Park KJ, An HJ, et al. Citrus unshiu flower inhibits LPS-induced iNOS and COX-2 via MAPKs in RAW 264.7 macrophages Orient J Chem 2015;31:1915–22.10.13005/ojc/310407Search in Google Scholar
14. Cho YH, Kim NH, Khan I, Yu JM, Jung HG, Kim HH, et al. Anti-inflammatory potential of quercetin-3-O-β-D-(“2”-galloyl)-glucopyranoside and quercetin isolated from Diospyros kaki calyx via suppression of MAP signaling molecules in LPS-induced RAW 264.7 macrophages. J Food Sci 2016;81:C2447–56.10.1111/1750-3841.13497Search in Google Scholar PubMed
15. Wu L, Li X, Wu H, Long W, Jiang X, Shen T, et al. 5-Methoxyl aesculetin abrogates lipopolysaccharide-induced inflammation by suppressing MAPK and AP-1 pathways in RAW 264.7 cells. Int J Mol Sci 2016;17:315.10.3390/ijms17030315Search in Google Scholar PubMed PubMed Central
16. Jin SE, Kim OS, Yoo SR, Seo CS, Kim Y, Shin HK, et al. Anti-inflammatory effect and action mechanisms of traditional herbal formula Gamisoyo-san in RAW 264.7 macrophages. BMC Complement Altern Med 2016;16:219.10.1186/s12906-016-1197-7Search in Google Scholar PubMed PubMed Central
17. Kim MJ, Kim SJ, Kim SS, Lee NH, Hyun CG. Hypochoeris radicata attenuates LPS-induced inflammation by suppressing p38, ERK, and JNK phosphorylation in RAW 264.7 macrophages. EXCLI J 2014;13:123–36.Search in Google Scholar
18. Yoon WJ, Moon JY, Kang JY, Kim GO, Lee NH, Hyun CG. Neolitsea sericea essential oil attenuates LPS-induced inflammation in RAW 264.7 macrophages by suppressing NF-kappaB and MAPK activation. Nat Prod Commun 2010;5:1311–6.10.1177/1934578X1000500835Search in Google Scholar
19. Yoon WJ, Moon JY, Song G, Lee YK, Han MS, Lee JS, et al. Artemisia fukudo essential oil attenuates LPS-induced inflammation by suppressing NF-kappaB and MAPK activation in RAW 264.7 macrophages. Food Chem Toxicol 2010;48:1222–9.10.1016/j.fct.2010.02.014Search in Google Scholar PubMed
20. Lee EH, Park HR, Shin MS, Cho SY, Choi HJ, Shin KS. Antitumor metastasis activity of pectic polysaccharide purified from the peels of Korean Citrus Hallabong. Carbohydr Polym 2014;111:72–9.10.1016/j.carbpol.2014.04.073Search in Google Scholar PubMed
21. Herath KH, Bing SJ, Cho J, Kim A, Kim GO, Lee JC, et al. Citrus hallabong [(Citrus unshiu×C. sinensis)×C. reticulata)] exerts potent anti-inflammatory properties in murine splenocytes and TPA-induced murine ear oedema model. Pharm Biol 2016;22:1–12.10.1080/13880209.2016.1194865Search in Google Scholar PubMed
22. Chang YH, Seo J, Song E, Choi HJ, Shim E, Lee O, et al. Bioconverted Jeju Hallabong tangor (Citrus kiyomi×ponkan) peel extracts by cytolase enhance antioxidant and anti-inflammatory capacity in RAW 264.7 cells. Nutr Res Pract 2016;10:131–8.10.4162/nrp.2016.10.2.131Search in Google Scholar PubMed PubMed Central
23. Lim H, Seo J, Chang YH, Han BK, Jeong JK, Park SB, et al. Anti-obesity effects of Jeju Hallabong tangor (Citrus kiyomi×ponkan) peel extracts in 3T3-L1 adipocytes. J Korean Soc Food Sci Nutr 2014;43:1688–94.10.3746/jkfn.2014.43.11.1688Search in Google Scholar
24. Park JY, Shin MS, Kim SN, Kim HY, Kim KH, Shin KS, et al. Polysaccharides from Korean Citrus hallabong peels inhibit angiogenesis and breast cancer cell migration. Int J Biol Macromol 2016;85:522–9.10.1016/j.ijbiomac.2016.01.015Search in Google Scholar PubMed
25. Kim MJ, Kim SS, Park KJ, An HJ, Choi YH, Lee NH, et al. Methyl jasmonate inhibits lipopolysaccharide-induced inflammatory cytokine production via mitogen-activated protein kinase and nuclear factor-κB pathways in RAW 264.7 cells. Die Pharmazie 2016;2016:540–3.Search in Google Scholar
26. Kim MJ, Hyun JM, Kim SS, Seong KC, Lim CK, Kang JS, et al. In vitro screening of subtropical plants cultivated in Jeju island for cosmetic ingredients. Orient J Chem 2016;32:807–15.10.13005/ojc/320206Search in Google Scholar
27. Yang EJ, Hyun JM, Lee NH, Hyun CG. In vitro screening of korean halophytes for cosmeceutical ingredients. Int J ChemTech Res 2016;9:541–7.Search in Google Scholar
28. Chanput W, Krueyos N, Ritthiruangdej P. Anti-oxidative assays as markers for anti-inflammatory activity of flavonoids. Int Immunopharmacol 2016;40:170–5.10.1016/j.intimp.2016.08.038Search in Google Scholar PubMed
29. Diaz P, Jeong SC, Lee S, Khoo C, Koyyalamudi SR. Antioxidant and anti-inflammatory activities of selected medicinal plants and fungi containing phenolic and flavonoid compounds. Chin Med 2012;7:26.10.1186/1749-8546-7-26Search in Google Scholar PubMed PubMed Central
©2018 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Research Articles
- Betaine treatment decreased serum glucose and lipid levels, hepatic and renal oxidative stress in streptozotocin-induced diabetic rats
- Oxidative stress and response of antioxidant system in Nostoc muscorum exposed to different forms of Zinc
- An investigation on Trakya region Oak (Quercus spp.) honeys of Turkey: their physico-chemical, antioxidant and phenolic compounds properties
- Effects of Shiranuhi flower extracts and fractions on lipopolysaccharide-induced inflammatory responses in murine RAW 264.7 cells
- Evaluation of resveratrol organogels prepared by micro-irradiation: fibroblast proliferation through in vitro wound healing
- Ecological and phytochemical attributes of endemic Ferula gummosa Boiss. at vegetative and generative stages
- Characterization of calmodulin in the clam Anodonta woodiana: differential expressions in response to environmental Ca2+ and Cd2+
- Foliar application effects of salicylic acid and jasmonic acid on the essential oil composition of Salvia officinalis
- Antioxidant and antimicrobial activities of four Astragalus species growing wild in Turkey
- Differences in structure, allergenic protein content and pectate lyase enzyme activity of some Cupressaceae pollen
- In vitro bioactivities and subacute toxicity study of O. basilicum, T. vulgaris and R. officinalis
- Wendlandia exserta: a pertinent source of antioxidant and antimicrobial agent
- Effects of epigallocatechin-3-gallate (EGCG) on a scleroderma model of fibrosis
Articles in the same Issue
- Frontmatter
- Research Articles
- Betaine treatment decreased serum glucose and lipid levels, hepatic and renal oxidative stress in streptozotocin-induced diabetic rats
- Oxidative stress and response of antioxidant system in Nostoc muscorum exposed to different forms of Zinc
- An investigation on Trakya region Oak (Quercus spp.) honeys of Turkey: their physico-chemical, antioxidant and phenolic compounds properties
- Effects of Shiranuhi flower extracts and fractions on lipopolysaccharide-induced inflammatory responses in murine RAW 264.7 cells
- Evaluation of resveratrol organogels prepared by micro-irradiation: fibroblast proliferation through in vitro wound healing
- Ecological and phytochemical attributes of endemic Ferula gummosa Boiss. at vegetative and generative stages
- Characterization of calmodulin in the clam Anodonta woodiana: differential expressions in response to environmental Ca2+ and Cd2+
- Foliar application effects of salicylic acid and jasmonic acid on the essential oil composition of Salvia officinalis
- Antioxidant and antimicrobial activities of four Astragalus species growing wild in Turkey
- Differences in structure, allergenic protein content and pectate lyase enzyme activity of some Cupressaceae pollen
- In vitro bioactivities and subacute toxicity study of O. basilicum, T. vulgaris and R. officinalis
- Wendlandia exserta: a pertinent source of antioxidant and antimicrobial agent
- Effects of epigallocatechin-3-gallate (EGCG) on a scleroderma model of fibrosis


