Abstract
Objective
The objective of the present study was to investigate the phenolic compositions, antioxidant, antimicrobial and cytotoxic activities of four Astragalus species, two of which are endemic to Turkish flora.
Methods
The total phenolic and flavonoid contents of methanol extracts obtained from four Astragalus species were detected using Folin-Ciocalteu and aluminum chloride colorimetric assays. Their phenolic compositions were identified by Liquid chromatography–mass spectrometry (LC-MS). The antioxidant activity was assayed with phosphomolybdenum, 2,2-diphenyl-1-picrylhydrazyl radical scavenging (DPPH), hydrogen peroxide scavenging, β-carotene bleaching activity, ferric-ion reducing power (FRAP) and cupric ions (Cu2+) reducing antioxidant capacity (CUPRAC) methods. Antimicrobial activities of the extracts were studied by agar well-diffusion assay. The cytotoxic effects of the extracts on MCF-7 (human breast cancer cell lines) were determined by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide) method.
Results
The extracts exerted moderate antioxidant and reducing activity with low phenolic contents. The main component in the extracts was determined as ferulic acid. The extracts demonstrated no antibacterial activity except P. aeruginosa. A. talasseus showed the highest cytotoxic activity on MCF-7 during 48 h.
Conclusion
It is believed that the results of this study will contribute to research recently increasing for the use of natural antioxidant and antimicrobial compounds in many industrial fields such as food, pharmacy and medicine.
Özet
Amaç
Bu çalışmanın amacı Türkiye florasına ait, 2’si endemik olmak üzere dört Astragalus türünün fenolik madde içeriği, antioksidan, antimikrobiyal ve sitotoksik aktivitelerini araştırmaktır.
Yöntem
Dört farklı Astragalus türünden elde edilen metanollü ekstrelerin toplam fenolik ve flavonoid madde miktarları Folin-Ciocalteu ve alüminyum klorid yöntemleri kullanılarak belirlenmiştir. Ekstrelerin fenolik bileşenleri Sıvı Kromatografisi- Kütle Spektrometresi kullanılarak belirlenmiştir. Antioksidan aktivite fosfomolibdenyum, 2,2-difenil-1-pikrilhidrazil radikal süpürücü (DPPH), hidrojen peroksit süpürücü, β-karoten beyazlatma aktivitesi, demir iyon indirgeyici güç (FRAP), indirgeyici güç ve bakır iyon (Cu2+) indirgeyici antioksidan kapasite (CUPRAC) yöntemleri kullanılarak belirlenmiştir. Ekstrelerin antimikrobiyal aktiviteleri agar difüzyon yöntemi ile çalışılmıştır. Sitotoksik aktiviteleri ise MTT yöntemi ile MCF-7 (insan meme kanser hücre hattı) kullanılarak belirlenmiştir.
Bulgular
Ekstreler düşük fenolik içerikleri ile orta seviyede antioksidan ve indirgeyici aktivite göstermişlerdir. Ekstrelerdeki ana bileşen ferulik asittir. Ekstreler P. aeruginosa hariç antibakteriyal aktivite göstermemiştir. A. talasseus MCF-7 üzerinde 48 saatlik uygulamada en yüksek sitotoksik aktiviteyi göstermiştir.
Sonuç
Sonuç olarak, çalışma sonuçlarının gıda, eczacılık ve tıp gibi birçok endüstriyel alanda doğal antioksidan ve antimikrobiyal bileşiklerin kullanımına yönelik son zamanlarda artan araştırmalara yardımcı olacağına inanılmaktadır.
Introduction
Humankind has used herbal products to obtain nutrients, medicines, oils and many other useful products throughout history. It is stated that there is a relation between a diet with high consumption of herbal products such as fruit and vegetables and a decrease in risk for many chronic diseases [1], [2]. The majority of the antioxidant capacity of a fruit or vegetable may be from phenolic compounds [3].
The oils and extracts obtained from plants have been used as natural agents in many industry such as food, pharmacy, alternative medicine and natural therapy because of their antimicrobial activity. In order to prolong the storage stability of foods, synthetic antioxidants are mainly used in industrial processing [4]. Recently, there has been a significant increase in the search for natural antioxidants to replace synthetic antioxidants in recent years [5]. Because the use of synthetic antioxidants which have many side effect such as cancer have limited. The antioxidative status is associated with reduced incidence of many diseases [6]. Thus, investigation and identification of new antioxidant compounds are very important.
The richest genus with 425 taxa is Astragalus L. (Fabaceae) in Turkish flora. Of these 425 taxa, 201 are endemic and the endemism rate is about 47% [7], [8], [9]. Researches on Astragalus species in Turkey have resulted in the isolation of cycloartane and triterpenoid saponins [10], [11], [12], [13], [14]. These compounds exert different biological activity such as immuno stimulant, antiviral, cardiovascular, anti-protozoal and cytotoxic [11], [15], [16]. The roots of Astragalus species are well-known in folk medicine due to their biological activities such as hepatoprotective, antioxidative, antibacterial, antiperspirant, antihypertensive, antidiabetic, diuretic and tonic [17], [18], [19], [20]. It has also been used in the treatment of diabetes mellitus, nephritis, leukemia and uterine cancer. In the district of Anatolia, located in South Eastern Turkey, an aqueous extract of the roots of Astragalus is traditionally used against leukemia and for its wound-healing properties [20].
Noteworthiness of Astragalus species are great due to have a high nutritional values, obtain gum glue as a pharmaceutical emulsifying agent, used as firewood and animal feedstuffs, and in erosion prevention [21].
Although biological activities of many Astragalus species have been investigated, the antioxidant and antimicrobial activities of A. gummifer, A. microcephalus, A. talasseus and A. acmophyllus have not previously been published as far as our literature survey could ascertain. With present study, it is aimed to examine the phenolic compositions, total phenolic and flavonoid contents, antioxidant, antimicrobial and cytotoxic activities of the methanol extracts obtained from the underground parts of four different Astragalus taxa, two of which are endemic in Turkey.
Materials and methods
Plant material
Locality information and collection time of the plants collected are listed below:
A. gummifer (Aksoy 2459), Kayseri-Talas, Ali Mountain, 38°40′20″N-35°33′ 854″ E, 1340 m, 15.08.2012.
A. microcephalus (Aksoy 2460), Kayseri-Talas, Ali Mountain, 38°40′20″ N-35°33′85″ E, 1340 m, 15.08.2012.
A. talasseus (Aksoy 2458), Kayseri-Talas, Ali Mountain, 38°40′20″ N-35°33′85″ E, 1340 m, 15.08.2012 (Endemic).
A. acmophyllus (Aksoy 2464), Kayseri-Erciyes Mountain, 38°32′06″ N-35°29′02″ E, 2850 m, 30.08.2012 (Endemic).
Extraction
Dried underground parts of the plants at room temperature were ground to a fine powder with a grinder. Then the powdered plant material (10 g) was extracted using a Soxhlet type xtractor with 100 mL methanol at 60°C for 6 h. Thereafter, the extract was filtered and evaporated to dryness under vacuum at 40°C with a rotary evaporator. The extract yield was calculated and stored at 4°C [22].
LC-MS analysis of phenolic compounds in the extracts
A liquid chromatography was equipped with electrospray ion sources mass spectrometer working with Mass Hunter software package. Zorbax SB-C18 (150×2.1 mm, 1.8 μm) column was used. The flow rate was 0.25 mL/min and the injection volume 10 μL. Positive and negative ion mode was performed. The nitrogen temperature was 350°C at a flow rate of 8 L/min. The mobile phase composition was: (5/95:h/h) methanol:water (eluent A) and methanol (eluent B) both containing 0.01% formic acid and 5 mM ammonium formate. The gradient program was as follows: 5% B (0–1 min), 30% B (1–3 min), 60% B (3–4 min), 60% B (4–5 min), 70% B (5–6 min), 80% B (6–8 min), 5% B (8.01 min), 5% B (8.01–10 min).
Gallic acid, cinnamic acid, caffeic acid, ferulic acid, chlorogenic acid, protocatechuic acid, ellagic acid, catechin hydrate, epicatechin, epigallocatechin gallate, rosmarinic acid, syringic acid, quercetin, quercetin-3-β-D-glucoside, myricetin, phloridzin hydrate, rutin, p-coumaric acid, o-coumaric acid, kaempferol, bergapten and psoralen were used as standard [22].
Determination of total phenolic
Folin-Ciocalteau assay was performed to detect of the total phenolic amount in the extract [23]. Forty microliter aliquot of the extract (1 mg/mL) was mixed with 200 μL of Folin-Ciocalteu reagent and 600 μL (20% Na2CO3) of sodium carbonate. The mixture was vortexed and incubated at room temperature for 2 h. Absorbance was then read at 765 nm by the spectrophotometer. The standard curve was formed with gallic acid. Total phenolic was expressed in terms of milligrams of gallic acid equivalents (mg GAE)/g extract.
Total flavonoid
Total flavonoid was assessed using Aluminum chloride colorimetric assay according to Pourmorad et al. [24]. The extract (0.5 mL) in methanol were separately mixed with 1.5 mL of methanol, 0.1 mL of 10% aluminum chloride, 0.1 mL of 1 M potassium acetate and 2.8 mL of distilled water. It remained at room temperature for 30 min; the absorbance of the reaction mixture was measured at 415 nm. The result is expressed as mg of quercetin equivalents (QE)/g extract.
Antioxidant activity
Total antioxidant activity
The antioxidant activity of methanolic extracts was determined by the phosphomolybdenum method [25]. 0.4 mL of the methanolic extract (1 mg/mL) was mixed with 4 mL of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The tubes were capped and incubated in water bath at 95°C for 90 min. After the samples had cooled to room temperature, the absorbance of the green phosphomolybdenum complex was measured at 695 nm. In the case of the blank, 0.4 mL of methanol was used in place of sample. The antioxidant activity was determined using a standard curve with ascorbic acid solutions as the standard. The antioxidant activity was expressed as mg of ascorbic acid equivalents (AAE)/g extract.
DPPH method
The ability of the extracts to scavenge DPPH radical was assessed spectrophotometrically [26]. Fifty microliter aliquots of the proper extract dilution at a concentration range of 0.1–2 mg/mL was mixed with and 1 mL of the methanolic DPPH solution (0.1 mM). Methanol was used as a control instead of extract. The mixtures were left for 30 min at room temperature in the dark and the absorbance at 517 nm measured using methanol as blank. IC50 (concentration causing 50% inhibition) value of the extract was determined graphically. The same procedure was repeated with BHT (butylated hydroxytoluene) as a positive control. The measurements were performed in triplicate and the results were averaged. Radical scavenging activity was expressed as percentage inhibition of DPPH radical and was calculated by following equation:
β-Carotene bleaching assay
Lipid peroxidation inhibitory effect in β-carotene bleaching system was assessed [27]. β-Carotene (10 mg) was dissolved in 10 mL of chloroform (CHCl3). An aliquot (0.2 mL) of this solution was added into a boiling flask containing 20 mg of linoleic acid and 200 mg of Tween 40. The chloroform was removed using a rotary evaporator at 40°C for 5 min. Distilled water (50 mL) was slowly added to the residue with vigorous agitation, to form an emulsion. The emulsion (5 mL) was added to a tube containing 0.2 mL of the extract solution (2 mg/mL). The test emulsion was incubated in a water bath at 50°C for 2 h, when the absorbance was measured at 470 nm. BHT was used as the positive control.
CUPRAC assay
The cupric ion reducing antioxidant activity of extract was detected [28]. One milliliter each of 10 mM CuCl2, 7.5 mM neocuproine and NH4Ac buffer (1 M, pH 7.0) solutions were added into a test tube. Then, 0.5 mL of different concentrations of extracts (0.2–1 mg/mL) were mixed. The mixture absorbance was recorded against a blank at 450 nm after 30 min incubation at room temperature. Trolox was used as the positive control.
FRAP assay
Ferric ions reducing capacity of the extract was examined [29]. FRAP is based on the reduction of ferric 2,4,6-tris(2-pyridyl)-1,3,5-triazine [Fe(III)-TPTZ] to the ferrous complex at low pH. FRAP reagent was made by mixing an equivalent volume of 300 mM acetate buffer (pH 3.6), 20 mM FeCl3·6H2O with 10 mM TPTZ in 40 mM HCl. Briefly, an aliquot of each plant extract was added to diluted FRAP reagent and incubated at 37°C during 30 min and the absorbance was read at 595 nm. The results were expressed as mmol/L of Fe2+.
H2O2 scavenging activity
The extract (25–500 μg/mL) was mixed with 43 mM H2O2 solution. After 10 min, the absorbance of the solution was read at 230 nm [30]. Gallic acid, BHA and BHT were used as control. The percentages of scavenged hydrogen peroxide of extract and standards were calculated using the following equation:
IC50 (concentration causing 50% inhibition) values of the extract and standards were determined graphically.
Reducing power
The reducing power of the methanolic extract was detected [31]. The extracts (0.5–10 mg/mL) were added to 2.5 mL potassium ferricyanide [K3Fe(CN)6] and was stored for 20 min at 50°C. Then, the solution was centrifuged at 1000 g for 10 min after trichloroacetic acid (2.5 mL) was added. The upper layer solution was added to FeCl3 (0.5 mL, 0.1%, w/v). The absorbance was read at 700 nm. BHT was used as control. Higher absorbance of the reaction mixture indicated greater reducing power.
Chelating activity on ferrous ion
Ferrous ions (Fe2+) chelating activity of the extract was examined [30]. Aliquots of the extract (5 mg/mL) were added to FeCl2 (2 mM, 0.1 mL). After incubation, ferrozine (5 mM and 0.2 mL) was added to the reaction solution. The absorbance was read at 562 nm. A lower absorbance indicates a higher chelating power. The chelating activity of the extract on Fe2+ was compared with that of ethylenediamine tetraacetic acid (EDTA). Chelating activity was calculated by the following formula:
Determination of antimicrobial activity
The following microorganisms were tested: Aeromonas hydrophila ATCC 7965, Bacillus cereus RSKK 863, Bacillus subtilis ATCC 6633, Escherichia coli ATCC 25922, Escherichiacoli O157:H7, Klebsiella pneumoniae ATCC 27736, Listeria monocytogenes 1/2B, Mycobacterium smegmatis RUT, Morganella morganii, Proteus mirabilis BC 3624, Pseudomonas aeruginosa ATCC 27853, Salmonella typhimurium NRRLE 4463, Staphylococcus aureus ATCC 29213, Yersinia enterocolitica ATCC 1501 and Candida albicans ATCC1223.
Agar-well diffusion assay was performed to detect of antimicrobial activity [22]. Suspensions of each microorganism were regulated to 106–107 colony-forming units (cfu)/mL and added to sterile growth medium. The mix was poured into Petri plates (9 cm). The wells (5 mm in diameter) were cut from the agar. The extracts were prepared at 1%, 2.5%, 5% and 10% concentrations in absolute methanol and 50 µL of extract solutions were applied to the wells. The growth inhibition zones were measured in millimeters. Inhibition zones of standard antibiotics namely Tetracycline TE-30 (10 mg/mL) and Oxacillin OX-5 (10 mg/mL) were compared.
MTT assay
MCF-7 (Human breast cancer) cell line was incubated in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with heat-inactivated 100 mL/L fetal bovine serum (Gibco), 100 U/mL penicillin, 100 mg/mL streptomycin. Fibroblast cell line was incubated in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 2 mM glutamine. The cells were cultured in a humidified atmosphere of 5% CO2 at 37°C, and the medium was changed every other day.
The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide) assay was used to examine effects of four Astragalus extracts on the cell growth and survival [32]. 1×104 cells were dispersed to a 96 well plate. After 24 h incubation, the cells were treated with extracts (10, 25, 50, 100, 200 and 400 μg/mL) and incubated for 24 and 48 h. Then 10 mL of MTT in PBS (5 mg/mL) was added into each well and the cells were further incubated for 4 h. The MTT formazan crystals were solubilized by adding 200 mL of DMSO to each well. Absorbance was measured at 570 nm with Micro-plate Reader. The absorbance of the control cells was taken as 100% viability. The results are expressed as percentages of viable cells versus the respective controls.
Statistical analysis
Data from the experiments were subjected to analysis of variance (ANOVA) using SPSS [33] for Windows. Percentage data were transformed using arcsine √x before ANOVA. Means were separated at the 5% significance level by the least significant difference (LSD) test. Bivariate correlations were analyzed by Pearson’s test using SPSS 10.0 on Windows.
Results and discussion
The yields of methanolic extracts ranged from 9.78 to 16.38%. While the extract obtained from A. acmophyllus had the highest yield, A. talasseus had the least extract yield (Table 1). Identification of the phenolic compositions of the extracts was carried out using Liquid chromatography–mass spectrometry (LC-MS)/MS. The amounts of phenolic compounds in the extracts were calculated as ppm and the results are given in Table 2.
The yields, total phenolic contents, total flavonoid contents and antioxidant activities of Astragalus species.
| Astragalus species | Yield (%) | Total phenolic (mg GAE/g) | Total flavonoid (mg QE/g) | Antioxidant activity (mgAAE/g) | β-Carotene % inhibition | DPPH IC50 (μg/mL) | H2O2 IC50 (μg/mL) | FRAP (mM/L) |
|---|---|---|---|---|---|---|---|---|
| A. gumnifer | 13.49 | 8.47±0.4b* | 1.01±0.0b | 131.37±0.2d | 61.19±2.99c | 195.19c | 189.51b | 0.76b |
| A. microcephalus | 10.84 | 13.49±0.0a | 2.19±0.0a | 150.67±0.4c | 74.63±2.99b | 86.67d | 149.86c | 0.97a |
| A. talasseus | 9.78 | 6.56±0.6c | 0.83±0.0c | 154.91±0.1b | 80.60±2.58a | 211.66b | 188.57b | 0.66c |
| A. acmophyllus | 16.38 | 5.49±0.3d | 0.76±0.0d | 178.25±0.3a | 54.23±0.86d | 253.88a | 220.03a | 0.60d |
*In each column, means of three independent experiments (±standard deviation) with different superscript letters are significantly different (p<0.05). Total phenolic content expressed as gallic acid equivalent (GAE), total flavonoid content expressed as quercetin equivalent (QE), total antioxidant activity expressed as ascorbic acid equivalent (AAE).
The quantity of some phenolic compounds (ppm) determined in Astragalus extracts by LC-MS.
| Compounds | A. gummifer | A. microcephalus | A. talasseus | A. acmophyllus | Total |
|---|---|---|---|---|---|
| Chlorogenic acid | – | – | 130 | 241 | 371 |
| Epicatechin | – | – | 101 | 210 | 311 |
| Catechin hidrate | – | – | 106 | 201 | 307 |
| Rutin | 114.52 | – | – | – | 114.52 |
| Quercetin | – | 293.5 | – | – | 293.5 |
| Kaempferol | – | 316.2 | – | – | 316.2 |
| Syringic acid | 418.08 | – | 317.1 | – | 735.18 |
| Sinnamic acid | – | – | 186 | 372 | 558 |
| Ferulic acid | 206.8 | 327.1 | 210 | 380 | 1123.9 |
| Total | 739.4 | 936.8 | 1050.1 | 1404 |
–, Not detected.
The total amounts of phenolic in the extracts evaluated by the Folin-Ciocalteu assay were indicated as gallic acid equivalent (GAE) and the results are given in Table 1. A. microcephalus (13.49±0.0 mg GAE/g extract) had the highest phenolic content, while A. acmophyllus (5.49±0.3 mg GAE/g extract) had the least amount of phenolic.
The amount of total flavonoid in the extracts measured by the aluminum chloride colorimetric assay was expressed as the quercetin equivalent (mg QE/g extract) and the results are given in Table 1. As shown in Table 1, A. microcephalus had the highest flavonoid content (2.19±0.0 mg QE/g extract), while A. acmophyllus (0.76±0.0 mg QE/g extract) had the least amount of flavanoid among the extracts.
The phenolic compositions of the Astragalus extracts tested were examined by LC-MS (Table 2). The amount of each compound is demonstrated as ppm. Phenolic compounds couldn’t be determined in the extracts not shown in the table. Chlorogenic acid, epicatechin, catechin hidrate, rutin, quercetin, kaempferol, syringic acid, sinnamic acid and ferulic acid were identified. The main component in the extracts was determined as ferulic acid (1123.9 ppm) followed by syringic acid (735.18 ppm) and sinnamic acid (558 ppm). The least abundant compound was quercetin (293.5 ppm).
The total antioxidant activity of the extracts measured by the phosphomolybdenum method is indicated by the ascorbic acid equivalent (mg AAE/g extract) and the results are shown in Table 1. A. gumnifer (131.37±0.2 mg AAE/g extract) had the lowest total antioxidant activity while A. acmophyllus (178.25±0.3 mg AAE/g extract) had the highest total antioxidant activity among the extracts.
The percent inhibition values of the extracts caused on the oxidation of the β-carotene-linoleic acid emulsion are given in Table 1. As shown in Table 1, A. talasseus had the highest activity (80.60%) and A. acmophyllus (54.23%) had the lowest activity among the extracts. % Inhibition values of the extracts were lower than the inhibition values of BHA (107.96±1.72%) and BHT (105.47±2.28%).
The antiradical activities were tested by the DPPH assay, and the results were expressed as % inhibition. The results are shown in Figure 1. The obtained values were compared with the standard antioxidant BHT. The concentration causing the 50% inhibition (IC50) was detected by plotting the graph including percent inhibition values versus concentrations for each extract (Table 1). A. acmophyllus had the lowest DPPH scavenging effect (IC50=253.88 μg/mL), A. microcephalus had the highest DPPH scavenging effect (IC50=86.67 μg/mL).
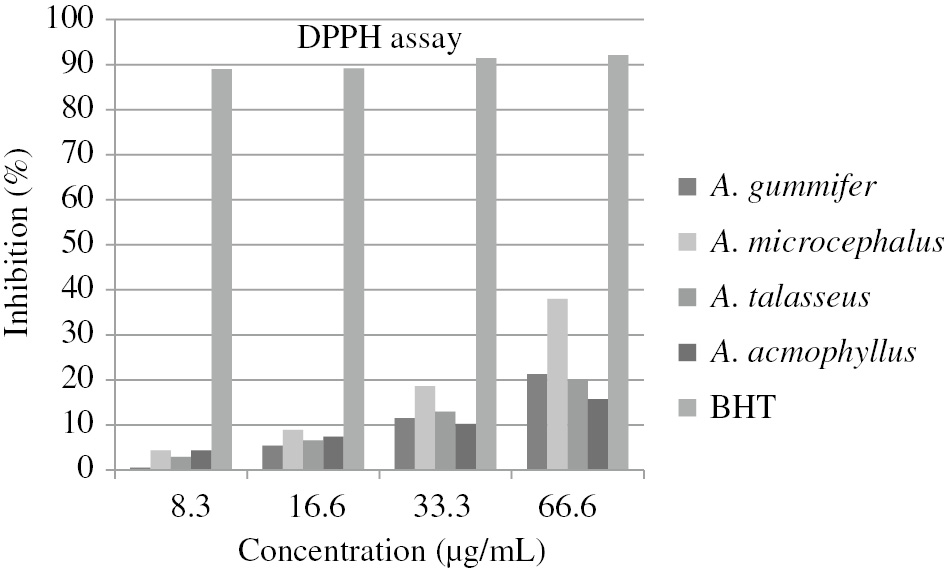
% Inhibition values of Astragalus extracts and BHT as positive control by DPPH assay.
The reduction activity of the copper (II)-neopurin complex of the extracts to the copper (I)-neucuproin complex was measured by the CUPRAC method [28]. The resulting complex gives maximum absorbance at 450 nm. The results are shown in Figure 2 as absorbance values at 450 nm and compared with the values of Trolox. As shown in Figure 2, A. microcephalus (0.83) exerted the highest absorbance value among the extracts at 1 mg/mL, whereas A. acmophyllus (0.32) showed the lowest absorbance value. Depending on the concentration, the absorbance and activity were increased. The absorbance values of the extracts were much lower than that of the trolox (2.85) used as control.
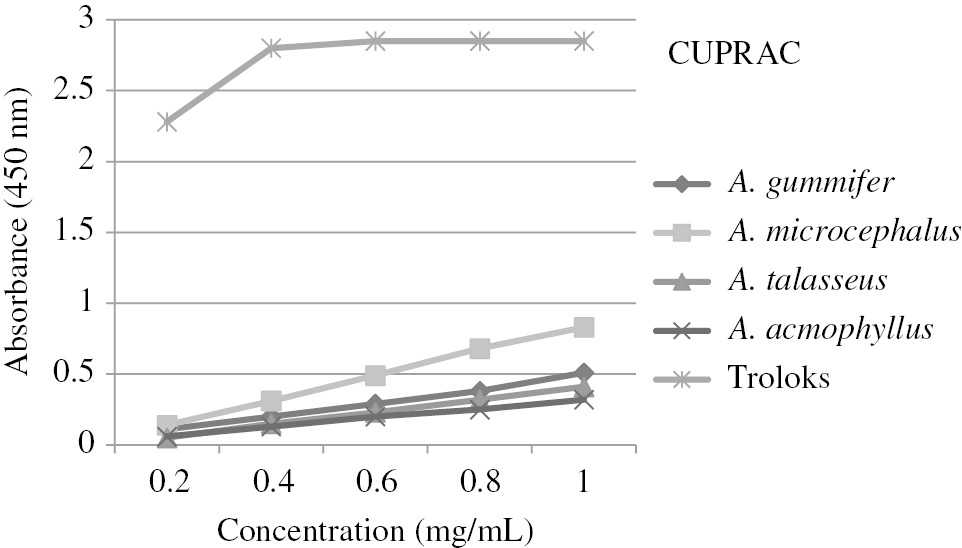
Antioxidant capacity of Astragalus extracts by CUPRAC method.
FRAP is commonly used to measure of reduction of ferric iron (Fe3+) to ferrous iron (Fe2+) in the presence of antioxidants [34]. The results are expressed as mM/L (Table 1). The obtained values were compared with the value of a standard L-ascorbic acid. A. microcephalus (0.97 mM/L) had the highest reducing power while A. acmophyllus had the least activity (0.60 mM/L) among the extracts. The iron ion-reducing powers of extracts were very lower than that of L-ascorbic acid (4.25 mM/L).
The hydrogen peroxide scavenging activity of the extracts was tested; the results are expressed as % inhibition and compared with the % inhibition values of BHT, BHA and Gallic acid. The % inhibition values of the extracts are shown in Figure 3. It was observed that hydrogen peroxide scavenging activity increased with increasing concentration. The concentrations (IC50) causing 50% inhibition were detected by plotting the graph including percent inhibition values versus concentrations for each extract and values were given in Table 1. A. acmophyllus (IC50=220.03 μg/mL) had the least hydrogen peroxide scavenging activity, while A. microcephalus (IC50=149.86 μg/mL) had the highest hydrogen peroxide scavenging activity. It has been observed that the scavenging activities of the extracts are much lower than that of the standards (IC50=31.09 for BHT, IC50=23.16 for BHA, and IC50=17.62 for gallic acid).
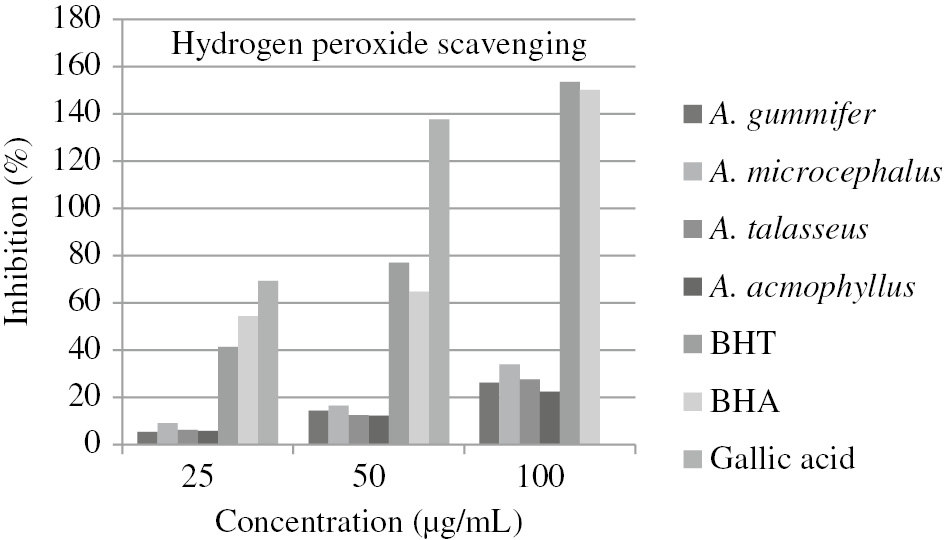
% Inhibition values of the Astragalus extracts and standards by H2O2 assay.
The reducing powers of the extracts have been measured. In this bioanalytical method used in antioxidant studies, the color of test solution transforms into green color due to the reducing activities of the antioxidant substances in the medium [35]. The results are shown in Figure 4 as absorbance values at 700 nm. The obtained values were compared with the values of standard antioxidant, BHT. A. microcephalus (2.172) had the highest absorbance value while A. gummifer had the least absorbance value (1.118) among the extracts at 10 mg/mL. Increased absorbance value indicates increased antioxidant activity. The reducing power of A. microcephalus was quite close to that of BHT (2.249) at this concentration.
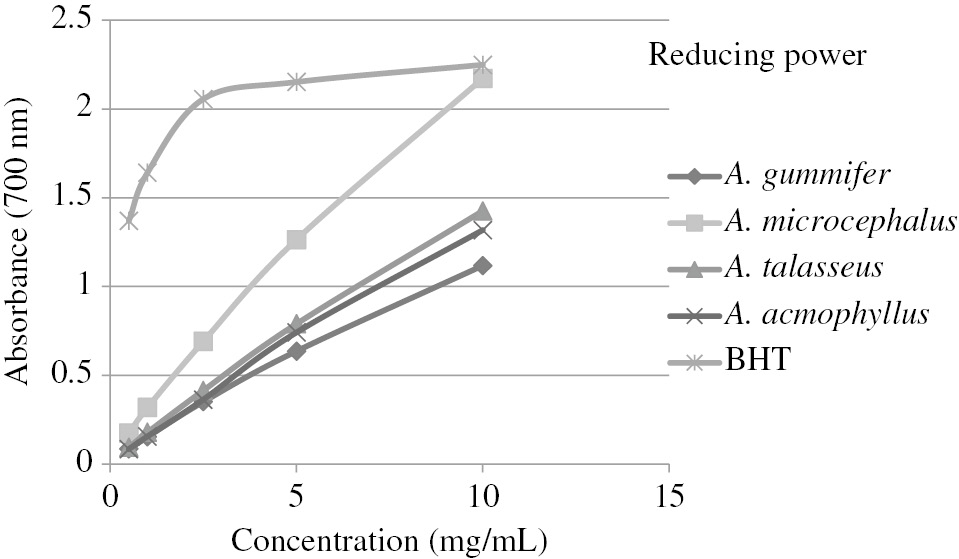
Reducing power of methanolic extracts from Astragalus species.
The metal chelating activity of the extracts was measured at 5 mg/mL and the results are indicated as % inhibition (Figure 5). A. acmophyllus had the highest activity (68.35%) while A. gummifer (43.88%) had the least activity among the extracts. % Inhibition values of A. gummifer, A. microcephalus, A. talasseus, A. acmophyllus and EDTA were found to be 43.88%, 65.47, 68.1, 68.35 and 99.45%, respectively.
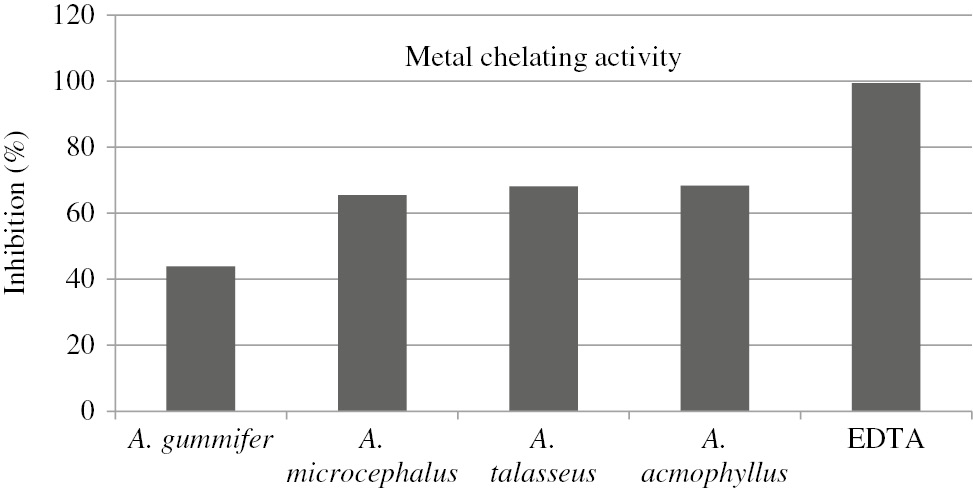
Chelating effects of Astragalus extracts on Fe2+ ion.
Asgarpanah et al. [36] showed that the methanolic extract obtained from aerial parts of A. squarrosus contained low phenolic (23.3 mg/g) and flavonoid (26.0 mg/g) contents thus had weak antiradical activity by DPPH assay. Thirty phenolic compounds in A. membranaceus were determined by Jun et al. [15]. Similarly, rutin, chlorogenic acid, kaempferol, ferulic acid and syringic acid were detected in Astragalus taxa tested in our study. Adıgüzel et al. [37] reported that the A. microcephalus, A. erinaceus, A. psoraloides, A. macrocephalus, and A. argyroides were strong DPPH scavenger (IC50=20.3–38.0 μg/mL), strong inhibitor on β-carotene-linoleic acid oxidation (23.8–88.1%) and extracts had inhibitory effect on the microorganisms tested. In our study, % inhibition on β- carotene-linoleic acid oxidation and IC50 values on DPPH assay of Astragalus taxa tested varied 54.23–80.60% and 86.67–253.88 μg/mL, respectively.
A. sinicus acetone extract exerted antiradical activity (95.1% at 10.0 mg/mL) and chelating effect (93.2%, at 10.0 mg/mL) with 279.9 and 175.2 mg/g total phenolic and flavonoid contents. Also, the reducing powers of ethanol, hexane, and water extract of A. sinicus were increased from 0.06 to 0.79, 0.08 to 0.69, and 0.09 to 0.39 OD 700 nm, when the concentrations were increased from 0.5 to 5 mg/mL, respectively [17]. According to our literature survey, ferric reducing power by FRAP and cupric reducing power by CUPRAC assay of Astragalus taxa studied were determined with this study at first time.
The antimicrobial activities of the extracts were studied by agar diffusion assay against 15 microorganisms at 1, 2.5, 5 and 10% concentrations (Table 3). The tested Astragalus extracts showed low antibacterial activity against only P. aeruginosa among the tested bacteria (6.5–8.0 mm). It was also observed that none of the extracts were effective on C. albicans.
Antibacterial activity of Astragalus extracts and standard antibiotics (mm, inhibition zones) against P. aeruginosa.
| % Concentration | A. gummifer | A. microcephalus | A. talasseus | A. acmophyllus | Tetracycline (10 mg/mL) | Oxacillin (10 mg/mL) |
|---|---|---|---|---|---|---|
| 10 | 8.0 | 8.0 | 7.0 | 7.0 | 18.0 | 12.0 |
| 5.0 | 7.0 | 7.0 | 7.0 | 6.5 | ||
| 2.5 | – | 7.0 | – | 6.5 | ||
| 1.0 | – | – | – | – |
–, Not detected.
Cytotoxic effects of methanol extracts from four Astragalus species on MCF-7 (Human Mammary Metastatic Carcinoma Cells) and Fibroblast cells were assessed by MTT assay. The extracts were applied to MCF-7 and Fibroblast cells for 24 and 48 h at 10, 25, 50, 100, 200 and 400 μg/mL. % Viability values were calculated. The results are graphically showed in Figure 6. When MCF-7 cells were treated for 24 h at 50 μg/mL, percentages of cell viability values were 72.94, 72.88, 66.74 and 72.92% for A. gummifer, A. microcephalus, A. talasseus and A. acmophyllus, respectively. Whereas, for 48 h of A. gummifer, A. microcephalus, A. talasseus and A. acmophyllus, the percentage of cell viability values were 87.51, 92.95, 58.91 and 64.55%, respectively. There was no inhibition on the growth of fibroblast cell lines, used as positive control, when incubated with all concentration of the extracts for 48 h. For 24 h incubation, only A. gummifer and A. talasseus extracts exhibited minor inhibition on the growth of fibroblast cell lines at 25–200 μg/mL. A. talasseus and A. acmophyllus exhibited better cytotoxic effects against MCF-7 cell lines tested for 48 h at 50–400 μg/mL. To best of our knowledge, this study demonstrated for the first time the cytotoxic potential on MCF-7 cell lines of four Astragalus taxa tested in here.
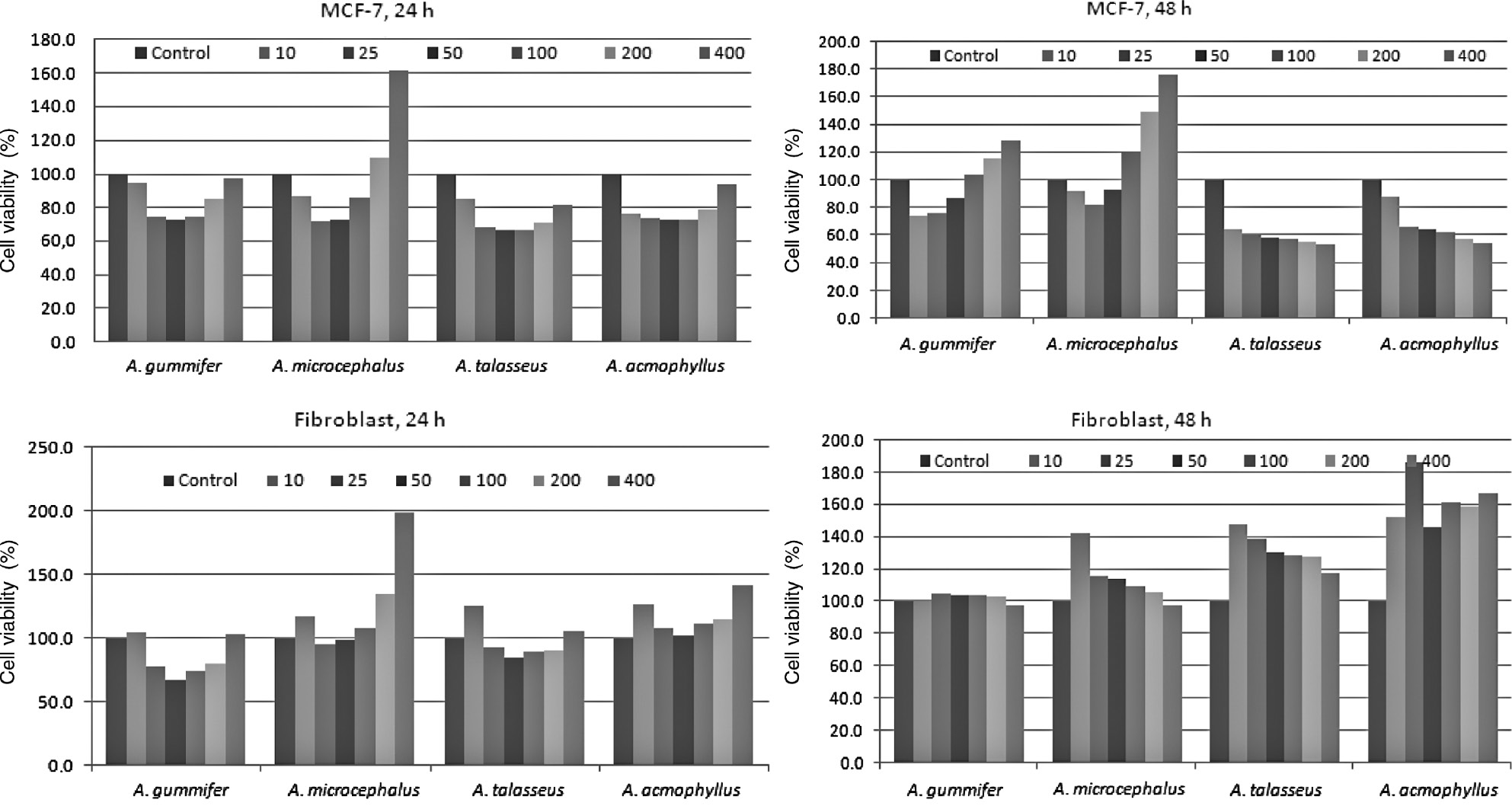
The cytotoxic effects of Astragalus extracts on proliferation of MCF-7 and Fibroblast cells for 24 and 48 h.
The antimicrobial activities of the several crude extracts from the aerial parts of A. verrucosus against S. aureus, Enterobacter agglomerans, E. coli, Salmonella infantis and P. aeruginosa were studied by Pistelli et al. [38] but no activity were observed. Türker and Yıldırım [39] showed that aquous, ethonaol and methanol extracts of A. brachypterus had strong antibacterial effects against Streptococcus pyogenes. The flavonoids from A. adsurgens had inhibitory effect on E. coli, B. cereus, S. aureus, Erwinia carotovora and Bacillus subtilis (MICs=7.8 to 31.3 μg/mL) and also exhibited moderate cytotoxic activity against HL-60 and SMMC-7721 cells (IC50=5 and 10 μg/mL) [18]. These differences may be attributed to the genotypic variation, climatic conditions, using different bacteria, different activity assay and different extract concentrations [40].
Ionkova et al. [41], showed the cytotoxic activities of saponins isolated from A. membranaceus against five different cancer cell lines. It has been detected that dichloromethane extracts of A. gombiformis showed strong cytotoxic effect against the human A549 lung epithelial carcinoma cell line (IC50=85±21.7 μg/mL) for 48 h and antibacterial activity [19].
In conclusion, from the results it is clear that Astragalus taxa tested in this study showed moderate antioxidant activity. The extracts demonstrated no antibacterial activity except P. aureginosa. To our knowledge, this is the first report on biological and chemical study of four Astragalus taxa tested. Astragalus extracts tested may be use food or medicinal applications that need natural antioxidant agents due to their antioxidant activities. Further researches on identify, isolate and characterize of the specific compounds from the extracts may be carry out to understand the action mechanisms.
Acknowledgement
This work was supported by the Research Fund of the University of Erciyes. Project number is FYL-2014-5072. We are grateful to Prof. Dr. Ahmet Aksoy for his helps for the collection and identification of plants.
Conflicts of interest statement: The authors have no conflict of interest.
References
1. Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer Int J 1992;18:1–29.10.1080/01635589209514201Search in Google Scholar PubMed
2. Ness AR, Powles JW. Fruit and vegetables, and cardiovascular disease: a review. Int J Epidemiol 1997;26:1–13.10.1093/ije/26.1.1Search in Google Scholar PubMed
3. Karadeniz F, Burdurlu HS, Koca N, Soyer Y. Antioxidant activity of selected fruits and vegetables grown in Turkey. Turk J Agric For 2005;29:297–303.Search in Google Scholar
4. Dung NT, Kim JM, Kang SC. Chemical composition, antimicrobial and antioxidant activities of the essential oil and the ethanol extract of Cleistocalyx operculatus (Roxb.) Merr and Perry buds. Food Chem Toxicol 2008;46:3632–9.10.1016/j.fct.2008.09.013Search in Google Scholar PubMed
5. Kumaran A, Karunakaran RJ. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT Food Sci Technol 2007;40:344–52.10.1016/j.lwt.2005.09.011Search in Google Scholar
6. Hossain MA, Rahman SM. Total phenolics, flavonoids and antioxidant activity of tropical fruit pineapple. Food Res Int 2011;44:672–6.10.1016/j.foodres.2010.11.036Search in Google Scholar
7. Davis PH. Flora of Turkey and The East Aegean Islands. Edinburgh: Edinburgh University Press, 1970:3, 49–168.Search in Google Scholar
8. Güner A, Özhatay N, Ekim T, Başer KH. Flora of Turkey and The East Aegean Islands, vol 11. Edinburgh: Edinburgh University Press, 2000:79–88.Search in Google Scholar
9. Ekici M, Aytaç Z, Akan M, Pınar M. A new species Astragalus L. (section: Onobrychoidei DC.: Fabaceae) from Turkey. Bot J Linn Soc 2008;157:741–7.10.1111/j.1095-8339.2008.00828.xSearch in Google Scholar
10. Calış I, Dönmez AA, Perrone A, Pizza C, Piacente S. Cycloartane glycosides from Astragalus campylosema Boiss. ssp. campylosema. Phytochemistry 2008;69:2634–8.10.1016/j.phytochem.2008.08.002Search in Google Scholar PubMed
11. Gülcemal D, Alankuş-Calışkan O, Perrone A, Ozgokçe F, Piacente S, Bedir E. Cycloartane glycosides from Astragalus aureus. Phytochemistry 2011;72:761–8.10.1016/j.phytochem.2011.02.006Search in Google Scholar PubMed
12. Gülcemal D, Masullo M, Bedir E, Festa M, Karayıldırım T, Alankuş-Çalışkan Ö, et al. Triterpene glycosides from Astragalus angustifolius. Planta Med 2012;78:720–9.10.1055/s-0031-1298337Search in Google Scholar PubMed
13. Polat E, Alankuş-Çalıskan Ö, Perrone A, Piacente S, Bedir E. Cycloartanetype glycosides from Astragalus amblolepis. Phytochemistry 2009;70:628–34.10.1016/j.phytochem.2009.03.006Search in Google Scholar PubMed
14. Polat E, Bedir E, Perrone A, Piacente S, Alankuş-Çalışkan Ö. Triterpenoid saponins from Astragalus wiedemannianus. Phytochemistry 2010;71:658–62.10.1016/j.phytochem.2009.11.013Search in Google Scholar PubMed
15. Jun MY, Kim EH, Lim JJ, Kim SH, Kim SH, Lim JD, et al. Variation of phenolic compounds contents in cultivated Astragalus membranaceus. Korean J Med Crop Sci 2012;20:447–53.10.7783/KJMCS.2012.20.6.447Search in Google Scholar
16. Benchadi W, Haba H, Lavaud C, Harakat D, Benkhaled M. Secondary metabolites of Astragalus cruciatus Link. and their chemotaxonomic significance. Rec Nat Prod 2013;7:105–13.Search in Google Scholar
17. Lim DH, Choi DB, Choi OY, Cho KA, Kim R, Choi HS, et al. Effect of Astragalus sinicus L. seed extract on antioxidant activity. J Ind Eng Chem 2011;17:510–6.10.1016/j.jiec.2011.02.040Search in Google Scholar
18. Chen J, Li Y, Yang LQ, Li YZ, Nan ZB, Gao K. Biological activities of flavonoids from pathogenic-infected Astragalus adsurgens. Food Chem 2012;131:546–51.10.1016/j.foodchem.2011.09.021Search in Google Scholar
19. Teyeb H, Zanina N, Neffati M, Douki W, Najjar MF. Cytotoxic and antibacterial activities of leaf extracts of Astragalus gombiformis Pomel (Fabaceae) growing wild in Tunisia. Turk J Biol 2012;36:53–8.10.3906/biy-1010-131Search in Google Scholar
20. Bedir E, Pugh N, Calıs I, Pasco DS, Khan IA. Immunostimulatory effects of cycloartane-type triterpene glycosides from Astragalus species. Biol Pharm Bull 2000;23:834–7.10.1248/bpb.23.834Search in Google Scholar PubMed
21. Uysal İ. Observations on the morphology, anatomy and ecology of endemic species Astragalus trojanus Stev. Erciyes Univ J Inst Sci Technol 1997;13:54–66.Search in Google Scholar
22. Albayrak S, Aksoy A, Sagdic O, Hamzaoglu E. Compositions, antioxidant and antimicrobial activities of Helichrysum (Asteraceae) species collected from Turkey. Food Chem 2010;119:114–22.10.1016/j.foodchem.2009.06.003Search in Google Scholar
23. Singleton VL, Rossi JA Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 1965;16:144–58.10.5344/ajev.1965.16.3.144Search in Google Scholar
24. Pourmorad F, Hosseinimehr SJ, Shahabimajd N. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr J Biotechnol 2006;5:1142–5.Search in Google Scholar
25. Prieto P, Pineda M, Aguilar M. Spectrofotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 1999;269:337–41.10.1006/abio.1999.4019Search in Google Scholar PubMed
26. Lee SK, Mbwambo ZH, Chung HS, Luyengi L, Games EJ, Metha RG. Evaluation of the antioxidant potential of natural products. Comb Chem High Throughput Screen 1998;1:35–46.10.2174/138620730101220118151526Search in Google Scholar
27. Cao L, Si JY, Liu Y, Sun H, Jin W, Li Z, et al. Essential oil composition, antimicrobial and antioxidant properties of Moslachinensis Maxim. Food Chem 2009;115:801–5.10.1016/j.foodchem.2008.12.064Search in Google Scholar
28. Apak R, Güçlü K, Özyurek M, Karademir SE, Ercağ E. The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. Int J Food Sci Nutr 2006;57:292–304.10.1080/09637480600798132Search in Google Scholar PubMed
29. Tuberoso CI, Montoro P, Piacente S, Corona G, Deiana M, Dessi MA, et al. Flavonoid characterization and antioxidant activity of hydroalcoholic extracts from Achillea ligustica All. J Pharm Biomed Anal 2009;50:440–8.10.1016/j.jpba.2009.05.032Search in Google Scholar
30. Peksel A, Arisan-Atac İ, Yanardag R. Evaluation of antioxidant and antiacetylcholinesterase activities of the extracts of Pistacia atlantica desf. Leaves. J Food Biochem 2010;34:451–76.10.1111/j.1745-4514.2009.00290.xSearch in Google Scholar
31. Aadil KR, Barapatre A, Sahu S, Jha H, Tiwary BN. Free radical scavenging activity and reducing power of Acacia nilotica wood lignin. Int J Biol Macromol 2014;67:220–7.10.1016/j.ijbiomac.2014.03.040Search in Google Scholar
32. Li M, Xu Y, Yang W, Li J, Xu X, Zhang X, et al. In vitro synergistic anti-oxidant activities of solvent-extracted fractions from Astragalus membranaceus and Glycyrrhiza uralensis. Food Sci Technol 2011;44:1745–51.10.1016/j.lwt.2011.02.017Search in Google Scholar
33. SPSS, SPSS Version 10.0. SPSS Inc, Chicago, Illinois, 2001.Search in Google Scholar
34. Zarena AS, Manohar B, Udaya-Sankar K. Optimization of supercritical carbon dioxide extraction of xanthones from mangosteen pericarp by response surface methodology. Food Bioprocess Technol 2012;5:1181–8.10.1007/s11947-010-0404-7Search in Google Scholar
35. Gülçin İ, Mshvildadze V, Gepdiremen A, Elias R. Antioxidant activity of a triterpenoid glycoside isolated from the berries of Hedera colchica:3-o-(β-d-glucopyranosyl)-hederagenin. Phytother Res 2006;20:130–4.10.1002/ptr.1821Search in Google Scholar
36. Asgarpanah J, Motamed SM, Farzaneh A, Ghanizadeh B, Tomraee S. Antioxidant activity and total phenolic and flavonoid content of Astragalus squarrosus Bunge. Afr J Biotechnol 2011;10:19176–80.10.5897/AJB11.3073Search in Google Scholar
37. Adıgüzel A, Sökmen M, Özkan H, Ağar G, Güllüce M, Şahin F. In vitro antimicrobial and antioxidant activities of methanol and hexane extract of Astragalus species growing in the eastern Anatolia region of Turkey. Turk J Biol 2009;33:65–71.10.3906/biy-0805-1Search in Google Scholar
38. Pistelli L, Bertoli A, Leporia E, Morellia I, Panizzi L. Antimicrobial and antifungal activity of crude extracts and isolated saponins from Astragalus errucosus. Fitoterapia 2002;73:336–9.10.1016/S0367-326X(02)00087-4Search in Google Scholar
39. Türker AU, Yıldırım AB. Evaluation of antibacterial and antitumor activities of some Turkish endemic plants. Trop J Pharm Res 2013;12:1003–10.10.4314/tjpr.v12i6.20Search in Google Scholar
40. Albayrak S, Aksoy A, Albayrak S, Sagdıc O. In vitro antioxidant and antimicrobial activity of some Lamiaceae species. Iran J Sci Technol 2013;A1:1–9.Search in Google Scholar
41. Ionkova I, Momekov G, Proksch P. Effects of cycloartane saponins from hairy roots of Astragalus membranaceus Bge., on human tumor cell targets. Fitoterapia 2010;81:447–51.10.1016/j.fitote.2009.12.007Search in Google Scholar PubMed
©2018 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Research Articles
- Betaine treatment decreased serum glucose and lipid levels, hepatic and renal oxidative stress in streptozotocin-induced diabetic rats
- Oxidative stress and response of antioxidant system in Nostoc muscorum exposed to different forms of Zinc
- An investigation on Trakya region Oak (Quercus spp.) honeys of Turkey: their physico-chemical, antioxidant and phenolic compounds properties
- Effects of Shiranuhi flower extracts and fractions on lipopolysaccharide-induced inflammatory responses in murine RAW 264.7 cells
- Evaluation of resveratrol organogels prepared by micro-irradiation: fibroblast proliferation through in vitro wound healing
- Ecological and phytochemical attributes of endemic Ferula gummosa Boiss. at vegetative and generative stages
- Characterization of calmodulin in the clam Anodonta woodiana: differential expressions in response to environmental Ca2+ and Cd2+
- Foliar application effects of salicylic acid and jasmonic acid on the essential oil composition of Salvia officinalis
- Antioxidant and antimicrobial activities of four Astragalus species growing wild in Turkey
- Differences in structure, allergenic protein content and pectate lyase enzyme activity of some Cupressaceae pollen
- In vitro bioactivities and subacute toxicity study of O. basilicum, T. vulgaris and R. officinalis
- Wendlandia exserta: a pertinent source of antioxidant and antimicrobial agent
- Effects of epigallocatechin-3-gallate (EGCG) on a scleroderma model of fibrosis
Articles in the same Issue
- Frontmatter
- Research Articles
- Betaine treatment decreased serum glucose and lipid levels, hepatic and renal oxidative stress in streptozotocin-induced diabetic rats
- Oxidative stress and response of antioxidant system in Nostoc muscorum exposed to different forms of Zinc
- An investigation on Trakya region Oak (Quercus spp.) honeys of Turkey: their physico-chemical, antioxidant and phenolic compounds properties
- Effects of Shiranuhi flower extracts and fractions on lipopolysaccharide-induced inflammatory responses in murine RAW 264.7 cells
- Evaluation of resveratrol organogels prepared by micro-irradiation: fibroblast proliferation through in vitro wound healing
- Ecological and phytochemical attributes of endemic Ferula gummosa Boiss. at vegetative and generative stages
- Characterization of calmodulin in the clam Anodonta woodiana: differential expressions in response to environmental Ca2+ and Cd2+
- Foliar application effects of salicylic acid and jasmonic acid on the essential oil composition of Salvia officinalis
- Antioxidant and antimicrobial activities of four Astragalus species growing wild in Turkey
- Differences in structure, allergenic protein content and pectate lyase enzyme activity of some Cupressaceae pollen
- In vitro bioactivities and subacute toxicity study of O. basilicum, T. vulgaris and R. officinalis
- Wendlandia exserta: a pertinent source of antioxidant and antimicrobial agent
- Effects of epigallocatechin-3-gallate (EGCG) on a scleroderma model of fibrosis


