The association between selected genetic variants and individual differences in experimental pain
-
Marie Udnesseter Lie
, Bendik Winsvold
Abstract
Objectives
The underlying mechanisms for individual differences in experimental pain are not fully understood, but genetic susceptibility is hypothesized to explain some of these differences. In the present study we focus on three genetic variants important for modulating experimental pain related to serotonin (SLC6A4 5-HTTLPR/rs25531 A>G), catecholamine (COMT rs4680 Val158Met) and opioid (OPRM1 rs1799971 A118G) signaling. We aimed to investigate associations between each of the selected genetic variants and individual differences in experimental pain.
Methods
In total 356 subjects (232 low back pain patients and 124 healthy volunteers) were genotyped and assessed with tests of heat pain threshold, pressure pain thresholds, heat pain tolerance, conditioned pain modulation (CPM), offset analgesia, temporal summation and secondary hyperalgesia. Low back pain patients and healthy volunteers did not differ in regards to experimental test results or allelic frequencies, and were therefore analyzed as one group. The associations were tested using analysis of variance and the Kruskal-Wallis test.
Results
No significant associations were observed between the genetic variants (SLC6A4 5-HTTLPR/rs25531 A>G, COMT rs4680 Val158Met and OPRM1 rs1799971 A118G) and individual differences in experimental pain (heat pain threshold, pressure pain threshold, heat pain tolerance, CPM, offset analgesia, temporal summation and secondary hyperalgesia).
Conclusions
The selected pain-associated genetic variants were not associated with individual differences in experimental pain. Genetic variants well known for playing central roles in pain perception failed to explain individual differences in experimental pain in 356 subjects. The finding is an important contribution to the literature, which often consists of studies with lower sample size and one or few experimental pain assessments.
Introduction
Assessments of experimental pain are assumed to be of clinical value in management of pain patients, but the underlying mechanisms for individual differences in experimental pain are not fully understood and needs to be better addressed. Assessments of experimental pain may include tests for pain sensitivity, e.g. pain threshold and pain tolerance, or tests that assess the dynamic function of pain modulation, e.g. conditioned pain modulation (CPM), offset analgesia, temporal summation and secondary hyperalgesia.
Increased pain sensitivity has been associated with numerous pain disorders [1], [2], [3] and is regarded as one of the characteristics in central sensitization of the nervous system [4]. CPM represents reduced pain perception of a painful stimulus (test-stimulus) when a second painful stimulus (conditioning stimulus) is inflicted and is assumed to measure inhibitory pain modulation [5]. CPM has also been associated with pain disorders [6] and has been shown to predict the development of pain [7], [8], [9] and treatment response [10], [11]. Offset analgesia is another measure of inhibitory pain modulation, where a disproportionate decrease in pain perception is seen after a small decrease in stimulus intensity [12]. Similar to CPM, offset analgesia has been associated with pain disorders [13], [14], [15]. Tests that reflect central sensitization in pain disorders are temporal summation, which represents an increase in pain perception despite no change in stimulation intensity [16], [17] and secondary hyperalgesia, which is present if the tissue beyond an area of tissue damage (primary hyperalgesia) becomes hypersensitive [18], [19].
One of the underlying mechanisms for individual differences in experimental pain is genetic susceptibility. Many genetic variants are assumed to be important for modulating pain perception, but genetic variants related to serotonin (5-HT), catecholamine and opioid signaling have been of particularly interest and extensively studied due to their physiological function [20]. However, results from studies examining association between these variants and individual differences in experimental pain in humans are conflicting [21], [22], [23], [24], [25] and more studies are needed to elucidate whether these genetic variants can explain individual differences in experimental pain. Therefore, the present study aimed to investigate associations between each of the selected genetic variants; SLC6A4 5-HTTLPR/rs25531 A>G, COMT rs4680 Val158Met and OPRM1 rs1799971 A118G, and individual differences in experimental pain.
Methods
Study design
The present study used data from a prospective cohort study of acute low back pain patients admitted to a hospital (n=232) [26], [27]. The present study was a cross-sectional study using socio-demographic data assessed through questionnaires, blood samples collected at hospital admission, and data from experimental pain testing performed six weeks after hospital admission. Similar data have been collected from healthy volunteers participating in studies at the same laboratory as the low back pain patients (n=124) [27], [28], [29]. The present study combined data from the low back pain patients and healthy volunteers.
A written informed consent was obtained prior to participation. The study was approved by the regional committee for medical and health research ethics in Norway (project number: 2010/2927, 2012/1108) and was conducted in accordance with the Declaration of Helsinki. Healthy volunteers received a gift certificate of NOK 250 for participation.
Study population
Patients were recruited from the Department of Neurology at Oslo University Hospital in Norway between January 2013 and June 2018. Inclusion criteria were age 18 years or older, acute low back pain with or without radiating pain, pain rated ≥4 on an 11 point numeric rating scale (NRS) (0=‘no pain’, 10=‘worst pain imaginable’). Healthy volunteers were recruited by advertisement at local hospitals and colleges/universities in Oslo, Norway. Inclusion criteria were men and women self-reported to be healthy, aged 18–60 years. Exclusion criteria for patients and healthy volunteers were non-Caucasian heritage (mother or father), inability to understand spoken or written Norwegian, not currently working, previous or current alcoholism or substance abuse, regular use of neuroleptics and tricyclic antidepressants, pregnancy, breastfeeding, psychiatric or somatic diseases making the person unsuitable for inclusion, spinal fracture, malignancy, infection, cauda equina syndrome, rapidly progressive neurologic deficits or chronic pain defined as pain rated ≥4 on an NRS for ≥3 month in the last two years.
Experimental pain testing
The experimental pain testing procedure consisted of standardized tests for sensitivity (pressure pain thresholds, heat pain threshold and heat pain tolerance) and for pain modulation (CPM, offset analgesia, temporal summation and secondary hyperalgesia). Subjects were blinded to the study hypothesis and readouts from the stimulation instruments. A pretest was performed to familiarize subjects with the stimulations and pain intensity rating procedures. Subjects continuously rated the pain intensity on a computerized 10 cm horizontal visual analog scale (VAS) (left end (0 cm): ‘no pain’, right end (10 cm): ‘worst pain imaginable’) by scrolling the wheel on a computer mouse in all constant heat stimulations if not otherwise described. See supporting information TableS1 for instrumental details of the different tests.
Pressure pain threshold
To assess pressure pain threshold, the experimenters manually increased pressure (5 N/s) on muscle trapezius with a 1 cm2 pressure algometer (AlgoMed, Medoc, Ramat Yishai, Israel). The subjects rated their pain by moving a knob along a 10 cm VAS on a box. The left side of the line represented ‘no pain’, and the right side line represented ‘worst pain imaginable’. The subjects were instructed to not move the knob until pain was first experienced. Assessments were performed bilaterally and an average value of the two assessments was used in the analyses.
Heat pain thresholds and tolerance
Heat pain threshold and heat pain tolerance were assessed with gradually increasing the temperature during stimulation on the distal volar aspect of the right forearm with a 30 × 30 mm Peltier thermode (baseline temperature: 32 °C, increase: rate 2 °C/s, decrease rate: 8 °C/s) (Pathway model ATS, Medoc, Ramat Yishai, Israel). When assessing heat pain threshold, subjects were instructed to stop the increase in temperature by clicking on a computer mouse when they felt the first sensation of pain. When assessing heat pain tolerance, subjects were instructed to click on the computer mouse when they could not tolerate the increasing temperature any longer. The temperature was automatically stopped at 52 °C for safety reasons. If the subject did not reach its threshold before 52 °C, this temperature was noted as the threshold. The tests were repeated three times and an average value was used in the analyses.
Pain6 calculation
A temperature aimed to reflect pain intensity equal to approximately 6 cm on 10 cm VAS (Pain6) was used during the tests for pain modulation. In order to estimate the Pain6 temperature for each individual, 2 °C was subtracted from an average of three tests of pain tolerance (see section heat pain thresholds and tolerance). The estimated temperature was thereafter tested with a 30 s heat stimulus with a 30 × 30 mm Peltier thermode (baseline temperature: 32 °C, increase rate: 1 °C/s, decrease rate: 8 °C/s) (Pathway model ATS, Medoc, Ramat Yishai, Israel) on the left thenar eminence. If the first 20 s of the stimulation was rated outside 4–9 cm on a 10 cm VAS, the temperature was adjusted accordingly.
Conditioned pain modulation
To assess CPM, a baseline test-stimulus was applied, followed by a 5-min break, before an identical test-stimulus was applied in parallel with a conditioning stimulus. The test-stimulus was a constant heat stimulation from a 30 × 30 mm Peltier thermode (baseline temperature: 32 °C, increase rate: 1 °C/s, decrease rate: 8 °C/s) (Pathway model ATS, Medoc, Ramat Yishai, Israel) with Pain6 temperature for 120 s on the right forearm. The conditioning stimulus was the opposite hand immersed in a 7 °C circulating water bath (LAUDA Alpha RA8, LAUDA-Brinkman LP., New Jersey, USA) with water up to the wrist and the hand held wide open for 120 s or until the pain forced the subject to withdraw the hand from the water bath. After 120 s, subjects were asked to rate the pain intensity of the conditioning stimulus on a 0–10 NRS. To avoid sensitization or habituation of the stimulated area, the area of the baseline test-stimulus and the test-stimulus in parallel with conditioning stimulus was not overlapping. Fifty of the healthy volunteers were part of a subproject and were randomized in regards to stimulation arm. A CPM effect was defined as the difference in average pain intensity between the test-stimulus alone and the test-stimulus in parallel with the conditioning stimulus. The CPM effect was also calculated as a percent change (CPM effect/test-stimulus alone × 100).
Offset analgesia
Two trials with heat stimulation with a 30 × 30 mm Peltier thermode (baseline temperature: 32 °C, increase rate: 1 °C/s, decrease rate: 8 °C/s) (Pathway model ATS, Medoc, Ramat Yishai, Israel) on the right forearm were used to assess offset analgesia. One trial had 30 s constant Pain6 temperature, while the other trial consisted of a three-temperature paradigm; first, heat stimulation was applied with Pain6 temperature for 5 s (T1). Next, the temperature was increased by 1 °C and kept constant for 5 s (T2) before the temperature returned to the initial temperature and kept constant for 20 s (T3). The stimulated area of the two trials was not overlapping to avoid sensitization or habituation of the stimulation area. The order and position of the trials were randomized, and the trials were separated by a 2-min break. Offset analgesia was calculated as the difference in pain ratings between T3-T2 during the three-temperature paradigm compared to the same time interval in the constant stimulation.
Temporal summation
Temporal summation was assessed by heat stimulation with a 30 × 30 mm Peltier thermode (baseline temperature: 32 °C, increase rate: 1 °C/s, decrease rate: 8 °C/s) (Pathway model ATS, Medoc, Ramat Yishai, Israel) on the right forearm with a constant Pain6 temperature for 120 s, except for 50 of the healthy volunteers who were part of a subproject and were randomized in regards to the stimulation arm. Temporal summation was defined as an increase in pain ratings (>0 cm) on a 10 cm VAS from the start (30–40 s) to the end (110–120 s) of the stimulation.
Secondary hyperalgesia
A 5-min heat stimulation of 45 °C with a 30 × 30 mm Peltier thermode (baseline temperature: 32 °C, increase rate: 1 °C/s, decrease rate: 8 °C/s) (Pathway model ATS, Medoc, Ramat Yishai, Israel) was used to create an area of primary hyperalgesia in the center of the volar aspect of the left forearm. After a 2-min break, a von Frey filament (Touch-Test TM Sensory Evaluator, Stoelting, Illinois, USA) was used to map the area of secondary hyperalgesia. The filament was pressed against the skin at 90° angle until the filament bowed, starting at a 5–6 cm distance from the heat stimulation area and repeated every 0.5 cm with 3–4 s intervals in eight directions 45° towards the heat stimulation area. The order of the directions was randomized. Subjects were instructed to look away from the arm and indicate when a prick had a clear change in sensation. This point was then marked with a colored pencil. After all directions were tested, the markings were transferred on to transparency film. The area of secondary hyperalgesia was extracted and calculated with Engauge Digitizer Software, version 10.8.
Genotyping
Blood samples were obtained in 4 ml EDTA tubes and frozen at −80 °C until DNA extraction was performed with QIAamp DNA Blood Kit (n=326) or QIAGEN Autopure LS (n=30) according to the manufacturer’s protocol (QIAGEN, Valencia, CA, USA). The genetic variants genotyped were SLC6A4 5-HTTLPR/rs25531 A>G, COMT rs4680 Val158Met and OPRM1 rs1799971 A118G. Genotypes were determined using fast quantitative real time polymerase chain reactions (qPCR) (Gene Amp, PCR System 9700, Applied Biosystems, California, USA). PCR amplifications were performed with 384-well plates containing genomic DNA, TaqPath ProAmp Master Mix and TaqMan SNP genotyping assay (Applied Biosystems, Foster City, CA) (see supporting information TableS2 for details of the genotyping). Negative controls containing water only were included in every run. Samples with undetermined genotypes were re-genotyped. The overall genotype call rate was 98%.
Regarding the SLC6A4 5-HTTLPR/rs25531, we performed gel electrophoresis to determine the long (529 bp) and short (486 bp) allele. Fragments were visualized with ultraviolet light after 2 h separation at 80 V in TAE buffer on a 2.5% agarose gel (MetaPhorTM Agarose, Lonza, Cologne, Germany), containing GelRed (Biotium Inc, California, USA). As previously described [30], the SLC6A4 5-HTTLPR and SLC6A4 rs25531 were divided into three groups; low (SA/SA), medium (SLG, LA/LG, SLA) or high (LA/LA) 5-HTT expression types.
Statistical analysis
Statistical analyses were conducted using SPSS Statistics version 25 (IBM, Armonk, NY). The distribution of sample characteristics and experimental pain test results were assessed in preliminary analyses by a Shapiro–Wilk test for normality and inspection of descriptive statistics, histograms, boxplots, and Q-Q plots.
Sample size calculations showed that with a two-sided significance level of 5 and 80% power, 228 subjects were needed to detect a 10% difference in pain scores between genotypes with a standard deviation of 20 cm on a 10 cm visual analogue scale (VAS, left end: ‘no pain’, right end: ‘worst pain imaginable’), assuming a genetic variant is present in 20% of the population. When offset analgesia and secondary hyperalgesia were added to the test protocol, new sample size calculations were made based on a standard deviation of 17, resulting in 168 subjects needed to detect a difference. No difference in individual differences in experimental pain have been observed between our two samples of low back pain patients and healthy volunteers ([27] and unreported studies), so to increase our sample size we chose to combine low back pain patients and healthy volunteers in the association analysis. To ensure that findings was due to associations between genetic variance and individual differences in experimental pain, low back pain patients and healthy volunteers were tested for systematic differences in sample characteristics, individual differences in experimental pain and genotype distributions. Similar comparison were done between patients who had almost or fully recovered from the acute back pain (defined as <3 VAS at the six weeks follow-up) and patients still in a pain state when the experimental tests were performed (defined as leg pain ≥3 VAS at the six weeks follow-up). Independent sample Student’s t-test was used for normally-distributed variables, Mann-Whitney U-test was used for variables with non-normal distribution, and Chi-square or Fisher’s exact test was used for categorical variables.
Paired sample Student’s t-test was used to determine if there was a CPM effect, temporal summation, and offset analgesia. Analysis of variance (ANOVA) or the non-parametric alternative Kruskal-Wallis test was used to determine the association between the selected genetic variants and individual differences in experimental pain.
Since earlier studies have shown that OPRM1 A118G may be sex-specific [31], [32], [33], a multivariate ANOVA was performed to investigate interactions between OPRM1 A118G and sex. Findings with p-values<0.01 were regarded as significant for all statistical analyses due to multiple testing.
Results
Sample characteristics
In total 356 subjects (232 back pain patients and 124 healthy volunteers) were genotyped and pain tested (Figure 1). Sample characteristics and gene frequencies are presented in Table 1, with details in Supplementary Table 3. Low back pain patients and healthy volunteers did not differ with regards to sample characteristics or distribution of genotypes, except for age (p<0.001), body-mass index (BMI) (p<0.001) and diastolic blood pressure (p<0.001) (Supporting information TableS3). No differences were found in sample characteristics and distribution of genotypes between patients with leg pain VAS <3 or VAS ≥3, except for age (p=0.002) and education (p=0.002) (Supporting information TableS3).
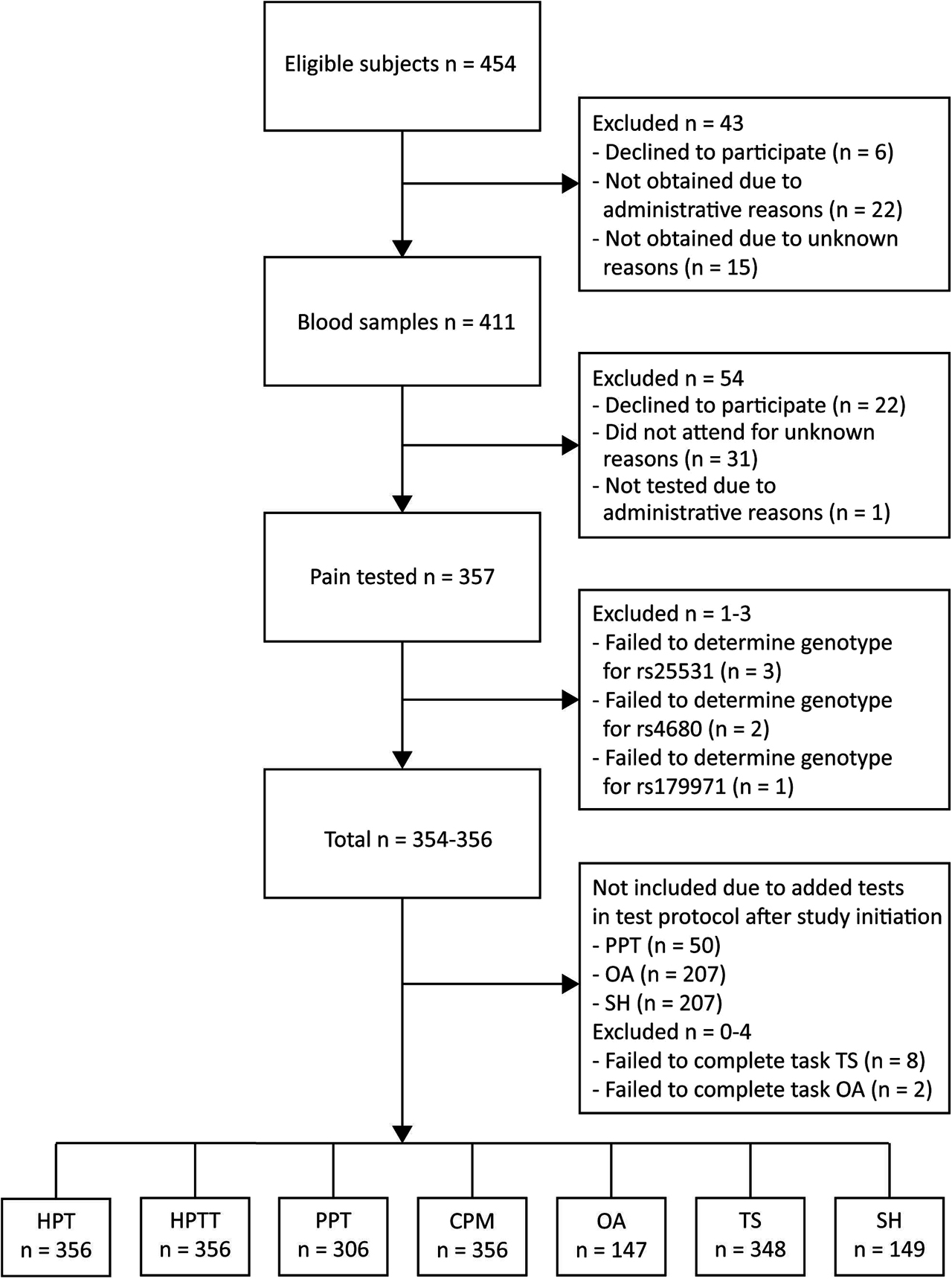
Flowchart.
PPT, pressure pain threshold; HPT, heat pain threshold; HPTT, heat pain tolerance; CPM, conditioned pain modulation; OA, offset analgesia; TS, temporal summation; SH, secondary hyperalgesia.
Sample characteristics.
| Variable | n | Value |
|---|---|---|
| Sex (males), n (%) | 357 | 208 (58.3) |
| Age (years), median (IQR) | 357 | 35 (26–45) |
| Education (>12 years), n (%) | 357 | 321 (89.9) |
| Left handed, n (%) | 346 | 38 (10.6) |
| BMI (kg/m2) mean (SD) | 351 | 25.2 (3.6) |
| Systolic blood pressure (mmHg), mean (SD) | 357 | 123.3 (13.3) |
| Diastolic blood pressure (mmHg), mean (SD) | 357 | 74.7 (9.7) |
| Current smoker, n (%) | 354 | 44 (12.3) |
| 5HTTLPR/rs25531 (SLC6A4), MAF | 354 | 0.3 |
| Val158Met (COMT), MAF | 355 | 0.4 |
| A118G (OPRM1), MAF | 356 | 0.1 |
-
IQR, inter quartile range; SD, standard deviation; MAF, minor allele frequency.
Experimental pain tests
Results from the assessments of pain modulation are presented in Figure 2A–D. There was a significant difference between pain ratings during baseline test-stimulus and pain ratings during test-stimulus in parallel with the conditioning stimulus (effect size=−2.5, SD=1.7, p<0.001), representing a CPM effect of −48.9%. In the offset analgesia paradigm, there was a significant difference between pain ratings during T3-T2 in the constant stimulation and pain ratings during T3-T2 in the three-temperature paradigm (effect size=−0.5, SD=1.8, p<0.001). Temporal summation of pain during constant heat stimulation was found, as there was a significant difference between pain ratings at the start and at the end of the constant heat stimulation (effect size=0.6, SD=2.1, p<0.001). For none of the experimental pain tests did test results differ between patients and healthy volunteers, or between patients with leg pain VAS<3 or VAS≥3 (Supplementary Table 3).
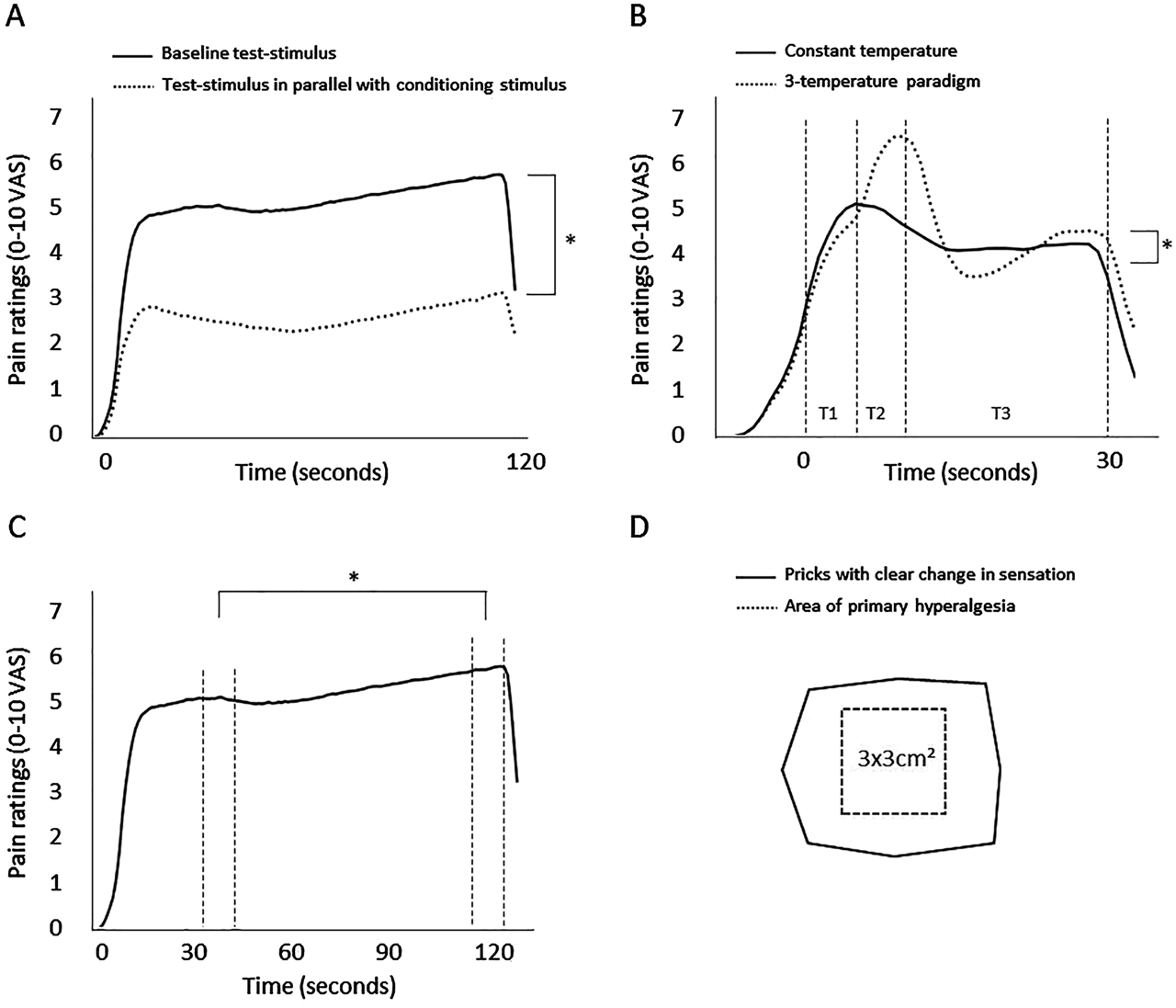
Results from the experimental pain assessments of pain modulation.
(A) A conditioned pain modulation (CPM) effect was present, with a decrease in pain ratings of the test-stimulus during conditioning stimulus (p<0.001), (B) Offset analgesia was present with a larger decrease in pain ratings in the three-temperature paradigm than in the constant paradigm (p<0.001), (C) Temporal summation of pain was observed with a significant increase in mean pain ratings during the continuous heat pain stimulation (p<0.001). The vertical lines marks the time periods that was compared, (D) Illustration of the area of secondary hyperalgesia for 97 of 149 subjects. In 52 of the subjects the direction of the transparency film, which the markings were transferred to, was unknown and could not be used in the illustration.
VAS, visual analog scale.
Genetic associations
There were no significant associations between any of the selected genetic variants, SLC6A4 5-HTTLPR/rs25531 A>G, COMT rs4680 Val158Met and OPRM1 rs1799971 A118G and individual differences in experimental pain assessed with pressure pain threshold, heat pain thresholds, heat pain tolerance, CPM, offset analgesia, temporal summation or secondary hyperalgesia (Figure 3 and Table 2). No significant interaction was found between OPRM1 A118G and sex in regards to individual differences in experimental pain (p=0.575).
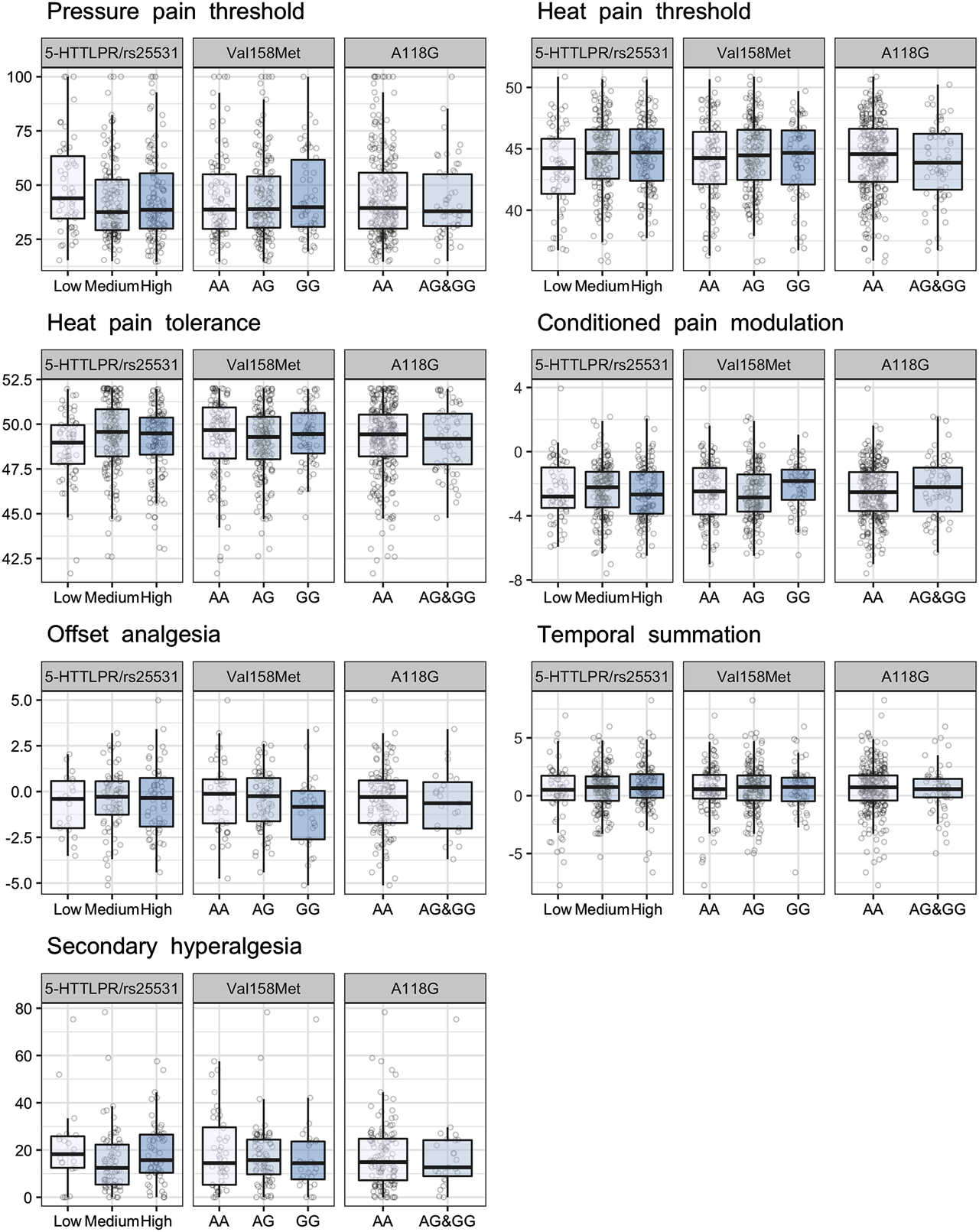
Associations between the selected genetic variants and individual differences in experimental pain. Findings with p-values≤0.01 were regarded as significant. There was no significant association between the selected genetic variants and individual differences in experimental pain.
Associations between the selected genetic variants and individual differences in experimental pain. Findings with p-values<0.01 were regarded as significant.
| Variant (gene) | Genotype | n | Mean (SD)/median (IQR) | r2/χ2 | p-Value | n | Mean (SD)/median (IQR) | r2/χ2 | p-Value | n | Mean (SD)/median (IQR) | r2/χ2 | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pressure pain thresholda | Heat pain thresholdb | Heat pain tolerancea | |||||||||||
| 5-HTTLPR/rs25531 (SLC6A4) | Lowc | 51 | 43.9 (34.3–65.5) | 4.3 | 0.12 | 63 | 43.3 (3.4) | 33.7 | 0.03 | 63 | 49.0 (47.7–50.0) | 6.7 | 0.04 |
| Mediumd | 141 | 37.5 (29.2–52.5) | 169 | 44.4 (3.1) | 169 | 49.6 (48.2–50.9) | |||||||
| Highe | 114 | 38.6 (29.8–55.7) | 124 | 44.5 (2.9) | 124 | 49.5 (48.3–50.4) | |||||||
| Val158Met (COMT) | AA | 98 | 38.7 (29.7–55.4) | 1.0 | 0.79 | 114 | 44.1 (3.2) | 2.9 | 0.82 | 114 | 49.7 (48.0–51.0) | 2.2 | 0.53 |
| AG | 152 | 38.9 (30.1–54 0) | 176 | 44.4 (3.0) | 176 | 49.3 (48.0–50.4) | |||||||
| GG | 54 | 39.8 (30.4–63.5) | 64 | 44.2 (3.2) | 64 | 49.5 (48.3–50.7) | |||||||
| A118G (OPRM1) | AA | 253 | 39.4 (29.9–56.0) | 0.3 | 0.60 | 294 | 44.3 (3.1) | 14.7 | 0.21 | 294 | 49.4 (48.2–50.5) | 0.1 | 0.75 |
| AG + GG | 53 | 37.9 (30.8–55.4) | 62 | 43.8 (3.1) | 62 | 49.2 (47.7–50.6) | |||||||
| Conditioned pain modulationb | Offset analgesiab | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HTTLPR/rs25531 (SLC6A4) | Lowc | 63 | −2.4 (1.8) | 1.0 | 0.73 | 21 | −0.7 (1.6) | 0.6 | 0.84 | ||||
| Mediumd | 169 | −2.4 (1.7) | 69 | −0.5 (1.7) | |||||||||
| Highe | 123 | −2.6 (1.7) | 56 | −0.5 (1.9) | |||||||||
| Val158Met (COMT) | AA | 114 | −2.4 (1.9) | 7.1 | 0.07 | 43 | −0.3 (2.0) | 4.3 | 0.25 | ||||
| AG | 176 | −2.6 (1.7) | 73 | −0.4 (1.6) | |||||||||
| GG | 63 | −2.1 (1.5) | 29 | −1.1 (1.9) | |||||||||
| A118G (OPRM1) | AA | 293 | −2.5 (1.7) | 4.7 | 0.21 | 123 | −0.5 (1.8) | 0.4 | 0.73 | ||||
| AG + GG | 62 | −2.2 (1.9) | 23 | −0.6 (1.8) | |||||||||
| Temporal summationb | Secondary hyperalgesiaa | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HTTLPR/rs25531 (SLC6A4) | Lowc | 62 | 0.2 (2.6) | 7.8 | 0.16 | 21 | 18.2 (12.3–26.0) | 4.2 | 0.12 | ||||
| Mediumd | 163 | 0.7 (1.8) | 71 | 12.4 (5.3–22.6) | |||||||||
| Highe | 122 | 0.8 (2.0) | 57 | 15.7 (10.4–26.6) | |||||||||
| Val158Met (COMT) | AA | 113 | 0.6 (2.2) | 0.3 | 0.97 | 44 | 14.5 (5.2–30.3) | 2.3 | 0.52 | ||||
| AG | 169 | 0.7 (2.0) | 75 | 15.7 (9.1–24.4) | |||||||||
| GG | 63 | 0.6 (1.9) | 29 | 14.4 (7.2–23.9) | |||||||||
| A118G (OPRM1) | AA | 288 | 0.7 (2.1) | 0.8 | 0.67 | 126 | 14.9 (7.1–24.9) | 0.1 | 0.75 | ||||
| AG + GG | 59 | 0.5 (2.0) | 23 | 12.6 (7.6–24.2) | |||||||||
-
SD, standard deviation; IQR, inter quartile range; r2, Analysis of variance (ANOVA) R-squared value; χ2, Kruskal-Wallis test chi-squared value.
-
aPresented with median (IQR) and Kruskal-Wallis χ2.
-
bPresented with mean (SD) and ANOVA r2.
-
cLow 5-HTT expression type (SA/SA).
-
dMedium 5-HTT expression type (SLG, LA/LG, SLA).
-
eHigh 5-HTT expression type (LA/LA).
Discussion
In the present study, we found no association between the selected genetic variants, SLC6A4 5-HTTLP/ rs25331 A>G, COMT Val158Met or OPRM1 A118G and individual differences in pressure pain threshold, heat pain threshold, heat pain tolerance, CPM, offset analgesia, temporal summation or secondary hyperalgesia. To our knowledge, the present study is one of the largest candidate gene study investigating associations between the selected genetic variants and individual differences in experimental pain. The present study is also the first to explore the association between the selected genetic variants and offset analgesia and secondary hyperalgesia.
The serotonin transporter (5-HTT), encoded by the SLC6A4 gene, plays a central role in the uptake of serotonin in the synaptic cleft. A length polymorphism (5-HTTLPR) in the promoter region of SLC6A4 results in two common variants; a short (S) and a long (L) allele [34], [35], [36]. The S allele leads to reduced 5-HTT expression, which may influence 5-HT signaling [37], [38]. In addition, a single nucleotide polymorphism (SNP) rs25531 A>G in the same promoter region is also associated with reduced 5-HTT expression [39]. Previous studies have shown a relationship between SLC6A4 5-HTTLPR/rs25531 A>G and individual differences in experimental pain, where the low 5-HTT expression type typically is associated with lower heat pain threshold [23], [40], and impaired CPM [24], but one study observed higher heat pain thresholds for the low expression type [41]. The present study did not show significant evidence to support these studies, but may point in the direction of a possible association between low expression type and lower heat pain threshold (p=0.03) and heat pain tolerance (p=0.04) due to the observed trend. Consistent with our results, some studies have shown no relationship between the SLC6A4 5-HTTLPR/rs25531 A>G and individual differences in experimental pain [25], [42], [43], [44]. A possible explanation for the diverse findings in the literature can be use of different test parameters when assessing experimental pain. Another explanation for conflicting results could be related to the complexity of the serotonergic system. A high concentration of serotonin in the synaptic cleft may impact the nearby postsynaptic 5-HT receptors, which results in increased signaling, or it may impact the presynaptic autoreceptors, which results in an increase of negative feedback and thereby decrease signaling [35]. However, this may depend on the location of localization of the 5-HTT relative to the 5-HT autoreceptors [45], [46]. Serotonin is also regulated by the seven different groups of 5HT-receptors, which mediate both excitatory and inhibitory neurotransmission.
Catechol-O-methyltransferase (COMT) encoded by the COMT gene, is an enzyme that promote degradation of catecholamines (dopamine, epinephrine, and norepinephrine). The SNP rs4680 G>A causes a substitution of the amino acid valine (Val) to methionine (Met) at codon 158, and reduces enzyme activity which results in higher levels of catecholamines [47]. The relationship between COMT Val158Met and individual differences in experimental pain has been studied in numerous animal and human pain models. Results are somewhat conflicting, with some studies reporting that the Met allele is associated with lower pressure pain thresholds, heat pain threshold, and temporal summation [21], [48], [49], [50], [51], while other studies find an opposite effect [22] or no association with individual differences in experimental pain [52], consistent with the present study’s results. However, the observed trend between COMT Val158Met and CPM (p=0.07) in the present study, may suggest a possible association between the Met allele and impaired CPM. The inconsistencies in the literature may be due to different sample selection [51], [53] and sample sizes, different choice of experimental tests or different tests protocols [22], [54].
Opioid signaling is regulated by the μ opioid receptor encoded by the OPRM1 gene. The SNP rs1799971 A>G causes a substitution of the amino acid asparagine to aspartic acid at codon 40, and removes a putative N-linked glycosylation site in the receptor, which may affect the function of the receptor [55], [56]. The G allele in OPRM1 A118G has been associated with higher pressure pain thresholds [57], [58], which is in contrast with the present study. Similar to the present study, some studies found no relationship between OPRM1 A118G and heat pain threshold and pressure pain threshold [59], [60], [61], [62]. The conflicting results could potentially be explained by sex-differences. An asparagine to aspartic amino acid substitution in OPRM1 A118G affects the glycosylation site of the receptor, which is important for cellular processes such as receptor folding, sorting, expression and ligand binding [63]. The level and type of glycosylation have shown to be different between female and male mice [33], [64], and some human studies have shown opposite effects of OPRM1 A118G in men and women [31], [32], [65]. For this reason we also analyzed the interaction of OPRM1 A118G and sex in regards to individual differences in experimental pain, but no such interactions were found.
Strength and limitations
The present study investigated pain sensitivity as well as anti- and pro-nociceptive functions of the pain system. We chose tests which have relatively large effects, with the outcome measure as a continuous endpoint, which enables differentiation between subjects and increase the power of the study. To date, there is no gold standard for assessing the dynamic function of the pain system. When using a genetic model to predict individual differences in experimental pain, one assumes that experimental pain response is a stable trait. However, results of experimental pain assessments has been shown to be influenced by psychological and environmental factors [66], and the reliability of the different tests range from poor to good depending on the methodology of the tests as well as statistics [67], [68]. Further research should establish gold standards for assessing experimental pain, which will likely lead to more consistent results between studies, and improve the chances to identify genetic risk factors.
The present study sample was heterogeneous, consisting of both healthy volunteers, patients that had recovered from acute low back pain, and patients still in pain after an acute low back pain episode six weeks earlier. Combining experimental data from patients and healthy volunteers are potentially problematic, but could be done because the groups did not differ with regards to experimental pain test results and genetic variant allele frequencies. In a genetic association study, factors such as age and sex are not considered potential confounders, since they do not affect the genetic variants, but sample heterogeneity can lead to reduced power, contributing to our negative results [69]. Although the sample size of the present study is small compared to association studies of clinical pain disorders, the sample size is relatively large compared to studies investigating association between the same selected genetic variants and individual differences in experimental pain. That the present study with 356 subjects does not find evidence to support findings from studies of smaller sample size emphasizes the limitations of experimental studies with a candidate gene approach and the importance of replication of findings before conclusions can be reached. Several experimental tests are often used in experimental pain studies, however few are adjusting for multiple testing. In the present study, a stricter significance level was used to decrease the probability of making a type I error, but remain power to detect significance for the experimental tests that typically are highly correlated.
We did only investigate the effect of three genetic variants and cannot exclude that other polymorphisms in these or other genes affect individual differences in experimental pain. However, the genetic variants studied were carefully selected based on their physiological function as well as previous research demonstrating their relationship to individual differences in experimental pain.
In conclusion, the selected genetic variants, SLC6A4 5-HTTLPR/rs25531 A>G, COMT rs4680 Val158Met and OPRM1 rs1799971 A118G, were not associated with individual differences in experimental pain.
Acknowledgments
The authors thank Magnus Dehli Vigeland for contribution to the manuscript and figures, Vibeke Siewers, Åse Kroken Paust and Kristoffer Pedersen for the data collection and follow-up of patients, the nurses at the Department of neurology for collecting blood samples, Maria Raae Andersen, Monica Wigemyr, Leif André Viken and Elena Petriu for the experimental pain testing, and Ingeborg Nymoen for laboratory work.
-
Research funding: The present study was funded by Oslo University Hospital.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: Authors state no conflict of interest.
-
Informed consent: Informed consent has been obtained from all individuals included in this study.
-
Ethical approval: The research related to human use complies with all the relevant national regulations, institutional policies and was performed in accordance with the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional review board or equivalent committee.
References
1. Andersen, S, Petersen, MW, Svendsen, AS, Gazerani, P. Pressure pain thresholds assessed over temporalis, masseter, and frontalis muscles in healthy individuals, patients with tension-type headache, and those with migraine--a systematic review. Pain 2015;156:1409–23. https://doi.org/10.1097/j.pain.0000000000000219.Search in Google Scholar PubMed
2. Staud, R, Robinson, ME, Vierck, CJJr, Cannon, RC, Mauderli, AP, Price, DD. Ratings of experimental pain and pain-related negative affect predict clinical pain in patients with fibromyalgia syndrome. Pain 2003;105:215–22. https://doi.org/10.1016/S0304-3959(03)00208-2.Search in Google Scholar PubMed
3. Fingleton, C, Smart, K, Moloney, N, Fullen, BM, Doody, C. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthr Cartil 2015;23:1043–56. https://doi.org/10.1016/j.joca.2015.02.163.Search in Google Scholar PubMed
4. Arendt-Nielsen, L, Morlion, B, Perrot, S, Dahan, A, Dickenson, A, Kress, HG, et al. Assessment and manifestation of central sensitisation across different chronic pain conditions. Eur J Pain 2018;22:216–41. https://doi.org/10.1002/ejp.1140.Search in Google Scholar PubMed
5. Yarnitsky, D, Arendt-Nielsen, L, Bouhassira, D, Edwards, RR, Fillingim, RB, Granot, M, et al. Recommendations on terminology and practice of psychophysical DNIC testing. Eur J Pain 2010;14:339. https://doi.org/10.1016/j.ejpain.2010.02.004.Search in Google Scholar PubMed
6. Lewis, GN, Rice, DA, McNair, PJ. Conditioned pain modulation in populations with chronic pain: a systematic review and meta-analysis. J Pain 2012;13:936–44. https://doi.org/10.1016/j.jpain.2012.07.005.Search in Google Scholar PubMed
7. O’Leary, H, Smart, KM, Moloney, NA, Doody, CM. Nervous system sensitization as a predictor of outcome in the treatment of peripheral musculoskeletal conditions: a systematic review. Pain Pract 2017;17:249–66. https://doi.org/10.1111/papr.12484.Search in Google Scholar PubMed
8. Yarnitsky, D, Crispel, Y, Eisenberg, E, Granovsky, Y, Ben-Nun, A, Sprecher, E, et al. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain 2008;138:22–8. https://doi.org/10.1016/j.pain.2007.10.033.Search in Google Scholar PubMed
9. Wilder-Smith, OH, Schreyer, T, Scheffer, GJ, Arendt-Nielsen, L. Patients with chronic pain after abdominal surgery show less preoperative endogenous pain inhibition and more postoperative hyperalgesia: a pilot study. J Pain Palliat Care Pharmacother 2010;24:119–28. https://doi.org/10.3109/15360281003706069.Search in Google Scholar PubMed
10. Yarnitsky, D, Granot, M, Nahman-Averbuch, H, Khamaisi, M, Granovsky, Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain 2012;153:1193–8. https://doi.org/10.1016/j.pain.2012.02.021.Search in Google Scholar PubMed
11. Nahman-Averbuch, H, Dayan, L, Sprecher, E, Hochberg, U, Brill, S, Yarnitsky, D, et al. Pain modulation and autonomic function: the effect of clonidine. Pain Med 2016;17:1292–301. https://doi.org/10.1093/pm/pnv102.Search in Google Scholar PubMed
12. Hermans, L, Calders, P, Van Oosterwijck, J, Verschelde, E, Bertel, E, Meeus, M. An overview of offset analgesia and the comparison with conditioned pain modulation: a systematic literature review. Pain Physician 2016;19:307–26.10.36076/ppj/2016.19.307Search in Google Scholar
13. Kobinata, H, Ikeda, E, Zhang, S, Li, T, Makita, K, Kurata, J. Disrupted offset analgesia distinguishes patients with chronic pain from healthy controls. Pain 2017;158:1951–9. https://doi.org/10.1097/j.pain.0000000000000989.Search in Google Scholar PubMed
14. Oudejans, LC, Smit, JM, van Velzen, M, Dahan, A, Niesters, M. The influence of offset analgesia on the onset and offset of pain in patients with fibromyalgia. Pain 2015;156:2521–7. https://doi.org/10.1097/j.pain.0000000000000321.Search in Google Scholar PubMed
15. Niesters, M, Hoitsma, E, Sarton, E, Aarts, L, Dahan, A. Offset analgesia in neuropathic pain patients and effect of treatment with morphine and ketamine. Anesthesiology 2011;115:1063–71. https://doi.org/10.1097/ALN.0b013e31822fd03a.Search in Google Scholar PubMed
16. Price, DD. Characteristics of second pain and flexion reflexes indicative of prolonged central summation. Exp Neurol 1972;37:371–87. https://doi.org/10.1016/0014-4886(72)90081-7.Search in Google Scholar PubMed
17. Price, DD, Browe, AC. Spinal cord coding of graded nonnoxious and noxious temperature increases. Exp Neurol 1975;48:201–21. https://doi.org/10.1016/0014-4886(75)90151-X.Search in Google Scholar
18. Hardy, JD, Wolff, HG, Goodell, H. Experimental evidence on the nature of cutaneous hyperalgesia. J Clin Invest 1950;29:115–40. https://doi.org/10.1172/JCI102227.Search in Google Scholar PubMed PubMed Central
19. Werner, MU, Petersen, KL, Rowbotham, MC, Dahl, JB. Healthy volunteers can be phenotyped using cutaneous sensitization pain models. PLoS One 2013;8:e62733. https://doi.org/10.1371/journal.pone.0062733.Search in Google Scholar PubMed PubMed Central
20. Mogil, JS. Pain genetics: past, present and future. Trends Genet 2012;28:258–66. https://doi.org/10.1016/j.tig.2012.02.004.Search in Google Scholar PubMed
21. Diatchenko, L, Nackley, AG, Slade, GD, Bhalang, K, Belfer, I, Max, MB, et al. Catechol-O-methyltransferase gene polymorphisms are associated with multiple pain-evoking stimuli. Pain 2006;125:216–24. https://doi.org/10.1016/j.pain.2006.05.024.Search in Google Scholar PubMed
22. Loggia, ML, Jensen, K, Gollub, RL, Wasan, AD, Edwards, RR, Kong, J. The catechol-O-methyltransferase (COMT) val158Met polymorphism affects brain responses to repeated painful stimuli. PLoS One 2011;6:e27764. https://doi.org/10.1371/journal.pone.0027764.Search in Google Scholar PubMed PubMed Central
23. Hooten, WM, Hartman, WR, Black, JL3rd, Laures, HJ, Walker, DL. Associations between serotonin transporter gene polymorphisms and heat pain perception in adults with chronic pain. BMC Med Genet 2013;14:78. https://doi.org/10.1186/1471-2350-14-78.Search in Google Scholar PubMed PubMed Central
24. Lindstedt, F. Conditioned pain modulation is associated with common polymorphisms in the serotonin transporter gene. PLoS One 2011;6:e18252. https://doi.org/10.1371/journal.pone.0018252.Search in Google Scholar PubMed PubMed Central
25. Potvin, S, Larouche, A, Normand, E, de Souza, JB, Gaumond, I, Marchand, S, et al. No relationship between the ins del polymorphism of the serotonin transporter promoter and pain perception in fibromyalgia patients and healthy controls. Eur J Pain 2010;14:742–6. https://doi.org/10.1016/j.ejpain.2009.12.004.Search in Google Scholar PubMed
26. Fjeld, O, Grotle, M, Siewers, V, Pedersen, LM, Nilsen, KB, Zwart, JA. Prognostic factors for persistent leg-pain in patients hospitalized with acute sciatica. Spine 2017;42:E272–9. https://doi.org/10.1097/BRS.0000000000001773.Search in Google Scholar PubMed
27. Fjeld, OR, Grotle, M, Matre, D, Pedersen, LM, Lie, MU, Smastuen, MC, et al. Predicting the outcome of persistent sciatica using conditioned pain modulation: 1-year results from a prospective cohort study. Scand J Pain 2019;20:69–75. https://doi.org/10.1515/sjpain-2019-0112.Search in Google Scholar PubMed
28. Lie, MU, Petriu, E, Matre, D, Hansson, P, Andersen, OK, Zwart, JA, et al. Psychophysical or spinal reflex measures when assessing conditioned pain modulation? Eur J Pain 2019;23:1879–89. https://doi.org/10.1002/ejp.1462.Search in Google Scholar PubMed
29. Lie, MU, Matre, D, Hansson, P, Stubhaug, A, Zwart, JA, Nilsen, KB. A tonic heat test stimulus yields a larger and more reliable conditioned pain modulation effect compared to a phasic heat test stimulus. Pain Rep 2017;2:e626. https://doi.org/10.1097/PR9.0000000000000626.Search in Google Scholar PubMed PubMed Central
30. Jacobsen, DP, Nielsen, MB, Einarsen, S, Gjerstad, J. Negative social acts and pain: evidence of a workplace bullying and 5-HTT genotype interaction. Scand J Work Environ Health 2018;44:283–90. https://doi.org/10.5271/sjweh.3704.Search in Google Scholar PubMed
31. Zubieta, JK, Dannals, RF, Frost, JJ. Gender and age influences on human brain mu-opioid receptor binding measured by PET. Am J Psychiatry 1999;156:842–8. https://doi.org/10.1176/ajp.156.6.842.Search in Google Scholar PubMed
32. Hasvik, E, Iordanova Schistad, E, Grovle, L, Julsrud Haugen, A, Roe, C, Gjerstad, J. Subjective health complaints in patients with lumbar radicular pain and disc herniation are associated with a sex – OPRM1 A118G polymorphism interaction: a prospective 1-year observational study. BMC Musculoskelet Disord 2014;15:161. https://doi.org/10.1186/1471-2474-15-161.Search in Google Scholar PubMed PubMed Central
33. Wang, Y-J, Huang, P, Blendy, JA, Liu-Chen, L-Y. Brain region- and sex-specific alterations in DAMGO-stimulated [(35) S]GTPγS binding in mice with Oprm1 A112G. Addict Biol 2014;19:354–61. https://doi.org/10.1111/j.1369-1600.2012.00484.x.Search in Google Scholar PubMed PubMed Central
34. Heils, A, Teufel, A, Petri, S, Stober, G, Riederer, P, Bengel, D, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem 1996;66:2621–4. https://doi.org/10.1046/j.1471-4159.1996.66062621.x.Search in Google Scholar PubMed
35. Mohammad-Zadeh, LF, Moses, L, Gwaltney-Brant, SM. Serotonin: a review. J Vet Pharmacol Ther 2008;31:187–99. https://doi.org/10.1111/j.1365-2885.2008.00944.x.Search in Google Scholar PubMed
36. Nakamura, M, Ueno, S, Sano, A, Tanabe, H. The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows ten novel allelic variants. Mol Psychiatry 2000;5:32–8. https://doi.org/10.1038/sj.mp.4000698.Search in Google Scholar PubMed
37. Lesch, KP, Bengel, D, Heils, A, Sabol, SZ, Greenberg, BD, Petri, S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996;274:1527–31. https://doi.org/10.1126/science.274.5292.1527.Search in Google Scholar PubMed
38. Jiang, JG, DeFrances, MC, Machen, J, Johnson, C, Zarnegar, R. The repressive function of AP2 transcription factor on the hepatocyte growth factor gene promoter. Biochem Biophys Res Commun 2000;272:882–6. https://doi.org/10.1006/bbrc.2000.2848.Search in Google Scholar PubMed
39. Hu, X-Z, Lipsky, RH, Zhu, G, Akhtar, LA, Taubman, J, Greenberg, BD, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet 2006;78:815–26. https://doi.org/10.1086/503850.Search in Google Scholar PubMed PubMed Central
40. Kunz, M, Hennig, J, Karmann, AJ, Lautenbacher, S. Relationship of 5-HTTLPR polymorphism with various factors of pain processing: subjective experience, motor responsiveness and catastrophizing. PLoS One 2016;11:e0153089. https://doi.org/10.1371/journal.pone.0153089.Search in Google Scholar PubMed PubMed Central
41. Lindstedt, F, Lonsdorf, TB, Schalling, M, Kosek, E, Ingvar, M. Perception of thermal pain and the thermal grill illusion is associated with polymorphisms in the serotonin transporter gene. PLoS One 2011;6:e17752. https://doi.org/10.1371/journal.pone.0017752.Search in Google Scholar PubMed PubMed Central
42. Schaldemose, EL, Horjales-Araujo, E, Demontis, D, Borglum, AD, Svensson, P, Finnerup, NB. No association of polymorphisms in the serotonin transporter gene with thermal pain sensation in healthy individuals. Mol Pain 2014;10:76. https://doi.org/10.1186/1744-8069-10-76.Search in Google Scholar PubMed PubMed Central
43. Tour, J, Lofgren, M, Mannerkorpi, K, Gerdle, B, Larsson, A, Palstam, A, et al. Gene-to-gene interactions regulate endogenous pain modulation in fibromyalgia patients and healthy controls-antagonistic effects between opioid and serotonin-related genes. Pain 2017;158:1194–203. https://doi.org/10.1097/j.pain.0000000000000896.Search in Google Scholar PubMed PubMed Central
44. Palit, S, Sheaff, RJ, France, CR, McGlone, ST, Potter, WT, Harkness, AR, et al. Serotonin transporter gene (5-HTTLPR) polymorphisms are associated with emotional modulation of pain but not emotional modulation of spinal nociception. Biol Psychol 2011;86:360–9. https://doi.org/10.1016/j.biopsycho.2011.01.008.Search in Google Scholar PubMed
45. Sprouse, JS, Aghajanian, GK. Electrophysiological responses of serotoninergic dorsal raphe neurons to 5-HT1A and 5-HT1B agonists. Synapse 1987;1:3–9. https://doi.org/10.1002/syn.890010103.Search in Google Scholar PubMed
46. Wei, H, Pertovaara, A. 5-HT(1A) receptors in endogenous regulation of neuropathic hypersensitivity in the rat. Eur J Pharmacol 2006;535:157–65. https://doi.org/10.1016/j.ejphar.2006.02.019.Search in Google Scholar PubMed
47. Lotta, T, Vidgren, J, Tilgmann, C, Ulmanen, I, Melen, K, Julkunen, I, et al. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry 1995;34:4202–10. https://doi.org/10.1021/bi00013a008.Search in Google Scholar PubMed
48. Zubieta, JK, Heitzeg, MM, Smith, YR, Bueller, JA, Xu, K, Xu, Y, et al. COMT val158Met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science 2003;299:1240–3. https://doi.org/10.1126/science.1078546.Search in Google Scholar PubMed
49. Martinez-Jauand, M, Sitges, C, Rodriguez, V, Picornell, A, Ramon, M, Buskila, D, et al. Pain sensitivity in fibromyalgia is associated with catechol-O-methyltransferase (COMT) gene. Eur J Pain 2013;17:16–27. https://doi.org/10.1002/j.1532-2149.2012.00153.x.Search in Google Scholar PubMed
50. Yao, P, Ding, YY, Wang, ZB, Ma, JM, Hong, T, Pan, SN. Effect of gene polymorphism of COMT and OPRM1 on the preoperative pain sensitivity in patients with cancer. Int J Clin Exp Med 2015;8:10036–9.Search in Google Scholar
51. Fernandez-de-Las-Penas, C, Ambite-Quesada, S, Palacios-Cena, M, Guillem-Mesado, A, Guerrero-Peral, A, Pareja, JA, et al. Catechol-O-Methyltransferase (COMT) rs4680 Val158Met polymorphism is associated with widespread pressure pain sensitivity and depression in women with chronic, but not episodic, tension-type headache. Clin J Pain 2019;35:345–52. https://doi.org/10.1097/AJP.0000000000000684.Search in Google Scholar PubMed
52. Hooten, WM, Hu, D, Cunningham, JM, Black, JL3rd. Effect of catechol-O-methyltransferase (rs4680) single-nucleotide polymorphism on opioid-induced hyperalgesia in adults with chronic pain. Mol Pain 2019;15:1744806919848929. https://doi.org/10.1177/1744806919848929.Search in Google Scholar PubMed PubMed Central
53. Kim, H, Neubert, JK, San Miguel, A, Xu, K, Krishnaraju, RK, Iadarola, MJ, et al. Genetic influence on variability in human acute experimental pain sensitivity associated with gender, ethnicity and psychological temperament. Pain 2004;109:488–96. https://doi.org/10.1016/j.pain.2004.02.027.Search in Google Scholar PubMed
54. Belfer, I, Segall, SK, Lariviere, WR, Smith, SB, Dai, F, Slade, GD, et al. Pain modality- and sex-specific effects of COMT genetic functional variants. Pain 2013;154:1368–76. https://doi.org/10.1016/j.pain.2013.04.028.Search in Google Scholar PubMed PubMed Central
55. Bond, C, LaForge, KS, Tian, M, Melia, D, Zhang, S, Borg, L, et al. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci U S A 1998;95:9608–13. https://doi.org/10.1073/pnas.95.16.9608.Search in Google Scholar PubMed PubMed Central
56. Kieffer, BL, Evans, CJ. Opioid receptors: from binding sites to visible molecules in vivo. Neuropharmacology 2009;56(1 Suppl):205–12. https://doi.org/10.1016/j.neuropharm.2008.07.033.Search in Google Scholar PubMed PubMed Central
57. Fillingim, RB. Individual differences in pain responses. Curr Rheumatol Rep 2005;7:342–7. https://doi.org/10.1007/s11926-005-0018-7.Search in Google Scholar PubMed
58. Peciña, M, Love, T, Stohler, C, Goldman, D, Zubieta, J-K. Effects of the mu opioid receptor polymorphism (OPRM1 A118G) on pain regulation, placebo effects and associated personality trait measures. Neuropsychopharmacology 2014;40:957–65. https://doi.org/10.1038/npp.2014.272.Search in Google Scholar PubMed PubMed Central
59. Hastie, BA, Riley, JL3rd, Kaplan, L, Herrera, DG, Campbell, CM, Virtusio, K, et al. Ethnicity interacts with the OPRM1 gene in experimental pain sensitivity. Pain 2012;153:1610–9. https://doi.org/10.1016/j.pain.2012.03.022.Search in Google Scholar PubMed PubMed Central
60. Leźnicka, K, Kurzawski, M, Cięszczyk, P, Safranow, K, Malinowski, D, Brzeziańska-Lasota, E, et al. Polymorphisms of catechol-O-methyltransferase (COMT rs4680:G>A) and μ-opioid receptor (OPRM1 rs1799971:A>G) in relation to pain perception in combat athletes. Biol Sport 2017;34:295–301. https://doi.org/10.5114/biolsport.2017.67856.Search in Google Scholar
61. Olesen, AE, Nielsen, LM, Feddersen, S, Erlenwein, J, Petzke, F, Przemeck, M, et al. Association between genetic polymorphisms and pain sensitivity in patients with hip osteoarthritis. Pain Pract 2017;18:587–596. https://doi.org/10.1111/papr.12648.Search in Google Scholar PubMed
62. Solak, O, Erdogan, MO, Yildiz, H, Ulasli, AM, Yaman, F, Terzi, ES, et al. Assessment of opioid receptor mu1 gene A118G polymorphism and its association with pain intensity in patients with fibromyalgia. Rheumatol Int 2014;34:1257–61. https://doi.org/10.1007/s00296-014-2995-1.Search in Google Scholar PubMed
63. Huang, P, Chen, C, Mague, SD, Blendy, JA, Liu-Chen, LY. A common single nucleotide polymorphism A118G of the μ opioid receptor alters its N-glycosylation and protein stability. Biochem J 2012;441:379–86. https://doi.org/10.1042/BJ20111050.Search in Google Scholar PubMed PubMed Central
64. Ding, N, Nie, H, Sun, X, Sun, W, Qu, Y, Liu, X, et al. Human serum N-glycan profiles are age and sex dependent. Age Ageing 2011;40:568–75. https://doi.org/10.1093/ageing/afr084.Search in Google Scholar PubMed
65. Olsen, MB, Jacobsen, LM, Schistad, EI, Pedersen, LM, Rygh, LJ, Roe, C, et al. Pain intensity the first year after lumbar disc herniation is associated with the A118G polymorphism in the opioid receptor mu 1 gene: evidence of a sex and genotype interaction. J Neurosci 2012;32:9831–4. https://doi.org/10.1523/JNEUROSCI.1742-12.2012.Search in Google Scholar PubMed PubMed Central
66. Hermans, L, Van Oosterwijck, J, Goubert, D, Goudman, L, Crombez, G, Calders, P, et al. Inventory of personal factors influencing conditioned pain modulation in healthy people: a systematic literature review. Pain Pract 2016;16:758–69. https://doi.org/10.1111/papr.12305.Search in Google Scholar PubMed
67. Kennedy, DL, Kemp, HI, Ridout, D, Yarnitsky, D, Rice, AS. Reliability of conditioned pain modulation: a systematic review. Pain 2016;157:2410–9. https://doi.org/10.1097/j.pain.0000000000000689.Search in Google Scholar PubMed PubMed Central
68. Graven-Nielsen, T, Vaegter, HB, Finocchietti, S, Handberg, G, Arendt-Nielsen, L. Assessment of musculoskeletal pain sensitivity and temporal summation by cuff pressure algometry: a reliability study. Pain 2015;156:2193–202. https://doi.org/10.1097/j.pain.0000000000000294.Search in Google Scholar PubMed
69. Pirinen, M, Donnelly, P, Spencer, CC. Including known covariates can reduce power to detect genetic effects in case-control studies. Nat Genet 2012;44:848–51. https://doi.org/10.1038/ng.2346.Search in Google Scholar PubMed
Supplementary Material
The online version of this article offers supplementary material (https://doi.org/10.1515/sjpain-2020-0091).
© 2020 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Editorial Comments
- Patients with shoulder pain referred to specialist care; treatment, predictors of pain and disability, emotional distress, main symptoms and sick-leave: a cohort study with a 6-months follow-up
- Inferring pain from avatars
- Systematic Review
- Repetitive transcranial magnetic stimulation of the primary motor cortex in management of chronic neuropathic pain: a systematic review
- Topical Reviews
- Exploring the underlying mechanism of pain-related disability in hypermobile adolescents with chronic musculoskeletal pain
- Pain management programmes via video conferencing: a rapid review
- Clinical Pain Research
- Prevalence of temporomandibular disorder in adult patients with chronic pain
- A cost-utility analysis of multimodal pain rehabilitation in primary healthcare
- Psychosocial subgroups in high-performance athletes with low back pain: eustress-endurance is most frequent, distress-endurance most problematic!
- Trajectories in severe persistent pain after groin hernia repair: a retrospective analysis
- Involvement of relatives in chronic non-malignant pain rehabilitation at multidisciplinary pain centres: part one – the patient perspective
- Observational Studies
- Recurrent abdominal pain among adolescents: trends and social inequality 1991–2018
- Cross-cultural adaptation and psychometric validation of the Hausa version of Örebro Musculoskeletal Pain Screening Questionnaire in patients with non-specific low back pain
- A proof-of-concept study on the impact of a chronic pain and physical activity training workshop for exercise professionals
- Intravenous patient-controlled analgesia vs nurse administered oral oxycodone after total knee arthroplasty: a retrospective cohort study
- Everyday living with pain – reported by patients with multiple myeloma
- Original Experimental
- The CA1 hippocampal serotonin alterations involved in anxiety-like behavior induced by sciatic nerve injury in rats
- A single bout of coordination training does not lead to EIH in young healthy men – a RCT
- Think twice before starting a new trial; what is the impact of recommendations to stop doing new trials?
- The association between selected genetic variants and individual differences in experimental pain
- Decoding of facial expressions of pain in avatars: does sex matter?
- Differences in personality, perceived stress and physical activity in women with burning mouth syndrome compared to controls
- Educational Case Reports
- Leiomyosarcoma of the small intestine presenting as abdominal myofascial pain syndrome (AMPS): case report
- Duloxetine for the management of sensory and taste alterations, following iatrogenic damage of the lingual and chorda tympani nerve
- Lead extrusion ten months after spinal cord stimulator implantation: a case report
- Short Communication
- Postoperative opioids and risk of respiratory depression – A cross-sectional evaluation of routines for administration and monitoring in a tertiary hospital
Articles in the same Issue
- Frontmatter
- Editorial Comments
- Patients with shoulder pain referred to specialist care; treatment, predictors of pain and disability, emotional distress, main symptoms and sick-leave: a cohort study with a 6-months follow-up
- Inferring pain from avatars
- Systematic Review
- Repetitive transcranial magnetic stimulation of the primary motor cortex in management of chronic neuropathic pain: a systematic review
- Topical Reviews
- Exploring the underlying mechanism of pain-related disability in hypermobile adolescents with chronic musculoskeletal pain
- Pain management programmes via video conferencing: a rapid review
- Clinical Pain Research
- Prevalence of temporomandibular disorder in adult patients with chronic pain
- A cost-utility analysis of multimodal pain rehabilitation in primary healthcare
- Psychosocial subgroups in high-performance athletes with low back pain: eustress-endurance is most frequent, distress-endurance most problematic!
- Trajectories in severe persistent pain after groin hernia repair: a retrospective analysis
- Involvement of relatives in chronic non-malignant pain rehabilitation at multidisciplinary pain centres: part one – the patient perspective
- Observational Studies
- Recurrent abdominal pain among adolescents: trends and social inequality 1991–2018
- Cross-cultural adaptation and psychometric validation of the Hausa version of Örebro Musculoskeletal Pain Screening Questionnaire in patients with non-specific low back pain
- A proof-of-concept study on the impact of a chronic pain and physical activity training workshop for exercise professionals
- Intravenous patient-controlled analgesia vs nurse administered oral oxycodone after total knee arthroplasty: a retrospective cohort study
- Everyday living with pain – reported by patients with multiple myeloma
- Original Experimental
- The CA1 hippocampal serotonin alterations involved in anxiety-like behavior induced by sciatic nerve injury in rats
- A single bout of coordination training does not lead to EIH in young healthy men – a RCT
- Think twice before starting a new trial; what is the impact of recommendations to stop doing new trials?
- The association between selected genetic variants and individual differences in experimental pain
- Decoding of facial expressions of pain in avatars: does sex matter?
- Differences in personality, perceived stress and physical activity in women with burning mouth syndrome compared to controls
- Educational Case Reports
- Leiomyosarcoma of the small intestine presenting as abdominal myofascial pain syndrome (AMPS): case report
- Duloxetine for the management of sensory and taste alterations, following iatrogenic damage of the lingual and chorda tympani nerve
- Lead extrusion ten months after spinal cord stimulator implantation: a case report
- Short Communication
- Postoperative opioids and risk of respiratory depression – A cross-sectional evaluation of routines for administration and monitoring in a tertiary hospital

