Betaine treatment decreased serum glucose and lipid levels, hepatic and renal oxidative stress in streptozotocin-induced diabetic rats
-
Betül Evran
, Buse Uğuralp
Abstract
Objective
The present study was aimed to investigate the effects of betaine (BET) on streptozotocin (STZ)-induced diabetes mellitus (DM) in rats. Additionally, the efficiency of BET was compared with metformin (MET), a standard oral antidiabetic drug.
Methods
STZ (55 mg/kg body weight; i.p.) was injected to male Wistar rats. Rats with DM were treated with BET (1 g/kg body weight/day;) or MET (500 mg/kg body weight/day;) for 4 weeks. Blood glycated hemoglobin (HbA1c), serum glucose, lipids, hepatic and renal function tests and urinary protein levels were examined. Reactive oxygen species (ROS) formation, malondialdehyde (MDA), glutathione (GSH) levels, and ferric reducing antioxidant power (FRAP) were also determined in liver and kidney.
Results
Glucose, HbA1c, and serum lipids increased and liver and kidney function tests were impaired in diabetic rats. Hepatic and renal ROS formation and MDA levels were elevated, hepatic, but not renal GSH and FRAP levels were decreased. BET decreased blood HbA1c, serum glucose and lipid levels and urine protein levels. BET diminished hepatic and renal prooxidant status.
Conclusion
Our results indicate that BET may be effective in decreasing STZ-induced high levels of blood HbA1c, and serum glucose and lipid levels and prooxidant status in liver and kidney tissues.
Özet
Amaç
Bu çalışmada betainin (BET) streptozotosin (STZ) ile diabet oluşturulan sıçanlarda etkisini araştırmak amaçlandı. Ayrıca BET’in etkisi standart oral antidiyabetik ilaç olan metforminle (MET) karşılaştırıldı.
Metod
STZ (55 mg/kg vücut ağırlığı;i.p.) erkek Wistar sıçanlara uygulandı. 4 hafta süreyle diyabetik sıçanlara BET (1/kg vücut ağırlığı/gün; diyetle) veya MET (500 mg/kg vücut ağırlığı/gün; içme suyunda) uygulaması yapıldı. Kanda glike hemoglobin (HbA1c), serumda glikoz, lipid ve karaciğer ve böbrek fonksiyon testleri ve idrarda protein düzeyleri ölçüldü. Ayrıca, karaciğer ve böbrek dokusunda reaktif oksijen türleri oluşumu, malondialdehit (MDA), glutatyon (GSH) düzeyleri ve antioksidan aktivite (FRAP) tayin edildi.
Bulgular
Diyabetik sıçanlarda glikoz, HbA1c ve serum lipit düzeyleri arttı, karaciğer ve böbrek fonksiyon testleri bozuldu. Karaciğer ve böbrekte ROS oluşumu ve MDA düzeyleri arttı, karaciğerde GSH ve FRAP düzeyleri azaldı. BET uygulaması kan HbA1c, serum glikoz ve lipit düzeylerini ve idrar protein düzeylerini azalttı. BET ayrıca karaciğer ve böbrekte prooksidan durumu baskıladı.
Sonuç
Sonuçlarımız BET’in STZ uygulamasıyla artmış olan kan HbA1c, serum glikoz ve lipit düzeyleri ile karaciğer ve böbrek dokularında prooksidan durumu azaltmada etkili olduğunu gösterdi.
Introduction
Diabetes mellitus (DM) is a chronic metabolic disease with high glucose levels caused by partial or complete insufficiency of insulin. Oxidative stress induced by hyperglycemia is accepted to play a major role in functional and structural defects seen in patients with DM and experimental models of diabetes [1], [2], [3]. Although exact mechanisms are unknown, autooxidation of glucose induced by hyperglycemia, formation of advanced glycation end products (AGEs), and activation of polyol pathway are considered to be responsible for the oxidative stress in DM [1], [2], [3].
Liver dysfunction and nephropathy are common complications of DM. Diabetic nephropathy is described with proteinuria, hypertension, and loss in renal function. It has been shown that DM diminishes prooxidant-antioxidant balance and causes inflammatory, apoptotic, and degenerative changes in liver [4], [5]. Similarly, DM was observed to increase oxidative stress and induce functional and structural defects in renal tissue [4], [6], [7], [8]. Based on these findings, several scientists have proposed antioxidant compounds to support treatment of DM. In this regard, effects of various antioxidant compounds (sulphydryl compounds, vitamin C and E, taurine, polyphenols) have been investigated [9].
Betaine (tri-methyl glycine; BET) is a choline metabolite formed in liver by the activities of mitochondrial choline dehydrogenase and betaine aldehyde dehydrogenase. Additionally, vegetables are alimentary sources of BET [10]. BET functions as an osmolite and shows antioxidant and antiinflammatory effects [10], [11]. Accordingly, BET treatment was reported to be useful against some oxidative stress-induced conditions such as atherosclerosis [12], liver [13], [14], [15], [16], and kidney [17], [18], [19], [20] disorders. In addition, it has been suggested that there is a relationship between the alteration of BET levels and development of DM complications [21], [22]. However, the effect of BET treatment on DM is poorly investigated [23], [24].
Metformin (MET) is an antidiabetic drug widely used in the treatment of DM [25]. MET was reported to ameliorate insulin resistance and have antioxidant, antiinflammatory and antiapoptotic effects [25], [26], [27]. These properties of MET contribute to its effect in preventing or ameliorating diabetic complications including hepatosteatosis [28], [29] and diabetic nephropathy [6], [28].
The present study aimed to investigate the ameliorative effects of BET on blood glycated hemoglobin (HbA1c), serum glucose and lipid levels, oxidative stress, and hepatic and renal dysfunction in STZ-induced diabetic rats and the efficiency of BET was compared with MET, an oral antihyperglycemic drug with antioxidant potential.
Methods
Chemicals
STZ, BET-hydrochloride and other chemicals were purchased from Sigma-Aldrich (USA). MET-HCl was obtained from Bilim Ilaç Sanayii (Istanbul, Turkey).
Animals
Male Wistar rats (3–4 months; 220–240 g body weight) were used for all experiments. Animals were obtained from Experimental Medical Research Institute of Istanbul University. They were kept in wire-bottomed stainless cages. All the animals were housed (three to four per cage) under a daily cycle of 12 h light and 12 h darkness, supplied with food and water ad libitum. Experimental settings such as feeding of animals and maintenance conditions were standardized for all the animals to minimize the variations. The experimental procedure used in this study met the guidelines of the Animal Care and Use Committee of the University of Istanbul.
STZ-induced diabetes model and treatments
Rats were divided into control and experimental groups. Six rats were randomly selected as control group and fed commercial food. Rats in experimental group were injected intraperitoneally with a single dose of STZ dissolved in citrate buffer (0.1 M; pH 4.5) at a dose of 55 mg/kg body weight (BW). Administration of STZ was performed after an overnight fasting. Three days later, blood glucose levels were determined in whole blood samples collected from the tip of the tail. Only rats with fasting blood glucose levels higher than 250 mg/dL [5], [7] were included in DM group.
Rats with DM were divided into three subgroups as follows: untreated (n=7),BET-treated (n=7), and MET-treated rats (n=7). Total food and water intake was recorded daily and diabetic rats were fed with BET containing diet or MET in drinking water for 4 weeks. The daily consumptions of BET and MET were equivalent to 1 g/kg BW and 500 mg/kg BW, respectively.
At the end of the treatments, rats were placed in individual metabolic cages for 24 h and urine samples were collected. They were centrifuged at 1750× g for 10 min at 4°C and frozen at −20°C. The day after, rats were sacrificed by collecting their blood into dry and heparin-containing tubes by cardiac puncture under sodium thiopental (50 mg/kg BW; i.p.) anesthesia. Serum samples were obtained by centrifugation at 1500× g for 10 min. Liver and kidney tissues were rapidly removed, washed in ice-cold saline and kept in ice. The materials were stored at −80°C until they were needed for analysis. In our study, all samples were analyzed at the same time in a single run for each assay to avoid inter-assay variations.
Biochemical analyses in blood and serum
Blood hemoglobin A1c (HbA1c) levels were measured in heparinized blood using a turbidimetric inhibition immunoassay (Roche Diagnostics, Mannheim, Germany). The levels of serum glucose, total cholesterol, triglyceride, total protein, albumin, urea nitrogen (BUN), creatinine, and activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH) were determined on an automated clinical chemistry analyzer (Roche Diagnostics, Mannheim, Germany).
Urinary protein determination
Urinary samples were centrifuged at 1750× g for 10 min, supernatants were diluted and protein levels were measured using Bradford reagent [30].
Reactive oxygen species (ROS) determination in liver and kidney tissues
Liver and kidney tissues were homogenized in ice-cold 0.15 M KCl (10%; w/v). ROS production in liver and kidney homogenates were measured fluorometrically [31]. Tissue homogenates were incubated with 100 μM 2′,7′-dichlorodihydrofluorescein diacetate at 37°C for 30 min. The fluorescence of 2′,7′-dichlorofluorescein was determined using a microplate fluorometer (Fluoroskan Ascent FL, Thermo scientific Inc, USA) with an excitation of 485 nm and emission of 538 nm. Results were expressed as relative fluorescence units (RFU).
Determination of malondialdehyde (MDA) levels in tissues
MDA levels in tissue homogenates were assessed using thiobarbituric acid in accordance with the method of Ohkawa et al. [32]. The breakdown product of 1,1,3,3-tetraethoxypropane was used as a standard.
Glutathione (GSH) determination in tissues
GSH levels were measured in tissue homogenates with 5,5-dithiobis-(2-nitrobenzoate) at 412 nm [33].
Ferric reducing antioxidant power (FRAP)determination in tissues
Total antioxidant capacity was analyzed using a ferric reducing antioxidant power (FRAP)-assay in postmitochondrial fraction [34]. Tissue homogenates were centrifuged at 600× g for 10 min and supernatants were recentrifuged at 10.000× g for 20 min at 4°C. FRAP assay was performed in the final supernatant fraction. This assay uses antioxidants as reductants in a redox-linked colorimetric method. At low pH, the ferric-tripyridyl-triazine complex is reduced to the ferrous form, which is monitored by measuring the change in absorbance at 593 nm. Protein levels in postmitochondrial fractions were determined using bicinchoninic acid [35].
In our laboratory settings, intra-assay coefficients of variations for FRAP, GSH, ROS and MDA assays were 3.2%, 6.1%, 4.6% and 10.4%, respectively.
Histopathologic analyses
Liver and kidney tissue samples were fixed in 10% formalin solution. After routine processing, tissue samples were embedded in paraffin blocks and 3 μm thick sections were obtained. Sections were stained with haematoxylen and eosin (H and E) and evaluated under light microscope.
Statistical analyses
The results were expressed as mean±SE. One-way analysis of variance (ANOVA) followed by Tukey’s honestly significant difference post-hoc test was used for equal variances. Kruskal-Wallis test was performed for unequal variances. In all cases, a difference was considered significant when p<0.05.
Results
Body weights, food and water consumption
Daily food and water intakes increased in STZ rats. MET decreased both food and water intakes in STZ rats, but BET treatment did not have any effect on these intakes. Final body weights were reduced in STZ rats as compared to control group. However, there were no changes in final body weights after BET or MET treatments in STZ rats (Table 1).
The effects of betaine (BET) and metformin (MET) treatments on final body weight, food and water intakes in diabetic rats induced by streptozotocin (STZ) (Means±SE).
| Parameters | Control (n=6) | STZ (n=7) | STZ+BET (n=7) | STZ+MET (n=7) |
|---|---|---|---|---|
| Food intake (g/day/rat) | 17.2±0.70a | 32.4±0.95 | 30.2±1.52 | 23.1±1.33a |
| Water intake (mL/day/rat) | 34.4±3.00a | 108±4.35 | 132±12.6 | 50.0±3.46a |
| Final body weight (g) | 256±11.3a | 182±11.6 | 182±6.67 | 177±4.99 |
ap<0.05 as compared to STZ group.
Blood HbA1c, serum glucose and lipid levels
STZ treatment caused significant increases in blood HbA1c, serum glucose, triglyceride, and cholesterol levels as compared to controls. BET treatment caused significant decreases in blood HbA1c, serum glucose, triglyceride, and cholesterol levels in STZ rats. MET treatment also decreased these parameters except cholesterol (Table 2).
The effects of betaine (BET) and metformin (MET) treatments on blood hemoglobin A1c (HbA1c), serum glucose, triglyceride and cholesterol levels in diabetic rats induced by streptozotocin (STZ) (Means±SE).
| Parameters | Control (n=6) | STZ (n=7) | STZ+BET (n=7) | STZ+MET (n=7) |
|---|---|---|---|---|
| HbA1c (%) | 3.53±0.02a | 8.89±0.35 | 7.21±0.31a | 6.14±0.20a |
| Glucose (mg/dL) | 115±5.11a | 374±17.9 | 308±13.9a | 293±12.2a |
| Triglyceride (mg/dL) | 36.7±3.38a | 57.3±4.31 | 31.9±6.18a | 36.0±3.22a |
| Cholesterol (mg/dL) | 43.7±2.51a | 76.9±3.62 | 55.9±6.90a | 75.9±7.75 |
ap<0.05 as compared to STZ group.
Hepatic and renal function tests in serum and urinary protein levels
Significant increases in serum AST, ALT, and LDH activities were observed in STZ rats. BET treatment did not alter these enzyme activities, but they decreased due to MET treatment. Increased BUN and decreased total protein and albumin levels in serum were detected in STZ rats, but creatinine levels remained unchanged. These parameters did not alter due to BET or MET treatments in STZ rats. Only, increases in albumin levels were detected following MET treatment. Additionally, both BET and MET treatments diminished high levels of urine protein in STZ rats (Table 3).
The effects of betaine (BET) and metformin (MET) treatments on alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) activities and urea nitrogen (BUN), creatinine, total protein and albumin levels as well as urinary protein levels in diabetic rats induced by streptozotocin (STZ) (Means±SE).
| Parameters | Control (n=6) | STZ (n=7) | STZ+BET (n=7) | STZ+MET (n=7) |
|---|---|---|---|---|
| ALT (U/L) | 37.8±1.89a | 81.6±6.80 | 67.0±7.67 | 55.7±3.48a |
| AST (U/L) | 83.2±4.03a | 143±21.4 | 128±8.61 | 95.1±8.01a |
| LDH (U/L) | 313±30.1a | 699±38.8 | 568±69.2 | 396±21.9a |
| BUN (mg/dL) | 17.2±1.49a | 79.7±6.81 | 76.4±11.4 | 68.0±3.82 |
| Creatinine (mg/dL) | 0.42±0.02 | 0.50±0.05 | 0.40±0.04 | 0.42±0.03 |
| Total protein (g/dL) | 6.32±0.12a | 5.46±0.20 | 5.60±0.17 | 5.82±0.12 |
| Albumin (g/dL) | 3.48±0.07a | 2.97±0.09 | 3.21±0.14 | 3.41±0.06a |
| Urinary protein (mg/mg creatinine) | 1.46±0.25a | 2.13±0.11 | 1.53±0.14a | 1.01±0.11a |
ap<0.05 as compared to STZ group.
ROS, MDA, FRAP, and GSH levels in the liver and kidneys
Significant increases in ROS and MDA levels and decreases in FRAP and GSH levels were observed in the liver of STZ rats. Both BET and MET treatments decreased liver ROS and MDA levels, but GSH and FRAP levels remained unchanged (Figure 1). In kidneys of STZ rats, ROS and MDA levels also increased, but there were no changes in FRAP and GSH levels. Both BET and MET treatments decreased kidney ROS and MDA levels. Additionally, kidney GSH levels increased after MET treatment in STZ rats (Figure 2).
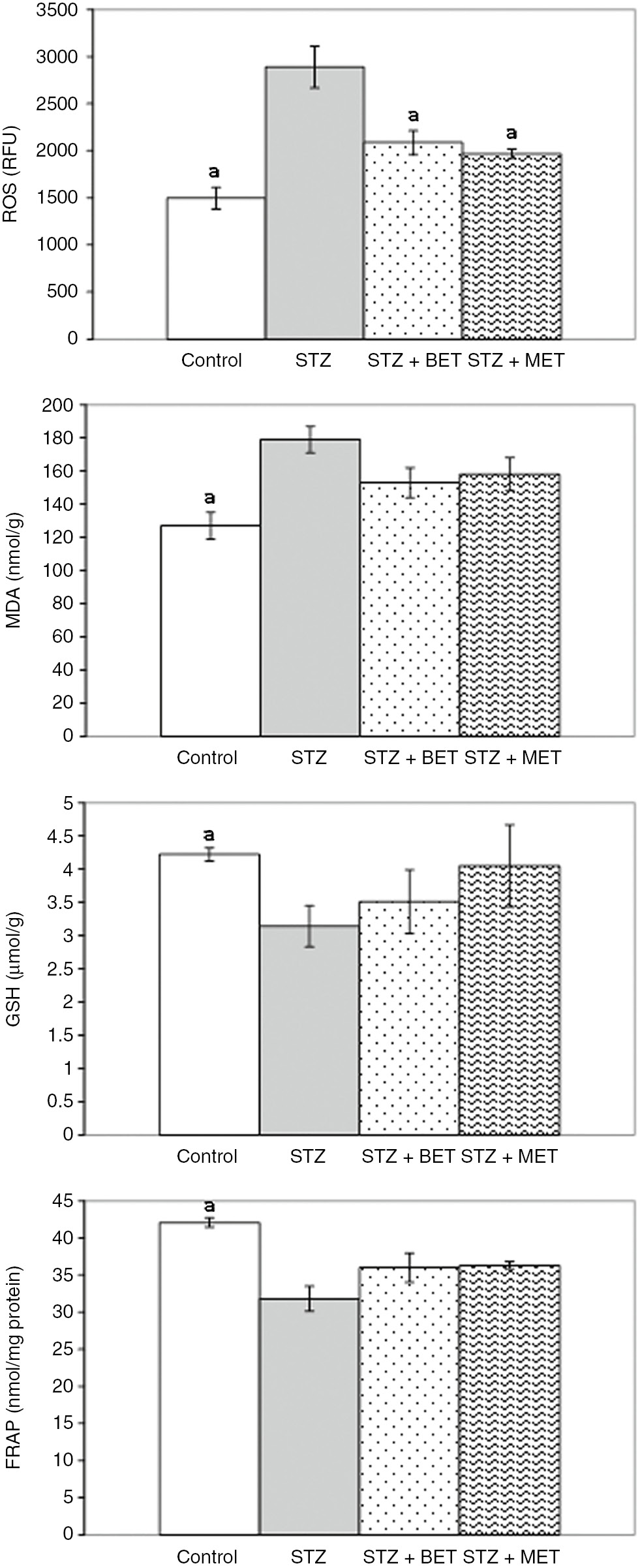
The effects of metformin (MET) and betaine (BET) treatments on liver reactive oxygen species (ROS), malondialdehyde (MDA), glutathione (GSH), and ferric reducing antioxidant power (FRAP) levels in diabetic rats induced by streptozotocin (STZ) (Means±SE). ap<0.05 as compared to STZ group.
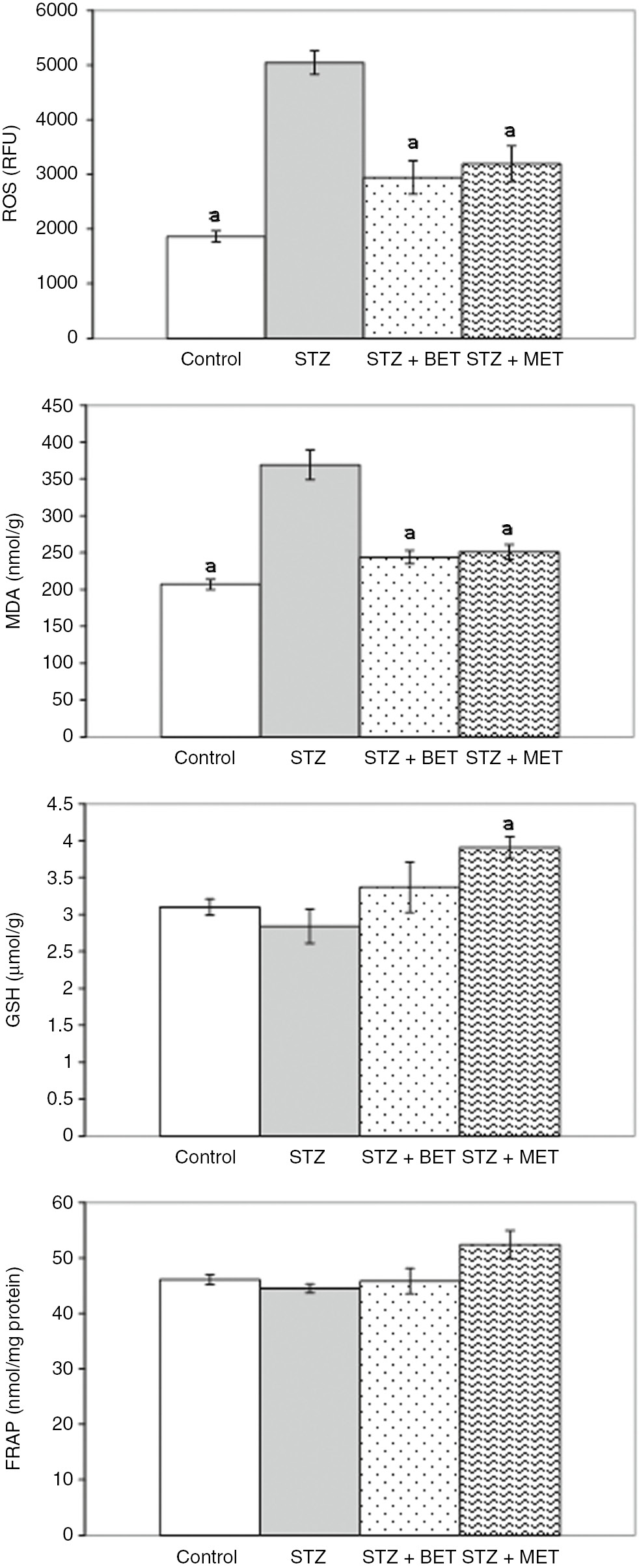
The effects of metformin (MET) and betaine (BET) treatments on kidney reactive oxygen species (ROS), malondialdehyde (MDA), glutathione (GSH), and ferric reducing antioxidant power (FRAP) levels in diabetic rats induced by streptozotocin (STZ) (Means±SE). ap<0.05 as compared to STZ group.
Liver and kidney histology
In livers of STZ-diabetic rats, mild mononuclear inflammatory cell infiltration was observed in portal areas. Mild proliferation in biliary duct epithelium and mild inflammatory cell infiltration in portal areas were also seen. BET or MET treatment did not significantly affect these findings (Figure 3). Normal kidney histology was observed in STZ-diabetic rats. BET or MET treatments did not alter histologic findings in kidney (Figure 4).
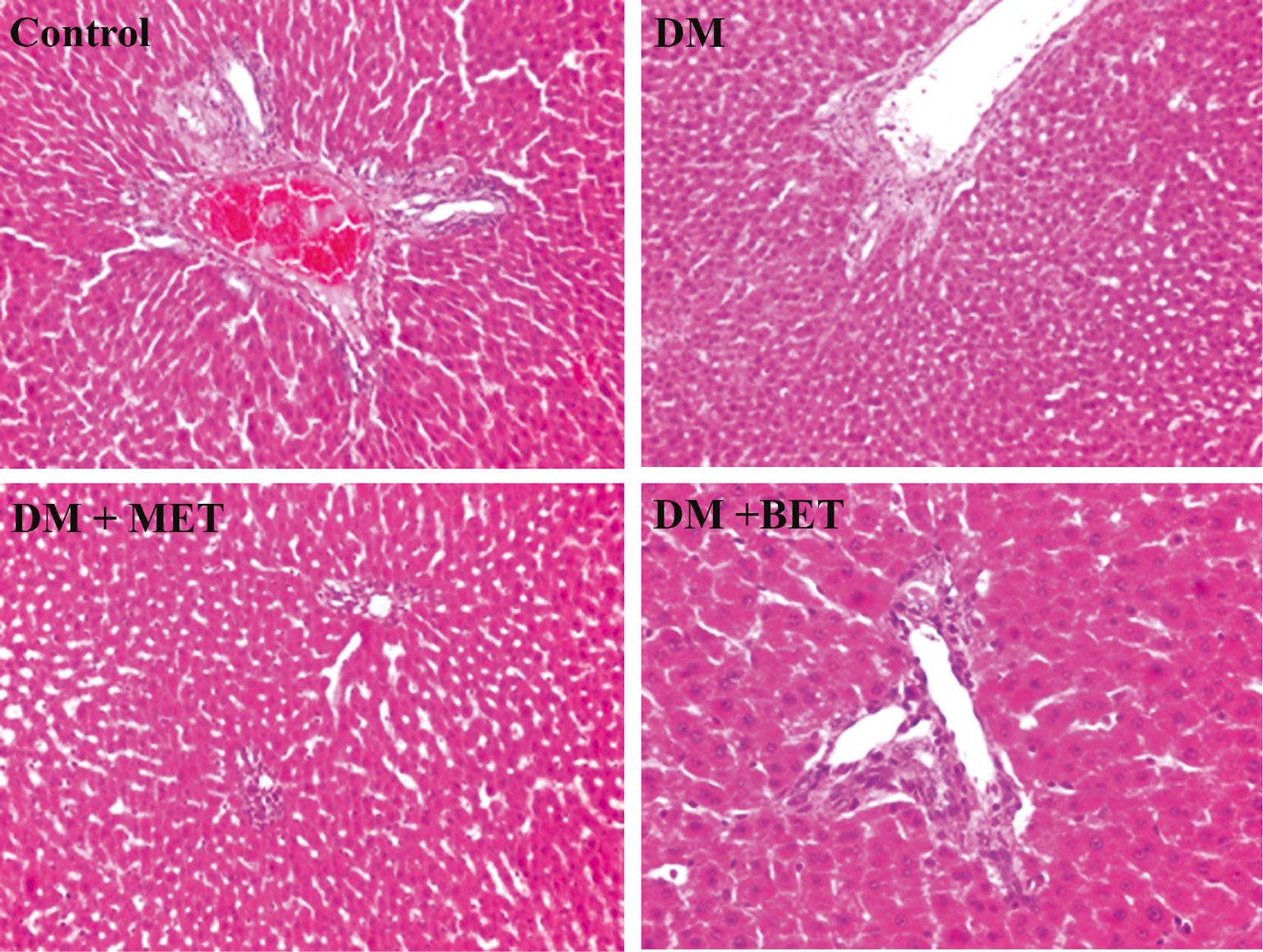
The effect of betaine (BET) and metformin treatments on liver histology in diabetic rats induced by streptozotocin (STZ) (H&E; ×100/×200).
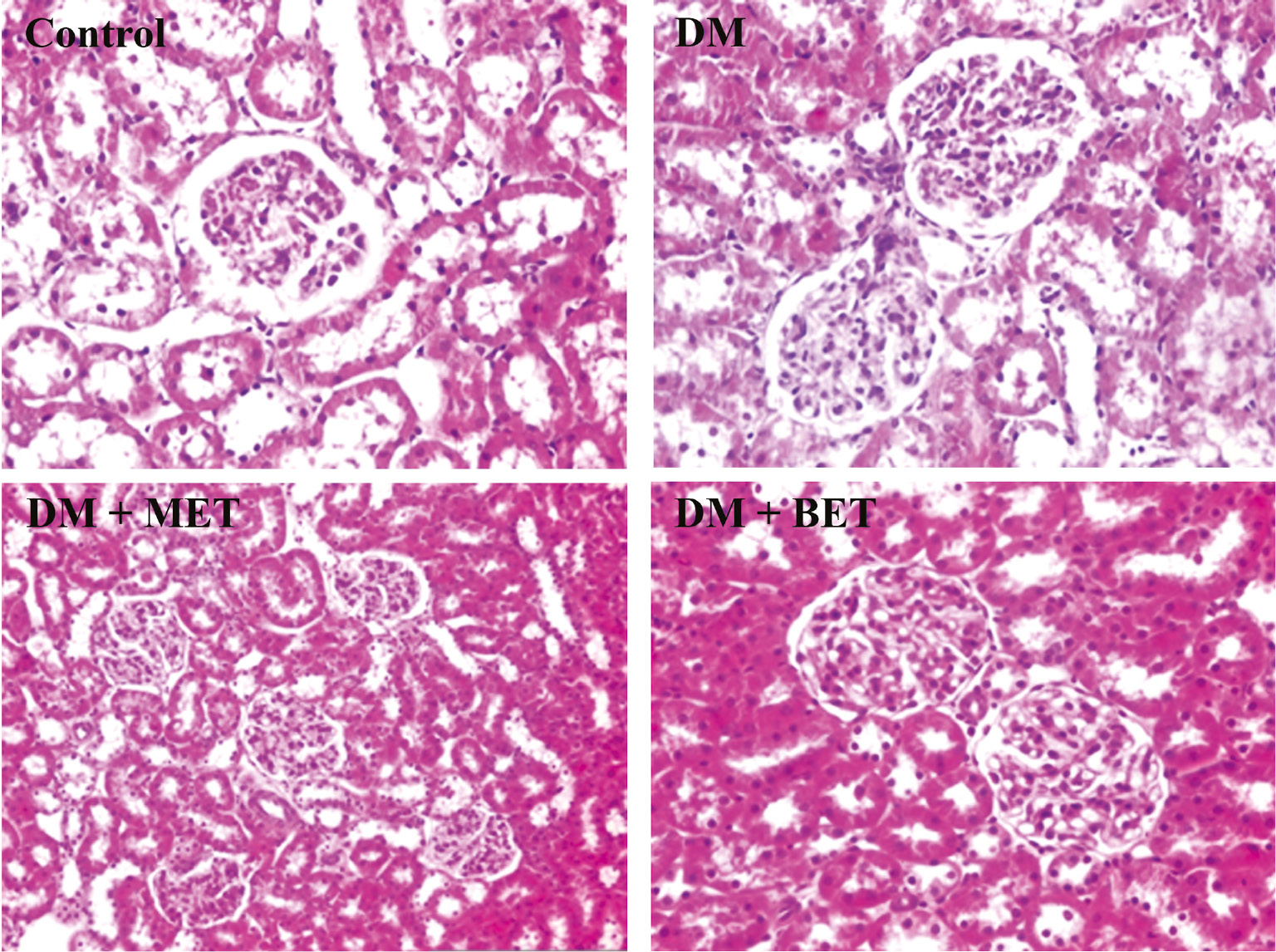
The effect of betaine (BET) and metformin treatments on kidney histology in diabetic rats induced by streptozotocin (STZ) (H&E; ×100/×200).
Discussion
STZ is a wide spectrum antibiotic frequently used to induce experimental DM. By damaging pancreatic β cells, STZ-induces a type 1-like DM. Thus, rat models of STZ-induced DM are convenient for studies on complications of DM [36]. A single STZ dose of 55–65 mg/kg is capable to induce functional and structural changes in different tissues of rats. In our study, rats were given 55 mg/kg STZ intraperitoneally. This treatment caused an increase in blood HbA1c, and serum glucose and lipid levels, and a disturbance in examined liver and kidney function tests. Increases in ALT, AST, and LDH activities together with hepatic histopathologic changes indicated the presence of hepatic dysfunction in STZ rats. On the other hand, elevated serum BUN levels, diminished total protein and albumin levels, and increased urinary protein levels were observed despite normal histologic appearance of kidney sections and unchanged creatinine levels. As is known, elevated BUN and creatinine levels are markers for renal injury. Elevated creatinine levels are seen only in advanced stage kidney damage whereas BUN levels increase earlier. Therefore, elevated BUN levels together with normal kidney histopathology and unchanged creatinine levels, as seen in our study, has been suggested to indicate a mild renal injury [37]. Under these conditions, significant increases in ROS production and lipid peroxide levels were detected in the liver and kidney of DM rats, indicating a prooxidant status in these tissues. These findings are in accordance with previous studies [4], [5], [6], [7], [8].
In the current study, the effect of BET treatment on blood glycemia, lipids, hepatic and renal function tests as well as oxidative stress in liver and kidney tissues were evaluated in STZ-induced diabetic rats. Additionally, the efficiency of BET was compared to that of MET. It has been reported that MET ameliorated insulin resistance and had antioxidant, antiinflammatory and antiapoptotic effects [25], [38]. These properties of MET may contribute to its preventive or ameliorative effect on complications of DM including hepatic [5], [28] and renal complications [6], [39]. In the current study, MET ameliorated hepatic function tests and decreased high levels of serum glucose and triglyceride, blood HbA1c, and urinary protein in STZ rats. Additionally, ROS and lipid peroxide levels diminished in the liver and kidney of MET-treated STZ rats. However, this treatment did not alter histopathologic appearance in these tissues.
On the other hand, BET has antioxidant and antiinflammatory effects. Although it does not interact directly with oxidants, the effect of BET on the metabolism of sulfur-containing substances in the liver was suggested to be responsible for its antioxidant activity [40]. BET administration decreases homocysteine levels, and increases S-adenosyl methionine (SAM) and GSH levels which have strong antioxidant effects in organism [10], [11]. Among them, SAM has a direct antioxidant activity, scavenges ROS and chelates iron ions and thereby inhibits hydroxyl radical generation [41]. Accordingly, BET was reported to protect tissues including liver and kidney against oxidative stress mediated injury. BET treatment was reported to be useful in the alleviation of alcoholic-[13] and non-alcoholic fatty liver [14], and necrotic [15] and fibrotic [16] lesions in the liver. Previous studies have also demonstrated protective effects of BET against carbon tetrachloride-[17], high fructose diet-[19] and cisplatin-[20]-induced nephrotoxicity.
Decreased liver [21] and kidney [42] levels of BET were reported in animal models of DM. In human studies, increased urinary excretion and decreased serum levels of BET were shown to be correlated with DM or its complications [22], [43], [44]. However, effects of BET supplementation in rats with DM have not been clearly elucidated yet. Jeong et al. [23] have found mildly decreased serum glucose and fructosamine levels, AST, ALT, and LDH activities together with amelioration in histopathology of pancreatic β cells in DM rats fed on 1% BET containing diet for 3 weeks. In another study, Jung et al. [24] have observed that 1% BET supplemented diet for 5 weeks resulted in decreases in insulin and triglyceride levels and amelioration of insulin resistance in db/db mice. They also found decreased lipid peroxide and increased GSH levels and catalase activities in the liver. However, BET treatment (500 mg/kg for 2 weeks with oral route) did not decrease blood glucose levels in STZ rats, but it prevented and/or delayed complications of diabetic retinopathy by inhibiting retinal neovascularization [45].
In the current study, BET treatment decreased blood HbA1c, serum glucose and lipid levels, and urine protein excretion and reduced the prooxidant status in liver and kidney tissues of DM rats. However, it did not ameliorate hepatic and renal function tests in serum and histopathologic findings in the liver or kidney. As a well known antidiabetic drug, MET was used in our study to compare the effects of BET. Our results are showing that BET exerts effects similar to that of MET in STZ-induced diabetic rats, but they do not imply any superiority. Therefore, investigating the effects of the co-treatment of BET and MET in STZ-treated rats can be interesting.
In conclusion, all of the above mentioned findings indicate that BET may be a candidate supplement in the treatment of DM and prevention of its complications.
Acknowledgement
The present work was supported by Research Fund of Istanbul University (Project No: 36345).
Conflict of interest statement: The authors report no conflict of interest.
Ethical Considerations: This study was approved by the Animal Care and Use Committee of the University of Istanbul (Project No: 2013/77).
References
1. Kassab A, Piwowar A. Cell oxidant stress delivery and cell dysfunction onset in type 2 diabetes. Biochimie 2012;94:1837–48.10.1016/j.biochi.2012.01.020Search in Google Scholar PubMed
2. De M Bandeira S, Da Fonseca LJ, Da S Guedes G, Rabelo LA, Goulart MO, Vasconcelos SM. Oxidative stress as an underlying contributor in the development of chronic complications in diabetes mellitus. Int J Mol Sci 2013;14:3265–84.10.3390/ijms14023265Search in Google Scholar PubMed PubMed Central
3. Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev 2013;93:137–88.10.1152/physrev.00045.2011Search in Google Scholar PubMed
4. Karaağaç N, Salman F, Doğru-Abbasoğlu S, Uysal M. Changes in prooxidant-antioxidant balance in tissues of rats following long-term hyperglycemic status. Endocr Res 2011;36:124–33.10.3109/07435800.2011.566237Search in Google Scholar PubMed
5. Ong KW,Hsu A, Song L, Huang D, Tan BK. Polyphenols-rich Vernonia amygdalina shows anti-diabetic effects in streptozotocin-induced diabetic rats. J Ethnopharmacol 2011;133:598–607.10.1016/j.jep.2010.10.046Search in Google Scholar PubMed
6. Alhaider AA, Korashy HM, Sayed-Ahmed MM, Mobark M, Kfoury H, Mansour MA. Metformin attenuates streptozotocin-induced diabetic nephropathy in rats through modulation of oxidative stress genes expression. Chem Biol Interact 2011;192:233–42.10.1016/j.cbi.2011.03.014Search in Google Scholar PubMed
7. Hao HH, Shao ZM, Tang DQ, Lu Q, Chen X, Yin XX, et al. Preventive effects of rutin on the development of experimental diabetic nephropathy in rats. Life Sci 2012;91:959–67.10.1016/j.lfs.2012.09.003Search in Google Scholar PubMed
8. İbrahim DS, El-Maksoud MA. Effect of strawberry (Fragaria x ananassa) leaves extract on diabetic nephropathy in rats. Int J Exp Path 2015;96:87–93.10.1111/iep.12116Search in Google Scholar PubMed PubMed Central
9. Dey A, Lakshmanan J. The role of antioxidants and other agents in alleviating hyperglycemia mediated oxidative stress and injury in liver. Food Funct 2013;4:1148–84.10.1039/c3fo30317aSearch in Google Scholar PubMed
10. Ueland PM, Holm PI, Hustad S. Betaine: a key modulator of one-carbon metabolism and homocysteine status. Clin Chem Lab Med 2005;43:1069–75.10.1515/CCLM.2005.187Search in Google Scholar PubMed
11. Day CR, Kempson SA. Betaine chemistry, roles, and potential use in liver disease. Biochim Biophys Acta 2016;1860:1098–106.10.1016/j.bbagen.2016.02.001Search in Google Scholar
12. Lv S, Fan R, Du Y, Hou M, Tang Z, Ling W, et al. Betaine supplementation attenuates atherosclerotic lesion in apolipoprotein E-deficient mice. Eur J Nutr 2009;48:205–12.10.1007/s00394-009-0003-4Search in Google Scholar
13. Balkan J, Öztezcan S, Küçük M, Çevikbaş U, Koçak-Toker N, Uysal M. The effect of betaine treatment on triglyceride levels and oxidative stress in the liver of ethanol-treated guinea pigs. Exp Toxicol Pathol 2004;55:505–9.10.1078/0940-2993-00347Search in Google Scholar
14. Kwon DY, Jung YS, Kim SJ, Park HK, Park JH, Kim YC. Impaired sulfur-amino acid metabolism and oxidative stress in nonalcoholic fatty liver are alleviated by betaine supplementation in rats. J Nutr 2009;139:63–8.10.3945/jn.108.094771Search in Google Scholar
15. Balkan J, Parıldar FH, Doğru-Abbasoğlu S, Aykaç-Toker G, Uysal M. The effect of taurine or betaine pretreatment on hepatotoxicity and prooxidant status induced by lipopolysaccharide treatment in the liver of rats. Eur J Gastroenterol Hepatol 2005;17:917–21.10.1097/00042737-200509000-00006Search in Google Scholar
16. Bingül İ, Başaran-Küçükgergin C, Aydın AF, Çoban J, Doğan-Ekici I, Doğru-Abbasoğlu S, et al. Betaine treatment decreased oxidative stress, inflammation, and stellate cell activation in rats with alcoholic liver fibrosis. Environ Toxicol Pharmacol 2016;45:170–8.10.1016/j.etap.2016.05.033Search in Google Scholar
17. Ozturk F, Ucar M, Ozturk IC, Vardi N, Batcioglu K. Carbon tetrachloride-induced nephrotoxicity and protective effect of betaine in Sprague-Dawley rats. Urology 2003;62:353–6.10.1016/S0090-4295(03)00255-3Search in Google Scholar
18. Go EK, Jung KJ, Kim JY, Yu BP, Chung HY. Betaine suppresses proinflammatory signaling during aging: The involvement of nuclear factor-kB via nuclear factor-inducing kinase/IkB kinase and mitogen-activated protein kinases. J Gerontol 2005;60:1252–64.10.1093/gerona/60.10.1252Search in Google Scholar PubMed
19. Fan CY, Wang MX, Ge CX, Wang X, Li JM, Kong LD. Betaine supplementation protects against high-fructose-induced renal injury in rats. J Nutr Biochem 2014;25:353–62.10.1016/j.jnutbio.2013.11.010Search in Google Scholar PubMed
20. Hagar H, El Medany A, Salam R, El Medany G, Nayal OA. Betaine supplementation mitigates cisplatin-induced nephrotoxicity by abrogation of oxidative/nitrosative stress and suppression of inflammation and apoptosis in rats. Exp Toxicol Pathol 2015;67:133–41.10.1016/j.etp.2014.11.001Search in Google Scholar PubMed
21. Wijeekoon EP, Hall B, Ratnam S, Brosnan ME, Zeisel SH, Brosnan JT. Homocysteine metabolism in ZDF (type 2) diabetic rats. Diabetes 2005;54:3245–51.10.2337/diabetes.54.11.3245Search in Google Scholar
22. Lever M, Slow S, McGregor DO, Dellow WJ, George PM, Chambers ST. Variability of plasma and urine betaine in diabetes mellitus and its relationship to methionine load test responses: an observational study. Cardiovasc Diabetol 2012;11:34.10.1186/1475-2840-11-34Search in Google Scholar
23. Jeong JJ, Kim YT, Seo WS, Yang HJ, Lee YS, Cha JY. Hypoglycemic and hepatoprotective effects of betaine on streptozotocin-induced diabetic rats. J Life Sci 2006;16:767–72.10.5352/JLS.2006.16.5.767Search in Google Scholar
24. Jung GY, Won SB, Kim J, Jeon S, Han A, Kwon YH. Betaine alleviates hypertriglycemia and tau hyperphosphorylation in db/db mice. Toxicol Res 2013;29:7–14.10.5487/TR.2013.29.1.007Search in Google Scholar
25. Cicero AF, Tartagni E, Ertek S. Metformin and its clinical use: new insights for an old drug in clinical practice. Arch Med Sci 2012;8:907–17.10.5114/aoms.2012.31622Search in Google Scholar
26. Bhat A, Sebastiani G, Bhat M. Systematic review: Preventive and therapeutic applications of metformin in liver disease. World J Hepatol 2015;7:1652–59.10.4254/wjh.v7.i12.1652Search in Google Scholar
27. Coperchini F, Leporati P, Rotondi M, Chiocato L. Expanding the therapeutic spectrum of metformin: fromd iabetes to cancer. J Endocrinol Invest 2015;38:1047–55.10.1007/s40618-015-0370-zSearch in Google Scholar
28. Waisundara VY, Hsu A, Huang D, Tan BK. Scutellaria baicalensis enhances the anti-diabetic activity of metformin in streptozotocin-induced diabetic Wistar rats. Am J Chin Med 2008;36:517–40.10.1142/S0192415X08005953Search in Google Scholar
29. Zheng T,Shu G, Yang Z, Mo S, Zhao Y, Mei Z. Antidiabetic effect of total saponins from Entadaphaseoloides (L.) Merr. İn type 2 diabetic rats. J Ethnopharmacol 2012;139:814–21.10.1016/j.jep.2011.12.025Search in Google Scholar
30. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54.10.1016/0003-2697(76)90527-3Search in Google Scholar
31. Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med 1999;27:612–6.10.1016/S0891-5849(99)00107-0Search in Google Scholar
32. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95:351–8.10.1016/0003-2697(79)90738-3Search in Google Scholar
33. Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med 1963;61:882–8.Search in Google Scholar
34. Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’’: the FRAP assay. Anal Biochem 1996;239:70–6.10.1006/abio.1996.0292Search in Google Scholar
35. Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Anal Biochem 1985;150:76–85.10.1016/0003-2697(85)90442-7Search in Google Scholar
36. Dey A, Swamaminathan K. Hyperglycemia-induced mitochondrial alterations in liver. Life Sci 2010;87:197–214.10.1016/j.lfs.2010.06.007Search in Google Scholar
37. Parvizi MR, Parviz M, Tavangar SM, Soltani N, Kadkhodaee M, Seifi B, et al. Protective effect of magnesium on renal function in STZ-induced diabetic rats. J Diabetes Metab Disord 2014;14:84.10.1186/s40200-014-0084-3Search in Google Scholar
38. Giannarelli R, Aragona M, Coppelli A, Del Prato S. Reducing insulin resistance with metformin: the evidence today. Diabetes Metab 2003;29:6S28–35.10.1016/S1262-3636(03)72785-2Search in Google Scholar
39. Tzeng TF, Liou SS, Chang CJ, Liu IM. The ethanol extract of Zingiber zerumbet attenuates streptozotocin-induced diabetic nephropathy in rats. Evid Based Complement Alternat Med 2013;2013:340645.10.1155/2013/340645Search in Google Scholar PubMed PubMed Central
40. Kim SK, Seo JM, Chae YR, Jung YS, Park JH, Kim YC. Alleviation of dimethyl nitrosamine-induced liver injury and fibrosis by betaine supplementation in rats. Chem Biol Interact 2009;177:204–11.10.1016/j.cbi.2008.09.021Search in Google Scholar PubMed
41. Caro AA, Cederbaum AI. Antioxidant properties of S-adenosyl-methionine in Fe2+ initiated oxidations. Free Radic Biol Med 2004;36:1303–16.10.1016/j.freeradbiomed.2004.02.015Search in Google Scholar PubMed
42. Zhao L, Gao H, Zhao Y, Lin D. Metabolomic analysis of the therapeutic effect of Zhibai Dihuang Pill in treatment of streptozotocin-induced diabetic nephropathy. J Ethnopharmacol 2012;142:647–56.10.1016/j.jep.2012.05.031Search in Google Scholar PubMed
43. Chen L, Chen YM, Wang LJ, Wei J, Tan YZ, Zhou JY, et al. Higher homocysteine and lower betaine increase the risk of microangiopathy in patients with diabetes mellitus varying the GG genotype of PEMT G774C. Diabetes Metab Res Rev 2013;29:607–17.10.1002/dmrr.2432Search in Google Scholar PubMed
44. Schartum-Hansen H, Ueland PM, Pedersen ER, Meyer K, Ebbing M, Bleie Ø, et al. Assesment of urinary betaine as a marker of diabetes mellitus in cardiovascular patients. PLoS One 2013;8:e69454.10.1371/journal.pone.0069454Search in Google Scholar PubMed PubMed Central
45. Kim YG, Lim HH, Lee SH, Shin MS, Kim CJ, Yang HJ. Betaine inhibits vascularization via suppression of Akt in the retinas of streptozotocin-induced hyperglycemic rats. Mol Med Rep 2015;12:1639–44.10.3892/mmr.2015.3613Search in Google Scholar PubMed PubMed Central
©2018 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Research Articles
- Betaine treatment decreased serum glucose and lipid levels, hepatic and renal oxidative stress in streptozotocin-induced diabetic rats
- Oxidative stress and response of antioxidant system in Nostoc muscorum exposed to different forms of Zinc
- An investigation on Trakya region Oak (Quercus spp.) honeys of Turkey: their physico-chemical, antioxidant and phenolic compounds properties
- Effects of Shiranuhi flower extracts and fractions on lipopolysaccharide-induced inflammatory responses in murine RAW 264.7 cells
- Evaluation of resveratrol organogels prepared by micro-irradiation: fibroblast proliferation through in vitro wound healing
- Ecological and phytochemical attributes of endemic Ferula gummosa Boiss. at vegetative and generative stages
- Characterization of calmodulin in the clam Anodonta woodiana: differential expressions in response to environmental Ca2+ and Cd2+
- Foliar application effects of salicylic acid and jasmonic acid on the essential oil composition of Salvia officinalis
- Antioxidant and antimicrobial activities of four Astragalus species growing wild in Turkey
- Differences in structure, allergenic protein content and pectate lyase enzyme activity of some Cupressaceae pollen
- In vitro bioactivities and subacute toxicity study of O. basilicum, T. vulgaris and R. officinalis
- Wendlandia exserta: a pertinent source of antioxidant and antimicrobial agent
- Effects of epigallocatechin-3-gallate (EGCG) on a scleroderma model of fibrosis
Articles in the same Issue
- Frontmatter
- Research Articles
- Betaine treatment decreased serum glucose and lipid levels, hepatic and renal oxidative stress in streptozotocin-induced diabetic rats
- Oxidative stress and response of antioxidant system in Nostoc muscorum exposed to different forms of Zinc
- An investigation on Trakya region Oak (Quercus spp.) honeys of Turkey: their physico-chemical, antioxidant and phenolic compounds properties
- Effects of Shiranuhi flower extracts and fractions on lipopolysaccharide-induced inflammatory responses in murine RAW 264.7 cells
- Evaluation of resveratrol organogels prepared by micro-irradiation: fibroblast proliferation through in vitro wound healing
- Ecological and phytochemical attributes of endemic Ferula gummosa Boiss. at vegetative and generative stages
- Characterization of calmodulin in the clam Anodonta woodiana: differential expressions in response to environmental Ca2+ and Cd2+
- Foliar application effects of salicylic acid and jasmonic acid on the essential oil composition of Salvia officinalis
- Antioxidant and antimicrobial activities of four Astragalus species growing wild in Turkey
- Differences in structure, allergenic protein content and pectate lyase enzyme activity of some Cupressaceae pollen
- In vitro bioactivities and subacute toxicity study of O. basilicum, T. vulgaris and R. officinalis
- Wendlandia exserta: a pertinent source of antioxidant and antimicrobial agent
- Effects of epigallocatechin-3-gallate (EGCG) on a scleroderma model of fibrosis


