Abstract
Objectives
High intensity and longer duration of acute postoperative pain are generally associated with a higher risk of developing chronic postoperative pain. Therefore, it is important to identify the preoperative predictors for acute postoperative pain. Preoperative evaluation of offset analgesia (OA) and the Pain Catastrophising Scale (PCS) may be potential predictors for acute postoperative pain. This study aimed to investigate the relationship between preoperative OA, PCS, and acute postoperative pain following orthognathic surgery.
Methods
Thirty patients (19 females) scheduled to undergo orthognathic surgery were included in this study. OA and PCS were evaluated preoperatively, and the patients reported their postoperative pain intensity using the visual analogue scale [0–100 mm] until it reached zero (number of days with pain). OA was induced on the dominant forearm via three consecutive painful heat pulses delivered for 5 s (T1=46 °C), 5 s (T2=47 °C), and 20 s (T3=46 °C). Subsequently, the associations between OA, PCS, and the number of days with pain were analysed.
Results
The median duration of postoperative pain was 10.3 days. Multiple linear regression analysis showed a significant (p=0.0019) predictive value of OA (p=0.008) for the number of days with pain. The PCS-magnification component was positively correlated with the number of days with pain (R=0.369, p=0.045), with no predictive values of PCS-total and PCS-subscale scores observed.
Conclusions
Preoperative evaluation of OA may be a new individualised, predictive tool for the number of days with acute postoperative pain following orthognathic surgery; hence, a possible biomarker for the patient’s vulnerability to developing chronic postoperative pain.
Ethical committee number
The study was approved by the Ethics Committee of Meikai University (A1624, A2113).
Trial registry number
This study was registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) Clinical Trial (Unique ID: UMIN000026719, UMIN000046957).
Introduction
Severe acute postoperative pain (POP) has been associated with an increased risk of developing chronic POP following various surgeries, including total hip arthroplasty, breast cancer surgery, inguinal herniorrhaphy, caesarean section, and thoracic surgery [1], [2], [3], [4]. Predicting acute POP is crucial as it may enable effective pre- and perioperative treatment to prevent the development of chronic POP.
The Pain Catastrophising Scale (PCS) is the most widely used instrument to assess pain catastrophising in chronic pain studies [4]. According to some studies [4], [5], [6], [7], [8], pain catastrophising is predictive of chronic POP; however, little is known about its ability to predict acute POP.
Quantitative Sensory Testing (QST) can be used to assess nerve function, and preoperative QST findings have been linked to the assessment of the development of chronic POP [9, 10]. Offset analgesia (OA) is a recently developed QST method that refers to the phenomenon of a disproportional reduction in pain intensity caused by a slight decrease from one temperature to another [11], [12], [13]. OA has attracted attention in recent years as a method for evaluating the potential aspect of descending pain inhibitory capacity [11–17]. However, the involvement of peripheral and central mechanisms in OA is currently debated [11], [12], [13]. Pain-free volunteers show a larger OA response compared with that of those with chronic pain [17, 18]. Unlike other QST assessment methods, OA has not been investigated as a predictor for acute POP.
The aim of this study was to investigate whether preoperative OA and PCS could predict acute POP (number of days with pain) following orthognathic surgery.
Methods
Participants
This study was approved by the Ethics Committee of Meikai University (A1624, A2113) and was conducted in accordance with the Declaration of Helsinki developed by the World Medical Association. Written informed consent was obtained from all patients before inclusion. In addition, this study was registered with the University Hospital Medical Information Network Clinical Trials Registry (unique ID: UMIN000026719, UMIN000046957) before conducting the research and adheres to the disclosure requirements of the institutional registry.
The inclusion criteria were: (1) scheduled orthognathic surgery, (2) age ≥16 years, and (3) ability to provide informed consent. The exclusion criteria were: (1) presence of psychiatric diseases, (2) use of any pain medication 24 h prior to the investigation, and (3) inability to provide informed consent. The patients were recruited between October 2019 and January 2022.
Preoperative evaluation
Experimental design
OA and PCS evaluations were performed the day before the surgery (Figure 1). OA evaluation was performed 15 min after PCS evaluation (Figure 1). All experiments were performed at a constant room temperature (25 °C). In addition, patients were interviewed to evaluate POP at 3 months, 6 months, and 1 year postoperatively.
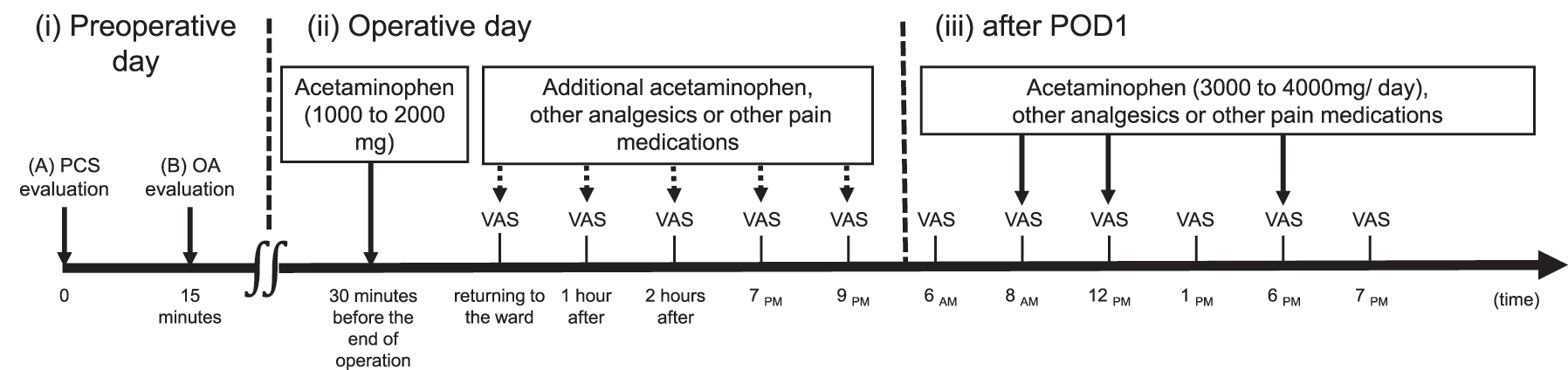
Schematic illustration of the study protocol on (i) preoperative day, (ii) operative day, and (iii) after POD1. (i) Preoperative day: (A) Patients completed PCS evaluation. (B) OA evaluation begins 15 min after the end of PCS evaluation. The thermal stimulus for OA is 32 °C (20 s) at baseline and 46 °C (5 s), 47 °C (5 s), and 46 °C (20 s). (ii) Operative day: during the operation, acetaminophen is administered at doses of 1,000–2,000 mg. Additional acetaminophen, other analgesics, or other pain medications are added based on the POP intensity evaluated using VAS (VAS-POP) (dashed arrows). VAS-POP is assessed immediately, 1 h, and 2 h after returning to the ward; and at 7 pm and 9 pm. (iii) After POD1: following POD1, VAS-POP is assessed at 6 am, 1 pm, and 7 pm. In addition, VAS-POP is assessed at 8 am, 12 pm, and 6 pm during acetaminophen administration. Other analgesics or pain medications are administered if the pain is not well-controlled. The dose of analgesics is adjusted and reduced according to the POP level. POD1, postoperative day 1; PCS, pain catastrophising scale; OA, offset analgesia; POP, postoperative pain; VAS, visual analogue scale.
OA evaluation
OA was induced on the dominant forearm via three consecutive heat pains at three coherent time intervals of 5 s (T1), 5 s (T2), and 20 s (T3=T1) using a developed quantitative thermal stimulator device (VICS, Tokyo, Japan) [19], [20], [21], [22], [23]. The temperatures at baseline, T1, T2, and T3 were 32 °C, 46 °C, 47 °C, and 46 °C, respectively [13, 23]. The OA paradigm was conducted, and the patients were asked to continuously rate the pain intensity using a custom-made electronic visual analogue scale (VAS) (0–100 mm), which was sampled and analysed using a personal computer [19], [20], [21], [22], [23]. The left endpoint (0) of the electronic VAS indicated ‘no pain’, whereas the right endpoint (100) indicated the ‘worst pain imaginable’. OA was evaluated by subtracting the intensity of the maximum and minimum pain ratings at T2 and T3, respectively, (maximum VAS during T2 – minimum VAS during T3) as previously described [14, 17, 23–27].
PCS evaluation
The Japanese version of the PCS questionnaire [28] comprising 13 items was used in this study. The participants rated the frequency with which they experienced different pain-related thoughts or feelings on a 5-point Likert scale, where 0 represents ‘not at all’, and 4 represents ‘all the time’. The scores of the three subscales of PCS (rumination, helplessness, and magnification) were also calculated in addition to the sum of all items as a total score.
General anaesthesia and surgical procedure
Orthognathic surgeries were performed under general anaesthesia with endotracheal intubation using balanced anaesthesia with propofol, remifentanil hydrochloride, and rocuronium bromide, and regional anaesthesia with 1 % lidocaine hydrochloride monohydrate containing 1/100,000 adrenaline. Acetaminophen (1,000–2,000 mg) was administered 30 min before the end of the surgery (Figure 1).
Postoperative protocol for pain management and evaluation of surgical pain
POP was managed with acetaminophen; 1,000–2,000 mg was administered the day after the surgery. After postoperative day 1 (POD1), 3,000 mg/day (1,000 mg at 8 am, 12 pm, and 6 pm) was administered. Loxoprofen sodium hydrate, additional acetaminophen, or other pain medications were administered if the pain was not well-controlled. The analgesic dose was adjusted and reduced according to the POP level. Depending on the POP, trigeminal nerve block (mandibular and mental nerve block) or local infiltration anaesthesia was performed using levobupivacaine hydrochloride postoperatively.
The POP intensity was evaluated using VAS (VAS-POP). On the operative day, VAS-POP was assessed immediately, 1 h, and 2 h after returning to the ward and at 7 pm and 9 pm. After POD1, VAS-POP was assessed at 6 am, 1 pm, and 7 pm (Figure 1). In addition, VAS-POP was assessed at 8 am, 12 pm, and 6 pm during acetaminophen administration. The administration of acetaminophen was discontinued if VAS-POP was <30/100 or if the patient did not request an additional prescription; this decision was made during the morning rounds.
The duration of acetaminophen administration and the total dose of analgesics administered were recorded. The postoperative (‘analgesic requirement period’) was defined as ‘the final analgesic administration time (day) − the end time of anaesthesia (day)’.
VAS-POP was assessed daily until discharge. If VAS-POP did not reach 0/100 at discharge, patients were requested to record it until it reached 0/100. Furthermore, patients were interviewed to evaluate POP at 3 months, 6 months, and 1 year postoperatively. The number of days until VAS-POP reached 0/100 was calculated as the ‘number of days with pain.’ Furthermore, the VAS-POP area under the curve (VASAUC) (mm × day) was calculated by summating the VAS-POP areas. The VASAUC for postoperative 24 h (VASAUC_24 h) (mm × day) was calculated by summating the VAS-POP areas for postoperative 24 h, and VAS-peak values for postoperative 24 h (VAS-peak_24 h) was checked from the recording. The analgesic requirement period, number of days with pain, VASAUC, VASAUC_24 h, and VAS-peak_24 h were calculated for all patients and the two groups according to the surgery type (Le Fort type I osteotomy (Le Fort 1) and bilateral sagittal split ramus osteotomy (SSRO) group [n=10]; bilateral SSRO group [n=15]).
Statistical analysis
Data regarding patient background, OA, PCS-total score, PCS-subscale scores, analgesic requirement period, number of days with pain, and VASAUC are presented as medians [interquartile range].
T-tests or Welch two-sample t-tests
T-tests or Welch two-sample t-tests were performed to determine the difference between the two groups according to the surgery type (Le Fort 1 and SSRO group [n=10]; SSRO group [n=15]) for the analgesic requirement period, number of days with pain, VASAUC, VASAUC_24 h, and VAS-peak_24 h.
Spearman’s rank correlation
Spearman’s rank correlation was used to evaluate the correlations between 1) OA, PCS-total score, and PCS-subscale scores vs. the analgesic requirement period; 2) OA, PCS-total score, PCS-subscale scores vs. number of days with pain; 3) OA, PCS-total score, PCS-subscale scores vs. VASAUC; and 4) VASAUC_24 h vs. number of days with pain.
Multiple linear regression analysis
Multiple linear regression analysis was performed for the “number of days with pain” and VASAUC as the outcome variable, and OA and PCS as the explanatory variables. The residuals of the multiple linear regression model were analysed by graphically plotting the residuals against the predicted values and plotting normal quantile-quantile (Q-Q) plots. Statistical analyses were performed using EZR software (Jichi Medical University, Tochigi, Japan) [29]. Statistical significance was set at p<0.05.
Results
Patients
In total, 32 out of 47 patients were recruited. Two patients, one who refused to receive analgesics and another patient with postoperative paralysis, were excluded from the analysis. Finally, 30 patients were analysed (Figure 2). Table 1 shows the patient’s demographic data and surgery type.
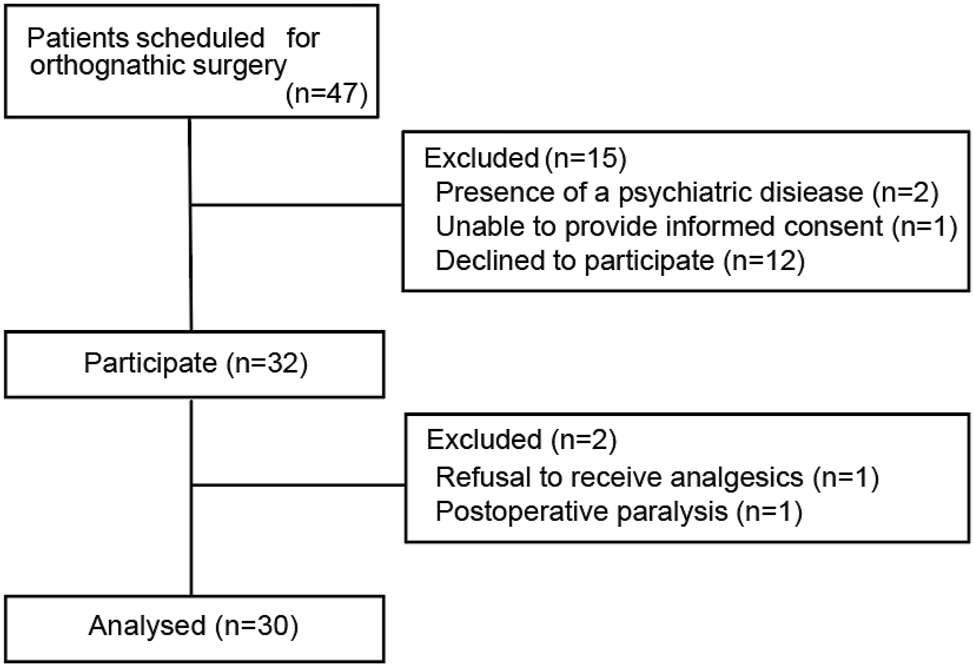
Flow chart of patient selection.
Patient background and surgery type.
| Patient background | |
|---|---|
| Sex (M/F) | 11/19 |
| Age, y | 23.0 [21.0–26.0] |
| Height, cm | 165.1 [159.4–170.1] |
| Body weight, kg | 56.2 [51.5–67.9] |
| Dominant hand (R/L) | 27/3 |
|
|
|
| Surgery type | |
|
|
|
| Le Fort 1 and SSRO | 10 |
| SSRO | 15 |
| Anterior maxillary osteotomy | 3 |
| Anterior maxillary osteotomy and SSRO | 1 |
| Mandibular body osteotomy | 1 |
-
F, female; M, male; L, left; R, right; SSRO, sagittal split ramus osteotomy. n=30; values are presented as numbers or medians [interquartile ranges].
OA, PCS, and POP parameters
The descriptive statistics of OA, PCS, and POP parameters are listed in Table 2. A frequency plot of individual OA is presented in Figure 3 to determine the individual variation. The highest number of days with pain was 36.5 days.
OA, PCS, and postoperative pain parameters.
| OA | 27.5 [14.0–36.8] |
| PCS-T | 23.0 [14.5–28.0] |
| PCS-R | 12.5 [10.0–15.0] |
| PCS-H | 5.0 [3.0–9.8] |
| PCS-M | 4.0 [2.0–6.8] |
| Analgesic requirement period, day | 7.6 [6.2–8.6] |
| Number of days with pain, day | 10.3 [7.7–14.0] |
| VASAUC, mm × day | 177.8 [127.4–259.7] |
| VASAUC_24 h, mm × day | 24.1 [16.9–41.9] |
| VAS-peak_24 h | 70.0 [51.3–78.8] |
-
OA, offset analgesia; PCS, pain catastrophising scale; PCS-H, pain catastrophising scale-helplessness; PCS-M, pain catastrophising scale-magnification; PCS-R, pain catastrophising scale-rumination; PCS-T, pain catastrophising scale-total. Number of days with pain denotes the duration for postoperative pain to reach 0/100 on the VAS. VASAUC, visual analogue scale area under the curve. n=30; values are presented as median [interquartile range].
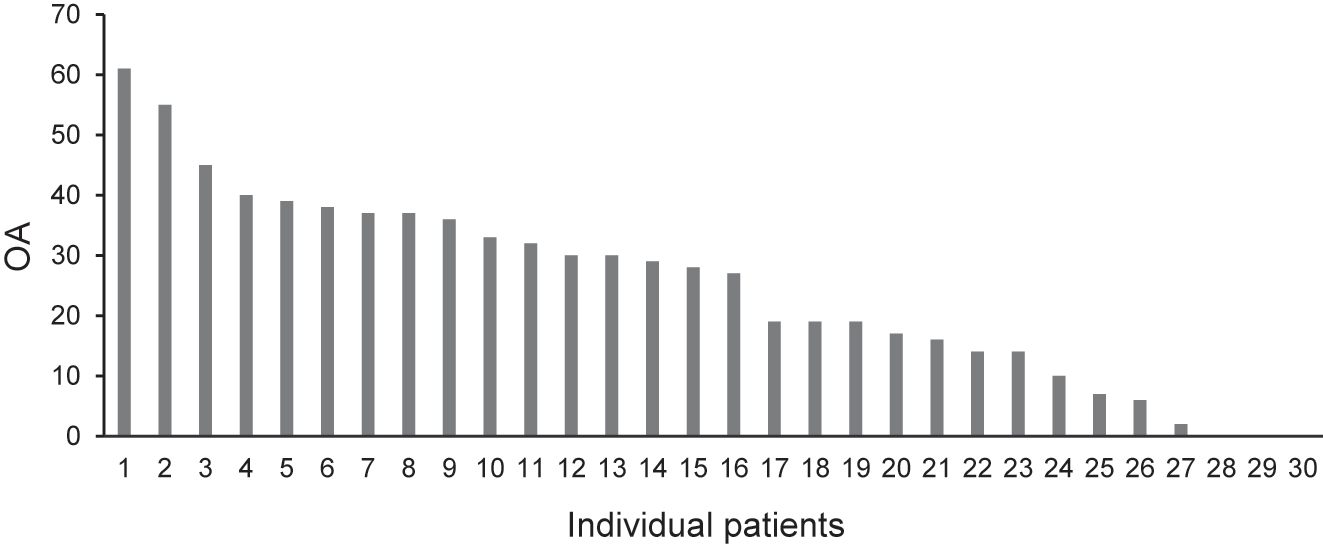
Frequency plot of individual OA for 30 patients. The OA scores indicate the difference (subtraction) between the maximum VAS value at 47 °C and the minimum VAS value when the OA is returned to 46 °C. OA, offset analgesia; VAS, visual analogue scale.
Perioperative analgesic procedure
During the surgery, 2,000 mg of acetaminophen was administered for bilateral SSRO with Le Fort 1 (n=10) and bilateral SSRO with an anterior maxillary osteotomy (n=1), whereas 1,000 mg of acetaminophen was administered for the other surgery types (n=19).
For postoperative analgesic consumption, acetaminophen was used as the first-choice drug. The median administration period was 8.0 [6.3–9.0] days, and the median total dose was 19,000 [14,375–22,375] mg. In 19 patients, the single dose was reduced from 1,000 mg to 500–600 mg after the POP was reduced compared with that just after the operation. The median number of days for dose reduction was 6.0 [4.5–7.0] days.
Regarding other analgesics, among 30 patients, 28 received other analgesics in addition to acetaminophen. Among them, 27 required the administration of loxoprofen sodium hydrate. In particular, one patient was required to switch from acetaminophen to loxoprofen sodium hydrate because of liver dysfunction, and another discontinued acetaminophen and switched to loxoprofen sodium hydrate because of its bitter taste. A single dose of loxoprofen sodium hydrate was 60–120 mg. The administration period of loxoprofen sodium hydrate was 3.0 [2.0–5.0] days. The total loxoprofen sodium hydrate dose was 390 [180–480] mg.
On the day of surgery, intravenous flurbiprofen axetil was administered to 19 patients. The single dose was 50 mg; one patient received the drug three times, whereas all other patients received the drug once. Pentazocine was administered to one patient on the operative day and POD1. One patient required a single dose of pentazocine (15 mg) three times on the operative day and POD1.
Local anaesthesia (0.5 % levobupivacaine hydrochloride) was administered on the operative day for POP control in two patients. A mandibular nerve block to the right and left sides was performed in one patient, whereas a mandibular nerve block to the right and left sides along with a mental nerve block to the right side was performed in another patient. The dose of 0.5 % levobupivacaine hydrochloride was 5 mL on each site.
POP following orthognathic surgery
The POP parameters for the surgery type (Le Fort 1 and SSRO group [n=10] and SSRO group [n=15]) are listed in Table 3. Student’s t-test and Welch two-sample t-test showed that there were no significant differences between the two groups in the analgesic requirement period (p=0.394), number of days with pain (p=0.31), VASAUC (p=0.479), VASAUC_24 h (p=0.475), and VAS-peak_24 h (p=0.824).
Postoperative pain parameters following orthognathic surgery.
| Le Fort 1 and SSRO group | SSRO group | |
|---|---|---|
| Analgesic requirement period, day | 7.5 [6.5–8.9] | 7.8 [6.1–8.9] |
| Number of days with pain, day | 12.4 [8.6–13.6] | 10.0 [7.8–13.1] |
| VASAUC, mm × day | 221.1 [127.5–357.9] | 210.5 [137.7–256.5] |
| VASAUC_24 h, mm × day | 22.8 [14.8–43.4] | 27.6 [21.9–40.9] |
| VAS-peak_24 h | 55.0 [50.0–77.5] | 60.0 [57.5–77.5] |
-
SSRO, sagittal split ramus osteotomy. Number of days with pain denotes the duration for postoperative pain to reach 0/100 on the VAS. VASAUC, visual analogue scale area under the curve. VASAUC_24 h denotes the VASAUC for postoperative 24 h. VAS-peak_24 h denotes VAS-peak values for post-operative 24 h. Le Fort 1 and SSRO group, n=10; SSRO group, n=15; values are presented as median [interquartile range].
Predicting postoperative pain
Normal Q-Q plots revealed that the VASAUC was normally distributed. The normal Q-Q plots revealed that the number of days with pain was not normally distributed; thus, a logarithmic translation was required. The log (number of days with pain) was normally distributed after logarithmic translation; however, the analgesic requirement period was not normally distributed before or after logarithmic translation.
Spearman’s rank correlation
Spearman’s rank correlation revealed that OA was inversely correlated with the number of days with pain (R=−0.539, p=0.002), indicating that lower preoperative OA was associated with a longer period of POP. A function in conjunction with a polynomial approximation (R2=0.2951) is presented in Figure 4.
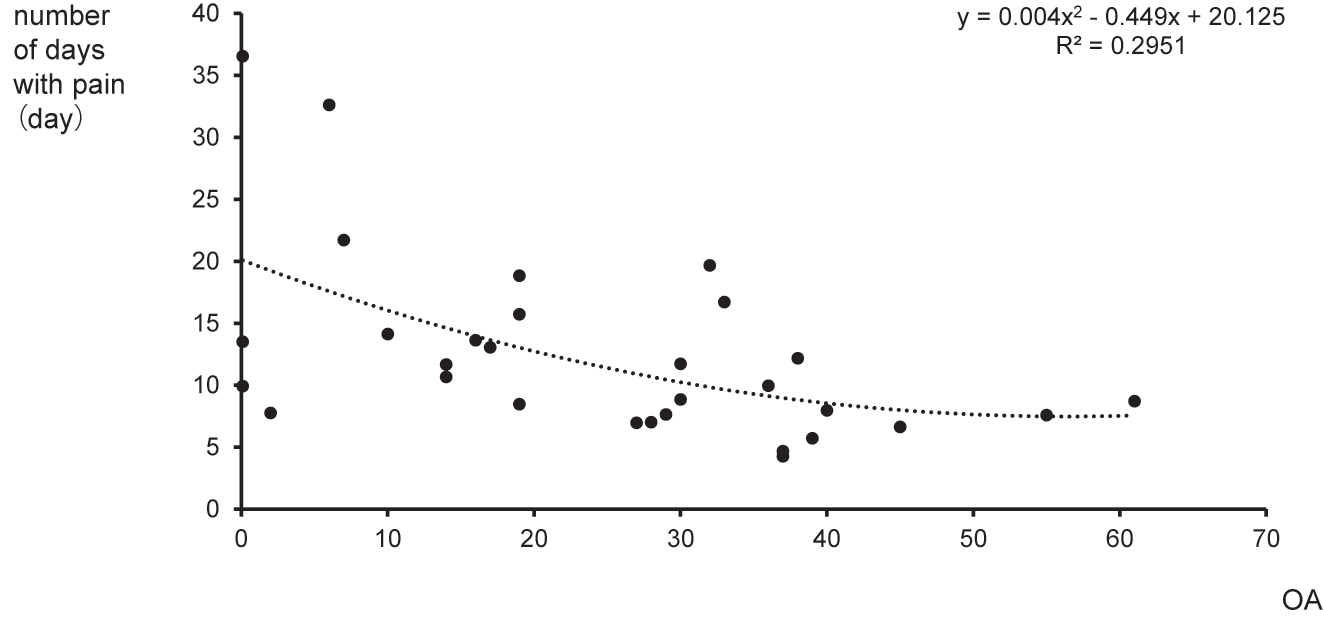
Scatter plot for OA vs. the number of days with pain. The plot shows OA vs. the number of days with pain with polynomial approximation (dotted line) (R2=0.2951). OA, offset analgesia.
PCS-magnification was positively correlated with the number of days with pain (R=0.369, p=0.045), indicating that higher preoperative PCS-magnification was associated with a longer period of POP. There were no significant correlations between the following: 1) OA, PCS-total score, and PCS-subscale scores vs. analgesic requirement period; 2) PCS-total score, PCS-rumination, and PCS-helplessness vs. the number of days with pain; and 3) OA, PCS-total score, PCS-subscale scores vs. VASAUC.
Similarly, there was no significant correlation between VASAUC_24 h and the number of days with pain (R=0.132, p=0.486).
Multiple linear regression analysis
Table 4 presents the results of the multiple linear regression model of the factors that explain the number of days with pain and VASAUC. Since the analgesic requirement period was not normally distributed, multiple linear regression analysis for the dependent variable (of the analgesic requirement period) was not performed. PCS-total and subscale scores were not significant predictors of log (number of days with pain) and VASAUC. In contrast, OA was a significant independent predictor of log (number of days with pain) but not of VASAUC (Table 4).
Multiple linear regression model of factors that explain the number of days with pain, and VASAUC in all patients.
| Dependent variable | Predictor | B (estimate) | 95 % CI | p-Value | Adjusted R2 |
|---|---|---|---|---|---|
| log (number of days with pain) | OA | –0.02 | –0.03 to 0.00 | 0.008a | 0.2788 |
| PCS-T | 0.01 | –0.01 to 0.03 | 0.26 | ||
| OA | –0.02 | –0.03 to −0.01 | 0.003a | 0.3077 | |
| PCS-R | 0.03 | –0.01 to 0.07 | 0.13 | ||
| OA | –0.02 | –0.03 to −0.01 | 0.003a | 0.3207 | |
| PCS-H | 0.02 | 0.00 to 0.05 | 0.09 | ||
| OA | –0.01 | –0.02 to 0.00 | 0.008a | 0.3251 | |
| PCS-M | 0.05 | –0.01 to 0.10 | 0.08 | ||
| VASAUC | OA | –0.46 | –3.77 to 2.85 | 0.78 | –0.0682 |
| PCS-T | 0.43 | –5.53 to 6.39 | 0.88 | ||
| OA | –0.49 | –3.64 to 2.66 | 0.75 | –0.0674 | |
| PCS-R | 1.23 | –10.98 to 13.44 | 0.84 | ||
| OA | –0.24 | –3.28 to 2.79 | 0.87 | 0.0072 | |
| PCS-H | 5.56 | –2.36 to 13.47 | 0.16 | ||
| OA | –0.09 | –3.30 to 3.12 | 0.95 | –0.0327 | |
| PCS-M | 8.03 | –8.87 to 24.93 | 0.34 |
-
Multiple linear regression with B representing regression coefficients with 95 % CI. Number of days with pain: denotes the duration for post-operative pain to reach 0/100 on the VAS. CI, confidence interval; OA, offset analgesia; PCS-H, pain catastrophising scale-helplessness; PCS-M, pain catastrophising scale-magnification; PCS-R, pain catastrophising scale-rumination; PCS-T, pain catastrophising scale-total; VASAUC, visual analogue scale area under the curve. n=30, ap<0.05.
As a result of multiple linear regression analysis, the derived prediction formula was as follows: log (number of days with pain)=(−0.01 × OA) + 2.53 (adjusted R2=0.3251, p=0.0019, OA; p=0.008).
Discussions
This study showed that a low preoperative OA value predicted the number of days with acute POP following orthognathic surgery. In addition, PCS-magnification was related to the number of days with POP.
OA and POP
OA was initially reported by Grill and Coghill in 2002 and is defined as a disproportionately large pain reduction after a small change (reduction) in temperature [11]. OA is assumed to be a filtering mechanism for nociceptive information, that is, an inhibitory temporal sharpening mechanism [11, 14]. A systematic literature review including healthy volunteers and patients reported that OA activates brain regions such as the periaqueductal grey, dorsolateral prefrontal cortex, insula, medulla, pons, and cerebellum [25]. Zhang et al. examined OA in patients with heterogeneous chronic pain disorders using functional magnetic resonance imaging (fMRI) and demonstrated that OA attenuation was associated with suppressed activation of the descending pain modulatory and reward systems [13]. Niesters et al. observed reduced or absent OA responses in patients with chronic neuropathic pain [30].
Diffuse noxious inhibitory controls and conditioned pain modulation (CPM) have been proposed to evaluate different aspects of descending inhibitory mechanisms [23, 25, 31], [32], [33]. CPM was suggested to be predictive of a lower risk of chronic post-thoracotomy pain [34] and pain after total knee arthroplasty [35]. In addition, patients with chronic pain after abdominal surgery showed lesser preoperative CPM and greater postoperative hyperalgesia [36]. Interestingly, CPM, but not OA, has been suggested to be predictive of the analgesic response to non-steroidal anti-inflammatory drugs, which indicates that the two assessments might represent different predictive components [24]. Acute POP is also predicted by perioperative CPM in patients undergoing orthognathic surgery [37]. Taken together, weakened endogenous pain modulation seems to be associated with more POP problems [34], [35], [36], [37], indicating that descending modulatory pathways play a predominant role. Therefore, it may also be important to evaluate whether OA can contain similar synergistic information to predict, in the first place, the development of acute POP.
These findings suggest that the modulatory mechanisms involved in OA are centrally mediated [12] and can influence the duration of POP, which is consistent with the findings of a previous study [37].
The scatter plot for OA vs. the number of days with pain (Figure 4) shows outliers. It is evident that there is much variability in most QST assessments [9, 38]. Accumulating evidence suggests that this variability is associated with POP after the surgery [10], which indicates that variability (and outliers) might hold important clinical value.
PCS and POP
Pain catastrophising has been defined as an exaggerated negative mental response to actual or anticipated painful experiences [5]. Psychological factors are consistently associated with chronic POP, including anxiety, depression, pain catastrophising, and general psychological distress [2]. Pain catastrophising has been identified as one of the strongest psychologic predictors of pain and pain outcomes [6, 39, 40].
PCS represents a cognitive aspect of pain [28], and its different components, individual interactions, and modelling of catastrophising have been recently reviewed [39]. Catastrophising has been associated with heightened pain experience in clinical and experimental studies [41], and POP intensity has been estimated based on the levels of preoperative anxiety and catastrophising [5, 8]; hence, PCS is predictive of chronic POP [4]. A previous study of breast surgery patients reported that PCS predicted POP in only patients with pre-existing chronic pain [7].
Nevertheless, the multiple linear regression analysis in the present study revealed that the PCS-total and PCS-subscale scores were not predictive factors for the number of days with pain.
The participants in the current study may not have experienced strong anxiety or catastrophising. Matsuoka et al. validated the Japanese version of the PCS and reported that the average values for healthy volunteers were as follows: PCS-total, 21.39 (9.92); PCS-rumination, 11.55 (4.49); PCS-helplessness, 6.16 (4.29); and PCS-magnification, 3.67 (2.73) [28]. The PCS scores obtained in the current study were similar to those of healthy Japanese volunteers [28].
The following reasons could have led to the similarity in PCS scores between the validated values for healthy volunteers from the study by Matsuoka and Sakano [28] and our findings: 1) the participants in the current study were healthy young patients without chronic pain; and 2) as the included patients were scheduled for an orthognathic surgery, which is a cosmetic surgery and not a surgery for cancer or chronic pain such as knee osteoarthritis, they might not have experienced strong anxiety or catastrophising. The fact that the obtained PCS was similar to that in healthy volunteers might be a possible reason why PCS was not a predictive factor of acute POP in the current study.
Although PCS-total and PCS-subscale scores were not predictive factors for the number of days with pain, the present findings demonstrated that patients with higher PCS-magnification scores experienced a longer duration of POP, indicating that there is a relationship between the preoperative PCS-magnification score and acute POP as reported by previous studies [1], [2], [3], [4], [5].
Clinical implication
A higher POP intensity may have led to a longer duration of POP [1], [2], [3], [4]. Nevertheless, our result revealed no significant correlation between acute pain intensity (VASAUC_24 h) and the number of days with pain, possibly due to intensive pain control with rescue analgesics and nerve blockade.
One of the most consistent predictors of chronic POP is the presence of severe acute pain in the first week after the surgery [4]. Poorly controlled acute POP has been suggested as a predictor of the development of chronic POP [1], [2], [3], [4]. Therefore, preoperative prediction and the detection of high-risk groups may pave the way for developing individualised management regimes that may increase the chances of preventing the development of chronic POP.
OA is a novel and easy pain assessment test for evaluating peripheral and central pain modulatory processes [12]. In addition, OA evaluation is easy to perform, and it is possible to perform tests at the bedside in a short time. Therefore, preoperative OA evaluation could be an attractive bedside test tool for identifying patients at risk of acute POP. Intensive care with tailor-made care for high-risk patients will help avoid the onset of chronic POP.
Limitations
The study population comprised only 30 patients. In addition, different types of surgery, such as Le Fort 1 and SSRO, or SSRO were performed. Therefore, the findings from the current study should be interpreted with caution, and larger studies should be conducted to validate these findings.
Although our result showed no significant correlation between the POP intensity and the duration of POP, it is possible that the POP intensity may have affected the results of this study.
A recent systematic review identified that temporal summation of pain is often an independent predictor for chronic POP when the temporal summation of pain is assessed in combination with other QST assessments [10]. However, the current study did not assess the temporal summation of pain. Future studies should assess whether OA is an independent predictor of acute or chronic POP.
Conclusions
This study demonstrated that the preoperative evaluation of OA predicts the number of days with acute POP following orthognathic surgery, and PCS-magnification is related to the number of days with POP.
Acknowledgements
The authors would like to thank all participants for their consent and cooperation.
-
Research funding: This study was supported by Grants-in-Aid for Scientific Research (KAKENHI) (Nos. 18K08826, 20K10189, and 21K08977) from the Japan Society for the Promotion of Science (JSPS). The Center for Neuroplasticity and Pain (CNAP) is supported by the Danish National Research Foundation (DNRF121).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: The authors declare that they have no competing interests.
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Ethical approval: This human-related research complied with all the relevant national regulations, institutional policies and is in accordance with the tenets of the Helsinki Declaration and has been approved by the author’s institutional review board, the Ethics Committee of Meikai University.
References
1. Fregoso, G, Wang, A, Tseng, K, Wang, J. Transition from acute to chronic pain: evaluating risk for chronic postsurgical pain. Pain Physician 2019;22:479–88.10.36076/ppj/2019.22.479Search in Google Scholar
2. Glare, P, Aubrey, KR, Myles, PS. Transition from acute to chronic pain after surgery. Lancet 2019;393:1537–46. https://doi.org/10.1016/s0140-6736(19)30352-6.Search in Google Scholar
3. Kehlet, H, Jensen, TS, Woolf, CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006;367:1618–25. https://doi.org/10.1016/s0140-6736(06)68700-x.Search in Google Scholar PubMed
4. VanDenKerkhof, EG, Peters, ML, Bruce, J. Chronic pain after surgery: time for standardization? A framework to establish core risk factor and outcome domains for epidemiological studies. Clin J Pain 2013;29:2–8. https://doi.org/10.1097/ajp.0b013e31824730c2.Search in Google Scholar
5. Pavlin, DJ, Sullivan, MJ, Freund, PR, Roesen, K. Catastrophizing: a risk factor for postsurgical pain. Clin J Pain 2005;21:83–90. https://doi.org/10.1097/00002508-200501000-00010.Search in Google Scholar PubMed
6. Reddi, D, Curran, N. Chronic pain after surgery: pathophysiology, risk factors and prevention. Postgrad Med J 2014;90:222–6. https://doi.org/10.1136/postgradmedj-2013-132215.Search in Google Scholar PubMed
7. Ruscheweyh, R, Viehoff, A, Tio, J, Pogatzki-Zahn, EM. Psychophysical and psychological predictors of acute pain after breast surgery differ in patients with and without pre-existing chronic pain. Pain 2017;158:1030–8. https://doi.org/10.1097/j.pain.0000000000000873.Search in Google Scholar PubMed
8. Sobol-Kwapinska, M, Bąbel, P, Plotek, W, Stelcer, B. Psychological correlates of acute postsurgical pain: a systematic review and meta-analysis. Eur J Pain 2016;20:1573–86. https://doi.org/10.1002/ejp.886.Search in Google Scholar PubMed
9. Petersen, KK. Predicting pain after standard pain therapy for knee osteoarthritis – the first steps towards personalized mechanistic-based pain medicine in osteoarthritis. Scand J Pain 2022;23:40–8. https://doi.org/10.1515/sjpain-2022-0082.Search in Google Scholar PubMed
10. Petersen, KK, Vaegter, HB, Stubhaug, A, Wolff, A, Scammell, BE, Arendt-Nielsen, L, et al.. The predictive value of quantitative sensory testing: a systematic review on chronic postoperative pain and the analgesic effect of pharmacological therapies in patients with chronic pain. Pain 2021;162:31–44. https://doi.org/10.1097/j.pain.0000000000002019.Search in Google Scholar PubMed
11. Grill, JD, Coghill, RC. Transient analgesia evoked by noxious stimulus offset. J Neurophysiol 2002;87:2205–8. https://doi.org/10.1152/jn.00730.2001.Search in Google Scholar PubMed
12. Ligato, D, Petersen, KK, Mørch, CD, Arendt-Nielsen, L. Offset analgesia: the role of peripheral and central mechanisms. Eur J Pain 2018;22:142–9. https://doi.org/10.1002/ejp.1110.Search in Google Scholar PubMed
13. Zhang, S, Li, T, Kobinata, H, Ikeda, E, Ota, T, Kurata, J. Attenuation of offset analgesia is associated with suppression of descending pain modulatory and reward systems in patients with chronic pain. Mol Pain 2018;14:1744806918767512. https://doi.org/10.1177/1744806918767512.Search in Google Scholar PubMed PubMed Central
14. Yelle, MD, Rogers, JM, Coghill, RC. Offset analgesia: a temporal contrast mechanism for nociceptive information. Pain 2008;134:174–86. https://doi.org/10.1016/j.pain.2007.04.014.Search in Google Scholar PubMed PubMed Central
15. Kurata, J. Neural mechanisms of offset analgesia. Adv Exp Med Biol 2018;1099:141–6. https://doi.org/10.1007/978-981-13-1756-9_12.Search in Google Scholar PubMed
16. Li, T, Zhang, S, Ikeda, E, Kobinata, H. Functional connectivity modulations during offset analgesia in chronic pain patients: an fMRI study. Brain Imaging Behav 2022;16:1794–802. https://doi.org/10.1007/s11682-022-00652-7.Search in Google Scholar PubMed
17. Kobinata, H, Ikeda, E, Zhang, S, Li, T, Makita, K, Kurata, J. Disrupted offset analgesia distinguishes patients with chronic pain from healthy controls. Pain 2017;158:1951–9. https://doi.org/10.1097/j.pain.0000000000000989.Search in Google Scholar PubMed
18. Shulman, J, Zurakowski, D, Keysor, J, Jervis, K, Sethna, NF. Offset analgesia identifies impaired endogenous pain modulation in pediatric chronic pain disorders. Pain 2020;161:2852–9. https://doi.org/10.1097/j.pain.0000000000001984.Search in Google Scholar PubMed
19. Oono, Y, Kohase, H. The elucidation of risk factors for acute and chronic postsurgical pain [in Japanese]. Pain Res 2021;36:24–34. https://doi.org/10.11154/pain.36.24.Search in Google Scholar
20. Oono, Y, Kono, R, Ando, S, Takagi, S, Kohaes, H. Development of the device for the evaluation of endogenous pain modulation with cold, heat and pressure stimulation [in Japanese]. Jpn J Orofac Pain 2021;13:117–27.Search in Google Scholar
21. Oono, Y, Kubo, H, Takagi, S, Wang, K, Arendt-Nielsen, L, Kohase, H. Painful cold-heat segmental pulse stimulation provokes the thermal pain illusion. Somatosens Mot Res 2022;39:1–9. https://doi.org/10.1080/08990220.2021.1986382.Search in Google Scholar PubMed
22. Oono, Y, Kubo, H, Takagi, S, Wang, K, Arendt-Nielsen, L, Kohase, H. Conditioned pain modulation is not associated with thermal pain illusion. Scand J Pain 2022;23:175–83. https://doi.org/10.1515/sjpain-2022-0037.Search in Google Scholar PubMed
23. Oono, Y, Kono, R, Takagi, S, Ide, Y, Nagasaka, H, Kohase, H, et al.. Photobiomodulation enhanced endogenous pain modulation in healthy volunteers. Lasers Med Sci 2022;38:16. https://doi.org/10.1007/s10103-022-03686-x.Search in Google Scholar PubMed PubMed Central
24. Petersen, KK, Simonsen, O, Olesen, AE, Mørch, CD, Arendt-Nielsen, L. Pain inhibitory mechanisms and response to weak analgesics in patients with knee osteoarthritis. Eur J Pain 2019;23:1904–12. https://doi.org/10.1002/ejp.1465.Search in Google Scholar PubMed
25. Hermans, L, Calders, P, Van Oosterwijck, J, Verschelde, E, Bertel, E, Meeus, M. An overview of offset analgesia and the comparison with conditioned pain modulation: a systematic literature review. Pain Physician 2016;19:307–26.10.36076/ppj/2016.19.307Search in Google Scholar
26. Nahman-Averbuch, H, Martucci, KT, Granovsky, Y, Weissman-Fogel, I, Yarnitsky, D, Coghill, RC. Distinct brain mechanisms support spatial vs temporal filtering of nociceptive information. Pain 2014;155:2491–501. https://doi.org/10.1016/j.pain.2014.07.008.Search in Google Scholar PubMed PubMed Central
27. Yelle, MD, Oshiro, Y, Kraft, RA, Coghill, RC. Temporal filtering of nociceptive information by dynamic activation of endogenous pain modulatory systems. J Neurosci 2009;29:10264–71. https://doi.org/10.1523/jneurosci.4648-08.2009.Search in Google Scholar
28. Matsuoka, H, Sakano, Y. Assessment of cognitive aspect of pain: development, reliability, and validation of Japanese version of pain catastrophizing scale [in Japanese]. Jpn J Psychosom Med 2007;47:95–102.Search in Google Scholar
29. Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013;48:452–8. https://doi.org/10.1038/bmt.2012.244.Search in Google Scholar PubMed PubMed Central
30. Niesters, M, Hoitsma, E, Sarton, E, Aarts, L, Dahan, A. Offset analgesia in neuropathic pain patients and effect of treatment with morphine and ketamine. Anesthesiology 2011;115:1063–71. https://doi.org/10.1097/aln.0b013e31822fd03a.Search in Google Scholar PubMed
31. Oono, Y, Nie, H, Matos, RL, Wang, K, Arendt-Nielsen, L. The inter- and intra-individual variance in descending pain modulation evoked by different conditioning stimuli in healthy men. Scand J Pain 2011;2:162–9. https://doi.org/10.1016/j.sjpain.2011.05.006.Search in Google Scholar PubMed
32. Oono, Y, Wang, K, Baad-Hansen, L, Futarmal, S, Kohase, H, Svensson, P, et al.. Conditioned pain modulation in temporomandibular disorders (TMD) pain patients. Exp Brain Res 2014;232:3111–9. https://doi.org/10.1007/s00221-014-3997-7.Search in Google Scholar PubMed
33. Yarnitsky, D, Arendt-Nielsen, L, Bouhassira, D, Edwards, RR, Fillingim, RB, Granot, M, et al.. Recommendations on terminology and practice of psychophysical DNIC testing. Eur J Pain 2010;14:339. https://doi.org/10.1016/j.ejpain.2010.02.004.Search in Google Scholar PubMed
34. Yarnitsky, D, Crispel, Y, Eisenberg, E, Granovsky, Y, Ben-Nun, A, Sprecher, E, et al.. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain 2008;138:22–8. https://doi.org/10.1016/j.pain.2007.10.033.Search in Google Scholar PubMed
35. Larsen, DB, Laursen, M, Edwards, RR, Simonsen, O, Arendt-Nielsen, L, Petersen, KK. The combination of preoperative pain, conditioned pain modulation, and pain catastrophizing predicts postoperative pain 12 months after total knee arthroplasty. Pain Med 2021;22:1583–90. https://doi.org/10.1093/pm/pnaa402.Search in Google Scholar PubMed
36. Wilder-Smith, OH, Schreyer, T, Scheffer, GJ, Arendt-Nielsen, L. Patients with chronic pain after abdominal surgery show less preoperative endogenous pain inhibition and more postoperative hyperalgesia: a pilot study. J Pain Palliat Care Pharmacother 2010;24:119–28. https://doi.org/10.3109/15360281003706069.Search in Google Scholar PubMed
37. Takashima, K, Oono, Y, Takagi, S, Wang, K, Arendt-Nielsen, L, Kohase, H. Acute postoperative pain after orthognathic surgery can be predicted by the preoperative evaluation of conditioned pain modulation and pain catastrophizing. Pain Rep 2022;7:e989. https://doi.org/10.1097/pr9.0000000000000989.Search in Google Scholar PubMed PubMed Central
38. Kennedy, DL, Kemp, HI, Ridout, D, Yarnitsky, D, Rice, ASC. Reliability of conditioned pain modulation: a systematic review. Pain 2016;157:2410–9. https://doi.org/10.1097/j.pain.0000000000000689.Search in Google Scholar PubMed PubMed Central
39. Petrini, L, Arendt-Nielsen, L. Understanding pain catastrophizing: putting pieces together. Front Psychol 2020;11:603420. https://doi.org/10.3389/fpsyg.2020.603420.Search in Google Scholar PubMed PubMed Central
40. Sullivan, MJL, Bishop, SR, Pivik, J. The pain catastrophizing scale: development and validation. Psychol Assess 1995;7:524–32. https://doi.org/10.1037/1040-3590.7.4.524.Search in Google Scholar
41. Sullivan, MJ, Thorn, B, Haythornthwaite, JA, Keefe, F, Martin, M, Bradley, LA, et al.. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain 2001;17:52–64. https://doi.org/10.1097/00002508-200103000-00008.Search in Google Scholar PubMed
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Editorial Comment
- What do we mean by “biopsychosocial” in pain medicine?
- Systematic Review
- The efficacy of manual therapy on HRV in those with long-standing neck pain: a systematic review
- Clinical Pain Research
- Development of a binary classifier model from extended facial codes toward video-based pain recognition in cancer patients
- Experience and usability of a website containing research-based knowledge and tools for pain self-management: a mixed-method study in people with high-impact chronic pain
- Effect on orofacial pain in patients with chronic pain participating in a multimodal rehabilitation programme – a pilot study
- Analysis of Japanese nationwide health datasets: association between lifestyle habits and prevalence of neuropathic pain and fibromyalgia with reference to dementia-related diseases and Parkinson’s disease
- Impact of antidepressant medication on the analgetic effect of repetitive transcranial magnetic stimulation treatment of neuropathic pain. Preliminary findings from a registry study
- Does lumbar spinal decompression or fusion surgery influence outcome parameters in patients with intrathecal morphine treatment for persistent spinal pain syndrome type 2 (PSPS-T2)
- Original Experimentals
- Low back-pain among school-teachers in Southern Tunisia: prevalence and predictors
- Economic burden of osteoarthritis – multi-country estimates of direct and indirect costs from the BISCUITS study
- Demographic and clinical factors associated with psychological wellbeing in people with chronic, non-specific musculoskeletal pain engaged in multimodal rehabilitation: –a cross-sectional study with a correlational design
- Interventional pathway in the management of refractory post cholecystectomy pain (PCP) syndrome: a 6-year prospective audit in 60 patients
- Original Articles
- Preoperatively assessed offset analgesia predicts acute postoperative pain following orthognathic surgery
- Oxaliplatin causes increased offset analgesia during chemotherapy – a feasibility study
- Effects of conditioned pain modulation on Capsaicin-induced spreading muscle hyperalgesia in humans
- Effects of oral morphine on experimentally evoked itch and pain: a randomized, double-blind, placebo-controlled trial
- The potential effect of walking on quantitative sensory testing, pain catastrophizing, and perceived stress: an exploratory study
- What matters to people with chronic musculoskeletal pain consulting general practice? Comparing research priorities across different sectors
- Is there a geographic and gender divide in Europe regarding the biopsychosocial approach to pain research? An evaluation of the 12th EFIC congress
Articles in the same Issue
- Frontmatter
- Editorial Comment
- What do we mean by “biopsychosocial” in pain medicine?
- Systematic Review
- The efficacy of manual therapy on HRV in those with long-standing neck pain: a systematic review
- Clinical Pain Research
- Development of a binary classifier model from extended facial codes toward video-based pain recognition in cancer patients
- Experience and usability of a website containing research-based knowledge and tools for pain self-management: a mixed-method study in people with high-impact chronic pain
- Effect on orofacial pain in patients with chronic pain participating in a multimodal rehabilitation programme – a pilot study
- Analysis of Japanese nationwide health datasets: association between lifestyle habits and prevalence of neuropathic pain and fibromyalgia with reference to dementia-related diseases and Parkinson’s disease
- Impact of antidepressant medication on the analgetic effect of repetitive transcranial magnetic stimulation treatment of neuropathic pain. Preliminary findings from a registry study
- Does lumbar spinal decompression or fusion surgery influence outcome parameters in patients with intrathecal morphine treatment for persistent spinal pain syndrome type 2 (PSPS-T2)
- Original Experimentals
- Low back-pain among school-teachers in Southern Tunisia: prevalence and predictors
- Economic burden of osteoarthritis – multi-country estimates of direct and indirect costs from the BISCUITS study
- Demographic and clinical factors associated with psychological wellbeing in people with chronic, non-specific musculoskeletal pain engaged in multimodal rehabilitation: –a cross-sectional study with a correlational design
- Interventional pathway in the management of refractory post cholecystectomy pain (PCP) syndrome: a 6-year prospective audit in 60 patients
- Original Articles
- Preoperatively assessed offset analgesia predicts acute postoperative pain following orthognathic surgery
- Oxaliplatin causes increased offset analgesia during chemotherapy – a feasibility study
- Effects of conditioned pain modulation on Capsaicin-induced spreading muscle hyperalgesia in humans
- Effects of oral morphine on experimentally evoked itch and pain: a randomized, double-blind, placebo-controlled trial
- The potential effect of walking on quantitative sensory testing, pain catastrophizing, and perceived stress: an exploratory study
- What matters to people with chronic musculoskeletal pain consulting general practice? Comparing research priorities across different sectors
- Is there a geographic and gender divide in Europe regarding the biopsychosocial approach to pain research? An evaluation of the 12th EFIC congress


